Abstract
Background
Intracranial vertebral artery dissecting aneurysms (VADAs) tend to recur despite successful stent-assisted coil embolization (SACE). Hemodynamics is useful in evaluating aneurysmal formation, growth and rupture. Our aim was to evaluate the hemodynamic patterns of VADA's recurrence.
Methods
Between September 2009 and November 2013, all consecutive patients with recurrent VADAs after SACE in our institutions were enrolled. Recurrence was defined as recanalization and/or regrowth. We assessed the hemodynamic alterations in wall shear stress (WSS) and velocity after the initial SACE and subsequently after retreatment of the aneurysms that recurred.
Results
Five patients were finally included. After the initial treatment, three cases showed recanalization and 2 cases showed regrowth. In the 2 regrew cases, the 2 original aneurysms maintained complete occlusion; however de-novo aneurysm regrowth was confirmed near the previous site. Compared with 3 recanalized aneurysms, the completely occluded aneurysms showed high mean reductions in velocity and WSS after initial treatment (77.6% versus 57.7% in velocity, 74.2% versus 52.4% in WSS), however, remaining high WSS at region near the previous lesion where the new aneurysm originated. After the second retreatment, there was no recurrence in all cases. Compared with the 3 aneurysms that recanalized, the 4 aneurysms that maintained complete occlusion showed higher reductions in velocity (62.9%) and WSS (71.1%).
Conclusion
Our series indicated that hemodynamics might have an important role in recurrence of VADAs. After endovascular treatment, sufficient hemodynamic reductions in aneurysm dome, orifice and parent vessel may be one of the key factors for preventing recurrence in VADAs.
Keywords: vertebral dissecting aneurysms, hemodynamics, recurrence, stent
Introduction
Intracranial vertebral artery dissecting aneurysms (VADAs) represent 3.3% of all intracranial aneurysms.1 VADAs' rupture, mass effect and/or posterior circulation ischemia result in high mortality and morbidity.2-4 Endovascular treatment of VADAs has been reported either by stent-assisted coil embolization (SACE) or parent artery occlusion.5-7 Although SACE in saccular aneurysms is associated with high rates of complete aneurysmal occlusion, on the contrary VADAs seem to recur and need retreatment despite initial successful occlusion.8
Computational Fluid Dynamic (CFD) is a valuable tool in evaluating aneurysmal formation, growth and rupture.9, 10 In our previous reports,11, 12 high wall shear stress (WSS) and flow velocity were found to be associated with recanalization of aneurysms embolized with coils alone. For VADAs treated with SACE, the relationship between hemodynamics and outcomes is still unclear.
The present study was designed as a self-controlled research in hemodynamic alterations of each patient after initial treatment and retreatment. It might be more interesting to explain the phenomenon that why did these VADAs recur after initial treatment and why did not after retreatment? Recurrent VADA with successful retreatment outcome is a valuable model to evaluate the hemodynamic risk factors in recurrence, which could remove confounding factors among different patients. As the strict inclusion criterion, such cases were rare and less reported in literature.
Therefore, we aimed to evaluate the correlation between hemodynamics by CFD and the incidence of recurrence in VADAs after endovascular treatment.
Material and Methods
The ethics committee of our hospital approved this study. Informed consent was obtained from each study patient.
Patient selection and geometrical reconstruction
Between September 2009 and November 2013, 112 consecutive patients presented with intracranial veterbrobasilar dissecting aneurysms underwent endovascular treatment in our hospital. Seventy-two patients were treated with SACE. Digital Subtraction Angiography (DSA) follow-up were available in 59 patients (81.9%). Recurrence was confirmed by angiographic follow-up in 10 patients (16.9%). Retreatments were needed in 6 patients with VADAs. Angiographic recurrence was defined as a substantial increase in the contrast medium–filled portion of the dissecting aneurysm compared with a control angiogram taken immediately after the initial treatment.8 Recurrences of dissecting aneurysms were divided into i) recanalization and ii) regrowth.13 Recanalization means opening of the previously embolized dissecting aneurysms, which has the same size, but the coils have been moved away from the original site due to blood flow and consequent compaction. Regrowth signifies that the dissecting aneurysm has become larger and the coil mass is no longer sufficient to obliterate it.13, 14 Extent of occlusion and criteria for recurrence were evaluated by three neuroradiologists at our institution. All the measurement and evaluation were made by DSA images.
