Abstract
A shift toward transgenic crops which produce combinations of insecticidal proteins has increased the interest (Syngenta Seeds, Inc., Minnetonka, MN) in studying the potential for interactions amongst those proteins. We present a general testing method which accommodates proteins with nonoverlapping spectrums of activity. Our sequential testing approach first investigates groups of the proteins with overlapping activity; e.g., proteins active against Lepidoptera or Coleoptera, respectively. The Colby method is used to test for interactions within each respective group. Subsequently, the mixture of proteins within each group is regarded as a single entity and tests for interactions between the groups (when combined) is conducted using analysis of variance. We illustrate the method using Cry1Ab, Vip3Aa20, and Cry1F (a mixture of proteins active against Lepidoptera), and mCry3A and eCry3.1Ab (a mixture of proteins active against Coleoptera). These insecticidal proteins are produced by Bt11 × MIR162 × TC1507 × MIR604 × 5307 maize. We detected no interactions between Cry1Ab, Vip3Aa20, and Cry1F in tests using larvae of two different lepidopteran species, and possible slight antagonism between mCry3A and eCry3.1Ab with a coleopteran test species. We detected no effect of (eCry3.1Ab + mCry3A) on the potency of (Cry1Ab + Vip3Aa20 + Cry1F) to lepidopteran larvae, and no effect of (Cry1Ab + Vip3Aa20 + Cry1F) on the potency of (mCry3A + eCry3.1Ab) to coleopteran larvae. We discuss implications of these results for characterization of Bt11 × MIR162 × TC1507 × MIR604 × 5307 maize, and the value of the method for characterizing other transgenic crops that produce several insecticidal proteins.
Keywords: Bacillus thuringiensis, synergism, Cry, stacked, trait
In recent years insect control with transgenic crops expressing insecticidal proteins from Bacillus thuringiensis Berliner (Bacillales: Bacillaceae) (Bt) has shifted toward using combinations of three or more insecticidal traits (Que et al. 2010, Carrière et al. 2015, Huang 2015). This approach of combining (stacking) insect control traits can have distinct advantages in terms of providing: 1) a reduced potential for insect resistance development, 2) a potentially broader array of target pest species control, and 3) simplified crop management plans as compared to transgenic crops which express one or only a couple insecticidal proteins. In particular, genetically engineered Zea mays L. (maize) products are increasingly using this combined insecticidal trait approach in global crop production to control various above- and below-ground insect pests. The Syngenta maize Events MIR604 Agrisure RW (Syngenta Seeds, Inc., Minnetonka, MN) and 5307 express the insecticidal proteins modified Cry3A (mCry3A) and eCry3.1Ab, respectively, which are active against certain coleopteran insect pests including the western corn rootworm (Diabrotica virgifera virgifera LeConte, WCRW) (Walters et al. 2008, 2010). These two insecticidal proteins are present together in Agrisure Duracade (Syngenta Seeds, Inc., Minnetonka, MN) maize by means of conventional breeding of Events MIR604 and 5307. Syngenta Events Bt11 and MIR162 maize, and Dow AgroSciences Event TC1507 Herculex (Dow AgroSciences, Inc., Indianapolis, IN) maize express the insecticidal proteins Cry1Ab, Vip3Aa20, and Cry1F, respectively, which are active against certain lepidopteran insect pests including the European corn borer (Ostrinia nubilalis Hübner, ECB) and the fall armyworm (Spodoptera frugiperda (J. E. Smith), FAW) (Koziel et al. 1993, Estruch et al. 1996, Herman et al. 2004, Wolt et al. 2005). Also through conventional breeding, Syngenta has created stacked maize hybrids containing all five of the above insecticidal proteins to provide control of both lepidopteran and coleopteran pest insects.
The characterization of a trait stack with multiple protein plant incorporated protectants (PIPs) should include relevant information on the registered single protein PIP components as well as discussion of any potential antagonistic, synergistic, or potentiating toxicological interactions of the multiple proteins in support of product registration (US EPA 2009a, Raybould et al. 2012). As the means to test for interactions amongst insecticidal proteins which target different orders of insect pests is not always evident, we have prescribed a bioassay method to test such a hypothesis (e.g., the insecticidal activity of a specified lepidopteran-active protein mixture is not affected by the presence of a given coleopteran-active protein mixture, and vice-versa).
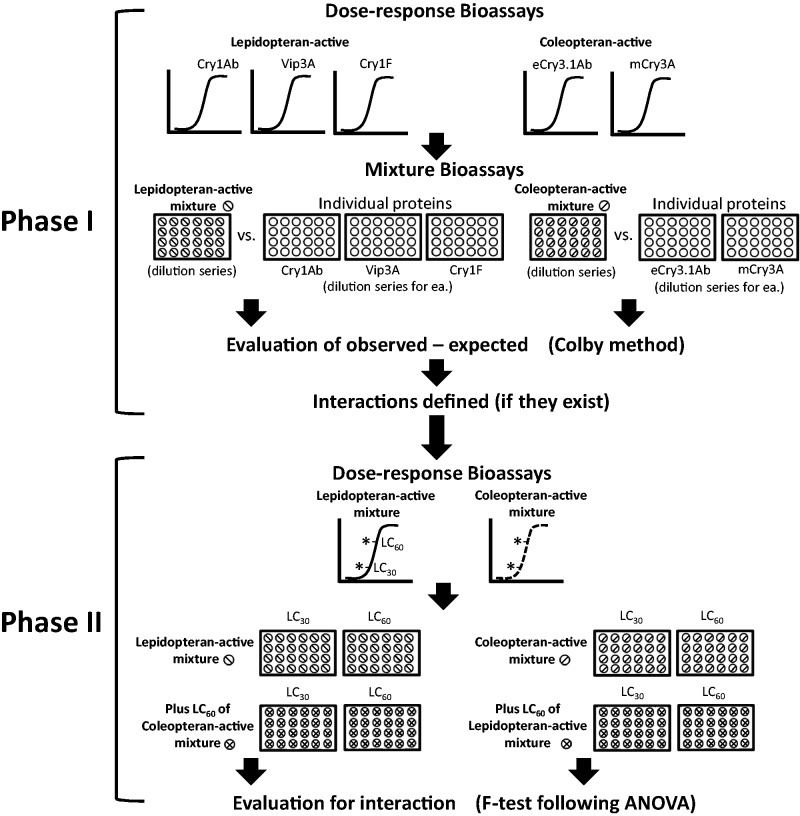
The testing method we describe herein is designed with two distinct phases (Fig. 1). This two-phase approach aptly addresses the question of protein-protein interactions in a modular fashion and provides a simple, yet thorough experimental design. A somewhat related, but experimentally different approach (use of six or more concentrations to generate dose-responses for single LC50 or IC50 comparisons, based on examination of the overlap of 95% CIs) has been used by others seeking regulatory approvals of transgenic crops which combine lepidopteran-active and coleopteran-acive traits (US EPA 2007). Although our method was devised for the purposes of a genetically modified trait stack risk assessment, it can be viewed as a general approach to testing complex mixtures (e.g., other insecticides) where the components have different spectrums of activity. In the first phase of experiments described, the potential interactions among the components of the respective lepidopteran-active (e.g., Cry1Ab + Vip3Aa20 + Cry1F) and coleopteran-active (e.g., eCry3.1Ab + mCry3A) protein mixtures are examined. In a second phase, the potential interaction of the lepidopteran-active and coleopteran-active protein mixtures in combination is then assessed for each type of sensitive target pest.
Fig. 1.
Testing for the interaction of insecticidal protein mixtures in both Lepidoptera and Coleoptera when the mixtures target susceptible pests across both orders.
For this example, in phase I, the interaction among the components of the lepidopteran-active insecticidal protein mixture (Cry1Ab + Vip3Aa20 + Cry1F) and the interaction among the components of the respective coleopteran-active insecticidal protein mixture (eCry3.1Ab + mCry3A) were each investigated using an approach based on the Colby method (Colby 1967).
