Abstract
Sertoli cell (SC) androgen receptor (AR) activity is vital for spermatogenesis. We created a unique gain-of-function transgenic (Tg) mouse model to determine the temporal role of SCAR expression in testicular development. The SC-specific rat Abpa promoter directed human Tg AR [Tg SC-specific AR (TgSCAR)] expression, providing strong premature postnatal AR immunolocalized to SC nuclei. Independent Tg lines revealed that TgSCAR dose dependently reduced postnatal and mature testis size (to 60% normal), whereas androgen-dependent mature seminal vesicle weights and serum testosterone levels remained normal. Total SC numbers were reduced in developing and mature TgSCAR testes, despite normal or higher Fshr mRNA and circulating FSH levels. Postnatal TgSCAR testes exhibited elevated levels of AR-regulated Rhox5 and Spinlw1 transcripts, and precocious SC function was demonstrated by early seminiferous tubular lumen formation and up-regulated expression of crucial SC tight-junction (Cldn11 and Tjp1) and phagocytic (Elmo1) transcripts. Early postnatal Amh expression was elevated but declined to normal levels in peripubertal-pubertal TgSCAR vs. control testes, indicating differential age-related regulation featuring AR-independent Amh down-regulation. TgSCAR induced premature postnatal spermatogenic development, shown by increased levels of meiotic (Dmc1 and Spo11) and postmeiotic (Capza3 and Prm1) germ cell transcripts, elevated meiotic-postmeiotic germ:Sertoli cell ratios, and accelerated spermatid development. Meiotic germ:Sertoli cell ratios were further increased in adult TgSCAR mice, indicating predominant SCAR-mediated control of meiotic development. However, postmeiotic germ:Sertoli cell ratios declined below normal. Our unique TgSCAR paradigm reveals that atypical SC-specific temporal AR expression provides a direct molecular mechanism for induction of precocious testicular development, leading to reduced adult testis size and decreased postmeiotic development.
Androgen actions are essential for the initial completion and then maintenance of spermatogenesis. Androgen-deficiency or loss of functional androgen receptor (AR) causes spermatogenic arrest and infertility (1–3). However, male germ cells in human and rodent postnatal testes lack functional AR expression (4–6), and mouse AR-deficient germ cells develop normally (7, 8), demonstrating the functional importance of the local AR-expressing somatic Leydig cell, peritubular cell, and Sertoli cell (SC) populations. SCs play a crucial role in fetal gonadal development and postnatal spermatogenesis, and total testicular SC number is considered a major determinant of final germ cell numbers found in mature testes (9). SCs display distinctive dynamic changes in AR expression levels during postnatal development, and between spermatogenic stages in mature testes, unlike constant AR expression in developing and adult Leydig and peritubular cell types. SCAR expression is absent in fetal-neonatal testes and becomes progressively stronger in SC nuclei (by immunodetection) during postnatal/peripubertal development in rodent (10–12), primate (13), and human (14) testes. Adult rodent (5, 6) and human (15) SCs display nuclear-specific AR expression patterns that vary prominently according to different stages of the associated seminiferous epithelium cycle. Therefore, AR expression itself suggests that acquisition of SC androgen sensitivity occurs in a carefully orchestrated manner in defined stages of postnatal and adult spermatogenic development.
Mouse models with SC-specific genetic disruption of AR function (SCAR knock-out, SCARKO) have demonstrated a vital role for SCAR in postmeiotic germ cell development (3, 16–18). Total testicular SC numbers remained normal in these loss-of-function models (12, 17), suggesting that the size of the SC population is independent of local SCAR action. However, dissecting the steroidal control of Sertoli and germ cell development may be complicated by overlapping and interconnected androgen and estradiol actions during testis development (19–23), which may confer compensatory pathways and levels of functional redundancy that impact interpretation of loss-of-function SCARKO approaches. Gain-of-function models provide an important alternative strategy to identify the hormonal regulation of SC and testicular development. For instance, selective steroidal delivery (20, 21, 24) or rescue of FSH actions by recombinant (25) or transgenic (Tg) FSH (26, 27) showed a major role for FSH, not androgen, in dictating SC postnatal proliferation and final numbers in the rodent. Unexpectedly, genetic disruption of the murine FSH receptor appears to have no effect on postnatal (<20 d old) SC numbers (28). The discrepancy in SC effects between gain vs. loss of function models may reflect a limitation of loss-of-function approaches, which are vulnerable to confounding effects of functional redundancy found in key developmental processes. On the other hand, gain-of-function approaches require careful consideration of the specific cellular sites of hormone receptor action, as well as hormonal specificity, such as in vivo aromatization of administered testosterone to estradiol in steroid replacement studies.
To directly determine the biological importance of the temporal onset of SC androgen actions during Sertoli and germ cell development and function in vivo, and to avoid issues of cellular or hormonal specificity and functional redundancy, we used a Tg strategy to selectively direct premature SCAR expression in mice. Our unique Tg SC-specific AR (TgSCAR) model used the rat androgen-binding protein (Abpa) gene promoter that directs SC expression of ABP (29) and other different transgenes (17, 30) and was predicted to confer early androgen sensitivity in developing SCs. Our current findings reveal that atypical Sertoli-specific temporal AR expression provides a direct molecular mechanism for induction of precocious testicular development and that the onset and level of local SCAR expression is pivotal for optimal testicular development and spermatogenesis.
Materials and Methods
Preparation of TgSCAR mice
Abpa.AR transgene
The human AR cDNA (AR) sequence in a 4.2-kb XhoI-BamHI fragment from pAR-IRES-EGFP, a gift from J. Issacs (31), was cloned into XhoI-BamHI sites of pBSSKII (Stratagene, La Jolla, CA), then recovered in a XhoI-NotI fragment and subcloned into XhoI-NotI sites of pBK-RSV (Stratagene). The 1.5-kb rat Abpa promoter was transferred as a XhoI-SalI fragment into the XhoI site of a pBK-RSV-AR clone. The full Abpa.AR transgene was confirmed by automated Dye-Deoxy DNA sequencing.
TgSCAR mice
Linearized Abpa.AR DNA was isolated by agarose gel electrophoresis after XhoI and MluI digestion to remove vector sequences, then microinjected [2 ng/μl in 10 mm Tris-HCl (pH 7.4) and 0.1 mm EDTA] as described (17) into fertilized oocytes from superovulated B6D2 F1 females mated with B6D2 F1 males at the ANZAC Research Institute. Tg Abpa.AR founders and offspring were identified by PCR analysis of genomic tail DNA using primers to Abpa (5′-GCATGGTCAGGGTCAGTGTC) and AR (5′-TTCCCTCTGTCGCCTCCTCT) sequences to amplify the junction between the Abpa promoter and human AR sequences. Hemizygous TgSCAR mice were used for breeding and experimental analysis. Animals were housed under controlled conditions (12-h light, 12-h dark cycle, 19–22 C) with ad libitum access to food and water. All animal procedures were approved by the Animal Welfare Committee of the Sydney South West Area Health Service and performed in accordance to the National Health and Medical Research Council code of practice for care and use of animals and the New South Wales Animal Research Act (1985).
Experimental animals, serum, and tissue collection
Day of birth was designated as postnatal day (PND)0, and Tg and wild-type (WT) littermates were killed on PND5, PND10, PND15, PND20, and PND30 to study testis development or collected at 70–80 d old to compare adult phenotypes. Serum was collected from ketamine/xylazine-anesthetized PND15 to adult animals by terminal cardiac exsanguination and aliquots stored at −20 C. Testes were removed and immediately frozen (liquid N2) for RNA analysis or fixed and incubated in Bouin's fixative for 4 h (immunohistochemistry) or 24 h (histology, stereology) and transferred to 70% ethanol. Control testes and serum were obtained from age- and strain-matched littermates.
Histology and stereology
Fixed testes were embedded in hydroxymethylmethacrylate resin (Technovit 7100; Kulzer and Co., Friedrichsdorf, Germany) as described for histology and stereology or paraffin embedded for immunohistochemistry (17). Tissue sections were cut using a Polycut S microtome (Reichert Jung, Nossloch, Germany). Thin sections (5–10 μm) were stained for histology using 0.5% toluidine blue or consecutively stained with periodic acid-Schiff, hematoxylin, and Scott's blue solution; thick sections (20 μm) for stereology were consecutively stained with periodic acid-Schiff, hematoxylin, and Scott's blue solution. Total testis Sertoli and germ cell populations were quantified by optical-disector stereology as described (27) using CASTGRID (Olympus, Aarhus, Denmark) software. Using light microscopy, average seminiferous tubular diameter was determined by measuring 30 round-shaped tubular cross-sections/testis and the percentage of PND15 tubular cross-sections with lumen formation determined by scoring 100 tubular cross-sections/testis.
Enriched SC, Leydig cell, and peritubular cell popluations
Testes were excised from TgSCAR and WT PND12 mice (n = 3/group) and cells isolated by minor modifications to previously described methods (31). Briefly, testes were decapsulated, minced, and placed in Hanks' balanced salt solution (BSS) containing 20 μg/ml deoxyribonuclease (DNase) and 2.5 mg/ml trypsin, at 34 C for 25 min in a shaking water bath (100 cycles/min). After treatment with 4.2 mg/ml soybean trypsin inhibitor (Sigma, St. Louis, MO), digested samples were filtered (2-mm mesh), then settled under gravity, forming supernatant enriched in Leydig cells and sediment enriched in tubule fragments. Leydig cell-enriched supernatants were collected, centrifuged, and cell pellets washed three times with BSS, then stored at −80 C. Tubule-enriched sediments were treated with 1 mg/ml collagenase type I (Sigma) at 34 C for 25 min in a water bath (100 cycles/min), then allowed to settle and supernatants enriched in peritubular cells separated from sediments enriched in tubule fragments containing SCs. Peritubular cell-enriched supernatants were centrifuged and pellets washed three times then stored at −80 C. Tubule fragments were resuspended in 2 ml of BSS and disrupted further by trituration using a Pasteur pipette, then filtered (100-μm mesh), and SC-enriched filtrates centrifuged and pellets washed three times then stored at −80 C.