The 3-dimesional (3D) DSA images of these 6 cases were collected and reviewed. One case was excluded from current study because of inadequate 3D image quality for CFD analysis. Finally, there were 5 cases included in our present hemodynamic study, demonstrated in Table 1. Enterprise stents (CORDIS ENTERPRISE™ Vascular Reconstruction Device; Cordis Neurovascular, Miami, FL, USA) were used in all these cases. For case 5, the retreatment was performed with two more stents in attempt to reconstruct the vessel lumen. However, the follow up after retreatment showed parent artery occlusion without any adverse event. Multiple factors, like in-stent thrombosis or platelet inhibition may be involved in parent vessel occlusion after retreatment. Therefore, we only performed simulation of initial treatment for case 5 without retreatment simulation.
Table 1.
The information of initial endovascular treatment, retreatment, angiographic follow up and straightening of vessels.
| Patient NO. | Initial treatment modality | Immediate angiographic result | Angiographic FU/Time (mons) | Retreatment method | Immediate result of retreatment | Final angiographic FU/Time (mons) | Straightening angle in parent artery (°) |
|---|---|---|---|---|---|---|---|
| 1 | SACE | NC | Recurrence/8 | SACE | CO | CO/10 | 37.16 |
| 2 | SACE | CO | Recurrence/7 | SACE | NC | CO/4 | 19.14 |
| 3 | SACE | NC | Recurrence/3 | Coiling | CO | CO/7 | 43.98 |
| 4 | SACE | PO | Recurrence/6 | SACE | NC | CO/11 | 17.74 |
| 5 | SACE | PO | Recurrence/5 | DS | CMR | PAO/11 | 15.23 |
SACE= stent-assisted coiling embolization; NC= near complete occlusion; CO= complete occlusion; PO= partial occlusion; FU= follow up; DS= double stents; CMR= contrast medium retention; PAO= parent artery occlusion
3D aneurysm geometries both before the initial treatment and retreatment were obtained from DSA images. 3D geometry surface displayed at an isosurface with a mean threshold value of 1300 Hounsfield unit (H). The threshold values before and after treatment were approximately similar for every patient. We firstly segmented and globally surface smoothed the images using Geomagic Studio version 12.0 (Geomagic, Research Triangle Park, NC) and saved the surface geometries as standard tessellation language (STL) format.15 Using virtual stent deployment in the parent artery16 and porous media method in the aneurysm sac,15,17 the post-treatment and post-retreatment aneurysm models were created for each case. The parameters setting in coil modeling were calculated based on the clinical coil usage. The volume of the coil was calculated, and the algebraic equation was as follows: volume of the coil= π × (diameter of coil/2)2 × the length of the coil. Packing density was defined as the ratio between the volume of the coils and volume of the aneurysms.18 Thus, we created four models for case 1, 2, 3 and 4: pre-treatment model, post-treatment model, pre-retreatment model and post-retreatment model. Three models, excluding post-retreatment model, were created for case 5.
CFD simulations and hemodynamic analysis
The virtual stent was merged with the aneurysm geometry in ICEM CFD version 14.0 (ANSYS Inc., Canonsburg, PA) to create finite volume tetrahedral elements for CFD simulation. The largest element size was 0.2mm and the element size on stent was set to be 0.025mm in order to sufficiently present the stent geometry, which was approximately 1/3 of the width of the strut of the Enterprise stent (0.078 mm; Cordis Neurovascular, Miami, FL, USA).15, 19 Mesh sizes ranged between 1.29 and 12.72 million elements in present study. ANSYS CFX 14.0 (ANSYS Inc., Canonsburg, Pa., USA) was then used to solve the flow governing Navier-Strokes equations with the assumption of laminar, incompressible and Newtonian blood flow. The density and dynamic viscosity of blood were specified as 1060 kg/m3 and 0.004 N·s/m2, respectively. The blood vessel wall was assumed to be rigid with no-slip boundary conditions. We used a pulsatile velocity profile obtained by Transcranial Doppler from a normal subject for the inlet flow conditions. The flow waveforms were scaled to achieve a mean inlet WSS of 15 dyne/cm under pulsatile conditions.15 Three cardiac cycle simulations were performed for numerical stability and the last cardiac cycle was collected as output. We then post-processed and visualized the results of these simulations with CFX post-processing tool. The spatial-averaged WSS at peak systole at aneurysm wall was calculated for each model of each case. The intra-aneurysmal flow velocity was also calculated at the peak systole and illustrated on a cutting plane across aneurysm and parent vessel. The flow reduction ratio is defined as (pre-treatment parameter- post-treatment parameter)/pre-treatment parameter. Angle measurements were performed as described by Kono et al.19 for evaluating the straightening of parent vessel.
Results
Hemodynamic alterations are shown in Table 2.
Table 2.
