Abstract
Purpose
Patients with stage IIIC breast cancer are classified as having pathologic nodal stage 3 (pN3) according to the 7th American Joint Committee on Cancer Tumor Node Metastasis (AJCC TNM) staging system. However, the prognosis of patients with this stage is still highly variable. This study was carried out to investigate the validity of metastatic axillary lymph node ratio (mALNR) as a predictor of long-term prognosis in stage IIIC breast cancer.
Methods
Medical records of 297 patients who underwent surgery with more than level II axillary dissection for breast cancer and who were diagnosed with pN3 by pathology between 1990 and 2010, were reviewed. Clinicopathologic variables were evaluated as prognostic factors of disease-free and overall survival by univariate and multivariate analyses.
Results
A preliminary analysis revealed the cutoff value of mALNR to be 0.65 (Low65 group vs. High65 group). The mean mALNR was 0.62 (0.16–1.0) and was the most significant independent predictor of disease-free and overall survival on multivariate analysis. The rates of recurrence were significantly different according to mALNR (Low65, 40.3%; High65, 63.0%; P < 0.001). The 10-year disease-free (Low65, 57.0%; High65, 35.0%) and overall (Low65, 64.2%; High65, 38.3%) survival rates decreased significantly with increased mALNR (P < 0.001).
Conclusion
Patients with stage IIIC breast cancer can be subdivided into subgroups with significantly different long-term prognoses. Our data suggest that the mALNR is an independent risk factor of recurrence and mortality. The mALNR is a valuable prognostic factor to predict the long-term prognosis of stage IIIC breast cancer patients.
Keywords: Breast neoplasms, Axilla, Lymph nodes, Survival, Prognosis
INTRODUCTION
The long-term prognosis for patients with breast cancer stage IIIC has been reported to be generally poor compared with those in other stages, with 5-year disease-free and overall survival rates of about 70% and 85%, respectively [1,2,3,4,5,6]. Patients with stage IIIC disease are considered to have pathologic nodal status N3 (pN3; metastases in 10 or more axillary lymph nodes; or in infraclavicular [level III axillary] lymph nodes; or in clinically detected ipsilateral internal mammary lymph nodes in the presence of 1 or more positive level I, II axillary lymph nodes; or in more than 3 axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected; or in ipsilateral supraclavicular lymph nodes), regardless of tumor size (T stage), according to the 6th and 7th American Joint Committee on Cancer Tumor Node Metastasis (AJCC TNM) staging system [7,8]. However, the nodal stage of breast cancer, which classifies disease according to the number of metastatic axillary nodes, is further subdivided into prognostic subgroups based on pathologic and molecular characteristics [9,10,11].
The metastatic axillary lymph node ratio (mALNR), defined as the number of positive axillary nodes divided by the number of dissected axillary nodes, is known to be a significant prognostic factor not only in breast cancer, but also in many other types of cancer [12]. Generally, patients with a large number of metastatic lymph nodes have high mALNR and poor prognosis [13,14].
Over the recent decades, the prognosis of advanced breast cancer, such as stage IIIC was improved according to development of multimodality treatment (including radical surgery, chemotherapy, radiotherapy, target therapy) [9,10]. Several studies have suggested the presence of heterogeneous subgroups with different pathologic and bio-molecular characteristics that could influence prognosis within the same stage [9,10,11,15]. Thus, patients with certain clinicopathologic features could be expected to have a better prognosis despite a high number of metastatic axillary nodes. Our study was designed to evaluate the prognostic value of mALNR in a group of stage IIIC (pN3) advanced breast cancer patients who received multimodality treatment including mastectomy with axillary dissection, and radiotherapy, endocrine therapy, and target therapy.
METHODS
We analyzed medical records from the breast cancer registry program of the Department of Surgery, Cheil General Hospital and Women's Healthcare Center, Dankook University College of Medicine, Seoul, Korea. Two hundred ninety-seven cases were collected from 3,787 patients who underwent surgery between 1990 and 2010 according to this database. Inclusion criteria were cases with pN3 nodal status (stage IIIC) breast cancer according to AJCC TNM staging system 6th and 7th edition. All patients had undergone mastectomy with more than level II or level III axillary dissection. Cases were excluded if follow-up data were unavailable. The median follow-up period was 60 months (range, 10–302 months). All cases received adjuvant treatment including standard chemotherapy, radiotherapy, endocrine therapy, and target therapy. However, we did not analyze the various types of chemotherapy regimens.
