Abstract
Background and Objectives
Acute triggers for VTE, which may include infection, are understudied, as is the timing and duration of VTE risk after infection. We hypothesized that there is an association between hospitalization with infection and short-term VTE risk that exceeds the known association between hospitalization and VTE.
Methods
VTE cases and infections were identified in the Atherosclerosis Risk in Communities (ARIC) cohort. A case-crossover design and conditional logistic regression were used to compare hospitalized infections among VTE cases (14, 30, 42, and 90 days before VTE) with corresponding control periods 1 year and 2 years prior. Since hospitalization is a known VTE trigger, study design and analytical techniques were used to isolate the impact of infection.
Results
There were 845 adjudicated incident VTE cases. Hospitalization with infection was more common in all case periods compared to equivalent control periods: 14 day OR (95% CI) = 1.7 (0.5, 5.8), 30 day OR (95% CI) = 2.7 (1.1, 6.4), 42 day OR (95% CI) = 2.2 (1.1, 4.7)), and 90 day OR (95% CI) = 1.2 (0.7, 2.0). The association was generally strongest in exposure periods closest to the VTE event and decreased as the time window before VTE increased.
Conclusions
These results support the hypothesis that hospitalized infection is a trigger of VTE. VTE preventive measures may prevent VTE events if used in the peri-infection period but clinical trials are needed.
Keywords: Infection, Pulmonary Embolism, Risk factors, Venous Thrombosis, Venous Thromboembolism
Introduction
Venous thromboembolism (VTE) consisting of deep-vein thrombosis (DVT) and pulmonary embolism (PE) is a common, life-threatening disease with over 500,000 hospitalized VTE cases annually in the United States.1 The Longitudinal Investigation of Thromboembolism Etiology (LITE) study, which is a community-based cohort of middle and older aged Americans, reported an incidence rate for VTE of 1.92 per 1000 person years.2 Study participants experiencing a first incident VTE in the late 1980s through early 2000s had a 28-day case fatality rate of 11%.2
Previous epidemiologic research has worked to identify both chronic and acute (triggering) risk factors for VTE. Established VTE triggers include surgery, trauma, immobility, malignancy and chemotherapy, hormonal therapy, and pregnancy and puerperium.3, 4 Infection has been investigated both as a chronic risk factor and acute precipitant or trigger of VTE. A 2012 systematic review by Tichelaar et al. summarized previous work on infection and risk of VTE and concluded that infections generally, and HIV, pneumonia, and urinary tract infections specifically, are associated with increased risk of VTE.5 Recent work has identified an association between cytomegalovirus and VTE.6, 7 A number of case reports have also identified a potential association between varicella and VTE.8, 9 Further, Zhu et al.’s 2009 study showed influenza vaccination is associated with reduced risk of VTE.10 Sepsis has also been shown to increase thrombosis and VTE risk.11, 12
Table 1 provides a summary of previous studies evaluating infection as a trigger of VTE. Rogers et al., for example, used a case-crossover design and found that infection occurred 2.9 times more often before a VTE hospitalization than in comparison periods.4 It has been proposed that infection triggers VTE through activating inflammatory, coagulation, and fibrinolysis processes associated with thrombosis, although immobility could also contribute.13
Table 1.