Hemodynamic alterations in VADAs after initial endovascular treatment and retreatment.
| Patient NO. | RR in WSS after IT (%) | RR in velocity after IT (%) | RR in WSS after RT (%) | RR in velocity after RT (%) |
|---|---|---|---|---|
| 1 | 63.4 | 63.5 | 59.3 | 50.0 |
| 2 | 84.9 | 91.6 | 88.4 | 76.1 |
| 3 | 65.8 | 62.8 | 61.8 | 56.3 |
| 4 | 54.5 | 53.9 | 74.8 | 69.2 |
| 5 | 37.0 | 56.4 | - | - |
VADAs= vertebral artery dissecting aneurysms; RR= reduction rate; IT= initial treatment; RT= retreatment.
At a mean follow up of 5.8 months after initial treatment, three cases (case 3, 4 and 5) showed recanalization and 2 cases showed regrowth (case 1 and 2). In case 1 and 2, although the 2 original aneurysms maintained complete occlusion, de-novo aneurysm regrowth was confirmed near the previous site in both cases (Figure 1 C). Compared with 3 recanalized aneurysms (case 3, 4 and 5), the 2 complete occluded aneurysms in the 2 regrew cases (case 1 and 2) showed much higher mean reductions in velocity and WSS after initial treatment (77.6% versus 57.7% in velocity, 74.2% versus 52.4% in WSS), however, remaining high WSS at region near the previous lesion where new aneurysm initiated (Figure 1 F and J).
Figure 1.
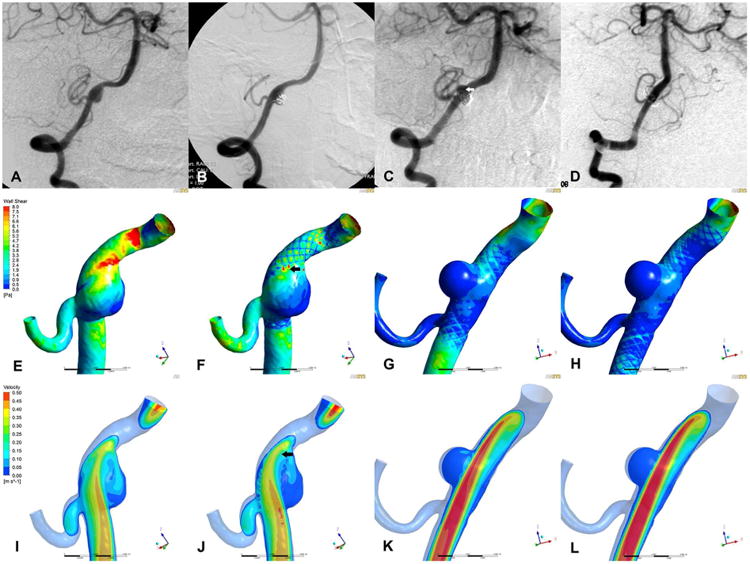
Angiograms and corresponding illustrations of hemodynamic characteristics in case 1.
A series of angiograms (A-D) were showed in the first row and corresponding hemodynamic results were demonstrated in the last two rows (WSS in middle row, E-H; velocity in the last row, I-L). The results of pre-treatment (A, E and I), post-treatment (B, F and J), pre-retreatment (C, G and K) and post-retreatment (D, H and L) models were listed in four columns from left to right. The original aneurysm was completely occluded with SAC (B) and maintained stable at 8 months follow up (C). However, a new aneurysm was found initiating from a different location near previous site (C, arrow). Retreatment was performed and complete occlusion was achieved (D). On the hemodynamic view, although the WSS and velocity was decreased in aneurysm after initial treatment (F and J), localized high WSS and flow impact were remained at parent vessel (F and J, arrows), and new aneurysm just grew at this region (C and F, arrows). After retreatment, both aneurysm and parent vessel showed further decrease in WSS (H).
After retreatment, there was no recurrence in all cases (mean follow-up of 8.6 months). The retreatment consisted in complete occlusion in case 1, 2, 3 and 4; parent artery occlusion in case 5. Compared with the 3 aneurysms recanalized after initial treatment, the 4 aneurysms that maintained complete occlusion after retreatment showed higher reductions in velocity (62.9% versus 57.7%) and WSS (71.1% versus 52.4%) after retreatment. Meanwhile, the parent vessels covered by stents also showed much lower WSS without significant high WSS region after retreatment.