The Colby method is based on an assumption of independent modes of action for the individual components tested (e.g., insecticidal proteins) and involves two concurrent experiments using the same sensitive insect species. The effects (in this case, mortality) of each insecticidal protein in isolation and from a mixture of the same insecticidal proteins are measured concurrently. The effects of the proteins in isolation are used to predict the effect for the concurrently tested mixture(s) of the proteins. The hypothesis is that no interactions exist among the components when calculating the expected mortality response (undergirding the subsequent mathematical comparisons of the predicted with the observed mortality for the combination of components). If the observed mortalities of the mixtures are consistently greater than predicted mortalities (typically tested across several dilutions or ratios), synergism would be inferred; if the observed mortalities of the mixtures are consistently less than predicted, antagonism would be inferred. Although the method provides a way to investigate the potential interactions, the biological relevance as well as any impact on the risk assessment needs to be considered further on a case-by-case basis, and taking into account what is already known about the function of the proteins being evaluated. For example, during the risk assessment, if there is no postulated mechanism for a synergistic interaction, and if dealing with insecticidal proteins that have a clearly established history of safe use, this knowledge may outweigh a statistical outcome in the laboratory suggesting a certain degree of interaction. Similarly, other parameters must be considered in the risk assessment such as the actual likelihood of a potential route of exposure or the existence of a high margin of exposure (Raybould et al. 2012).
In phase I of these experiments, two series of insect feeding bioassays were performed for the lepidopteran-active protein mixture, one series with ECB larvae and one with FAW larvae (Fig. 1). This was necessary since ECB larvae are effectively controlled by Cry1Ab or Cry1F (Siegfried et al. 2007, 2014), but not by Vip3A (Yu et al. 1997, Lee et al. 2003), whereas conversely, FAW larvae are effectively controlled byVip3A or Cry1F, but not by Cry1Ab (Siebert et al. 2008, Niu et al. 2014). The effects of the coleopteran-active protein mixture (eCry3.1Ab and mCry3A) were investigated with only one series of insect feeding bioassays in phase I using larvae of the Colorado potato beetle (Leptinotarsa decemlineata, CPB), because CPB are susceptible to both insecticidal proteins. Although corn rootworm larvae (especially WCRW) are the primary target pests for the eCry3.1Ab and mCry3A proteins (i.e. not the CPB), the CPB was chosen as the test organism or pest model as it has been previously noted that WCRW is an extremely challenging pest model to work with for examining the potency of insecticidal proteins in lab bioassays. Other groups have encountered difficulties obtaining precise mortality responses, sometimes leading researchers to the use of growth inhibition as an endpoint parameter for this test organism (Moellenbeck et al. 2001, Herman et al. 2002, English et al. 2003, Walters et al. 2008). Using larvae from the CPB as a susceptible test organism instead of from the WCRW therefore provided a reliable way to more accurately describe mortality in artificial diet bioassays for assessing the potential interaction of eCry3.1Ab and mCry3A.
In phase II of our experimental approach, the effect of the coleopteran-active protein mixture on the activity of the lepidopteran-active protein mixture and the effect of the lepidopteran-active protein mixture on the activity of the coleopteran-active protein mixture were each investigated using two doses selected to give intermediate activity (∼LC30 and LC60). A very similar approach was recently used to investigate the potential for interaction of a single lepidopteran-active protein with a single coleopteran-active protein (Raybould et al. 2012). In this present work, for each combinatorial bioassay, a sensitive insect species (i.e., ECB to measure lepidopteran activity, and CPB to measure coleopteran activity) was exposed to the higher (∼LC60) or the lower concentration (∼LC30) of the insecticidal protein mixture alone, and in combination with the higher concentration of the other protein mixture for which it is insensitive (i.e., the insecticidal protein mixture that targets the other insect order). Use of both a lower and higher dose provides a more robust comparison than using just a single dose as it supports an assessment of the potential interaction at more than one concentration of the protein mixtures, yet it remains simple enough to conduct the necessary concurrent bioassays for statistical comparisons which follow. In addition, by using two doses which are in a targeted intermediate activity range, there is an increased ability to detect any significant synergistic or antagonistic interaction of the insecticidal protein mixtures as compared to use of only one higher or lower dose combination for the interaction test mixture.
The results of these types of insecticidal protein interaction studies in target insects may be used to support current approaches to risk assessment regarding the potential occurrence of interactions in nonsensitive nontarget species, including humans and other animals. The interaction studies can be a component of such assessments, along with other important environmental considerations specific to how the insecticidal protein traits are deployed (Raybould et al. 2012).
Materials and Methods
Preparation of Insecticidal Proteins
Vip3A, eCry3.1Ab, and mCry3A proteins were each prepared from Escherichia coli expression systems, purified by liquid chromatography, and converted into respective lyophilized powders. The microbially produced preparations were determined to contain 86.5% Vip3A, 89.6% eCry3.1Ab, and 71.4% mCry3A by weight, respectively. All three protein preparations were stored at −20 ± 8°C until further use. Similarly, Cry1Ab was prepared from an E.coli expression system and purified by liquid chromatography, but first was treated with trypsin to generate a trypsin-resistant core (Chestukhina et al. 1982, Oppert 1999, Schnepf et al. 1998, Lightwood et al. 2000). The final preparation of solubilized truncated Cry1Ab was determined to contain 107 μg Cry1Ab/ml (at 97.1% of the total protein) and was stored at 5 ± 3 °C. Microbially produced Cry1F was prepared from a recombinant microbial Pseudomonas fluorescens expression system and purified into a single lyophilized powder prior to this study by Dow AgroSciences (Indianapolis, IN, USA). This preparation was determined to contain 35% Cry1F by weight and was stored at −80 ± 10°C until further use. Table 1 summarizes the corresponding susceptible insects (and order) for these different insecticidal proteins used.
Table 1.
Summary of insecticidal proteins and corresponding susceptible insects used
| Insecticidal protein | Susceptible insect tested | Order |
|---|---|---|
| Cry1Ab | ECB | Lepidoptera |
| Cry1F | ECB, FAW | Lepidoptera |
| Vip3Aa20 | FAW | Lepidoptera |
| eCry3.1Ab | CPB | Coleoptera |
| mCry3A | CPB | Coleoptera |
Bioassay Preparation
Diet incorporation bioassays were conducted as described in Graser and Walters (2015). In brief, for the ECB and FAW bioassays, a soy-wheat germ-based artificial lepidopteran diet (Frontier Scientific Services Inc., Newark, DE, USA) was freshly prepared for each bioassay according to the manufacturer’s instructions and maintained in liquefied form at ∼52.5 ± 2.5 °C in a water bath. For the CPB bioassays, a casein-based artificial diet (Frontier Scientific Services, Inc.) was also freshly prepared for each bioassay and similarly maintained in liquefied form in a water bath. To prevent bacterial and fungal growth, antibiotics were added (Graser and Walters 2015) to each liquefied diet type (after equilibration to the water bath temperature) prior to mixing with samples and addition to respective treatment or control plates. For both diet types, each treatment or buffer control solution was thoroughly mixed with artificial diet at a ratio of 1:1 (v:v) (e.g., 3 ml of treatment solution:3 ml insect diet). The mixed diets were dispensed at 100 µl per well into 24-well assay plates and allowed to solidify. Insect larvae from respective test species (one larva per well) were added, plates were sealed with clear polyolefin film and maintained at ambient temperature and humidity in the dark.
Phase I Insecticidal Bioassays
Testing for Interaction of Cry1Ab, Cry1F and Vip3Aa20 Insecticidal Proteins
Diet incorporation bioassays were used to measure the insecticidal effect of Cry1Ab, Vip3Aa20, and Cry1F individually, and in a combination of all three proteins on first instar ECB and FAW larvae. For each protein, Cry1Ab, Vip3Aa20, and Cry1F (prepared in 50 mM CAPS, pH 10.0; purified water; and 20 mM CAPS, pH 10.5, respectively), the starting concentrations used as a mixture in the interaction bioassay were first determined using individual dose–response bioassays for the ECB and FAW larvae (Supp Tables 1 and 2 [online only]). The concentrations of respective Cry1Ab, Vip3Aa20, and Cry1F components that give the desired response were determined to be 200, 100, and 400 ng/ml diet for the ECB bioassay, and 200, 400, and 2,000 ng/ml diet, respectively, for the FAW bioassay. These respective starting concentrations were based on a concentration of the most active protein component(s) capable of high mortality when undiluted and which would support a five-dose dilution series resulting in partial activity. In addition, for the component that was the least active for each insect species (e.g., Vip3Aa20 for ECB, and Cry1Ab for FAW), a starting concentration was selected at a uniform protein input of one-half the concentration of the most active component (e.g., Cry1Ab for ECB, and Vip3Aa20 for FAW) in the respective tests. For convenience, the mixture of these proteins at the above starting concentrations is referred to as “Mixture A” for ECB bioassays or “Mixture B” for FAW bioassays, respectively. Mixtures A and B were each diluted by half, one-fourth, one-eighth, and one-sixteenth of their starting concentration resulting in a total of five treatment levels for each. The buffer used for the combined treatment dilutions consisted of equal volumes of 50 mM CAPS (pH 10.0), purified water, and 20 mM CAPS (pH 10.5) and was also used as a negative buffer control. Three replicate plates were used for each treatment or control being tested. Mortality was assessed at 120 h after treatment initiation.
