Abstract
Purpose
It is believed that blood from the superior mesenteric vein and splenic vein mixes incompletely in the portal vein and maintains a streamline flow influencing its anatomic distribution. Although several experimental studies have demonstrated the existence of streamlining, clinical studies have shown conflicting results. We investigated whether streamlining of portal vein affects the lobar distribution of colorectal liver metastases and estimated its impact on survival.
Methods
Data of patients who underwent hepatectomy for colorectal liver metastases were retrospectively collected. The chi-square test was used for analyzing the distribution of metastasis. Cox analysis was used to identify risk factors of survival. Fisher exact test was used for subgroup analysis comparing hepatic recurrence.
Results
A total of 410 patients were included. The right-to-left ratio of liver metastases were 2.20:1 in right-sided colon cancer and 1.39:1 in left-sided cancer (P = 0.017). Cox analyses showed that margin < 5 mm (P < 0.001; 95% confidence interval [CI], 1.648–4.884; hazard ratio [HR], 2.837), age ≥ 60 years (P = 0.004; 95% CI, 1.269–3.641; HR, 2.149), N2 status (P < 0.001, 95% CI, 1.598–4.215; HR, 2.595), tumor size ≥ 45 mm (P = 0.014; 95% CI, 1.159–3.758; HR, 2.087) and other metastasis (P = 0.012; 95% CI, 1.250–5.927; HR, 2.722) were risk factors of survival. However, in 70 patients who underwent right hemihepatectomy for solitary metastasis, left-sided colorectal cancer was a risk factor (P = 0.019; 95% CI, 1.293–17.956; HR, 4.818), and was associated with higher recurrence than right-sided cancer (43.1% and 15.8%, respectively, P = 0.049).
Conclusion
This study showed significant difference in lobar distribution of liver metastases between right colon cancer and left colorecral cancer. Furthermore, survival of left-sided colorectal cancer was poorer than that of right-sided cancer in patients who underwent right hemihepatectomy for solitary metastasis. These findings can be helpful for clinicians planning treatment strategy.
Keywords: Colorectal liver metastasis, Streamline flow, Portal vein, Colon cancer, Liver metastasis
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in males and the second most common in females, causing 608,700 global deaths in 2008 [1]. Liver is the most common site of metastasis, and nearly 50% of stage IV CRC cases are metastasized only to the liver [2]. Although stage IV cancer is generally considered uncontrollable, the idea of resecting the primary CRC and liver metastasis for potential cure has gained wide acceptance as a safe and successful treatment [3,4].
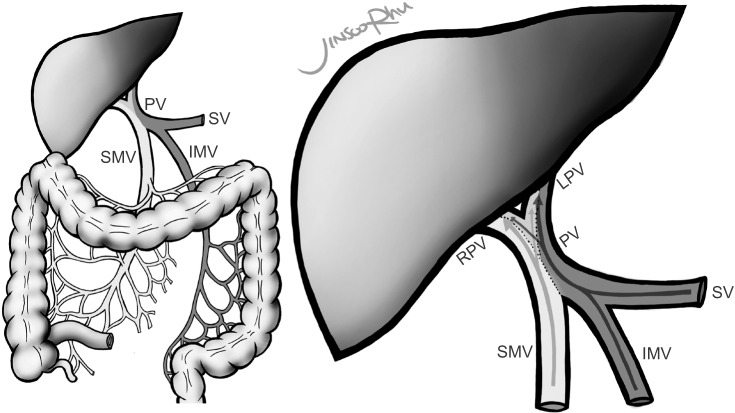
One of the areas of interest in CRC is the route of metastasis. The venous drainage from the right and transverse colon flows into the superior mesenteric vein (SMV), while venous drainage from the left colon and upper rectum flows into the inferior mesenteric vein (IMV). The portal vein (PV) is formed by the confluence of the SMV and the splenic vein (SV). Based on the double origin of the PV, studies have demonstrated the segregation of blood streams within the PV, the so called “streamline flow” [5,6]. It has been theorized that venous blood flows from the SMV and the IMV mix incompletely in the PV, resulting in disproportionate lobar distribution within the liver (Fig. 1). Copher and Dick [5] reported an experiment visualizing separate streams of blood flow of the PV after injecting trypan blue into its tributaries in dogs. As a human study, Moore and Bridenbaugh [7] performed portal venography and suggested that streamline flow exists, based on the finding that the SV flows mainly into the left lobe of the liver, whereas the SMV flows mainly into the right. In 2012, a study calculating the diameters of the main venous structures also suggested the existence of streamline flow [8].
