Abstract
Testosterone is a final product of androgenic hormone biosynthesis, and Leydig cells are known to be the primary source of androgens. In the mammalian testis, two distinct populations of Leydig cells, the fetal and the adult Leydig cells, develop sequentially, and these two cell types differ both morphologically and functionally. It is well known that the adult Leydig cells maintain male reproductive function by producing testosterone. However, it has been controversial whether fetal Leydig cells can produce testosterone, and the synthetic pathway of testosterone in the fetal testis is not fully understood. In the present study, we generated transgenic mice in which enhanced green fluorescence protein was expressed under the control of a fetal Leydig cell-specific enhancer of the Ad4BP/SF-1 (Nr5a1) gene. The transgene construct was prepared by mutating the LIM homeodomain transcription factor (LHX9)-binding sequence in the promoter, which abolished promoter activity in the undifferentiated testicular cells. These transgenic mice were used to collect highly pure fetal Leydig cells. Gene expression and steroidogenic enzyme activities in the fetal Leydig cells as well as in the fetal Sertoli cells and adult Leydig cells were analyzed. Our results revealed that the fetal Leydig cells synthesize only androstenedione because they lack expression of Hsd17b3, and fetal Sertoli cells convert androstenedione to testosterone, whereas adult Leydig cells synthesize testosterone by themselves. The current study demonstrated that both Leydig and Sertoli cells are required for testosterone synthesis in the mouse fetal testis.
Physiologically, steroid hormones are classified into five groups: androgens, estrogens, progestins, glucocorticoids, and mineralocorticoids. Among these, testosterone (an androgen, 17β-hydroxyandrost-4-en-3-one) is synthesized predominantly in the testes using cholesterol as the starting material. The multiple reactions in testosterone synthesis are mediated successively by steroidogenic acute regulatory protein (StAR), cholesterol side-chain cleavage P450 (CYP11A1), 3β-hydroxysteroid dehydrogenase (type II in humans and type I in mice), 17α-hydroxylase/17,20-lyase P450 (CYP17A1), and 17β-hydroxysteroid dehydrogenase type III (HSD17B3) (1–5).
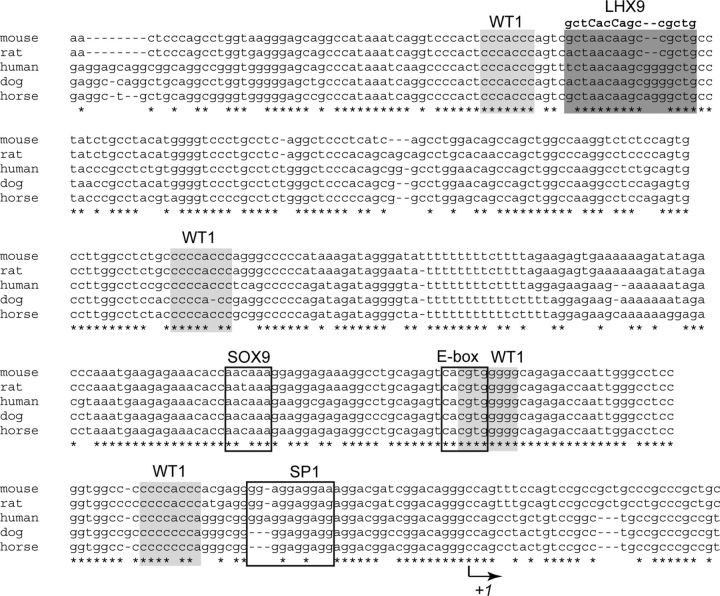
Ad4BP/SF-1 (NR5A1) (6, 7) is expressed in the steroidogenic and reproductive tissues/cells, such as the gonads, adrenal cortex, pituitary gonadotropes, and ventromedial hypothalamic nucleus (8–10). All these tissues/cells require Ad4BP/SF-1 for their development and for establishment of specific functions (11, 12). Therefore, the tissue/cell-specific expression of Ad4BP/SF-1 has been investigated by focusing on the promoter and enhancer functions. The promoter region of Ad4BP/SF-1 contains binding sites for SRY (sex determining region Y)-box 9 (SOX9 (13), the LIM homeobox transcription factor (LHX9) (14), specificity protein 1 (15), E-box binding protein (16–18), and Wilms' tumor-1 protein (WT1) (14) (Fig. 1). As for the putative enhancers responsible for tissue/cell-specific expression of Ad4BP/SF-1, multiple regions have been identified: a region in the fourth intron has been shown to display fetal adrenal-specific expression (19), two regions in the sixth intron to display pituitary gonadotrope-specific (20) and ventromedial hypothalamic nucleus-specific expression (21), and a region upstream of the first exon to display fetal Leydig cell-specific expression (22). We can conclude that these tissue/cell-specific enhancers together with the promoter regions are responsible for elaborating the expression of the Ad4BP/SF-1 gene.
Fig. 1.
Binding sites for transcription factors in the upstream region of Ad4BP/SF-1 genes. Nucleotide sequences of the Ad4BP/SF-1 gene promoter in mouse, rat, human, dog, and horse were obtained from the University of California, Santa Cruz, genome browser (http://genome.ucsc.edu/), and the sequences were aligned using the multiple sequence alignment program, MAFFT (47, 48). Potential binding sites for WT-1 and LHX9 are indicated by light- and dark-shaded boxes respectively, and binding sites for SOX9, E-box binding protein, and specificity protein 1 are indicated by open boxes. The mutated sequence at the LHX9 binding site is indicated (substituted nucleotides are indicated by upper case letters). Nucleotides conserved in all animal species are labeled with asterisks. The transcription initiation site is indicated by an arrow.
Leydig cells in the fetal testis are different from those in the adult testis in terms of their origin and function (4, 23). For instance, testosterone is synthesized in Leydig cells in the adult testes. By contrast, O'Shaughnessy et al. (24) showed that mouse fetal Leydig cells prepared from neonatal testes produce androstenedione, which thereafter is converted to testosterone by the cells within the seminiferous tubules. This study indicated for the first time that at least two different cell types are involved in testosterone production in the mouse neonatal testis. Interestingly, however, Weisser et al. (25) recently reported that rat postnatal fetal Leydig cells produce testosterone by themselves. Therefore, it is still controversial whether fetal Leydig cells have an ability to produce testosterone or not. One of the likely reasons that this controversy remains to be solved is because there is no established method to obtain fetal Leydig cells with high purity. In the present study, we generated transgenic mice in which enhanced green fluorescence protein (EGFP) is expressed under the control of a fetal Leydig cell-specific enhancer of the Ad4BP/SF-1 gene. We prepared high-quality purifications of fetal Leydig cells from these transgenic mice and evaluated their gene expression and steroidogenic activity.
