Abstract
Transient receptor potential (TRP) cation channels are widely expressed and function in many physiologically important processes. Perturbations in the expression or mutations of the channels have implications for diseases. Many thyroid disorders, as excessive growth or disturbed thyroid hormone production, can be a result of dysregulated TSH signaling. In the present study, we found that of TRP canonicals (TRPCs), only TRPC2 was expressed in Fischer rat thyroid low-serum 5% cells (FRTL-5 cells). To investigate the physiological importance of the channel, we developed stable TRPC2 knockdown cells using short hairpin RNA (shTRPC2 cells). In these cells, the ATP-evoked entry of calcium was significantly decreased. This led to increased cAMP production, because inhibitory signals from calcium to adenylate cyclase 5/6 were decreased. Enhanced cAMP signaling projected to Ras-related protein 1-MAPK kinase 1 (MAPK/ERK kinase 1) pathway leading to phosphorylation of ERK1/2. The activated ERK1/2 pathway increased the expression of the TSH receptor. In contrast, secretion of thyroglobulin was decreased in shTRPC2 cells, due to improper folding and glycosylation of the protein. We show here a novel role for TRPC2 in regulating thyroid cell function.
Transient receptor potential canonical (TRPC) channels have been studied extensively since the discovery of the first members of the family (1, 2). The TRPCs are the closest mammalian homologs to the drosophila TRP channel and function as nonselective cation channels that are permeable for calcium (3). The ability of TRPCs to function in receptor-operated calcium entry downstream of phospholipase C activation is well documented. The involvement of TRPCs in store-operated calcium entry (SOCE) is debatable (reviewed in Refs. 4, 5).
Detailed information regarding the physiological importance of several TRPCs is available (reviewed in Ref. 5). Information regarding TRPC2 is, however, limited. TRPC2 channels are expressed in rodents, with well-documented effects in the vomeronasal organ (6–8). Studies on TRPC2 knockout mice show that TRPC2 is involved in pheromone sensing in the vomeronasal organ and is important for sexual and social behavior (6, 8, 9). Furthermore, TRPC2 is important for evoking sustained calcium increase in sperm during the acrosome reaction (10). TRPC2 also evokes calcium signals in erythroblasts in response to stimulation with erythropoietin (11). Thus, there is evidence for TRPC2 participating in both receptor-operated calcium entry and SOCE (10–14).
The main function of thyroid epithelial cells is to produce T3 and T4. These are made from the large glycoprotein thyroglobulin (Tg), which is heavily processed before thyroid hormones are released. First, Tg undergoes processing in the endoplasmic reticulum (ER) and Golgi. Second, iodine is taken up from the blood by the sodium-iodine symporter (NIS), and Tg is iodinated by thyroid peroxidase (TPO). Finally, Tg is broken down, and T4 and T3 are released into the bloodstream (reviewed in Ref. 15). In regulating thyroid cell function, cAMP is perhaps the most important intracellular signaling molecule. However, investigations have shown that changes in intracellular-free calcium [Ca2+]i also regulate a multitude of central processes, including regulation of iodide efflux (16) and proliferation and DNA synthesis (17, 18). Furthermore, the effects evoked by the TSH may also be modified by changes in [Ca2+]i (16, 19). The regulation of TSH receptor (TSHR) expression (20) and the expression and dimerization of Tg (21, 22) are dependent on changes in [Ca2+]i. Many agonists, e.g. ATP and TSH, evoke potent changes in [Ca2+]i through activation of G protein-coupled receptors (23–26). Part of these changes in [Ca2+]i occur through inositol 1,4,5-trisphosphate-evoked release of calcium sequestered in the ER and entry of extracellular calcium due to SOCE (27). Furthermore, receptor-mediated calcium entry pathways are also present, because the Fischer rat thyroid low-serum 5% (FRTL-5) cells express several P2X ionotropic receptors for ATP (18).
The existence of TRPC channels in thyroid cells has not been shown. In the present study, we show that FRTL-5 cells express exclusively TRPC2 channels. We used short hairpin RNA (shRNA) to make stable cell lines expressing control shRNA (shC) or shRNA against TRPC2 (shTRPC2). We observed that the ATP-evoked calcium entry was markedly reduced in shTRPC2 cells, compared with shC cells. The production of cAMP was increased, partly due to up-regulation of the TSHR, and partly due to removal of calcium's inhibitory effect on adenylyl cyclases (ACs) in shTRPC2 cells. We propose a mechanism where communication between the cAMP and the calcium pathways regulates the expression of the TSHR. In addition, we show that TRPC2 is important for posttranslational processing and secretion of Tg.
Materials and Methods
Materials
ATP, puromycin, tunicamycin, forskolin, insulin, Gly-His-Lys, ethidium bromide, poly-l-lysine, 3-isobutyl-1-methylxanthine, and Coon's modified Ham's F12 were from Sigma-Aldrich Corp. (St. Louis, MO). Bovine TSH was obtained from A. F. Parlow (the National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Peptide Program, Torrance, CA). 1,4-Diamino-2,3-dicyano-1,4-bis(2-aminophenylthio) butadiene (U0126) was from Calbiochem (Darmstadt, Germany). Endoglycosidase H (Endo H) was from New England Biolabs (Ipswich, MA). Transferrin, hydrocortisone, somatostatin, fura 2-AM, 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM), RiboGreen RNA Quantitation Reagent, SuperScript III Reverse Transcriptase, and OptiMEM were from Life Technologies (Grand Island, NY). Oligo(dT)15 primers was from Promega (Madison, WI). DynaZyme EXT DNA polymerase was from Finnzymes (Helsinki, Finland). ABsolute QPCR ROX Mix reference dye was from Abgene (Rochester, NY). FuGENE HD was from Roche (Basel, Switzerland). BTX Cuvettes Plus were from Harvard Apparatus (Holliston, MA). Aurum Total RNA Mini kit was from Bio-Rad Laboratories (Hercules, CA). Direct Cyclic AMP Enzyme Immunoassay kit was from AssayDesigns, Inc. (Ann Arbor, MI). A/G PLUS-agarose beads was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Western Lightning Plus-ECL substrate and Protran nitrocellulose membrane were from PerkinElmer, Inc. (Waltham, MA). All other chemicals were of reagent grade.
Antibodies and respective dilutions used were: rabbit anti-TRPC2 (1:1000; Novus Biologicals, Littleton, CO); goat anti-TSHR (N-19) (1:500), mouse anti-TSHR (3B12) (1:200), goat anti-Tg (N-15) (1:1000), mouse antithyroid transcription factor (TTF)-1 (F-12) (1:1000), mouse antipaired box gene 8 (Pax8) (PAX8R1) (1:1000), mouse anti-TPO (MoAb47) (1:1000), and goat anti-NIS (D-16) (1:1000) (all from Santa Cruz Biotechnology, Inc.); rat anti-heat-shock cognate protein 70 (Hsc70) (IB5) (1:5000; Stressgen Biotechnology, San Diego, CA); mouse anti-phospho (p)ERK1/2 (E10) (1:1000) and rabbit anti-ERK1/2 (137F5) (1:3000) (Cell Signaling Technology, Danvers, MA); antigoat horseradish peroxidase (HRP)-linked secondary antibody (1:3000; Santa Cruz Biotechnology, Inc.), antimouse (1:3000) and antirat (1:3000) HRP-linked secondary antibodies (Cell Signaling Technology); and antirabbit HRP-linked secondary antibody (1:3000; Bio-Rad Laboratories).