Testing for Interaction of eCry3.1Ab and mCry3A Insecticidal Proteins
Diet incorporation bioassays were used to measure the effects of eCry3.1Ab and mCry3A, individually and in combination, on CPB larvae. For each insecticidal protein (prepared in 10 mM ammonium bicarbonate, pH 10.0; and purified water, respectively) the starting concentrations used as a mixture in the interaction bioassay were first determined using individual dose–response bioassays for the CPB larvae where it was found that the eCry3.1Ab and mCry3A insecticidal proteins each have a similar mortality response against the CPB larvae (Supp Table 3 [online only]). The starting concentrations of eCry3.1Ab and mCry3A were determined to be 4 µg/ml diet. Similar to the strategy used for the lepidopteran-active insecticidal proteins described earlier, these respective starting concentrations were based on a concentration capable of high mortality when undiluted and which would support a five-dose dilution series (half, one-fourth, one-eighth, and one-sixteenth of the starting concentration) resulting in partial activity. The buffer used for the combined treatment dilutions consisted of equal volumes of purified water and 10 mM ammonium bicarbonate (pH 10.0) and was also used as a negative buffer control. Three replicate plates were used for each treatment or control being tested. Mortality was assessed at 120 h after treatment initiation.
Phase II Insecticidal Bioassays
Dose–Response of the Lepidopteran-Active Protein Mixture using ECB to Establish Two Intermediate Mortality Doses (Projected LC30 and LC60) for the Combined Lepidopteran- and Coleopteran-Active Mixture Interaction Testing
Vip3Aa20 and Cry1F solutions were prepared in purified water and 20 mM CAPS (pH 10.5), respectively, then combined with Cry1Ab in 50 mM CAPS (pH 10.0) to obtain a single mixture for insect bioassay. As both the ECB and the FAW experiments confirmed no interaction of the lepidopteran-active proteins Cry1Ab, Cry1F and Vip3Aa20, the dose–response for this mixture in Phase II was only conducted with ECB.
A similar series of concentrations was used as described for Phase I bioassays, except the highest concentration (designated 1X) of the lepidopteran-active protein mixture was increased to 400 ng Cry1Ab + 200 ng Vip3Aa20 + 800 ng Cry1F per ml diet. This was then serially diluted to generate eight doses from 1X to X/128 (Supp Table 4 [online only]).
Diet incorporation bioassays were set up as described for Phase I bioassays, with each treatment consisting of the protein mixture combined with insect diet at a ratio of 1:1 (v:v). A negative control for the bioassay consisted of the dilution buffer combined 1:1 with the diet. Mortality was assessed at 120 h after treatment initiation. Two concentrations showing intermediate levels of response, one approximating the 30% mortality response (ECB low dose) and one approximating the 60% mortality response (ECB high dose) were identified based on the dose–response data for use in the subsequent protein interaction experiments.
Dose–Response of the Coleopteran-Active Protein Mixture Using CPB to Establish Two Intermediate Mortality Doses (Projected LC30 and LC60) for the Combined Lepidopteran- and Coleopteran-Active Mixture Interaction Testing
A solution of eCry3.1Ab was prepared in 10 mM ammonium bicarbonate (pH 10.0) and combined with an equal volume of mCry3A solution prepared in purified water to obtain a single mixture for insect bioassay. A similar series of concentrations was used as described for Phase I bioassays, except the highest concentration (designated 1X) of the coleopteran-active protein mixture was increased to 8 µg eCry3.1Ab + 8 µg mCry3A per ml diet. This was then serially diluted to generate eight doses from 1X to X/128 (Supp Table 5 [online only]).
Diet incorporation bioassays were set up as described for Phase I, with each treatment consisting of the protein mixture combined with insect diet at a ratio of 1:1 (v:v). A negative control for the bioassay consisted of the dilution buffer combined 1:1 with the diet. Mortality was assessed at 120 hours after treatment initiation. Two concentrations showing intermediate levels of response, one around the 30% mortality response (CPB low dose) and one around the 60% mortality response (CPB high dose) were identified based on the dose–response data for use in the subsequent protein interaction experiments.
Testing for Effects of the Coleopteran-Active Protein Mixture on the Mortality of ECB Exposed to the Lepidopteran-Active Protein Mixture
The effect of the coleopteran-active protein mixture on the insecticidal toxicity of the lepidopteran-active protein mixture was examined using ECB bioassays. Two doses of the lepidopteran-active protein mixture, alone and in combination with the high dose of the coleopteran-active protein mixture (Supp Table 6 [online only]), were used in three independent bioassays (giving three 24-well plates for each treatment or control being tested).
The mortality responses of ECB exposed to the lepidopteran-active protein mixture alone were compared with the responses of ECB exposed to the lepidopteran-active protein mixture combined with the coleopteran-active protein mixture. The protein interaction bioassays were set up using the same procedures and conditions described in Phase I ECB bioassays. The CPB high dose (alone) was used as a negative control to exclude any nonspecific effects of the coleopteran-active protein mixture on ECB. Additional negative controls included the buffers used in the preparations of both the lepidopteran-active protein mixture and the combination of the lepidopteran-active protein mixture + the coleopteran-active protein mixture. A positive control using CPB high dose against CPB was included to confirm the biological activity of the coleopteran-active protein mixture. Mortality was assessed at 120 h after treatment initiation.
Testing for Effects of the Lepidopteran-Active Protein Mixture on the Mortality of CPB Exposed to the Coleopteran-Active Protein Mixture
The effect of the lepidopteran-active protein mixture on the insecticidal toxicity of the coleopteran-active protein mixture was examined using CPB bioassays. Two doses of the coleopteran-active protein mixture, alone and in combination with the high dose of the lepidopteran-active protein mixture (Supp Table 7 [online only]), were used in three independent bioassays (giving three 24-well plates for each treatment or control being tested).
The mortality responses of CPB exposed to the coleopteran-active protein mixture alone were compared with the responses of CPB exposed to the coleopteran-active protein mixture combined with the lepidopteran-active protein mixture. The protein interaction bioassays were set up using the same procedures and conditions described in Phase I CPB bioassays. The ECB high dose (alone) was used as a negative control to exclude any nonspecific effects of the lepidopteran-active protein mixture on CPB. Additional negative controls included the buffers used in the preparations of both the coleopteran-active protein mixture and the combination of the lepidopteran-active protein mixture + the coleopteran-active protein mixture. A positive control using ECB high dose against ECB was included to confirm the biological activity of the lepidopteran-active protein mixture. Mortality was assessed at 120 h after treatment initiation.
Data Analyses to Determine the Interaction Among a Mixture of Cry1Ab + Vip3Aa20 + Cry1F or a Mixture of eCry3.1Ab + mCry3A Using the Colby Method
In phase I insecticidal protein bioassays, responses to the individual proteins were evaluated as percent mortalities and used to predict the mortality for protein combinations according to the Colby method (Colby 1967). The percent mortality was corrected according to the mortality observed in a negative control using Abbott’s formula1 (Abbott 1925). The presence of any synergistic or antagonistic interaction between the proteins within a mixture (e.g., lepidopteran-active Cry1Ab + Vip3Aa20 + Cry1F or coleopteran-active eCry3.1Ab + mCry3A) was evaluated by comparing the predicted with the observed responses following exposure to the respective protein mixture. Predicted responses were calculated based on an assumption of independent modes of action. If component A alone gives x% effect and component B alone gives y% effect, then under the assumption of independent modes of action, the predicted response to A + B is x + y – (xy/100). For Cry1Ab, Vip3Aa20, and Cry1F, if component A alone gives x% effect, component B alone gives y% effect and component C alone gives z% effect, then under the assumption of independent action, the predicted response to A + B + C is x + y + z – [(xy + xz + yz)/100] + (xyz/10,000). Three independent bioassays were conducted for ECB, FAW, or CPB larvae with the individual and combined protein treatments tested concurrently in each assay, then used to obtain the respective averages for observed versus expected mortalities.
