Abstract
Poor inhibitory control is a well-established cognitive correlate of adults with ADHD. However, the simple reaction time (RT) task used in a majority of studies records performance errors only via the presence or absence of a single key press. This all-or-nothing response makes it impossible to capture subtle differences in underlying processes that shape performance. Subsequently, all-or-nothing tasks may underestimate the prevalence of executive function deficits in ADHD. The current study measured inhibitory control using a standard Go/No-Go RT task and a more sensitive continuous grip force task among adults with (N = 51, 22 female) and without (N = 51, 29 female) ADHD. Compared to adults without ADHD, adults with ADHD made more failed inhibits in the classic Go/No-Go paradigm and produced greater and more variable force during motor inhibition. The amount of force produced on failed inhibits was a stronger predictor of ADHD-related symptoms than the number of commissions in the standard RT task. Adults with ADHD did not differ from those without ADHD on the mean force and variability of force produced in Go trials. These findings suggest that the use of a precise and continuous motor task, such as the force task used here, provides additional information about the nature of inhibitory motor control in adults with ADHD.
Keywords: grip force, motor control, attention-deficit/hyperactivity disorder (ADHD), response inhibition, go/no-go task
I. INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is a common childhood-onset disorder characterized by age-inappropriate, chronic, pervasive, and impairing levels of inattention and/or hyperactivity-impulsivity (American Psychiatric Association. and American Psychiatric Association. DSM-5 Task Force., 2013). ADHD persists into adulthood in up to 65% of cases (Faraone et al., 2006; Simon et al., 2009; Turgay et al., 2012), affects the ability to gain and maintain employment (Kessler et al., 2009; Kupper et al., 2012), and is associated with an increased risk for substance abuse (Wilens et al., 1995; Upadhyaya, 2008; Groenman et al., 2013), obesity (Cortese et al., 2008; Nazar et al., 2012; Albayrak et al., 2013; Nazar et al., 2014), workplace injuries (Swensen et al., 2004; Breslin and Pole, 2009; Hodgkins et al., 2011), and traffic accidents (Barkley et al., 1993; Jerome et al., 2006a, b; Barkley and Cox, 2007; Merkel et al., 2013). Though less often discussed, motor impairments are prominent among children with ADHD (Barkley, 1998) and up to 50% of pediatric ADHD patients are also comorbid for developmental coordination disorder (Kadesjo and Gillberg, 1999; Pitcher et al., 2003; Gillberg et al., 2004). Similarly, adults with ADHD have impaired visuomotor memory in gripping tasks (Neely et al., 2016), visuomotor adaptation in reaching tasks (Kurdziel et al., 2015), deficits in oculomotor control (Feifel et al., 2004; Carr et al., 2006), increased postural sway (Hove et al., 2015), and impaired timing in finger tapping tasks (Valera et al., 2010). These findings are important because motor processes have clearer neural correlates than many of the cognitive constructs associated with ADHD. Thus, the motor system provides a good avenue to examine the neurobiology of ADHD.
Inhibitory control is the process of suppressing competing responses to select the most appropriate response. The ability to suppress inappropriate behaviors in favor of appropriate alternatives is paramount to adapting behavior in changing circumstances and is thereby a critical component for controlling behavior at all levels, including movement. Although numerous studies report poor inhibitory control in ADHD (Nigg et al., 2002; Aron and Poldrack, 2005; Alderson et al., 2007; Wodka et al., 2007; Suskauer et al., 2008; Gilbert et al., 2011; Bari and Robbins, 2013), the type of task used in the majority of studies (e.g. go-no-go or stop signal reaction time, RT, task) records performance via the presence or absence of an all-or-nothing key press. Such an approach confounds cognitive, sensory, and motor processes into a single dichotomous response. As a result, we may be overlooking critical processes that provide insight into the neural mechanisms of the disorder. For example, a great deal of motor activity can be produced even when an individual does not ultimately press a key in a standard RT task. The current study overcomes this barrier by using a continuous and precise measure of motor output in a grip force variant of the classic go/no-go task. We used force output as a measure of activity in the motor system. In order to test the validity of this measure, participants completed a continuous grip force go/no-go task with both low and high force amplitude conditions as well as a standard go/no-go task that used an all-or-nothing keypress. Trials were presented rapidly, creating a prepotency to respond. In this context, the inhibition of a prepotent response requires effortful cognitive control, whereas allowing the motor response to proceed occurs in a more automatic fashion (Bargh et al., 1996; Muraven and Baumeister, 2000). We reasoned that greater activity in the motor system would be reflected by the production of larger forces during no-go trials. We included low and high force amplitude conditions as a means to examine response planning. In particular, the amount of force produced on a no-go trial may reflect a pre-planned response and scale to the target amplitude. Therefore, deficits in response planning would be indicated by force output (on no-go trials) that does not scale to the target amplitude.
2. METHODS
2.1. Participants
We recruited young adults, ages 18 to 25, who identified as currently having ADHD or as having never been diagnosed with ADHD. Participants were community recruited through advertisements in State College, Pennsylvania. Exclusion criteria included: (1) previous concussions that resulted in a loss of consciousness for more than 10 minutes; (2) previous diagnosis of seizures, epilepsy, encephalitis, meningitis or an autism spectrum disorder; (3) previous diagnosis of a musculoskeletal or neurological disorder; and (4) previous diagnosis of any disorder involving psychosis.
2.1.1. Adults with ADHD
Adults with ADHD met DSM-V criteria including cross situational severity and impairment as determined by a semi-structured interview, the Conners’ Adult ADHD Diagnostic Interview (CAADID; Multi-Health Systems Inc.). Adults had > 5 symptoms of inattention or hyperactivity, that were impairming in at least two settings (e.g. family and work). In total, 53 young adults met the criteria for ADHD. However, two participants did not complete the go/no-go task as instructed. These individuals were unable to keep pace with the speeded trial presentation in the motor task. The final ADHD group (N = 51, 29 females) had a mean age of 21.10 + 1.71 years. Adults taking a psychostimulant (N=22) completed the laboratory session after a 24-hour washout period. No participants were taking medications known to affect motor control at the time of testing, including antipsychotics, stimulants, or anticonvulsants (Reilly et al., 2008).
