Abstract
Endoplasmic reticulum (ER) stress is a regulatory mechanism that allows cells to adapt to a series of metabolic, redox, and other environmental changes. The role of ER stress was first identified in the maintenance of proteostasis. It has since been shown that ER stress is also critical to the regulation of lipid homeostasis, membrane turnover, and autophagy. ER stress initiates an intrinsic signaling network, the unfolded protein response, one component of the multifold and complex cellular signaling process system, which leads to major changes in the profiles of transcription factors. The unfolded protein response affects several other signaling routes through direct connections and also by indirect means. It directly influences hormone formation and life/death decisions at a cellular level; this relationship also involves connections to nutrient and environmental sensing-biotransformation processes. In conclusion, ER stress represents an integrated complex organelle response that makes an essential contribution to the maintenance of intracellular homeostasis.
Intracellular metabolic homeostasis is a result of a complex interplay between different subcellular compartments. The endoplasmic reticulum (ER), being an essential regulator in lipid and protein synthesis, is one of the central hubs of the cross-compartmental signaling network (1, 2).
Various metabolic conditions require an enlarged capacity in the ER-localized pathways of intermediary metabolism, and this frequently results in proliferation of the organelle. Two characteristic examples of ER metamorphosis are the enhanced synthesis of secretory proteins (eg, in plasma cells) and the induction of enzymes of biotransformation (eg, in hepatocytes and endocrine cells). Although the former represents a volume stress, that is, an increased claim for the ER lumen, the latter generates a surface stress, an enhanced requirement for the ER membrane and membrane proteins. Both conditions can be resolved by proliferation of the appropriate subdomains of the ER: rough ER and/or smooth ER. The regulatory processes of proteostasis and signalostasis are frequently integrated with the adaptation of lipid biosynthesis. Although the molecular mechanism of both the unfolded protein response (UPR) and biotransformation is fairly well known, the lipid aspect of the process is less well characterized.
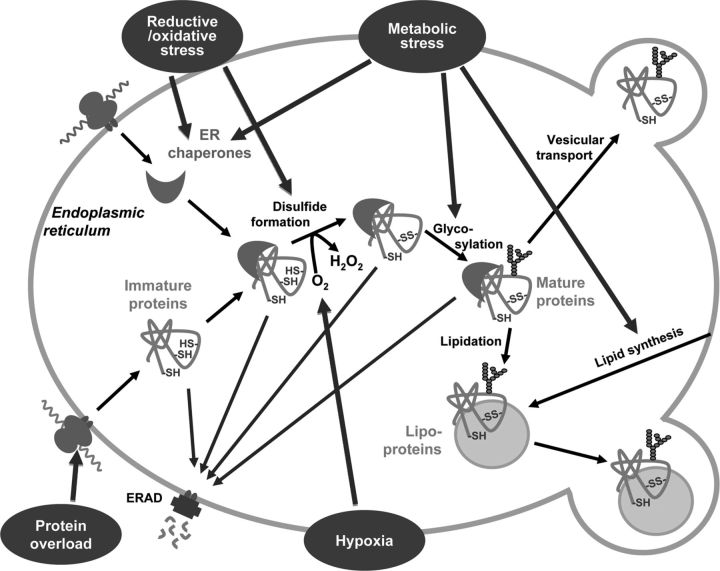
The ER is a separate compartment with a complex network of membranes. Essentially, the ER is engaged in synthetic processes. The translation of secretory and membrane proteins takes place on ER-bound ribosomes, and posttranslational modifications, including folding and transport of the proteins, also occur in this organelle. These posttranslational modifications require several redox constituents, carbohydrate precursors, and lipids for disulfide bond formation, glycoprotein formation, and lipidation, respectively. Thus, changes in intraluminal redox homeostasis affect protein folding (for a recent review, see Ref. 3); access to carbohydrate precursors and lipids also determines the ER maturation of proteins (Figure 1.). Besides its defining role in protein synthesis, the ER hosts several lipid-processing enzymes; hence, its proper functioning also determines lipid metabolism. Furthermore, the ER is an integral part of the intracellular endomembrane system and provides the lipids and proteins needed for de novo membrane generation (for a recent review, see Ref. 4). Considering the diverse roles of the ER, metabolic disturbances and glucolipotoxicity that influence the delicate balance of the ER can be predicted to have far-reaching and general consequences (for a recent review, see Ref. 5).
Figure 1.
ER stressors influence ER proteostasis. Proteins formed in the ER are folded and posttranslationally modified in the luminal compartment. Various cofactors are required for posttranslational modifications. After the synthesis of the polypeptide chain by the ribosomes, oxygen is needed for disulfide formation, various carbohydrates for protein glycosylation, and lipids for lipidation. During the folding process, the proteins are accompanied by different ER chaperones in the luminal compartment. Increased demand for protein folding due to protein overload or various other ER stressors, eg, redox stress, hypoxia, and metabolic stress, can change supplies of cofactor, thereby influencing the folding process. Improperly folded immature proteins can be degraded by the ERAD, whereas mature proteins are exported to secretory vesicles.
Biotransformation including drug metabolism is a classic biosynthetic process that prepares low–molecular-weight compounds for secretion. It has been suggested that the original physiological targets/substrates/inducers of drug metabolism enzymes and transporters are signal molecules (6). Most biotransformation reactions are localized in ER membranes or in the luminal compartment. The substrates for these biotransformations are lipid-soluble endobiotics and xenobiotics, which are mainly converted by ER membrane-bound drug metabolism enzymes and translocated by ER membrane-bound drug metabolism transporters. The expression of these enzymes and transporters is frequently regulated in a coordinated way by various transcription factor gene batteries. The biotransformation process can result in inactivation of existing signal molecules and formation of novel signal molecules. Biotransformation is therefore intertwined with cellular signaling. The induction state of drug metabolism enzymes can determine several signaling processes, all of which can be ER homeostasis dependent.