Statistical Analysis to Determine the Interaction for Combinations of the Lepidopteran- and Coleopteran-Active Protein Mixtures
In phase II insecticidal protein bioassays, using a combination of the lepidopteran- and coleopteran-active protein mixtures, data were subjected to analysis of variance using the following model:
where Yijk, observed % mortality; U, overall mean; Di, dose effect of the active ingredient; Ij, inactive ingredient effect; Tk, effect of test; DIij, dose × inactive ingredient interaction; eijk, residual error.
For each protein interaction bioassay, F-tests were used to assess the statistical significance of the effects of dose (treatment concentration), inactive ingredient, and dose × inactive ingredient interaction at the customary 0.05 probability level. The software used for the statistical analysis was SAS, version 9.2 (SAS Institute, Inc., Cary NC).
Results
Phase I Insecticidal Bioassays
Testing for Interaction of Lepidopteran-Active Cry1Ab, Cry1F, and Vip3Aa20 Insecticidal Proteins
The insecticidal activity of Cry1Ab, Vip3Aa20 and Cry1F individually and in a mixture was assessed for ECB (Table 2) and FAW larvae (Table 3). Dose-responses were evident for the individual proteins as well as the mixtures, except, as expected, in the case of individually tested Vip3Aa20 against ECB (Table 2), or individually tested Cry1Ab against FAW (Table 3). These results provided additional internal negative controls as little or no activity was expected for those respective tests based on the insect susceptibility as described previously. Average mortality in the buffer negative control assays was below 3 or 5% for ECB and FAW, respectively (data not shown).
Table 2.
Insecticidal activity of Cry1Ab, Vip3Aa20 and Cry1F against ECB at 120 h after treatment
| Test protein | Protein conc. (ng/ml) | % Mortality (observed)a | % Mortality (expected) |
|---|---|---|---|
| Cry1Ab | 200 | 66.7 | N/A |
| 100 | 43.1 | ||
| 50 | 45.1 | ||
| 25 | 13.9 | ||
| 12.5 | 5.6 | ||
| Vip3Aa20 | 100 | 0.0 | N/A |
| 50 | 2.8 | ||
| 25 | 7.0 | ||
| 12.5 | 0.0 | ||
| 6.3 | 2.8 | ||
| Cry1F | 400 | 62.5 | N/A |
| 200 | 37.5 | ||
| 100 | 6.9 | ||
| 50 | 1.4 | ||
| 25 | 4.2 | ||
| Test mixtureb | A | 80.0 | 87.5 |
| A/2 | 85.7 | 65.4 | |
| A/4 | 52.8 | 52.5 | |
| A/8 | 22.1 | 15.1 | |
| A/16 | 5.7 | 12.0 |
All data were corrected for buffer control mortality using Abbott’s formula.
The test mixture A was composed of 200, 100, and 400 ng/ml diet of Cry1Ab, Vip3Aa20, and Cry1F, respectively, as described in the Methods section.
Table 3.
Insecticidal activity of Cry1Ab, Vip3Aa20 and Cry1F against FAW at 120 h after treatment
| Test protein | Protein concn. (ng/ml) | % Mortality (observed)a | % Mortality (expected) |
|---|---|---|---|
| Cry1Ab | 200 | 0.0 | N/A |
| 100 | 0.0 | ||
| 50 | 4.4 | ||
| 25 | 5.8 | ||
| 12.5 | 0.0 | ||
| Vip3Aa20 | 400 | 60.9 | N/A |
| 200 | 51.5 | ||
| 100 | 44.1 | ||
| 50 | 14.5 | ||
| 25 | 5.8 | ||
| Cry1F | 2,000 | 76.8 | N/A |
| 1,000 | 53.6 | ||
| 500 | 37.7 | ||
| 250 | 15.0 | ||
| 125 | 13.0 | ||
| Test mixtureb | B | 91.3 | 90.9 |
| B/2 | 85.5 | 77.5 | |
| B/4 | 71.0 | 66.7 | |
| B/8 | 47.1 | 31.5 | |
| B/16 | 20.3 | 18.0 |
All data were corrected for buffer control mortality using Abbott’s formula.
The test mixture B was composed of 200, 400, and 2,000 ng/ml diet of Cry1Ab, Vip3Aa20, and Cry1F, respectively, as described in the Methods section.
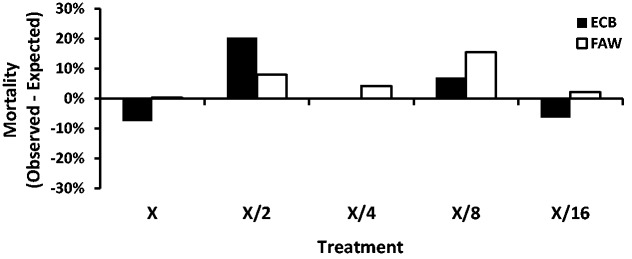
The results of the bioassays for the proteins individually were used to calculate the respective expected mortality in the mixture treatments for either ECB or FAW larvae (Tables 2 and 3) using Colby’s formula. The difference between the observed mortality and expected mortality (Fig. 2) was used to determine any interaction of Cry1Ab, Vip3Aa20, and Cry1F in combination.
Fig. 2.
Difference between the observed and expected mortality for ECB or FAW on diets containing mixtures of Cry1Ab + Vip3Aa20 + Cry1F 120 hours after treatment. X refers to the A or B undiluted mixture as described in the Methods section for ECB or FAW, respectively. X/2 to X/16 refers to serial dilutions for each respective mixture.
The difference between the observed and expected mortalities was very small across all dilutions (Fig. 2) with the greatest differential at just over 20%, and for only 1 out of 9 comparisons. In addition, there was no consistent pattern in the difference between observed and expected mortalities for either ECB or FAW larvae across the respective serial dilution series (Fig. 2). These results do not indicate any synergism or antagonism between Cry1Ab, Vip3Aa20, and Cry1F for either susceptible species.
Testing for Interaction of Coleopteran-Active eCry3.1Ab and mCry3A Insecticidal Proteins
The insecticidal activity of eCry3.1Ab and mCry3A individually and in a mixture was assessed for CPB larvae (Table 4). Dose-responses were evident for the individual proteins as well as the mixtures, but the mortality observed tended to be in the lower half of the response range. Average mortality in the buffer negative control assays was below 7% (data not shown).
Table 4.
Insecticidal activity of eCry3.1Ab and mCry3A against CPB at 120 h after treatment
| Test protein | Protein concn. (µg/ml) | % Mortality (observed)a | % Mortality (expected) |
|---|---|---|---|
| eCry3.1Ab | 4 | 46.3 | N/A |
| 2 | 38.8 | ||
| 1 | 40.3 | ||
| 0.5 | 29.9 | ||
| 0.25 | 17.9 | ||
| mCry3A | 4 | 41.8 | N/A |
| 2 | 46.3 | ||
| 1 | 20.9 | ||
| 0.5 | 25.4 | ||
| 0.25 | 23.9 | ||
| Test mixtureb | X | 53.8 | 73.1 |
| X/2 | 52.3 | 74.4 | |
| X/4 | 38.8 | 51.6 | |
| X/8 | 23.9 | 43.2 | |
| X/16 | 20.9 | 39.8 |
All the data were corrected for control mortality using Abbott’s formula.
The test mixture X was composed of 4 μg/ml diet of eCry3.1Ab plus 4 μg/ml diet of mCry3A, as described in the Methods section.
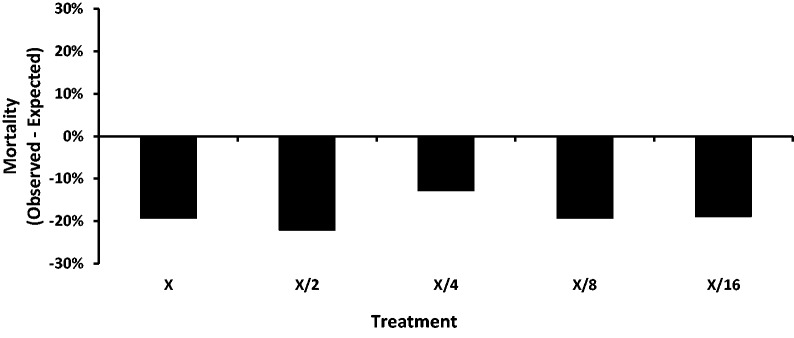
The results of the bioassays for the proteins individually were used to calculate the expected mortality in the mixture treatments for CPB larvae (Table 4) using the Colby formula as described previously. The difference between the observed mortality and expected mortality (Fig. 3) was used to determine any interaction of eCry3.1Ab and mCry3A in combination.