Case 1 and 2
Regrowth was confirmed in both cases at angiographic follow up after initial SACE. The de-novo aneurysm was found initiating from a different location near previous site. According to our simulation result, after initial SACE, the WSS and flow velocity were markedly decreased in both original aneurysms without any flow impact on aneurysm wall under the effect of stent and coils (Figure 1 F and J). The reduction rates of both parameters were 63.4% and 63.5% in case 1, and 84.9% and 91.6% in case 2 (Table 2). However, localized high WSS and flow impact were remained at parent vessel in both cases after stent deployment. Matched between WSS contour map and angiographic image, the new lesions just grew at such regions (Figure 1 F and J, arrows). The WSS and velocity at this region were 4.2 and 5.8 times higher than the mean values at parent artery in case 1 (8.91 versus 2.10 Pa and 0.0554 versus 0.0096 m/s, respectively), and those parameters were 2.0 and 3.5 times higher than those at parent artery in case 2 (9.49 versus 4.85 Pa and 0.0811 versus 0.0232 m/s, respectively). After retreatment, both de-novo aneurysms showed much lower WSS and velocity (Figure 1 H and L). The reduction rates of WSS in de-novo aneurysms were 59.3% in case 1 and 88.4% in case 2, and the reduction rates of velocity in de-novo aneurysms were 50.0% and 76.1% respectively. Additionally, the parent arteries covered by stents also showed significant lower WSS after retreatment.
Case 3
A 29 years patient underwent SACE of a right VADA. Angiographic follow up showed a stable occlusion after retreatment. Before initial treatment, the high WSS and concentrated flow jet could be found at inflow zone near the aneurysm neck (Figure 2 E and I). After initial SACE, the WSS at aneurysm dome was significantly reduced (65.8%), however, there was remaining high WSS near the neck (Figure 2 F, arrow). Intra-aneurysmal flow velocity was greatly reduced by coil mass (62.8%). The inflow jet near the aneurysm neck was high after stent placement (Figure 2 J, arrow). Although the reductions in WSS and velocity of aneurysm were 65.8% and 62.8% respectively, no obvious flow diversion could be observed at neck area after initial treatment. After retreatment, both parameters were further decreased (61.8% and 56.3% respectively) and no inflow jet could be found near the neck (Fig. 2 H and L). The outcome of retreatment was stable maintaining complete occlusion at last follow up.
Figure 2.
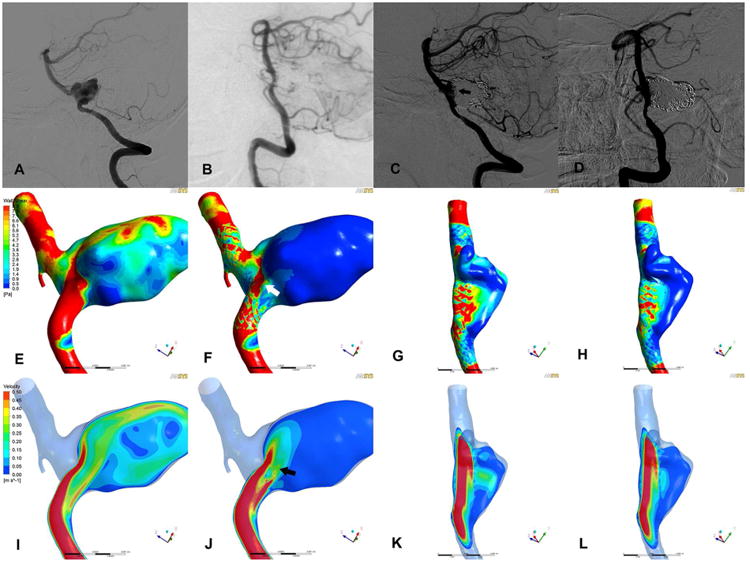
Angiograms and corresponding illustrations of hemodynamic characteristics in case 3.
The results of pre-treatment (A, E and I), post-treatment (B, F and J), pre-retreatment (C, G and K) and post-retreatment (D, H and L) were demonstrated in four columns. A series of angiograms (A-D) were showed in the first row and corresponding hemodynamic results were demonstrated in the last two rows (WSS in middle row, E-H; velocity in the last row, I-L). The aneurysm was completely occluded after initial treatment (B). However, recanalization was confirmed at 3 months follow up (C, arrow). In simulation results, the WSS and velocity in aneurysm were significantly reduced (F and J) after initial treatment, however, remaining high WSS and inflow jet near the neck (F and J, arrows) that were associated with recanalization. After retreatment, no inflow jet could be found near the neck (H and L)
Case 4
A 49 years patient underwent SACE of a right VADA. The aneurysm still maintained incomplete occlusion at follow up angiography. Retreatment was performed with additional SACE and the lesion was completely occluded. The velocity distribution on a cutting plane before treatment showed the inflow jet directly impact on the distal portion of aneurysm wall where the WSS was high. The intra-aneurysmal blood flow was reduced after the initial SACE. However, the reductions were not significant compared with reductions after retreatment (54.5% and 53.9%). After retreatment, the WSS and flow velocity in aneurysm were markedly reduced (74.8% and 69.2%) and no inflow impingement was found in aneurysm sac. The last angiographic follow-up showed the aneurysm was complete occlusion and parent vessel was successfully reconstructed.