The endomembrane system permits the integration of the connected nutrient, pathogen, and xenogenic sensing systems, a phenomenon enabled by the differential redox homeostasis of the luminal compartment and cytosol (for a recent review, see Ref. 3). Moreover, redox active thiol and pyridine nucleotide pools are uncoupled in the ER lumen. The high oxidized to reduced glutathione ratio, which ensures the oxidative conditions of the ER, is combined with a reduced nicotinamide adenine dinucleotide phosphate (NADPH) pool providing reducing power. This unique redox homeostasis favors biosynthesis, a characteristic of ER-related processes.
The most powerful physiological regulatory systems that affect ER homeostasis are ER stress and its related signaling network, UPR. However, overwhelming disturbance of ER homeostasis may result in a pathological switch. Recent advances show that ER stress, resulting from altered ER homeostasis, is a common pathomechanism of different diseases such as type 2 diabetes, metabolic syndrome, retinitis pigmentosa, nonalcoholic fatty liver disease, atherosclerosis, etc (2, 7, 8). Because ER stress initiates a complex signaling response, ER is also a subcellular source of signals. The ER-related signaling, the UPR, has various links to other signaling routes mediated either by plasma membrane receptors or intracellular receptors. Taken together, ER signaling is an essential sensing, control, and adaptation mechanism in the whole cellular, and also intercellular, signaling network (9, 10).
UPR in the Regulation of Proteostasis and in the Formation of ER Membranes
Perturbations in ER homeostasis, especially a change in cellular energy, redox state, or Ca2+ homeostasis, interfere with protein folding in the ER. Regardless of whether ER stress is due to disturbed ER functions or to increased protein load, the deficit in folding capacity results in an accumulation of imperfectly mature polypeptides in the ER lumen (Figure 1). This accumulation is sensed by ER membrane embedded sensors PKR-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6), and triggers the UPR (9, 10).
The UPR is primarily a protective and adaptive mechanism aiming to restore the balance between folding capacity and demand. Therefore, it involves both attenuation of translation and the enhanced expression of ER chaperones and the components of ER-associated protein degradation (ERAD). If the adaptive mechanisms fail, the UPR switches from prosurvival to proapoptotic mode. Induction of CCAAT/enhancer binding protein (C/EBP) homologous protein (CHOP), and the activation of c-Jun amino-terminal kinase (JNK), apoptosis signal-regulating kinase 1 (ASK1), and procaspase-12 (or procaspase-4) lead to activation of the caspase cascade.
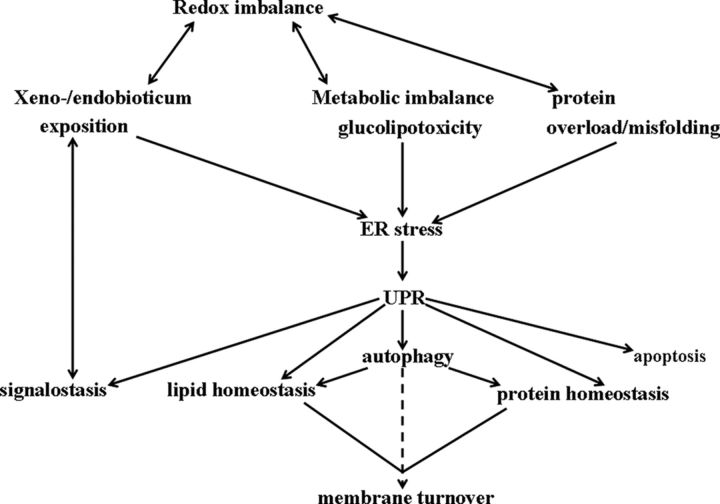
The UPR is not restricted to changes in protein homeostasis. The size and shape of cell organelles are continually adjusted to the needs of the cell, which are in turn modulated by the ever-changing environment. The growing mass of ER proteins evidently requires the expansion of the ER membrane network, and hence the UPR is always accompanied by stimulated synthesis of membrane lipids. Enhanced membrane synthesis, and the consequent increase in ER size, can be considered as an integral yet distinct part of the complex cellular response to ER stress (Figure 2). Recent findings in the yeast Saccharomyces cerevisiae indicate that the expansion of ER volume is a key factor in the ability of cells to cope with ER stress. This membrane expansion, in the form of the generation of new ER sheets, has been shown to require UPR signaling and to be driven by lipid biosynthesis (11). Stress stimuli leading to membrane- and lipid-related aberrancies have been shown to activate the IRE1 branch of the UPR in yeast cells (12).
Figure 2.
ER stressors affect ER membrane formation. Various stressors cause ER stress directly or indirectly and subsequently UPR. Many of these are connected to redox imbalance. Through alterations in protein and lipid homeostasis and in autophagy, the UPR can affect ER membrane turnover. The UPR is also connected to cellular signaling, which is in turn connected to the actual state of biotransformation, xeno-/endobiotics exposition.
UPR and Lipid Homeostasis
A series of publications have indicated the complex role of the UPR in the maintenance of protein and lipid homeostasis and, contrastingly, in the pathomechanism of disturbances due to glucolipotoxicity (Figure 2). Excess of fatty acids results in ER-related lipid droplet (LD) formation, which requires a concerted regulation of ER membrane formation and composition. Moreover, protein lipidation and the starting steps of lipoprotein secretion also occur in the ER (for a recent review, see Ref. 5) (Figure 1). The role of ER stress in this aspect has mainly been studied in the liver and in adipose tissue. Among the various branches of the UPR, the function of IRE1 has particularly been explored.