We separately examined the mALNR (number of positive axillary nodes/number of dissected axillary nodes) as a predictor of long-term prognosis. To find a cutoff value of the mALNR, we analyzed the prognoses in patients with a variety of mALNR values. Using univariate analyses for both disease-free and overall survival according to mALNR, the survival difference was most significant when a cutoff value of 0.65 was used. Therefore, patients with a mALNR less than 0.65 were grouped as Low65, and the others were grouped as High65.
All statistical analyses were conducted using SPSS ver. 19,0 (IBM Corp., Armonk, NY, USA). Prognostic parameters considered were age (≤35 years vs. >35 years), menopause status (premenopause or postmenopause), neoadjuvant chemotherapy (not administered vs. administered), operation method (partial vs. total), axillary dissection (level II vs. level III), tumor size (≤2 cm vs. > 2 cm), histologic grade (1 vs. 2 & 3), nuclear grade (1 & 2 vs. 3), estrogen receptor (ER) (−: negative vs. +: positive), progesterone receptor (PR) (− vs. +), Her2 (− vs. +), p53 (− vs. +), and mALNR (Low65 vs. High65). To evaluate correlations between mALNR and clinical-pathologic parameters, data were cross-tabulated (chi-square test/2×2 table; Pearson or Fisher exact test). Univariate analyses with disease-free and overall survival were evaluated using