Abstract
Recent studies have shown that the quantity of donor-reactive memory T cells is an important factor in determining the relative heterologous immunity barrier posed during transplantation. Here, we hypothesized that the quality of T cell memory also potently influences the response to costimulation blockade-based immunosuppression. Using a murine skin graft model of CD8+ memory T cell-mediated costimulation blockade resistance, we elicited donor-reactive memory T cells using three distinct types of pathogen infections. Strikingly, we observed differential efficacy of a costimulation and integrin blockade regimen based on the type of pathogen used to elicit the donor-reactive memory T cell response. Intriguingly, the most immunosuppression-sensitive memory T cell populations were composed primarily of central memory cells that possessed greater recall potential, exhibited a less differentiated phenotype, and contained more multi-cytokine producers. These data therefore demonstrate that the memory T cell barrier is dependent on the specific type of pathogen infection via which the donor-reactive memory T cells are elicited, and suggest that the immune stimulation history of a given transplant patient may profoundly influence the relative barrier posed by heterologous immunity during transplantation.
Introduction
Costimulation blockade (CoB) with belatacept (a second generation CTLA4-Ig) in renal transplantation has the benefit of improved long-term renal allograft function and less metabolic toxicity (1, 2). However, belatacept has been associated with a higher incidence and severity of acute rejection. The mechanisms responsible for this CoB resistant rejection have not been clearly defined, but it has been increasingly recognized that the immune history and alloreactive memory T cell precursor frequency of a transplant recipient may be major determinants of the success or failure of more selective immunosuppressive strategies (3–6).
There is abundant pre-clinical evidence that CoB alone can induce tolerance in mice (7, 8), but this strategy has been less capable of tolerance induction in more immunologically complex and antigen experienced nonhuman primates and humans (9–11). To underscore this point, while memory T cells comprise approximately 2% of the T cell compartment in specific pathogen free experimental mice, they comprise 40–50% of the T cell pool of nonhuman primates and adult humans (12–14). Thus antigen stimulation history and the pre-existing memory T cell repertoire may potentially play a central role in mediating CoB resistant rejection, as memory T cells possess reduced activation thresholds and decreased reliance on costimulatory signals (4, 5).
In transplant recipients, donor-reactive memory T cells arise from prior exposure to foreign MHC via prior blood transfusion, transplantation or pregnancy. Additionally, heterologous immune mechanisms whereby memory T cells generated in response to infectious pathogens become cross-reactive with donor antigens provide another potential source of CoB resistant alloreactive memory T cells in transplant recipients (15–18). Experimental evidence has implicated memory T cells as mediators of CoB resistant rejection (17, 19) and higher pre-transplant frequencies of donor-specific memory have been shown to correlate with inferior transplant outcomes (3, 20, 21). Furthermore, Nadazdin et al. recently showed that high alloreactive memory T cell precursor frequency impairs tolerance induction to kidney allografts in nonhuman primates (22).
In an effort to facilitate the use of CoB by selectively targeting donor-reactive memory T cells, our group has previously shown that neutralizing memory T cells by targeting integrin molecules that are differentially expressed on this subset of T cells could overcome the barrier of CoB resistant rejection (23, 24). Additionally, in a murine model of donor-specific memory CD8+ T cells that mediate CoB resistance, rejection was abrogated when coupling either anti-LFA-1 or anti-VLA-4 therapy to costimulatory blockade (25), thus validating that a combined costimulation and integrin blockade approach specifically inhibits graft rejection mediated by donor-specific CD8+ memory T cells.
It has become increasingly apparent that a large degree of heterogeneity exists amongst memory T cell phenotypes, function, distribution, longevity and protective capacity (26). For example, central (TCM) and effector (TEM) memory T cells have been classically characterized based on the differential expression of homing receptors (27), but analysis of actual post-activation populations illustrates much greater diversity in survival, recall potentials and subsets defined by other markers (28, 29). Moreover, current thinking holds that the route of exposure, dose, replication rate, recurrence, and tropism of the infectious challenge may impact qualitative aspects of memory T cell development (30). Therefore we hypothesized that the ultimate quality of T cell memory formed in response to pathogen stimulation can influence the host response to proven selective immunosuppressive strategies.
In this study we show that pathogen stimulation in the form of pre-transplant acute, latent, or persistent infections produces quantitatively similar but phenotypically and functionally distinct donor-reactive CD8+ memory T cell populations. Importantly, genetically identical mice that experienced different “immune histories” (i.e. had been infected with different pathogens) exhibited differential graft survival following combined costimulation/integrin blockade. Our data suggest that qualitative differences may account for the differences in allograft survival and altered susceptibility profiles observed in response to immunosuppression with combined CoB and anti-LFA-1 or anti-VLA-4 therapy. These findings highlight the influence of pathogen stimulation history on the differentiation of donor-reactive memory T cells and the generation of immune modulation strategy-dependent variability in allograft survival.
Methods
Mice
C57BL/6 mice were obtained from the National Cancer Institute and ovalbumin (OVA)-specific TCR transgenic OT-I mice (31) purchased from Taconic Farms were bred to Thy1.1+ background. C57BL/6 mice that constitutively express membrane-bound OVA under the beta-actin promoter (mOVA mice) (32) were a gift from M. Jenkins (University of Minnesota). All animals were housed in pathogen-free facilities and maintained in accordance with Emory University Institutional Animal Care and Use Committee guidelines.
T cell adoptive transfers and pathogen stimulation
For adoptive transfers of OVA-specific T cells, spleen and mesenteric lymph nodes isolated from Thy1.1+ OT-I mice were stained with mAbs for CD4, CD8, Thy1.1, and Vα2 for quantification by TruCount bead analysis (BD Biosciences). After resuspension in PBS, 104 Thy1.1+ OT-I T cells were injected intravenously into naïve C57BL/6 mice. OT-I TCR transgenic cells were used to standardize the T cell precursor frequency, TCR fine specificity, and TCR affinity for OVA antigen between animals and treatment arms. 48 hours after adoptive transfer, mice were inoculated with one of three recombinant pathogens engineered to express the OVA epitope: 104 CFU of Listeria monocytogenes (LM)-OVA (33), 105 PFU of murine gammaherpesvirus 68 (gHV)-OVA (34), or 105 PFU of mouse polyoma virus (PyV)-OVA (35). LM and gHV were given intraperitoneally (i.p.) and PyV via footpad injection.
OT-I CD8+ T cell depletion
Thirty days after OT-I adoptive transfer and pathogen stimulation, anti-Thy1.1 mAb (19E12, Bio X Cell) was administered i.p. for Thy1.1+ T cell depletion (36). Mice were injected 7 (500 μg), 4 (250 μg), and 1 (250 μg) day before skin transplantation, followed by skin grafting on day 0. Depletion was maintained with weekly 250 μg injections. Anti-Thy1.1 antibody-mediated depletion of OT-I CD8+Thy1.1+ T cells was robust and consistent between anatomic compartments (Figure 1S).
Figure 1. The magnitude and distribution of the antigen-specific T cell response is equivalent after pathogen stimulation with LM, gHV, or PyV.