Fig. 1. “Streamline flow of the PV” is a theory stating that blood from the superior mesenteric vein (SMV) and inferior mesenteric vein (IMV) mix incompletely in the PV, resulting in disproportionate lobar distribution within the liver. The SMV distributes mainly to the right lobe compared to the IMV, which supplies both hemilivers similarly. PV, portal vein; SV, splenic vein; SMV, superior mesenteric vein; IMV, inferior mesenteric vein; RPV, right PV; LPV, left PV.
Clinical studies investigating such a streamline flow have been published [9,10,11,12,13]. Dionne published a study analyzing the pattern of hematogenous metastasis of rectal cancer, finding no difference in the lobar distribution within the liver [9]. However, the study only included rectal cancer, which drains mainly into the systemic circulation, not the PV. A postmortem study by Schulz et al. [11] also reported no evidence of different lobar distribution based on autopsy findings that showed 1,571 metastatic tumors in 26 livers. However, the study had a limitation that the subjects were mortality cases, which reflects the progressiveness, limiting its interpretation. Recent studies of Shirai et al. [12] and Konopke et al. [10] showed positive results in the analysis of 195 tumors from 85 patients and 264 tumors from 179 patients, respectively. In contrast, Wigmore et al. [13] found no relationship after analyzing 708 metastases in 207 patients. The overall results of these previous studies illustrate the uncertainty of the clinical significance of the ‘theoretical’ streamline flow.
Therefore, we investigated whether streamline flow of the PV affects the lobar distribution of metastases by analyzing patients with colorectal liver metastasis (CLM) who underwent hepatectomy. Furthermore, we aimed to determine a clinical implication of the streamline flow by estimating its impact on survival.
METHODS
Patients
Data were retrospectively collected from the database of the institution. The included patients underwent curative hepatectomy for CLM from January 1995 to December 2012 at Samsung Medical Center, Seoul, Korea.
Patients who only underwent biopsy were excluded. Patients who underwent radiofrequency ablation were also excluded due to possible misdiagnosis. For the same reason, patients who were pathologically negative for CLM after hepatectomy were also excluded.
Primary tumor of the colon
Patients who had CRC located between the cecum and upper rectum were included, while mid-to-lower rectal cancers were excluded because of the different venous outflow. We only included patients in whom rectal cancer was located 11 cm or more from the anal verge. Tumor location was based on review of medical and operative records. Patients with cancer located between the cecum and transverse colon were included in the right colon group, while patients with cancer located between the splenic flexure and upper rectum were included in the left colorectum group. Staging was based on the guidelines of the American Joint Committee on Cancer 7th edition, and the data was based on review of pathology reports.
Liver metastasis
The location of metastasis was determined based on Cantlie's line, after reviewing preoperative imaging, operation records, and pathology reports. When a tumor extended beyond Cantlie's line, the center core was the key for judging the location. Only the first hepatectomy was included when a patient underwent multiple hepatectomies due to recurrence.
Prognostic factors of survival
To evaluate the prognostic factors of CLM, data related to survival were reviewed. Patient age, sex, T stage, N stage, pre- and postoperative CEA levels, presence of additional metastatic organs at the time of hepatectomy, size and number of metastatic tumor, resection margin, type of operation, recurrences, death, and follow-up duration were collected. Preand postoperative CEA levels included those of both colectomy and hepatectomy. Follow-up duration was defined as months between hepatectomy and final follow-up or death.
The continuous variables were divided into 2 groups at the point where they had the minimal P-value. Therefore, age was divided based on a cutoff of 60 years, while dominant tumor size and margin were divided based on 45 mm and 5 mm, respectively. The number of metastases was divided into single and multiple metastases. Serum CEA levels before both colectomy and hepatectomy were divided based on 10 ng/mL, while postoperative levels were divided based on 5 ng/mL.
Statistical analysis
Chi-square test with Yates' continuity correction was used in analyzing the difference in lobar distribution of liver metastasis of CRC. Cox proportional hazard ratio was used in analyzing risk factors of survival.
In the subgroup analysis, all the factors shown to be related to survival were included along with primary tumor location and were analyzed with Cox analysis. Fisher exact test was used for analyzing the relation of primary tumor location and hepatic recurrence in the subgroup analysis. Statistical analyses were performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
A total of 571 patients underwent curative hepatectomy for CLM. Of these, 161 had mid-to-lower rectal cancer, leaving 410 patients to be included in the study. Most of the patients included in the study experienced metastases only in the liver, while 28 patients also had lung metastases.