the Kaplan-Meier method, and comparisons between groups were analyzed by the log-rank test. Multivariate analysis was performed on significant prognostic parameters according to univariate analysis using the Cox proportional hazard model in forward stepwise regression to evaluate the independent power of each variable. Statistical significance was set at P-value < 0.05 (95% level of confidence).
RESULTS
Comparison of clinicopathologic features according to mALNR
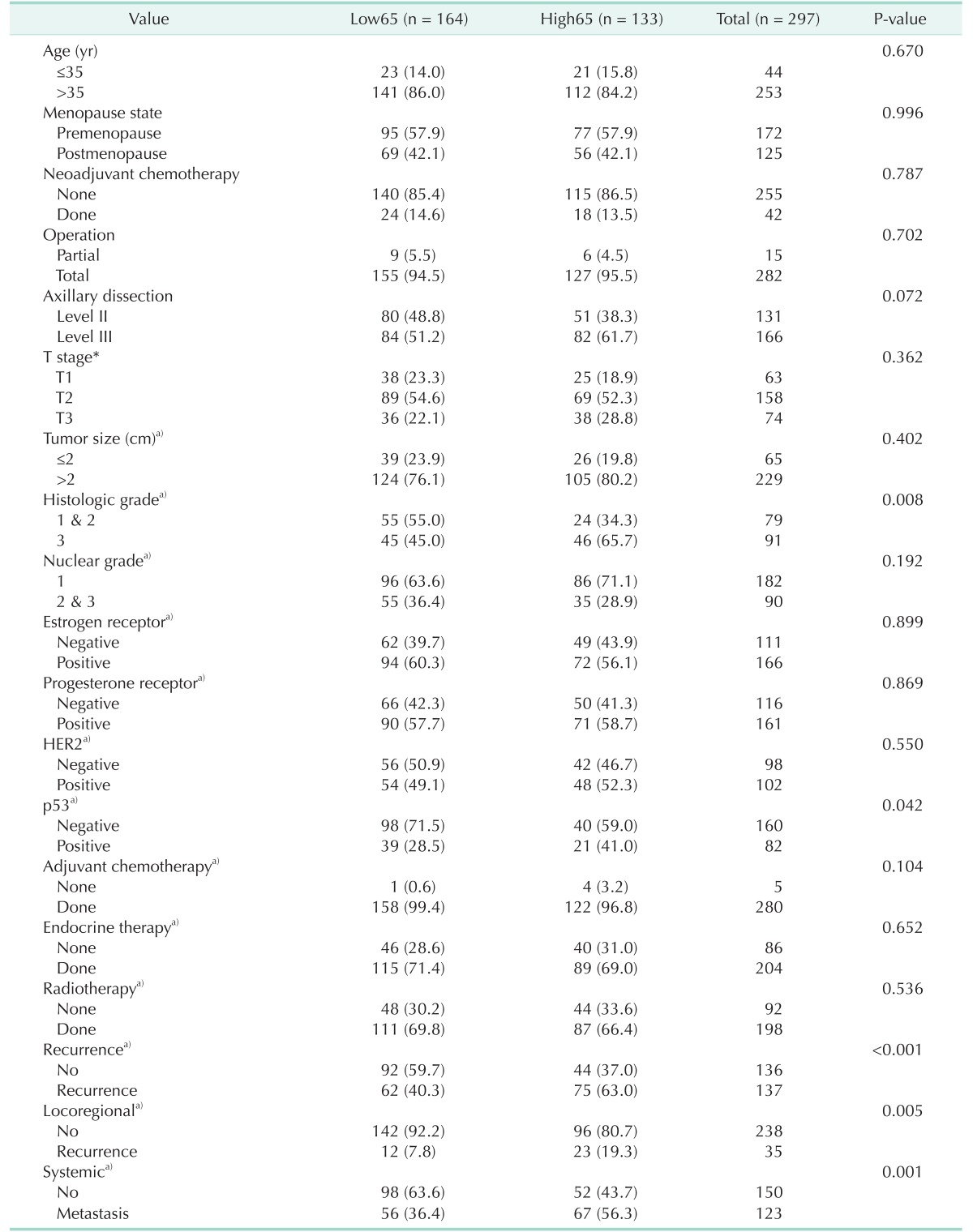
The mean age (±standard deviation; SD) of the 297 patients was 47.3 ± 9.9 years (range, 24–77 years). As shown in Table 1, with the exception of histologic grade (P = 0.008), p53 (P = 0.042), and recurrence (P < 0.001), the clinicopathologic features were well balanced between the two groups of patients.
Table 1. Clinicopathologic features and recurrence data according to metastatic axillary lymph node ratio.
Values are presented as number (%).
mALNR, metastatic axillary lymph node ratio; Low65, patients with mALNR ≤ 0.65; High65, patients with mALNR > 0.65.
a)Missing case was excluded.
The total numbers of metastatic and dissected axillary lymph nodes of the 297 patients were 6,148 and 10,113, respectively. The overall mALNR (total metastatic axillary nodes/total dissected axillary nodes of the 297 patients) was 0.61. The mean (±standard deviation) number