Plasmid constructs
The yellow fluorescent protein (YFP) plasmid (pEYFP-N1) was from CLONTECH (Mountain View, CA). Truncated TRPC2 [dominant-negative TRPC2 (TRPC2DN)] was provided by Genevieve Bart (University of Eastern Finland, Kuopio, Finland). MAPK kinase (MEK)1-S218E/S222D and MEK1-K97M have previously been described (28). Phosphodiesterase 4D3 (PDE4D3) construct (29) was provided by Wito Richter (University of California, San Francisco, CA). Rap1A17N was provided by Jean de Gunzburg (Institut Curie, Paris, France). The receptor-transporting protein 1 (RTP1) plasmid was provided by Debra Fadool (Florida State University, Tallahassee, FL).
The rat green fluorescent protein-TRPC2 construct was made as follows. Rat TRPC2 cDNA was a gift from Catherine Dulac (Harvard University, Cambridge, MA). The cDNA was cut out from the pBluescript cloning vector with BamHI and NcoI. After blunting the NcoI site, the fragment was subcloned into pEGFP-C3 (CLONTECH) cut with BglII and SmaI. By using the BamHI site, the initiator methionine was deleted from the TRPC2 gene, and the fusion generated the EGFP-TRPC2 construct.
Cell culture
FRTL-5 cells, originally from the Interthyr Foundation (Bethesda, MD), were grown in Coon's modified Ham's F12 medium supplemented with 5% calf serum, 50 U/ml penicillin and 50 μg/ml streptomycin, and six hormones (6H) (10 μg/ml insulin, 5 μg/ml transferrin, 10 nm hydrocortisone, 10 ng/ml tripeptide Gly-His-Lys, 0.3 mU/ml TSH, and 10 ng/ml somatostatin). The PC-12 cell line was grown in DMEM supplemented with 2 mm l-glutamine, 10% fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. The cell cultures were maintained in a water-saturated atmosphere of 5% CO2 and 95% air at 37 C.
Primary thyroid cell isolation
Rat thyroid cells were isolated according to Eggo et al. (30). In brief, rat thyroid tissue was digested with 0.2% collagenase, and follicles were plated on poly-l-lysine-coated culture dishes in Coon's modified Ham's F12 medium supplemented with 1% FBS, 0.3 mU/ml TSH, and 1 μg/ml insulin to allow cells to attach. After 3 d, the medium was changed to serum-free Coon's modified Ham's F12 medium supplemented with 0.3 mU/ml TSH and 1 μg/ml insulin to promote thyroid cell survival and prevent the survival of contaminating cells. Cells were used for experiments after 2 wk in serum-free conditions. In some experiments, mRNA was isolated directly from freshly dissected rat thyroid tissue.
Transfection and generation of stable cell lines
FRTL-5 cells were plated on 12-well plates. The day after, transfections were carried out with FuGENE HD and SureSilencing shRNA plasmids (SABiosciences, Frederick, MD) according to the manufacturers' instructions. Forty-eight-hour posttransfection untransfected cells were killed with 1 μg/ml puromycin. Puromycin was included in the growth media from here on. The knockdown of TRPC2 was measured on mRNA level with quantitative PCR (qPCR) and on protein level with Western blotting.
Transient transfections were done as follows. Cells from a 100-mm culture dish were detached, and 4 × 106 cells were pelleted and resuspended in 400 μl of OptiMEM together with 20 μg of plasmid DNA. The cells were then electroporated in BTX cuvettes (975 μF and 240 V) using a Bio-Rad Gene Pulser Xcell (Bio-Rad Laboratories). The electroporated cells were used for experiments after 48–72 h.
Qualitative RT-PCR and qPCR
RNA was isolated using the Aurum Total RNA Mini kit according to the manufacturer's instructions. RNA quality and integrity was tested by absorbance spectrometry and agarose gel electrophoresis, respectively. RNA concentrations were determined using the RiboGreen RNA Quantitation Reagent. Reverse transcriptase reactions were performed on 0.25 μg of RNA using SuperScript III Reverse Transcriptase and Oligo(dT)15 primers following the manufacturers' instructions.
The TRPC primer sequences for qualitative RT-PCR were obtained from Babich et al. (31), all other primers used were designed. All primers used are listed in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org. The PCR was performed in 50-μl reactions (5 μl of cDNA, 1 mm primers, 200 nm each of dATP, dCTP, dGTP, and dCTP, and 0.5 U of DynaZyme EXT DNA polymerase on a Mastercycler gradient thermal cycler (Eppendorf, Hamburg, Germany) with activation step at 94 C for 5 min followed by 30 cycles with strand separation at 94 C for 30 sec and annealing for 60 sec (temperatures listed in Supplemental Table 1) followed by elongation at 72 C for 60 sec. Negative controls, where reverse transcriptase was omitted from the cDNA reaction, were performed to rule out that the RNA samples were contaminated with genomic DNA. The PCR products were separated by gel electrophoresis and visualized with ethidium bromide under UV light.
qPCR assays were designed using the Universal ProbeLibrary Assay Design Center (Roche). The used primer sequences and probes are listed in Supplemental Table 1. qPCR reactions of 10 μl were used, containing 100 nm universal probe library probe or 200 nm normal length probe (for β-actin or glyceraldehyde-3-phosphate dehydrogenase), 300 nm forward and reverse primers, 1× ABsolute QPCR ROX Mix, and cDNA. The Absolute QPCR ROX Mix contains Thermo-Start DNA Polymerase, proprietary reaction buffer, dNTPs and ROX reference dye. The qPCR was performed with the Applied Biosystems 7900HT Fast Sequence Detection System (Applied Biosystems, Foster City, CA) with a 15-min activation step at 95 C and 40 cycles with a strand separation step in 95 C for 15 sec and an annealing and elongation step at 60 C for 1 min. The amplification results were analyzed using SDS and RQ Manager programs (Applied Biosystems).
Calcium measurements
Cells cultured on poly-l-lysine-coated coverslips were washed twice with HEPES-buffered salt solution (HBSS) consisting of 118 mm NaCl, 4.6 mm KCl, 1 mm CaCl2, 10 mm glucose, and 20 mm HEPES (pH 7.4) and incubated with 2 μm fura 2-AM for 30 min at room temperature and then washed with HBSS. The coverslip was placed in a perfusion chamber mounted on a Zeiss Axiovert 35 microscope (Zeiss, Oberkochen, Germany). For experiments in calcium-free HBSS, CaCl2 was omitted and 150 μm EGTA added. The 340- and 380-nm excitation filters were used and emission was measured at 510 nm. Light was obtained from an XBO 75W/2 xenon lamp. The filter wheel was controlled by a Lambda 10-2 control device (Sutter Instruments, Novato, CA), and images were collected with a SensiCam CCD camera (PCO/CD Imaging, Kelheim, Germany). The images were processed using Axon Imaging Workbench 5.1 software (Axon Instruments, Foster City, CA). The F340/F380 ratio was used as a measure of intracellular calcium concentration.