Materials and Methods
Generation and observation of transgenic mice
The transgenic construct, SmAc-1.8-Ad4BP-EGFP, contains a 1.8-kb fetal Leydig cell-specific enhancer and a 5.8-kb upstream fragment from the initiator methionine codon in the second exon of the murine Ad4BP/SF-1 gene (22). SmAc-1.8-Ad4BP(LBmut)-EGFP was constructed by mutating an LHX9-binding site in the Ad4BP/SF-1 gene promoter (Figs. 1 and 2A). Transgenic mice were generated as described by Hogan et al. (26). The presence of the transgene in mice was examined by PCR with the primers specific for EGFP (20). Transgenic embryos were harvested at embryonic day (E)10.5, E12.5, and E18.5 and observed by using the fluorescent stereomicroscope, SZX16/SZX2-ILLT (Olympus, Tokyo, Japan). All protocols for the animal experiments were approved by the Animal Care and Use Committee of Kyushu University.
Fig. 2.
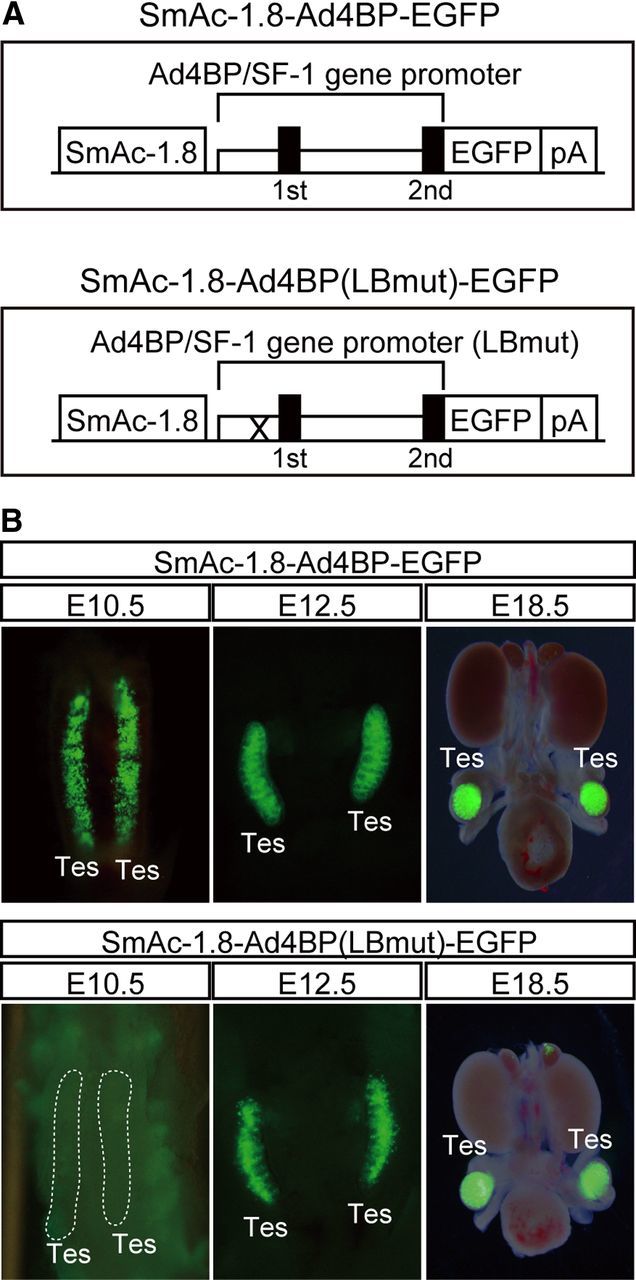
Disappearance of EGFP expression from indifferent gonads through a mutation at the Lhx9-binding site of the Ad4BP/SF-1 gene promoter. A, Two DNA constructs, SmAc-1.8-Ad4BP-EGFP and SmAc-1.8-Ad4BP(LBmut)-EGFP, were used for transgenic mouse studies. SmAc-1.8-Ad4BP-EGFP consists of a 1.8-kb fetal Leydig cell-specific enhancer (SmAc-1.8); the Ad4BP/SF-1 gene promoter contains a 5.8-kb region upstream from the initiator methionine in the second exon (Ad4BP/SF-1 gene promoter). EGFP is the reporter gene, and poly(A) signal is represented as pA. The DNA composition of SmAc-1.8-Ad4BP(LBmut)-EGFP is the same as SmAc-1.8-Ad4BP-EGFP, except that the LHX9-binding site in the Ad4BP/SF-1 gene promoter is mutated (indicated by X). B, EGFP expression in the testes of the transgenic mice carrying SmAc-1.8-Ad4BP-EGFP (upper panels) and SmAc-1.8-Ad4BP(LBmut)-EGFP (lower panels) at E10.5, E12.5, and E18.5 fetuses is shown. Tes, Testis.
Immunohistochemistry
An immunofluorescence study was performed using a BZ-9000 microscope (Keyence, Osaka, Japan). Anti-EGFP rabbit polyclonal antibody (Medical and Biological Laboratories, Nagoya, Japan), anti-EGFP rat monoclonal antibody (Nacalai Tesque, Kyoto, Japan), anti-Ad4BP/SF-1 rabbit antiserum (27), anti-3β-HSD rabbit antiserum (28), and anti-Ad4BP/SF-1 rat monoclonal antibody (20, 29) were used as primary antibodies. The anti-HSD17B3 rat monoclonal antibody was produced as described (20, 29), and the specificity of this antibody is shown in Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org. ALEXA Fluoro 488 goat antirabbit IgG, ALEXA Fluoro 488 goat antirat IgG, ALEXA Fluoro 555 goat antirabbit IgG, and ALEXA Fluoro 555 goat antirat IgG were used as secondary antibodies (Life Technologies, Carlsbad, CA). The reagent 4′6′-diamidino-2-phenylindole was used for nuclear staining.
Preparation of fetal Leydig and Sertoli cells
Fetal Leydig and fetal Sertoli cells were prepared from the testes of SmAc-1.8-Ad4BP(LBmut)-EGFP transgenic mice and Sox9-EGFP (30) knock-in mice fetuses at E18.5, respectively. The testes were incubated in Earle's balanced salt solution (Sigma, St. Louis, MO) containing 0.1 U/ml collagenase (GIBCO, Grand Island, NY) and 1 U/ml dispase (GIBCO) at 37 C for 30 min. During the incubation, the testes were dispersed by pipetting gently several times. The gonads were incubated for a further 15 min with 0.2 mg/ml deoxyribonuclease I (Roche, Basel, Switzerland). After centrifugation at 300 × g for 5 min at room temperature, the cells were suspended in PBS and stained with 7-amino-actinomycin D (Sigma). The cells were recovered by centrifugation, resuspended in PBS containing 1 mm EDTA, and filtered by a cell strainer (BD Biosciences, San Jose, CA). EGFP-positive cells were recovered by fluorescence-activated cell sorting (FACS) using the cell sorter JSAN (Bay Bioscience Co. Ltd., Kobe, Japan).
Percoll density gradient centrifugation
For preparation of adult Leydig cells, Percoll density gradient centrifugation was performed as previously described by Niedziela and Lerchl (31). Briefly, 20 decapsulated testes from 9-wk-old mice were incubated for 10 min at 34 C with gentle stirring in 50 ml DMEM (Nacalai Tesque) supplemented with 0.1% BSA (Roche), 100 U/ml penicillin, 100 μg/ml streptomycin, 0.025% soybean trypsin inhibitor (Sigma), and 0.5 mg/ml collagenase (GIBCO). The resulting solution was filtered through a 70-μm cell strainer (BD Biosciences). Centrifugation was performed using a discontinuous density gradient of Percoll (20, 30, 50, and 60%; GE Healthcare UK Ltd., Amersham Place, UK). After centrifugation at 25,000 × g for 45 min at 4 C, Leydig cells formed a distinct band between the 30 and 50% layers. For biochemical analyses, the isolated cells were plated, and any unattached cells were washed out after 12 h. For RNA extraction, the isolated cells were subjected to FACS, and contaminated small cells (including spermatids) were eliminated based on size.