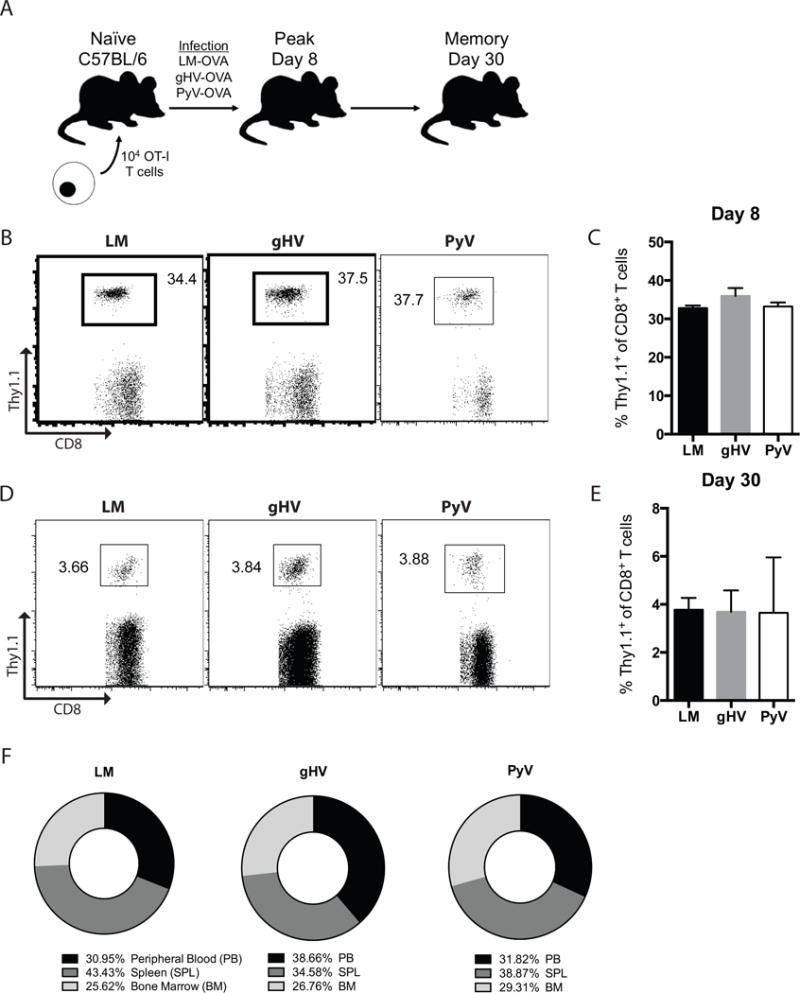
(A) Schematic of the mouse system used. 104 OT-I CD8+ T cells were adoptively transferred into naïve C57BL/6 mice that were infected 48 hours later with OVA-expressing LM, gHV, or PyV. Peripheral blood lymphocytes were then analyzed for antigen-specific CD8+Thy1.1+ T cells to measure the peak effector and memory responses 8 and 30 days after infection, respectively. (B) Frequencies of antigen-specific CD8+Thy1.1+ effector T cells 8 days after infection. Flow cytometry plots are representative and gated on CD8+ lymphocytes. (C) Summary data of the frequency of antigen-specific CD8+Thy1.1+ T cells following pathogen stimulation (n = 10 per group). (D) Frequencies of antigen-specific CD8+Thy1.1+ memory T cells 30 days after infection. Flow cytometry plots are representative and gated on CD8+ lymphocytes. (E) Summary data of the frequency of antigen-specific CD8+Thy1.1+ T cells following pathogen stimulation (n = 10 per group). (F) Mean percent distribution of total pathogen-primed OT-I memory cells in each designated anatomic compartment 30 days after infection (n = 3 per group, p > 0.999 between all groups). Summary data represent mean (SE).
Skin transplantation and immunosuppression
30 days after pathogen stimulation, full thickness tail skin grafts were transplanted onto the dorsal thorax of inoculated recipient mice (37). Skin graft recipients then received either no treatment, CoB consisting of human CTLA4-Ig (500 μg, Bristol-Myers Squibb) and hamster anti-mouse CD154 mAb (500 μg, MR-1, Bio X Cell), rat anti-LFA-1 mAb alone (250 μg, M17/4, Bio X Cell), rat anti-VLA-4 mAb alone (250 μg, PS/2, Bio X Cell), or CoB plus anti-LFA-1 or anti-VLA-4. All mAbs were administered i.p. on post-transplant days 0, 2, 4 and 6. Anti-LFA-1 and anti-VLA-4 mAbs were non-depleting (25).
Flow cytometry and intracellular cytokine staining (ICCS)
For phenotypic analysis, peripheral blood lymphocytes were stained for CD8, Thy1.1, CD44, CD62L, CD27, CD43, OX40, CD137, ICOS, 2B4, PD-1, KLRG1, LFA-1 and VLA-4. For ICCS, lymphocytes from graft-draining axillary lymph nodes were stimulated with 10 nM OVA peptide 257–264 (SIINFEKL) (Emory University Core Facility) in the presence of 10 μg/ml of Brefeldin A for 4 hours. An ICCS kit was used according to the manufacturer’s instructions and stained for IFN-γ, TNF, and IL-2. All reagents were purchased from BD Biosciences. All samples were run on an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo Software (Tree Star).
Statistical Analysis
Survival data were plotted on Kaplan-Meier curves and the log-rank (Mantel-Cox) test was used for survival analyses. Wilcoxon matched-pairs signed rank test was used for fraction of total distribution analyses. The Mann–Whitney U nonparametric test was performed for all other analyses. Statistical significance was attributed to p values < 0.05.
Results
Antigen-specific CD8+ T cell responses are similar in magnitude and tissue distribution following pathogen stimulation
We first set out to quantify the antigen-specific memory T cell response following pathogen stimulation with LM, gHV or PyV. While LM is an acutely cleared intracellular bacterium known to elicit potent memory responses (38), gHV (murine homologue of human Epstein-Barr virus) and PyV (mouse homologue of human BK and JC viruses) cause latent and persistent infections, respectively, and are controlled by effector and memory T cell subsets (39, 40). Since these pathogens primarily stimulate MHC class I restricted T cell responses (33–35) and CoB resistance is primarily mediated by CD8+ T cells (17, 25, 37, 41), we studied this response by adoptively transferring congenically labeled Thy1.1+ naïve CD8+ OT-I T cells into naïve C57BL/6 mice (Figure 1A). Mice were infected with OVA-expressing LM, gHV, or PyV, and peripheral blood lymphocytes were analyzed for the magnitude of the CD8+Thy1.1+ T cell response at days 8 and 30 post-infection. Both the 30–40% peak effector response observed on day 8 (Figures 1B, C) and the 3–4% contracted memory response on day 30 (Figures 1D, E) were quantitatively equivalent amongst the three groups. The tissue distribution of OT-I cells amongst the peripheral blood, spleen and bone marrow 30 days after pathogen infection was not significantly different between LM-, gHV- or PyV-infected mice (Figure 1F).
Pathogen stimulation history influences donor-specific CD8+ T cell susceptibility to a costimulation and LFA-1 blockade-based regimen
To test whether pathogen stimulation history impacts the susceptibility of CoB-resistant donor-reactive memory T cells to combined CD28/CD154/LFA-1 blockade, we re-evaluated the efficacy of our previously effective regimen against LM-induced CD8+ memory T cell-mediated CoB resistant rejection (25). Using the OT-I transgenic mouse system, naïve B6 mice were adoptively transferred OT-I T cells, infected with OVA-expressing LM, gHV or PyV, and then grafted with skin from mOVA mice 30 days after infection (Figure 2A). All three infected groups of mice received either CoB alone, anti-LFA-1 alone, or CoB plus anti-LFA-1 therapy. Skin graft survival was most prolonged in LM-infected mice treated with combined costimulation and LFA-1 blockade (p < 0.001, Figure 2B). CoB and anti-LFA-1 therapy more modestly prolonged allograft survival in PyV-infected mice (p = 0.003, Figure 2D), and failed to prolong skin graft survival in mice exposed to gHV (p = 0.125, Figure 2C). Donor-specific memory T cell mediated rejection was equally resistant to CoB alone and anti-LFA-1 therapy alone independent of infection history (Figures 2B–D). Group median survival times (MSTs) are outlined in Table 1.