2.1.2. Adults without ADHD
Age- and sex- matched controls reported < 3 total symptoms and < 2 symptoms per ADHD dimension. Self-report of anxiety and/or depression was not exclusionary. A total of 73 young adults met the criteria for healthy control. The included participants (N=51, 22 females, 21.00 + 1.70 years) were chosen as age- and sex-matched controls for the ADHD group.
2.2. Procedures
The experimental task was completed as part of a larger battery of experimental and standardized measures that took place in one 3-hour session. After a complete description of the study, written informed consent was obtained from the participant. All procedures were approved by the Institutional Review Board at The Pennsylvania State University, and were consistent with the Declaration of Helsinki. All participants received monetary compensation for their participation in the study.
In advance of the laboratory session, all participants completed a brief medical history, the long form of the Connors Adult ADHD Rating Scales (CAARS), the Achenbach Adult Self Report (ASR) (Achenbach, 2003), and the Edinburgh Handedness Inventory (Newcombe et al., 1971). Symptoms of ADHD were assessed with the self-report, long version (S:L) of the CAARS-S:L, which has 66 items and 8 factor-derived subscales: Inattention/Memory Problems, Hyperactivity/Restlessness, Impulsivity/Emotional Lability, Problems with Self-Concept, DSM-IV Inattentive Symptoms, DSM-IV Hyperactive/Impulsive Symptoms, DSM-IV ADHD Symptoms Total, and an ADHD Index. Symptoms of conditions that commonly co-occur with ADHD (e.g. anxiety, depression, and conduct problems) were evaluated with the ASR, a 123-item rating scale scored with a 3-point Lickert scale. The ASR has excellent psychometric properties and uses age-based normative data to identify normal, borderline clinical, and clinical ranges of behavior (Achenbach, 2003). Handedness was assessed with the Edinburgh Handedness Inventory (Muth et al., 1971). The 10-item inventory asks participants to indicate which hand they would use to complete common tasks, such as striking a match, throwing, or using scissors. Handedness is determined using a laterality quotient (LQ = (R-L)/(R+L) * 100), where a score of 100 reflected complete right-hand dominance, and a score of −100 reflected complete left-hand dominance.
In the laboratory session, participants completed a semi-structured interview, the CAADID, which was updated to reflect the criteria of the DSM-5. Other tests conducted during the lab visit included portions of the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) (Wechsler, 2008) to estimate intelligence quotient (IQ), the Purdue Pegboard Test (Buddenberg and Davis, 2000) to assess coordination, and dynamometer tests for maximum pinch grip strength.
2.2.1. Go/No-Go force task
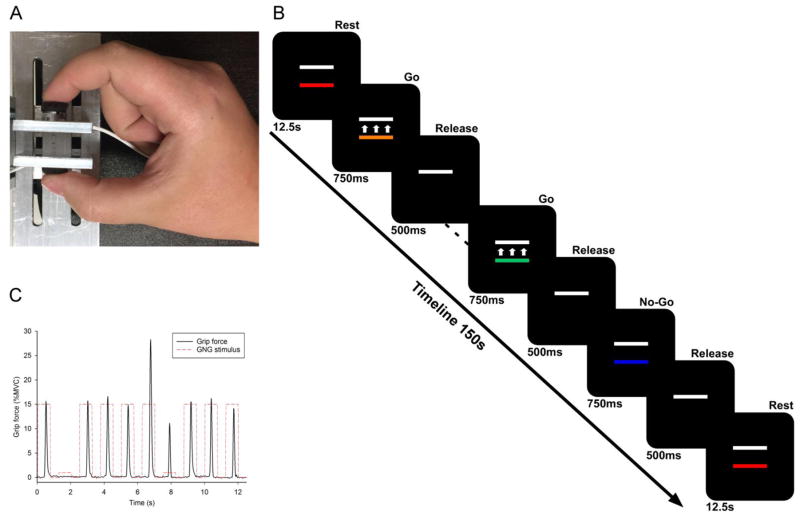
Stimuli were presented on a 102 cm Samsung television screen with 1920 x 1080 resolution and 120 Hz refresh rate. Participants were comfortably seated in a chair (JedMed Straight Back Chair, St. Louis, MO) facing the center of the TV screen a horizontal distance of 127 cm. Their dominant arm rested at approximately 100 degrees of flexion on a height-adjustable table. As shown in Figure 1A, participants used their thumb and index finger to form a pinch grip against a custom-built force apparatus with identical button load cells on either side (Measurement Specialties, Hampton, VA). Total force produced by the thumb and index finger was measured. Voltage outputs were sent to a transducer coupler to be amplified (Coulbourn Instruments Type B V72-25B), transmitted via a 16-bit A/D converter, and digitized at 62.5 Hz. Digitized voltage signals were transformed to Newtons with a resolution of 0.0016 N. The summed force output from the load cells was presented on the television screen in real time. Voltage data acquisition, voltage-to-force transformation, and stimuli presentation were all conducted using customized programs written in LabVIEW (National Instruments, Austin, TX).
Figure 1.
(A) Participants used their thumb and index finger to grip against a custom-built force apparatus with identical button load cells. (B) The visual display consisted of two rectangular bars on a black background. A stationary, white target bar represented the target force amplitude. For each run, the target was normalized to 15% (low amplitude) or 60% (high amplitude) of the participant’s maximum voluntary contraction. A second colored bar moved up with increasing applied force and down with decreasing force. (C) The experimental time-series force data for 10 trials from an exemplar participant.