The expression of genes involved in fatty acid and cholesterol synthesis is stimulated by dietary carbohydrates through the activation of the IRE1/XBP1 (X-box binding protein 1) branch of the UPR. It has been shown that the conditional disruption of XBP1 is followed by decreased hepatic lipogenesis (13–16). Furthermore, in hepatocytes, IRE1/XBP1-induced protein disulfide isomerase (PDI) expression is connected to very-low-density lipoprotein assembly, lipidation, and secretion (Figure 1), which is in turn connected to the regulation of protein folding and also to hepatic lipid homeostasis (15).
Activation of C/EBP and peroxisome proliferator-activated receptor-γ (PPARγ) transcription factors via PERK-mediated eukaryotic initiation factor 2α phosphorylation is also suggested to contribute to the induction of lipogenic genes (17). Accordingly, inactivation of the PERK branch has been associated with a profound decrease in lipogenic enzyme expression in the mammary gland (18) and with reduced hepatic lipogenesis in animals fed a high-fat diet (19).
Sterol Regulatory Element-Binding Proteins in Sterol Response
Sterol regulatory element-binding proteins (SREBPs) are transcription factor precursors and the principal regulators of lipid synthesis. Inactive SREBPs are associated with SREBP cleavage activating protein (SCAP) and are retained in the ER membrane by another membrane protein, Insig, in the presence of sterols. Lower sterol levels cause the dissociation of Insig, which allows the vesicular transport of SREBP and SCAP to the Golgi. Once in the Golgi apparatus, SREBP is cleaved by S1P and S2P proteases to yield a soluble cytoplasmic portion of SREBP, which can act as a transcription factor (20, 21).
Activated SREBPs up-regulate the synthesis of enzymes involved in lipogenesis and sterol biosynthesis. SREBP-1 comprises two isoforms, SREBP-1a and SREBP-1c, generated by alternative splicing. SREBP-1c is responsible for the transcriptional regulation of de novo lipogenesis and is expressed mainly in the liver. The ubiquitous SREBP-2 is implicated in the regulation of cholesterol metabolism (22).
Insulin preferentially induces proteolytic activation of SREBP-1c, thus promoting lipogenesis, but does not affect SREBP-2 processing. This difference may be explained by the existence of Insig isoforms and their preference toward various SREBP-SCAP complexes. Insulin causes repression of the hepatic isoform Insig-2a and induction of Insig-1, thereby stimulating the ER-to-Golgi transport of the SREBP-1c–SCAP complex (23).
Effect of UPR Branches on the SREB-Mediated Regulation of Lipid Homeostasis
The first recognized connection between the regulation of lipogenesis and the UPR pathway was the analogous activation process of SREBP and the ER stress sensor ATF6 (24). Both membrane proteins undergo limited proteolysis in the Golgi by the same proteases during activation. Moreover, during ER stress provoked by homocysteine in HepG2 cells or in mice exhibiting homocysteinemia, the UPR pathway and the SREBP-1 were activated concomitantly (25). Similarly, an elevated level of ER stress markers and SREBP-1c and a consequent induction of lipogenic enzymes were reported in the liver of alcohol-fed animals (26). ER stress induced by thapsigargin treatment in HeLa and MCF7 cells resulted in SREBP-2 activation via the conventional, sterol-dependent pathway that normally regulates SREBP (27). A recent study highlighted the direct link between ER stress and the activation of hepatic lipogenesis and subsequent hepatic steatosis in vivo. According to the study, ER stress induces SREBP-1c activation and the subsequent transcription of lipogenic enzymes, leading to hepatic steatosis (28). On the other hand, attenuation of ER stress response by 78 kDa glucose-regulated protein overexpression reduces ER stress markers and inhibits SREBP-1c cleavage and the expression of SREBP-1c and SREBP-2 target genes in the liver of obese ob/ob mice (28).
ER Stress and LDs
The recently revealed dynamic interaction between the ER and LDs puts the relationship between lipid and protein homeostasis into a new perspective. It has been demonstrated in S. cerevisiae that LDs are functionally connected to the ER membrane. Membrane proteins are reversibly translocated between the two organelles apparently without vesicular transport (29). Accumulation of LDs has been stimulated by various conditions, causing, for example, ER stress in yeast cells (30). The phenomenon was shown to be independent of Ire1p; LD accumulation did not influence cell viability. ER stress-induced LD accumulation has also been found in a human hepatoma cell line. According to the study, tunicamycin and thapsigargin promotes lipogenesis and LD formation in Huh-7 cells. Expression of activated XBP1, a key transcriptional regulator involved in the UPR, in these hepatoma cells causes a remarkable induction of PPARγ and C/EBPα, which might explain the enhancement of neutral lipid synthesis (31). In line with these findings, tunicamycin has been shown to promote nonalcoholic steatohepatitis in mice, emphasizing the link between protein maturation, lipid homeostasis, and inflammation (32). These findings support the understanding that LDs cannot be simply considered as stores of triglycerides and cholesterol; rather, they are ubiquitous cellular organelles participating in a variety of cellular functions. Further investigation is required to reveal the exact function of LDs in protein and lipid trafficking, protein degradation, and cell signaling and to elucidate the pathological role of ER stress and consequent intracellular lipid deposition in the development of insulin resistance and pancreatic β-cell dysfunction.
UPR and Autophagy
Compensatory changes in the ER can be also be combined with autophagy (Figure 2), a fact not surprising given the multiple role of this lysosomal metabolic pathway and the close connection between ER and autophagy (33, 34). It has been revealed that autophagy is a complementary mechanism of proteasomal protein degradation; removal of the aggregated proteins relies completely on autophagy (35). Additionally, the role of autophagy is not constrained to stress adaptation and survival mechanisms because it is responsible for degradation of glycogen in neonates (36); moreover, recent studies have revealed that lipid metabolism is intricately regulated by autophagy (37–39).