Quantitative RT-PCR analyses
cDNAs were synthesized from RNA samples by using Superscript II reverse transcriptase (Life Technologies) and oligo (deoxythymidine)15 primer (Promega, Madison, WI) according to the manufacturer's instructions. Quantitative RT-PCR was performed by using the THUNDERBIRD SYBR quantitative PCR mix (TOYOBO Co. Ltd., Osaka, Japan); the primers are listed in Table 1. Three or four distinct pools of each cell type were used for cDNA preparation, and gene expression in each sample was examined in triplicate. The values were standardized using β-actin (Actb).
Table 1.
Primers used in quantitative RT-PCR analyses
| Gene name | Primer (forward/reverse) | Primer sequence (5′–3′) |
|---|---|---|
| Actb (β-actin) | Forward | AGGGTGTGATGGTGGGAATGG |
| Reverse | TGGCTGGGGTGTTGAAGGTCT | |
| Nr5a1 (Ad4BP/SF-1) | Forward | AAGCCACTCTGTAGGACCAAGC |
| Reverse | TGTAAATCTGACGCGAAAGCAG | |
| Sox9 | Forward | CCTTGAGCCTTAAATCCCGGCTGC |
| Reverse | CTTTGCTTGCCCCTGGCTTTCCTT | |
| Amh (Mis) | Forward | GAACCTCTGCCCTACTCGGG |
| Reverse | AAGTCCACGGTTAGCACCAAA | |
| Hsd3b1 | Forward | CAAGTGTGCCAGCCTTCATCT |
| Reverse | TTCATGATTCTGTTCCTCGTGG | |
| Hsd3b6a | Forward | TTTTTTTGAGGTATTGACAAGTATTTATTG |
| Reverse | TCCCCATTCAGAGCATGTATAGC | |
| Cyp11a1 (P450SCC) | Forward | CGAATCGTCCTAAACCAAGAG |
| Reverse | CACTGATGACCCCTGAGAAAT | |
| StAR | Forward | TACATCCAGCAGGGAGAGGTG |
| Reverse | CAGCGCACGCTCACGAAGTCT | |
| Cyp17a1 | Forward | GGGTGACCCCAAGGTGGTGGTCTTTCT |
| Reverse | TGGGATCCGGGACGTTAGATTCGG | |
| Hsd17b3 | Forward | ATGGAGTCAAGGAGGAAAGGC |
| Reverse | GGCTGTAAAGAGGCCAGGG | |
| Ddx4 (Mvh, VASA) | Forward | GCACACGTTGAATACAGCGGGGAT |
| Reverse | TGGGAGGAAGAACAGAAGAACAGG | |
| Pdgfra | Forward | CAAACCCTGAGACCACAATGG |
| Reverse | TGATGCCCACATAGCCTTCAT | |
| Maf (c-Maf) | Forward | CCGTTGTACATGCACTGAAGGCGT |
| Reverse | TGCCCCAAAGCAGGCATCATTGTT | |
| Mafb | Forward | CCGCGAGGCTTATTCCAAG |
| Reverse | AGCCAGAAGTGTCCAGACGG |
Gene names (and aliases), direction of the primers (forward or reverse), and primer sequences are shown.
Primers for Hsd3b6 are originally designed by O'Shaughnessy et al. (38).
Biochemical analyses of steroidogenic enzyme activities
Steroidogenic enzyme activities of the cells were determined as described previously (32, 33). The purified fetal Leydig, fetal Sertoli, and adult Leydig cells were cultured separately or together using collagen type I-coated dishes (Asahi Techno Glass, Chiba, Japan) in DMEM containing 10% fetal bovine serum (GIBCO) and penicillin/streptomycin (GIBCO) in 5% CO2 at 37 C. After 48 h culture, [7-3H]-pregnenolone or [1-3H]-androstenedione (PerkinElmer, Boston, MA; 5.0 × 107 counts/min) was added to the culture media. Six hours after adding [7-3H]-pregnenolone or 1 h after adding [1-3H]-androstenedione, the cells and culture media were recovered together in ethyl acetate. Extracted steroidal molecules were subjected to HPLC analyses by using a reverse-phase LiChrospher 100 RP-18 columns (4.0 × 250 mm; Kanto, Tokyo, Japan). The column was eluted with a 30-min linear gradient of 40–70% of acetonitrile at a flow rate of 0.7 ml/min, followed by an isocratic elution with 70% acetonitrile. The eluate was counted in a flow scintillation analyzer (Radiomatic 525TR; PerkinElmer).
Results
Effect of mutation at the Lhx9-binding site in the Ad4BP/SF-1 gene promoter
We have previously identified a fetal Leydig cell-specific enhancer in the Ad4BP/SF-1 gene and established a transgenic mouse line with a DNA construct in which EGFP was used as a reporter gene (SmAc-1.8-Ad4BP-EGFP in Fig. 2A) (22). It is well known that fetal Leydig cells differentiate initially in the interstitial space of the fetal testes at E12.5 in mice (3–5). Consistent with this finding, the EGFP gene expression could be detected in the interstitial space at E12.5 (Fig. 2B). Surprisingly however, EGFP was detected even in sexually indifferent gonads at E10.5, by which point fetal Leydig cells have not yet differentiated.
While investigating this unexpected expression of EGFP, we noticed that the proximal upstream region of Ad4BP/SF-1 gene contains binding sites for multiple transcription factors such as WT1, LHX9 (14), E-box-binding proteins (15–18), and SOX9 (13) (Fig. 1). Furthermore, knockout studies have demonstrated that the loss of Wt1 or Lhx9 gene largely down-regulated Ad4BP/SF-1 gene expression in the indifferent gonads (14, 34), suggesting that these sites are implicated in reporter gene expression. To explore this discovery, we generated an additional transgenic mouse with a DNA construct carrying a mutation in the LHX9-binding site (SmAc-1.8-Ad4BP(LBmut)-EGFP in Fig. 2A). As expected, the expression of EGFP in the sexually indifferent gonad disappeared at E10.5 but was maintained in the testes at E12.5 and E18.5 (Fig. 2B).