The mean follow-up period was 39.9 months (median, 30.5 months). The mean age was 58.8 years (median, 60.0 years), ranging from 26 to 82 years. The mean number of liver metastasis was 1.59, ranging from 1 to 6. The mean size of liver metastasis was 3.29 cm (median, 2.60 cm), ranging from 0.4 to 19 cm. The mean resection margin was 1.39 cm (median, 1.00 cm), ranging from positive margin to 10 cm. Data on margins were absent in 64 patients.
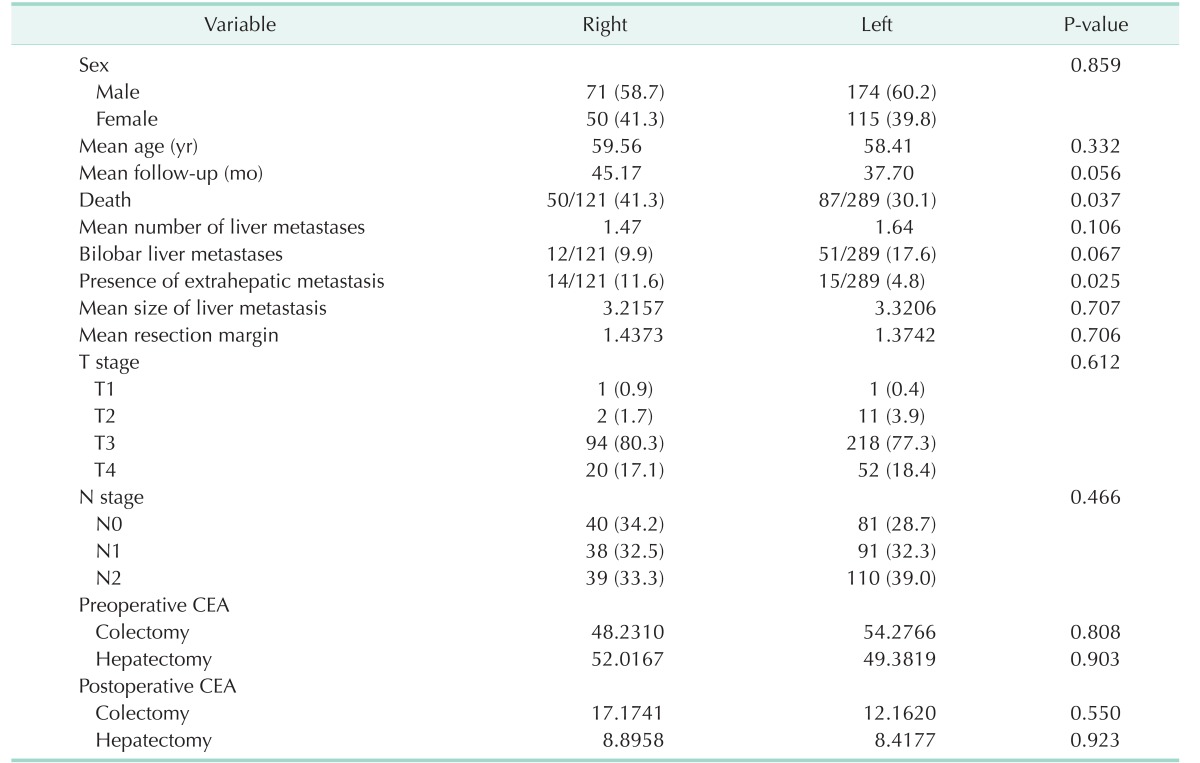
Table 1 summarizes the characteristics of the patients and shows no significant differences in demographics or characteristics between right-sided colon cancer and left-sided CRC except death and presence of other metastasis at the time of hepatectomy. There were 50 (41.3%) and 88 deaths (30.4%) from right and left CRC, respectively (P = 0.044). Fourteen patients with right CRC (11.6%) experienced other metastases, while 15 patients with left CRC (4.8%) experienced such metastases (P = 0.025). However, age, number of metastases, size of metastatic tumor, margin, follow-up period, CEA level, and CA 19-9 level were not significantly different between the 2 groups.
Table 1. Demographics and characteristics of patients with colorectal liver metastases based on site of primary colorectal cancer.
Values are presented as number (%) unless otherwise indicated.
Six hepatic surgeons performed the surgeries during the period. A total of 33 cases were performed as a laparoscopic hepatectomy. Preoperative CT and MRI were performed, and intraoperative ultrasonography was used for complete resection in selected patients. The principle of resection was to obtain an adequate margin while preserving the liver as much as possible. Among 63 patients who experienced bilobar metastases, 33 underwent minor resection; 15 underwent right hemihepatectomy with minor resection of left-side metastasis; 6 underwent left hemihepatectomy with minor resection of right-side metastasis; 5 underwent extended left hemihepatectomy; and 4 underwent extended right hemihepatectomy. Every patient received adjuvant chemotherapy.