Hormone assays
Mouse serum FSH levels were measured by immunofluorometric assay as described previously (32). Serum testosterone was measured after hexane-ethylacetate extraction using liquid chromatography tandem mass spectrometry (0.1 ng/ml quantification limit) as described for mouse samples (33). All samples were measured in duplicate in a single batch.
Immunodetection of AR
Immunofluorescent detection of AR was performed on PND5 testes sections (5-μm thick). Briefly, sections were deparaffinized, rehydrated, and treated with 2% H2O2 (10 min). Microwave-induced antigen retrieval was performed in 10 mm citrate buffer (pH 6). Testes sections were incubated for 1 h with anti-AR antibody (N-20, sc-816; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:100 in 1% BSA in PBS. After three 5-min washes with PBS, sections were incubated for 30 min with biotinylated goat antirabbit IgG (1:400, Vectastain-Elite ABC kit; Vector Laboratories, Burlingame, CA), followed by three 5-min washes with PBS, then incubated with horseradish peroxidase-conjugate (Invitrogen, Carlsbad, CA) as instructed for 60 min. After two 5-min washes with PBS, sections were incubated with Alexa Fluor 488 tyramide conjugate in PBS (TSA kit; Invitrogen) for 10 min, then rinsed and incubated with 4′,6-diamidino-2-phenylindole (DAPI) (1:100; Sigma). Sections were mounted with ProLong Gold antifade (Invitrogen) reagent and signal detected by confocal microscopy (FV1000; Olympus). Immunochemical detection of AR was performed in adult testes sections as described above, except signal was visualized with Vectastain-Elite ABC kit as recommended (Vector Laboratories) using 3,3′-diaminobenzidine tetrahydrochloride chromogenic substrate (Dako, Carpinteria, CA), counterstained with Harris's hematoxylin.
RNA extraction, cDNA synthesis, and quantitative real-time PCR (qPCR)
Total RNA was extracted from whole testes using TRIzol (Sigma) according to manufacturer's protocol and residual genomic DNA removed by ribonuclease-free DNase I (0.5 U/μg RNA; Invitrogen). cDNA was obtained using oligo-dT primers and reverse transcriptase (Superscript III; Invitrogen) as recommended. qPCR was performed using a Rotor-Gene 6000 (Corbett Research, Sydney, Australia) and SensiMix SYBR kit (Bioline, Alexandria, Australia) according to manufacturer. Target transcript primer sequences and relevant accession numbers are listed in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org. Amplicon products were assessed by melting curve analysis (Rotor-Gene 6000 software), and all reaction efficiencies were equivalent (95–100%). Relative target and internal control transcript copy number was quantitated by qPCR using the standard curve method (undiluted DNA standard assigned arbitrary unit), then target normalized to expression of two internal sample controls, Hmbs and Hprt1, using GeNorm software analysis (34), which verified Hmbs and Hprt1 as stable housekeepers. ActB and Rps9 were also tested as internal controls by GeNorm Software. To compare SC-specific transcript expression levels, a two-step adjustment of relative gene expression was used to account for the marked expansion of non-Sertoli (mostly germ) cell numbers during testis development, which greatly dilutes any measured SC-specific gene expression using whole testis RNA samples, as detailed previously (21). First, an adjustment corrected for relative differences in testis size (weight representing total cell numbers), providing relative values of total target levels for whole testis RNA. A second adjustment then corrected this relative total expression by total SC numbers (calculated for each group by stereology) to obtain an estimate of relative transcript expression/SC. All samples were tested in duplicate.
For enriched primary SC, Leydig cell, and peritubular cell populations described above, total RNA was extracted from cell pellets by RNeasy micro kit (QIAGEN, Valencia, CA) according to manufacturer protocols, and residual genomic DNA was removed by ribonuclease-free DNase I (0.5 U/μg RNA; Invitrogen). cDNA was prepared using oligo-dT primers and reverse transcriptase Superscript III (Invitrogen), then PCR performed using primers (Supplemental Table 1) to detect Fshr, Lhr, and Acta2 expression as markers for SC, Leydig cell, and peritubular cell, respectively, and Actb as sample internal loading control. Amplicons were visualized by agarose gel electrophoresis stained with SYBR safe (Invitrogen).
Statistical analysis
Statistical analysis was performed using SPSS, version 19.0 (SPSS, Inc., Chicago, IL) or NCSS 2007 (NCSS, Kaysville, UT). Comparisons of Tg and developmental effects were determined by two-way ANOVA (log transformation was required for serum FSH). Data for Tg vs. WT samples at equal time points used unpaired t tests, and comparison of Tg or WT samples at different time points used one-way ANOVA. Nonlinear regression analysis was performed using suitable preset 4-parameter sigmoid models for individual curve fits (Sigmaplot version 10; Systat Software, Chicago, IL). Differences were regarded significant when P < 0.05. All data are presented as mean ± sem.
Results
SC expression of TgSCAR
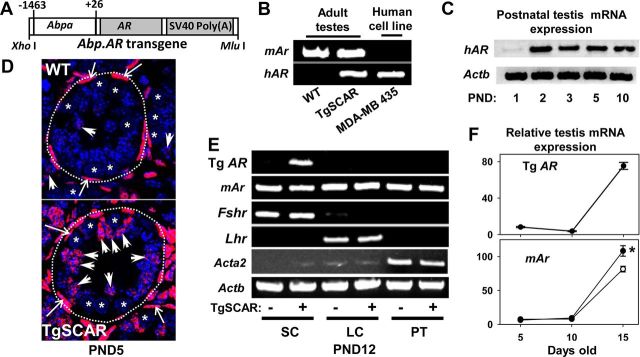
Tg mice were prepared using the Abpa.AR DNA construct (Fig. 1A), which contains the rat Abpa P1 promoter sequence previously shown to direct SC-specific transgene expression in mice (17, 29, 30). Five Tg founders were genotyped and used to establish independent TgSCAR lines. All hemizygous TgSCAR mice used for breeding were fertile. Testicular Tg AR mRNA expression levels in PND5 and adult males were compared in three Tg lines, designated TgSCAR-1, TgSCAR-2, and TgSCAR-3, using primers designed to detect human AR but not mouse Ar transcripts (Fig. 1B). The independent Tg lines displayed distinct expression levels of testis Tg AR mRNA by qPCR, with Tg AR mRNA levels in TgSCAR-3 > TgSCAR-2 > TgSCAR-1 mice (see Supplemental Fig. 1A). Postnatal expression of TgSCAR was detected as early as PND2 in TgSCAR-3 testes (Fig. 1C). Immunodetection for AR revealed strong positive intratubular staining localized in DAPI-stained SC nuclei in PND5 TgSCAR-3 testes contrasting with the sparse and very weak SC staining (endogenous mouse AR) in age-matched WT littermates (Fig. 1D). Similar AR immunostaining was found in TgSCAR-2 testes (Supplemental Fig. 1B). Germ cells and cytoplasmic areas surrounding DAPI-stained nuclei had no detectable ectopic Tg AR expression. Localization of TgSCAR expression was further demonstrated with Tg AR mRNA detected by RT-PCR in enriched SC, but not Leydig cell and peritubular cell, populations isolated from PND12 TgSCAR-3 testes (Fig. 1E), noting Tg AR expression was absent in WT (non-Tg) Sertoli samples as expected. In contrast, endogenous mouse Ar mRNA was detected in all three SC-, Leydig cell-, and peritubular cell-enriched preparations (Fig. 1E), using primers that did not detect human AR mRNA (Fig. 1B). Expression patterns of Fshr, Lhr, and Acta2 mRNA used as SC, Leydig cell, and peritubular cell markers, respectively, confirmed enrichment of these somatic cell populations known to express endogenous AR. Further comparison showed that testicular TgSCAR mRNA expression levels were markedly elevated at PND15 relative to PND5–PND10, similar to the expression pattern of endogenous mouse Ar mRNA, which was significantly elevated (25%, P < 0.01) in TgSCAR-3 compared with WT PND15 testes (Fig. 1F).
Fig. 1.
Detection of Tg human AR in TgSCAR-3 mice. A, The Tg DNA construct consisted of the human AR cDNA (AR) sequence flanked by the rat Abpa P1 promoter and simian virus 40 (SV40) polyadenylation signal sequence, as described in Materials and Methods. B, Species-specific detection of Tg human AR (hAR) or endogenous mouse Ar (mAr) mRNA in WT or TgSCAR-3 adult mouse testes by RT-PCR, using the AR-expressing human MDA-MB-435 cell line as control. C, Postnatal onset of testicular Tg hAR mRNA expression detected as early as PND2 in TgSCAR-3 mice by RT-PCR, using Actb as sample control. D, Anti-AR antibody detection by immunofluorescent microscopy using testis sections from PND5 TgSCAR-3 and WT mice. Anti-AR staining (pink-red; green to red pseudocoloring used for Alexa Fluor 488) overlapped with DAPI-labeled nuclei (blue). Strong AR staining was clearly detected in the irregular columnar shaped SC nuclei (some highlighted by large arrowheads) of TgSCAR testes, contrasting with sparse staining in WT testes. Strong peritubular expression of AR (long arrows) was found in WT and TgSCAR testes. Dashed circular lines highlight circumference of seminiferous tubules. Germ cells (asterisks) and cytoplasmic regions around DAPI-stained nuclei were devoid of AR. Shown at same magnification (original ×100). E, Testicular primary somatic AR-expressing cells were enriched from PND12 TgSCAR (+) and non-Tg WT littermate (−) testes using trypsin-collagenase digestion as described in Materials and Methods. TgSCAR mRNA was detected in enriched SC, but not Leydig cell (LC) and peritubular cell (PC), preparations by RT-PCR, demonstrating Sertoli-specific expression, unlike the mouse Ar mRNA found in all cell preparations. F, top, Relative testicular levels of Tg human AR mRNA increased with age in postnatal TgSCAR mice. Bottom, Endogenous mouse Ar increased with age and was significantly higher in PND15 TgSCAR (solid circle) vs. WT (open circle) testes. Values are mean ± sem (n = 6–10/group). *, P < 0.05.