Before the force task, each participant’s maximum voluntary contraction (MVC) was measured using a pinch grip dynamometer (Lafayette Hydraulic Pinch Gauge, Model J00111, Lafayette, IN). The average of three, five-second trials determined each participant’s maximum voluntary contraction (MVC) in Newtons. MVC was then used to create target force amplitudes for each participant. During the experiment, the visual display consisted of two rectangular bars on a black background (Figure 1C). A stationary, white target bar represented the target force. For each run, the target was normalized to 15% (low amplitude) or 60% (high amplitude) of the participant’s MVC. A second colored bar moved up with increasing applied force and down with decreasing force. The distance moved by this bar was proportional to the amount of force produced. When no force was produced, the moveable bar was stationary. The moveable bar changed colors from trial to trial and could be red, green, aqua, orange, yellow, or blue. Participants completed 100 consecutive trials to the same target amplitude and trials were presented rapidly, creating a prepotency to respond. In this context, the inhibition of a prepotent response requires effortful cognitive control, whereas allowing the motor response to proceed occurs in a more automatic fashion (Bargh et al., 1996; Muraven and Baumeister, 2000).
Each participant completed two runs of 100 trials (25% no-go) at low and high amplitude force conditions, for a total of four runs. Each run started and ended with 12.5s of rest, during which the movable bar was red, indicating to the participant that they should not press on the load cells. Every trial onset was cued by the color of the movable bar. Go trials were cued by the colors green, aqua, orange, or yellow. No-Go trials were cued by the color blue (Figure 1B). Go and No-Go trials were randomly presented. In Go trials, participants were instructed to press as quickly and accurately as possible to match the movable bar to the amplitude prescribed by the target bar, and to release when the movable bar disappeared. In No-Go trials, participants were to refrain from pressing the load cells. Trials were 750ms in duration, followed by a 500ms inter-trial period of rest, during which only the white target bar was visible. Each participant practiced the task immediately prior to the experimental session.
2.2.2. Standard Go/No-Go task
The Go/No-Go RT task was identical to the Go/No-Go force task, with the exception that there was no white target bar and the colored bar was stationary in the middle of the screen. Visual stimuli were presented on a 23-inch monitor (Dell, Round Rock, TX) using E-Prime (Psychology Software Tools, Inc., Sharpsburg, PA). Stimuli were colored bars just as in the force task. Participants were instructed to press the space-bar on a keyboard in Go trials as quickly as possible, and to refrain from pressing the space bar in No-Go trials, cued by the color blue. No feedback concerning accuracy was provided. Participants completed four runs of 100 trials (25% no-go) in the standard RT task.
2.3. Data analysis
2.3.1 Force data analysis
The force time series data was digitally filtered using a tenth-order low-pass Butterworth filter with a 15 Hz cut-off frequency. Figure 1C displays the time-series force data for 10 trials from an exemplar participant. A custom-written MATLAB program was used to place markers at four time points for each trial: onset of force, beginning and end of force interval, and offset of force. Each trial was then visually inspected to confirm correct placement of the time points. A second MATLAB program read in the four time points and associated force output in order to calculate the mean force amplitude for each trial, for each participant. Mean force amplitude was calculated as the average force output during the time between the second and third time-points for each trial. Standard deviation of force was calculated as the variability of mean force across Go and No-Go trials. We then obtained an average value for mean force output and standard deviation of force output for all Go and No-Go trials in the low and high amplitude force conditions, for each participant. These values were submitted to 2 (group: ADHD, CTRL) by 2 (amplitude: low, high) mixed model analysis of variance (ANOVA). All statistical tests were evaluated at an alpha of .05. It is important to note that No-Go trials were not categorized according to behavioral outcome. In other words, mean force was calculated for all No-Go trials and trials were not labeled post hoc as successes or failures. In other words, mean force represents how hard individuals were pressing on the load cells and thus provides a continuous measure of motor output for both Go and No-Go trials.
2.3.2. Data analysis for standard Go/No-Go RT task
E-Prime output variables included percentage of no-go failures, i.e., “commissions”, and reaction time for go successes (RT-go). Within-subject means were computed for each trial type, for each run of 100 trials. A grand mean for each participant, for each trial type, was then computed using these data. Means were submitted to three separate independent samples t-tests.
3. RESULTS
3.4.1. Participants
As reported in Table 1, independent univariate ANOVAs demonstrated that there were no differences in age or estimated IQ between the ADHD and control groups. The ADHD group reported significantly more ADHD-related symptoms in adulthood as measured by the CAADID and CAARS. As expected (Hinshaw et al., 2012; Anastopoulos et al., 2016), adults with ADHD self-reported more internalizing (e.g. anxiety or depression) and externalizing (e.g., aggression, rule-breaking behavior) difficulties on the ASR (ps < .001). Importantly, group means were within normal limits (T-scores < 59). As such, any group effects are unlikely to be attributed to the presence of a co-occurring condition instead of ADHD. Eighty-seven participants were confirmed to be right-hand dominant using the Edinburgh Handedness Inventory. The results for the left-handed (n = 7) and mixed-handed (n = 8) individuals did not differ from those who were right handed and thus they were included in the analysis. Of note, we conducted a post hoc power analysis using GPower (Faul et al., 2009) to confirm that the sample size (N = 102) was sufficient to detect effects and interactions. Specifically, given the lowest ηp2 observed in our Results (ηp2 = 0.02), the associated power is 0.953.