Infection as a trigger of VTE in previous studies
| Citation | Study Design | Infection Studied | Measure of Association, (95% CI)* |
|---|---|---|---|
| Masrouha et al, 201614 | Prospective Cohort | Preoperative Pneumonia | DVT – OR = 1.67 (1.32, 2.11) PE – OR = 2.18 (1.48, 3.22) |
| Chen et al, 201515 | Retrospective Cohort | Pneumonia | DVT – HR = 1.78 (1.39, 2.28) PE – HR = 1.97 (1.43, 2.72) |
| Dalager-Pedersen et al, 201416 | Prospective Cohort | Community Acquired Bacteremia | OR = 1.9 (1.4, 2.7) |
| Paran et al, 20136 | Prospective Cohort | Cytomegalovirus | OR = 2.49 (1.53, 4.06) |
| Rogers et al, 20124 | Case-Crossover | All infections | IRR = 2.9 (2.13, 3.94) |
| Schmidt et al, 201217 | Case-Control | Systemic respiratory tract infections, urinary tract infections, skin infections, intra-abdominal infections, and septicemia | IRR = 3.3 (2.9, 3.8) |
| Ribeiro et al, 201218 | Case-Control | Pneumonia | OR = 5.0 (3.9–6.3) |
| Clayton et al, 201119 | Case-Control | Respiratory Infections | DVT – OR = 2.64 (1.62, 4.29) PE – OR = 2.50 (1.33, 4.72) |
| Cimminiello et al, 201020 | Case-Control | All Infections | OR = 1.86 (1.16, 2.97) |
| Tichelaar et al, 201021 | Case-Control | All Infections | OR = 2.5 (1.4, 4.8) |
| Gangireddy et al, 200722 | Retrospective Cohort | Pneumonia | OR = 2.7 (2.10, 3.50) |
| Urinary Tract Infections | OR = 1.8 (1.3, 2.50) | ||
| Smeeth et al, 200623 | Case-Crossover | Urinary Tract Infections | IR = 2.10 (1.56, 2.82) |
| Systemic Respiratory Tract Infections | IR = 1.91 (1.49, 2.44) |
HR – Hazard Ratio, OR – Odds Ratio, IRR – Incidence Rate Ratio, IR – Cumulative Incidence Ratio
Recognition of infection as a VTE trigger is growing but better understanding of infection as a VTE trigger is needed. In summarizing previous work related to pneumonia and VTE, Violi et al. concluded that data point to an association between pneumonia and VTE but “prospective studies are still lacking” and “it is unclear if pneumonia per se carries a risk of venous thrombosis or…some peculiar clinical characteristics are associated with an enhanced risk of venous thrombosis”.11 Previous studies often relied on case-control or retrospective designs and failed to properly account for potential confounding factors such as the seasonality of infection. Further, previous studies used different definitions for infection and evaluated risk over different time periods, making the timing and duration of VTE risk after infection unclear. Herein, we explore the relationship between hospitalization with infection and short-term VTE risk. An improved understanding of infection-triggered VTE risk could help patients with infections and their care providers take steps to reduce their elevated VTE risk.
We used data collected in the long-running, prospective Atherosclerosis Risk in Communities (ARIC) cohort to study this association further. We hypothesized that in the ARIC study there is an association between hospitalization with infection and short-term VTE risk that exceeds the known general association between hospitalization and VTE.
Methods
Study Population
LITE is a prospective study of VTE occurrence in 2 pooled, multicenter, longitudinal population-based cohort studies: the Atherosclerosis Risk in Communities (ARIC) study and the Cardiovascular Health Study (CHS). The LITE study design, methods, and VTE incidence rates have been described in detail elsewhere.24 For this analysis, only ARIC data were included as it had longer follow-up. The ARIC Study cohort comprises 15,792 adults aged 45–64 years at recruitment in 1987–1989. Subsequent exams took place 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), and 2011–2013 (visit 5). Visit 6 (2016–2017) is currently underway. Cohort participants were selected from four U.S. communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. A detailed discussion of ARIC study design and objectives is provided elsewhere.25
Study Design
We conducted a case-crossover study in which each ARIC participant with PE or DVT during follow-up served as his/her own control. The case-crossover design affords the ability to isolate potential triggering exposures that vary over time within persons and better control for potential confounding that might occur between persons. Since hospitalization is a known VTE trigger, we attempted to isolate the impact of infection using two analytical approaches discussed below.
