Abstract
Defense elicitors are products that activate acquired defense responses in plants, thus rendering the plants less susceptible to attack by a broad range of pests. We demonstrated previously under laboratory conditions that foliar applications of the defense elicitors Actigard (acibenzolar-S-methyl), Employ (harpin protein), or ODC (chitosan) to potted pear trees (Pyrus communis L.) each caused an increase in mortality of Cacopsylla pyricola (Förster) (Hemiptera: Psyllidae) nymphs and altered the settling and oviposition behavior of the adults. In this study, we monitored C. pyricola populations over a 3-yr period on orchard-grown trees treated with water (untreated control), Actigard, Employ, or ODC. Fewer nymphs were observed on trees treated with elicitors compared with untreated trees in both 2014 and 2016. A similar but statistically nonsignificant pattern was observed in 2015 when nearly 30% fewer nymphs were observed on trees treated with elicitors versus untreated controls. Observed reductions in psyllid numbers by defense elicitors were modest and do not warrant the use of these products alone for managing C. pyricola. However, these products are often used for management of fire blight, and our observations that elicitors also reduce C. pyricola populations may be useful for system-wide integrated pest management approaches.
Keywords: systemic acquired resistance, induced defense, salicylic acid, phloem-feeder
Plants challenged by pathogens or insects often respond with an increase in chemical and structural defenses. These acquired defenses are dependent in part upon either jasmonate or salicylate signaling compounds and provide plants with broad-spectrum protection against subsequent pest attack (Stout 2007). Acquired defenses can also be artificially activated by treating plants with chemical elicitors. In fact, several chemical compounds identified as defense elicitors are active ingredients of commercial products marketed for the management of plant pathogens (Reignault and Walters 2007). Actigard (Syngenta, Wilmington, DE) contains acibenzolar-S-methyl (ASM), which is a functional analogue of the defense signaling compound salicylic acid. Employ (Plant Health Care, Pittsburgh, PA) contains a harpin protein of the fire blight pathogen, Erwinia amylovora (Burrill), and elicits endogenous production of salicylic acid in plants. The product ODC (AgriHouse Brands, Berthoud, CO) contains chitosan, which is a component of fungal cell walls. All three products activate defense responses associated with salicylate-dependent defenses and render plants less susceptible to a broad range of biotrophic pathogens (Reignault and Walters 2007).
Although commercial elicitors of salicylate-dependent defenses are marketed for control of pathogens, plant responses to treatment with active components of these products have been found to reduce performance of certain insects. For example, ASM, harpin protein, and chitosan each elicit plant defenses that reduce populations of phloem feeding insects including aphids (Hemiptera: Aphididae) and psyllids (Hemiptera: Psyllidae) (Cooper et al. 2004, Dong et al. 2004, Boughton et al. 2006, Li et al. 2006, Gao et al. 2007, Avila et al. 2012, Zhang et al. 2012, Cooper and Horton 2015). It is therefore possible that the use of defense elicitors to control plant pathogens in crops may also contribute to integrated pest management of certain insects.
Pear psylla, Cacopsylla pyricola (Förster) (Hemiptera: Psyllidae) is an important pest of pear (Pyrus communis L.; Rosaceae) in North American and Europe. This psyllid causes economic losses in pear primarily by producing copious amounts of sticky honeydew, which russets fruit, promotes the growth of sooty mold, and complicates manual harvest of fruit (Westigard and Zwick 1972). Management tactics vary considerably among growers and regions, but typically involve several applications of dormant oil to reduce or delay oviposition by overwintered females, and several applications of neonicotinoids, pyrethroids, insect growth regulators, or kaolin throughout the season. Our recent study (Cooper and Horton 2015) demonstrated under laboratory conditions that applications of Actigard, Employ, or ODC to small (<1-m tall) potted pear trees each led to reduced survival of the pear psylla. Nymphal mortality was about 20–30% greater on treated trees than on untreated trees, and adults preferred to settle and oviposit on untreated trees compared with treated trees in choice assays (Cooper and Horton 2015). Moreover, the plant responses elicited by these products were systemic, in that they led to reduced psyllid performance even on leaves that had been shielded from foliar applications. The mechanisms responsible for reduced psylla performance on trees treated with elicitors are not currently understood, but may involve both antixenotic and antibiotic factors (Cooper and Horton 2015). Although results of this previous study demonstrated that defense elicitors reduce psyllid numbers on potted trees (Cooper and Horton 2015), it is as yet unknown whether these elicitors would lead to reduced psyllid numbers on large orchard-grown trees.