Effect of TgSCAR on body and testis weight, serum hormone levels, and fertility
Body and testis weights
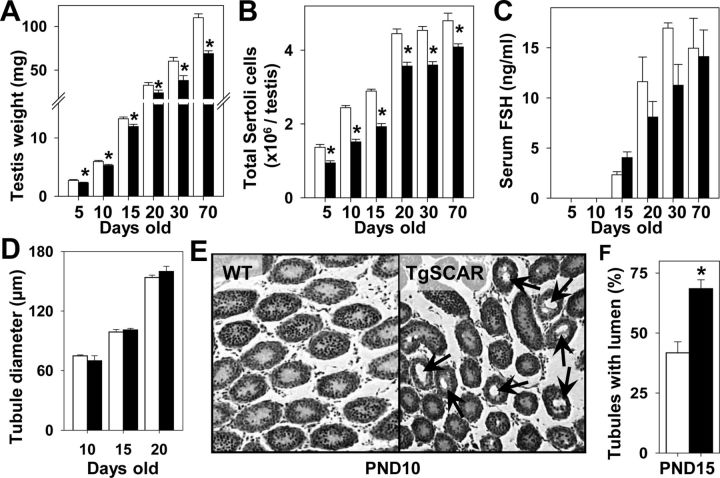
Initial analysis compared TgSCAR effects on body and testis weights in the TgSCAR-1, TgSCAR-2, and TgSCAR-3 lines. Total body weights of TgSCAR males in all three lines remained equivalent to those of age-matched postnatal and adult controls (data not shown). In contrast, testis size was significantly smaller in all postnatal (from PND5 to PND10) and adult TgSCAR males relative to WT age-matched littermates (Supplemental Fig. 2A). Testes in mature TgSCAR-1, TgSCAR-2, and TgSCAR-3 mice were 87, 79, and 63% of normal size, respectively (Supplemental Fig. 2A), indicating increasing levels of TgSCAR expression dose dependently reduced testis size. More detailed analyses of TgSCAR actions were performed using the significantly smaller (two-way ANOVA, P < 0.01) testes from TgSCAR-3 males (Fig. 2A), hereafter described as TgSCAR mice. All tested adult TgSCAR males were fertile and produced equivalent litter sizes compared with WT males (7.9 ± 0.3 vs. 8.3 ± 0.4 pups/litter, n = 7 WT vs. n = 8 TgSCAR male breeders).
Fig. 2.
Testicular size, SC numbers, and serum FSH levels. A, Decreased testes weights were found in postnatal (from PND5) to mature TgSCAR (black bars) compared with WT (white bars) mice. B, Total SC numbers were decreased in TgSCAR relative to WT littermates from PND5 to maturity. C, Circulating FSH levels (measured ≥PND15) significantly increased (two-way ANOVA, P < 0.05) during the development of male mice, with no significant interaction of TgSCAR (two-way ANOVA). Serum FSH levels were equivalent in TgSCAR relative to age-matched WT males (one-way ANOVA). D, Seminiferous tubular diameter was equivalent in TgSCAR (black) and WT (white) PND10–PND20 testes. E, Seminiferous tubular lumen formation (indicated by arrows) was detected in TgSCAR but not WT PND10 testes. F, The percentage of tubules with lumen was significantly increased in TgSCAR (black) compared with WT (white) PND15 testes. Values are mean ± sem [n = 6–10/group (A–C); n = 3/group (D–F)]. *, P < 0.05.
Hormone levels
Serum volumes limited hormone analysis to PND15-to-adult mice. Serum FSH levels significantly increased (two-way ANOVA, P < 0.05) during the development of male mice (Fig. 2C), but there was no significant interaction effect with TgSCAR (two-way ANOVA, P = 0.05). Further comparison found no difference between serum FSH levels in PND15 (one-way ANOVA, P = 0.09) or adult TgSCAR relative to age-matched WT mice. Serum testosterone levels remained normal in TgSCAR compared with WT adult males (2.8 ± 1.8 vs. 2.6 ± 1.4 ng/ml, n = 7–8/group). Androgen-dependent seminal vesicle weights were equivalent in TgSCAR and WT adult males (269.7 ± 8.3 vs. 280.9 ± 17.8 mg, n = 7/group).
Effect of TgSCAR on tubular lumen formation, SC number, and gene expression
SC numbers
Stereological analysis revealed that total SC numbers were significantly reduced (two-way ANOVA, P < 0.05) in TgSCAR relative to WT testes (Fig. 2B). In PND5 and adult TgSCAR testes, SC numbers were 70 and 85% of normal age-matched values, respectively. Analysis of nonlinear curves fits (sigmoidal 4-parameter) (Supplemental Fig. 2B) indicated that SCs numbers reached 50% of adult (70 do) levels at PND15 and PND11 for TgSCAR and WT testes, respectively, and then reached 90% of adult levels at PND21 and PND22 for TgSCAR and WT testes, respectively. Total SC numbers significantly increased from PND5 to PND20 in both TgSCAR and WT mice, noting a striking increase during PND15–PND20 contributed to 40 and 32% of total SC numbers obtained in PND20 TgSCAR and WT testes, respectively. Furthermore, significant SC expansion continued beyond PND20, with SC numbers increasing another 15% (P < 0.05) and 10% (P < 0.005) to reach numbers found in TgSCAR and WT adult testes, respectively (Fig. 2B).
Seminiferous tubular lumen diameter
Seminiferous tubular lumen formation was used as a marker for SC fluid secretion and functional maturation (35, 36). Total diameters of seminiferous tubules were equivalent in PND10, PND15, and PND20 TgSCAR compared with age-matched WT testes (Fig. 2D). However, tubule lumen formation was readily detected in TgSCAR but not WT PND10 testes (Fig. 2E) and was significantly increased (64%, P < 0.05) in TgSCAR relative to WT PND15 testes (Fig. 2F), demonstrating accelerated lumen formation in postnatal TgSCAR males. No association between total tubular diameter and lumen formation supports early work, indicating that seminiferous tubule diameters are not necessarily correlated with lumen formation (37).
SCAR-regulated transcripts
To determine TgSCAR effects on AR-regulated transcripts during SC maturation, relative SC expressions of AR-dependent Rhox5 and Spinlw1 genes (17) were compared in postnatal-to-adult TgSCAR and WT males. Relative expression levels were normalized to testis Hmbs and Hprt1 expression levels, which both displayed consistent expression patterns during postnatal-to-adult testis development, and between TgSCAR and WT samples, noting Actb and Rps9 were not stable and not suitable controls during testis development (data not shown). Relative SC expression levels of Rhox5 and Spinlw1 increased with age and were significantly elevated (two-way ANOVA, P < 0.001) in TgSCAR compared with WT testes (Fig. 3A). Elevated AR-dependent Rhox5 and Spinlw1 expression was also found in postnatal TgSCAR-2 mice (Supplemental Fig. 3). In contrast, expression of SC-specific Amh declined with age (two-way ANOVA, P < 0.0001) and showed a significant interaction with TgSCAR (P < 0.005). Time-point analysis (one-way ANOVA) showed that Amh mRNA was significantly higher in PND5 (60%, P < 0.01) and PND10 (55%, P < 0.01) TgSCAR relative to age-matched WT testes and then declined to similar expression levels in PND15 and PND30 TgSCAR vs. matched WT mice (Fig. 3A). GATA1 may repress anti-Müllerian hormone (AMH) expression in early postnatal testes (38). SC Gata1 mRNA levels displayed a significant age effect with a significant TgSCAR interaction (two-way ANOVA, P < 0.01). Relative Gata1 expression levels were significantly reduced (57%, one-way ANOVA, P < 0.01) in TgSCAR vs. WT PND5 testes, showing an inverse relationship with higher Amh expression in early TgSCAR testes, but were higher in PND10-to-adult TgSCAR compared with age-matched WT testes (Fig. 3). In silico analysis identified just one low-scoring potential androgen-response element (ARE) in the proximal Amh promoter (screening 4-kb 5′ of the start codon), whereas several proximal and distal predicted AREs were found upstream of Gata1 (see Supplemental Table 2).
Fig. 3.