VTE case ascertainment
The outcome of interest was VTE as defined for ARIC between 1987 and 2011 (n = 845). VTE cases among ARIC participants were identified through participant report and surveillance of local hospital discharge lists for cohort members. For each hospitalization identified, ICD-9 codes were used to identify possible VTE cases. Information retrieved from the hospital records was assigned a VTE classification separately by 2 physicians; differences between the 2 physicians were adjudicated by discussion. Details on quality assurance for ascertainment and classification of VTE cases are found elsewhere.24 The hospital admission date abstracted from the patient medical record was considered the VTE date. VTE was further classified as all PEs and all DVTs, and all PEs but restricting DVTs to those occurring in the leg because upper extremity DVTs were relatively few and almost always the result of venous catheters.
Main independent variable: hospitalization with infection
The exposure of interest was hospitalization with infection during case periods of 14, 30, 42, or 90 days prior to VTE diagnosis. Case periods were selected to address the timing and duration of VTE risk after infection similar to study methodology used by Cowan et al26 and Elkind et al.27 We employed two exposure classification schemes. First, exposure was dichotomized as hospitalization with infection or no hospitalization with infection (referent) which includes participants not hospitalized and those with hospitalizations that did not include an infection. Second, exposure was classified as hospitalization with infection, hospitalization without infection (referent), or no hospitalization. For the control periods, we used equivalent length periods exactly 1 and 2 years before VTE diagnosis, to account for the seasonality of infection rates. Hospitalization with infection was assessed using hospital discharge ICD-9 codes. The following ICD-9 codes adapted from methodology used by Rogers et al.4 for infections were included:
0xx.xx, 11x.xx, 245.0x, 254.1x, 320.xx, 321.xx, 322.xx, 323.xx, 324.xx, 357.0x, 360.0x, 370.55, 373.13, 376.01, 379.09, 380.1x, 382.0x, 382.1x, 382.2x, 382.3x, 382.4x, 383.0x, 386.33, 391.xx, 420.90, 420.99, 421.xx, 422.92, 429.89, 46x.xx, 475.xx, 478.22, 478.24, 478.29, 48x.xx, 491.1x, 510.xx, 511.1x, 513.xx, 519.01, 519.2x, 522.5x, 522.7x, 523.3x, 527.3x, 528.3x, 536.41, 540.xx, 566.xx, 567.0x, 567.1x, 567.2x, 567.30x, 569.5x, 569.61, 572.0x, 575.0x, 575.12, 577.0x, 590.xx, 595.0x, 597.0x, 599.0x, 601.2x, 604.0x, 607.1x, 607.2x, 608.0x, 608.4x, 616.10, 616.3x, 616.4x, 68x.xx, 711.xx, 727.89, 728.0x, 728.86, 730.xx, 785.4x, 785.52, 790.7x, 995.91, 995.92, 996.6x, 997.62, 998.5x, 999.3x, V09.xx
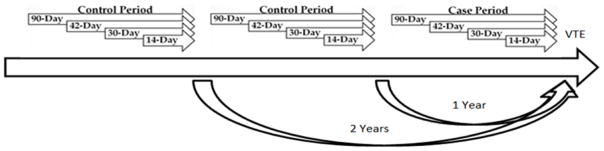
Codes in any position among the discharge diagnoses were counted. A washout period of 2 days was used to exclude infections diagnosed within two days of a VTE diagnosis that may have been diagnosed secondarily at the time of VTE hospitalization. The hospital discharge date for infection was considered the infection date and the infection date must have preceded the VTE diagnosis date. The study design is summarized in Figure 1.
Figure 1.
Case-crossover study design employed to study VTE in relation to triggering by hospitalized infection, ARIC. Presence of hospitalized infection was assessed for each of the 12 time periods, and case period was compared to control periods.
Analysis
Two separate analyses were performed based on the two exposure classifications described above. First, the prevalence of hospitalization with infection 14, 30, 42, and 90-days prior to VTE was compared to the corresponding time periods exactly 1 and 2 years prior to the VTE event. Conditional logistic regression was used to estimate odds ratios (OR) of VTE and 95% confidence intervals (CIs) for each time period (14, 30, 42, and 90 days). We controlled for the number of hospitalizations in the 9 months preceding each case or control period to account for potential decline in overall health status due to age and immobility associated with hospitalization. We further controlled for the total number of hospitalizations in the 90-day exposure period to account for non-infection triggering due to hospitalization and isolate the impact of infection.