Kaplan-Meier survival curve showed 1-year survival rate (YSR), 2 YSR, and 5 YSR of 95.2%, 81.9%, and 55.8%, respectively.
Lobar distribution of CLM
The lobar distribution of CLM is summarized in Table 2. Right-sided colon cancer was diagnosed in 121 patients; 11 in the cecum, 67 in the ascending colon, 18 in the hepatic flexure, and 25 in the transverse colon. Left-sided CRC was diagnosed in 289 patients; 8 in the splenic flexure, 14 in the descending colon, 192 in the sigmoid colon, 42 in the rectosigmoid colon, and 33 in the upper rectum.
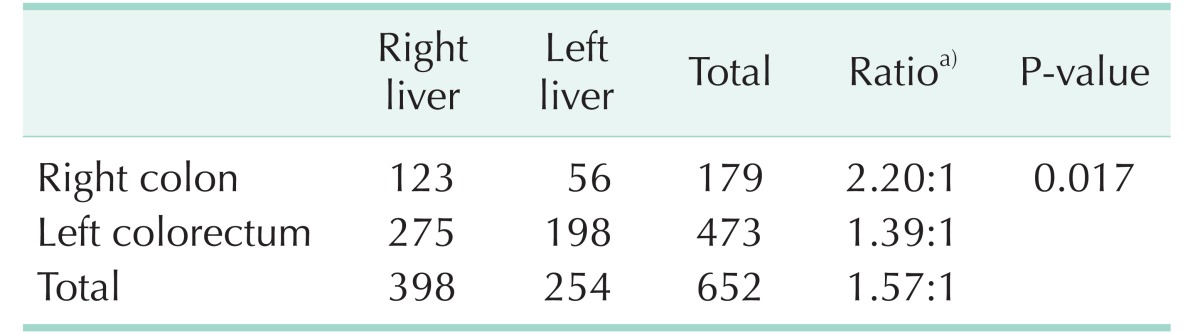
Table 2. The difference in lobar distribution of liver metastasis of colon cancer according to primary tumor site.
a)Right:left.
A total of 652 metastases were histologically confirmed in the specimens. There were 398 metastases (61.0%) found in the right hemiliver, whereas 254 metastases (39.0%) were confirmed in the left. The total ratio of right to left hemilivers was 1.57:1.
In right-sided colon cancer, 123 and 56 metastases were found in the right and left hemilivers, respectively. On the other hand, left-sided CRCs had 275 metastases to the right hemiliver and 198 metastases to the left hemiliver. The ratio of right and left hemilivers was 2.20:1 in right-sided colon cancer and 1.39:1 in left-sided CRC. Chi-square test with Yates' continuity correction showed a significant difference in lobar distribution between right and left CRCs (P = 0.017).
Prognostic factors of survival
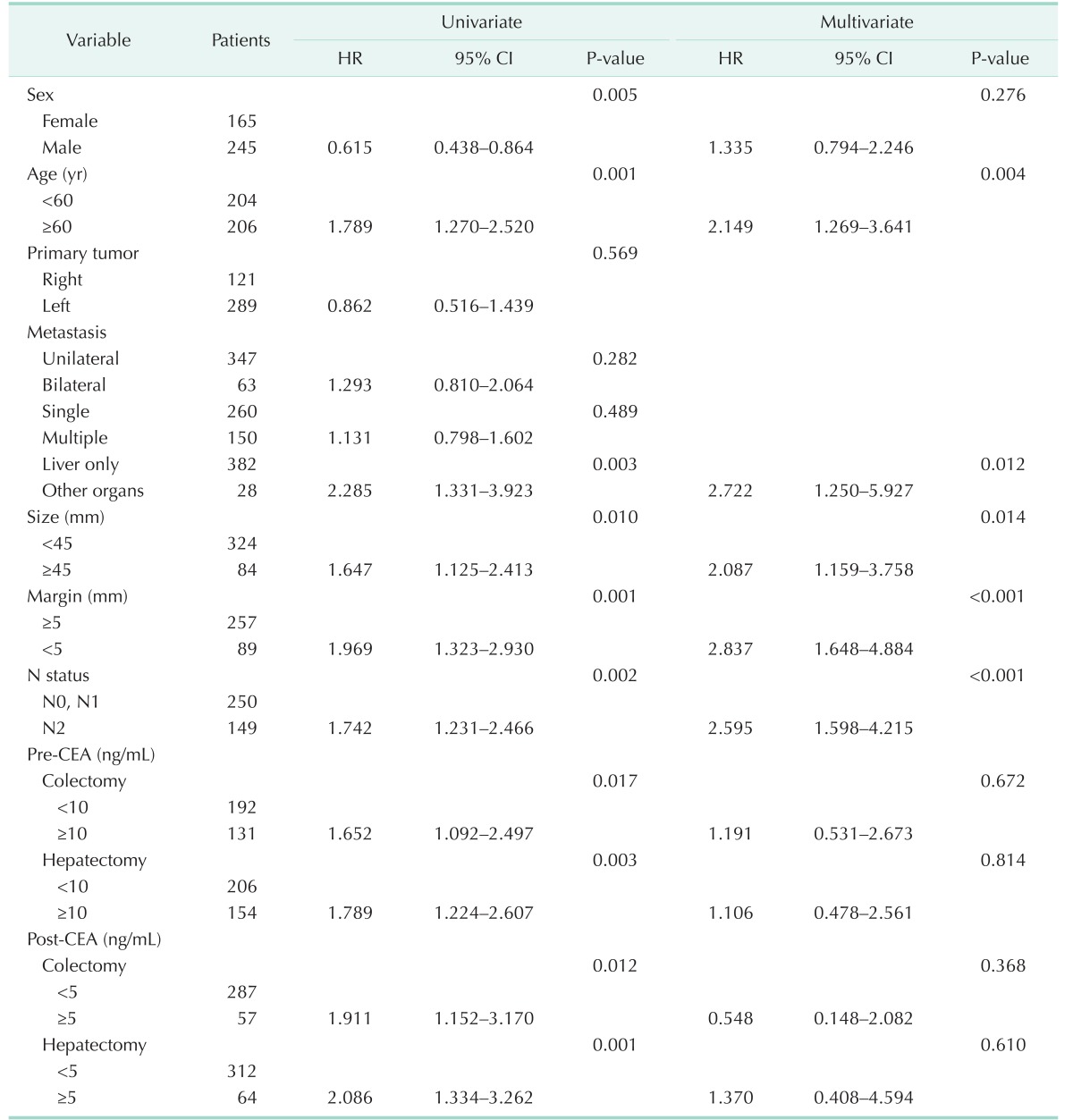