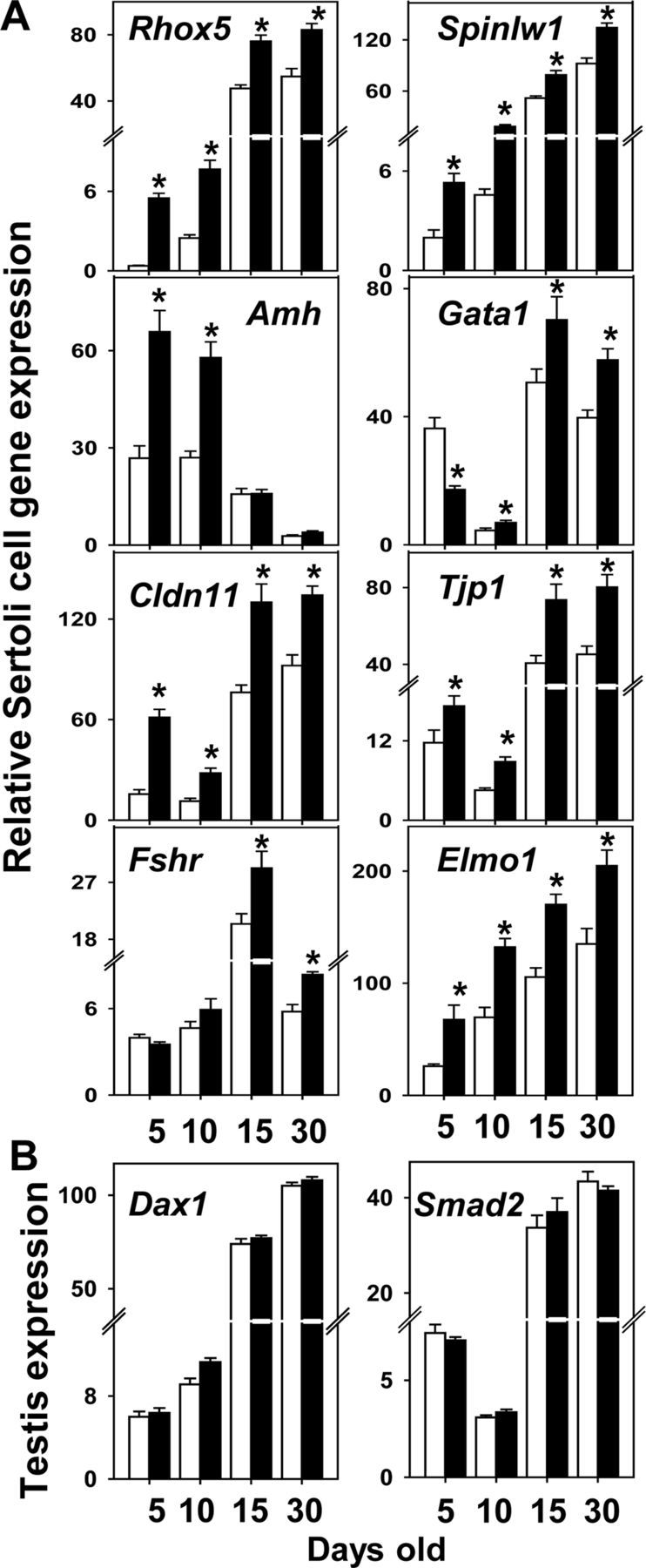
Expression of SC transcripts in TgSCAR testes. A, Relative SC expression levels of AR-regulated Rhox5 and Spinlw1 transcripts were significantly higher in postnatal TgSCAR (black bars) compared with age-matched WT (white bars) mice, determined by qPCR using whole testis mRNA then adjusting total expression levels for SC number as described in Materials and Methods. SC Amh and Gata1 mRNA levels were up- and down-regulated, respectively, in TgSCAR vs. WT PND5 mice, showing an inverse relationship in early postnatal development. SC Amh expression declined to normal levels in later PND15 and PND30 TgSCAR males. TgSCAR expression elevated SC expression of Cldn11 and Tjp1, encoding tight-junction proteins, as well as Elmo1 mRNA, vital for SC-mediated phagocytosis. In contrast, expression levels of SC Fshr mRNA remained normal in early postnatal development and then elevated in peripubertal TgSCAR males. B, Total testicular levels of Smad2 and Dax1 mRNA were equivalent in WT and TgSCAR males across the ages examined. Relative levels were not corrected for SC number, because both transcripts may be expressed in other testis cell types. Data are expressed as mean ± sem (n = 6–9/group). Significant differences (P < 0.05) relative to age-matched control are indicated by asterisks.
SC functional markers
To study TgSCAR effects on SC development, we compared expression levels of markers associated with SC functional maturation. SC Cldn11 and Tjp1 mRNA, encoding important tight-junction factors, displayed similar age-related patterns of mRNA expression in WT testes, both greatly increased (two-way ANOVA, P < 0.001) at PND15–PND30 (Fig. 3A). Relative SC Cldn11 and Tjp1 mRNA expression levels were significantly elevated (two-way ANOVA, P < 0.05) in postnatal-to-adult TgSCAR testes (Fig. 3A). Analysis of the Cldn11 promoter identified five potential proximal/distal AREs (see Supplemental Table 2), whereas similar analysis found none in the Tjp1 proximal promoter, and two ARE-like elements more than 3 kb upstream of Tjp1 exon I (Supplemental Table 2). SC expression levels of Elmo1, encoding a crucial phagocytic factor for SC function (39), increased with age and was significantly elevated (two-way ANOVA, P < 0.002) in TgSCAR relative to WT mice (Fig. 3A). Four predicted AREs were found in the Elmo1 promoter region (Supplemental Table 2), one resembling a divergent direct repeat ARE proposed to be more AR selective in vitro (40).
SCs exclusively express Fshr (41), which was found at equivalent levels in PND5–PND10 TgSCAR and WT mice but was significantly elevated (two-way ANOVA, P < 0.001) in older PND15–PND30 TgSCAR vs. WT testes (Fig. 3A). DAX-1 (dose-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1) is an important SC factor but also expressed in Leydig cells (42); so Dax1 mRNA levels could not be corrected for relative SC numbers. Testis Dax1 mRNA showed an equivalent age-related rise and levels of relative expression in TgSCAR and WT mice (Fig. 3B). Testicular expression levels of Smad2, recently proposed to be up-regulated by SCAR activity (43), also remained equivalent in TgSCAR and WT males at all ages examined (Fig. 3B).
Effect of TgSCAR on germ cell transcripts and numbers during development
Germ cell transcripts
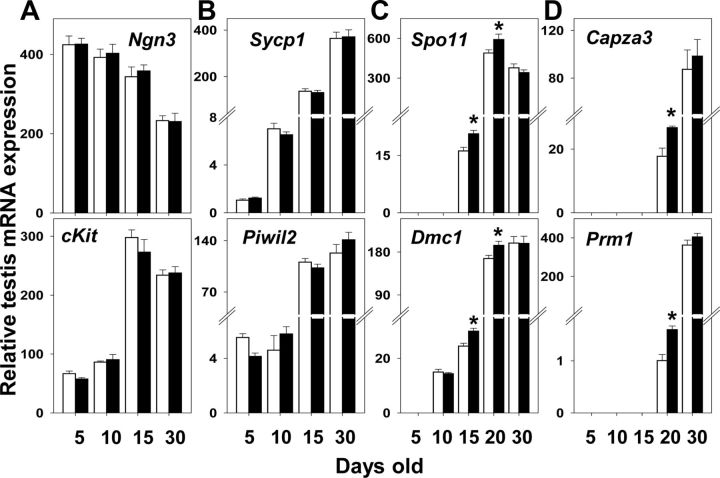
The effect of TgSCAR on germ cell-specific transcripts was examined during postnatal-pubertal development. Relative testicular expression levels of spermatogonial markers Ngn3, specifically expressed in undifferentiated spermatogonia (44), and cKit, which is first expressed in differentiating type A spermatogonia (45), were equivalent in TgSCAR and WT samples (Fig. 4A). Likewise, testicular levels of Piwil2 mRNA detected as early as the spermatogonia stage (PND5), and Sycp1 mRNA also found at PND5 and predominantly expressed from the zygotene (PND10) spermatocyte stage (46), remained normal in TgSCAR mice (Fig. 4B). However, the relative testicular expression levels of Dmc1 mRNA, absent in spermatogonia (PND5) but found in spermatocytes (47), and Spo11 mRNA, which is strongly expressed from the pachytene spermatocyte stage (48), were significantly elevated at PND15 and PND20 in TgSCAR compared with matched WT testes (Fig. 4C). Expression levels of postmeiotic spermatid markers Prm1 (49) and Capza3 (50) were also significantly elevated in TgSCAR relative to WT PND20 mice (Fig. 4D).
Fig. 4.
Effect of TgSCAR on postnatal germ cell transcripts. Relative testis expression levels of germ cell transcripts found in mitotic spermatogonia (Ngn3 and ckit), meiotic spermatocytes (Sycp1, Piwil2, Dmc1, and Spo11), or postmeiotic spermatids (Capza3 and Prm1) were determined by qPCR as described in Materials and Methods. Spermatogonial (A) and early meiotic (B) markers were expressed at normal levels in TgSCAR testes. Expression levels of meiotic Dmc1 and Spo11 (C) and postmeiotic Capza3 and Prm1 (D) markers were significantly elevated during PND15–PND20 in TgSCAR vs. WT testes. Values are mean ± sem (n = 6–9/group). Asterisks indicate significant differences (P < 0.05) between age-matched TgSCAR and WT groups.