Both Actigard and Employ applied at bloom are often used to control fire blight in pear orchards (Brisset et al. 2000, Balajoo et al. 2012). The use of these products against fire blight may also contribute to the integrated pest management of pear psylla if they are found to be effective against this pest. We tested the effects of foliar applications of Actigard, Employ, and ODC on wild C. pyricola populations in a 3-yr study and report that the use of these products is associated with modest reductions in C. pyricola nymphs and adults.
Materials and Methods
Foliar Applications
Experiments were conducted over three growing seasons (2014–2016) in a Bartlett pear orchard located at the USDA-ARS experimental farm near Moxee, WA. The orchard was planted in 2001 with 5-m × 5-m spacing. The orchard was divided into six plots, each separated by a row of untreated trees. Each plot included four experimental trees separated by at least one untreated tree, and each tree within a block was randomly assigned a foliar treatment: Employ, Actigard, ODC, or untreated control. Trees received the same treatment assignments each of the 3 yrs of the study. Foliar treatments were applied according to the product labels (Table 1) using a gas-powered backpack sprayer (model SR420, Stihl Inc., Virginia Beach, VA). Spray volumes were estimated using spray times with a sprayer rate of 32 ml/second. Treatments were applied every 4 wks according to the product labels. In 2014, treatments were applied on 10 April, 8 May, 5 June, and 3 July. In 2015, treatments were applied on 16 April, 16 May, 11 June, and 9 July. In 2016, treatments were applied on 14 April, 12 May, 9 June, and 7 July.
Table 1.
Foliar application rates of defense elicitors
| Treatment | Product label rate1 Product/0.4 ha | Mix rate2 | Application volume3 |
|---|---|---|---|
| Control | N/A | Water | 1 L/tree |
| Actigard 50WG | 9.3 to 28.4 g | 1.2 g/10.7 L water | 890 ml/tree |
| Employ | 56.7 to 113.4 g | 6.9 g/6.2 L water | 445 ml/tree |
| ODC | 100 to 500 ml | 8.8 ml/13.3 L water | 2.2 L/tree |
Commercial application rates according to the manufacturer's label.
Product mixtures for treatment of individual experimental trees based on the maxium product label rate.
Volume of mixed product applied to each individual experimental tree.
Insect Sampling
Populations of C. pyricola were monitored weekly beginning 1 wk before the first application of defense elicitors and ending in mid-August consistent with harvest dates for Bartlett in Washington. Nymphs were sampled in situ by counting the number of nymphs on the 10 most terminal leaves on one shoot from each of the four cardinal directions around each tree (four shoots per tree). Adult psyllids were sampled by tapping a branch from each of the four cardinal directions three times with a 0.5-m-long radiator hose to dislodge the insects onto a 0.2-m2 white beat tray. As for nymphs, this provided four samples per tree.
Statistical Analyses
Statistical analyses comparing C. pyricola nymphal or adult counts among treatments were performed using repeated measures (PROC GLIMMIX, SAS Institute 2013). Data collected in 2014, 2015, and 2016 were analyzed separately with the number of nymphs or the number of adults per tree (sum of four sampling units per tree) as the dependent variables. Week, treatment, and the week-by-treatment interaction were included as the fixed effects. Week and block were included as the random variables. Based on fit statistics and normal and quantile–quantile plots, analyses were performed assuming negative binomial distributions using the DIST = NB option of the MODEL statement (Gbur et al. 2012). An autoregressive covariance structure was specified using the TYPE = AR(1) option of the RANDOM statement. Where differences among treatments were indicated, mean number of insects on trees treated with each elicitor were compared with those on control trees using paired contrast statements (DIFF = CONTROL option of the LSMEANS statement).