Potential risk factors of survival were analyzed with Cox analysis. In the univariable analysis, sex, age, presence of other metastasis, size, margin, N2 nodal status, and perioperative CEA level were related to survival. Multivariable analysis was performed with the factors that were related to survival in the univariable analysis. In the multivariable analysis, age ≥ 60 years (P = 0.004; hazard ratio [HR], 2.149), additional distant metastasis (P = 0.012; HR, 2.722), dominant tumor size ≥ 45 mm (P = 0.014; HR, 2.087), margin < 5 mm (P < 0.001; HR, 2.837), and N2 status (P < 0.001; HR, 2.595) were significant risk factors related to survival.
The results of univariable and multivariable Cox analyses comparing risk factors of survival are summarized in Table 3.
Table 3. Univariable and multivariable analyses with Cox proportional hazard ratio comparing potential risk factors of survival in colorectal liver metastasis.
Multivariable analysis was performed with the factors related to survival in the univariable analysis.
HR, hazard ratio; CI, confidence interval.
Subgroup analysis: right hemihepatectomy for solitary metastasis
If the lobar distribution of CLM is different between right colon cancer and left CRC, we hypothesized that patients with certain conditions would have different rates of recurrence and survival depending on primary tumor location. For instance, in patients who underwent right hemihepatectomy and thus only have left PV and left hemiliver, the possibility of having undetected metastasis in the remnant liver might be higher in those with a primary tumor located in the left colorectum compared to the right colon. Therefore, we performed a subgroup analysis for this specific group.
A total of 70 patients underwent right hemihepatectomy for solitary liver metastasis. Nineteen patients had right CRC, while 51 patients had left CRC. Death occurred in 5 of 19 patients (26.3%) and 21 of 51 patients (41.2%) with right and left CRC, respectively. The median follow-up period was 34 months.