Figure 2. Pathogen stimulation history influences skin allograft survival and donor-specific CD8+ T cell susceptibility to CoB/anti-LFA-1 therapy.
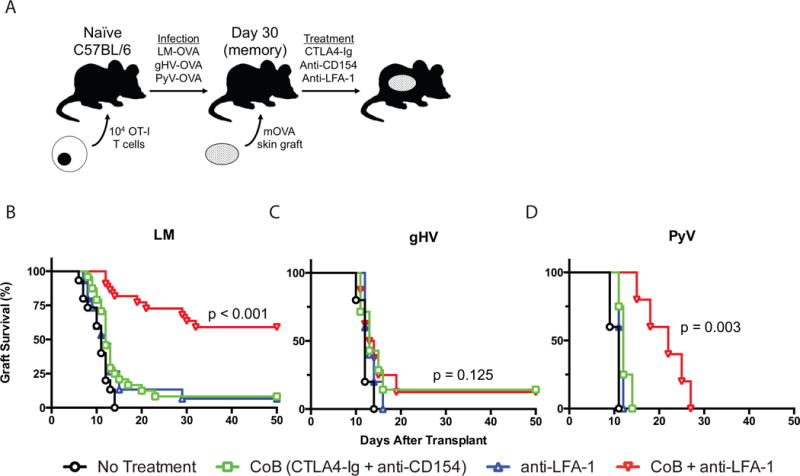
(A) Schematic of the mOVA transplant mouse system used. 104 OT-I CD8+ T cells were adoptively transferred into naïve C57BL/6 mice that were infected 48 hours later with OVA-expressing LM, gHV, or PyV. 30 days after infection, mice were transplanted mOVA skin grafts and treated with CoB (CTLA4-Ig + anti-CD154), anti-LFA-1, or CoB + anti-LFA-1. (B-D) Kaplan-Meier curves of mOVA skin allograft survival following infection with LM (B), gHV (C), or PyV (D). CoB + anti-LFA-1 therapy significantly prolonged skin allograft survival in LM-infected mice (MST 101 days, p < 0.001)(B) and PyV-infected mice (MST 22 days, p = 0.003)(D), but not in gHV-infected mice (MST 13.5 days, p = 0.125)(C). N = 4–24 animals per group.
Table 1.
Treatment groups and skin allograft survival
| Treatment | Mice per Group (n) |
Median allograft Survival (days) |
||
|---|---|---|---|---|
|
| ||||
| LM | gHV | PyV | ||
|
|
||||
| No treatment | 5–15 | 11 | 12 | 11 |
| CoB (CTLA4-Ig + anti-CD154) | 4–24 | 12 | 13 | 12 |
| Anti-LFA-1 | 5–15 | 12 | 13 | 12 |
| Anti-VLA-4 | 5 | 14 | 14 | 11 |
| CoB + anti-LFA-1 | 5–22 | 101** | 13.5 | 22* |
| CoB + anti-VLA-4 | 5–21 | 100** | 20.5** | 14 |
p < 0.01,
p < 0.001
Combined costimulation and integrin blockade against VLA-4 elicits an altered donor-specific memory T cell susceptibility profile
In order to determine whether the impact of T cell stimulation history on allograft survival in response to CoB and anti-LFA-1 therapy was particular to LFA-1, we similarly targeted the integrin VLA-4. Mice with adoptively transferred OT-I T cells were infected with OVA-expressing LM, gHV or PyV and then transplanted mOVA skin grafts (Figure 3A). Immunosuppression consisted of either CoB alone, anti-VLA-4 alone, or combination CoB plus anti-VLA-4 therapy previously effective against LM-elicited CoB-resistant CD8+ donor-specific T cells (25). CoB plus anti-VLA-4 exhibited an altered susceptibility profile compared to the anti-LFA-1-based regimen (Table 1, Figure 2). Similar to the results observed with CoB/anti-LFA-1, this combination regimen most effectively prolonged skin allograft survival in recipients that had been previously infected with LM (p < 0.001, Figure 3B); however in contrast to CoB/anti-LFA-1, CoB/anti-VLA-4 modestly prolonged graft survival in recipients that had been previously infected with gHV (p < 0.001, Figure 3C) but failed to prolong graft survival in recipients that had prior PyV infection (p = 0.232, Figure 3D). Group MSTs are outlined in Table 1.
Figure 3. Donor-specific CD8+ T cells exhibit an altered susceptibility profile to CoB/VLA-4 therapy after pathogen stimulation.
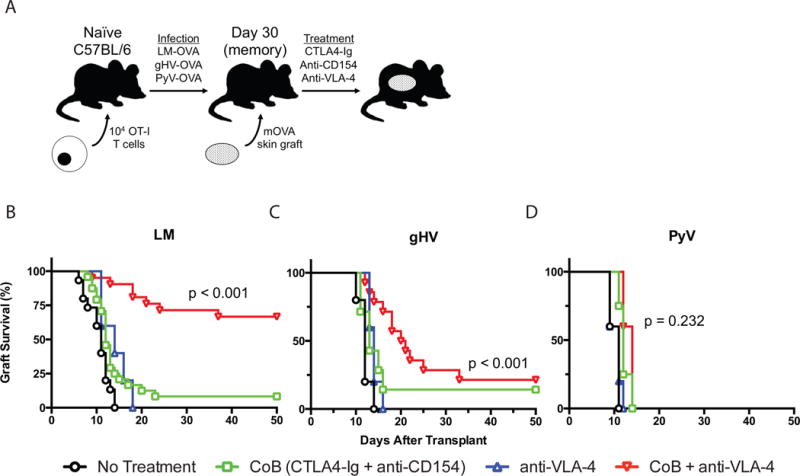
(A) Schematic of the mOVA transplant mouse system used. 104 OT-I CD8+ T cells were adoptively transferred into naïve C57BL/6 mice that were infected 48 hours later with OVA-expressing LM, gHV, or PyV. 30 days after infection, mice were transplanted mOVA skin grafts and treated with CoB (CTLA4-Ig + anti-CD154), anti-VLA-4, or CoB + anti-VLA-4. (B-D) Kaplan-Meier curves of mOVA skin allograft survival following infection with LM (B), gHV (C), or PyV (D). CoB + anti-VLA-4 therapy significantly prolonged skin allograft survival in LM-infected mice (MST 100 days, p < 0.001)(B) and gHV-infected mice (MST 20.5 days, p < 0.001)(C), but not in PyV-infected mice (MST 14 days, p = 0.232)(D). N = 4–24 animals per group.