Results and Discussion
Repeated-measures analyses did not reveal significant week-by-treatment interactions for either nymphs or adults regardless of sampling year (Table 2), but counts of nymphs and adults varied by sampling week within each year (Table 2). Nymphal populations exhibited two generation peaks, which occurred in late April to early May and in June of each year (Figs. 1A–3A). The second generation of nymphs was three to four times larger than the first generation in all 3 yrs (Figs. 1A–3A). Adult populations also exhibited two generation peaks, which occurred about 2 wks after observed peaks in nymphal populations (Figs. 1B–3B). The relative size of the two peaks varied among years. In 2014, the second generation of adults was numerically larger than the first generation, but the second generation was small compared to the first generation in both 2015 and 2016 (Figs. 1B–3B).
Table 2.
Statistical analyses examining the effects of foliar applications of defense elicitors on C. pyricola populations
| Variable | 2014 | 2015 | 2016 |
|---|---|---|---|
| Nymphs | |||
| Week | F18, 72=40.6; P<0.001 | F16, 64=24.9; P<0.001 | F18, 72=32.6; P<0.001 |
| Treatment | F3, 12=5.7; P=0.013 | F3, 12=1.6; P=0.253 | F3, 12=4.8; P=0.020 |
| Week × Treatment | F54, 216=1.4; P=0.056 | F48, 192=0.8; P=0.818 | F54, 216=1.1; P=0.295 |
| Adults | |||
| Week | F18, 72=58.1; P<0.001 | F16, 64=43.8; P<0.001 | F18, 72=7.3; P<0.001 |
| Treatment | F3, 12=6.3; P=0.008 | F3, 12=0.4; P=0.763 | F3, 12=2.6; P=0.097 |
| Week × Treatment | F54, 216=0.8; P=0.806 | F48, 192=0.7; P=0.900 | F54, 216=0.9; P=0.643 |
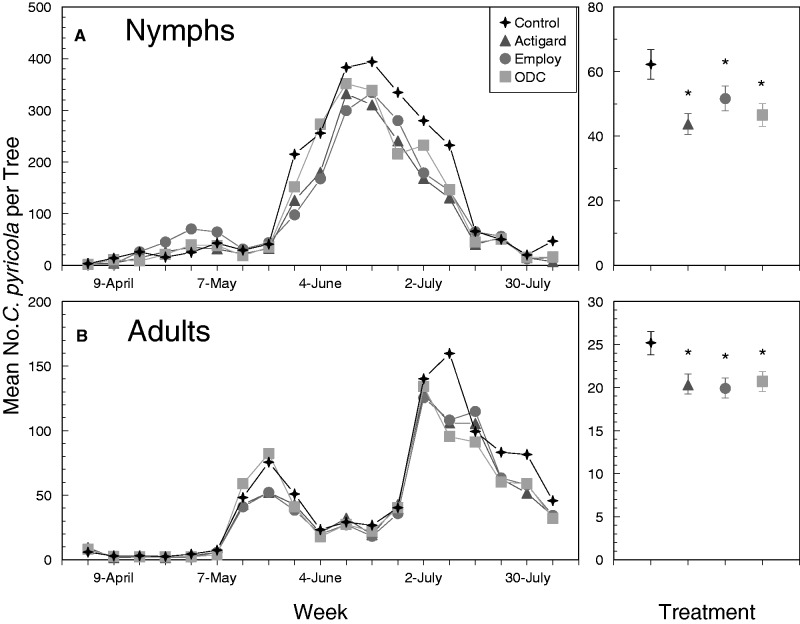
Fig. 1.