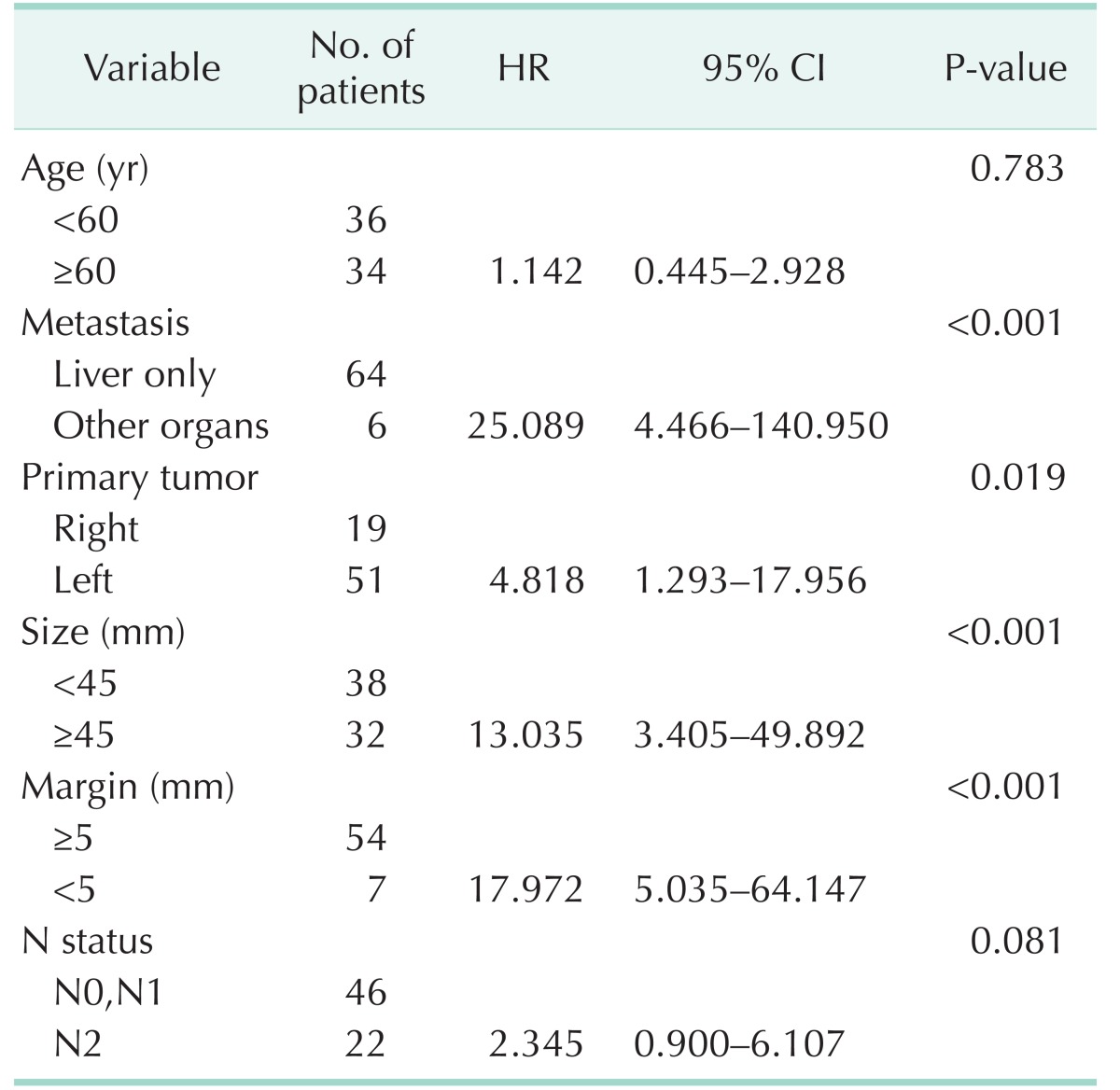
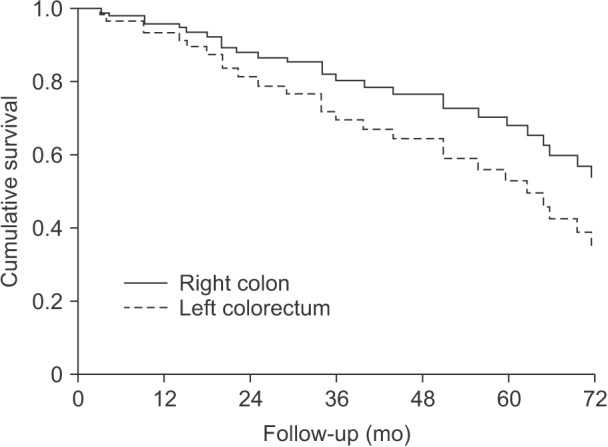
Factors associated with survival in the previous analysis were included in the subgroup analysis along with primary tumor location. The results are summarized in Table 4. Left-sided CRC was a significant risk factor of survival compared to right-sided colon cancer (P = 0.019; HR, 4.818). Other significant factors included size ≥ 45 mm (P < 0.001; HR, 13.035), margin < 5 mm (P < 0.001; HR, 17.972), and additional distant metastasis (P < 0.001; HR, 25.089). The survival curve of these patients based on primary tumor location is visualized in Fig. 2.