Second, we used conditional logistic regression to compare the trichotomized (3-level) exposure – occurrence of hospitalization with infection, no hospitalization, hospitalization without infection (referent) – at intervals of 14, 30, 42, and 90 days prior to the VTE with two preceding control periods (1 year and 2 years prior to the VTE). We controlled for the number of hospitalizations in the 9 months preceding each case or control period to account for potential decline in overall health status due to age and immobility associated with hospitalization.
Both analyses were designed to isolate the effect of infection and estimate the effect of infection independent of the known association between hospitalization and VTE. Both analyses were conducted using all PEs and DVTs (All VTE cases) (n=845), and using all PEs but restricting DVTs to those occurring in the leg (n=755).
Results
Participant characteristics at the time of VTE diagnosis are provided in Table 2 for both all VTE cases and for PE and leg DVT cases. The majority of total VTE cases were female (56%) and white (62%), and the average age (±SD) was 70.5 (7.8) when the VTE events occurred.
Table 2.
At-Event Characteristics of ARIC Participants Who Developed VTE, 1987–2011
| Characteristic | All VTE (n=845) | PE and Leg DVT (n= 755) |
|---|---|---|
| Age, years, mean ± SD | 70.5 ± 7.9 | 70.5 ± v7.8 |
| Sex, Count (%) | ||
| Male | 375 (44.4%) | 332 (44.0%) |
| Female | 470 (55.6%) | 423 (56.0%) |
| Race, Count (%) | ||
| White | 526 (62.3%) | 480 (63.6%) |
| Black | 318 (37.6%) | 274 (36.3%) |
| Asian | 1 (0.1%) | 1 (0.1%) |
Of the 845 total VTE cases, 75 had a hospitalization with an infection in the 90 days preceding the VTE event. Given that a hospitalization can contain more than 1 infection code, these hospitalizations contained 184 infection discharge diagnosis codes. Pneumonia (14.7%), urinary tract infections (13.6%), and septicemia/bacteremia (12.5%) were the most common infection types. Table 3 contains the frequency of infection types prior to VTE events. A total of 36 VTE cases had a hospitalization with infection during at least one of the 90-day control periods. For the 90-day exposure periods, the mean number of hospitalizations was 0.46, 0.11, and 0.09 for the case period, first control period, and second control period respectively. In the 9 months preceding each case or control period, the mean number of hospitalizations was 0.42, 0.28, and 0.22 for the case period, first control period, and second control period respectively.
Table 3.
Types of hospital discharge infections in the 90 days prior to developing VTE, ARIC, 1987–2011
| Infection Type | Case Period - Count | Percent of Total |
|---|---|---|
| Pneumonia | 27 | 14.7% |
| Other Respiratory Infections | 9 | 4.9% |
| Urinary Tract Infection | 25 | 13.6% |
| Other Genitourinary Infections | 3 | 1.6% |
| Septicemia/Bacteremia | 23 | 12.5% |
| Gastrointestinal Tract Infections | 16 | 8.7% |
| Infections due to Surgical or Medical Care | 14 | 7.6% |
| Other Drug-Resistant Infections | 5 | 2.7% |
| Candidiasis | 12 | 6.5% |
| Other Mycoses | 1 | 0.5% |
| Staphylococcus infections | 8 | 4.3% |
| Other Infections | 41 | 22.3% |
| Total | 184 | 100% |
The results from the primary analysis with exposure dichotomized as hospitalization with infection or no hospitalization with infection are shown in Table 4. Hospitalization with infection was more common in all case periods compared to equivalent control periods: 14 day OR (95% CI) = 1.7 (0.5, 5.8), 30 day OR (95% CI) = 2.7 (1.1, 6.4), 42 day OR (95% CI) = 2.2 (1.1, 4.7)), and 90 day OR (95% CI) = 1.2 (0.7, 2.0). Overall, the association appeared to be generally strongest in exposure periods closest to the VTE event and decreased as the time window before VTE increased.