Case 5
A 53 years patient underwent SACE of a left VADA. The aneurysm was still not occluded at follow up after initial treatment and parent vessel occlusion was found at second follow up after additional stents deployment. Before treatment, a concentrated inflow jet could be observed on the cutting plane and directly impacted on the distal portion of aneurysm where the WSS was relatively high compared to entire aneurysm. After initial treatment, the main stream of inflow jet was redirected back to the parent artery, however still remaining flow impingement. The flow reductions in WSS and velocity in aneurysm were only 37.0% and 56.4% respectively. At angiographic follow up, the inflow stream was still observed at residual sac without complete occlusion.
Additionally, the straightening of parent vessel could be observed in all cases on the follow up angiogram after SACE. Stent placement straightened vessels by mean 26.65° at mean 5.8 months after treatment.
Discussion
In the present analysis, high WSS and flow velocity in the aneurysmal wall or in the parent artery segment close to the aneurysm were associated with recurrence of VADAs after SACE. Reductions in those factors at the level of the diseased vessel segment seem to be associated with stable occlusion.
Hemodynamic analysis by CFD is helpful in evaluating aneurysmal formation, growth and rupture.9, 10 However, there are limited data on the correlation between hemodynamics and recurrence of VADAs after SACE.19-21 A CFD study 21 reported a patient presented with subarachnoid hemorrhage resulting from bilateral VADAs. Preoperative simulations were performed to predict the rupture side and estimate the increase in WSS on an aneurysm in case of trapping of contralateral one, which might cause further enlargement or rupture. However, the treatment strategy of SACE was not simulated for analysis. We have previously reported that the high WSS and flow velocity were associated with aneurysm recurrence after endovascular treatment.11, 12 However, all aneurysms in those studies were not VADA and were not treated with SACE. In another study,19 CFD was performed on 16 vertebral artery aneurysms treated with SACE and followed with conventional catheter angiography. The authors compared the hemodynamic effect of stent struts by straightening the vessel and therefore reducing flow velocity in aneurysms. However, the hemodynamic effect of coils was not evaluated in their study. In the present study, we performed CFD simulations to evaluate the different outcomes after initial treatment and retreatment in the same patient, which could eliminate variability among individuals.
Techniques in stent and coil simulations
In addition to the patient-specific aneurysm models used in this study, we also developed a novel virtual stenting workflow to simulate the intracranial stent deployment16 and used the porous media method to simulate coil embolization.17 With all these techniques, the hemodynamic simulation could be more accurate and our results may provide helpful information in successful management of such lesions. With development of CFD techniques, more and more researchers estimated the hemodynamic effect of intracranial stent or coils using different modeling techniques.17, 19, 22-27 However, the simulation results may be influenced by methodological diversity. In the study by Tremmel et al,26 the stent was conformed to fit into parent vessel and deployed across aneurysm neck, which was thought to be less accurate in simulations compared with real status of stent deployment. Some other studies evaluated hemodynamic alterations by stent placement in animal models and the stent geometry was reconstructed using micro-CT.22, 27 Such stent model was much more accurate, however, it was less likely to apply to large cohort study. Used in our present study, the virtual stenting technique might be a better option for CFD simulation of aneurysm outcome after SACE.
In addition to stent, coil is another factor that significantly influences on the intra-aneurysmal flow condition. However, some CFD researches evaluated hemodynamic effect on aneurysm outcome; they did not include modeling of the coils into simulation.11, 12, 19 On the other hand, some studies assessed hemodynamic effect of coils in aneurysm sac.17, 23, 24 However, in those studies, the relationship between such effect of coils and aneurysm outcome was unknown. In our present study, the coiled aneurysm sac was modeled as a porous medium region of which the porosity and permeability were calculated from a series of coils used in clinical practice. As long as we know, the present study was the first hemodynamic research of aneurysm outcome using advanced combined stent and coil simulation techniques.