Pathogen-primed OVA-specific CD8+ OT-I memory T cells are required for differential resistance to immunosuppression
To confirm whether OT-I memory T cells mediate differential immunosuppression resistance in this mOVA transplant model, we adoptively transferred OT-I T cells into naïve B6 mice and infected them with OVA-expressing LM, gHV or PyV. Thirty days post-infection, mice within each group served as either untreated controls or received a depleting anti-Thy1.1 mAb to eliminate all OVA-specific CD8+Thy1.1+ memory T cells (Figure 4A). The anti-Thy1.1 mAb effectively depleted OT-I CD8+ memory generated via infection with any of the three pathogens (Figures 4B, C). Following depletion, control and anti-Thy1.1-treated mice were transplanted mOVA skin grafts and treated with CoB/anti-LFA-1 therapy. The mice depleted of OVA-specific CD8+ memory T cells following LM, gHV or PyV infection uniformly accepted the mOVA skin grafts (Figure 4D). As above, non-depleted control LM-infected mice also accepted their skin grafts under CoB/anti-LFA-1 therapy, but in stark contrast, both non-depleted control gHV- and PyV-infected mice rejected their mOVA skin grafts despite CoB/anti-LFA-1 therapy (Figure 4D).
Figure 4. Pathogen-primed CD8+ OT-I T cells are required for differential CoB/anti-integrin resistance.
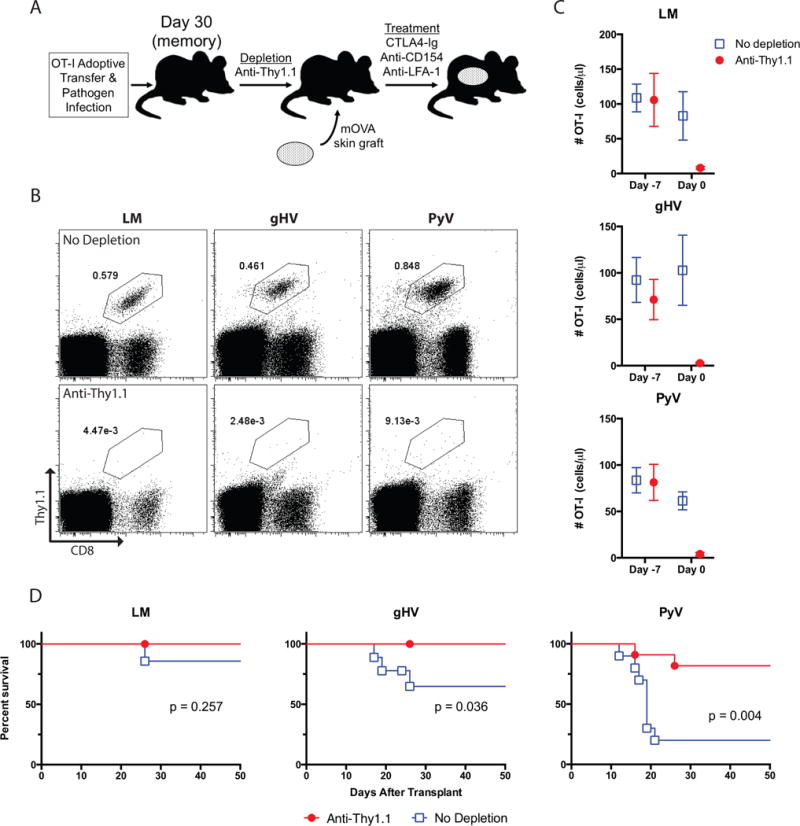
(A) Schematic of depletion regimen. OT-I T cells were adoptively transferred into naïve B6 mice and infected with OVA-expressing LM, gHV or PyV. Thirty days post-infection, mice within each group either received depleting anti-Thy1.1 mAb or were left untreated as controls. Mice were then transplanted mOVA skin grafts seven days after initiation of depletion and treated with CoB/anti-LFA-1 therapy. (B) Frequency of antigen-specific OT-I memory T cells on day 0 pre-transplant in control and anti-Thy1.1 depleted mice. Flow cytometry plots are representative and gated on lymphocytes. (C) Summary data of the number of antigen-specific OT-I T cells before (day -7) and after (day 0) administration of anti-Thy1.1 mAb in the peripheral blood (n = 8–12 per group). (D) Kaplan-Meier curves of mOVA skin allograft survival following infection with LM, gHV or PyV. CoB + anti-LFA-1 therapy significantly prolonged skin allograft survival in anti-Thy1.1-depleted gHV- and PyV-infected mice compared to non-depleted controls (p = 0.036 and p = 0.004, respectively). N = 8–12 animals per group.
LFA-1 and VLA-4 surface expression on donor-specific memory T cells does not account for differences in susceptibility profiles
One possible explanation for the variable susceptibility patterns between LM-, gHV-, and PyV-primed animals could be differential expression of LFA-1 and/or VLA-4. To test this we measured the surface expression of LFA-1 and VLA-4 on CD8+Thy1.1+ memory T cells. LFA-1 and VLA-4 were both increased on memory relative to naïve T cells (Figures 5A, B); but there were no differences in surface expression of LFA-1 or VLA-4 amongst all three pathogen-stimulated groups (Figures 5C, D).
Figure 5. Donor-specific CD8+ T cell surface expression of LFA-1 and VLA-4 does not explain altered susceptibility profiles to CoB/integrin therapy.
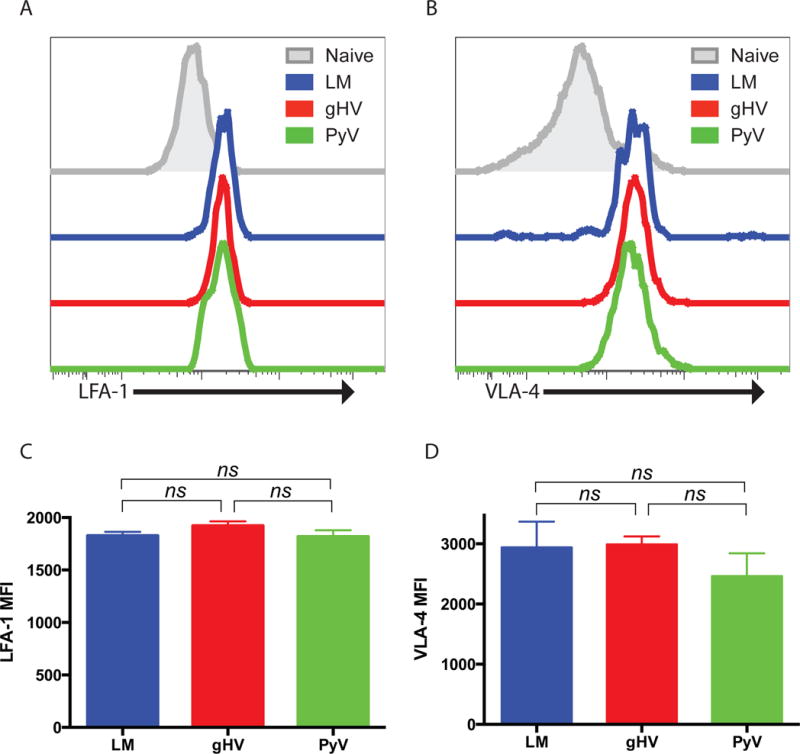
Naïve C57BL/6 mice were adoptively transferred 104 OT-I T cells, infected with OVA-expressing LM, gHV or PyV, and peripheral blood lymphocytes analyzed for surface expression of LFA-1 and VLA-4 30 days after infection. (A & B) Representative surface expression of LFA-1 (A) and VLA-4 (B) on CD8+Thy1.1+ memory T cells compared to naïve T cells. (C & D) Summary data of LFA-1 (C) and VLA-4 (D) MFI on CD8+Thy1.1+ T cells (n = 8–10 per group). Summary data represent mean (SE).