Table 4.
Association of Dichotomized Recent Hospitalization with Infection and Risk of VTE, ARIC, 1987–2011
| All VTEs | Case (n) | Control* (n) | Crude OR, 95% CI | Adjusted† OR, 95% CI |
|---|---|---|---|---|
| 14 Days | ||||
| No Hospitalization | 830 | 1684 | Ref | Ref |
| Hospitalization with Infection | 15 | 6 | 5.72 (2.07, 15.81) | 1.74 (0.52, 5.83) |
| 30 Days | ||||
| No Hospitalization | 806 | 1680 | Ref | Ref |
| Hospitalization with Infection | 39 | 10 | 9.39 (4.38, 20.14) | 2.65 (1.10, 6.39) |
| 42 Days | ||||
| No Hospitalization | 795 | 1676 | Ref | Ref |
| Hospitalization with Infection | 50 | 14 | 8.10 (4.31, 15.22) | 2.23 (1.06, 4.69) |
| 90 Days | ||||
| No Hospitalization | 770 | 1654 | Ref | Ref |
| Hospitalization with Infection | 75 | 36 | 5.59 (3.52, 8.90) | 1.15 (0.65, 2.03) |
Control periods were equivalent length periods exactly 1 and 2 years before VTE diagnosis
Adjusted for 9 month and 90 day hospitalization totals
The secondary analysis with exposure categorized as hospitalization with infection, hospitalization without infection, and no hospitalization (Table 5) yielded slightly smaller ORs as the dichotomized exposure analysis and with less precision. Hospitalization with infection was more common in all case periods compared to equivalent control periods: 14 day OR (95% CI) = 1.6 (0.5, 5.1), 30 day OR (95% CI) = 1.7 (0.7, 4.0), 42 day OR (95% CI) = 1.5 (0.8, 3.2), and 90 day OR (95% CI) = 1.3 (0.8, 2.2). Similar results were found when the analysis was restricted to PEs and leg DVTs (supplementary tables 1 and 2).
Table 5.
Association of Trichotomized Recent Hospitalization Categories and Risk of VTE, ARIC, 1987–2011
| All VTEs | Case (n) | Control* (n) | Unadjusted OR, 95% CI | Adjusted† OR, 95%CI |
|---|---|---|---|---|
| 14 Days | ||||
| No Hospitalization | 789 | 1659 | 0.29 (0.17, 0.47) | 0.25 (0.15, 0.43) |
| Hospitalization without Infection | 41 | 25 | Ref | Ref |
| Hospitalization with Infection | 15 | 6 | 1.80 (0.59, 5.52) | 1.60 (0.51, 5.06) |
| 30 Days | ||||
| No Hospitalization | 698 | 1640 | 0.14 (0.09, 0.21) | 0.13 (0.08, 0.19) |
| Hospitalization without Infection | 108 | 40 | Ref | Ref |
| Hospitalization with Infection | 39 | 10 | 1.87 (0.80, 4.35) | 1.69 (0.72, 3.99) |
| 42 Days | ||||
| No Hospitalization | 655 | 1621 | 0.14 (0.10, 0.20) | 0.14 (0.10, 0.20) |
| Hospitalization without Infection | 140 | 55 | Ref | Ref |
| Hospitalization with Infection | 50 | 14 | 1.57 (0.77, 3.20) | 1.53 (0.75, 3.15) |
| 90 Days | ||||
| No Hospitalization | 563 | 1555 | 0.16 (0.12, 0.21) | 0.16 (0.12, 0.22) |
| Hospitalization without Infection | 207 | 99 | Ref | Ref |
| Hospitalization with Infection | 75 | 36 | 1.29 (0.76, 2.18) | 1.27 (0.75, 2.16) |
Control periods were equivalent length periods exactly 1 and 2 years before VTE diagnosis
Adjusted for 9 month hospitalization total
Discussion
This case-crossover study within a population-based cohort demonstrated that VTE risk is higher after hospitalization with infection independent of the known association between hospitalization and VTE. Patients with infection had higher odds of VTE in the 90 days after their hospitalization with infection compared to equivalent control periods 1 and 2 years previously. This supports our hypothesis that there is an association between hospitalization with infection and subsequent short-term VTE risk that exceeds the known association between hospitalization and VTE. Thus hospitalized infection appears to trigger VTE events.