Electrophysiology
FRTL-5 cells were plated at low density on 13-mm coverslips. Recordings were done at room temperature (23–25 C). Coverslips were placed in a chamber of 200 μl and continuously perfused at flow rate of 700 μl/min with an extracellular solution comprised of 150 mm NaCl, 5.4 mm CsCl, 3.0 mm CaCl2, and 5 mm HEPES (pH 7.40 with NaOH). Patch pipettes (3–8 mΩ) were filled with a solution containing 135 mm Cs-methanesulfonate, 10 mm CsCl, 4.25 mm Na2ATP, 0.5 mm LiGTP, 125 μm EGTA, and 10 mm HEPES (pH 7.20 with CsOH). The liquid junction potential of 15 mV was estimated with JPCalc software (32) and was subtracted off-line.
Whole-cell membrane currents (33) were recorded with an EPC-9 amplifier and Pulse software (HEKA Elektronik, Lambrecht, Germany). Cells were stimulated with a series of rectangular voltage pulses (ranging from −135 to +105 mV, increment 10 mV, and duration 100 msec) that were preceded and followed by steps of 100 and 50 msec to −95 mV. Protocol was applied at 2-sec intervals, during which cells were held at −15 mV. Whole-cell capacitance and series resistance were 80% compensated for, and currents were sampled at 20 kHz and filtered at 10 kHz. Analysis and graphics were done using PulseFit (HEKA Elektronik) and Origin (OriginLab, Northampton, MA) software. Mean current level in the end of voltage pulse series was measured (time window 10 msec).
cAMP production
Cells were plated on 24-well culture dishes and grown in 6H medium for 24 h. Experiments were done on cells grown in normal growth media (6H) or on cells grown deprived of TSH for 3 d in TSH-free media (5H). The cells were stimulated for 30 min with the indicated concentrations of either forskolin or TSH in a humidified 5% CO2 atmosphere at 37 C. In experiments with BAPTA-AM, the cells were preincubated with 10 μm BAPTA-AM for 30 min before the experiment. A phosphodiesterase inhibitor (3-isobutyl-1-methylxanthine, 100 μm) was included in all experiments. The incubation was terminated by three washes with cold PBS. Cells were lysed in 0.1 m HCl containing 0.1% Triton X-100 for 10 min. cAMP concentrations in the samples were determined with Direct Cyclic AMP Enzyme Immunoassay kit according to the manufacturer's instructions. Levels of cAMP in the samples were normalized to protein concentration.
Tg immunoprecipitation
Cells were plated on 100-mm culture dishes and grown in 6H medium. After 48 h, equal amounts of media were collected and incubated with goat anti-Tg antibody. The samples were incubated under rotation overnight at 4 C. The day after, protein A/G PLUS-agarose beads were added and the samples incubated for 2 h. The beads where pelleted by centrifugation (1000 × g, 2 min) and washed three times with immunoprecipitation wash buffer [50 mm Tris (pH 7.5), 250 mm NaCl, 0.1% Nonidet P-40, and 0.05% sodium deoxycholate]. After the last wash, the beads where resuspended in Laemmli buffer and boiled for 5 min. The beads were pelleted by centrifugation and the supernatant run on a 6% SDS-PAGE gel. Proteins were then transferred onto a nitrocellulose membrane and incubated with goat anti-Tg primary antibody and mouse antigoat HRP-linked secondary antibody. The amount of secreted Tg was normalized against mature and immature forms of intracellular Tg from respective cell lysate.
Western blotting
Cells were washed three times with ice-cold PBS and scraped in lysis buffer [10 mm Tris/HCl (pH 7.7), 150 mm NaCl, 7 mm EDTA, 0.5% Nonidet P-40, 0.2 mm phenylmethylsulfonylfluoride, and 0.5 μg/ml leupeptin]. The lysates were incubated on ice for 15 min and then centrifugated at 10,000 × g for 15 min. Proteins were separated on 6–10% SDS-PAGE, depending on the size of the protein of interest, and then transferred onto a nitrocellulose membrane. The membranes were incubated with primary antibody of interest and HRP-linked secondary antibody. Protein bands were detected using Western Lightning Plus enhanced chemiluminescence substrate. All Western blottings were reblotted and normalized to a loading control (Hsc70 or ERK1/2). The ImageJ program (34) was used to quantify band intensities.
Statistics
Results are expressed as means ± sem from at least three independent experiments. Student's t test was used when two means were compared. One-way ANOVA and Bonferroni's post hoc test were used when three or more means were compared. Two-way ANOVA was used for comparing concentration responses. A P value less than 0.05 was considered statistically significant.
Results
Expression and knockdown of TRPC2 in FRTL-5 cells
The identity of the channels mediating receptor-evoked calcium entry is unclear in thyroid cells. Therefore, we investigated which TRPC channels are present in rat thyroid FRTL-5 cells. An electrophysiological screening of the currents in these cells using monovalent and divalent cation solutions, and the TRPC antagonists 2-aminoethoxyphenyl borate and SKF96365, suggested the presence of TRPC cation channels (Supplemental Fig. 1). In line with these results, we found that FRTL-5 cells only express TRPC2 on mRNA level (Fig. 1A). Primary rat thyroid cells also expressed TRPC2, which suggests that TRPC2 could be of importance also in vivo. In addition to TRPC2, TRPC5 was also expressed in primary thyroid cells. To determine what functions TRPC2 may have in FRTL-5 cells, stable cell lines expressing a negative control construct (shC) or a shRNA construct directed against TRPC2 (shTRPC2) were generated. The knockdown of TRPC2 in shTRPC2 cells was verified by qPCR (Fig. 1B) and Western blotting (Fig. 1B, inset).
Fig. 1.