of metastatic axillary nodes was 20.7 ± 11.6 per patient (range, 1–72), and the mean number of dissected axillary nodes was 34.1 ± 13.2 per patient (range, 11–87). The mean numbers of metastatic and dissected axillary nodes were 14.2 and 34.2 in patients in the Low65 group (n = 164), respectively, and 28.8 and 33.8 in patients in the High65 group (n = 1,338) (Table 2).
Table 2. Comparison of axillary lymph node status.
ALN, axillary lymph node; mALNR, metastatic ALN; SD, standard deviation; Low65, patients with mALNR ≤ 0.65; High65, patients with mALNR > 0.65.
a)By Student t-test.
Recurrence and survival rates according to mALNR
The 10-year overall survival rate of the 297 study patients was 52.6% (95% CI, 45.9–59.3).
The rates of recurrence (40.3% in Low65 group and 63.0% in high65 group) (including locoregional and systemic) were significantly different according to the mALNR (Table 1).
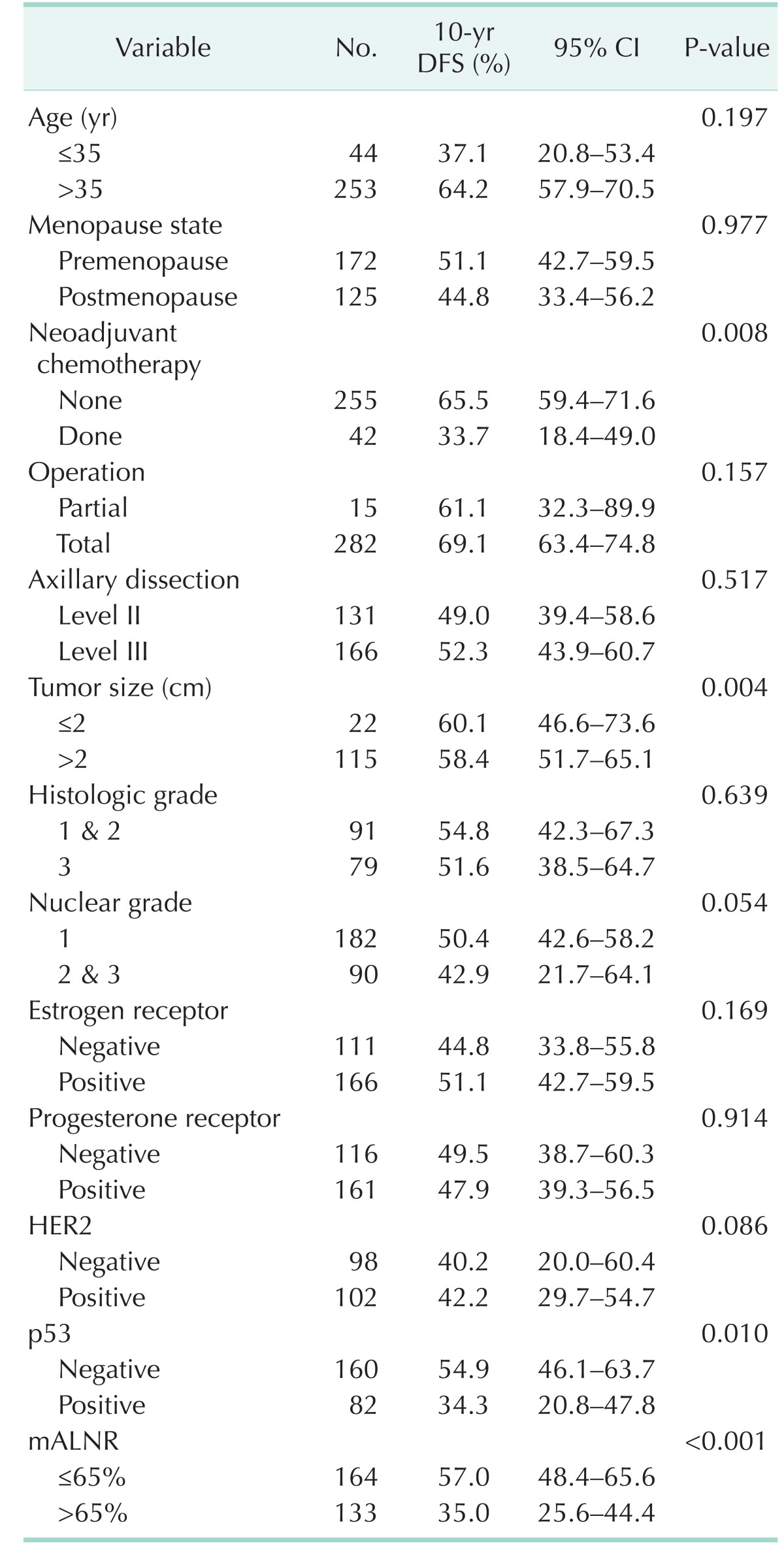
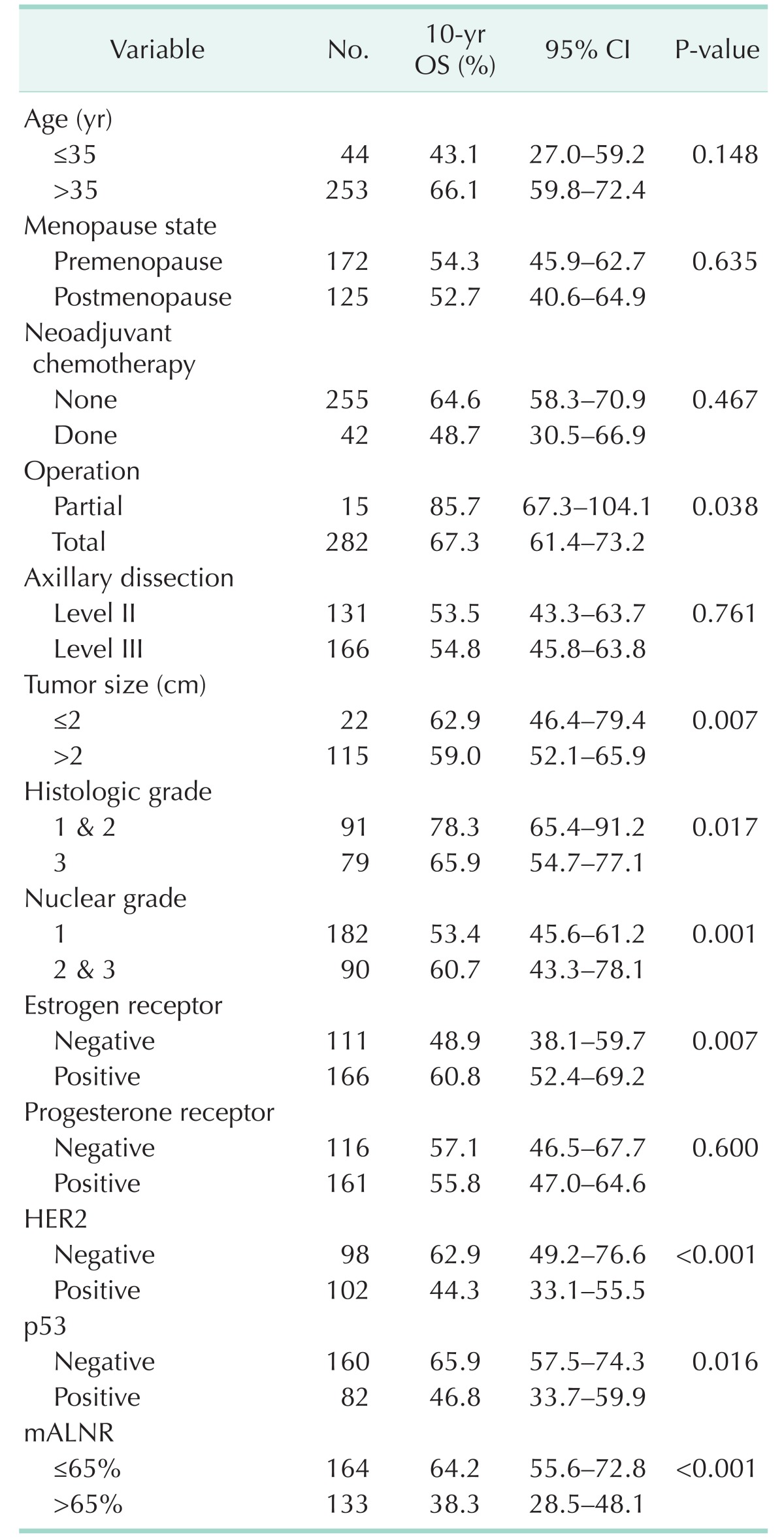
Univariate analysis for recurrence showed that neoadjuvant chemotherapy, tumor size, p53, and mALNR were significant predictors of disease-free survival (Table 3). The 10-year disease-free survival rate was 57.0% for the Low65 group and 35.0% for the High65 group (P < 0.001). Univariate analysis for mortality showed that operation (P = 0.038), tumor size (P = 0.007), histologic