We employed two analytical approaches to control for potential confounding and isolate the association of hospitalization with infection on VTE risk. Both approaches produced similar effect estimates but the trichotomized analysis had less precision and no statistically significant results. VTE risk appears to be around 2 times higher in the 42 days following a hospitalization with infection compared to no infection. The association between hospitalization with infection and VTE was graded, such that VTE risk generally appeared highest in the exposure periods most proximal to the VTE event and decreased as the time window before VTE increased. These results corroborate previous work that found that infection may function as a VTE trigger.
Besides the possibility that infection leads to VTE through decreased mobility, Epaulard et al. summarized existing studies that showed that the inflammatory response to infection and the coagulation and fibrinolysis processes likely share common pathways, explaining why infection is associated with thrombosis.13 That is, the risk of VTE due to infection arises through a link between inflammation and coagulation activation.13
Our study has a number of strengths, including a large sample size from a community cohort, a rigorous methodology to adjudicate VTE events, and a crossover design to control for potential confounding. It also has limitations. The vast majority of VTE events were symptomatic since events were captured via hospital records and were clinically diagnosed and verified by the investigators without specific screening for asymptotic VTE events. We were unable to look at VTE triggering by infection type due to insufficient numbers and corresponding low power. Like all case crossover studies, our study may suffer from survival bias as we did not consider infections in participants who did not have a VTE event. Our study only considered the relationship between infection and VTE among those who survived any infections and later had a VTE event. Confounding by age is possible, generally, because as participants age their risk of both VTE and hospitalization with infection increase. However, to reduce potential confounding by age, only control periods 1 and 2 years prior to VTE events were examined. We also adjusted for possible confounding by the number of hospitalizations. Other confounders such as medication use that may vary between the exposure and control periods were not ascertained. Relatively few infections prior to VTE events yielded OR estimates that were imprecise, with wide confidence intervals, particularly in the trichotomized analysis. We certainly under-ascertained infections, especially minor ones, by including only hospitalizations with infections. If minor infections similarly increase VTE risk, our missing them would most likely lead to non-differential misclassification of the exposure in both case and control periods, which would typically bias ORs towards the null for dichotomized hospitalization with infection.
Our study has a number of potential implications. Hospitalized patients with an infection should be considered potential candidates for VTE prophylaxis. Our results support the inclusion of infection information in the VTE risk assessment of hospitalized such as the Padua Prediction Score.28 Identification of hospitalization with infection as a VTE trigger may prompt more aggressive treatment with standard preventive strategies, including such as leg mobilization or compression or anticoagulants, during and immediately following hospitalization with infection to reduce the increased risk of acute VTE. This time period immediately following infection has been referred to in previous trigger research as a “treatable moment” that may be used to reduce otherwise elevated VTE risk.27 Evidence-based infection control efforts such as influenza vaccination may be considered because of their ability to not only reduce infection but may also reduce VTE.10
Conclusion
VTE patients had higher odds of a hospitalization with infection within 90 days prior to their VTE event compared to equivalent control periods 1 and 2 years previous. There may be a role for infection in VTE prevention decision making, though clinical trials and a cost-benefit analysis should be considered.
Highlights.
Hospitalized infection appears to trigger VTE events
The triggering effect of infection on VTE decreases over time after an infection
VTE preventive measures may prevent VTE events in the peri-infection period
Hospitalized patients with an infection may be considered for VTE prophylaxis
Acknowledgments
We thank the staff and participants of the ARIC study for their important contributions.