Donor-specific memory T cell susceptibility to dual costimulation/integrin blockade correlates with a TCM phenotype, greater recall potential, and altered PD-1/KLRG1 expression
To determine the mechanism underlying the variable immunosuppression-related susceptibility profiles amongst CD8+ memory T cells following pathogen stimulation, we next sought to phenotypically characterize donor-reactive CD8+ OT-I T cells generated by each pathogen. The most treatment-susceptible LM-stimulated memory T cells expressed a predominant TCM (CD44hiCD62Lhi) phenotype, while the more resistant gHV- and PyV-stimulated memory T cells displayed majority TEM (CD44hiCD62Llo) phenotypes (Figure 6A). A reciprocal relationship between the TCM and TEM compartments was observed amongst all three groups (Figures 6B, C). LM-stimulated OT-I T cells were composed of more TCM and less TEM than gHV- and PyV-stimulated cells (p = 0.007 and p < 0.001, respectively). Significant differences were also observed between gHV- and PyV-induced TCM and TEM compartments (p = 0.006 and p = 0.024, respectively).
Figure 6. Donor-specific CD8+ memory T cells most susceptible to CoB/integrin blockade exhibit a TCM phenotype and are CD43loCD27hi.
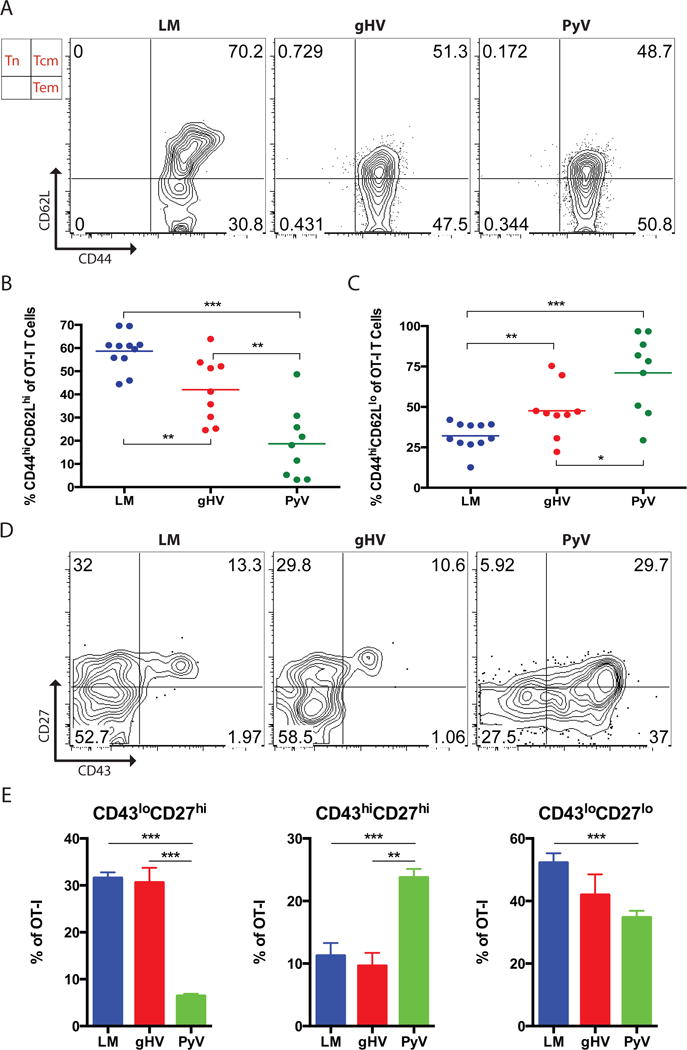
Naive C57BL/6 mice were adoptively transferred 104 OT-I T cells and then infected with OVA-expressing LM, gHV or PyV. Peripheral blood lymphocytes were then characterized 30 days after infection. (A) Analysis of CD44 and CD62L expression on antigen-specific memory T cells. Flow cytometry plots are representative and gated on CD8+Thy1.1+ T cells. (B & C) Summary data of the frequency of CD8+ antigen-specific CD44hiCD62Lhi TCM (B) and CD44hiCD62Llo TEM (C) T cells (n = 8–10 per group). (D) Analysis of CD43 and CD27 expression on antigen-specific memory T cells. Flow cytometry plots are representative and gated on CD8+Thy1.1+ T cells. (E) Summary data of the frequency of CD43loCD27hi, CD43hiCD27hi, and CD43loCD27lo T cells (n = 8–10 per group). Summary data represent mean (SE). * p < 0.05, ** p < 0.01, *** p < 0.001.
We then evaluated CD8+Thy1.1+ memory T cells for expression of CD27 and CD43, activation markers that define subpopulations of memory T cells that differ in their capacities to mount recall responses (29). LM-and gHV-induced memory T cells contained the greatest frequency of CD43loCD27hi cells (greatest recall potential) compared to PyV-stimulated T cells (Figures 6D, E). Conversely, PyV-stimulated memory consisted of the most CD43hiCD27hi cells (lower recall potential) when compared to T cell memory derived from LM and gHV infection. Differences between LM- and gHV-induced memory by CD27 and CD43 were not observed.
We did not observe any differences in OX40, ICOS or 2B4 expression on OT-I memory T cells between the three pathogen-infected groups (Figure 7A). We did observe differences in CD137, PD-1 and KLRG1. Analysis of PD-1 by KLRG1 on pathogen-primed memory T cells indicated that PD-1loKLRG1lo/hi cells comprise the majority of LM-induced donor-specific memory, while PD-1hiKLRG1lo/hi cells comprise the majority of PyV-primed memory (Figures 7B, C). gHV-infected memory manifested intermediate compositions of these phenotypes. More specifically, LM-induced memory was almost entirely composed of PD-1lo cells with the greatest percentage of the least differentiated subset of PD-1loKLRG1lo T cells amongst the pathogen-infected groups (p < 0.001, Figure 7C). In stark contrast, PyV-infected memory consisted almost entirely of PD-1hi cells, where PD-1hiKLRG1lo cells were the most dominant subset within PyV-primed memory and between all of the pathogen-infected groups (p < 0.001).
Figure 7. Donor-specific CD8+ memory T cells exhibit a less differentiated PD-1/KLRG1 phenotype.