grade (P = 0.017), nuclear grade (P = 0.001), ER (P = 0.007), Her2 (P < 0.001), p53 (P = 0.016), and mALNR (P < 0.001) were significant predictors of overall survival (Table 4). The 10-year overall survival rate was 64.2% for the Low65 group and 38.3% for the High65 group (P < 0.001).
Table 3. Univariate analysis of clinicopathologic parameters of disease-free survival (DFS) after operation.
CI, confidence interval; mALNR, metastatic axillary lymph node ratio.
Table 4. Univariate analysis of clinicopathologic parameters of overall survival (OS) after operation.
CI, confidence interval; mALNR, metastatic axillary lymph node ratio.
Multivariate analyses of recurrence and mortality
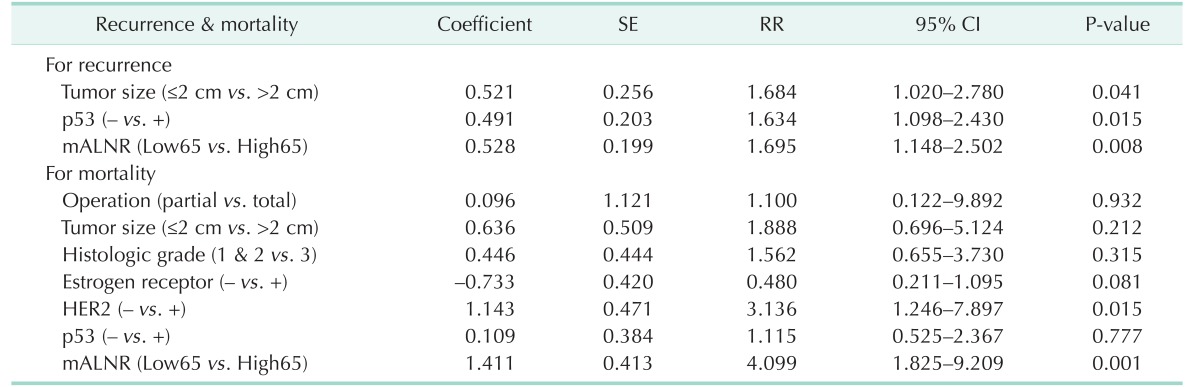
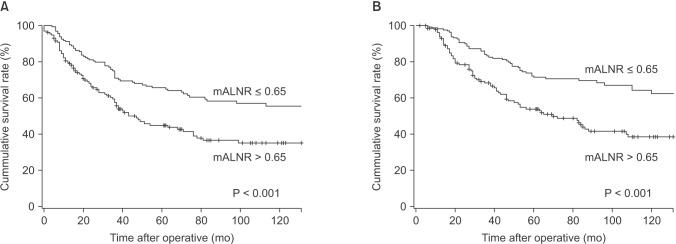
Multivariate analysis using the Cox proportional hazards model included prognostic parameters that were significant on the univariate analysis. Tumor size (P = 0.041), p53 (P = 0.015), and mALNR (P = 0.008) were independent prognostic factors of 10-year disease-free survival on multivariate analysis (Table 5). Her2 (P = 0.015) and mALNR (P = 0.001) were independent prognostic factors of long-term overall survival on multivariate analysis (Table 5). mALNR was the most significant and independent poor prognostic factor on multivariate analysis. Survival curves according to mALNR are shown in Fig. 1.