Autophagy seems to have a direct bidirectional connection with the ER membrane. On one hand, the double-layer lipid membrane of autophagosomes most likely originates from two main sources, the ER and mitochondria (40, 41). On the other hand, an increasing number of publications indicate that the ER stress-induced special form of autophagy, referred to as reticulophagy, is essential to counterbalance ER expansion during the UPR (42, 43). These results clearly demonstrate the interdependency of the birth and death of these organelles.
Besides the extensive lipid content exchange between the ER and autophagosome, functional connections between the UPR and autophagy have also been demonstrated. Induction of autophagy upon ER stress has been confirmed by various approaches, and consequences of this connection to cell physiology have also been suggested by experimental data. Cells subjected to ER stress exhibit levels of autophagy marker protein LC3-II (microtuble-associated protein light chain 3) similar to those measured in starved cells (44), and formation of autophagosomes has also been detected by microscopic studies in ER-stressed cells (45). Inspection of luminal contents of autophagosomes have identified enclosed portions of the ER and aggregated proteins, indicating the role of reticulophagy during UPR (43, 46). These observations imply that reticulophagy is crucial to maintaining the homeostasis of the ER and operates in parallel to ERAD to aid elimination of aggregated proteins when the ERAD system is overwhelmed in cells subjected to ER stress. The physiological consequences of failed cooperation between UPR and autophagy could include the transition from obesity to diabetes (47).
Involvement of autophagy in lipid metabolism has highlighted its role in the development of obesity, metabolic syndrome, and atherosclerosis (33). Given the central function of ER in lipid metabolism, it is reasonable to assume that autophagy and ER work together in a delicately balanced manner in the metabolism of lipids; however, no experimental data have yet proved this connection.
Although the relevant data are continuously accumulating, the selective entrapping of portions of the ER and the signaling mechanism between the UPR and autophagy remain largely unknown. Activation of Atg1/ULK (autophagy related 1/UNC-51-like) kinase is an early, central step in autophagy induction, and indeed, stimulation of Atg1 has been detected upon ER stress in yeast cells, although the mechanism of its activation has still to be assigned (42). Although Atg1/ULK kinase is involved in the initiation of autophagy, formation of LC3-II is inevitable for expansion of the autophagosomal membrane. The signaling of LC3-II formation in ER stress conditions reveals a direct connection with the UPR. Conversion of LC3-I into the functionally active LC3-II depends on eIF2α phosphorylation (44).
Connections Between the Regulation of Biotransformation, Signal Metabolism, Lipid Homeostasis, and the Formation of ER Membranes: The Redox Relationship
Detoxification of various endobiotics and xenobiotics is carried out by biotransformation enzymes (and transporters); the whole process is frequently also called drug metabolism. Substrates of drug metabolism are usually lipophilic molecules. The metabolism of these lipid-soluble compounds may interfere with lipid homeostasis.
Enzymes and transporters that catalyze and mediate drug metabolism are frequently ER membrane-bound proteins, whose turnover is controlled by transcription factors. Regulation of biotransformation and lipid homeostasis share several transcription factors: constitutive androstane receptor (CAR), liver X receptor (LXR), pregnane X receptor (PXR), PPARs, etc, (48, 49). Some of these are ligand-dependent transcription factors, and their ligands are substrates of certain drug-metabolizing enzymes. The gene batteries of these transcription factors encode biotransformation phase I, II, and III enzymes/transporters and proteins involved in redox homeostasis.
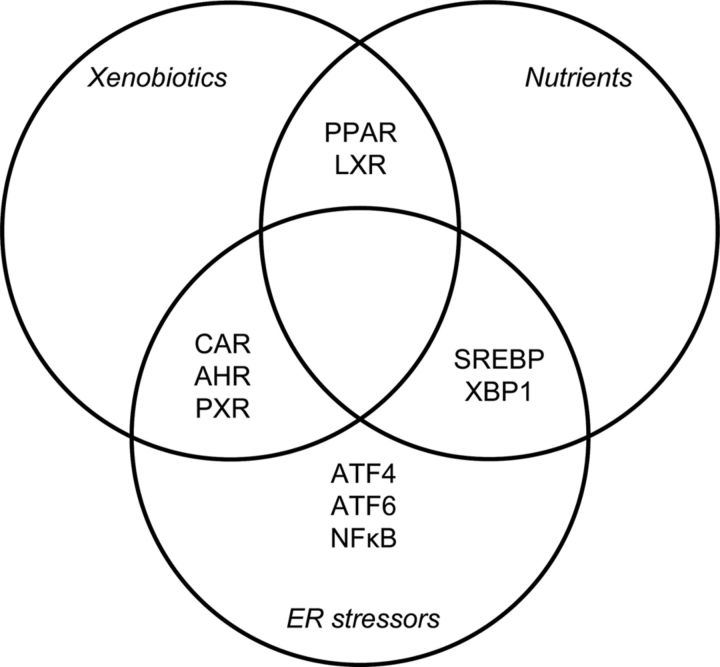
In various cases, proliferation of hepatic ER membranes upon stimulation by different xenobiotics has also been shown (for a review, see Ref. 50). A classic inducer of ER membrane proliferation in the liver is phenobarbital, whose effect is mediated by CAR and PXR (for a recent review, see Refs. 51 and 52). Thus, the regulation of ER membrane synthesis and biotransformation may also be related to one another (Figures 2 and 3). The ligands of several transcription factors may in certain cases induce ER stress; for example, PPARγ ligands induce ER stress in pancreatic β-cells (53).
Figure 3.
Interrelationship among xenobiotics, nutrients, and ER stressors at the level of transcription factors modulating ER functions.
The regulation of biotransformation, lipid homeostasis, and ER membrane homeostasis also share some common regulators/inducers (Figure 3). Various low–molecular-weight compounds interfere with ER homeostasis. Several observations have been published on drug-induced UPR through different mechanisms, for instance by redox stress. UPR induced by the redox stressor acetaminophen is part of drug-induced liver injury (54). Recently, the IRE1α-XBP1 branch has been shown to affect the stability of certain P450 enzymes involved in acetaminophen metabolism (55).