Hemodynamics of VADAs recurrence
As demonstrated in Table 2 and figures, after initial treatment, the reductions in WSS and flow velocity in aneurysm were insufficient (case 4 and 5) or high WSS and flow impingement remained at some region of diseased vessel (case 1 and 2) or aneurysm orifice (case 3). The angiographic follow up of these cases showed recurrence. On the contrary, after retreatment, the WSS and flow velocity in aneurysms were further decreased and the direct inflow jet was redirected with additional stent and coils. The follow up after retreatment confirmed stable outcome. Although the different retreatment methods were used, the homogeneous alterations in hemodynamics were induced, which lead to further stable outcome. Our results indicated that hemodynamic reduction was the key point in outcome no matter what method used in treatment. The self-controlled comparison might eliminate the confounding factors among individuals and make the results more accurate. In addition to stent struts and coil mass, the straightening of vessel caused by stent placement could also effect on intra-aneurysmal flow condition.19 Other groups also revealed that stent-induced angular remodeling significant altered perianeurysmal hemodynamics.28, 29 In our study, the straightening of vessel was also observed in all cases with a mean of 26.65 degrees. Combined with stent struts, coils and straightening of vessels, our results suggested that sufficient reductions in WSS and flow velocity in aneurysms were benefit for outcome of VADAs after SACE.
The potential hemodynamic mechanism of recurrence was complex and still unclear. The regions of elevated WSS and blood flow were thought to cause endothelia degeneration and vessel wall dilatation and predisposed the vessel to further aneurysm initiation or growth.9, 30 Meanwhile, the remaining high flow impact at aneurysm neck could induce coil mass compaction or persist unocclusion under long term flow impingement,31 which presented as recanalization or incomplete occlusion at angiographic follow up.
Limitations
In our study, the case number was small. As we demonstrated, the inclusion criterion was strict and such cases were rare in clinical practice. We tried to compare the hemodynamic alterations caused by initial treatment and retreatment in the same patient as self-control. Therefore, we thought our result could still provide valuable information in reducing recurrence rate of VADAs in clinical practice without including other controls. Larger study from multicenter database was still needed in the future. Additionally, our results might not be applicable to aneurysms at other locations because our cases were all VADAs. Rigid wall, laminar flow, general boundary conditions and Newtonian blood assumptions were used in our CFD simulations. Such assumptions might introduce bias and patient-specific settings would make more accurate results. The geometrical structure of the Enterprise stent struts was simplified as approximation of actual stent and the porous media method was used to mimic an ideal coil mass without actual coil configuration. The angle measurement might introduce bias. Other factors, such as pathological factors, are also likely to be involved in the process of VADAs recurrence after SAC. The hemodynamics could not fully explain the mechanism of recurrence. The definition and evaluation of recurrence might introduce bias and the pathology might have different ways among recanalization, regrowth and de-novo aneurysm growth. Multidisciplinary study is needed in the future.
Conclusion
To the best of our knowledge, this is the first study to describe the relationship between hemodynamics and outcome in VADAs. Our series seems to indicate that hemodynamics may have an important role in recurrence of VADAs. After endovascular treatment, sufficient hemodynamic reductions in aneurysm dome, orifice and parent vessel may be one of the key factors for preventing recurrence in VADAs. Larger studies from multicenter database are needed.
Supplementary Material
Highlights.
The relationship between hemodynamics and outcomes were demonstrated within a series of vertebral artery dissecting aneurysms treated with stent-assisted coiling embolization.
The present study was designed as a self-controlled research in hemodynamic alterations of each patient after initial treatment and retreatment.
The present study was the first hemodynamic research of aneurysm outcome using advanced combined virtual stenting and coil simulation techniques.
After endovascular treatment, sufficient hemodynamic reductions in aneurysm dome, orifice and parent vessel may be one of the key factors for preventing recurrence in VADAs.
Acknowledgments
None.
Funding: This work was supported by National Natural Science Foundation of China (grant 81301003, 81171079, 81371315, 81471167 and 81220108007), Special Research Project for Capital Health Development (Grant No.2014-1-1071) and National Institutes of Health (R01 NS091075).