Characterization of EGFP-positive cells in the two transgenic mouse lines
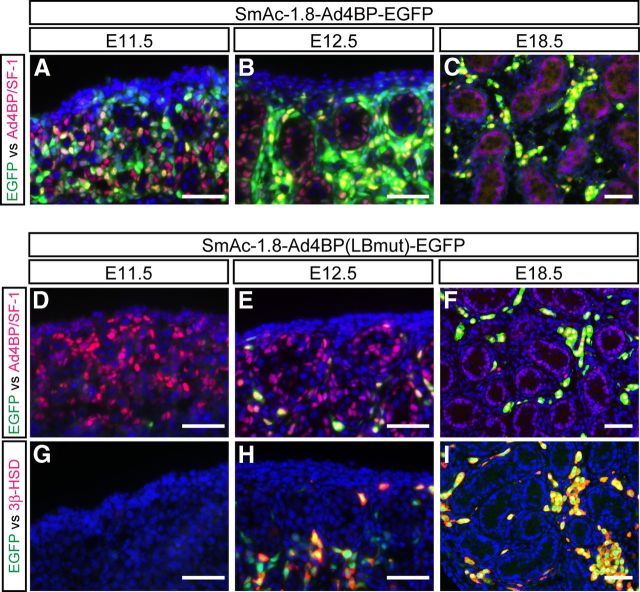
The developing fetal gonads from the two lines of the transgenic mice carrying SmAc-1.8-Ad4BP-EGFP or SmAc-1.8-Ad4BP(LBmut)-EGFP were subjected to immunohistochemical analyses. EGFP was expressed in many mesenchymal cells in the E11.5 gonads of SmAc-1.8-Ad4BP-EGFP transgenic mice, and many of them displayed strong immunoreactivity to Ad4BP/SF-1, whereas some of them did not. In addition, cells immunoreactive to Ad4BP/SF-1, but not EGFP, were also present (Fig. 3A). In the testes at E12.5, when fetal Leydig cells are initially differentiated, EGFP was expressed in most but not all interstitial cells including the cells immunoreactive to Ad4BP/SF-1. However, EGFP single-positive cells were also present in the interstitial space (Fig. 3B). In the testes at E18.5, EGFP and Ad4BP/SF-1 signals mostly overlapped (Fig. 3C). As described previously (22), these EGFP-positive cells were fetal Leydig cells because they were also immunoreactive to 3β-hydroxysteroid dehydrogenase (3β-HSD). In the testes of SmAc-1.8-Ad4BP(LBmut)-EGFP transgenic mice, EGFP was not expressed in the fetal testes at E11.5 (Fig. 3, D and G). EGFP and Ad4BP/SF-1 double-positive cells emerged at E12.5 (Fig. 3E), and most of the EGFP-positive cells were immunoreactive to 3β-HSD (Fig. 3H). Thereafter the EGFP-positive cells localized at the interstitial space had increased in number by E18.5, and the EGFP signals were tightly confined to the 3β-HSD-positive fetal Leydig cells at E18.5 (Fig. 3, F and I).
Fig. 3.
Expression of EGFP in the testes of the two lines of transgenic mice. Gonads were prepared from the transgenic mice carrying SmAc-1.8-Ad4BP-EGFP (upper panels) and SmAc-1.8-Ad4BP(LBmut)-EGFP (lower panels) at E11.5, E12.5, and E18.5. They were subjected to immunofluorescence analyses by using the combination of the antibodies to EGFP (green) and Ad4BP/SF-1 (red) or EGFP (green) and 3β-HSD (red). 4′6′-Diamidino-2-phenylindole (blue) was used to stain nuclei. Bars, 50 μm.
Characterization of weakly and strongly EGFP-positive cells
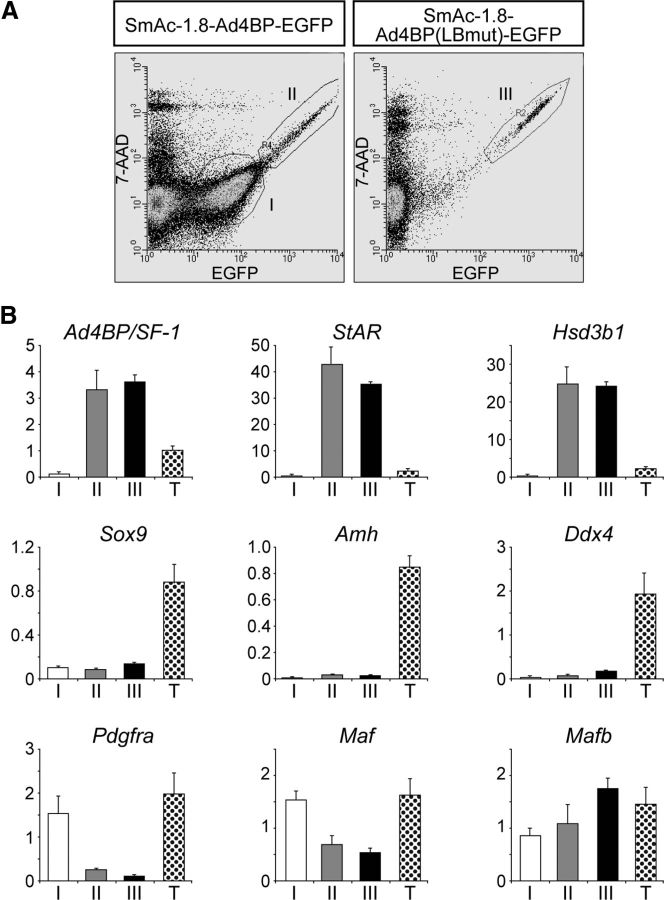
The testicular cells were prepared from the E18.5 testes of the two transgenic mouse lines carrying SmAc-1.8-Ad4BP-EGFP or SmAc-1.8-Ad4BP(LBmut)-EGFP and subjected to FACS. As indicated by II and III in Fig. 4A, cell populations with higher expression of EGFP were observed to a similar extent in both transgenic lines. In addition, many cells expressing EGFP at lower levels were observed only in the transgenic mice carrying SmAc-1.8-Ad4BP-EGFP (indicated by I in Fig. 4A). These weakly EGFP-positive cells could not be clearly detected by immunofluorescence analyses (Fig. 3C), probably because the amount of EGFP expressed in fraction I was 10 times less than that in fractions II and III.
Fig. 4.
Characterization of weakly and strongly EGFP-positive cells prepared from transgenic mouse testes. A, The testicular cells (E18.5) from the transgenic mice carrying SmAc-1.8-Ad4BP-EGFP (left panel) and SmAc-1.8-Ad4BP(LBmut)-EGFP (right panel) were subjected to FACS after staining with 7-amino-actinomycin D. Fractions I and II contain weakly and strongly EGFP-positive cells, respectively, from SmAc-1.8-Ad4BP-EGFP transgenic mice, whereas fraction III contains strongly EGFP-positive cells from SmAc-1.8-Ad4BP(LBmut)-EGFP transgenic mice. B, Expression of gonadal cell marker genes in fractions I, II, and III was examined using quantitative RT-PCR. Total RNA was prepared from cell fractions I, II, III, and whole testes (T) and was subjected to quantitative RT-PCR for the genes indicated. The amount of mRNA relative to Actb (encoding β-actin) is plotted. Values represent means ± sd.