Funding:
The ARIC Study was supported by National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. The LITE study was funded by National Heart, Lung, and Blood Institute (NHLBI) grant R01 HL59367. L.T.C. is supported by an NHLBI training grant T32 HL007779.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease C and Prevention. Venous thromboembolism in adult hospitalizations - United States, 2007–2009. MMWR Morbidity and mortality weekly report. 2012;61:401–4. [PubMed] [Google Scholar]
- 2.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. The American journal of medicine. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Lippi G, Franchini M, Favaloro EJ. Unsuspected triggers of venous thromboembolism--trivial or not so trivial? Seminars in thrombosis and hemostasis. 2009;35:597–604. doi: 10.1055/s-0029-1242713. [DOI] [PubMed] [Google Scholar]
- 4.Rogers MA, Levine DA, Blumberg N, Flanders SA, Chopra V, Langa KM. Triggers of hospitalization for venous thromboembolism. Circulation. 2012;125:2092–9. doi: 10.1161/CIRCULATIONAHA.111.084467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tichelaar YI, Kluin-Nelemans HJ, Meijer K. Infections and inflammatory diseases as risk factors for venous thrombosis. A systematic review. Thrombosis and haemostasis. 2012;107:827–37. doi: 10.1160/TH11-09-0611. [DOI] [PubMed] [Google Scholar]
- 6.Paran Y, Shalev V, Steinvil A, Justo D, Zimmerman O, Finn T, Berliner S, Zeltser D, Weitzman D, Raz R, Chodick G. Thrombosis following acute cytomegalovirus infection: a community prospective study. Ann Hematol. 2013;92:969–74. doi: 10.1007/s00277-013-1715-3. [DOI] [PubMed] [Google Scholar]
- 7.Schimanski S, Linnemann B, Luxembourg B, Seifried E, Jilg W, Lindhoff-Last E, Schambeck CM. Cytomegalovirus infection is associated with venous thromboembolism of immunocompetent adults--a case-control study. Ann Hematol. 2012;91:597–604. doi: 10.1007/s00277-011-1334-9. [DOI] [PubMed] [Google Scholar]
- 8.Rabah F, El-Banna N, Abdel-Baki M, Beshlavi I, Macaraig D, Bhuyan D, Al-Hinai M, Al-Mashaikhi N, Wasifuddin SM, Tomas E, Pathare A. Postvaricella thrombosis-report of two cases and literature review. The Pediatric infectious disease journal. 2012;31:985–7. doi: 10.1097/INF.0b013e31825c7993. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara M, Bertocco F, Ferrara D, Capozzi L. Thrombophilia and varicella zoster in children. Hematology. 2013;18:119–22. doi: 10.1179/1607845412Y.0000000055. [DOI] [PubMed] [Google Scholar]
- 10.Zhu T, Carcaillon L, Martinez I, Cambou JP, Kyndt X, Guillot K, Vergnes MC, Scarabin PY, Emmerich J. Association of influenza vaccination with reduced risk of venous thromboembolism. Thrombosis and haemostasis. 2009;102:1259–64. doi: 10.1160/TH09-04-0222. [DOI] [PubMed] [Google Scholar]
- 11.Violi F, Cangemi R, Calvieri C. Pneumonia, thrombosis and vascular disease. Journal of thrombosis and haemostasis : JTH. 2014;12:1391–400. doi: 10.1111/jth.12646. [DOI] [PubMed] [Google Scholar]
- 12.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thrombosis research. 2012;129:290–5. doi: 10.1016/j.thromres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Epaulard O, Foote A, Bosson JL. Chronic Infection and Venous Thromboembolic Disease. Seminars in thrombosis and hemostasis. 2015;41:644–9. doi: 10.1055/s-0035-1556729. [DOI] [PubMed] [Google Scholar]
- 14.Masrouha KZ, Musallam KM, Rosendaal FR, Hoballah JJ, Jamali FR. Preoperative Pneumonia and Postoperative Venous Thrombosis: A Cohort Study of 427,656 Patients Undergoing Major General Surgery. World journal of surgery. 2016 doi: 10.1007/s00268-016-3409-1. [DOI] [PubMed] [Google Scholar]