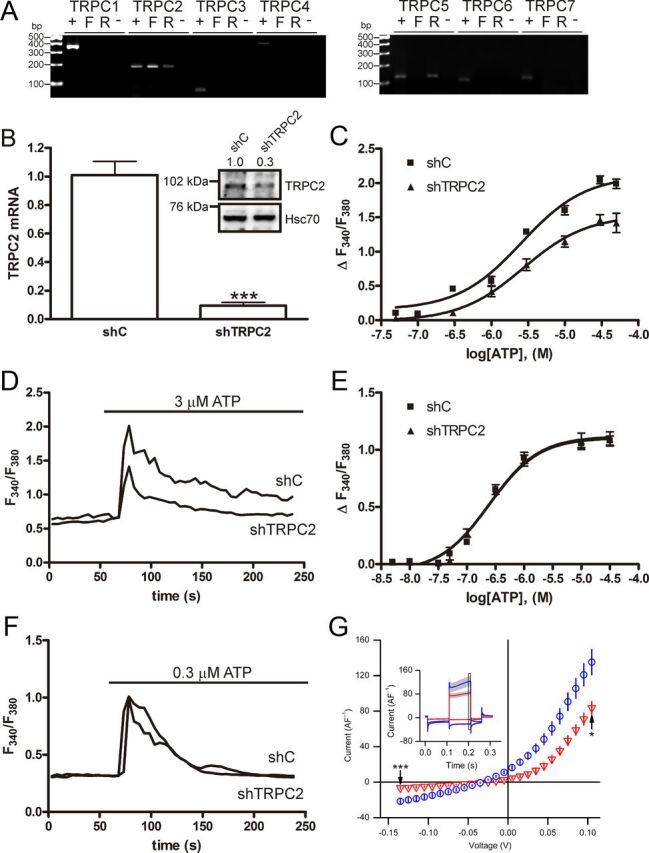
Expression and knockdown of TRPC2 in FRTL-5 cells. A, RT-PCR screen of TRPC isoforms in FRTL-5 cells and primary thyroid cells isolated from rat. +, Positive control RNA used for the primers. Rat testis RNA was used as positive control for all TRPCs except for TRPC3, where RNA from PC-12 cells was used. F, FRTL-5 cell RNA; R, rat thyroid RNA; −, negative control with FRTL-5 cell RNA that has not been reverse transcribed. B, Relative expression of TRPC2 in shC-expressing cells and cells expressing a shTRPC2 construct (shTRPC2), as measured by qPCR (the bars give the mean ± sem, n = 3, *** P < 0.001). The inset shows TRPC2 protein expression in shC and shTRPC2 cells, relative band densities are included above the Western blotting (n = 3, P < 0.05). C, Concentration-response curves for ATP in a calcium-containing buffer (P < 0.001). D, Example traces of cells stimulated in a calcium-containing buffer with the EC50 concentration of ATP. E, Concentration-response curves for ATP in a calcium-free buffer. F, Example traces of cells treated with the EC50 concentration for ATP in a calcium-free buffer. The data give the mean ± sem of at least 50 cells. G, Electrophysiological comparison of shTRPC2 (red triangles, n = 13) and shC (blue circles, n = 16) cell lines revealed that the suppression of TRPC2 expression reduced both the inward and outward whole-cell membrane current. The mean current amplitude in shTRPC2 cells was significantly smaller both at the −135 and +105 mV levels (marked with black arrows; **, P < 0.01 and ***, P < 0.001). In the inset, averaged current traces (±se) generated by the voltage stimulus series at −135 and +105 mV levels are shown for shC (blue) and shTRPC2 cells (red). The 10-msec analysis window is indicated with a black square.
TRPC2 has been shown to be activated downstream of purinergic receptor activation (10). Because FRTL-5 cells express several purinergic receptors (18), we used ATP as an agonist to determine whether TRPC2 is functional in FRTL-5 cells. When stimulating shC and shTRPC2 cells with ATP in a calcium-containing buffer, calcium elevation was markedly attenuated in shTRPC2 cells (Fig. 1, C and D). In contrast, no differences in calcium peak amplitude were seen in calcium-free buffer (Fig. 1, E and F). Whole-cell patch-clamp recordings demonstrated that resting membrane currents were smaller in shTRPC2 cells than in shC cells. The mean inward current at −135 mV was almost fully attenuated in shTRPC2 cells. Outward currents at +105 mV were suppressed in shTRPC2 cells (Fig. 1G). Thus, FRTL-5 cells express functional TRPC2 channels that show constitutive activity and function in a receptor-operated fashion downstream of purinergic receptor activation. Furthermore, the results indicate that FRTL-5 cells are a good model for studying the physiological importance of endogenous TRPC2.
Because there was a dramatic decrease of calcium entry when reducing the expression of TRPC2, we wanted to confirm that shTRPC2 cells still were expressing thyroid-specific transcription factors and had not dedifferentiated. The expression of TTF-1, Pax8, TPO, and NIS did not differ between shC and shTRPC2 cells (Supplemental Fig. 2, A–D).
cAMP production is negatively regulated by TRPC2
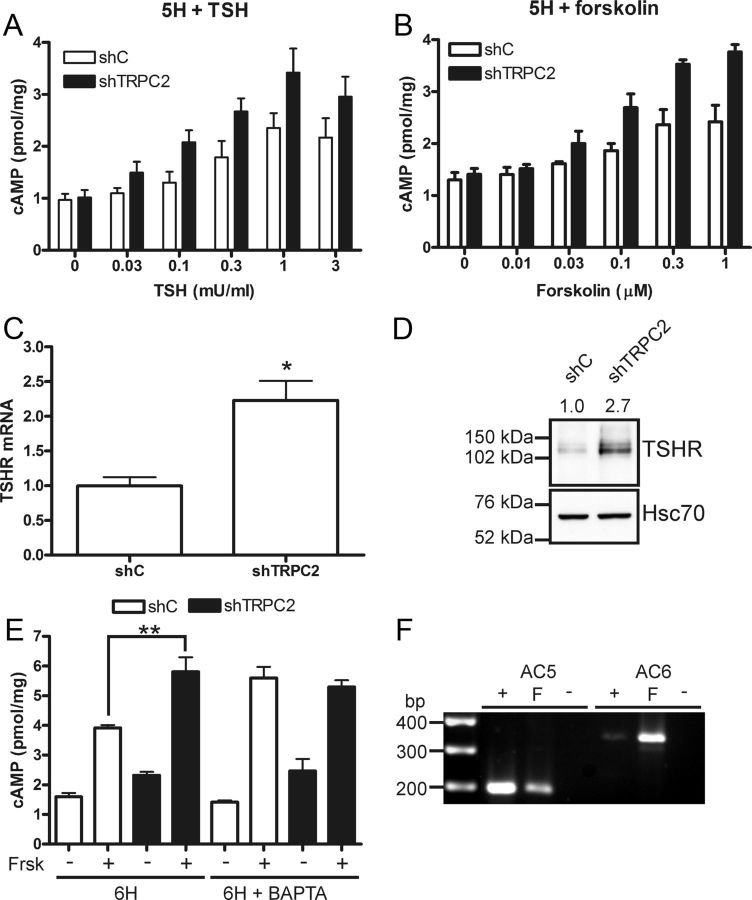
The cAMP pathway is of great importance in thyroid cells. Stimulating the TSHR will activate Gαs, which subsequently will activate ACs and production of cAMP (reviewed in Ref. 35). We next investigated whether TRPC2 is involved in regulating the production of cAMP. To measure cAMP production, the cells were cultured for 3 d without TSH in the growth media. Interestingly, more cAMP was produced after stimulation with TSH in a concentration-dependent manner in shTRPC2 cells, compared with shC cells (Fig. 2A). A similar potentiation of the response to the direct AC stimulator, forskolin, was seen in shTRPC2 cells (Fig. 2B). Even without previous TSH deprivation, forskolin-induced cAMP production was increased in shTRPC2 cells (Supplemental Fig. 2E).