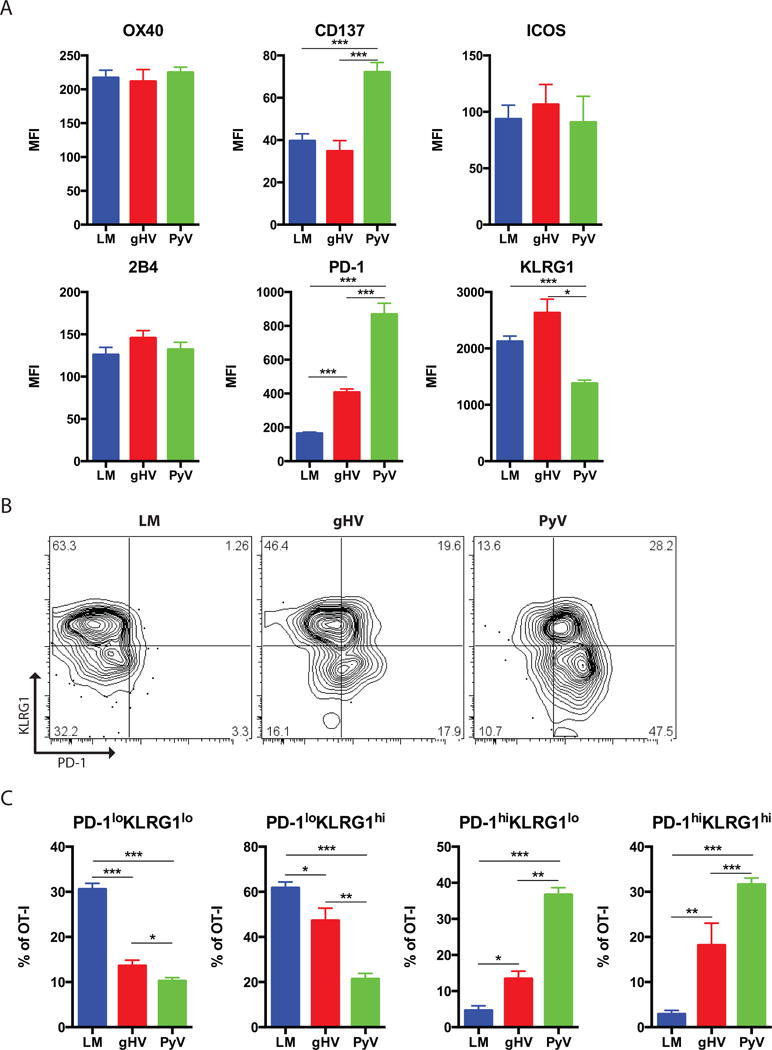
Naive C57BL/6 mice were adoptively transferred 104 OT-I T cells and then infected with OVA-expressing LM, gHV or PyV. Peripheral blood lymphocytes were then characterized 30 days after infection. (A) Summary data of the MFI on antigen-specific CD8+Thy1.1+ memory T cells for the designated surface markers (n = 8–10 per group). (B) Analysis of PD-1 and KLRG1 expression on antigen-specific memory T cells. Flow cytometry plots are representative and gated on CD8+Thy1.1+ T cells. (C) Summary data of the frequency of PD1loKLRG1lo, PD1loKLRG1hi, PD1hiKLRG1lo, and PD1hiKLRG1hi T cells (n = 8–10 per group). Summary data represent mean (SE). * p < 0.05, ** p < 0.01, *** p < 0.001.
Pathogen stimulation history influences the functional characteristics of donor-specific CD8+ memory T cells following skin transplantation
To evaluate for functional differences between the pathogen-induced donor-reactive memory T cells, analysis of their cytokine producing ability was performed. Mice were adoptively transferred OT-I T cells, infected with LM, gHV or PyV and then transplanted with mOVA skin grafts. Lymphocytes from graft-draining axillary lymph nodes harvested from recipient mice 7 days after transplant were then stimulated with cognate antigen and stained for intracellular cytokines. Flow cytometric analysis revealed that LM generated more donor-specific IFN-γ+TNF+ multi-cytokine producers than gHV (p = 0.008) and PyV (p = 0.036) (Figures 8A, B). The percentage of IFN-γ+IL-2+ multi-cytokine producers amongst CD8+Thy1.1+ T cells following antigen stimulation was also greatest amongst LM-infected mice (p = 0.008 and p = 0.036, respectively, Figures 8C, D). PyV infection led to fewer IFN-γ+TNF+ and IFN-γ+IL-2+ producers than gHV (p = 0.036).
Figure 8. Pathogen stimulation history impacts cytokine production amongst donor-specific CD8+ T cells.
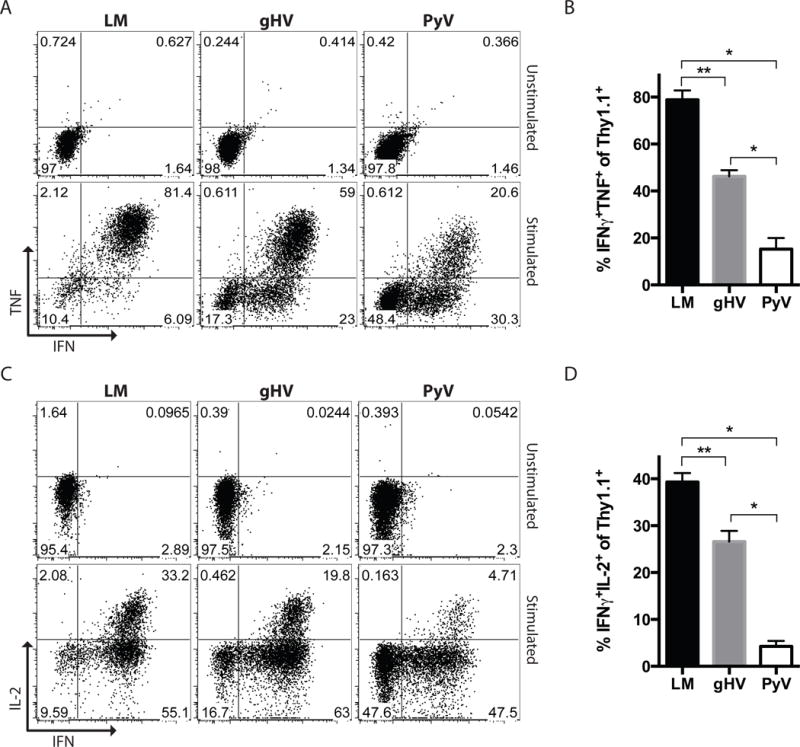
Naïve C57BL/6 mice were adoptively transferred 104 OT-I T cells, infected with LM, gHV or PyV, and transplanted skin from mOVA mice. 7 days after transplant, lymphocytes from graft-draining axillary lymph nodes were isolated and restimulated ex-vivo with SIINFEKL peptide. (A) IFN-γ and TNF production by donor-reactive CD8+Thy1.1+ T cells after antigen stimulation. Flow cytometry plots are representative and gated on CD8+Thy1.1+ lymphocytes. (B) Summary data of the frequencies of IFN-γ+TNF+ donor-reactive CD8+Thy1.1+ T cells (n = 3–5 per group). (C) IFN-γ and IL-2 production by donor-reactive CD8+Thy1.1+ T cells after antigen stimulation. Flow cytometry plots are representative and gated on CD8+Thy1.1+ lymphocytes. (B) Summary data of the frequencies of IFN-γ+IL-2+ donor-reactive CD8+Thy1.1+ T cells (n = 3–5 per group). Summary data represent mean (SE). * p < 0.05, ** p < 0.01.
Discussion
Immunologic memory has been recognized as a barrier to improved allograft outcomes and tolerance induction in transplantation – particularly with more selective agents like belatacept (3, 4, 6, 11). While it has been established that the quantity of donor-reactive memory can significantly impact tolerance induction (17, 22), the data presented here suggest that the actual quality of T cell memory may have an equally critical impact on the outcome of CoB-based immunosuppressive strategies. Determinants of memory T cell quality include factors related to the nature of the antigen, antigen-specific precursor frequency, and the surrounding inflammatory environment. Blair et al. have shown that the availability and competition for antigen, along with the duration of antigen presentation, can regulate memory T cell differentiation, with more prolonged antigen exposure favoring a TEM phenotype (42, 43). Moreover, initial antigen-specific precursor frequency can also impact the programming of memory T cells as well as their functional requirement for costimulatory signals (44). Still other studies have shown that T cell expansion and memory formation are critically dependent upon the cytokine milieu (45, 46), for example increased IL-12 exposure favors the development of KLRG1hi TEM (47). Based on these studies, we speculate that the differential generation of costimulation/integrin resistant memory T cells may be related to the ability of OVA-expressing LM, gHV and PyV to elicit a range of inflammatory cytokine levels with variable periods of antigen availability, as LM is an acutely cleared bacterial pathogen, and gHV and PyV cause latent and persistent viral infections, respectively (38–40). Overall, the quality of memory we observed to be generated by these pathogens is consistent with known effects of their individual attributes on memory T cell differentiation.