Table 5. Independent prognostic factors of disease-free and overall survival by multivariate analysis (Cox regression model).
SE, standard error; RR, relative risk; CI, confidence interval for relative risk (odds ratio); mALNR, metastatic axillary lymph node ratio; Low65, patients with mALNR ≤ 0.65; High65, patients with mALNR > 0.65.
Fig. 1. (A) Cumulative survival curves for recurrence according to metastatic axillary lymph node ratio (mALNR). The 10-year survival rate was different between the Low65 group (mALNR ≤ 0.65; 57.0%) and the High65 group (mALNR > 0.65; 35.0%), P < 0.001. (B) Cumulative survival curves for mortality according to metastatic axillary lymph node ratio. The 10-year survival rate was different between the Low65 group (64.2%) and the High65 group (38.3%), P < 0.001. Low65, patients with mALNR ≤ 0.65; High65, patients with mALNR > 0.65.
DISCUSSION
Stage IIIC breast cancer is generally associated with a poor prognosis (5-year survival rate of 40%) [3,16,17,18,19]. This study found long-term, 10-year disease-free and overall survival rates of 44.5% and 52.6%, respectively. These high survival rates might be due to the aggressive adjuvant treatment, including chemotherapy, endocrine therapy, and targeted therapy, performed in all eligible patients. For example, patients with HER2 positive breast cancer diagnosed before 2009 did not receive Trastuzumab because it was not covered by Korean insurance. Additional optimal targeted therapies, such as trastuzumab administration on the basis HER2 status, might be needed to improve clinical outcomes in patients with stage IIIC breast cancer [1].
In this study, young age (≤35 years) showed a tendency related to poor prognosis in both recurrence and mortality. Young age might have prognostic significance in advanced breast cancer. However, when examining increased age, menopausal status was not shown to be related to prognosis in this study.
Recently, many studies have suggested that mALNR can predict prognosis more precisely, and that mALNR should be incorporated into the next version of the staging system, regardless of the number of metastatic axillary nodes (based AJCC TNM breast cancer staging system) [12]. Several studies have reported that a significant cutoff value of mALNR for breast cancer is about 0.5–0.7 [13]. This cutoff value is useful for the prediction of recurrence and mortality. In this study, the most significant difference in locoregional and systemic recurrence was identified when 0.65 was used as a cutoff level. Further validation of this cutoff value is required.
When examining the results of our study, it is clear that the mALNR is not only the most significant prognostic factor, but also a useful tool for risk-stratifying patients with stage IIIC cancer. This finding is very important because most patients with more than 9 metastatic axillary nodes are categorized as pN3 disease. However, in our study, patients classified based on this nodal staging system did not have similar prognoses. Thus, this classification system might not be ideal for risk stratification of patients. In our study, when the cutoff value of the mALNR was set at 0.65, 164 patients has mALNR ≤0.65 and 133 >0.65. The 10-year disease-free survival rate was 57.0% and 35.0%, respectively. Among our patients, the recurrence rate was significantly different between the two groups: 40.3% vs. 63.0%, respectively (P < 0.001). This indicates that a substantial proportion of patients classified as having the same pN3 stage will experience recurrence after radical surgery, and that mALNR can be a better predictor of recurrence than the current TNM stage.