Fig. 3.
Difference between observed and expected mortality of CPB on diet containing a mixture of eCry3.1Ab and mCry3A after 120 h. X refers to the undiluted mixture as described in the Methods. X/2 to X/16 refers to serial dilutions for the mixture.
The difference between the observed and expected mortalities for the combination of eCry3.1Ab and mCry3A was very small across all dilutions (Fig. 3) with the greatest differential at just under 22%. There was a consistent pattern in the difference between observed and expected mortalities of lower than expected mortality in the CPB exposed to the protein mixture as compared with the individual protein treatments. This result suggests some slight degree of antagonistic interaction between eCry3.1Ab and mCry3A for the CPB larvae.
Phase II Insecticidal Bioassays
Dose–Response of the Lepidopteran-Active Protein Mixture Using ECB to Establish Two Intermediate Mortality Doses (Projected LC30 and LC60) for the Combined Lepidopteran- and Coleopteran-Active Mixture Interaction Testing
The mortalities for the lepidopteran-active protein mixture (concentrations ranking from 1X to X/128) were assessed and a clear dose–response was evident with a decrease in activity corresponding to the dilution series (Fig. 4), but with no effect at the lowest concentration (X/128). The negative control showed a low mortality of 4.3% (data not shown).
Fig. 4.
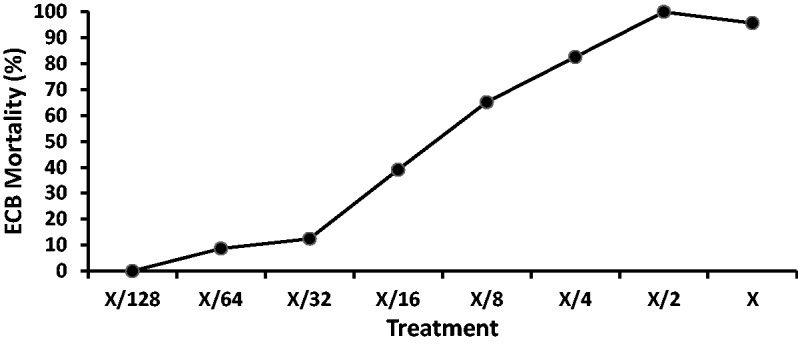
Percent mortality of ECB exposed to the lepidopteran-active protein mixture treatments. Treatment X contains 400 ng Cry1Ab + 200 ng Vip3Aa20 + 800 ng Cry1F per ml diet as described in the Methods section.
Two concentrations of the lepidopteran-active protein mixture that showed intermediate levels of response, one around the 30% mortality response (ECB low dose) and one around the 60% mortality response (ECB high dose) were identified at X/16 (25 ng Cry1Ab + 12.5 ng Vip3Aa20 + 50 ng Cry1F/ml diet) and X/8 (50 ng Cry1Ab + 25 ng Vip3Aa20 + 100 ng Cry1F/ml diet), respectively (Fig. 4).
Dose–Response of the Coleopteran-Active Protein Mixture Using CPB to Establish Two Intermediate Mortality Doses (Projected LC30 and LC60) for the Combined Lepidopteran- and Coleopteran-Active Mixture Interaction Testing
The mortalities for the coleopteran-active protein mixture (concentrations ranking from 1X to X/128) were assessed and a clear dose-response was evident corresponding to the dilution series across the X to X/16 concentrations tested (Fig. 5). The X/32, X/64, and X/128 dilutions also showed minimal mortality, similar to the X/16 concentration. The negative control showed a low mortality of 4.2% (data not shown).
Fig. 5.
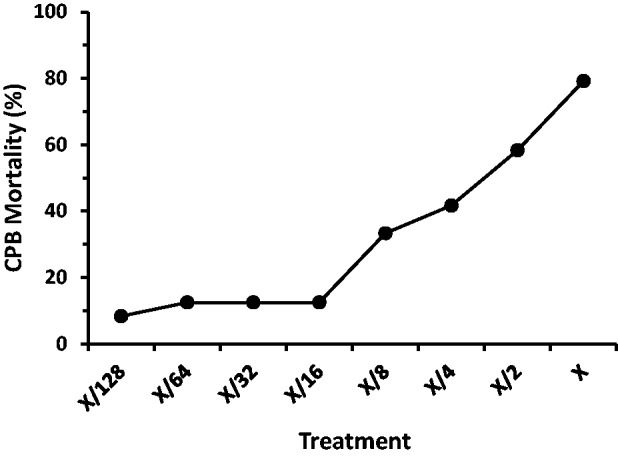
Percent mortality of CPB exposed to the coleopteran-active protein mixture treatments. Treatment X contains 8 µg mCry3A + 8 µg eCry3.1Ab per ml diet as described in the Methods section.
Two concentrations of the coleopteran-active protein mixture that showed intermediate levels of response, one around the 30% mortality response (CPB low dose) and one around the 60% mortality response (CPB high dose) were identified at X/8 (1 µg mCry3A + 1 µg eCry3.1Ab/ml diet) and X/2 (4 µg mCry3A + 4 µg eCry3.1Ab/ml diet), respectively (Fig. 5).
Testing for Effects of the Coleopteran-Active Protein Mixture on the Mortality of ECB Exposed to the Lepidopteran-Active Protein Mixture
The percent mortality of ECB for the lepidopteran-active protein mixture alone was near the targeted mortality for the ECB low dose (26.8%, with ∼30% mortality as the target, based on the preliminary dose–response), and slightly above the targeted mortality for the ECB high dose (76%, with ∼60% mortality as the target) across the three independent bioassays (Table 5). Similar results were observed when these data were compared with the respective ECB low dose or ECB high dose in combination with the coleopteran-active protein mixture (i.e. plus the CPB high dose) (Tables 5 and 6).
Table 5.
Effect of the coleopteran-active protein mixture on the insecticidal activity of the lepidopteran-active protein mixture using ECB
| Treatmenta | Mortality (%) |
|||
|---|---|---|---|---|
| Test 1 | Test 2 | Test 3 | Mean | |
| ECB low dosea | 25.0 | 26.1 | 29.2 | 26.8 |
| ECB low dose + CPB high dose | 20.8 | 45.8 | 20.8 | 29.1 |
| ECB high dose | 70.8 | 69.6 | 87.5 | 76.0 |
| ECB high dose + CPB high dose | 58.3 | 73.9 | 79.2 | 70.5 |
| CPB high dose | 4.3 | 4.2 | 16.7 | 8.4 |
| Negative control 1 | 4.2 | 4.2 | 8.3 | 5.6 |
| Negative control 2 | 4.2 | 0.0 | 4.2 | 2.8 |
Respective low and high dose treatments as defined in preceding sections earlier. Negative control 1 equals 20 mM CAPS pH 10.5 buffer, negative control 2 equals mixture of 20 mM CAPS (pH 10.5) + 10 mM ammonium bicarbonate (pH 10.0) + purified water.
Table 6.
Statistical analysis of the ECB bioassay to investigate the effect of the coleopteran-active protein mixture on the insecticidal activity of the lepidopteran-active protein mixture
| Dose | x Inactiv ingredient | Mean % mortality |
|---|---|---|
| ECB low dose | Absent | 26.8 |
| ECB low dose | CPB high dose | 29.1 |
| ECB high dose | Absent | 76.0 |
| ECB high dose | CPB high dose | 70.5 |
| Mean of dose across inactive ingredienta | ||
| ECB low dose | 28.0 | |
| ECB high dose | 73.2 | |
| Mean of inactive ingredient across doseb | ||
| Absent | 51.4 | |
| CPB high dose | 49.8 | |
| F-test probabilitiesc | ||
| Dose (active ingredient) | <0.001 | |
| Inactive ingredient | 0.792 | |
| Dose × Inactive ingredient | 0.514 | |
| Standard deviation | 9.8 |
The percent mortality for each respective ECB dose was averaged across the absence or presence of the inactive ingredient (CPB high dose).
The percent mortality for either the absence or presence of the inactive ingredient (CPB high dose), respectively, was averaged across the two ECB doses.