Aryl hydrocarbons enter quinone-quinol redox cycles, which can lead to oxidative stress in various cells. These molecules influence both redox state and drug metabolism and can accumulate under various (sometimes pathological) circumstances (56, 57), ultimately resulting in ER stress (58). The PERK-dependent branch of the UPR results in activation of Nrf2 (nuclear factor-like 2), which is a transcription factor in the regulation of antioxidant defense and cellular survival. Its gene battery contains, among others, genes of UDP-glucuronosyltransferases, which are phase II biotransformation enzymes. Thus, Nrf2 participates in the regulation of redox homeostasis and drug metabolism at the same time. A tight coupling of aryl hydrocarbon receptor (AHR) and Nrf2-dependent regulation in the prevention of quinone-induced oxidative stress and ER stress has recently been reviewed (59).
AHR can also be used as an example to demonstrate the connection between drug metabolism and cellular signaling. The metabolism of drug metabolism substrate signal molecules is dependent on the actual induction state of biotransformation. The regulatory role of AHR in eicosanoid signal metabolism (60) and in UPR (59) has been shown (Figure 3). It is noteworthy that induction of gulonolactone oxidase involved in vitamin C synthesis, a connection to antioxidant homeostasis, is also AHR-dependent (61). Nevertheless, the different induction states of the AHR gene battery lead to alterations in drug metabolism.
Low–molecular-weight pro-oxidants can cause ER stress by generating reactive oxygen species. Redox cycles in the metabolism of aryl compounds can affect drug metabolism as well. Changes in intermediary and xenobiotic metabolism due to starvation or diabetes mellitus may also influence the redox state and reactive oxygen species production in different cells (57), therefore contributing to redox stress-related effects and the concomitant UPR (62). Consequently, the induction state and expression of drug metabolism enzymes are also dependent on these changes. Endocrine disrupters can also induce ER stress as redox stressors and as ligands of intracellular receptors, eg, AHRs (63).
Low–molecular-weight compounds can influence ER homeostasis. Metabolites of intermediary metabolism directly affect cofactor supplies to protein folding, lipid production, and drug metabolism. Changes in the intraluminal NADPH to NADP ratio reveal a direct link between metabolic state and redox state. Glucose-6-phosphate is a source of electrons for NADP reduction in the ER. In contrast to cytosol, the direct link between the redox state of the pyridine nucleotide pool and the reduced thiol pool is missing in the ER due to the lack of the NADPH-dependent glutathione reductase in the luminal compartment of the ER (64). Consequently, there is a separate reduced pyridine nucleotide pool in the ER supporting the essentially anabolic function of the organelle in intracellular metabolic homeostasis (5). At the same time, the luminal compartment of the ER is an oxidizing environment from the point of view of thiol oxidation, which is needed for oxidative protein folding. Oxygen is the ultimate electron acceptor in the electron transfer chain for disulfide bridge formation in protein folding, and it is also required for oxygenations in drug metabolism. Thus, hypoxia and excessive biotransformation are ER stressors (Figure 1).
ER Stress and Signaling Routes: Cross Talk Between Internal and External Signaling
The outcome of the UPR is a complex change in ER homeostasis. Environmental changes may determine ER homeostasis and modulate the UPR. There is a complex cross talk between internal and external signaling among various plasma membrane receptors and sensors involved in the UPR. This network is coupled to a series of plasma membrane receptor-mediated signaling routes. Among these is the basic anabolic growth factor signaling route; insulin signaling is an especially important pathway. As mentioned above, insulin signaling is connected to SREBPs, which are essential components of the sterol response. Moreover, c-Jun amino-terminal kinase (JNK) and IkB kinase are activated through the IRE1 route. They phosphorylate insulin receptor substrate at serine and consequently block insulin signaling. This is one mechanism which leads to insulin resistance (for a review, see Ref. 2). Insulin signaling is also connected to activation of mammalian target of rapamycin (mTOR). Through the same serine phosphorylation of insulin receptor substrate, the UPR prevents activation of mTOR; in this way, ER stress mediates a negative feedback regulation in the maintenance of proteostasis. Thus, UPR prevents overload of ER protein folding by inhibiting activation of mTOR; increased production of proteins induced by nutrient availability is prevented by insulin signaling (65).
Additionally, the UPR interferes with TNFα signaling (2). Thus, both mobilizing and growth signaling pathways are connected to the UPR, whereas inflammation is connected to metabolic and redox homeostasis. Inflammation contributes to the final balance and homeostatic state among the different signaling routes, demonstrating the role of physiological coordination in the inflammatory network. Evidence of cross talk between inflammatory toll-like receptor (TLR) signaling and the UPR has also been demonstrated, indicating a putative pathological link between internal and external signaling (66).
The redox homeostasis of ER lumen also affects cellular signaling by changing the hormonal status. Cortisone-cortisol conversion (prereceptorial glucocorticoid activation) is catalyzed by the luminal enzyme 11β-hydroxysteroid dehydrogenase type 1 in a reversible reaction (67). The actual direction of the conversion is strictly dependent on the NADPH to NADP+ ratio, because reductase activity requires a very high ratio of NADPH to NADP+ (68). Because NADPH is thought to be mainly generated by another luminal enzyme, hexose-6-phosphate dehydrogenase, a direct connection exists between metabolism (in terms of glucose-6-phosphate supply), redox homeostasis (in terms of NADPH generation), and hormonal output (69, 70).
Differentiation of B lymphocytes into various antibody-secreting cells offers an attractive example to demonstrate the role of the UPR in the complex integration of extracellular stimuli and intrinsic signals. It also shows the indispensable role of the UPR (IRE1/XBP1 pathway) in lipid supply and protein synthesis for membrane biogenesis (71). In accordance with these observations, differentiation of preadipocytes into various LD-containing adipocytes due to fatty acid overload also indicates the complex function of the UPR in the coordinated regulation of protein and lipid synthesis (2).