As shown above, high mALNR appeared to be a significant prognostic factor in both disease-free survival and overall survival. As reported in previous studies, maximal axillary dissection is recommended in breast cancer patients with advanced axillary nodal metastasis [13,20]. Our study also found that mALNR was most significantly related to prognosis including recurrence and mortality. The metastatic lymph node ratio in other various cancer types is also thought to be an independent prognostic factor [21,22,23]. Similar to previous studies, we found that a higher mALNR was associated with poor long-term prognosis in breast cancer.
The high rate of recurrence in patients with a high mALNR emphasizes the importance of aggressive adjuvant treatment. This importance was recently emphasized in several studies [15,24,25]. These studies reported reduction in locoregional and systemic recurrence and increase in disease-free survival with adjuvant regional radiotherapy. This suggests that adjuvant radiotherapy is an essential treatment component in advanced breast cancer patients with axillary metastasis. The incidence of locoregional recurrence in our study was 12.8% (35 of 273), which is comparable with previous reports [2,15,19,26]. Even with multimodality treatment, a considerable proportion (approximately 5%–13%) of patients still experience locoregional recurrence [2,15,19,26]. In our study, high mALNR was the most important predictor of locoregional recurrence.
Several inherent limitations of this study should be described. First, it is possible that unrecognized biases might influence the results because of this retrospective study. Second, the specific sub analysis according to variable clinic-pathological parameters cannot be considered definitive, especially for the target anti-HER2 therapy (Trastzumab). Third, recent researches about lymph node ratio have been limited because there is no clear consensus about the cutoff points and many researchers have therefore used their own criteria. Therefore, further studies and follow-up are needed.
In conclusion, stage IIIC breast cancer is a disease with significant variation in long-term prognosis. The mALNR might serve as a more valuable prognostic indicator for patients with stage IIIC breast cancer than the traditional TNM stage. A high mALNR was the most significant independent prognostic factor influencing long-term prognosis including disease-free and overall survival. Furthermore, in patients with a high mALNR, aggressive adjuvant treatment and close follow-up might be required.
ACKNOWLEDGEMENTS
Special thanks to Sung Soo Kang, Hae Kyung Lee, Chan Seok Yoon (Cheil General Hospital, Dankook University College of Medicine), and Ji Hyun Lee (Department of Surgery, CHA Gangnam Medical Center, CHA University) for helping with the study.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Kim YY, Park HK, Lee KH, Kim KI, Chun YS. Prognostically distinctive subgroup in pathologic N3 breast cancer. J Breast Cancer. 2016;19:163–168. doi: 10.4048/jbc.2016.19.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buzdar AU, Kau SW, Hortobagyi GN, Ames FC, Holmes FA, Fraschini G, et al. Clinical course of patients with breast cancer with ten or more positive nodes who were treated with doxorubicin-containing adjuvant therapy. Cancer. 1992;69:448–452. doi: 10.1002/1097-0142(19920115)69:2<448::aid-cncr2820690229>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 3.Donegan WL, Lewis JD. Clinical diagnosis and staging of breast cancer. Semin Oncol. 1978;5:373–384. [PubMed] [Google Scholar]
- 4.Nemoto T, Vana J, Bedwani RN, Baker HW, McGregor FH, Murphy GP. Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer. 1980;45:2917–2924. doi: 10.1002/1097-0142(19800615)45:12<2917::aid-cncr2820451203>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Kuru B, Camlibel M, Dinc S, Gulcelik MA, Alagol H. Prognostic significance of axillary node and infraclavicular lymph node status after mastectomy. Eur J Surg Oncol. 2003;29:839–844. doi: 10.1016/j.ejso.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Newman LA. Epidemiology of locally advanced breast cancer. Semin Radiat Oncol. 2009;19:195–203. doi: 10.1016/j.semradonc.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, et al. Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 8.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Basaran G, Devrim C, Caglar HB, Gulluoglu B, Kaya H, Seber S, et al. Clinical outcome of breast cancer patients with N3a (≥10 positive lymph nodes) disease: has it changed over years? Med Oncol. 2011;28:726–732. doi: 10.1007/s12032-010-9516-1. [DOI] [PubMed] [Google Scholar]