Significance level of α = 0.05; df = 11.
The positive control (CPB high dose against CPB) had an average of 61.8% mortality (Table 7), confirming the biological activity of the coleopteran-active protein mixture for this interaction testing. In contrast, the negative control diet treated with CPB high dose (alone) (Table 5) showed an average of only 8.4% mortality, which confirmed the absence of nonspecific effects derived from eCry3.1Ab and mCry3A on the ECB larvae. The two buffer negative control diets showed an average of only 5.6 or 2.8% mortality, respectively. Although the dose effect for the lepidopteran-active protein mixture on ECB mortality (i.e., the ECB low dose vs. high dose) was significant, as intended, there were no statistically significant effects with the addition of the inactive ingredient (CPB high dose) and no statistically significant “Dose × Inactive ingredient” interactions detected (Table 6). The results of this experiment demonstrate that the coleopteran-active protein mixture had no effect on the level of mortality produced by the lepidopteran-active protein mixture against ECB.
Table 7.
Effect of the lepidopteran-active protein mixture on the insecticidal activity of the coleopteran-active protein mixture using CPB
| CPB mortality (%) |
||||
|---|---|---|---|---|
| Treatmenta | Test 1 | Test 2 | Test 3 | Mean |
| CPB low dosea | 66.7 | 45.8 | 41.7 | 51.4 |
| CPB low dose + ECB high dose | 58.3 | 54.2 | 33.3 | 48.6 |
| CPB high dose | 75.0 | 62.5 | 47.8 | 61.8 |
| CPB high dose + ECB high dose | 87.5 | 70.8 | 87.5 | 81.9 |
| ECB high dose | 0.0 | 4.2 | 8.3 | 4.2 |
| Negative control 1 | 0.0 | 8.3 | 4.2 | 4.2 |
| Negative control 2 | 4.2 | 8.3 | 8.3 | 6.9 |
Respective low dose and high dose treatments as defined in preceding sections earlier. Negative control 1 equals mixture of 10 mM ammonium bicarbonate (pH 10.0) + purified water, negative control 2 equals mixture of 20 mM CAPS (pH 10.5) + 10 mM ammonium bicarbonate (pH 10.0) + purified water.
Testing for Effects of the Lepidopteran-Active Protein Mixture on the Mortality of CPB Exposed to the Coleopteran-Active Protein Mixture
The percent mortality of CPB for the coleopteran-active protein mixture alone was near the targeted mortality for the CPB high dose (61.8%, with ∼60% mortality as the target, based on the preliminary dose–response), and slightly above the targeted mortality for the CPB low dose (51.4%, with ∼30% mortality as the target) across the three independent bioassays (Table 7). Similar results were observed when these data were compared with the respective CPB low dose or CPB high dose in combination with the lepidopteran-active protein mixture (i.e., plus the ECB high dose) (Tables 7 and 8).
Table 8.
Statistical analysis of the CPB bioassay to investigate the effect of the lepidopteran-active protein mixture on the insecticidal activity of the coleopteran-active protein mixture
| Dose | x Inactive Ingredient | Mean % mortality |
|---|---|---|
| CPB low dose | Absent | 51.4 |
| CPB low dose | ECB high dose | 48.6 |
| CPB high dose | Absent | 61.8 |
| CPB high dose | ECB high dose | 81.9 |
| Mean of dose across inactive ingredienta | ||
| CPB low dose | 50.0 | |
| CPB high dose | 71.9 | |
| Mean of inactive ingredient across doseb | ||
| Absent | 56.6 | |
| ECB high dose | 65.3 | |
| F-test probabilitiesc | ||
| Dose (active ingredient) | 0.006 | |
| Inactive ingredient | 0.147 | |
| Dose x Inactive ingredient | 0.070 | |
| Standard deviation | 9.0 |
The percent mortality for each respective CPB dose was averaged across the absence or presence of the inactive ingredient (ECB high dose).
The percent mortality for either the absence or presence of the inactive ingredient (ECB high dose), respectively, was averaged across the two CPB doses.
Significance level of α = 0.05; df = 11.
The positive control (ECB high dose against ECB) had an average of 76.0% mortality (Table 5), confirming the biological activity of the lepidopteran-active protein mixture for this interaction testing. In contrast, the negative control diet treated with ECB high dose (alone) (Table 7) showed an average of only 4.2% mortality, which confirmed the absence of nonspecific effects derived from Cry1Ab, Vip3Aa20, and Cry1F on the CPB larvae. The two buffer negative control diets showed an average of only 4.2 or 6.9% mortality, respectively. Although the dose effect for the coleopteran-active protein mixture on CPB mortality was significant, as expected (i.e., the CPB low dose vs. high dose), there were no statistically significant effects with the addition of the inactive ingredient (ECB high dose) and no statistically significant “Dose × Inactive ingredient” interactions detected (Table 8). The results of this experiment demonstrate that the lepidopteran-active protein mixture had no effect on the level of mortality produced by the coleopteran-active protein mixture against CPB.
Discussion
An efficient and streamlined methodology was constructed to investigate potential interactions among multiple insecticidal proteins that employ different modes of action and/or are active against different insect spectra or orders (e.g., insect pests from the orders Lepidoptera or Coleoptera). This approach builds on previous methods and examples that involved fewer insecticidal proteins and/or less complex combinations (e.g., Raybould et al. 2012) and captures a way forward for future more complex interaction testing needs. For the context of these type of investigations, we adopted the most widely interpreted definition of a “different” mode of action which is used for resistance management practices, that it is related to two insecticidal proteins displaying differential in vitro binding to the gut membranes of the target pest (e.g., a lack of heterologous competition in a binding assay, or the demonstration of unique binding sites being present) (Schnepf et al. 1998, Bravo et al. 2011, Hernández-Rodríguez et al. 2013, Lucena et al. 2014). Although we illustrated the feasibility of this interaction testing method using lepidopteran-active protein mixtures and coleopteran-active protein mixtures, one could modify this framework for other mixture needs.
In phase I of our interaction testing approach, the Colby method was used as a straightforward tool for testing mixtures where the distinct modes of action for the insecticidal proteins has been established. When using the Colby method, it has been noted that it is most accurate when responses to the individual components are near the 50% response level (Gisi et al. 1985); this guidance was contained within our dilution series design that generated multiple response points within ±25% of that target response level for each protein and the mixtures tested (Tables 2–4). Although the Colby method has been criticized in some applications, in terms of its ability to quantify the degree of any interaction which is found (Morse 1978, Nespeca 1997), or its potential to be too liberal in terms of generating false positives in the detection of synergism (Foucquier and Guedj 2015), it remains quite useful to reliably detect gross effects which is really the focus of the interaction testing for risk assessment purposes. In addition, any tendency toward false positives by the Colby method actually provides a highly rigorous test of the hypothesis under consideration for risk assessment (i.e., that there is no synergism).
There was no evidence for synergistic or antagonistic effects upon combining Cry1Ab, Vip3Aa20, and Cry1F in bioassays using the two sensitive Lepidoptera test organisms, ECB or FAW. These results corroborate the hypothesis of no antagonism or synergism among Cry1Ab, Vip3Aa20, and Cry1F present in a mixture when compared with the individual components. A tendency toward lower mortality was shown with regard to the eCry3.1Ab and mCry3A protein mixture on CPB than would be expected from the effects of the proteins alone, which may suggest some slight antagonistic interaction with regard to CPB when these proteins are presented in combination. The nature of this slight effect is uncertain, however, it is evident that the combination of eCry3.1Ab with mCry3A proteins in MIR604 × 5307 maize hybrids Agrisure Duracade (Syngenta Seeds, Inc., Minnetonka, MN) results in a stack which is highly effective against the WCRW (Hibbard et al. 2011, Frank et al. 2015).
In phase II of the interaction testing design, the ECB bioassay results indicate that the coleopteran-active protein mixture does not affect the activity of the lepidopteran-active protein mixture against ECB. In addition, the CPB bioassay results indicate that the lepidopteran-active protein mixture does not affect the activity of the coleopteran-active protein mixture against CPB. Thus, these two results together, using two different test species, provide robust testing and corroboration of the hypothesis of no antagonism or synergism among Cry1Ab, Vip3Aa20, Cry1F, eCry3.1Ab, and mCry3A.