- 15.Chen YG, Lin TY, Huang WY, Lin CL, Dai MS, Kao CH. Association between pneumococcal pneumonia and venous thromboembolism in hospitalized patients: A nationwide population-based study. Respirology. 2015;20:799–804. doi: 10.1111/resp.12501. [DOI] [PubMed] [Google Scholar]
- 16.Dalager-Pedersen M, Sogaard M, Schonheyder HC, Thomsen RW, Baron JA, Nielsen H. Venous thromboembolism after community-acquired bacteraemia: a 20-year danish cohort study. PloS one. 2014;9:e86094. doi: 10.1371/journal.pone.0086094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt M, Horvath-Puho E, Thomsen RW, Smeeth L, Sorensen HT. Acute infections and venous thromboembolism. Journal of internal medicine. 2012;271:608–18. doi: 10.1111/j.1365-2796.2011.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribeiro DD, Lijfering WM, Van Hylckama Vlieg A, Rosendaal FR, Cannegieter SC. Pneumonia and risk of venous thrombosis: results from the MEGA study. Journal of thrombosis and haemostasis : JTH. 2012;10:1179–82. doi: 10.1111/j.1538-7836.2012.04732.x. [DOI] [PubMed] [Google Scholar]
- 19.Clayton TC, Gaskin M, Meade TW. Recent respiratory infection and risk of venous thromboembolism: case-control study through a general practice database. International journal of epidemiology. 2011;40:819–27. doi: 10.1093/ije/dyr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cimminiello C, Filippi A, Mazzaglia G, Pecchioli S, Arpaia G, Cricelli C. Venous thromboembolism in medical patients treated in the setting of primary care: a nationwide case-control study in Italy. Thrombosis research. 2010;126:367–72. doi: 10.1016/j.thromres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Tichelaar YI, Knol HM, Mulder AB, Kluin-Nelemans JC, Lijfering WM. Association between deep vein thrombosis and transient inflammatory signs and symptoms: a case-control study. Journal of thrombosis and haemostasis : JTH. 2010;8:1874–6. doi: 10.1111/j.1538-7836.2010.03939.x. [DOI] [PubMed] [Google Scholar]
- 22.Gangireddy C, Rectenwald JR, Upchurch GR, Wakefield TW, Khuri S, Henderson WG, Henke PK. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. Journal of vascular surgery. 2007;45:335–341. doi: 10.1016/j.jvs.2006.10.034. discussion 341–2. [DOI] [PubMed] [Google Scholar]
- 23.Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367:1075–9. doi: 10.1016/S0140-6736(06)68474-2. [DOI] [PubMed] [Google Scholar]
- 24.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Archives of internal medicine. 2002;162:1182–9. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 25.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 26.Cowan LT, Alonso A, Pankow JS, Folsom AR, Rosamond WD, Gottesman RF, Lakshminarayan K. Hospitalized Infection as a Trigger for Acute Ischemic Stroke: The Atherosclerosis Risk in Communities Study. Stroke; a journal of cerebral circulation. 2016;47:1612–7. doi: 10.1161/STROKEAHA.116.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elkind MS, Carty CL, O’Meara ES, Lumley T, Lefkowitz D, Kronmal RA, Longstreth WT., Jr Hospitalization for infection and risk of acute ischemic stroke: the Cardiovascular Health Study. Stroke; a journal of cerebral circulation. 2011;42:1851–6. doi: 10.1161/STROKEAHA.110.608588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, De Bon E, Tormene D, Pagnan A, Prandoni P. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. Journal of thrombosis and haemostasis : JTH. 2010;8:2450–7. doi: 10.1111/j.1538-7836.2010.04044.x. [DOI] [PubMed] [Google Scholar]