Postnatal germ cell populations
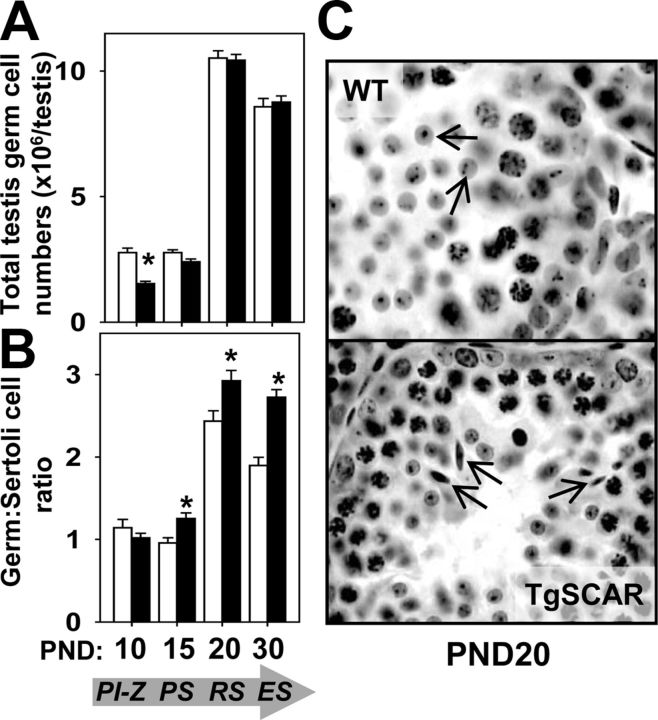
To further examine TgSCAR actions during spermatogenic development, detailed stereological analysis determined the total numbers of distinct germ cell populations representing the most advanced level of normal germinal epithelia development at each time point: PND10, preleptotene-zygotene spermatocytes; PND15, pachytene spermatocytes; PND20, round spermatids; and PND30, elongated spermatids. Total numbers of preleptotene-zygotene spermatocytes at PND10 were significantly decreased (55%, P < 0.01) in TgSCAR compared with WT testes (Fig. 5A). However, TgSCAR testes exhibited a normal germ:Sertoli cell ratio for these early meiotic germ cells (Fig. 5B). In contrast, total numbers of the more advanced meiotic pachytene spermatocytes at PND15, and postmeiotic round (PND20) and elongated (PND30) spermatids, were equivalent in TgSCAR and age-matched WT testes (Fig. 5A). Therefore, these later meiotic-postmeiotic germ cell populations produced significantly higher germ:Sertoli cell ratios in TgSCAR relative to WT testes (Fig. 5B), indicating that postnatal TgSCAR SCs carried more developing meiotic-postmeiotic germ cells relative to age-matched WT SCs. Histological examination revealed the presence of elongated spermatids in TgSCAR but not WT PND20 testes, demonstrating more advanced development of germ cells in immature TgSCAR mice (Fig. 5C).
Fig. 5.
Effect of TgSCAR on postnatal germ cell numbers. A, Total germ cell populations were determined by optical disector stereology, with germ cell types selected to represent the most advanced stage of normal development found at the indicated days of postnatal development: PND10, Preleptotene-zygotene spermatocytes (PI-Z); PND15, pachytene spermatocytes (PS); PND20, round spermatids (RS); PND30, elongated spermatids (ES), indicated in the arrow below. Total numbers of PI-Z spermatocytes were significantly reduced, whereas total PS, RS, and ES numbers were equivalent in TgSCAR compared with age-matched WT testes. B, Germ cell to SC ratios were compared during postnatal development at the indicated ages. The PI-Z:SC ratio was normal in PND10 TgSCAR testes, whereas the later meiotic PS:SC, postmeiotic RS:SC, and ES:SC ratios were all significantly elevated at PND15, PND20, and PND30, respectively, in TgSCAR relative to WT testes. Values are mean ± sem (n = 5–6/group). Asterisks indicate significant differences (P < 0.05) between age-matched WT and TgSCAR groups. C, Histological comparison of PND20 testis sections showed the presence of RS (top panel, arrows) in WT males compared with more advanced ES (bottom panel, arrows) in TgSCAR males.
Stage-dependent AR expression in TgSCAR and WT mature testes
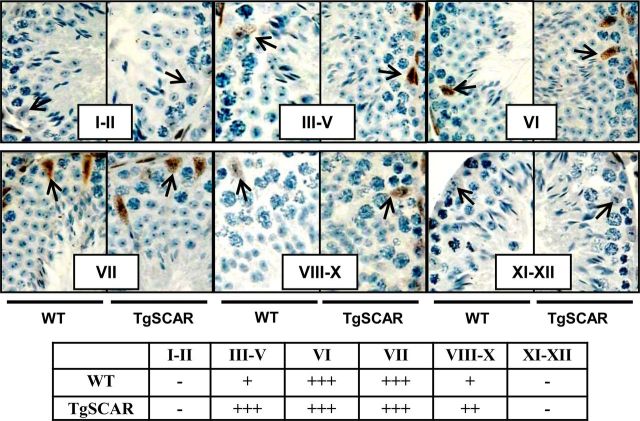
To determine the pattern of TgSCAR expression in seminiferous tubules of mature mice, immunodetection of AR was performed in parallel with morphological staging of spermatogenic tubules based on Harris's hematoxylin nuclear staining as previously described (51). No AR expression was observed in stages I–II and XI–XII tubules in both TgSCAR and WT testes (Fig. 6). In stage III–V tubule sections, AR was strongly expressed in TgSCAR compared with very weak expression in WT testes. The AR expression level was very strong in stages VI–VII for both TgSCAR and WT testes, and then moderate AR expression levels were exhibited in stages VIII–X for TgSCAR relative to very weak expression in corresponding sections from WT littermates (Fig. 6). The proposed AR-dependent stages VI–VII (6) were found to represent the same percentage of tubule cross-sections (8.4 ± 0.4% vs. 9.3 ± 0.2%, n = 4/group) in TgSCAR and WT testes.
Fig. 6.
Immunodetection of stage-specific AR expression in adult TgSCAR vs. WT mice. Immunohistochemical detection of AR (brown staining) in testis sections was performed in parallel to the morphological staging (roman numerals) of seminiferous epithelia by light microscopy as described in Materials and Methods. Stage-specific AR staining was found in SC nuclei in TgSCAR and WT sections. Strong staining in stages VI–VII was detected in seminiferous tubules of TgSCAR and WT males. Moderate-strong AR staining was found in stage III–V and VIII–X tubules from TgSCAR testes, vs. weak expression in stage-matched WT sections. No AR was detected in stage I–II and XI–XII tubules from TgSCAR and WT testes. Arrows indicate SC nuclei. Table below summarizes the level of relative AR expression found in distinct stages: −, No expression; +, weak; ++, moderate; +++, strong expression.
Effect of TgSCAR on germ cell numbers in mature testes
Overall, testicular histology was comparable between adult TgSCAR and WT testes (Fig. 6). Detailed stereological analysis revealed changes to germ cell populations in adult TgSCAR compared with WT testes. The total numbers of spermatogonia were significantly decreased (60%, P < 0.01) in TgSCAR vs. WT testes (Fig. 7A), although the spermatogonia:SC ratio was equivalent in TgSCAR and WT testes (Fig. 7B). Despite the smaller size of TgSCAR compared with WT testes, absolute numbers of meiotic spermatocytes were normal in TgSCAR and WT testes (Fig. 7A), which was explained by the higher than normal spermatocyte:SC ratios in TgSCAR testes (Fig. 7B). In contrast, the total number and the germ:Sertoli cell ratio for the postmeiotic round and elongated spermatids were all significantly reduced in TgSCAR compared with WT testes (Fig. 7).
Fig. 7.
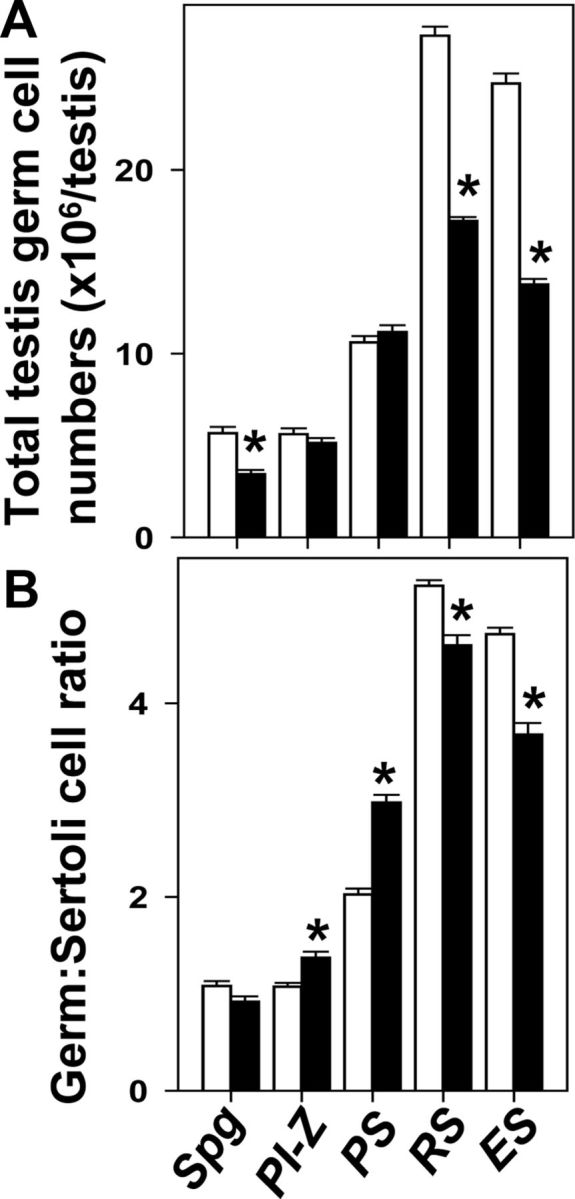
Germ cell populations in mature TgSCAR vs. WT testes. A, Total testicular germ cell populations were determined by optical disector stereology in TgSCAR and WT littermates. Total mitotic spermatogonia (Spg) and postmeiotic round spermatid (RS) and elongated spermatid (ES) numbers were significantly reduced in TgSCAR relative to WT testes. In contrast, total preleptotene-zygotene spermatocyte (PI-Z) and pachytene spermatocyte (PS) numbers were normal in mature TgSCAR testes. B, Comparison of total germ cell to SC ratios showed that the mitotic Spg:SC ratio was normal in adult TgSCAR testes, whereas the meiotic PI-Z:SC and PS:SC ratios were significantly higher than normal in TgSCAR testes. In contrast, TgSCAR testes exhibited reduced postmeiotic RS:SC and ES:SC ratios compared with WT values. Values are mean ± sem (n = 5–6/group). Asterisks indicate significant differences (P < 0.05) between age-matched TgSCAR and WT groups.