Taken together, we can see that ER stress-induced UPR as a signaling response is in a complex relationship with both plasma membrane and intracellular signaling networks.
Concluding Remarks
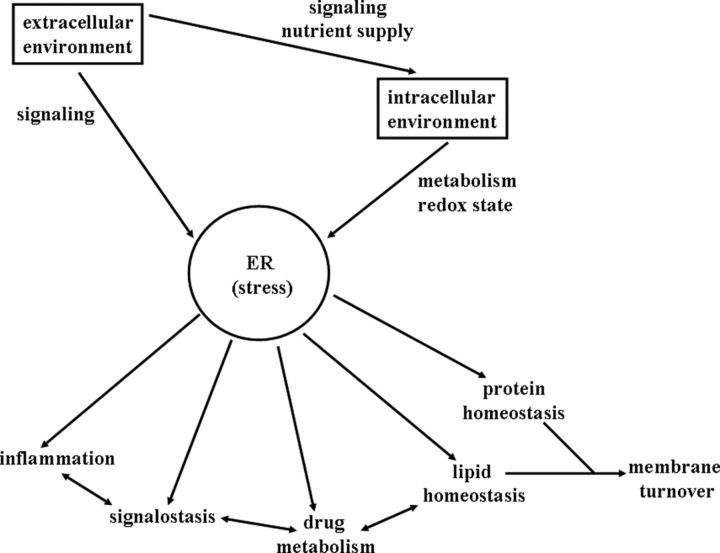
The ER is a mainly biosynthetic compartment that has a dominant role in maintaining protein, lipid, and redox homeostasis, controlling secretory processes and forming secretory products. As such, the ER is part of a cell defense system, contributing to the elimination of erroneous endogenous secretory products, a form of quality control in protein synthesis, and also to the inactivation and elimination of various endobiotics and xenobiotics. In the case of signal molecules, inactivation can represent part of the off signal. In addition, the ER consumes excess saturated fatty acids by forming lipid droplets. The ER, as an internal signaling source by the UPR, determines the cellular signaling network, which also has several connections to mitochondria-initiated signaling routes. Thus, ER directly influences certain plasma membrane receptor signaling pathways and also some nuclear receptors and other transcription factors. Because the ER can initiate apoptosis through ER stress-induced UPR, the ER is instrumental in cell death/survival determination. The coordinated regulation of lipid homeostasis, redox homeostasis, drug metabolism, and intracellular signaling in the ER is an adaptive mechanism that can respond to environmental changes (Figure 4).
Figure 4.
Functions of the ER stress in the integration of responses to stimuli from the extracellular and intracellular environments.
The ER is part of the endomembrane system. Membrane formation and turnover is also connected to the UPR. Regulation of protein, lipid, and drug metabolism is interconnected though signaling cross talks with regard to membrane formation in the ER. Through protein and lipid autophagy, membrane turnover can also be regulated by ER stress. Overall, there is an organelle-level integration of intracellular homeostasis that can adapt to extracellular and intracellular challenges.
Acknowledgments
Thanks are due to Andrew Symons for his help in preparation of the manuscript.
This work was supported by the Hungarian Scientific Research Fund (OTKA 104113 and 106060) and by the New Széchenyi Plan (TÁMOP-4.2.1./B-09/1/KMR-2010-0001).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AHR
- aryl hydrocarbon receptor
- ATF6
- activating transcription factor 6
- Atg1
- autophagy related 1
- CAR
- constitutive androstane receptor
- C/EBP
- CCAAT/enhancer binding protein
- ER
- endoplasmic reticulum
- ERAD
- ER-associated protein degradation
- IRE1α
- inositol-requiring enzyme 1α
- LC3
- microtuble-associated protein light chain 3
- LD
- lipid droplet
- LXR
- liver X receptor
- mTOR
- mammalian target of rapamycin
- NADPH
- reduced nicotinamide adenine dinucleotide phosphate
- Nrf2
- nuclear factor-like 2
- PERK
- PRK-like ER kinase
- PPARγ
- peroxisome proliferator-activated receptor-γ
- PRK
- double-stranded RNA-activated protein kinase
- PXR
- pregnane X receptor
- SCAP
- SREBP cleavage activating protein
- SREBP
- sterol regulatory element-binding protein
- UPR
- unfolded protein response
- XBP1
- X-box binding protein 1.