Mean number of C. pyricola nymphs per shoot (A) and adults per beat sheet sample (B) in 2014. Dates provided on the x-axis indicate days on which foliar applications were applied. Figures on the right show the overall effects of treatment regardless of sampling week. Error bars denote SEs and an asterisks indicate that values are significantly different from the untreated control treatment.
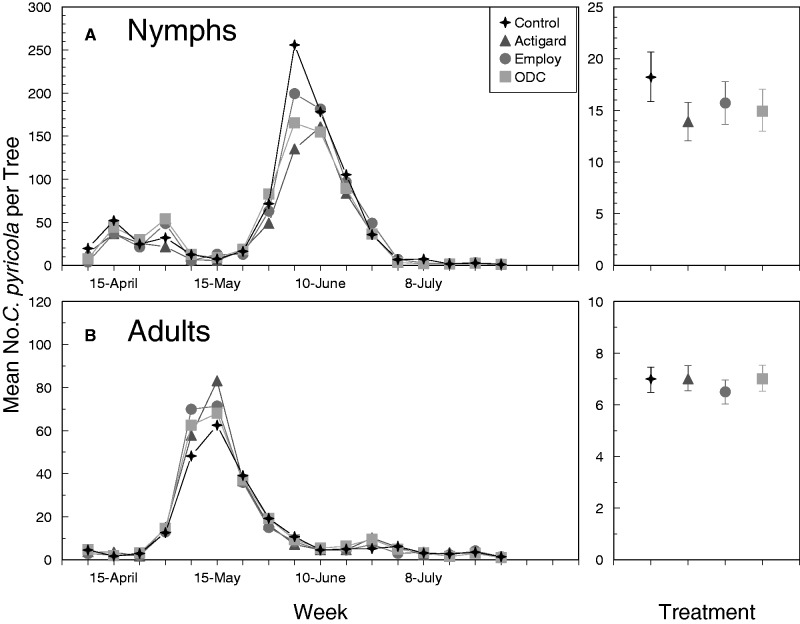
Fig. 3.
Mean number of C. pyricola nymphs per shoot (A) and adults per beat sheet sample (B) in 2016. Dates provided on the x-axis indicate days on which foliar applications were applied. Figures on the right show the overall effects of treatment regardless of sampling week. Error bars denote SEs and an asterisks indicate that values are significantly different from the untreated control treatment.
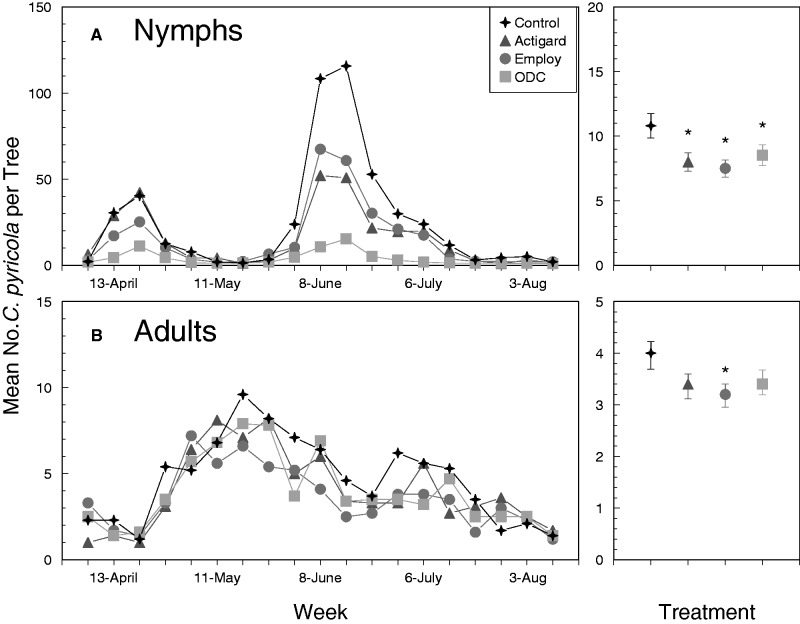
Analyses revealed significant differences in numbers of nymphs among foliar treatments in 2014 and 2016, but not in 2015 (Table 2). The lack of significant week-by-treatment interactions indicated that the effects of foliar applications on nymphs were similar among weeks. In 2014, significantly fewer nymphs were observed on trees treated with Actigard, Employ, or ODC than on untreated controls pooled over all sampling dates (Fig. 1A, right panel). Although not statistically significant in 2015, nearly 20–30% fewer nymphs were observed on trees treated with Actigard, Employ, or ODC than on untreated controls (Fig. 2A, right panel). Paired contrasts suggested marginally significant reductions (α < 0.1) of nymphs on trees treated with Actigard in 2015 compared with untreated controls (t = 2.1; df = 1, 192; P = 0.058; Fig. 2A, right panel). In 2016, similar to the response observed in 2014, significantly fewer nymphs (Fig. 3A, right panel) were recorded from trees treated with Actigard, Employ, or ODC than on untreated controls in 2016. Overall, results from the three sampling years were consistent and suggested that treating trees with defense elicitors leads to a modest reduction in populations of C. pyricola nymphs. These reductions were most obvious during the second-generation population peak, when numbers of nymphs were 20–30% greater on untreated trees than on trees treated with elicitors. Results of these field trials were also consistent with our previous laboratory study, which indicated that treating pear with foliar applications of Actigard-, Employ-, or ODC-induced systemic defenses that increased mortality of psyllid nymphs (Cooper and Horton 2015).
Fig. 2.