- 10.Lee JS, Kim SI, Choi SY, Park HS, Lee JS, Park S, et al. Factors influencing the outcome of breast cancer patients with 10 or more metastasized axillary lymph nodes. Int J Clin Oncol. 2011;16:473–481. doi: 10.1007/s10147-011-0207-5. [DOI] [PubMed] [Google Scholar]
- 11.Geara FB, Nasr E, Tucker SL, Charafeddine M, Dabaja B, Eid T, et al. Breast cancer patients with 10 or more involved axillary lymph nodes treated by multimodality therapy: influence of clinical presentation on outcome. Int J Radiat Oncol Biol Phys. 2007;68:364–369. doi: 10.1016/j.ijrobp.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006;24:2910–2916. doi: 10.1200/JCO.2005.03.1526. [DOI] [PubMed] [Google Scholar]
- 13.Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–1068. doi: 10.1200/JCO.2008.18.6965. [DOI] [PubMed] [Google Scholar]
- 14.Ahn SH, Kim HJ, Lee JW, Gong GY, Noh DY, Yang JH, et al. Lymph node ratio and pN staging in patients with node-positive breast cancer: a report from the Korean breast cancer society. Breast Cancer Res Treat. 2011;130:507–515. doi: 10.1007/s10549-011-1730-9. [DOI] [PubMed] [Google Scholar]
- 15.Kim SW, Choi DH, Huh SJ, Park W, Nam SJ, Kim SW, et al. Lymph node ratio as a risk factor for locoregional recurrence in breast cancer patients with 10 or more axillary nodes. J Breast Cancer. 2016;19:169–175. doi: 10.4048/jbc.2016.19.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones SE, Moon TE, Bonadonna G, Valagussa P, Rivkin S, Buzdar A, et al. Comparison of different trials of adjuvant chemotherapy in stage II breast cancer using a natural history data base. Am J Clin Oncol. 1987;10:387–395. doi: 10.1097/00000421-198710000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Montero AJ, Rouzier R, Lluch A, Theriault RL, Buzdar AU, Delaloge S, et al. The natural history of breast carcinoma in patients with > or = 10 metastatic axillary lymph nodes before and after the advent of adjuvant therapy: a multiinstitutional retrospective study. Cancer. 2005;104:229–235. doi: 10.1002/cncr.21182. [DOI] [PubMed] [Google Scholar]
- 18.Duraker N, Caynak ZC, Bati B. Is there any prognostically different subgroup among patients with stage IIIC (any TN3M0) breast carcinoma? Ann Surg Oncol. 2008;15:430–437. doi: 10.1245/s10434-007-9558-6. [DOI] [PubMed] [Google Scholar]
- 19.Diab SG, Hilsenbeck SG, de Moor C, Clark GM, Osborne CK, Ravdin PM, et al. Radiation therapy and survival in breast cancer patients with 10 or more positive axillary lymph nodes treated with mastectomy. J Clin Oncol. 1998;16:1655–1660. doi: 10.1200/JCO.1998.16.5.1655. [DOI] [PubMed] [Google Scholar]
- 20.Duraker N, Bati B, Caynak ZC, Demir D. Lymph node ratio may be supplementary to TNM nodal classification in node-positive breast carcinoma based on the results of 2,151 patients. World J Surg. 2013;37:1241–1248. doi: 10.1007/s00268-013-1965-1. [DOI] [PubMed] [Google Scholar]
- 21.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophillymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Hong J, Mao Y, Chen X, Zhu L, He J, Chen W, et al. Elevated preoperative neutrophil-to-lymphocyte ratio predicts poor disease-free survival in Chinese women with breast cancer. Tumour Biol. 2016;37:4135–4142. doi: 10.1007/s13277-015-4233-1. [DOI] [PubMed] [Google Scholar]
- 23.Krenn-Pi lko S, Langsenlehner U, Stojakovic T, Pichler M, Gerger A, Kapp KS, et al. The elevated preoperative derived neutrophil-to-lymphocyte ratio predicts poor clinical outcome in breast cancer patients. Tumour Biol. 2016;37:361–368. doi: 10.1007/s13277-015-3805-4. [DOI] [PubMed] [Google Scholar]
- 24.Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med. 2015;373:317–327. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 25.Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med. 2015;373:307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jabro G, Wazer DE, Ruthazer R, Lum R, Sklar N, Goldman D, et al. The importance of local-regional radiotherapy with conventional or high-dose chemotherapy in the management of breast cancer patients with > or = 10 positive axillary nodes. Int J Radiat Oncol Biol Phys. 1999;44:273–280. doi: 10.1016/s0360-3016(99)00009-7. [DOI] [PubMed] [Google Scholar]