Total RNAs prepared from the three cell fractions and whole testes (E18.5) were subjected to quantitative RT-PCR analyses. As shown in Fig. 4B, Ad4BP/SF-1 mRNA was enriched in fractions II and III, but not in fraction I. Likewise, Leydig cell markers such as Star and Hsd3b1 were highly expressed in the fractions II and III, but not in fraction I. Sertoli cell markers such as Sox9 (SRY-box containing gene 9) and Amh (anti-Mullerian hormone) were not enriched in any of the three populations, whereas their expressions were clearly detectable in the whole testis RNA. The germ cell marker, homolog of a DEAD (Asp-Glu-Ala-Asp) family gene (Ddx4, VASA), was mostly undetectable in the three fractions. Taken together, these marker gene expressions indicated that the cells in fractions II and III were predominantly comprised of fetal Leydig cells and that fraction I was comprised of cells other than Leydig, Sertoli, and germ cells.
To characterize fraction I, the expression of the Pdgfra gene, which encodes for platelet-derived growth factor receptor (PDGFR)-α was examined. Previous studies have indicated that PDGFRα is expressed in the interstitial cells of human (35) and mouse fetal testes (36). Our study suggests that Pdgfra is not expressed in fetal Leydig cells but is expressed in fraction I, possibly in interstitial cells other than Leydig cells (Fig. 4B). A previous study has reported that avian oncogene homolog (Maf), also known as c-Maf, and Mafb are expressed in a distinct progenitor population of fetal Leydig cells, and Mafb is expressed also in a part of fetal Leydig cells (37). Consistent with this previous study, Maf was more abundantly expressed in fraction I than in fetal Leydig cells, whereas Mafb was higher in the fetal Leydig cells than in fraction I.
Characterization of fetal Leydig cells
The generation of transgenic mice in the present study made it possible for the first time to prepare fetal Leydig cells with high purity. We therefore compared gene expression in fetal Leydig cells with that the equivalents in adult Leydig and fetal Sertoli cells. Quantitative RT-PCR studies have demonstrated that Ad4BP/SF-1 is strongly expressed in fetal and adult Leydig cells and is weakly expressed in fetal Sertoli cells. StAR, Cyp11a1, Hsd3b1, Cyp17a1, all of which are known as markers of both fetal and adult Leydig cells, are not expressed in fetal Sertoli cells (Fig. 5A). Among these markers, we noted that the expression of Cyp17a1 in fetal Leydig cells was approximately 20% of that in adult Leydig cells. The expression of the Sertoli cell markers, Sox9 and Amh, was higher in Sertoli cells than in fetal and adult Leydig cells. Hsd3b6 is known to be a marker of adult but not fetal Leydig cells (38), and the expression patterns reflected this as expected.
Fig. 5.
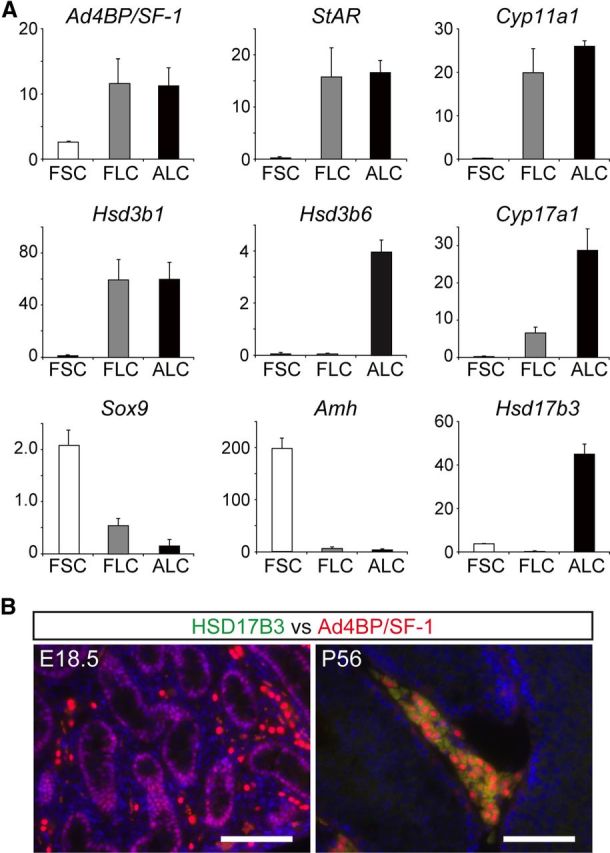
Differential gene expression among fetal Leydig, adult Leydig, and fetal Sertoli cells. A, Fetal Sertoli cells (FSCs) and fetal Leydig cells (FLCs) were isolated from E18.5 testes and adult Leydig cells (ALCs) were prepared from 9-wk-old male mice. Total RNA was prepared from each cell type and subjected to quantitative RT-PCR for the genes indicated. The amount of mRNA relative to Actb (encoding β-actin) is plotted. Values are the means ± sd. B, The expression of HSD17B3 was examined in the fetal and adult testes. Sections of fetal (E18.5) and adult testes (P56) were used for immunohistochemical staining using rat monoclonal antibody for mouse HSD17B3. Bars, 100 μm.
The Hsd17b3 gene encodes the enzyme HSD17B3, which mediates the conversion of androstenedione (4-androstene-3,17-dione) to testosterone and which is highly expressed in adult Leydig cells (38). However, its gene expression in the fetal testis has been controversial. In situ hybridization in mouse unexpectedly revealed that Hsd17b3 is expressed in cells within the testicular cords (possibly Sertoli cells) but not in the interstitial cells (24). In contrast, Hsd17b3 expression was recently detected in the fetal Leydig cells of neonatal rats (25). The present study has clearly demonstrated that Hsd17b3 is not expressed in mouse fetal Leydig cells, but expressed in fetal Sertoli cells, although the amount of mRNA was approximately 10% of that in the adult Leydig cells. This expression was further examined at the protein level using an antibody against HSD17B3. As shown in Fig. 5B, immunohistochemical analyses detected HSD17B3 in the adult Leydig cells. However, possibly due to its significantly low expression, HSD17B3 was not detected in the fetal Sertoli cells.
Functional interplay between fetal Leydig and Sertoli cells in testosterone production
The fetal testes can synthesize testosterone. In light of the results above, we can assume that fetal Sertoli cells as well as fetal Leydig cells are implicated in testosterone synthesis in the fetal testis (Fig. 6A), yet this ability is considerably weak in the fetal testis. To confirm this, fetal Leydig, fetal Sertoli, and adult Leydig cells were incubated with [7-3H]-pregnenolone (3β-hydroxypregn-5-en-20-one). As shown in Fig. 6B, the adult Leydig cells converted pregnenolone to progesterone (pregn-4-ene-3,20-dione), androstenedione, and testosterone. In contrast, the fetal Leydig cells produced progesterone, androstenedione, and 17α-hydroxyprogesterone (17-hydroxypregn-4-ene-3,20-dione) but not testosterone. This result appeared to be consistent with the absence of HSD17B3 in fetal Leydig cells. Moreover, the conversion from progesterone to androstenedione mediated by CYP17A1 in the fetal Leydig cells was considerably lower than that in the adult Leydig cells, probably corresponding to the expression level of CYP17A1 in the fetal Leydig cells. Testosterone production from [1-3H]-androstenedione was examined using fetal Leydig cells and fetal Sertoli cells. As expected, the fetal Leydig cells did not yield any product, whereas the fetal Sertoli cells converted the substrate to testosterone (Fig. 6B). Finally, we examined whether testosterone is produced when fetal Leydig cells are cocultured with fetal Sertoli cells. Although in small quantities, testosterone production was observed as expected (Fig. 6B).