Fig. 2.
cAMP production is inhibited by TRPC2. A, Concentration-response curve with the indicated concentrations for TSH-induced (30 min) production of cAMP in TSH-starved (5H) shC and shTRPC2 cells. Data are given as the mean ± sem, n = 4–5, P < 0.01. B, Concentration-response curve with the indicated concentrations for forskolin-induced (30 min) production of cAMP in TSH-starved (5H) shC and shTRPC2 cells. Data are presented as the mean ± sem, n = 4, P < 0.001. C, The TSHR is up-regulated on mRNA level, bars indicate means ± sem, n = 3; *, P < 0.05. D, The TSHR is up-regulated on protein level in shTRPC2 cells. The relative band densities is included above the Western blotting (n = 3, P < 0.05). E, Chelation of intracellular calcium with BAPTA (10 μm, 30-min preincubation) in normal growth media (6H) removes differences between shC and shTRPC2 cells in forskolin (Frsk)-induced (10 μm, 30 min) cAMP production (n = 3; **, P < 0.01). F, A PCR screen for ACs inhibited by calcium in FRTL-5 cells. +, Positive control RNA from rat brain; F, FRTL-5 cell RNA; −, a PCR done on FRTL-5 cell RNA that has not been reverse transcribed.
An increased cAMP production in response to TSH suggests that the TSHR may be up-regulated. Indeed, TSHR was up-regulated on both mRNA (Fig. 2C) and protein level (Fig. 2D). However, the up-regulation of TSHR in shTRPC2 cells does not readily explain the results obtained with forskolin. Because calcium entry is decreased in the shTRPC2 cells and TRPC2 has basal activity, we next studied whether calcium has an inhibitory effect on cAMP production. Forskolin-induced cAMP production was measured in the presence of the calcium chelator BAPTA to determine whether the suppressed calcium responses seen in shTRPC2 cells are the cause of increased cAMP signals in these cells. In the presence of BAPTA, the forskolin-induced cAMP production increased in shC cells to the same level as seen in shTRPC2 cells, whereas the shTRPC2 cells were not affected by BAPTA (Fig. 2E). BAPTA potently inhibits the robust ATP-evoked calcium transients in shC and shTRPC2 cells (Supplemental Fig. 2, F and G). Taken together, there are ACs constitutively inhibited by calcium present in FRTL-5 cells, and calcium signals originating from TRPC2 are suppressing their activity. There are several AC isoforms that are regulated by calcium, we therefore studied which ACs are expressed in FRTL-5 cells. On mRNA level, we found that FRTL-5 cells express AC5 and AC6 (Fig. 2F), both of which are inhibited by calcium (reviewed in Ref. 36). In addition, FRTL-5 cells express several AC isoforms that are not inhibited by calcium, namely AC1, AC4, AC7, and AC9 (results not shown).
ERK1/2 regulates the expression of the TSHR
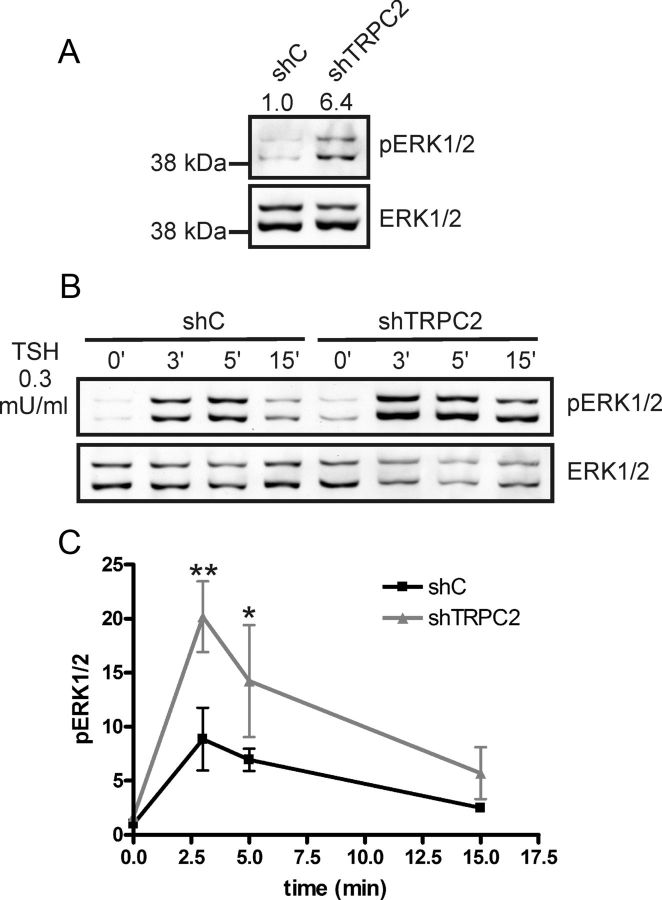
In FRTL-5 thyroid cells, cAMP activates ERK1/2 via a small guanosine triphosphatase Rap1-dependent cascade (37). We therefore investigated whether TRPC2 knockdown has an effect on the phosphorylation of ERK1/2. The basal phosphorylation of ERK1/2 was increased in shTRPC2 cells (Fig. 3A). In serum and hormone-starved cells, the phosphorylation of ERK1/2 was more strongly increased in shTRPC2 than in shC cells also when stimulated with 0.3 mU/ml TSH (Fig. 3, B and C). This TSH concentration did not elicit calcium transients in shC or shTRPC2 cells (results not shown).
Fig. 3.
The phosphorylation of ERK1/2 is increased in shTRPC2 cells. A, The phosphorylation of ERK1/2 is increased in shTRPC2 compared with shC cells under normal growth conditions. Relative band densities are included above the Western blotting (n = 4, P < 0.001). B, Serum and hormone-starved cells stimulated with TSH (0.3 mU/ml) for the indicated time points. C, Quantification of results in B, significant differences at 3 and 5 min (n = 3; *, P < 0.05 and **, P < 0.01).
