Nucl. Acids Res. (2017) doi: 10.1093/nar/gkw1365
The Authors wish it to apologise for errors in Figure 7D and Funding. A new figure and Funding statement are provided below. These errors have also been corrected in the original article.
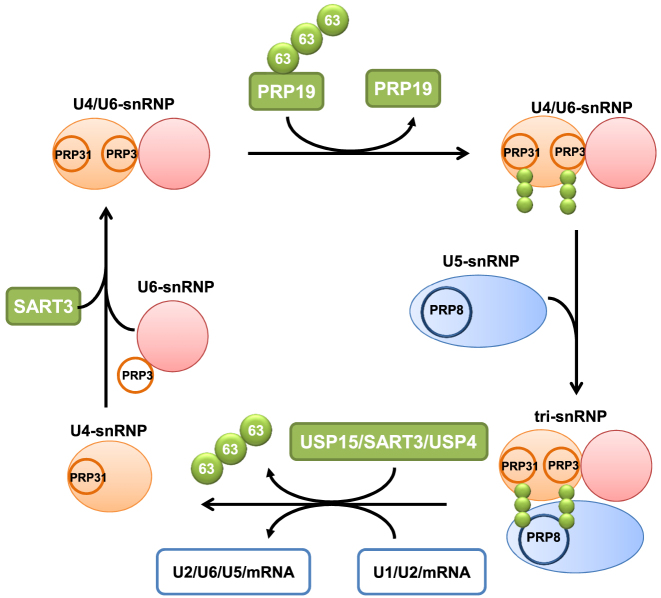
Figure 7D.
Working model of USP15 and USP4 mediated regulation of the spliceosome. Both PRP31 and PRP3, the U4/U6 snRNP components, are ubiquitinated by PRP19 with K63-linked ubiquitin chain. Ubiquitinated PRP31 and PRP3 in turn are recognized by U5 snRNP component PRP8 via its JAMM domain, and this may help the formation of the stabilized U4/U6.U5 tri-snRNP complex. Followed by successful docking of the U4/U6.U5 tri-snRNP complex at the spliceosome, PRP31 and PRP3 are deubiquitinated by the USP15–SART3–USP4 complex, thereby decreasing the affinity towards PRP8 possibly resulting in the dissociation of the U4 snRNA from the tri-snRNP complex. Release of U4 snRNP facilitates pre-mRNA splicing of cell cycle regulatory genes, especially Bub1 and α-tubulin, by the active spliceosome complex.
FUNDING
National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) [20110021713 and 2015R1A2A2A04005596]. Funding for open access charge: National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) [20110021713 and 2015R1A2A2A04005596].