These results are not surprising, given the general trends for these insecticidal protein types. Observation of synergistic interactions amongst the Bt-derived insecticidal proteins does not appear to be routine for how these lepidopteran-active and coleopteran-active proteins act in nature, with relatively few reports of this from amongst the over 480 Cry1, Cry2, Cry3 and Vip3 proteins identified to date (Crickmore et al. 2016). Furthermore, previous work indicated no interaction with a combination of Cry1Ab and Vip3A proteins (US EPA 2009b). In contrast, these types of interactions have been well-documented and routinely included in descriptions of the function of mosquitocidal Bt proteins (Wirth et al. 1997, Bravo et al. 2011, Palma et al. 2014). In addition, in those cases where a synergistic interaction has been reported for the lepidopteran-active and coleopteran-active Bt proteins, it is often in the range of just a few-fold or less. Results from laboratory interaction testing that suggest a more minimal increase in activity could be due to real effects or still represent random variation in the susceptible pest insect bioassay data. In either case, the biological relevance would need to be evaluated for Bt-derived insecticidal proteins in the context of a risk assessment. That is, those interactions which can be reproducibly established for insecticidal proteins in a trait stack must be evaluated for biological relevance in the context of other risk assessment parameters (e.g., likelihood of a potential route of exposure or the actual margins of exposure) which often supersede the postulated synergistic interaction that might occur (Raybould et al. 2012).
A FIFRA Scientific Advisory Panel stated it was “not aware of any instances where a ‘new’ toxin has been created by unexpected interaction between two known proteins” (US EPA 2005). In addition, a 2009 U.S. EPA Scientific Advisory Panel observed that “with respect to Cry1 and Cry3 proteins used in Bt crops, given their proven safety record, unless a >10-fold degree of synergism is observed, there would seem to be no need to test for human health or nontarget effects” (US EPA 2009c). Nevertheless, the results of these types of insecticidal protein interaction studies do support the weight-of-evidence approach for a risk assessment regarding the potential occurrence of interactions in nonsensitive nontarget species, including humans and other animals (Raybould et al. 2012).
Absence of interactions between Cry1Ab, Vip3Aa20, and Cry1F corroborate the hypothesis that the responses of organisms exposed to these proteins via cultivation of Bt11 × MIR162 × TC1507 × MIR604 × 5307 maize hybrids can be reliably predicted from the effects of the component events. Similarly, slight antagonism between eCry3.1Ab and mCry3A corroborates the hypothesis that any adverse effect of a mixture of these proteins would be no greater than, and potentially less than, the sum of the individual effects of these proteins. Finally, absence of interactions between (Cry1Ab + Vip3A + Cry1F) and (eCry3.1Ab + mCry3A) corroborate the hypothesis that the responses of organisms exposed to these proteins via cultivation of Bt11 × MIR162 × TC1507 × MIR604 × 5307 maize hybrids can be reliably predicted from the effects of the component events.
If product development continues to increase the number of insecticidal proteins in stacks, it is important to note that the results from the testing of insecticidal protein mixtures which establishes them as having no interaction can be used in support of any further stack assessments which additionally include other combinations of insecticidal proteins. The future assessment of more complex stacks that express additional insecticidal proteins would only need to confirm that there is no new interaction between the previous mixture (or a subset of those proteins) and the newly added protein component. The previous conclusions regarding interactions for those proteins tested would not be nullified by added components, nor would it necessitate that all of the previously tested components remain present in the new complex mixture. In this way, prior data can be useful to draw inferences for risk assessment as long as the proteins are present in the “lower order” stack as well as the new complex mixture.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
Disclosure of Potential Conflicts of Interest
All authors are employees of Syngenta Crop Protection, LLC.
Supplementary Material
Acknowledgements
We gratefully acknowledge the assistance of Dr. Keith Ward (Syngenta Jealott’s Hill International Research Center) for help with the statistical analyses. We also thank the protein production team at the Syngenta Jealott’s Hill International Research Center for providing the Cry1Ab, Vip3Aa20, mCry3A, and eCry3.1Ab purified protein test substances used in this work. In addition, we acknowledge the assistance of Jared Conville (Syngenta RTP Innovation Center) for help in supplying the insect larvae.
Footnotes
1 Abbott’s formula for control mortality: (% treatment mortality − % control mortality) / (100 − % control mortality) × 100.
References Cited
- Abbott W. S. 1925. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265–267. [Google Scholar]
- Bravo A., Likitvivatanavong S., Gill S. S., Soberón M. 2011. Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 41: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrière Y., Crickmore N., Tabashnik B. E. 2015. Optimizing pyramided transgenic Bt crops for sustainable pest management. Nat. Biotech. 33: 161–168. [DOI] [PubMed] [Google Scholar]
- Chestukhina G. G., Kostina L. I., Mikhailova A. L., Tyurin S. A., Klepikova F. S., Stepanov V. M. 1982. The main features of Bacillus thuringiensis delta-endotoxin molecular structure. Arch. Microbiol. 132: 159–162. [Google Scholar]
- Colby S. R. 1967. Calculating synergistic and antagonistic response of herbicide combinations. Weeds 15: 20–22. [Google Scholar]
- Crickmore N., Baum J., Bravo A., Lereclus D., Narva K., Sampson K., Schnepf E., Sun M., Zeigler D. R. 2016. Bacillus thuringiensis toxin nomenclature. (http://www.btnomenclature.info/) (accessed 19 April 2016).
- English L. H., Brussock S. M., Malvar T. M., Bryson J. W., Kulesza C. A., Walters F. S., Slatin S. L., Von Tersch M. A., Romano C. 2003. Nucleic acid compositions encoding modified Bacillus thuringiensis coleopteran-toxic crystal proteins. U.S. Patent No. 6,642,030. [Google Scholar]
- Estruch J. J., Warren G. W., Mullins M. A., Nye G. J., Craig J. A., Koziel M. G. 1996. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl. Acad. Sci. USA 93: 5389–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucquier J., Guedj M. 2015. Analysis of drug combinations: current methodological landscape. Pharm. Res. Per. 3: e00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. L., Kurtz R., Tinsley N. A., Gassmann A. J., Meinke L. J., Moellenbeck D., Gray M. E., Bledsoe L. W., Krupke C. H., Estes R. E., Weber P., Hibbard B. E. 2015. Effect of seed blends and soil-insecticide on western and northern corn rootworm emergence from mCry3A + eCry3.1Ab Bt maize. J. Econ. Entomol. 108: 1260–1270. [DOI] [PubMed] [Google Scholar]
- Gisi U., Binder H., Rimbach E. 1985. Synergistic interactions of fungicides with different modes of action. Trans. Br. Mycol. Soc. 85: 299–306. [Google Scholar]
- Graser G., Walters F. S. 2015. A standardized lepidopteran bioassay protocol to investigate the bioactivity of insecticidal proteins produced in transgenic crops, 2nd edn, pp. 259–270 InMacDonald J., Kolotilin I., Menassa R. (eds.), Recombinant proteins from plants. Series: Methods in Molecular Biology. Springer, New York. [DOI] [PubMed] [Google Scholar]
- Herman R. A., Scherer P. N., Young D. L., Mihaliak T. M., Meade T., Woodsworth A. T., Stockhoff B. A., Narva K. E. 2002. Binary insecticidal crystal protein from Bacillus thuringiensis, strain PS149B1: Effects of individual protein components and mixtures in laboratory bioassays. J Econ Entomol. 95: 635–639. [DOI] [PubMed] [Google Scholar]
- Herman R. A., Phillips A. M., Collins R. A., Tagliani L. A., Claussen F. A., Graham C. D., Bickers B. L., Harris T. A., Prochaska L. M. 2004. Compositional equivalency of Cry1F corn event TC6275 and conventional corn (Zea mays L.). J Agric Food Chem. 52: 2726–2734. [DOI] [PubMed] [Google Scholar]