Our results suggest that susceptibility to dual blockade and improved skin allograft survival in the LM-infected mice could be related to the induction of a predominant TCM phenotype amongst allograft-specific memory T cells. TCM and TEM differ in their homing receptors and immediate effector function (27). TCM express more CD62L, produce more IL-2, and have greater proliferative capacity than their effector counterparts (48). TEM are generally considered to have a more limited lifespan with weaker proliferative potential but rapid effector function (49). In our study, the most immunosuppression-sensitive LM-induced memory T cell compartment was primarily composed of CD44hiCD62Lhi TCM and functionally exhibited the greatest TNF and IL-2 multi-cytokine production (Figures 6, 8). Consistent with this TCM-like phenotype, this treatment-susceptible LM-induced memory T cell population was predominantly composed of CD43loCD27hi cells (Figure 6), a phenotype that has previously been associated with greater recall and proliferative potential (26, 29, 50).
Analysis of PD-1 and KLRG1 on pathogen-primed memory cells revealed differential pathogen-specific phenotypic signatures. While little published data exists describing the relationship between concomitant PD-1 and KLRG1 expression on CD8+ memory T cells, their independent expression has been relatively well studied. PD-1 is considered a negative inhibitor of the immune response that is upregulated on activated T cells and is a marker of T cell exhaustion (51). PD-1 blockade restores CD8+ T cell proliferation, cytokine secretion, and cytolytic activity (52). The co-inhibitory receptor KLRG1 is expressed on antigen-experienced T cells and has been postulated to be a marker of senescent cells that lack proliferative capacity. KLRG1 expression increases with age and differentiation; with the highest percentage of expression on memory cells and highly differentiated end stage cells (53, 54). PD-1 and KLRG1 expression levels generally parallel each other and are proportionally related to the degree of T cell differentiation (55), but the temporal relationship between these markers of exhaustion and senescence and how they relate to susceptibility to immunosuppression following transplantation are unknown. In the context of the differential allograft survival and memory T cell susceptibility to immunosuppression observed in this study, we posit that PD-1hiKLRG1lo memory cells possibly represent antigen-experienced cells that are intermediately differentiated – cells that have recently encountered antigen (PD-1hi) but are not yet senescent (KLRG1hi). This PD-1hiKLRG1lo phenotype may be associated with memory T cells that are differentiated enough to exhibit resistance to costimulation/integrin blockade, but not too differentiated to effectively mediate allograft rejection; while pathogen-primed PD-1loKLRG1lo memory T cells may be more susceptible to blockade by virtue of their less differentiated state.
Taken together, we find that the most immunosuppression-sensitive memory was more polyfunctional and TCM-like, with greater recall potential and a less differentiated PD-1loKLRG1lo phenotype. Conversely, the more immunosuppression-resistant memory T cells were less capable of cytokine production and majority TEM, with lower recall potential and a more differentiated PD-1hiKLRG1lo phenotype. We postulate that the degree of memory T cell maturity and activation along the differentiation pathway inversely confers the amount of reliance on costimulation “checkpoints” needed to mediate an immune response; and that this pathogen-dependent quality of T cell memory influences its susceptibility or resistance to the specific immunosuppression used.
An interesting facet of the data presented here is that the pathogen-dependent susceptibility profiles were different when anti-LFA-1 vs. anti-VLA-4 was employed (Table 1, Figures 2, 3). While the mechanism underlying this differential susceptibility to anti-LFA-1 vs. anti-VLA-4 therapy remains unknown, we showed that it is not related to differences in expression levels of LFA-1 or VLA-4 on T cells primed with the three different pathogens (Figure 5), or to alterations in the physical distribution of memory OT-I T cells preceding LFA-1 vs. VLA-4 antagonism (Figure 1). However, we previously demonstrated that despite the fact that LFA-1 and VLA-4 both belong to the integrin family of adhesion molecules, targeting them in transplantation results in divergent effects on memory T cells. Specifically, while anti-VLA-4 in the setting of CoB dramatically suppressed trafficking of memory T cells to the allograft, LFA-1 antagonism both impaired trafficking and additionally attenuated donor-reactive T cell effector function during the memory recall response (25).
In this study we show that OVA-specific OT-I memory T cells are necessary for the observed differential resistance between mice infected with the three types of pathogens (Figure 4). It is probable that pathogen-specific factors impact both the quality of donor-specific memory and other immune parameters within the host that might be influencing graft outcome. It is also possible that pathogen-dependent differences in memory T cell quality engendered upon initial priming of OT-I T cells could lead to differential trafficking and distribution of memory subsets in our model. While we did not observe any differences in the distribution of total antigen-specific memory T cells between groups within secondary lymphoid organs (Figure 1), differences in the composition of memory subsets (e.g. TCM vs. TEM) might suggest that the relative representation of circulating vs. resident memory T cells could differ between groups, and could impact memory T cell susceptibility to the immunosuppressive regimens used in this study. However, these possibilities would still support the concept that different pathogen infections can result in the generation of memory T cells that are qualitatively different in terms of their resistance to a costimulation/integrin blockade-based regimen following transplantation.
In summary, this study highlights that the quality of pathogen-derived donor-reactive CD8+ T cell memory has potential to impact the clinical response to alternative immunosuppressive regimens following organ transplantation. Our data focus on the pre-transplant status of donor-specific memory and support the conclusion that phenotypic and functional differences amongst T cell memory influence allograft survival and the susceptibility profiles to costimulation and integrin blockade-based therapies, with the clinical implication that the overall composition and character of a transplant recipient’s pre-existing alloreactive T cell memory will ultimately influence the outcomes of novel immunosuppressive strategies, particularly belatacept-based therapies. We conceive that in the future, assessment of the quality, in addition to quantity, of a potential transplant recipient’s donor-reactive memory T cell compartment could inform clinicians on the optimal immune modulation strategy to employ.
Supplementary Material
Naïve C57BL/6 mice were adoptively transferred 104 OT-I CD8+Thy1.1+ T cells and infected with OVA-expressing pathogen. After OT-I memory formation, a depleting anti-Thy1.1+ mAb was administered on days 0, 3, 7 and 14. (A) Summary data of the depletion of OT-I memory T cells compared to a naïve B6 control mouse without Thy1.1+ OT-I cells (n = 5, * denotes mAb dosing on the X-axis). (B) Summary data of the number of OT-I memory T cells in the peripheral blood (PB), spleen (SPL), bone marrow (BM), and lymph nodes (LN) on day 49 compared to a naïve B6 control mouse without Thy1.1+ OT-I T cells (n = 5). Summary data represent the mean (SE).
Acknowledgments
The authors would like to thank Dr. Samuel Speck (Emory University) for the contribution of gHV-OVA and Dr. Michael Bevan (University of Washington) for the contribution of LM-OVA. R01 AI073707 (M.L.F.), R01 AI104699 (M.L.F.), R37 AI040519 (C.P.L.), and an ASTS Fellowship in Transplantation Grant (I.R.B.) supported this work.