3.4.2. Go/No-Go force task
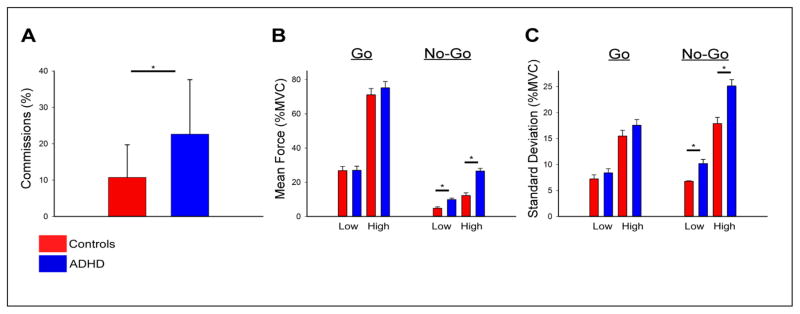
Mean force was evaluated by a repeated-measures ANOVA for Trial Type (Go, No-Go) and Amplitude (Low, High) with Group (Control, ADHD) as a between-subjects variable. Main effects were observed for amplitude, F(1, 100) = 888.96, p < .001, ηp2= 0.90, trial type, F(1, 100) = 320.21, p < .001, ηp2= 0.06, and group, F(1, 100) = 6.12, p = .015 ηp2= 0.88. Interactions were significant for group by amplitude, F(1, 100) = 11.15, p = .001, ηp2= 0.88, and amplitude by trial type, F(1, 100) = 385.36, p < .001, ηp2= 0.79. No interaction was observed for group by trial type, (F(1, 100) = 3.40, p = .068, ηp2 = .033, or group by amplitude by trial type, F(1, 100) = 2.31, p = .132, ηp2= 0.02. Although the three-way interaction did not reach statistical significance, we conducted four independent samples t-tests to examine group differences for each trial type and amplitude, based on our a priori hypotheses. The results of the t-tests demonstrated that mean force produced in the Go trials did not differ between groups (p’s > .444). In contrast, however, mean force produced in the No-Go trials was higher for adults with ADHD compared to controls for the low (t(100) = −4.63, p < .001) and high (t(100) = −6.12, p < .001) amplitude conditions, reflecting the significant group by amplitude interaction. As shown in Figure 2B, these data are also consistent with our conclusion that adults with ADHD produce higher mean force in No-Go trials, but not Go trials, compared to controls.
Figure 2.
(A) Adults with ADHD made more commission errors in No-Go trials in the standard RT Go/No-Go task. (B) Adults with ADHD produced more force in No-Go, but not Go, trials compared to healthy controls, for both the low and high amplitude conditions. (C) Adults with ADHD produced more variable force in No-Go, but not Go, trials compared to healthy controls, for both the low and high amplitude conditions.
Standard deviation of force was evaluated by a repeated-measures ANOVA for Trial Type (Go, No-Go) and Amplitude (Low, High) with Group (Control, ADHD) as a between-subjects variable. Main effects were observed for amplitude, F(1, 100) = 592.59, p < .001, ηp2 = 0.86, trial type, F(1,100) = 40.64, p < .001, ηp2 = 0.29, and group, F(1, 100) = 8.39, p = .005, ηp2= 0.08. Interactions were significant for group by amplitude, F(1,100) = 7.20, p = .009, ηp2 = 0.07, group by trial type, F(100), 17.36, p < 001, ηp2 = 0.15, and group by trial type by amplitude, F(1, 1000) = 6.158, p = .015, ηp2 = 0.06. We then conducted four independent samples t-tests to examine group differences for each trial type and amplitude, based on our a priori hypotheses. The results demonstrated that the standard deviation produced in the Go trials did not differ between groups (ps > .167). In contrast, and as shown in Figure 2C, the standard deviation for No-Go trials was higher for adults with ADHD compared to controls for the low (t(100) = −2.87, p < .005) and high (t(100) = −4.35, p < .001) amplitude conditions. This pattern mirrors the findings noted for mean force.
3.4.3. Standard Go/No-Go task
There was a main effect of group on the total number of commission errors, t(100) = −4.86, p < .001, d = 0.96, in which adults with ADHD had more commissions (22.6, SD=15.0) compared to controls (10.7, SD=9.0). There were no significant effects of group on mean RT for go-trials, t(100) = 1.03, p = .306 (grand mean = 348.1 ms, SD=45.0).
3.4.4. Clinical associations
Correlation and multiple regression analyses were conducted to examine the relationship between the CAARS S:L ADHD Index scores and three predictors: mean force output in no-go trials in the low and high amplitude conditions and number of commission errors in the no-go trials in the standard RT task. As shown in Table 2, each predictor is positively and significantly correlated with ADHD Index scores, demonstrating that those who produced higher forces and those who made more commission errors had higher ADHD Index scores. We first used hierarchical regression to determine whether the predictors contribute a significant amount of variability to the regression equation. Mean force for the low and high amplitude conditions was entered at Stage one and number of commission errors was entered at Stage two. The hierarchical multiple regression revealed that at Stage one, mean force in the low and high amplitude conditions contributed significantly to the regression model, F(2, 99) = 10.88, p < .001, and accounted for 18% of the variance in the ADHD Index score. Adding the number of commission errors to the model explained an additional 0.01% of the variation and the change in R2 was not significant, F(1, 98) = 0.08, p = .776. Next, we reversed the order of the predictors in the model. The number of commission errors was entered at Stage one and mean force for the low and high amplitude conditions was entered at Stage two. The hierarchical multiple regression revealed that at Stage one, number of commissions contributed significantly to the regression model, F(1, 100) = 8.33, p = .005, and accounted for 7.7% of the variance in the ADHD Index score. Adding mean force output in the low and high amplitude conditions to the model explained an additional 10.4% of the variation and the change in R2 was significant, F(2, 98) = 6.22, p = .003. Together, these results demonstrate that the standard RT task does not predict unique variance over and above that of what is predicted by the force task, but that the force task contributes for unique variance over and above that is what is predicted by the standard RT task alone.
Table 2.
Summary statistics, correlations, and multiple regression results.
| Variables | Mean | Standard deviation | Correlation with CAARS ADHD Index | Multiple regression weights
|
|
|---|---|---|---|---|---|
| b | β | ||||
| CAARS ADHD Index score | 48.58 | 12.55 | |||
| Mean force high amplitude | 19.38% | 13.74% | .393* | 35.87 | .393 |
| Mean force low amplitude | 7.35% | 6.10% | .385* | ||
| Errors of commission | 16.68 | 13.68 | .277* | ||
Notes: Pearson correlations with p <.001 are noted with an asterisk (*).