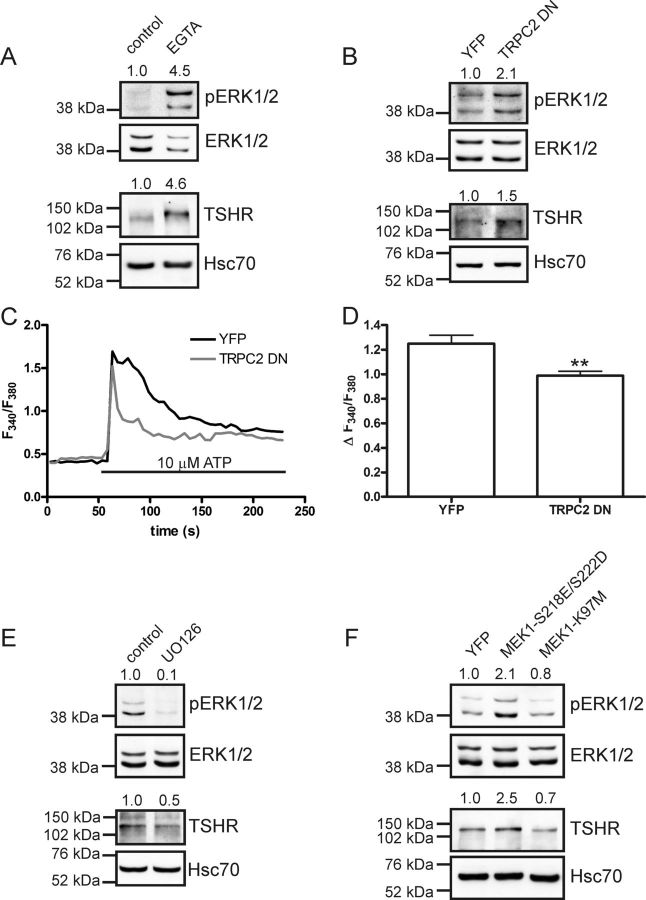
To investigate whether there is a link between calcium entry, pERK1/2, and increased expression of the TSHR, we chelated extracellular calcium with EGTA (5 mm, 24 h). Chelating extracellular calcium resulted in an increased phosphorylation of ERK1/2 and also an increase in TSHR protein in native FRTL-5 cells (Fig. 4A). Furthermore, by transiently transfecting a truncated form of TRPC2 (TRPC2DN) into native FRTL-5 cells, we could increase both pERK1/2 and the TSHR (Fig. 4B). The TRPC2DN construct had a similar effect on ATP-induced calcium entry as knockdown of TRPC2 (Fig. 4, C and D, compare with Fig. 1, C and D). To test the hypothesis that the MEK/ERK1/2 pathway regulates the expression of TSHR in FRTL-5 cells, we treated native FRTL-5 cells with the MEK-inhibitor U0126 (10 μm, 24 h). With this treatment, both ERK1/2 phosphorylation and TSHR expression were reduced (Fig. 4E). Furthermore, we used a constitutively active mutant (MEK1-S218E/S222D) or a catalytically inactive mutant (MEK1-K97M) of MEK1. Expression of the constitutively active mutant increased ERK1/2 phosphorylation and TSHR expression, whereas the catalytically inactive mutant was without any significant effect (Fig. 4F).
Fig. 4.
Calcium and the MEK/ERK1/2 pathway regulate TSHR expression in FRTL-5 cells. A, Treating FRTL-5 cells with EGTA (5 mm, 24 h) elevated pERK (n = 4, P < 0.001) and also increased TSHR expression (n = 4, P < 0.001). B, Transient expression of truncated (dominant-negative) TRPC2 construct (TRPC2DN) increased both pERK and TSHR (n = 4, P < 0.05 and n = 6, P < 0.01, respectively). C, The TRPC2DN construct reduced ATP-induced calcium transients as compared with YFP-transfected controls. D, Quantification of results from C, bars indicate means ± sem, n ≥ 35; **, P < 0.01. E, Inhibiting MEK with U0126 (10 μm, 24 h) reduced both pERK1/2 (n = 3, P < 0.05) and the TSHR (n = 4, P < 0.01) in native FRTL-5 cells. F, Transient expression of a constitutively active MEK1 mutant (MEK1–S218E/S222D) in FRTL-5 cells increased pERK (n = 3, P < 0.05), whereas transient expression of a catalytically inactive mutant (MEK1–K97M) had no significant effect. Relative band densities are included above the representative Western blottings.
Enhanced cAMP signaling in shTRPC2 cells increases the expression of the TSHR
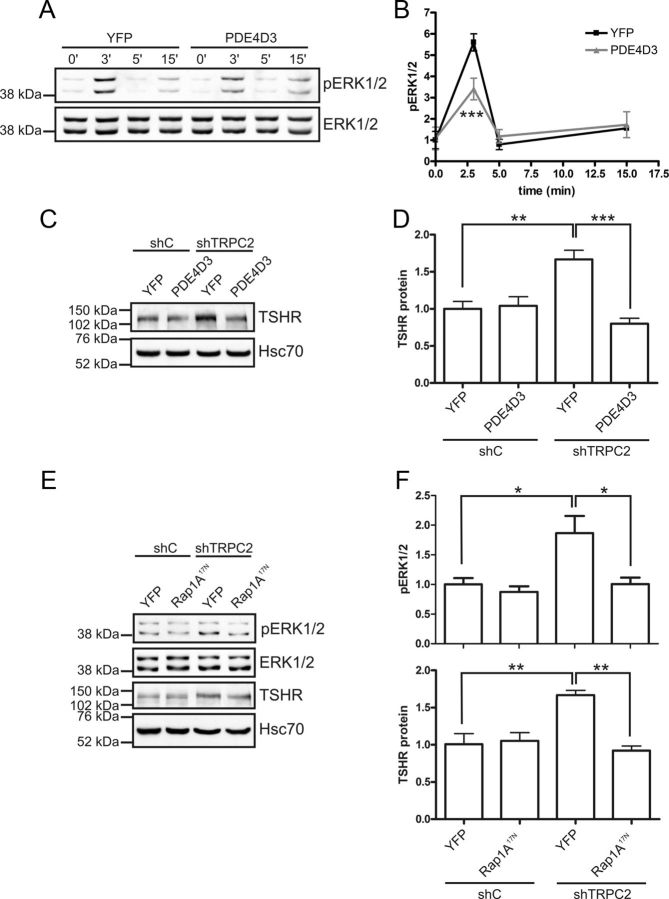
With the aforementioned pieces of the puzzle in place, we inhibited different effectors of the cAMP pathway to gain direct evidence for their involvement in regulating the expression of the TSHR. First, we overexpressed the cAMP-specific PDE4D3 to investigate whether increased breakdown of cAMP would reduce the expression of the TSHR. Heterologously overexpressed PDE4D3 has been shown to effectively reduce cAMP levels (38). Overexpression of PDE4D3 did not affect pERK1/2 in shC or shTRPC2 cells under normal growth conditions (results not shown). However, PDE4D3 overexpression reduced the phosphorylation of ERK1/2 in serum and hormone-starved shTRPC2 cells that were acutely stimulated with TSH (Fig. 5, A and B). Overexpression of PDE4D3 did not have any significant effects in shC cells (results not shown). TSHR expression was reduced to control level by transiently expressing PDE4D3 in shTRPC2 cells (Fig. 5, C and D). We then turned our focus on Rap1, a guanosine triphosphatase that can be activated downstream of cAMP. We used the dominant- negative form of Rap1A (Rap1A17N) to inhibit Rap1A. In line with our hypothesis, Rap1A17N reduced both pERK1/2 and TSHR in shTRPC2 cells but was without an effect in shC cells (Fig. 5, E and F).
Fig. 5.