References
- 1. Rutkowski DT , Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol. 2010;189:783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hotamisligil G. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bánhegyi G , Margittai E , Szarka A , Mandl J , Csala M. Crosstalk and barriers between the electron carriers of the endoplasmic reticulum. Antioxid Redox Signal. 2012;16:772–780. [DOI] [PubMed] [Google Scholar]
- 4. Csala M , Kereszturi É , Mandl J , Bánhegyi G. The endoplasmic reticulum as the extracellular space inside the cell: role in protein folding and glycosylation. Antioxid Redox Signal. 2012;16:1100–1108. [DOI] [PubMed] [Google Scholar]
- 5. Fu S , Watkins SM , Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. [DOI] [PubMed] [Google Scholar]
- 6. Nebert DW. Proposed role of drug-metabolizing enzymes: regulation of steady state levels of the ligands that effect growth, homeostasis, differentiation, and neuroendocrine functions. Mol Endocrinol. 1991;5:1203–1214. [DOI] [PubMed] [Google Scholar]
- 7. Mandl J , Mészáros T , Bánhegyi G , Hunyady L , Csala M. Endoplasmic reticulum: nutrient sensor in physiology and pathology. Trends Endocrinol Metabol. 2009;20:194–201. [DOI] [PubMed] [Google Scholar]
- 8. Malhi H , Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ron D , Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. [DOI] [PubMed] [Google Scholar]
- 10. Zhang K , Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schuck S , Prinz WA , Thorn KS , Voss C , Walter P. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol. 2009;187:525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Promlek T , Ishiwata-Kimata Y , Shido M , Sakuramoto M , Kohno K , Kimata Y. Membrane aberrancy and unfolded proteins activate the endoplasmic reticulum stress sensor Ire1 in different ways. Mol Biol Cell. 2011;22:3520–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee AH , Scapa EF , Cohen DE , Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang K , Wang S , Malhotra J , et al. The unfolded protein response transducer IRE1α prevents ER stress-induced hepatic steatosis. EMBO J. 2011;30:1357–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang S , Chen Z , Lam V , et al. IRE1α-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab. 2012;16:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. So JS , Hur KY , Tarrio M , et al. Silencing of lipid metabolism genes through IRE1α-mediated mRNA decay lowers plasma lipids in mice. Cell Metab. 2012;16:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee AH , Glimcher LH. Intersection of the unfolded protein response and hepatic lipid metabolism. Cell Mol Life Sci. 2009;66:2835–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bobrovnikova-Marjon E , Hatzivassiliou G , Grigoriadou C , et al. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:16314–16319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oyadomari S , Harding HP , Zhang YH , Oyadomari M , Ron D. Dephosphorylation of translation initiation factor 2α enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Radhakrishnan A , Ikeda Y , Kwon HJ , Brown MS , Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci U S A. 2007;104:6511–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun LP , Seemann J , Goldstein JL , Brown MS. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Proc Natl Acad Sci U S A. 2007;104:6519–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shimano H. SREBPs: physiology and pathophysiology of the SREBP family. FEBS J. 2009;276:616–621. [DOI] [PubMed] [Google Scholar]
- 23. Yellaturu CR , Deng X , Park EA , Raghow R , Elam MB. Insulin enhances the biogenesis of nuclear sterol regulatory element-binding protein (SREBP)-1c by posttranscriptional down-regulation of Insig-2A and its dissociation from SREBP cleavage-activating protein (SCAP).SREBP-1c complex. J Biol Chem. 2009;284:31726–31734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ye J , Rawson RB , Komuro R , et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. [DOI] [PubMed] [Google Scholar]
- 25. Werstuck GH , Lentz SR , Dayal S , et al. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest. 2001;107:1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Esfandiari F , Villanueva JA , Wong DH , French SW , Halsted CH. Chronic ethanol feeding and folate deficiency activate hepatic endoplasmic reticulum stress pathway in micropigs. Am J Physiol Gastrointest Liver Physiol. 2005;289:54–63. [DOI] [PubMed] [Google Scholar]
- 27. Colgan SM , Tang D , Werstuck GH , Austin RC. Endoplasmic reticulum stress causes the activation of sterol regulatory element binding protein-2. Int J Biochem Cell Biol. 2007;39:1843–1851. [DOI] [PubMed] [Google Scholar]
- 28. Kammoun HL , Chabanon H , Hainault I , et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacquier N , Choudhary V , Mari M , Toulmay A , Reggiori F , Schneiter R. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Sci. 2011;124:2424–2437. [DOI] [PubMed] [Google Scholar]
- 30. Fei W , Wang H , Fu X , Bielby C , Yang H. Conditions of endoplasmic reticulum stress stimulate lipid droplet formation in Saccharomyces cerevisiae. Biochem J. 2009;424:61–67. [DOI] [PubMed] [Google Scholar]
- 31. Lee JS , Mendez R , Heng HH , Yang ZQ , Zhang K. Pharmacological ER stress promotes hepatic lipogenesis and lipid droplet formation. Am J Transl Res. 2012;4:102–113. [PMC free article] [PubMed] [Google Scholar]
- 32. Lee JS , Zheng Z , Mendez R , Ha SW , Xie Y , Zhang K. Pharmacologic ER stress induces non-alcoholic steatohepatitis in an animal model. Toxicol Lett. 2012;211:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh R , Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scheper W , Nijholt DA , Hoozemans JJ. The unfolded protein response and proteostasis in Alzheimer disease. Preferential activation of autophagy by endoplasmic reticulum stress. Autophagy. 2011;7:910–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mizushima N , Levine B , Cuervo AM , Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kondomerkos DJ , Kalamidas SA , Kotoulas OB. An electron microscopic and biochemical study of the effects of glucagon on glycogen autophagy in the liver and heart of newborn rats. Microsc Res Tech. 2004;63:87–93. [DOI] [PubMed] [Google Scholar]