4. DISCUSSION
We report three novel findings. First, on Go trials of the force task, force output and variability was not different for adults with ADHD compared to adults without ADHD. Second, on No-Go trials in the force task, adults with ADHD produced greater and more variable force compared to adults without ADHD. Third, mean force output on No-Go trials was a stronger predictor of the CAARS S:L ADHD Index compared to performance in the standard RT task.
The lack of a group difference for force output and force variability on Go trials demonstrates that adults with ADHD appropriately scaled their motor output to the goals of the task in both low and high amplitude Go trials. The finding that force output and variability scaled to the target amplitude in No-Go trials, suggests that the motor system in adults with ADHD do not have difficulty calibrating response parameters to the demands of the upcoming task. That is, work in non-human primates that involves the Go/No-Go task (Wise et al., 1983; Kalaska and Crammond, 1995) and cerebellar internal models for action (Wolpert et al., 1995; Wolpert and Miall, 1996; Wolpert et al., 1998; Imamizu et al., 2000; Ito, 2005) have found that the motor system integrates sensory information with the goals of the task to estimate the parameters of the action. The cerebellum is well-positioned for such a task since the majority of its inputs are from primary motor cortex, prefrontal cortex, and posterior parietal cortex (Ramnani, 2012). Thus, it is unlikely that the fine motor deficits that are often observed in ADHD are due to difficulty creating an internal model of force amplitude. Further, the finding that force amplitude scaled to the parameters of the task, even in No-Go trials, suggests that responses were planned in advance of the visual cue. Such a movement strategy is expected in a paradigm with rapid stimulus-response requirements (Favilla et al., 1990; Ghez et al., 1991; Leocani et al., 2000).
That being said, adults with ADHD did produce greater and more variable force during No-Go trials compared to adults without ADHD. One interpretation is that the excess force produced in No-Go trials is a reflection of hyperactivity in the motor system. Indeed, the idea that excess muscular tension is related to hyperactivity is not a new concept (Braud et al., 1975); however, to the best of our knowledge this has not been empirically tested. We suggest that the amount of force produced on No-Go trials provides a precise and continuous measure of inhibitory control. In particular, an individual with “good” inhibitory control produces low levels of force, whereas an individual with “poor” inhibitory control produces higher levels of force. The result is a continuum of inhibitory control performance that can be examined in relation to other behavioral, clinical, or physiological measures. Adults with ADHD produced more variable force during No-Go trials compared to adults without ADHD. Variability is inherent to biological systems and is considered to reflect noise in the system. A traditional perspective on variability in the motor system is that optimal performance is characterized by low variability (Selen et al., 2006). However, an alternate view is that low variability leads to rigid and stereotyped movement, whereas excess variability leads to unstable movement (Stergiou et al., 2006). Both scenarios lead to less than optimal performance. In the present work, we suggest that increased force variability on No-Go trials is indicative of excess neuromuscular noise and unstable performance. Neuromuscular noise is the result of several physiological mechanisms, such as motor unit synchronization and recruitment, the level of antagonistic coactivation, and the integrity of feedback loops (for a review see (Oomen and van Dieen, 2016)). Future studies that can disentangle the physiological components of the task will be able to provide greater information about the role of neuromuscular noise in the pathology of ADHD.
More broadly speaking, adults with ADHD have impaired visuomotor memory in gripping tasks (Neely et al., 2016), visuomotor adaptation in reaching tasks (Kurdziel et al., 2015), deficits in oculomotor control (Feifel et al., 2004; Carr et al., 2006), increased postural sway (Hove et al., 2015), and impaired timing finger tapping tasks (Valera et al., 2010). The force task employed here provides a precise and continuous measure of motor output, which in future studies could be correlated with behavioral or neuroimaging data to help pinpoint the neurological cause of the impairment. Further, the force amplitude manipulation provides a means to evaluate whether adults with ADHD can appropriately plan and scale the amplitude of their response. Previous work demonstrates the importance of the basal ganglia and prefrontal cortex for force production (Vaillancourt et al., 2003; Vaillancourt et al., 2004; Vaillancourt et al., 2006; Spraker et al., 2007; Prodoehl et al., 2009; Spraker et al., 2009; Coombes et al., 2011; Neely et al., 2013a; Neely et al., 2013b). The networks for force production overlap with networks critical for inhibitory control, including the subthalamic nucleus of the basal ganglia, the supplementary motor area, and the inferior frontal cortex (Aron and Poldrack, 2005). More importantly, these brain regions are implicated in neural models of ADHD characterized by large-scale networks including anterior cingulate cortex, posterior parietal cortex, thalamus, prefrontal cortex, striatum, and cerebellum (Bush et al., 2005; Seidman et al., 2005; Makris et al., 2009; Liston et al., 2011; Cortese et al., 2012). Thus, the use of precision force tasks, which have well understood neural correlates, may provide a good framework to examine the neurobiology of ADHD.
5. CONCLUSION
These current work represents a novel examination of inhibitory control in young adults. We demonstrated that the force variant of the Go/No-Go task is a precise and continuous measure of inhibitory control. As such, the resulting continuum of performance can be examined in relation to other behavioral, clinical, or physiological measures to provide greater information about the pathophysiology of inhibitory motor control. The results of the current study demonstrate that adults with ADHD have inhibitory control deficits in both the standard RT and force variant of the Go/No-Go task. Importantly, however, performance on the force task was a better predictor of ADHD symptomatology than the standard RT task. This suggests that a precisely controlled motor task may provide a more nuanced approach to examine motor control as it relates to ADHD-related symptoms. Our future work aims to identify patterns of performance in motor and cognitive tasks in a community population of young adults with ADHD who may be more impaired than the college-attending population studied in this work. We focus on young adults because late adolescence and young adulthood is a period when individuals establish their career trajectory, have rapidly changing social patterns and relationships, and establish their independence. Further, it is well-established that young adults with ADHD have adverse outcomes. These outcomes may be related to problems in the motor system. Thus, it is critical we understand the neural basis of ADHD to inform optimal therapeutics that can be used to improve functional outcomes in young adults with ADHD.