- Hernández-Rodríguez C. S., Hernández-Martínez P., Van Rie J., Escriche B., Ferré J. 2013. Shared midgut binding sites for Cry1A.105, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinia nubilalis and Spodoptera frugiperda. PLoS One 8: 0491–0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard B. E., Frank D. L., Kurtz R., Boudreau E., Ellersieck M. R., Odhiambo J. F. 2011. Mortality impact of Bt transgenic corn roots expressing eCry3.1Ab, mCry3A, and eCry3.1Ab plus mCry3A on western corn rootworm larvae in the field. J. Econ. Entomol. 104: 1584–1591. [DOI] [PubMed] [Google Scholar]
- Huang F. 2015. Resistance management for Bt maize and above-ground Lepidopteran targets in the USA: from single gene to pyramided traits, pp. 173–185. InSoberón M., Gao Y., Bravo A A. (eds.), Bt resistance: characterization and strategies for GM crops producing Bacillus thuringiensis toxins. CABI, Boston. [Google Scholar]
- Koziel M. G., Beland G. L., Bowman C., Carozzi N. B., Crenshaw R., Crossland L., Dawson J., Desai N., Hill M., Kadwell S., et al. 1993. Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis. BioTechnology 11: 194–200. [Google Scholar]
- Lee M. K., Walters F. S., Hart H., Palekar N., Chen J. S. 2003. The mode of action of the Bacillus thuringiensis vegetative insecticidal protein Vip3Aa20 differs from that of Cry1Ab δ-endotoxin. Appl. Environ. Microbiol. 69: 4648–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightwood D. J., Ellar D. J., Jarrett P. 2000. Role of proteolysis in determining potency of Bacillus thuringiensis Cry1Ac δ-endotoxin. Appl. Environ. Microbiol. 66: 5174–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucena W. A., Pelegrini P. B., Martins-de-Sa D., Fonseca F. C., Gomes J. E., Jr, de Macedo L. L., da Silva M. C., Oliveira R. S., Grossi-de-Sa M. F. 2014. Molecular approaches to improve the insecticidal activity of Bacillus thuringiensis Cry toxins. Toxins 6: 2393–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellenbeck D. J., Peters M. L., Bing J. W., Rouse J. R., Higgins L. S., Sims L., Nevshemal T., Marshall L., Ellis R. T., Bystrak P. G., Lang B. A., Stewart J. L., Kouba K., Sondag V., Gustafson V., Nour K., Xu D., Swenson J., Zhang J., Czapla T., Schwab G., Jayne S., Stockhoff B A., Narva K., Schnepf H. E., Stelman S. J., Poutre C., Koziel M., Duck N. 2001. Insecticidal proteins from Bacillus thuringiensis protect corn from corn rootworms. Nat Biotechnol. 19: 668–672. [DOI] [PubMed] [Google Scholar]
- Morse P. M. 1978. Some comments on the assessment of joint action in herbicide mixtures. Weed Sci. 26: 58–71. [Google Scholar]
- Nespeca M. 1997. Interactive Effects of Imazapyr plus Triclopyr ester and Imazapyr plus Glyphosate Mixtures on Woody Weed Seedlings. M.S. thesis, Virginia Tech. University
- Niu Y., Yang F., Dangal V., Huang F. 2014. Larval survival and plant injury of Cry1F-susceptible, -resistant, and -heterozygous fall armyworm (Lepidoptera: Noctuidae) on non-Bt and Bt corn containing single or pyramided genes. Crop Prot. 59: 22–28. [Google Scholar]
- Oppert B. 1999. Protease interactions with Bacillus thuringiensis insecticidal toxins. Arch. Insect Biochem. Physiol. 42: 1–12. [DOI] [PubMed] [Google Scholar]
- Palma L., Muñoz D., Berry C., Murillo J., Caballero P. 2014. Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins. 6: 3296–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que Q., Chilton M. D. M., de Fontes C. M., He C., Nuccio M., Zhu T., Wu Y., Chen J. S., Shi L. 2010. Trait stacking intransgenic crops: challenges and opportunities. GM Crops 1: 220–229. [DOI] [PubMed] [Google Scholar]
- Raybould A., Graser G., Hill K., Ward K. 2012. Ecological risk assessments for transgenic crops with combined insect-resistance traits: the example of Bt11 x MIR604 maize. J. Appl. Entomol. 136: 27–37. [Google Scholar]
- Schnepf E., Crickmore N., Van Rie J., Lereclus D., Baum J., Feitelson J., Zeigler D. R., Dean D. H. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62: 775–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert M. W., Babock J. M., Nolting S., Santos A. C., Adamczyk J. J., Jr, Neese P. A., King J. E., Jenkins J. N., McCarty J., Lorenz G. M., et al. 2008. Efficacy of Cry1F insecticidal protein in maize and cotton for control of fall armyworm (Lepidoptera: Noctuidae). Fl. Entomol. 91: 555–565. [Google Scholar]
- Siegfried B. D., Spencer T., Crespo A. L., Storer N. P., Head G. P., Owens E. D., Guyer D. 2007. Ten years of monitoring for Bt resistance in the European corn borer: What we know, what we don't know and what we can do better. Am. Entomol. 53: 208–214. [Google Scholar]
- Siegfried B. D., Rangasamy M., Wang H., Spencer T., Haridas C. V., Tenhumberg B., Sumerford D. V., Storer N. P. 2014. Estimating the frequency of Cry1F resistance in field populations of the European corn borer (Lepidoptera: Crambidae). Pest Manag. Sci. 70: 725–733. [DOI] [PubMed] [Google Scholar]
- US EPA U. S. Environmental Protection Agency. 2005. Biopesticides Registration Action Document – Bacillus thuringiensis var. aizawai Cry1F and the genetic material (from the insert of plasmid PGMA281) necessary for its production in cotton and Bacillus thuringiensis var. kurstaki Cry1Ac and the genetic material (from the insert of plasmid PMYC3006) necessary for its production in cotton. (http://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-006512_1-Sep-05.pdf) (accessed 22 December 2015).
- US EPA U. S. Environmental Protection Agency. 2007. Memorandum. 9 Pages. Mika J. Hunter. Microbial Pesticides Branch. Review of “Evaluation of Potential Interactions between the Bacillus thuringiensis Proteins Cry1A.105, Cry2Ab2, and Cry3Bb1” for Monsanto's MON 89034 X MON 88017 Maize. MRID 469513-05 & 469513-06. DP# 335188. Document (PDF) (347 KB PDF). (https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/006514/006498-006514-006515-2007-12-14a.pdf) (accessed 22 December 2016).
- US EPA U. S. Environmental Protection Agency. 2009a. Position paper on scientific issues associated with the data required to register Plant-Incorporated Protectants (https://www.regulations.gov/#!documentDetail;=EPA-HQ-OPP 2008. 0835-003) (accessed 15 June 2016).
- US EPA U. S. Environmental Protection Agency. 2009b. Biopesticides Registration Action Document – Bacillus thuringiensis Vip3Aa20 Insecticidal Protein and the Genetic Material Necessary for Its Production (via Elements of Vector pNOV1300) in Event MIR162 Maize (OECD Unique Identifier: SYN-IR162-4), p. 76. (http://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-006599_3-Apr-09.pdf) (accessed 19 April 2016).
- US EPA U. S. Environmental Protection Agency. 2009c. SAP Minutes No. 2009-04. A set of scientific issues considered by the Environmental Protection Agency regarding: the data required to register plant-incorporated protectants. (http://archive.epa.gov/scipoly/sap/meetings/web/pdf/022526finalreport.pdf) (accessed 22 December 2015).
- Walters F. S., deFontes C. M., Hart H., Warren G. W., Chen J. S. 2010. Lepidopteran-active variable region sequence imparts coleopteran activity in eCry3.1Ab, an engineered Bacillus thuringiensis hybrid insecticidal protein. Appl. Environ. Microbiol. 76: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters F. S., Stacy C. M., Lee M. K., Palekar N., Chen J. S. 2008. An engineered chymotrypsin/cathepsin G site in Domain I renders Bacillus thuringiensis Cry3A active against Western Corn Rootworm larvae. Appl. Environ. Microbiol. 74: 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M. C., Georghiou G. P., Federici B. A. 1997. CytA enables CryIV endotoxins of Bacillus thuringiensis to overcome high levels of CryIV resistance in the mosquito, Culex quinquefasciatus. Proc. Natl. Acad. Sci. 94: 10536–10540. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolt J. D., Conlan C. A., Majima K. 2005. An ecological risk assessment of Cry1F maize pollen impact to pale grass blue butterfly. Environ. Biosafety Res. 4: 243–251. [DOI] [PubMed] [Google Scholar]
- Yu C. G., Mullins M. A., Warren G. W., Koziel M. G., Estruch J. J. 1997. The Bacillus thuringiensis vegetative insecticidal protein Vip3A lyses midgut epithelium cells of susceptible insects. Appl. Environ. Microbiol. 63: 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.