The cAMP pathway up-regulates TSHR in shTRPC2 cells. A and B, In serum and hormone-starved cells, TSH-induced phosphorylation of ERK1/2 is reduced in shTRPC2 cells that overexpress PDE4D3 (n = 3; ***, P < 0.001). C and D, Reducing cAMP by overexpressing PDE4D3 decreased TSHR expression in shTRPC2 cells. The data are presented as means ± sem, n = 5; **, P < 0.01 and ***, P < 0.001. E and F, Introducing a dominant-negative form of Rap1A (Rap1A17N) into shTRPC2 cells decreases basal pERK1/2 (n = 4, P < 0.05) and TSHR to control level (n = 6, P < 0.01).
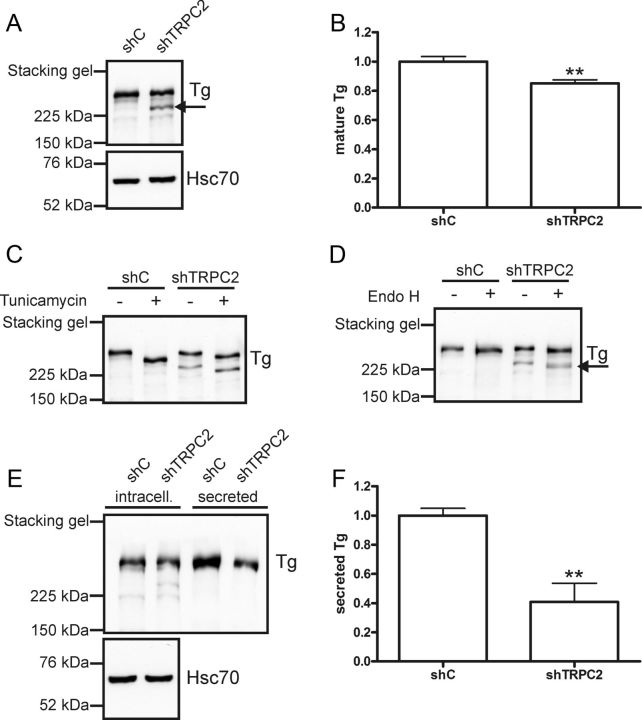
TRPC2 is important for correct posttranslational modification and secretion of Tg
The major function for thyroid epithelial cells is to produce thyroid hormones T3 and T4. FRTL-5 cells do not produce any detectable amounts of thyroid hormones. However, they produce and secrete the precursor to thyroid hormones, Tg. Therefore, we wanted to investigate whether knockdown of TRPC2 affects Tg expression or secretion. When measuring intracellular Tg protein, we detected an additional band with a lower molecular weight in the shTRPC2 cells (Fig. 6A). This band was likely an immature form of Tg, because Tg is heavily glycosylated during maturation. The total Tg content was equal in shC and shTRPC2 cells, indicating that there is no change in total Tg expression in the shTRPC2 cells. In agreement with this, there was no change in Tg mRNA expression between shC and shTRPC2 cells (Supplemental Fig. 2H). However, when comparing only the larger molecular weight band, there was a significant decrease (Fig. 6B).
Fig. 6.
Tg maturation and secretion is perturbed in shTRPC2 cells. A, Western blotting with an anti-Tg antibody revealed two distinct bands, the smaller molecular weight band in cell lysates from shTRPC2 cells probably reflects an immature form of Tg (shown by an arrow). B, When comparing only the mature form, there was a significant decrease of Tg in shTRPC2 cells (n = 7; **, P < 0.01). C, Tunicamycin (1 μg/ml, 16 h) evoked a mobility shift of both mature and immature forms of Tg. D, Endo H (500 U/20 μg protein, 1 h, 37 C) evoked a mobility shift of the immature form of Tg in shTRPC2 cells only (shown by an arrow). E, Less Tg was immunoprecipitated from the media of shTRPC2 cells. F, Quantification of results from E, bars indicate means ± sem, n = 4; **, P < 0.01. Immunoprecipitated Tg was normalized to intracellular Tg from corresponding cell lysate.
Calcium is necessary for the maturation and dimerization of Tg (39). Because there was a decrease in mature Tg in shTRPC2 cells, we investigated the reason for this. To test whether the immature form of Tg is glycosylated, the cells were treated with tunicamycin (1 μg/ml, 16 h). Tunicamycin blocks GlcNAc phosphotransferase, which catalyzes the first step in N-linked glycoprotein synthesis. Tunicamycin treatment resulted in a mobility shift of all bands, indicating that even the additional lower molecular weight band in shTRPC2 cells was glycosylated (Fig. 6C). Endo H is commonly used to show that sugar residues added to the protein of interest in the ER have been processed by Golgi α-mannosidase II, i.e. that a protein has reached the Golgi. Glycoproteins are resistant to Endo H treatment after they have been processed in the Golgi. We treated cell lysates from shC and shTRPC2 cells with Endo H. Only the additional smaller molecular weight band in the shTRPC2 cells was sensitive to Endo H treatment (Fig. 6D), indicating that this immature form of Tg is retained in the ER. Based on these results, part of the Tg seemed to be retained in the ER, which could affect the amount of Tg secreted from the cells. To test this hypothesis, media were collected from shC and shTRPC2 cells, and secreted Tg was immunoprecipitated with an anti-Tg antibody (Fig. 6E). Densitometrical values of secreted Tg were normalized to respective densitometrical value of intracellular Tg. Indeed, less Tg was secreted from shTRPC2 cells as compared with shC cells (Fig. 6F).
Effects seen in shTRPC2 cells are reversed by coexpression of GFP-TRPC2 with RTP1
To conclude that the effects seen in the shTRPC2 cells are specific for TRPC2 knockdown, we reintroduced TRPC2 to see whether the effects were reversed. Previous results have shown that the transmembrane chaperone protein RTP1 increases plasma membrane insertion of TRPC2 (40). Transcripts for RTP1 were detected in FRTL-5 cells (Supplemental Fig. 2I). Transient expression of RTP1 in shC and shTRPC2 cells had no effects on ATP-induced calcium transients (Fig. 7, A and B). YFP had no effects on calcium transients in shTRPC2 cells (results not shown). However, the ATP-evoked calcium responses were increased to control levels by transient expression of GFP-TRPC2 alone or with RTP1 in shTRPC2. We then investigated whether the effects on pERK1/2 and TSHR also were rescued. Expressing RTP1 alone had no effect on pERK1/2 or TSHR (Fig. 7, C–F). However, a significant decrease in pERK was seen in shTRPC2 cells coexpressing RTP1 with GFP-TRPC2 (Fig. 7, C and D). In agreement with our hypothesis that the phosphorylation of ERK1/2 regulates TSHR expression, we saw a reduction in TSHR protein to control level when coexpressing RTP1 with GFP-TRPC2 in shTRPC2 cells (Fig. 7, E and F). The immature form of Tg seen in shTRPC2 cells was also significantly reduced by coexpressing RTP1 with GFP-TRPC2 (Fig. 7, G and H).
Fig. 7.