Mean number of C. pyricola nymphs per shoot (A) and adults per beat sheet sample (B) in 2015. Dates provided on the x-axis indicate days on which foliar applications were applied. Figures on the right show the overall effects of treatment regardless of sampling week. Error bars denote SEs and an asterisks indicate that values are significantly different from the untreated control treatment.
Significantly more adults were collected from untreated trees than from trees treated with Actigard, Employ, or ODC pooled over all sampling weeks in 2014 (Fig. 1B, right panel). As observed for nymphs, the differences in treatments were most obvious during the second-generation peak, which occurred in July 2014 (Fig. 1B). This pattern was not observed in 2015 (Fig. 2B) or 2016 (Fig. 3B), when the second generation was substantially reduced compared to that of 2014. Although the overall treatment effect was not significant at α = 0.05 in 2016 (Table 2; P = 0.097), paired contrasts indicated that significantly fewer adults were collected from trees treated with Employ than from untreated trees (t = 2.67; P = 0.020), and marginally fewer adults were collected from trees treated with Actigard than from untreated trees (t = 1.96; df = 1, 192; P = 0.073) (Fig. 3B). Our previous laboratory study did not indicate that defense elicitors led to decreased adult survival, but adults did tend to settle and oviposit on untreated trees more often than on trees treated with Actigard, Employ, or ODC in choice assays (Cooper and Horton 2015). It is possible that differences among treatments observed in 2014 were due to reduced numbers of nymphs developing to adults on treated trees, and due to movement of adults to adjacent untreated trees. It is unclear whether treatment differences attributed to adult preference would be replicated if an entire orchard were treated with an elicitor product.
Results of our study demonstrate that foliar applications of Actigard, Employ, or ODC may reduce C. pyricola nymphs under field conditions. These results are consistent with those of our previous laboratory bioassays (Cooper and Horton 2015), and with other reports that elicitors of salicylic acid-dependent defenses reduce performance of other phloem-feeding insects (Dong et al. 2004, Cooper et al. 2004, Li et al. 2006, Boughton et al. 2006, Gao et al. 2007, Zhang et al. 2012). The modest reduction in C. pyricola nymphs observed in our study does not warrant the use of elicitors alone for the control of psyllids. However, elicitors are often used to manage fire blight and knowledge that these elicitors may also partially suppress C. pyricola populations could be useful for system-wide integrated pest management approaches. More trials are required to evaluate the efficacy of these products applied to entire orchards and used in a spray schedule typical for fire blight management.