- 37. Singh R , Kaushik S , Wang Y , et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singh R , Xiang Y , Wang Y , et al. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009;119:3329–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kovsan J , Blüher M , Tarnovscki T , et al. Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab. 2011;96:E268–E277. [DOI] [PubMed] [Google Scholar]
- 40. Hayashi-Nishino M , Fujita N , Noda T , Yamaguchi A , Yoshimori T , Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol. 2009;11:1433–1437. [DOI] [PubMed] [Google Scholar]
- 41. Hailey DW , Rambold AS , Satpute-Krishnan P , et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yorimitsu T , Nair U , Yang Z , Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–30304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bernales S , McDonald KL , Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kouroku Y , Fujita E , Tanida I , et al. ER stress (PERK/eIF2α phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. [DOI] [PubMed] [Google Scholar]
- 45. Ding WX , Ni HM , Gao W , et al. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–4710. [DOI] [PubMed] [Google Scholar]
- 46. Teckman JH , Perlmutter DH. Retention of mutant α1-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am J Physiol Gastrointest Liver Physiol. 2000;279:G961–G974. [DOI] [PubMed] [Google Scholar]
- 47. Quan W , Hur KY , Lim Y , et al. Autophagy deficiency in β-cells leads to compromised unfolded protein response and progression from obesity to diabetes in mice. Diabetologia. 2012;55:392–403. [DOI] [PubMed] [Google Scholar]
- 48. Honkakoski P , Negishi M. Regulation of cytochrome P450 (CYP) genes by nuclear receptors. Biochem J. 2000;347:321–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roth A , Looser R , Kaufmann M , et al. Regulatory cross-talk between drug metabolism and lipid homeostasis: constitutive androstane receptor and pregnane X receptor increase insig-1 expression. Mol Pharmacol. 2008;73:1282–1289. [DOI] [PubMed] [Google Scholar]
- 50. Schulte-Hermann R. Induction of liver growth by xenobiotic compounds and other stimuli. CRC Crit Rev Toxicol. 1974;3:97–158. [DOI] [PubMed] [Google Scholar]
- 51. Ross J , Plummer SM , Rode A , et al. Human constitutive androstane receptor (CAR) and pregnane X receptor (PXR) support the hypertrophic but not the hyperplastic response to the murine nongenotoxic hepatocarcinogens phenobarbital and chlordane in vivo. Toxicol Sci. 2010;116:452–466. [DOI] [PubMed] [Google Scholar]
- 52. Wang YM , Ong SS , Chai SC , Chen T. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Metab Toxicol. 2012;8:803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weber SM , Chambers KT , Bensch KG , Scarim AL , Corbett JA. PPARγ ligands induce ER stress in pancreatic β-cells: ER stress activation results in attenuation of cytokine signaling. Am J Physiol Endocrinol Metab. 2004;287:1171–1177. [DOI] [PubMed] [Google Scholar]
- 54. Nagy G , Kardon T , Wunderlich L , et al. Acetaminophen induces ER dependent signaling in mouse liver. Arch Biochem Biophys. 2007;459:273–279. [DOI] [PubMed] [Google Scholar]
- 55. Hur KY , So JS , Ruda V , et al. IRE1α activation protects mice against acetaminophen-induced hepatotoxicity. J Exp Med. 2012;209:307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bánhegyi G , Garzó T , Antoni F , Mandl J. Accumulation of phenols and catechols in isolated mouse hepatocytes in starvation or after pretreatment with acetone. Biochem Pharmacol. 1988;37:4157–4162. [DOI] [PubMed] [Google Scholar]
- 57. Mandl J , Bánhegyi G , Kalapos MP , Garzó T. Increased oxidation and decreased conjugation of drugs in the liver caused by starvation. Altered metabolism of certain aromatic compounds and acetone. Chem Biol Interact. 1995;96:87–101. [DOI] [PubMed] [Google Scholar]
- 58. Wang D , Wei Y , Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. [DOI] [PubMed] [Google Scholar]
- 59. Bock KW. Ah receptor- and Nrf2-gene battery members: modulators of quinone-mediated oxidative and endoplasmic reticulum stress. Biochem Pharmacol. 2012;83:833–838. [DOI] [PubMed] [Google Scholar]
- 60. Nebert DW , Karp CL. Endogenous functions of the aryl hydrocarbon receptor (AHR): intersection of cytochrome P450 1 (CYP1)-metabolized eicosanoids and AHR biology. J Biol Chem. 2008;283:36061–36065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Braun L , Kardon T , El Koulali K , Csala M , Mandl J , Bánhegyi G. Different induction of gulonolactone oxidase in aromatic hydrocarbon-responsive or -unresponsive mouse strains. FEBS Lett. 1999;463:345–349. [DOI] [PubMed] [Google Scholar]
- 62. Bánhegyi G , Benedetti A , Csala M , Mandl J. Stress on redox. FEBS Lett. 2007;581:3634–3640. [DOI] [PubMed] [Google Scholar]
- 63. Diamanti-Kandarakis E , Bourguignon JP , Giudice LC , et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Piccirella S , Czegle I , Lizák B , et al. Uncoupled redox systems in the lumen of the endoplasmic reticulum. Pyridine nucleotides stay reduced in an oxidative environment. J Biol Chem. 2006;281:4671–4677. [DOI] [PubMed] [Google Scholar]
- 65. Ozcan U , Ozcan L , Yilmaz E , et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Woo CW , Cui DY , Arellano J , et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by Toll-like receptor signaling. Nat Cell Biol. 2009;11:1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bánhegyi G , Benedetti A , Fulceri R , Senesi S. Cooperativity between 11β-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase in the lumen of the endoplasmic reticulum. J Biol Chem. 2004;279:27017–27021. [DOI] [PubMed] [Google Scholar]
- 68. Dzyakanchuk AA , Balázs Z , Nashev LG , Amrein KE , Odermatt A. 11β-Hydroxysteroid dehydrogenase 1 reductase activity is dependent on a high ratio of NADPH/NADP+ and is stimulated by extracellular glucose. Mol Cell Endocrinol. 2009;301:137–141. [DOI] [PubMed] [Google Scholar]
- 69. Walker EA , Ahmed A , Lavery GG , et al. 11β-Hydroxysteroid dehydrogenase type 1 regulation by intracellular glucose 6-phosphate provides evidence for a novel link between glucose metabolism and hypothalamo-pituitary-Adrenal axis function. J Biol Chem. 2007;282:27030–27036. [DOI] [PubMed] [Google Scholar]
- 70. Bánhegyi G , Csala M , Benedetti A. Hexose-6-phosphate dehydrogenase: linking endocrinology and metabolism in the endoplasmic reticulum. J Mol Endocrinol. 2009;42:283–289. [DOI] [PubMed] [Google Scholar]
- 71. Brewer JW , Jackowski S. UPR-mediated membrane biogenesis in B cells. Biochem Res Int. 2012;2012:738471. [DOI] [PMC free article] [PubMed] [Google Scholar]