Fig. 6.
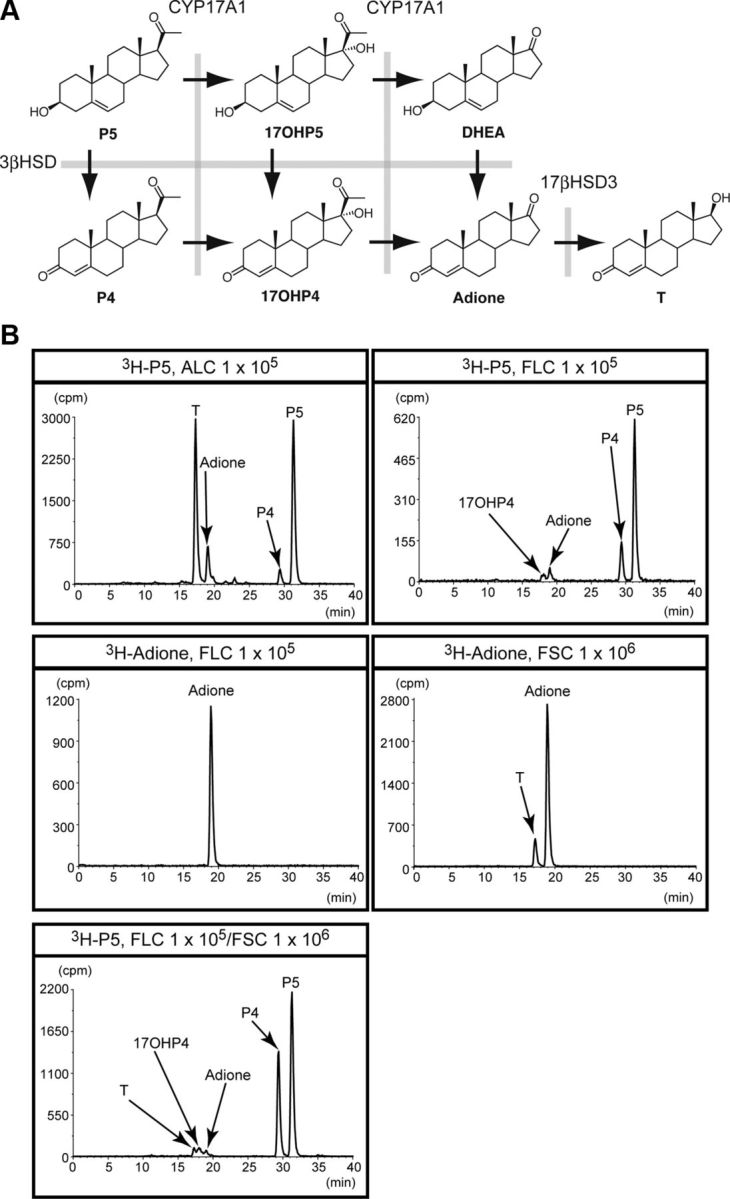
Steroidogenic activities of fetal Leydig, adult Leydig, and fetal Sertoli cells. A, Synthetic pathway from pregnenolone to testosterone is shown. 3β-HSD, CYP17A1, and 17β-hydroxysteroid dehydrogenase type III (17βHSD3) are involved in the pathway. T, Testosterone; Adione, androstenedione; P4, progesterone; 17OHP4, 17α-hydroxyprogesterone; P5, pregnenolone; DHEA, dehydroepiandrosterone ((3S,8R,9S,10R,13S,14S)-3-hydroxy-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one). B, Indicated numbers of fetal Leydig (FLCs), adult Leydig (ALCs), and fetal Sertoli cells (FSCs) were incubated in the presence of 3H-pregnenolone (3H-P5) or 3H-androstenedione (3H-Adione). Steroids extracted from whole medium and cells were subjected to HPLC analysis. Arrows show the peaks of the steroids. The study was performed three times with different cell preparations, and representative HPLC chromatograms are indicated. The horizontal line indicates elution time (minutes), whereas the vertical line indicates 3H count.
Discussion
Establishment of transgenic mice with EGFP-labeled fetal Leydig cells
We previously identified a fetal Leydig cell-specific enhancer in the upstream region of the Ad4BP/SF-1 gene (22). This enhancer clearly induced EGFP expression in the fetal Leydig cells. However, in the present study, we found that EGFP is expressed in the sexually indifferent gonad (E10.5) before the differentiation of fetal Leydig cells. Based on this unexpected expression, we hypothesized that EGFP was induced by the regulatory elements in the promoter region of Ad4BP/SF-1 because the promoter region was shown to induce lacZ (14) and Cre (39) expression in the sexually indifferent gonad. Indeed, multiple WT1-binding sites and an LHX9-binding site are localized in the region (14). Among these sites, we noticed that LHX9 seems to be expressed in the undifferentiated somatic cell population of the fetal gonads (34, 40) and hence hypothesized that the LHX9-binding site might contribute to induce reporter gene expression in the undifferentiated interstitial cells. Therefore, we established another transgenic mouse line with a construct carrying a mutation in the LHX9-binding site [SmAc-1.8-Ad4BP(LBmut)-EGFP]. Expectedly, EGFP expression in the indifferent gonad and interstitial cells disappeared, except in fetal Leydig cells.
Characterization of EGFP-labeled cells
As mentioned above, we established two lines of transgenic mice using a wild-type promoter or a mutated promoter at the LHX9-binding site. We found that the fetal testes from the transgenic mice with the wild-type promoter contained both weakly and strongly EGFP-positive cell populations. Evaluation of marker gene expression revealed that the strongly positive cells corresponded to fetal Leydig cells, whereas the weakly positive cells likely correspond to interstitial cells (excluding fetal Leydig cells).
Fetal Leydig cells increase in number throughout the fetal stage. Nevertheless, it has been experimentally shown that they scarcely proliferate (41–43). Therefore, it has been suggested that progenitor cells for fetal Leydig cells are already present in the interstitial space. With this proposition in mind, it is particularly intriguing that Pdgfra is expressed exclusively in the weakly EGFP-positive cells. In situ hybridization signals of Pdgfra distribute in the interstitial space (36), and thus, it can be concluded that Pdgfra is expressed in interstitial cells except for fetal Leydig cells. Moreover, gene disruption of Pdgfra led to a decrease in fetal Leydig cells (36), and this phenotype raised the possibility that the PDGFRα-positive cells contain precursor cells for fetal Leydig cells. Further study of the weak-EGFP cell population isolated here would give us clues to understand the mechanisms underlying fetal Leydig cell differentiation.