Table 1.
Participant characteristics
| Variables | Group
|
Significant Group Differences | |
|---|---|---|---|
| Control | ADHD | ||
| Sample size | 51 | 51 | |
| Females | 22 | 29 | |
| Right-handed | 43 | 44 | |
| Mixed-handed | 4 | 4 | |
| Left-handed | 4 | 3 | |
| Age, yrs | 21.0 (1.7) | 21.1 (1.8) | ADHD = CTRL, p = .779 |
| FSIQ | 106.3 (11.1) | 105.3 (11.5) | ADHD = CTRL, p = .630 |
| CAADID | |||
| Number of inattention symptoms endorsed in adulthood | 0.02 (0.14) | 6.45 (1.95) | ADHD > CTRL, (F1, 100) = 557.20 p < .001 |
| Number of hyperactive/impulsive symptoms endorsed in adulthood | 0.14 (0.40) | 4.90 (2.33) | ADHD > CTRL, (F1, 100) = 232.47 p < .001 |
| Total number of symptoms endorsed in adulthood | 0.16 (0.42) | 11.35 (3.16) | ADHD > CTRL, (F1, 100) = 664.47 p < .001 |
| CAARS-S:L | |||
| DSM-IV ADHD Symptoms Total T-score | 43.1 (10.0) | 66.4 (15.9) | ADHD > CTRL, (F1, 100) = 78.64, p < .001 |
| ADHD Index T-score | 40.2 (7.0) | 57.0 (11.1) | ADHD > CTRL, (F1, 100) = 83.36, p < .001 |
| ASR | |||
| Internalizing Composite T-score | 44.1 (10.8) | 56.3 (13.0) | ADHD > CTRL, (F1, 100) = 26.38, p < .001 |
| Aggressive Behavior T-score | 51.5 (3.6) | 56.9 (7.6) | ADHD > CTRL, (F1, 100) = 20.87, p < .001 |
| Rule-breaking Behavior T-score | 52.0 (3.5) | 58.9 (8.7) | ADHD > CTRL, (F1, 100) = 27.98, p < .001 |
Note: Values are means and standard deviations (in parentheses).
HIGHLIGHTS.
Adults with and without ADHD completed a force-variant of the Go/No-Go task.
Adults with ADHD produced more force than adults without ADHD on failed inhibits.
The amount of force was a better predictor of ADHD compared to the standard task.
Precise and continuous motor tasks can be used to study behavioral deficits in ADHD.
Acknowledgments
This publication was supported, in part, by Grant UL1 TR002014 and KL2 TR002015 from the National Center for Advancing Translational Sciences (NCATS) to KAN. This publication was supported, in part, by Grant R01 MH084947 to CHP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest:
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TMR, LA . Manual for the ASEBA Adult Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2003. [Google Scholar]
- Albayrak O, et al. Common obesity risk alleles in childhood attention-deficit/hyperactivity disorder. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2013;162:295–305. doi: 10.1002/ajmg.b.32144. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, Kofler MJ. Attention-Deficit/Hyperactivity disorder and behavioral inhibition: A meta-analytic review of the stop-signal paradigm. J Abnorm Child Psych. 2007;35:745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association., American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders : DSM-5. 5. Arlington, Va: American Psychiatric Association; 2013. [Google Scholar]
- Anastopoulos AD, DuPaul GJ, Weyandt LL, Morrissey-Kane E, Sommer JL, Rhoads LH, Murphy KR, Gormley MJ, Gudmundsdottir BG. Rates and Patterns of Comorbidity Among First-Year College Students With ADHD. J Clin Child Adolesc Psychol. 2016:1–12. doi: 10.1080/15374416.2015.1105137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biological psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Chen M, Burrows L. Automaticity of social behavior: direct effects of trait construct and stereotype-activation on action. J Pers Soc Psychol. 1996;71:230–244. doi: 10.1037//0022-3514.71.2.230. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Progress in neurobiology. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Attention-deficit hyperactivity disorder. Scientific American. 1998;279:66–71. doi: 10.1038/scientificamerican0998-66. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Cox D. A review of driving risks and impairments associated with attention-deficit/hyperactivity disorder and the effects of stimulant medication on driving performance. J Safety Res. 2007;38:113–128. doi: 10.1016/j.jsr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Guevremont DC, Anastopoulos AD, DuPaul GJ, Shelton TL. Driving-related risks and outcomes of attention deficit hyperactivity disorder in adolescents and young adults: a 3- to 5-year follow-up survey. Pediatrics. 1993;92:212–218. [PubMed] [Google Scholar]
- Breslin FC, Pole JD. Work injury risk among young people with learning disabilities and attention-deficit/hyperactivity disorder in Canada. American journal of public health. 2009;99:1423–1430. doi: 10.2105/AJPH.2008.140855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddenberg LA, Davis C. Test-retest reliability of the Purdue Pegboard Test. Am J Occup Ther. 2000;54:555–558. doi: 10.5014/ajot.54.5.555. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Carr LA, Nigg JT, Henderson JM. Attentional versus motor inhibition in adults with attention-deficit/hyperactivity disorder. Neuropsychology. 2006;20:430–441. doi: 10.1037/0894-4105.20.4.430. [DOI] [PubMed] [Google Scholar]
- Coombes SA, Corcos DM, Vaillancourt DE. Spatiotemporal tuning of brain activity and force performance. Neuroimage. 2011;54:2226–2236. doi: 10.1016/j.neuroimage.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Angriman M, Maffeis C, Isnard P, Konofal E, Lecendreux M, Purper-Ouakil D, Vincenzi B, Bernardina BD, Mouren MC. Attention-deficit/hyperactivity disorder (ADHD) and obesity: a systematic review of the literature. Critical reviews in food science and nutrition. 2008;48:524–537. doi: 10.1080/10408390701540124. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychological medicine. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Favilla M, Gordon J, Hening W, Ghez C. Trajectory control in targeted force impulses. VII. Independent setting of amplitude and direction in response preparation. Exp Brain Res. 1990;79:530–538. doi: 10.1007/BF00229322. [DOI] [PubMed] [Google Scholar]