Abbreviations
- CoB
costimulation blockade
- TCM
central memory T cells
- TEM
effector memory T cells
- OVA
ovalbumin
- LM
Listeria monocytogenes
- gHV
gammaherpesvirus 68
- PyV
polyoma virus
- i.p.
intraperitoneal
- ICCS
intracellular cytokine staining
- SIINFEKL
ovalbumin peptide 257–264
- MST
median survival time
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Costimulation blockade with belatacept in renal transplantation. The New England journal of medicine. 2005;353(8):770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 2.Vanrenterghem Y, Bresnahan B, Campistol J, Durrbach A, Grinyo J, Neumayer HH, et al. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies) Transplantation. 2011;91(9):976–983. doi: 10.1097/TP.0b013e31820c10eb. [DOI] [PubMed] [Google Scholar]
- 3.Brook MO, Wood KJ, Jones ND. The impact of memory T cells on rejection and the induction of tolerance. Transplantation. 2006;82(1):1–9. doi: 10.1097/01.tp.0000226082.17507.da. [DOI] [PubMed] [Google Scholar]
- 4.Valujskikh A, Li XC. Frontiers in nephrology: T cell memory as a barrier to transplant tolerance. Journal of the American Society of Nephrology: JASN. 2007;18(8):2252–2261. doi: 10.1681/ASN.2007020151. [DOI] [PubMed] [Google Scholar]
- 5.Ford ML, Kirk AD, Larsen CP. Donor-reactive T-cell stimulation history and precursor frequency: barriers to tolerance induction. Transplantation. 2009;87(9 Suppl):S69–74. doi: 10.1097/TP.0b013e3181a2a701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford ML, Larsen CP. Transplantation tolerance: memories that haunt us. Sci Transl Med. 2011;3(86):86ps22. doi: 10.1126/scitranslmed.3002504. [DOI] [PubMed] [Google Scholar]
- 7.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257(5071):789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 8.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381(6581):434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 9.Kirk AD. Crossing the bridge: large animal models in translational transplantation research. Immunological reviews. 2003;196:176–196. doi: 10.1046/j.1600-065x.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 10.Kean LS, Gangappa S, Pearson TC, Larsen CP. Transplant tolerance in non-human primates: progress, current challenges and unmet needs. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(5 Pt 1):884–893. doi: 10.1111/j.1600-6143.2006.01260.x. [DOI] [PubMed] [Google Scholar]
- 11.Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunological reviews. 2009;229(1):294–306. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cossarizza A, Ortolani C, Paganelli R, Barbieri D, Monti D, Sansoni P, et al. CD45 isoforms expression on CD4+ and CD8+ T cells throughout life, from newborns to centenarians: implications for T cell memory. Mech Ageing Dev. 1996;86(3):173–195. doi: 10.1016/0047-6374(95)01691-0. [DOI] [PubMed] [Google Scholar]
- 13.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 14.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. Journal of immunology. 2002;168(1):29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 15.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. Journal of immunology. 2002;169(7):3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 16.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. Journal of immunology. 2003;170(8):4077–4086. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 17.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amir AL, D’Orsogna LJ, Roelen DL, van Loenen MM, Hagedoorn RS, de Boer R, et al. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115(15):3146–3157. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]
- 19.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2002;2(6):501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 20.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. Journal of immunology. 1999;163(4):2267–2275. [PubMed] [Google Scholar]
- 21.Poggio ED, Augustine JJ, Clemente M, Danzig JM, Volokh N, Zand MS, et al. Pretransplant cellular alloimmunity as assessed by a panel of reactive T cells assay correlates with acute renal graft rejection. Transplantation. 2007;83(7):847–852. doi: 10.1097/01.tp.0000258730.75137.39. [DOI] [PubMed] [Google Scholar]
- 22.Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011;3(86):86ra51. doi: 10.1126/scitranslmed.3002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nature medicine. 2009;15(7):746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest. 2010;120(12):4520–4531. doi: 10.1172/JCI43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitchens WH, Haridas D, Wagener ME, Song M, Kirk AD, Larsen CP, et al. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(1):69–80. doi: 10.1111/j.1600-6143.2011.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31(6):859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annual review of immunology. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 28.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. Journal of immunology. 2006;176(4):2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 29.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. The Journal of experimental medicine. 2007;204(7):1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. Journal of immunology. 2006;177(2):831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 31.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 32.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003;3(11):1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 33.Dudani R, Chapdelaine Y, Faassen Hv H, Smith DK, Shen H, Krishnan L, et al. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. Journal of immunology. 2002;168(11):5737–5745. doi: 10.4049/jimmunol.168.11.5737. [DOI] [PubMed] [Google Scholar]
- 34.Braaten DC, Sparks-Thissen RL, Kreher S, Speck SH, Virgin HWt. An optimized CD8+ T-cell response controls productive and latent gammaherpesvirus infection. Journal of virology. 2005;79(4):2573–2583. doi: 10.1128/JVI.79.4.2573-2583.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews NP, Pack CD, Lukacher AE. Generation of antiviral major histocompatibility complex class I-restricted T cells in the absence of CD8 coreceptors. Journal of virology. 2008;82(10):4697–4705. doi: 10.1128/JVI.02698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. The Journal of experimental medicine. 2007;204(9):2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104(12):1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerksiek KM, Pamer EG. T cell responses to bacterial infection. Current opinion in immunology. 1999;11(4):400–405. doi: 10.1016/S0952-7915(99)80067-3. [DOI] [PubMed] [Google Scholar]
- 39.Virgin HW, Speck SH. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Current opinion in immunology. 1999;11(4):371–379. doi: 10.1016/s0952-7915(99)80063-6. [DOI] [PubMed] [Google Scholar]
- 40.Swanson PA, 2nd, Lukacher AE, Szomolanyi-Tsuda E. Immunity to polyomavirus infection: the polyomavirus-mouse model. Seminars in cancer biology. 2009;19(4):244–251. doi: 10.1016/j.semcancer.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, et al. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. Journal of immunology. 2006;177(11):7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 42.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci U S A. 2007;104(38):15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blair DA, Turner DL, Bose TO, Pham QM, Bouchard KR, Williams KJ, et al. Duration of antigen availability influences the expansion and memory differentiation of T cells. Journal of immunology. 2011;187(5):2310–2321. doi: 10.4049/jimmunol.1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. The Journal of experimental medicine. 2007;204(2):299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. The Journal of experimental medicine. 2005;202(5):637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. Journal of immunology. 2006;177(3):1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 47.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 49.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 50.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nature medicine. 2002;8(4):379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 51.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 52.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100(10):3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 54.Thimme R, Appay V, Koschella M, Panther E, Roth E, Hislop AD, et al. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. Journal of virology. 2005;79(18):12112–12116. doi: 10.1128/JVI.79.18.12112-12116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nature reviews Immunology. 2011;11(4):289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Naïve C57BL/6 mice were adoptively transferred 104 OT-I CD8+Thy1.1+ T cells and infected with OVA-expressing pathogen. After OT-I memory formation, a depleting anti-Thy1.1+ mAb was administered on days 0, 3, 7 and 14. (A) Summary data of the depletion of OT-I memory T cells compared to a naïve B6 control mouse without Thy1.1+ OT-I cells (n = 5, * denotes mAb dosing on the X-axis). (B) Summary data of the number of OT-I memory T cells in the peripheral blood (PB), spleen (SPL), bone marrow (BM), and lymph nodes (LN) on day 49 compared to a naïve B6 control mouse without Thy1.1+ OT-I T cells (n = 5). Summary data represent the mean (SE).