Discussion
We established a unique gain-of-function Tg mouse model to directly investigate the temporal role of AR expression in SC function and testis development. This Tg model provided dose-dependent premature AR expression selectively targeted to the testicular SCs. Our findings have revealed for the first time that advanced SCAR expression alone can accelerate SC maturation and spermatogenic development, which ultimately reduces the SC population and testis size.
SC-specific and premature postnatal TgSCAR expression was directly shown by strong immunodetection of AR in SC nuclei in PND5 TgSCAR testes contrasting with sparse SCAR detected in age-matched WT testes. In addition, Tg AR mRNA was restricted to enriched SC, but not Leydig cell or peritubular cell, populations, extending previous findings that the rat Abpa promoter directs SC-specific expression of heterologous transgenes (17, 30, 52). Comparison of independent TgSCAR lines with distinct levels of Tg AR mRNA expression showed that TgSCAR dose dependently reduced testicular size from early postnatal development to adulthood, with the highest TgSCAR levels producing testes 40% smaller than normal. TgSCAR did not appear to alter testicular androgen production, because the weight of androgen-dependent seminal vesicles remained normal, consistent normal levels of serum testosterone.
Expression of TgSCAR reduced the number of developing SCs throughout the postnatal-pubertal period, detected as early as PND5, and ultimately decreased SC numbers in mature testes, providing a basis for the smaller testes of TgSCAR mice. Intriguingly, our detailed stereological analysis revealed that SC numbers rapidly increased during late postnatal-peripubertal development (PND15–PND20) in both TgSCAR and WT testes and significantly increased beyond PND20. The overall increase beyond PND15 represented 40–50% of the SC population found in mature TgSCAR or WT mice or approximately one additional division for each SC present at PND15. Supporting our findings, data from an earlier study indicate that SC numbers reach maximal levels in PND28–PND31 mice (53). Combined, these findings based on the enumeration of total testicular cell numbers, using validated stereological approaches, indicate that significant SC proliferation must occur during late postnatal-pubertal development. Moreover, these findings challenge the prevailing view that rodent SCs cease to proliferate around PND16 in midpostnatal life (9), based on DNA-labeling of S-phase cells as a proxy to measure proliferation in rats (35, 54) and mice (55), which appears to significantly underestimate peripubertal-pubertal stage SC proliferation in mice.
In contrast to reduced SC numbers found in TgSCAR testes, mice lacking functional SCAR (SCARKO) exhibited normal SC numbers (12, 17), as did LH receptor-deficient males (27). These knockout models suggested that FSH, not LHR or SCAR, activity was a major determinant defining the ultimate size of the SC population (27). Serum FSH or SC Fshr expression levels were not reduced in PND15–PND20 TgSCAR males, suggesting that FSH actions were maintained in TgSCAR mice. However, our gain-of-function TgSCAR model shows that temporal SCAR expression can significantly impact total SC numbers despite the presence of FSH. We propose that the reduced SC population in TgSCAR testes reflects AR induction of premature SC maturation, which reduces the pool of immature SCs available for FSH-induced mitotic expansion.
Precocious SC maturation in TgSCAR mice was demonstrated by the accelerated postnatal formation of seminiferous tubular lumen, produced by fluid secretion from maturing SCs (36, 37). Furthermore, TgSCAR induced age-related changes to SC-specific transcripts associated with functional maturation. TgSCAR markedly up-regulated postnatal SC expression of Rhox5 and Spinlw1 genes, which contain functional (56) and predicted (17) AREs and are both expressed during SC postnatal development (17, 57, 58), demonstrating a premature rise in functional Sertoli AR activity. Elevated levels of SC transcripts with major roles in tight-junction or phagocytic function provided further in vivo evidence for an accelerated maturation of TgSCAR SCs. Postnatal Cldn11 and Tjp1 mRNA levels, encoding two key SC tight-junction proteins (59, 60), displayed a marked up-regulation at PND15–PND30 in both TgSCAR and WT testes, coinciding with tight-junction formation and blood-testis-barrier formation (36), which presumably occurs with ongoing SC expansion from PND15 to PND30. Both were significantly up-regulated in TgSCAR compared with WT testes, Cldn11 considerably more than Tjp1 expression, which may be explained by the presence of five vs. two predicted AREs in their respective promoter regions. TgSCAR-regulated Cldn11 expression is consistent with its down-regulation in postnatal SCARKO testes (61) and recent work showing up-regulated Cldn11 expression during androgen-induced murine spermatogenic development (62). TgSCAR also increased postnatal SC expression of Elmo1, which is vital for SC-mediated phagocytic clearance of apoptotic germ cells (39). The elevated age-related rise of postnatal Elmo1 mRNA in TgSCAR SCs, together with four potential AREs in the Elmo1 promoter region, one resembling a predicted AR-selective direct repeat element (40), provides evidence that Elmo1 may be an AR-regulated target in developing SCs.
Conversely, SC AMH levels normally decline during human and rodent testis development (11, 63, 64), proposed to be due to negative regulation by AR actions during the onset of puberty (11). Unexpectedly, SC Amh mRNA was higher in TgSCAR relative to normal prepubertal (PND5–PND10) mice and then declined to normal levels during peripubertal-pubertal development (PND15–PND30) in TgSCAR mice, suggesting age-related differential AR regulation. The precise postnatal SC pathways down-regulating AMH in vivo remain unknown. An inverse relationship between elevated SC Amh and reduced Gata1 expression in TgSCAR vs. WT PND5 testes supports an early repressor role proposed for GATA1 (38). However, this inverse Amh-Gata1 relationship was not present beyond PND5 in TgSCAR vs. WT mice. Adding more complexity, potential AREs found upstream of Gata1 present the possibility of direct AR regulation of GATA1. We propose that SCAR does not directly dictate the temporal down-regulation of AMH expression, which is consistent with the absence of a strong ARE in the Amh promoter, and relatively normal age-dependent decline of AMH in postnatal SCARKO testes (12).
Recently, testicular Smad3 levels were proposed to regulate SCAR expression, which in turn was predicted to elevate SC Smad2 expression to control temporal postnatal development (43). Testicular Smad2 expression remained normal throughout postnatal development in TgSCAR males, despite striking changes to several SCAR-regulated genes. Although our findings do not rule out a Smad3/Smad2 pathway in testis development, they demonstrate that AR-regulated SC maturation can occur independently of changes to Smad2 expression. Another key factor, DAX-1 required for normal SC and spermatogenic development (42), had normal levels of gene expression during postnatal development in TgSCAR testes, suggesting that it also represents a Sertoli AR-independent gene.
Premature SC maturation in TgSCAR testes was associated with accelerated postnatal spermatogenic development. Normal expression levels of spermatogonia and early spermatocyte markers, and normal germ:Sertoli cell ratios for preleptotene-zygotene spermatocytes in PND10 TgSCAR testes suggested that temporal mitotic-to-early meiotic germ cell development proceeds independently of SCAR regulation. In contrast, elevated levels of later meiotic (Dmc1 and Spo11) and postmeiotic (Capza3 and Prm1) germ cell transcripts in postnatal TgSCAR testes were explained by higher than normal pachytene spermatocyte:SC and spermatid:SC ratios in TgSCAR vs. WT postnatal testes. In addition, the premature appearance of elongated spermatids in postnatal TgSCAR testes provided definitive evidence for accelerated spermatogenic development in TgSCAR mice. These findings show that postnatal SCAR activity regulates temporal germ cell development from the meiotic pachytene stage, extending the proposal that the meiotic phase is highly androgen-regulated during the initiation of spermatogenesis (24, 26).
Adult SCs displayed similar stage-specific (III–X) expression patterns of AR by immunohistochemistry in both TgSCAR and WT seminiferous epithelia, which indicates that Tg AR expression was localized within the stages normally expressing endogenous mouse AR (6). Prominent AR expression centered around stages VI–VII in TgSCAR testes also coincides with the reported peak expression level of rat ABP mRNA in stages VII–VIII (65), suggesting the Abpa promoter directs gene expression similar to stage-specific AR expression in vivo. The anti-AR antibody could not distinguish between human Tg or endogenous mouse AR, but stronger staining of Sertoli nuclei in stage III–V and VIII–X tubules of TgSAR vs. WT testes suggested higher AR levels in TgSCAR SCs. Although TgSCAR had no impact on the percentage of AR-dependent stage VI–VII tubules, it induced distinct changes to germ cell development in adult testes. Normal spermatogonia:SC ratios in mature TgSCAR testes suggested that the SCAR has no major influence on the mitotic germ cell population, beyond defining the number of supportive SCs, consistent with work suggesting that spermatogonia are largely regulated by FSH not androgen actions (27). In contrast, normal numbers of preleptotene-zygotene and pachytene spermatocytes in the small-sized adult TgSCAR testes resulted in higher than normal meiotic germ:Sertoli cell ratios. The capacity of TgSCAR SCs to carry more meiotic germ cells may reflect local AR-mediated germ cell survival (66), which warrants further investigation. Despite the relative increase in meiotic germ cells, total numbers and germ:Sertoli cell ratios of the postmeiotic spermatids were reduced in mature TgSCAR vs. WT males. It is possible that elevated SCAR activity may increase meiotic germ cell survival beyond a threshold required for normal development or support of the subsequent spermatid populations.