Steroid synthesis in fetal testis
Both fetal and adult testes synthesize testosterone to induce virilization and maintain male reproductive functions. Genes required for testosterone synthesis are all expressed in the adult Leydig cells, and thus, the population can independently synthesize testosterone. In the fetal stage, however, it is controversial whether fetal Leydig cells produce testosterone by themselves or not. In situ hybridization revealed that the Hsd17b3 gene encoding HSD17B3 is expressed within the testicular cords in mice, and consistent with this observation, interstitial cells isolated with fine forceps from the testes in the early postnatal stage could not produce testosterone (24). In contrast, a study with rat fetal Leydig cells isolated from testes on postnatal day (P) 8 by FACS using an anti-LH receptor (LHR) antibody showed that these cells express HSD17B3 and are capable of producing testosterone (25). This discrepancy raised two possibilities; one is that the cell population used in these previous studies contained cells other than fetal Leydig cells. Data obtained from contaminated cells might have led the two groups of authors to contradictory conclusions. An alternative possibility is that the rat, but not mouse fetal Leydig cells actually express HSD17B3 and produce testosterone at P8. If so, the functions of the fetal Leydig cells might be completely different between mouse and rat, or the fetal Leydig cells might change their characteristics during early postnatal development.
We examined steroidogenic activity of the fetal Leydig cells purified from the transgenic mice generated in our study. We found that fetal Leydig cells can produce androstenedione but not testosterone due to lack of HSD17B3 expression. In addition, androstenedione was converted to testosterone in the fetal Sertoli cells, in which HSD17B3 is expressed. However, the amount of testosterone synthesized by coculturing of the fetal Leydig cells with fetal Sertoli cells was much less than that synthesized by the adult Leydig cells. This is easily attributable to the significantly low expression levels of HSD17B3 in fetal Sertoli cells and of CYP17A1 in fetal Leydig cells. At the same time, these results may suggest that the mouse fetal testis synthesizes predominantly androstenedione rather than testosterone. However, Mahendroo et al. (44) demonstrated that the cultured mouse whole fetal testis synthesizes more testosterone than androstenedione. This higher activity of testosterone synthesis in whole testes than cultured cells is possibly due to more effective transfer of androstenedione from Leydig cells to Sertoli cells in the tissue than in the coculture of the cells.
As an alternative pathway for androgen production, the backdoor pathway has been studied in vertebrate animal species (2, 45), and recently it was clearly demonstrated that this pathway is essential for masculinization of human fetuses (46). Therefore, we examined whether the backdoor pathway is active in the fetal Leydig and Sertoli cells. However, we failed to detect any intermediary metabolites of the backdoor pathway in cell culture assays, strongly suggesting that the backdoor pathway is not active in mouse fetal testes. Showing a good correlation with our results, Mahendroo et al. (44) also demonstrated that the backdoor pathway is not active in mouse fetal testes. Taken together, it seems possible to conclude that the backdoor pathway is not activated in mouse fetal testes.
Fetal Leydig cells are thought to play a crucial role in male development. However, because of the difficulty in preparing pure fetal Leydig cells from fetal testes, the nature of their contribution remained to be elucidated. In the present study, we generated transgenic mouse lines in which fetal Leydig cells were labeled specifically with EGFP and thereby made it possible to collect fetal Leydig cells of high purity as well as undifferentiated interstitial cells. Moreover, the DNA construct carrying the fetal Leydig-specific enhancer coupled with a mutated promoter enables us to express any gene in a cell-specific manner. The experimental approach established in our study can be used as a powerful tool in the study of fetal Leydig cells from developmental, cell-biological, or endocrinological standpoints.
Acknowledgments
This work was supported by Grants 22132002, 23116707, and 23590339 from the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT)/Japan Society for the Promotion of Science (JSPS) KAKENHI.
Present address for T.A.: Graduate School of Frontier Biosciences, Osaka University, 2-2 Yamada-oka, Suita, Osaka 565-0871, Japan.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- CYP17A1
- 17α-Hydroxylase/17,20-lyase P450
- E
- embryonic day
- EGFP
- enhanced green fluorescence protein
- FACS
- fluorescence-activated cell sorting
- 3β-HSD
- 3β-hydroxysteroid dehydrogenase
- HSD17B3
- 17β-hydroxysteroid dehydrogenase type III
- LHR
- LH receptor
- LHX9
- LIM homeobox protein 9
- P
- postnatal day
- PDGFR
- platelet-derived growth factor receptor
- SOX9
- SRY (sex determining region Y)-box 9
- StAR
- steroidogenic acute regulatory protein
- WT1
- Wilms' tumor-1 protein.
References
- 1. Miller WL , Auchus RJ. 2011. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32:81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scott HM , Mason JI , Sharpe RM. 2009. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev 30:883–925 [DOI] [PubMed] [Google Scholar]
- 3. Habert R , Lejeune H , Saez JM. 2001. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol 179:47–74 [DOI] [PubMed] [Google Scholar]
- 4. Griswold SL , Behringer RR. 2009. Fetal Leydig cell origin and development. Sex Dev 3:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Shaughnessy PJ , Baker PJ , Johnston H. 2006. The foetal Leydig cell—differentiation, function and regulation. Int J Androl 29:90–95, discussion 105–108 [DOI] [PubMed] [Google Scholar]
- 6. Luo X , Ikeda Y , Schlosser DA , Parker KL. 1995. Steroidogenic factor 1 is the essential transcript of the mouse Ftz-F1 gene. Mol Endocrinol 9:1233–1239 [DOI] [PubMed] [Google Scholar]
- 7. Morohashi K , Honda S , Inomata Y , Handa H , Omura T. 1992. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem 267:17913–17919 [PubMed] [Google Scholar]
- 8. Morohashi K. 1997. The ontogenesis of the steroidogenic tissues. Genes Cells 2:95–106 [DOI] [PubMed] [Google Scholar]
- 9. Parker KL , Schimmer BP. 1997. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev 18:361–377 [DOI] [PubMed] [Google Scholar]
- 10. Val P , Lefrançois-Martinez AM , Veyssière G , Martinez A. 2003. SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo X , Ikeda Y , Parker KL. 1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- 12. Shinoda K , Lei H , Yoshii H , Nomura M , Nagano M , Shiba H , Sasaki H , Osawa Y , Ninomiya Y , Niwa O , Morohashi K , Li E. 1995. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn 204:22–29 [DOI] [PubMed] [Google Scholar]
- 13. Shen JH , Ingraham HA. 2002. Regulation of the orphan nuclear receptor steroidogenic factor 1 by Sox proteins. Mol Endocrinol 16:529–540 [DOI] [PubMed] [Google Scholar]
- 14. Wilhelm D , Englert C. 2002. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev 16:1839–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woodson KG , Crawford PA , Sadovsky Y , Milbrandt J. 1997. Characterization of the promoter of SF-1, an orphan nuclear receptor required for adrenal and gonadal development. Mol Endocrinol 11:117–126 [DOI] [PubMed] [Google Scholar]