- Feifel D, Farber RH, Clementz BA, Perry W, Anllo-Vento L. Inhibitory deficits in ocular motor behavior in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2004;56:333–339. doi: 10.1016/j.biopsych.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Ghez C, Hening W, Gordon J. Organization of voluntary movement. Curr Opin Neurobiol. 1991;1:664–671. doi: 10.1016/s0959-4388(05)80046-7. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Isaacs KM, Augusta M, Macneil LK, Mostofsky SH. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology. 2011;76:615–621. doi: 10.1212/WNL.0b013e31820c2ebd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg C, Gillberg IC, Rasmussen P, Kadesjo B, Soderstrom H, Rastam M, Johnson M, Rothenberger A, Niklasson L. Co-existing disorders in ADHD -- implications for diagnosis and intervention. European child & adolescent psychiatry. 2004;13(Suppl 1):I80–92. doi: 10.1007/s00787-004-1008-4. [DOI] [PubMed] [Google Scholar]
- Groenman AP, Oosterlaan J, Rommelse N, Franke B, Roeyers H, Oades RD, Sergeant JA, Buitelaar JK, Faraone SV. Substance use disorders in adolescents with attention deficit hyperactivity disorder: a 4-year follow-up study. Addiction. 2013;108:1503–1511. doi: 10.1111/add.12188. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Owens EB, Zalecki C, Huggins SP, Montenegro-Nevado AJ, Schrodek E, Swanson EN. Prospective follow-up of girls with attention-deficit/hyperactivity disorder into early adulthood: continuing impairment includes elevated risk for suicide attempts and self-injury. J Consult Clin Psychol. 2012;80:1041–1051. doi: 10.1037/a0029451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkins P, Montejano L, Sasane R, Huse D. Risk of injury associated with attention-deficit/hyperactivity disorder in adults enrolled in employer-sponsored health plans: a retrospective analysis. The primary care companion to CNS disorders. 2011:13. doi: 10.4088/PCC.10m01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove MJ, Zeffiro TA, Biederman J, Li Z, Schmahmann J, Valera EM. Postural sway and regional cerebellar volume in adults with attention-deficit/hyperactivity disorder. Neuroimage Clin. 2015;8:422–428. doi: 10.1016/j.nicl.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- Ito M. Bases and implications of learning in the cerebellum--adaptive control and internal model mechanism. Prog Brain Res. 2005;148:95–109. doi: 10.1016/S0079-6123(04)48009-1. [DOI] [PubMed] [Google Scholar]
- Jerome L, Segal A, Habinski L. What we know about ADHD and driving risk: a literature review, meta-analysis and critique. Journal of the Canadian Academy of Child and Adolescent Psychiatry = Journal de l’Academie canadienne de psychiatrie de l’enfant et de l’adolescent. 2006a;15:105–125. [PMC free article] [PubMed] [Google Scholar]
- Jerome L, Segal A, Habinski L. What we know about ADHD and driving risk: a literature review, meta-analysis and critique. J Can Acad Child Adolesc Psychiatry. 2006b;15:105–125. [PMC free article] [PubMed] [Google Scholar]
- Kadesjo B, Gillberg C. Developmental coordination disorder in Swedish 7-year-old children. J Am Acad Child Adolesc Psychiatry. 1999;38:820–828. doi: 10.1097/00004583-199907000-00011. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ. Deciding not to GO: neuronal correlates of response selection in a GO/NOGO task in primate premotor and parietal cortex. Cereb Cortex. 1995;5:410–428. doi: 10.1093/cercor/5.5.410. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Lane M, Stang PE, Van Brunt DL. The prevalence and workplace costs of adult attention deficit hyperactivity disorder in a large manufacturing firm. Psychological medicine. 2009;39:137–147. doi: 10.1017/S0033291708003309. [DOI] [PubMed] [Google Scholar]
- Kupper T, Haavik J, Drexler H, Ramos-Quiroga JA, Wermelskirchen D, Prutz C, Schauble B. The negative impact of attention-deficit/hyperactivity disorder on occupational health in adults and adolescents. International archives of occupational and environmental health. 2012;85:837–847. doi: 10.1007/s00420-012-0794-0. [DOI] [PubMed] [Google Scholar]
- Kurdziel LB, Dempsey K, Zahara M, Valera E, Spencer RM. Impaired visuomotor adaptation in adults with ADHD. Exp Brain Res. 2015;233:1145–1153. doi: 10.1007/s00221-014-4190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123( Pt 6):1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Liston C, Malter Cohen M, Teslovich T, Levenson D, Casey BJ. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biol Psychiatry. 2011;69:1168–1177. doi: 10.1016/j.biopsych.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Monuteaux MC, Seidman LJ. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev Neurosci. 2009;31:36–49. doi: 10.1159/000207492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel RL, Jr, Nichols JQ, Fellers JC, Hidalgo P, Martinez LA, Putziger I, Burket RC, Cox DJ. Comparison of On-Road Driving Between Young Adults With and Without ADHD. J Atten Disord. 2013 doi: 10.1177/1087054712473832. [DOI] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: does self-control resemble a muscle? Psychol Bull. 2000;126:247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Muth OH, Weswig PH, Whanger PD, Oldfield JE. Effect of feeding selenium-deficient ration to the subhuman primate (Saimiri sciureus) Am J Vet Res. 1971;32:1603–1605. [PubMed] [Google Scholar]
- Nazar BP, Pinna CM, Suwwan R, Duchesne M, Freitas SR, Sergeant J, Mattos P. ADHD Rate in Obese Women With Binge Eating and Bulimic Behaviors From a Weight-Loss Clinic. J Atten Disord. 2012 doi: 10.1177/1087054712455503. [DOI] [PubMed] [Google Scholar]