Abbreviations
- VADAs
vertebral artery dissecting aneurysms
- SACE
stent-assisted coil embolization
- WSS
wall shear stress
- CFD
computational fluid dynamic
- DSA
digital subtraction angiography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Santos-Franco JA, Zenteno M, Lee A. Dissecting aneurysms of the vertebrobasilar system. A comprehensive review on natural history and treatment options. Neurosurg Rev. 2008;31:131–140. doi: 10.1007/s10143-008-0124-x. discussion 140. [DOI] [PubMed] [Google Scholar]
- 2.Jin SC, Kwon DH, Choi CG, Ahn JS, Kwun BD. Endovascular strategies for vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. 2009;30:1518–1523. doi: 10.3174/ajnr.A1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park KW, Park JS, Hwang SC, Im SB, Shin WH, Kim BT. Vertebral artery dissection: Natural history, clinical features and therapeutic considerations. J Korean Neurosurg Soc. 2008;44:109–115. doi: 10.3340/jkns.2008.44.3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995;36:905–911. doi: 10.1227/00006123-199505000-00003. discussion 912-903. [DOI] [PubMed] [Google Scholar]
- 5.Peluso JP, van Rooij WJ, Sluzewski M, Beute GN, Majoie CB. Endovascular treatment of symptomatic intradural vertebral dissecting aneurysms. AJNR Am J Neuroradiol. 2008;29:102–106. doi: 10.3174/ajnr.A0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rho MH, Park HJ, Chung EC, Choi YJ, Lee SY, Won YS, et al. Various techniques of stent-assisted coil embolization of wide-necked or fusiform artherosclerotic and dissecting unruptured vertebrobasilar artery aneurysms for reducing recanalization: Mid-term results. Acta Neurochir (Wien) 2013;155:2009–2017. doi: 10.1007/s00701-013-1866-y. [DOI] [PubMed] [Google Scholar]
- 7.Suh SH, Kim BM, Park SI, Kim DI, Shin YS, Kim EJ, et al. Stent-assisted coil embolization followed by a stent-within-a-stent technique for ruptured dissecting aneurysms of the intracranial vertebrobasilar artery. Clinical article. J Neurosurg. 2009;111:48–52. doi: 10.3171/2009.2.JNS081418. [DOI] [PubMed] [Google Scholar]
- 8.Kim BM, Shin YS, Kim SH, Suh SH, Ihn YK, Kim DI, et al. Incidence and risk factors of recurrence after endovascular treatment of intracranial vertebrobasilar dissecting aneurysms. Stroke. 2011;42:2425–2430. doi: 10.1161/STROKEAHA.111.617381. [DOI] [PubMed] [Google Scholar]
- 9.Meng H, Wang Z, Hoi Y, Gao L, Metaxa E, Swartz DD, et al. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke. 2007;38:1924–1931. doi: 10.1161/STROKEAHA.106.481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang J, Natarajan SK, Tremmel M, Ma D, Mocco J, Hopkins LN, et al. Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke. 2011;42:144–152. doi: 10.1161/STROKEAHA.110.592923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Wang S, Chen J, Yu H, Zhang Y, Jiang F, et al. Influence of hemodynamics on recanalization of totally occluded intracranial aneurysms: A patient-specific computational fluid dynamic simulation study. J Neurosurg. 2012;117:276–283. doi: 10.3171/2012.5.JNS111558. [DOI] [PubMed] [Google Scholar]
- 12.Luo B, Yang X, Wang S, Li H, Chen J, Yu H, et al. High shear stress and flow velocity in partially occluded aneurysms prone to recanalization. Stroke. 2011;42:745–753. doi: 10.1161/STROKEAHA.110.593517. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Wang Y, Li C, Wang Y, Mu S, Yang X. Retreatment and outcomes of recurrent intracranial vertebral artery dissecting aneurysms after stent assisted coiling: A single center experience. PloS One. 2014;9:e113027. doi: 10.1371/journal.pone.0113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Islak C. The retreatment: Indications, technique and results. Eur J Radiol. 2013;82:1659–1664. doi: 10.1016/j.ejrad.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Jing L, Wang C, Paliwal N, Wang S, Zhang Y, et al. Effect of hemodynamics on outcome of subtotally occluded paraclinoid aneurysms after stent-assisted coil embolization. J Neurointerv Surg. 2015 doi: 10.1136/neurintsurg-2015-012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paliwal N, Yu H, Xu J, Xiang J, Siddiqui AH, Yang X, et al. Virtual stenting workflow with vessel-specific initialization and adaptive expansion for neurovascular stents and flow diverters. Comput Methods Biomech Biomed Engin. 2016:1–9. doi: 10.1080/10255842.2016.1149573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang SZY, Lu G, Yang X, Zhang X, Ding G. Hemodynamic performance of coil embolization and stentassisted coil embolization treatments: A numerical comparative study based on subject-specific models of cerebral aneurysms. Sci China Phys Mech Astron. 2011;54:2053–2063. [Google Scholar]