Acknowledgments
We thank Heather Headrick, Pauline Anderson, Glenda Torres, Delia Ramos, and Piedad Alcala for technical assistance, and the useful comments provided by reviewers. Funding was provided by the Fresh and Processed Pear Research Committees. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture. USDA is an equal opportunity provider and employer.
References Cited
- Avila C. A., Arevalo-Soliz . M., Jia L., Navarre D. A., Chen Z., Howe G. A., Meng Q. W., Smith J. E., Goggin F. L. 2012. Loss of function of FATTY ACID DESATURASE 7 in tomato enhances basal aphid resistance in a salicylate-dependent manner. Plant Physiol. 158: 2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balajoo O. M., Kesahavarzi M., Zahabi A., Danesh Y. R., Haghjuyan R. 2012. Protective effect of acibenzolar-S- methyl on fire blight severity in quince and characterization of the Erwinia amylovora strains involved. J. Plant Pathol. 94: 211–214. [Google Scholar]
- Boughton A., Hoover K., Felton G. W. 2006. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 120: 175–188. [Google Scholar]
- Brisset M. N., Cesbron S., Thomson S. V., Paulin J. P. 2000. Acibenzolar-S-methyl induces the accumulation of defense-related enzymes in apple and protects from fire blight. Eur. J. Plant Pathol. 106: 529–536. [Google Scholar]
- Cooper W. R., Horton D. R. 2015. Effects of elicitors of host plant defenses on pear psylla, Cacopsylla pyricola. Entomol. Exp. Appl. 157: 300–306. [Google Scholar]
- Cooper W. R., Jia L., Goggin G. L. 2004. Acquired and R-gene mediated resistance against the potato aphid in tomato. J. Chem. Ecol. 30: 2527–2542. [DOI] [PubMed] [Google Scholar]
- Dong H. P., Peng . L., Meng X. D., Bonasera J. M., Chen G. Y., Beer S. V., Dong H. S. 2004. Downstream divergence of the ethylene signaling pathway for harpin-stimulated Arabidopsis growth and insect defense. Plant Physiol. 136: 3628–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Anderson J. P., Klingler J. P., Nair R. M., Edwards O. R., Singh K. B. 2007. Involvement of the octadecanoid pathway in bluegreen aphid resistance in Medicago truncatula. Mol. Plant Microbe Interact. 20: 82–93. [DOI] [PubMed] [Google Scholar]
- Gbur E. E., Stroup . W., McCarter K. S., Durham S., Young L. J., Christman M., West M., Kramer M. 2012. Analysis of generalized linear mixed models in agricultural and natural resources science. American Society of Agronomy, Madison, WI. [Google Scholar]
- Li Q., Xie Q. G., Smith-Becker J., Navarre D. A., Kaloshian I. 2006. Mi-1 mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Mol. Plant Microbe Interact. 19: 655–664. [DOI] [PubMed] [Google Scholar]
- Reignault P., Walters D. 2007. Topical application of inducers for disease control.In, Walters D., Newton A., Lyon G. (eds), Induced Resistance for Plant Defense: A Sustainable Approach to Crop Protection. Blackwell Publishing, Ames, IA, pp. 179–200. [Google Scholar]
- SAS Institute Inc. 2013. SAS release 9.4 ed. SAS Institute; Cary, NC. [Google Scholar]
- Stout M. J. 2007. The molecular bases of plant resistance and defense responses to aphid feeding: current status. Entomol. Exp. Appl. 122: 1–16. [Google Scholar]
- Westigard P. H., Zwick R. W. 1972. The pear psylla in Oregon. Oregon State University Agricultural Experiment Station, Technical Bulletin 122.
- Zhang C. H., Hi, Chen L., Wang X., Lu B., Zhang S., Liang Y., Liu R., Qian J., Sun W., You Z., Dong H. 2012. Harpin-induced expression and transgenic overexpression of the phloem protein gene AtPP2-A1 in Arabidopsis repress phloem feeding of the green peach aphid Myzus persicae. BMC Plant Biology. 11: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]