- Nazar BP, Suwwan R, de Sousa Pinna CM, Duchesne M, Freitas SR, Sergeant J, Mattos P. Influence of attention-deficit/hyperactivity disorder on binge eating behaviors and psychiatric comorbidity profile of obese women. Compr Psychiatry. 2014;55:572–578. doi: 10.1016/j.comppsych.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Neely K, Chennavasin A, Yoder A, Williams GKR, Huang-Pollock CL, Loken E. Memory-guided force output is associated with self-reported ADHD symptoms in young adults. Experimental Brain Research. doi: 10.1007/s00221-016-4718-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely KA, Coombes SA, Planetta PJ, Vaillancourt DE. Segregated and overlapping neural circuits exist for the production of static and dynamic precision grip force. Hum Brain Mapp. 2013a;34:698–712. doi: 10.1002/hbm.21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely KA, Chennavasin AP, Yoder A, Williams GK, Loken E, Huang-Pollock CL. Memory-guided force output is associated with self-reported ADHD symptoms in young adults. Exp Brain Res. 2016 doi: 10.1007/s00221-016-4718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely KA, Planetta PJ, Prodoehl J, Corcos DM, Comella CL, Goetz CG, Shannon KL, Vaillancourt DE. Force control deficits in individuals with Parkinson’s disease, multiple systems atrophy, and progressive supranuclear palsy. PLoS One. 2013b;8:e58403. doi: 10.1371/journal.pone.0058403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe F, Oldfield RC, Ratcliff GG, Wingfield A. Recognition and naming of object-drawings by men with focal brain wounds. J Neurol Neurosurg Psychiatry. 1971;34:329–340. doi: 10.1136/jnnp.34.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Butler KM, Huang-Pollock CL, Henderson JM. Inhibitory processes in adults with persistent childhood onset ADHD. Journal of consulting and clinical psychology. 2002;70:153–157. doi: 10.1037//0022-006x.70.1.153. [DOI] [PubMed] [Google Scholar]
- Oomen NM, van Dieen JH. Effects of age on force steadiness: A literature review and meta-analysis. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Pitcher TM, Piek JP, Hay DA. Fine and gross motor ability in males with ADHD. Developmental medicine and child neurology. 2003;45:525–535. doi: 10.1017/s0012162203000975. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Corcos DM, Vaillancourt DE. Basal ganglia mechanisms underlying precision grip force control. Neurosci Biobehav Rev. 2009;33:900–908. doi: 10.1016/j.neubiorev.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N. Frontal lobe and posterior parietal contributions to the cortico-cerebellar system. Cerebellum. 2012;11:366–383. doi: 10.1007/s12311-011-0272-3. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Lencer R, Bishop JR, Keedy S, Sweeney JA. Pharmacological treatment effects on eye movement control. Brain and cognition. 2008;68:415–435. doi: 10.1016/j.bandc.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Selen LP, van Dieen JH, Beek PJ. Impedance modulation and feedback corrections in tracking targets of variable size and frequency. J Neurophysiol. 2006;96:2750–2759. doi: 10.1152/jn.00552.2006. [DOI] [PubMed] [Google Scholar]
- Simon V, Czobor P, Balint S, Meszaros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194:204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- Spraker MB, Corcos DM, Vaillancourt DE. Cortical and subcortical mechanisms for precisely controlled force generation and force relaxation. Cereb Cortex. 2009;19:2640–2650. doi: 10.1093/cercor/bhp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol. 2007;98:821–834. doi: 10.1152/jn.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. J Neurol Phys Ther. 2006;30:120–129. doi: 10.1097/01.npt.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Fotedar S, Blankner JG, Pekar JJ, Denckla MB, Mostofsky SH. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. Journal of cognitive neuroscience. 2008;20:478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen A, Birnbaum HG, Ben Hamadi R, Greenberg P, Cremieux PY, Secnik K. Incidence and costs of accidents among attention-deficit/hyperactivity disorder patients. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2004;35:346, e341–349. [PubMed] [Google Scholar]
- Turgay A, Goodman DW, Asherson P, Lasser RA, Babcock TF, Pucci ML, Barkley R Group ATPMW. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73:192–201. doi: 10.4088/JCP.10m06628. [DOI] [PubMed] [Google Scholar]
- Upadhyaya HP. Substance use disorders in children and adolescents with attention-deficit/hyperactivity disorder: implications for treatment and the role of the primary care physician. Primary care companion to the Journal of clinical psychiatry. 2008;10:211–221. doi: 10.4088/pcc.v10n0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Thulborn KR, Corcos DM. Neural basis for the processes that underlie visually guided and internally guided force control in humans. J Neurophysiol. 2003;90:3330–3340. doi: 10.1152/jn.00394.2003. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Corcos DM. Intermittent visuomotor processing in the human cerebellum, parietal cortex, and premotor cortex. J Neurophysiol. 2006;95:922–931. doi: 10.1152/jn.00718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Thulborn KR, Corcos DM. Subthalamic nucleus and internal globus pallidus scale with the rate of change of force production in humans. Neuroimage. 2004;23:175–186. doi: 10.1016/j.neuroimage.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Valera EM, Spencer RM, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, Biederman J, Seidman LJ. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:359–367. doi: 10.1016/j.biopsych.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV) San Antonio, TX: NCS Pearson; 2008. [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Prince J. Pharmacotherapy of adult attention deficit/hyperactivity disorder: a review. Journal of clinical psychopharmacology. 1995;15:270–279. doi: 10.1097/00004714-199508000-00006. [DOI] [PubMed] [Google Scholar]
- Wise SP, Weinrich M, Mauritz KH. Motor aspects of cue-related neuronal activity in premotor cortex of the rhesus monkey. Brain Res. 1983;260:301–305. doi: 10.1016/0006-8993(83)90685-6. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Mahone EM, Blankner JG, Larson JC, Fotedar S, Denckla MB, Mostofsky SH. Evidence that response inhibition is a primary deficit in ADHD. Journal of clinical and experimental neuropsychology. 2007;29:345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC. Forward Models for Physiological Motor Control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]