TgSCAR had no detectable effect on fertility, despite inducing smaller testes with less postmeiotic development. However, these findings raise the possibility that aberrant temporal or excessive SCAR expression may impact key parameters (testis size, postmeiotic development) required for optimal fertility in other species, noting the spermatogenic output of laboratory mice far exceeds that necessary for reproductive success (67). Precocious testis development due to increased androgen levels is well established, such as hyperactive steroidogenic function from activating mutations of the LHR (68) or stimulatory G protein α-subunit (69). Our novel model has revealed a new molecular pathway for precocious testicular development, in which premature and excessive SCAR activity leads to accelerated SC and germ cell maturation, independent of FSH and testosterone, producing smaller adult testes with less SCs and developing spermatids.
In summary, our novel TgSCAR gain-of-function model directly shows a key role for carefully regulated onset and expression levels of SCAR during the induction or maintenance of spermatogenic development. Our new paradigm provides unique opportunity to directly differentiate vital AR-regulated genes and regulatory pathways involved in optimal SC maturation and meiotic-postmeiotic germ cell development.
Acknowledgments
We thank Mamdouh Khalil and the ANZAC Animal facility and Sarah Lamb for technical assistance.
This work was supported by National Health and Medical Research Council (Australia) Project Grants 464857 and 632753.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMH
- Anti-Müllerian hormone
- AR
- androgen receptor
- ARE
- androgen-response element
- BSS
- balanced salt solution
- DAPI
- 4′,6-diamidino-2-phenylindole
- DAX-1
- dose-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1
- DNase
- deoxyribonuclease
- PND
- postnatal day
- qPCR
- quantitative real-time PCR
- SC
- Sertoli cell
- SCARKO
- SCAR knock-out
- Tg
- transgenic
- TgSCAR
- Tg SC-specific AR
- WT
- wild type.
References
- 1. Quigley CA , De Bellis A , Marschke KB , el-Awady MK , Wilson EM , French FS. 1995. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 16:271–321 [DOI] [PubMed] [Google Scholar]
- 2. Cattanach BM , Iddon CA , Charlton HM , Chiappa SA , Fink G. 1977. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature 269:338–340 [DOI] [PubMed] [Google Scholar]
- 3. Chang C , Chen YT , Yeh SD , Xu Q , Wang RS , Guillou F , Lardy H , Yeh S. 2004. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA 101:6876–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sar M , Lubahn DB , French FS , Wilson EM. 1990. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology 127:3180–3186 [DOI] [PubMed] [Google Scholar]
- 5. Bremner WJ , Millar MR , Sharpe RM , Saunders PT. 1994. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology 135:1227–1234 [DOI] [PubMed] [Google Scholar]
- 6. Zhou Q , Nie R , Prins GS , Saunders PT , Katzenellenbogen BS , Hess RA. 2002. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl 23:870–881 [PubMed] [Google Scholar]
- 7. Johnston DS , Russell LD , Friel PJ , Griswold MD. 2001. Murine germ cells do not require functional androgen receptors to complete spermatogenesis following spermatogonial stem cell transplantation. Endocrinology 142:2405–2408 [DOI] [PubMed] [Google Scholar]
- 8. Lyon MF , Glenister PH , Lamoreux ML. 1975. Normal spermatozoa from androgen-resistant germ cells of chimaeric mice and the role of androgen in spermatogenesis. Nature 258:620–622 [DOI] [PubMed] [Google Scholar]
- 9. Sharpe RM , McKinnell C , Kivlin C , Fisher JS. 2003. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125:769–784 [DOI] [PubMed] [Google Scholar]
- 10. You L , Sar M. 1998. Androgen receptor expression in the testes and epididymides of prenatal and postnatal Sprague-Dawley rats. Endocrine 9:253–261 [DOI] [PubMed] [Google Scholar]
- 11. Al-Attar L , Noël K , Dutertre M , Belville C , Forest MG , Burgoyne PS , Josso N , Rey R. 1997. Hormonal and cellular regulation of Sertoli cell anti-Müllerian hormone production in the postnatal mouse. J Clin Invest 100:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan KA , De Gendt K , Atanassova N , Walker M , Sharpe RM , Saunders PT , Denolet E , Verhoeven G. 2005. The role of androgens in Sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology 146:2674–2683 [DOI] [PubMed] [Google Scholar]
- 13. McKinnell C , Saunders PT , Fraser HM , Kelnar CJ , Kivlin C , Morris KD , Sharpe RM. 2001. Comparison of androgen receptor and oestrogen receptor β immunoexpression in the testes of the common marmoset (Callithrix jacchus) from birth to adulthood: low androgen receptor immunoexpression in Sertoli cells during the neonatal increase in testosterone concentrations. Reproduction 122:419–429 [DOI] [PubMed] [Google Scholar]
- 14. Chemes HE , Rey RA , Nistal M , Regadera J , Musse M , González-Peramato P , Serrano A. 2008. Physiological androgen insensitivity of the fetal, neonatal, and early infantile testis is explained by the ontogeny of the androgen receptor expression in Sertoli cells. J Clin Endocrinol Metab 93:4408–4412 [DOI] [PubMed] [Google Scholar]
- 15. Suárez-Quian CA , Martínez-García F , Nistal M , Regadera J. 1999. Androgen receptor distribution in adult human testis. J Clin Endocrinol Metab 84:350–358 [DOI] [PubMed] [Google Scholar]
- 16. De Gendt K , Swinnen JV , Saunders PT , Schoonjans L , Dewerchin M , Devos A , Tan K , Atanassova N , Claessens F , Lécureuil C , Heyns W , Carmeliet P , Guillou F , Sharpe RM , Verhoeven G. 2004. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101:1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lim P , Robson M , Spaliviero J , McTavish KJ , Jimenez M , Zajac JD , Handelsman DJ , Allan CM. 2009. Sertoli cell androgen receptor DNA binding domain is essential for the completion of spermatogenesis. Endocrinology 150:4755–4765 [DOI] [PubMed] [Google Scholar]
- 18. Holdcraft RW , Braun RE. 2004. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 131:459–467 [DOI] [PubMed] [Google Scholar]
- 19. Baines H , Nwagwu MO , Furneaux EC , Stewart J , Kerr JB , Mayhew TM , Ebling FJ. 2005. Estrogenic induction of spermatogenesis in the hypogonadal (hpg) mouse: role of androgens. Reproduction 130:643–654 [DOI] [PubMed] [Google Scholar]
- 20. Lim P , Allan CM , Notini AJ , Axell AM , Spaliviero J , Jimenez M , Davey R , McManus J , MacLean HE , Zajac JD , Handelsman DJ. 2008. Oestradiol-induced spermatogenesis requires a functional androgen receptor. Reprod Fertil Dev 20:861–870 [DOI] [PubMed] [Google Scholar]
- 21. Allan CM , Couse JF , Simanainen U , Spaliviero J , Jimenez M , Rodriguez K , Korach KS , Handelsman DJ. 2010. Estradiol induction of spermatogenesis is mediated via an estrogen receptor-α mechanism involving neuroendocrine activation of follicle-stimulating hormone secretion. Endocrinology 151:2800–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walczak-Jedrzejowska R , Kula K , Oszukowska E , Marchlewska K , Kula W , Slowikowska-Hilczer J. 2011. Testosterone and oestradiol in concert protect seminiferous tubule maturation against inhibition by GnRH-antagonist. Int J Androl 34:e378–e385 [DOI] [PubMed] [Google Scholar]
- 23. Atanassova NN , Walker M , McKinnell C , Fisher JS , Sharpe RM. 2005. Evidence that androgens and oestrogens, as well as follicle-stimulating hormone, can alter Sertoli cell number in the neonatal rat. J Endocrinol 184:107–117 [DOI] [PubMed] [Google Scholar]
- 24. Singh J , O'Neill C , Handelsman DJ. 1995. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology 136:5311–5321 [DOI] [PubMed] [Google Scholar]
- 25. Singh J , Handelsman DJ. 1996. Neonatal administration of FSH increases Sertoli cell numbers and spermatogenesis in gonadotropin-deficient (hpg) mice. J Endocrinol 151:37–48 [DOI] [PubMed] [Google Scholar]
- 26. Haywood M , Spaliviero J , Jimemez M , King NJ , Handelsman DJ , Allan CM. 2003. Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology 144:509–517 [DOI] [PubMed] [Google Scholar]
- 27. Allan CM , Garcia A , Spaliviero J , Zhang FP , Jimenez M , Huhtaniemi I , Handelsman DJ. 2004. Complete Sertoli cell proliferation induced by follicle-stimulating hormone (FSH) independently of luteinizing hormone activity: evidence from genetic models of isolated FSH action. Endocrinology 145:1587–1593 [DOI] [PubMed] [Google Scholar]
- 28. O'Shaughnessy PJ , Monteiro A , Abel M. 2012. Testicular development in mice lacking receptors for follicle stimulating hormone and androgen. PLoS One 7:e35136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Esteban C , Gérard A , Larrib S , Torán N , Gérard H , Reventós J. 1997. Sertoli cell-specific expression of rat androgen-binding protein in transgenic mice: effects on somatic cell lineages. Mol Cell Endocrinol 132:127–136 [DOI] [PubMed] [Google Scholar]