Table 4. Multivariable analysis with Cox proportional hazard ratio comparing risk factors of survival in patients who underwent right hemihepatectomy for solitary liver metastasis.
HR, hazard ratio; CI, confidence interval.
Fig. 2. In 70 patients who underwent right hemihepatectomy for solitary liver metastasis of colorectal cancer, left-sided colorectal cancer was a significant risk factor of survival, while right-sided colon cancer was not (P = 0.019; hazard ratio, 4.818).
To support our hypothesis regarding hidden metastasis in the remnant liver, we compared the ratio of hepatic recurrence in these patients. Three of 19 patients (15.8%) in the right colon cancer group experienced hepatic recurrence, while 22 of 51 patients (43.1%) in the left CRC group demonstrated hepatic recurrence. Fisher exact test showed that the difference was statistically significant (P = 0.049).
Likewise, we tried to analyze the effect of primary tumor location in patients who underwent left hemihepatectomy for solitary metastasis. However, it was impossible to draw out any significant result because of the small number of patients.
DISCUSSION
This study showed significant difference in lobar distribution of CLM based on primary tumor location, including 410 resectable cases, which is the largest number to date. Furthermore, this is the first study to show primary tumor location as a factor related to survival. Due to the relatively higher rate of metastasis to the left hemiliver, left-sided CRC has a higher risk of undetected metastasis in the left hemiliver at the time of right hemihepatectomy compared to right-sided colon cancer. Our data also showed that patients who underwent right hemihepatectomy for solitary metastasis had different rates of hepatic recurrence depending on primary tumor location.
The concept of streamline flow suggests that venous flows from the SMV and IMV mix incompletely in the PV, resulting in a disproportionate blood supply to the right and left hemilivers. The initial studies that were designed to support such a hypothesis were experimental studies visualizing portal flow to the liver [5,7].
Based on prior studies, some investigators have attempted to demonstrate a statistical relationship between primary location of CRC and lobar involvement of the liver, assuming that streamline flow delivers cancer cells unequally to the right and left hemilivers. The first published autopsy cases presented disappointing results [9,14]. However, clinical studies have since shown a possible relationship between primary tumor location and liver metastasis. In the study of Shirai et al. [12], right-sided colon cancer showed right lobe predominance in liver metastasis, 29 to the right and 3 to the left, while left-sided CRC had a right to left ratio of approximately 2:1. Two later studies by Wigmore et al. [13] and Konopke et al. [10] demonstrated conflicting results.
Our study was based on curiosity whether the conceptual streamline flow can lead to difference in lobar distribution of liver metastasis. The right to left ratio of liver metastases was 2.20:1 in right colon cancer and 1.39:1 in left CRC. A previous study that calculated the liver volume of 1,000 living donors in Korea showed that the proportion of right liver volume was 65.3%, while that of left liver volume was 34.7%, a right to left ratio of liver volume of 1.88:1 [15]. If liver metastasis is distributed equally based on liver volume, the ratio should have resulted similar to 1.88:1.
In addition to the streamline flow of PV, our study also showed the importance of obtaining an adequate resection margin. Patients who had <5 mm of margin had poorer survival than the patients who had a margin ≥5 mm. This finding is consistent with previous studies, except that they suggested a safety margin >1 cm [2,16,17,18,19]. Nevertheless, the result emphasizes the importance of the quality of surgery, which is the only surgeon-dependent significant prognostic factor. Age, N2 stage, tumor size, and presence of other metastasis were also important factors related to survival in our study. These factors were traditionally considered as important patient-related factors related to survival [2,19,20]. Although some studies have reported the importance of CEA level [2,16,19], our study only showed a relationship in the univariable analysis.
Our findings are noteworthy because, in part, they increase the understanding of the initial pathway of hematogenous metastasis of CRC. The subgroup analysis included 70 patients, which is approximately one-sixth of the study population. Although we should be cautious in interpreting the result, it is a unique finding suggesting that, even in patients with the same TNM stage who underwent the same surgery, prognosis can be different based on primary tumor location. This significant finding can help surgical and medical oncologists plan their treatment strategy for individual patients.