- 18.Jing L, Zhong J, Liu J, Yang X, Paliwal N, Meng H, et al. Hemodynamic effect of flow diverter and coils in the treatment of large and giant intracranial aneurysms. World Neurosurg. 2016;89:199–207. doi: 10.1016/j.wneu.2016.01.079. [DOI] [PubMed] [Google Scholar]
- 19.Kono K, Shintani A, Terada T. Hemodynamic effects of stent struts versus straightening of vessels in stent-assisted coil embolization for sidewall cerebral aneurysms. PloS One. 2014;9:e108033. doi: 10.1371/journal.pone.0108033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jou LD, Wong G, Dispensa B, Lawton MT, Higashida RT, Young WL, et al. Correlation between lumenal geometry changes and hemodynamics in fusiform intracranial aneurysms. AJNR Am J Neuroradiol. 2005;26:2357–2363. [PMC free article] [PubMed] [Google Scholar]
- 21.Kono K, Shintani A, Fujimoto T, Terada T. Stent-assisted coil embolization and computational fluid dynamics simulations of bilateral vertebral artery dissecting aneurysms presenting with subarachnoid hemorrhage: Case report. Neurosurgery. 2012;71:E1192–1200. doi: 10.1227/NEU.0b013e318270603a. discussion E1200-1191. [DOI] [PubMed] [Google Scholar]
- 22.Huang Q, Xu J, Cheng J, Wang S, Wang K, Liu JM. Hemodynamic changes by flow diverters in rabbit aneurysm models: A computational fluid dynamic study based on micro-computed tomography reconstruction. Stroke. 2013;44:1936–1941. doi: 10.1161/STROKEAHA.113.001202. [DOI] [PubMed] [Google Scholar]
- 23.Morales HG, Kim M, Vivas EE, Villa-Uriol MC, Larrabide I, Sola T, et al. How do coil configuration and packing density influence intra-aneurysmal hemodynamics? AJNR Am J Neuroradiol. 2011;32:1935–1941. doi: 10.3174/ajnr.A2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schirmer CM, Malek AM. Critical influence of framing coil orientation on intra-aneurysmal and neck region hemodynamics in a sidewall aneurysm model. Neurosurgery. 2010;67:1692–1702. doi: 10.1227/NEU.0b013e3181f9a93b. discussion 1702. [DOI] [PubMed] [Google Scholar]
- 25.Shobayashi Y, Tateshima S, Kakizaki R, Sudo R, Tanishita K, Vinuela F. Intra-aneurysmal hemodynamic alterations by a self-expandable intracranial stent and flow diversion stent: High intra-aneurysmal pressure remains regardless of flow velocity reduction. J Neurointerv Surg. 2013;5(3):iii38–42. doi: 10.1136/neurintsurg-2012-010488. [DOI] [PubMed] [Google Scholar]
- 26.Tremmel M, Xiang J, Natarajan SK, Hopkins LN, Siddiqui AH, Levy EI, et al. Alteration of intra-aneurysmal hemodynamics for flow diversion using enterprise and vision stents. World Neurosurg. 2010;74:306–315. doi: 10.1016/j.wneu.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Deng B, Fang Y, Yu Y, Cheng J, Wang S, et al. Hemodynamic changes caused by flow diverters in rabbit aneurysm models: Comparison of virtual and realistic fd deployments based on micro-ct reconstruction. PloS One. 2013;8:e66072. doi: 10.1371/journal.pone.0066072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao B, Baharoglu MI, Cohen AD, Malek AM. Y-stent coiling of basilar bifurcation aneurysms induces a dynamic angular vascular remodeling with alteration of the apical wall shear stress pattern. Neurosurgery. 2013;72:617–629. doi: 10.1227/NEU.0b013e3182846d9f. discussion 628-619. [DOI] [PubMed] [Google Scholar]
- 29.Gao B, Baharoglu MI, Malek AM. Angular remodeling in single stent-assisted coiling displaces and attenuates the flow impingement zone at the neck of intracranial bifurcation aneurysms. Neurosurgery. 2013;72:739–748. doi: 10.1227/NEU.0b013e318286fab3. discussion 748. [DOI] [PubMed] [Google Scholar]
- 30.Chatziprodromou I, Tricoli A, Poulikakos D, Ventikos Y. Haemodynamics and wall remodelling of a growing cerebral aneurysm: A computational model. J Biomech. 2007;40:412–426. doi: 10.1016/j.jbiomech.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Kai Y, Hamada J, Morioka M, Yano S, Kuratsu J. Evaluation of the stability of small ruptured aneurysms with a small neck after embolization with guglielmi detachable coils: Correlation between coil packing ratio and coil compaction. Neurosurgery. 2005;56:785–792. doi: 10.1227/01.neu.0000156790.28794.ea. discussion 785-792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