- 30. Haywood M , Tymchenko N , Spaliviero J , Koch A , Jimenez M , Gromoll J , Simoni M , Nordhoff V , Handelsman DJ , Allan CM. 2002. An activated human follicle-stimulating hormone (FSH) receptor stimulates FSH-like activity in gonadotropin-deficient transgenic mice. Mol Endocrinol 16:2582–2591 [DOI] [PubMed] [Google Scholar]
- 31. Litvinov IV , Antony L , Isaacs JT. 2004. Molecular characterization of an improved vector for evaluation of the tumor suppressor versus oncogene abilities of the androgen receptor. Prostate 61:299–304 [DOI] [PubMed] [Google Scholar]
- 32. Jimenez M , Spaliviero JA , Grootenhuis AJ , Verhagen J , Allan CM , Handelsman DJ. 2005. Validation of an ultrasensitive and specific immunofluorometric assay for mouse follicle-stimulating hormone. Biol Reprod 72:78–85 [DOI] [PubMed] [Google Scholar]
- 33. McNamara KM , Harwood DT , Simanainen U , Walters KA , Jimenez M , Handelsman DJ. 2010. Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol 121:611–618 [DOI] [PubMed] [Google Scholar]
- 34. Vandesompele J , De Preter K , Pattyn F , Poppe B , Van Roy N , De Paepe A , Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Haaster LH , de Jong FH , Docter R , de Rooij DG. 1993. High neonatal triiodothyronine levels reduce the period of Sertoli cell proliferation and accelerate tubular lumen formation in the rat testis, and increase serum inhibin levels. Endocrinology 133:755–760 [DOI] [PubMed] [Google Scholar]
- 36. Russell LD , Bartke A , Goh JC. 1989. Postnatal development of the Sertoli cell barrier, tubular lumen, and cytoskeleton of Sertoli and myoid cells in the rat, and their relationship to tubular fluid secretion and flow. Am J Anat 184:179–189 [DOI] [PubMed] [Google Scholar]
- 37. Bressler RS , Lustbader IJ. 1978. Effect of testosterone on development of the lumen in seminiferous tubules of the rat. Andrologia 10:291–298 [DOI] [PubMed] [Google Scholar]
- 38. Beau C , Rauch M , Joulin V , Jégou B , Guerrier D. 2000. GATA-1 is a potential repressor of anti-Müllerian hormone expression during the establishment of puberty in the mouse. Mol Reprod Dev 56:124–138 [DOI] [PubMed] [Google Scholar]
- 39. Elliott MR , Zheng S , Park D , Woodson RI , Reardon MA , Juncadella IJ , Kinchen JM , Zhang J , Lysiak JJ , Ravichandran KS. 2010. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature 467:333–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barbulescu K , Geserick C , Schüttke I , Schleuning WD , Haendler B. 2001. New androgen response elements in the murine pem promoter mediate selective transactivation. Mol Endocrinol 15:1803–1816 [DOI] [PubMed] [Google Scholar]
- 41. Sprengel R , Braun T , Nikolics K , Segaloff DL , Seeburg PH. 1990. The testicular receptor for follicle stimulating hormone: structure and functional expression of cloned cDNA. Mol Endocrinol 4:525–530 [DOI] [PubMed] [Google Scholar]
- 42. Jeffs B , Ito M , Yu RN , Martinson FA , Wang ZJ , Doglio LT , Jameson JL. 2001. Sertoli cell-specific rescue of fertility, but not testicular pathology, in Dax1 (Ahch)-deficient male mice. Endocrinology 142:2481–2488 [DOI] [PubMed] [Google Scholar]
- 43. Itman C , Wong C , Hunyadi B , Ernst M , Jans DA , Loveland KL. 2011. Smad3 dosage determines androgen responsiveness and sets the pace of postnatal testis development. Endocrinology 152:2076–2089 [DOI] [PubMed] [Google Scholar]
- 44. Yoshida S , Sukeno M , Nakagawa T , Ohbo K , Nagamatsu G , Suda T , Nabeshima Y. 2006. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 133:1495–1505 [DOI] [PubMed] [Google Scholar]
- 45. Yoshinaga K , Nishikawa S , Ogawa M , Hayashi S , Kunisada T , Fujimoto T , Nishikawa S. 1991. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development 113:689–699 [DOI] [PubMed] [Google Scholar]
- 46. Meuwissen RL , Offenberg HH , Dietrich AJ , Riesewijk A , van Iersel M , Heyting C. 1992. A coiled-coil related protein specific for synapsed regions of meiotic prophase chromosomes. EMBO J 11:5091–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Habu T , Taki T , West A , Nishimune Y , Morita T. 1996. The mouse and human homologs of DMC1, the yeast meiosis-specific homologous recombination gene, have a common unique form of exon-skipped transcript in meiosis. Nucleic Acids Res 24:470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shannon M , Richardson L , Christian A , Handel MA , Thelen MP. 1999. Differential gene expression of mammalian SPO11/TOP6A homologs during meiosis. FEBS Lett 462:329–334 [DOI] [PubMed] [Google Scholar]
- 49. Kleene KC , Distel RJ , Hecht NB. 1984. Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev Biol 105:71–79 [DOI] [PubMed] [Google Scholar]
- 50. Geyer CB , Inselman AL , Sunman JA , Bornstein S , Handel MA , Eddy EM. 2009. A missense mutation in the Capza3 gene and disruption of F-actin organization in spermatids of repro32 infertile male mice. Dev Biol 330:142–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ahmed EA , de Rooij DG. 2009. Staging of mouse seminiferous tubule cross-sections. Methods Mol Biol 558:263–277 [DOI] [PubMed] [Google Scholar]
- 52. Reventos J , Sullivan PM , Joseph DR , Gordon JW. 1993. Tissue-specific expression of the rat androgen-binding protein/sex hormone-binding globulin gene in transgenic mice. Mol Cell Endocrinol 96:69–73 [DOI] [PubMed] [Google Scholar]
- 53. Vergouwen RP , Huiskamp R , Bas RJ , Roepers-Gajadien HL , Davids JA , de Rooij DG. 1993. Postnatal development of testicular cell populations in mice. J Reprod Fertil 99:479–485 [DOI] [PubMed] [Google Scholar]
- 54. Orth JM. 1982. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec 203:485–492 [DOI] [PubMed] [Google Scholar]
- 55. Vergouwen RP , Jacobs SG , Huiskamp R , Davids JA , de Rooij DG. 1991. Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil 93:233–243 [DOI] [PubMed] [Google Scholar]
- 56. Schauwaers K , De Gendt K , Saunders PT , Atanassova N , Haelens A , Callewaert L , Moehren U , Swinnen JV , Verhoeven G , Verrijdt G , Claessens F. 2007. Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc Natl Acad Sci USA 104:4961–4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lindsey JS , Wilkinson MF. 1996. Pem: a testosterone- and LH-regulated homeobox gene expressed in mouse Sertoli cells and epididymis. Dev Biol 179:471–484 [DOI] [PubMed] [Google Scholar]
- 58. Sivashanmugam P , Hall SH , Hamil KG , French FS , O'Rand MG , Richardson RT. 2003. Characterization of mouse Eppin and a gene cluster of similar protease inhibitors on mouse chromosome 2. Gene 312:125–134 [DOI] [PubMed] [Google Scholar]
- 59. Byers S , Graham R , Dai HN , Hoxter B. 1991. Development of Sertoli cell junctional specializations and the distribution of the tight-junction-associated protein ZO-1 in the mouse testis. Am J Anat 191:35–47 [DOI] [PubMed] [Google Scholar]
- 60. Mazaud-Guittot S , Meugnier E , Pesenti S , Wu X , Vidal H , Gow A , Le Magueresse-Battistoni B. 2010. Claudin 11 deficiency in mice results in loss of the Sertoli cell epithelial phenotype in the testis. Biol Reprod 82:202–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Willems A , De Gendt K , Allemeersch J , Smith LB , Welsh M , Swinnen JV , Verhoeven G. 2010. Early effects of Sertoli cell-selective androgen receptor ablation on testicular gene expression. Int J Androl 33:507–517 [DOI] [PubMed] [Google Scholar]
- 62. McCabe MJ , Allan CM , Foo CF , Nicholls PK , McTavish KJ , Stanton PG. 2012. Androgen initiates Sertoli cell tight junction formation in the hypogonadal (hpg) mouse. Biol Reprod 87:1–8 [DOI] [PubMed] [Google Scholar]
- 63. Tran D , Picard JY , Campargue J , Josso N. 1987. Immunocytochemical detection of anti-Müllerian hormone in Sertoli cells of various mammalian species including human. J Histochem Cytochem 35:733–743 [DOI] [PubMed] [Google Scholar]
- 64. Rajpert-De Meyts E , Jørgensen N , Graem N , Müller J , Cate RL , Skakkebaek NE. 1999. Expression of anti-Müllerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab 84:3836–3844 [DOI] [PubMed] [Google Scholar]
- 65. Chen Y , Ye S , Huang Y , Zheng W. 2000. Stage-dependent expression of androgen binding protein mRNA in sertoli cell of rat testis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 22:287–289 [PubMed] [Google Scholar]
- 66. Hill CM , Anway MD , Zirkin BR , Brown TR. 2004. Intratesticular androgen levels, androgen receptor localization, and androgen receptor expression in adult rat Sertoli cells. Biol Reprod 71:1348–1358 [DOI] [PubMed] [Google Scholar]
- 67. Meistrich ML. 1982. Quantitative correlation between testicular stem cell survival, sperm production, and fertility in the mouse after treatment with different cytotoxic agents. J Androl 3:58–68 [Google Scholar]
- 68. Shenker A , Laue L , Kosugi S , Merendino JJ , Minegishi T , Cutler GB. 1993. A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature 365:652–654 [DOI] [PubMed] [Google Scholar]
- 69. Diaz A , Danon M , Crawford J. 2007. McCune-Albright syndrome and disorders due to activating mutations of GNAS1. J Pediatr Endocrinol Metab 20:853–880 [DOI] [PubMed] [Google Scholar]