Although our study showed a meaningful result in the lobar distribution of CLM, more data from other institutions are needed to verify its clinical significance. Our study has a limitation in that it was a retrospective study. The difference in lobar distribution cannot be directly attributed to streamline flow. The small number of patients in the subgroup analysis limits the validity of the result. Therefore, additional studies from other institutions and a well-designed experimental study of portal venous flow are needed to reach a valid conclusion.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Tocchi A, Mazzoni G, Brozzetti S, Miccini M, Cassini D, Bettelli E. Hepatic resection in stage IV colorectal cancer: prognostic predictors of outcome. Int J Colorectal Dis. 2004;19:580–585. doi: 10.1007/s00384-004-0594-4. [DOI] [PubMed] [Google Scholar]
- 3.Morise Z, Sugioka A, Fujita J, Hoshimoto S, Kato T, Hasumi A, et al. Does repeated surgery improve the prognosis of colorectal liver metastases? J Gastrointest Surg. 2006;10:6–11. doi: 10.1016/j.gassur.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Lee H, Heo JS, Cho YB, Yun SH, Kim HC, Lee WY, et al. Hepatectomy vs radiofrequency ablation for colorectal liver metastasis: a propensity score analysis. World J Gastroenterol. 2015;21:3300–3307. doi: 10.3748/wjg.v21.i11.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copher GH, Dick BM. “Streamline” phenomena in the portal vein and selective distribution of portal blood in the liver. Arch Surg. 1928;17:408–419. [Google Scholar]
- 6.Desai AG, Park CH, Schilling JF. “Streaming” in portal vein. Its effect on the spread of metastases to the liver. Clin Nucl Med. 1985;10:556–559. [PubMed] [Google Scholar]
- 7.Moore GE, Bridenbaugh RB. Roentgen demonstration of the venous circulation in the liver; portal venography. Radiology. 1951;57:685–690. doi: 10.1148/57.5.685. [DOI] [PubMed] [Google Scholar]
- 8.Incedayı M, Arıbal S, Sivrioglu AK, Sonmez G, Ozturk E, Yalcın B, et al. Are hepatic portal venous system components distributed equally in the liver? A multidetector computerized tomography study. Balkan Med J. 2012;29:419–423. doi: 10.5152/balkanmedj.2012.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dionne L. The pattern of blood-borne metastasis from carcinoma of rectum. Cancer. 1965;18:775–781. doi: 10.1002/1097-0142(196506)18:6<775::aid-cncr2820180615>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Konopke R, Distler M, Ludwig S, Kersting S. Location of liver metastases reflects the site of the primary colorectal carcinoma. Scand J Gastroenterol. 2008;43:192–195. doi: 10.1080/00365520701677755. [DOI] [PubMed] [Google Scholar]
- 11.Schulz W, Hagen C, Hort W. The distribution of liver metastases from colonic cancer. A quantitative postmortem study. Virchows Arch A Pathol Anat Histopathol. 1985;406:279–284. doi: 10.1007/BF00704297. [DOI] [PubMed] [Google Scholar]
- 12.Shirai Y, Wakai T, Ohtani T, Sakai Y, Tsukada K, Hatakeyama K. Colorectal carcinoma metastases to the liver. Does primary tumor location affect its lobar distribution? Cancer. 1996;77:2213–2216. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2213::AID-CNCR5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Wigmore SJ, Madhavan K, Redhead DN, Currie EJ, Garden OJ. Distribution of colorectal liver metastases in patients referred for hepatic resection. Cancer. 2000;89:285–287. doi: 10.1002/1097-0142(20000715)89:2<285::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Strohmeyer T, Schultz W. The distribution of metastases of different primary tumors in the liver. Liver. 1986;6:184–187. doi: 10.1111/j.1600-0676.1986.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 15.Um EH, Hwang S, Song GW, Jung DH, Ahn CS, Kim KH, et al. Calculation of standard liver volume in Korean adults with analysis of confounding variables. Korean J Hepatobiliary Pancreat Surg. 2015;19:133–138. doi: 10.14701/kjhbps.2015.19.4.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cady B, Stone MD, McDermott WV, Jr, Jenkins RL, Bothe A, Jr, Lavin PT, et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg. 1992;127:561–568. doi: 10.1001/archsurg.1992.01420050085011. [DOI] [PubMed] [Google Scholar]
- 17.Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hagerstrand I, Ranstam J, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. doi: 10.1002/bjs.1800730917. [DOI] [PubMed] [Google Scholar]
- 18.Gayowski TJ, Iwatsuki S, Madariaga JR, Selby R, Todo S, Irish W, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116:703–710. [PMC free article] [PubMed] [Google Scholar]
- 19.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Association Francaise de Chirurgie. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 20.Fortner JG, Silva JS, Cox EB, Golbey RB, Gallowitz H, Maclean BJ. Multivariate analysis of a personal series of 247 patients with liver metastases from colorectal cancer. II. Treatment by intrahepatic chemotherapy. Ann Surg. 1984;199:317–324. doi: 10.1097/00000658-198403000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]