- 16. Nomura M , Bärtsch S , Nawata H , Omura T , Morohashi K. 1995. An E box element is required for the expression of the ad4bp gene, a mammalian homologue of ftz-f1 gene, which is essential for adrenal and gonadal development. J Biol Chem 270:7453–7461 [DOI] [PubMed] [Google Scholar]
- 17. Daggett MA , Rice DA , Heckert LL. 2000. Expression of steroidogenic factor 1 in the testis requires an E box and CCAAT box in its promoter proximal region. Biol Reprod 62:670–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris AN , Mellon PL. 1998. The basic helix-loop-helix, leucine zipper transcription factor, USF (upstream stimulatory factor), is a key regulator of SF-1 (steroidogenic factor-1) gene expression in pituitary gonadotrope and steroidogenic cells. Mol Endocrinol 12:714–726 [DOI] [PubMed] [Google Scholar]
- 19. Zubair M , Ishihara S , Oka S , Okumura K , Morohashi K. 2006. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol 26:4111–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shima Y , Zubair M , Komatsu T , Oka S , Yokoyama C , Tachibana T , Hjalt TA , Drouin J , Morohashi K. 2008. Pituitary homeobox 2 regulates adrenal4 binding protein/steroidogenic factor-1 gene transcription in the pituitary gonadotrope through interaction with the intronic enhancer. Mol Endocrinol 22:1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shima Y , Zubair M , Ishihara S , Shinohara Y , Oka S , Kimura S , Okamoto S , Minokoshi Y , Suita S , Morohashi K. 2005. Ventromedial hypothalamic nucleus-specific enhancer of Ad4BP/SF-1 gene. Mol Endocrinol 19:2812–2823 [DOI] [PubMed] [Google Scholar]
- 22. Shima Y , Miyabayashi K , Baba T , Otake H , Oka S , Zubair M , Morohashi K. 2012. Identification of an enhancer in the Ad4BP/SF-1 gene specific for fetal Leydig cells. Endocrinology 153:417–425 [DOI] [PubMed] [Google Scholar]
- 23. O'Shaughnessy PJ , Fowler PA. 2011. Endocrinology of the mammalian fetal testis. Reproduction 141:37–46 [DOI] [PubMed] [Google Scholar]
- 24. O'Shaughnessy PJ , Baker PJ , Heikkilä M , Vainio S , McMahon AP. 2000. Localization of 17β-hydroxysteroid dehydrogenase/17-ketosteroid reductase isoform expression in the developing mouse testis—androstenedione is the major androgen secreted by fetal/neonatal Leydig cells. Endocrinology 141:2631–2637 [DOI] [PubMed] [Google Scholar]
- 25. Weisser J , Landreh L , Söder O , Svechnikov K. 2011. Steroidogenesis and steroidogenic gene expression in postnatal fetal rat Leydig cells. Mol Cell Endocrinol 341:18–24 [DOI] [PubMed] [Google Scholar]
- 26. Hogan B , Beddington F , Lacy E. 1994. Manipulating the mouse embryo. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- 27. Morohashi K , Zanger UM , Honda S , Hara M , Waterman MR , Omura T. 1993. Activation of CYP11A and CYP11B gene promoters by the steroidogenic cell-specific transcription factor, Ad4BP. Mol Endocrinol 7:1196–1204 [DOI] [PubMed] [Google Scholar]
- 28. Fatchiyah , Zubair M , Shima Y , Oka S , Ishihara S , Fukui-Katoh Y , Morohashi K. 2006. Differential gene dosage effects of Ad4BP/SF-1 on target tissue development. Biochem Biophys Res Commun 341:1036–1045 [DOI] [PubMed] [Google Scholar]
- 29. Yokoyama C , Komatsu T , Ogawa H , Morohashi K , Azuma M , Tachibana T. 2009. Generation of rat monoclonal antibodies specific for Ad4BP/SF-1. Hybridoma (Larchmt) 28:113–119 [DOI] [PubMed] [Google Scholar]
- 30. Nel-Themaat L , Vadakkan TJ , Wang Y , Dickinson ME , Akiyama H , Behringer RR. 2009. Morphometric analysis of testis cord formation in Sox9-EGFP mice. Dev Dyn 238:1100–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Niedziela M , Lerchl A. 1999. Isolation method of Leydig cells from mature male Djungarian hamsters (Phodopus sungorus) and their steroidogenic activity in vitro. Andrologia 31:157–161 [PubMed] [Google Scholar]
- 32. Matsunaga M , Ukena K , Baulieu EE , Tsutsui K. 2004. 7α-Hydroxypregnenolone acts as a neuronal activator to stimulate locomotor activity of breeding newts by means of the dopaminergic system. Proc Natl Acad Sci USA 101:17282–17287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Doi M , Takahashi Y , Komatsu R , Yamazaki F , Yamada H , Haraguchi S , Emoto N , Okuno Y , Tsujimoto G , Kanematsu A , Ogawa O , Todo T , Tsutsui K , van der Horst GT , Okamura H. 2010. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med 16:67–74 [DOI] [PubMed] [Google Scholar]
- 34. Birk OS , Casiano DE , Wassif CA , Cogliati T , Zhao L , Zhao Y , Grinberg A , Huang S , Kreidberg JA , Parker KL , Porter FD , Westphal H. 2000. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature 403:909–913 [DOI] [PubMed] [Google Scholar]
- 35. Basciani S , Mariani S , Arizzi M , Ulisse S , Rucci N , Jannini EA , Della Rocca C , Manicone A , Carani C , Spera G , Gnessi L. 2002. Expression of platelet-derived growth factor-A (PDGF-A), PDGF-B, and PDGF receptor-α and -β during human testicular development and disease. J Clin Endocrinol Metab 87:2310–2319 [DOI] [PubMed] [Google Scholar]
- 36. Brennan J , Tilmann C , Capel B. 2003. Pdgfr-α mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev 17:800–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DeFalco T , Takahashi S , Capel B. 2011. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol 352:14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Shaughnessy PJ , Willerton L , Baker PJ. 2002. Changes in Leydig cell gene expression during development in the mouse. Biol Reprod 66:966–975 [DOI] [PubMed] [Google Scholar]
- 39. Chaboissier MC , Kobayashi A , Vidal VI , Lützkendorf S , van de Kant HJ , Wegner M , de Rooij DG , Behringer RR , Schedl A. 2004. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 131:1891–1901 [DOI] [PubMed] [Google Scholar]
- 40. Tang H , Brennan J , Karl J , Hamada Y , Raetzman L , Capel B. 2008. Notch signaling maintains Leydig progenitor cells in the mouse testis. Development 135:3745–3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Byskov AG. 1986. Differentiation of mammalian embryonic gonad. Physiological Rev 66:71–117 [DOI] [PubMed] [Google Scholar]
- 42. Kerr JB , Risbridger GP , Knell CM. 1988. Stimulation of interstitial cell growth after selective destruction of foetal Leydig cells in the testis of postnatal rats. Cell Tissue Res 252:89–98 [DOI] [PubMed] [Google Scholar]
- 43. Orth JM. 1982. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec 203:485–492 [DOI] [PubMed] [Google Scholar]
- 44. Mahendroo M , Wilson JD , Richardson JA , Auchus RJ. 2004. Steroid 5α-reductase 1 promotes 5α-androstane-3α,17β-diol synthesis in immature mouse testes by two pathways. Mol Cell Endocrinol 222:113–120 [DOI] [PubMed] [Google Scholar]
- 45. Auchus RJ. 2004. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab 15:432–438 [DOI] [PubMed] [Google Scholar]
- 46. Flück CE , Meyer-Böni M , Pandey AV , Kempná P , Miller WL , Schoenle EJ , Biason-Lauber A. 2011. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am J Hum Genet 89:201–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Katoh K , Misawa K , Kuma K , Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Katoh K , Toh H. 2008. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9:286–298 [DOI] [PubMed] [Google Scholar]