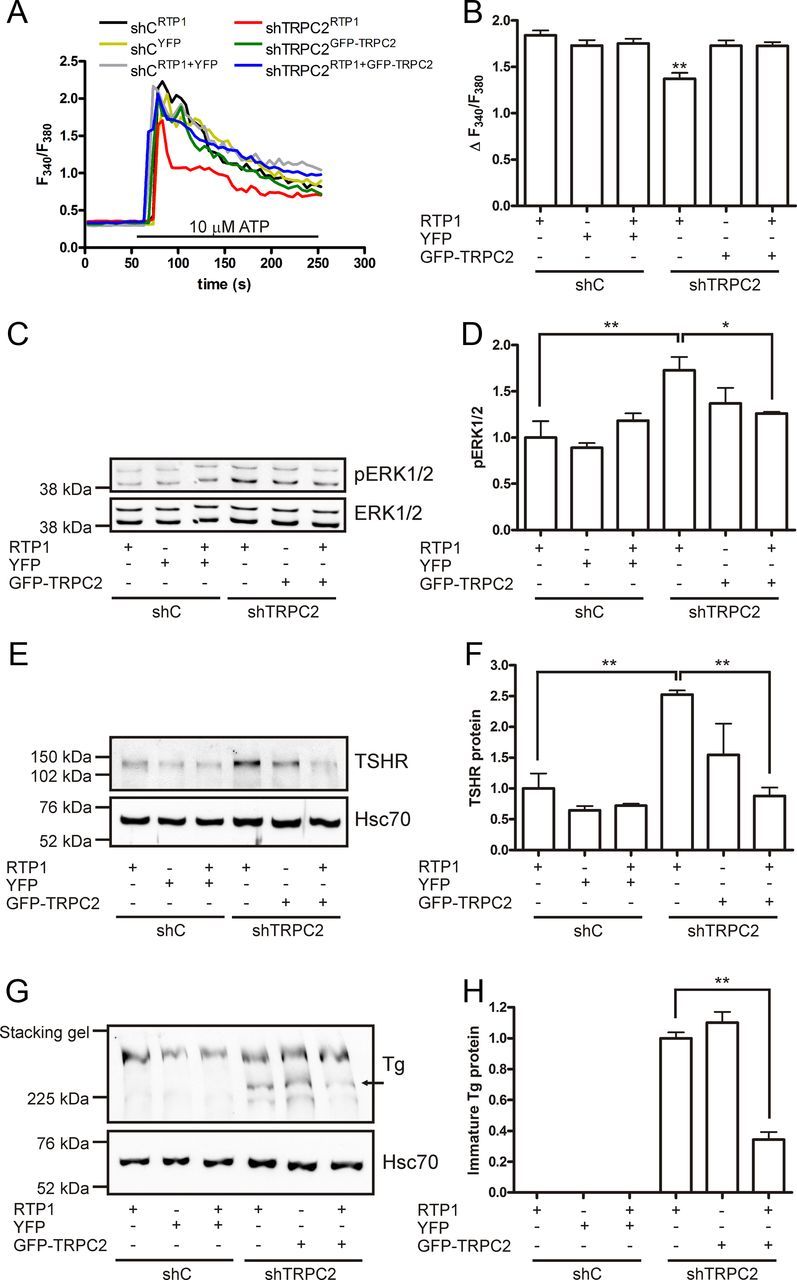
Effects seen by knocking down TRPC2 are reversible. A, Transient expression of RTP1 alone in shC (shCRTP1) and shTRPC2 (shTRPC2RTP1) cells had no effect on ATP-induced calcium transients. In contrast, upon transient transfection of GFP-TRPC2 alone (shTRPC2GFP-TRPC2), or together with RTP1 (shTRPC2RTP1+GFP-TRPC2), the ATP-induced calcium responses were increased to the same level as in control cells. B, Quantification of the calcium transients seen in A. shTRPC2RTP1 cells had significantly smaller ATP-induced calcium transients compared with all other transfections (n ≥ 48; **, P < 0.01). C and D, A decrease in pERK to control level is seen in shTRPC2RTP1+GFP-TRPC2 cells (n = 3; *, P < 0.05). E and F, The TSHR is also decreased to control level in shTRPC2RTP1+GFP-TRPC2 cells (n = 3; **, P < 0.01). G and H, The immature form of Tg is reduced in shTRPC2RTP1+GFP-TRPC2 cells when compared with shTRPC2RTP1 cells (n = 4; **, P < 0.01).
Discussion
In the present report, we show that, of the TRPC family of cation channels, only TRPC2 is expressed in rat thyroid FRTL-5 cells. Transcripts of TRPC2 were found in both primary rat thyroid cells and in freshly isolated rat thyroids (result not shown). We also detected transcripts for TRPC5 in the primary cells. This could be due to endogenous expression of TRPC5 in thyroid cells or alternatively that our preparations were contaminated with a small amount of other cell types. In TRPC2−/− mice, no abnormalities in regard to thyroid function have been reported (6, 8). In agreement with this, we could not detect a transcript for TRPC2 in thyroid glands isolated from mice (results not shown). TRPC2 was functional in FRTL-5 cells, because calcium entry in response to ATP was drastically reduced in shTRPC2 cells as compared with shC cells. Electrophysiological measurements strengthen this notion: the reduction in inward current amplitude in shTRPC2 cells was evident, suggesting that a TRPC2-dependent, nonselective cation current mainly carried by sodium, and calcium was significantly reduced. The expression of the thyroid-specific transcription factors TTF-1 and Pax8 were not altered, indicating that knocking down TRPC2 did not dedifferentiate FRTL-5 cells. Moreover, neither NIS nor TPO expression was affected in shTRPC2 cells.
By reducing the expression of TRPC2 with shRNA, we saw a marked decrease in calcium entry and an increase in agonist-induced production of cAMP. The TSHR was up-regulated on both mRNA and protein level in shTRPC2, and this partly explains the increased cAMP production. However, forskolin increased the cAMP production as well, which suggests that an AC is more active. We found that FRTL-5 cells express AC5 and AC6, of which both are inhibited by calcium. When intracellular calcium was chelated, we saw no differences in cAMP production, which imply that calcium transients mediated by TRPC2 are attenuating cAMP production in FRTL-5 cells. Interestingly, the phosphorylation of ERK1/2 was increased in the shTRPC2 cells, which prompted us to investigate whether there is a link between cAMP, ERK1/2, and the expression of TSHR.
Expression of a constitutively active mutant of MEK1 increased the expression of the TSHR in FRTL-5 cells. We therefore propose a new mechanism involving ERK1/2, as a positive regulator of TSHR expression (Fig. 8). The phosphorylation of ERK1/2 is increased through cAMP/Rap1, whereas TRPC2 negatively regulates this through putative inhibition of AC5 and AC6. Reducing intracellular cAMP by overexpressing PDE4D3 or inhibiting Rap1 reduced the TSHR expression, which further shows that the cAMP pathway is responsible for the up-regulation of the TSHR in the shTRPC2 cells. Taken together, we propose a model where deprivation of calcium will remove inhibitory action on ACs, thus positively regulating pERK1/2, which will increase the expression of the TSHR. Earlier studies have shown that calcium decreases the expression of TSHR (20) and that insulin, IGF-I, or serum are required for the negative autoregulation of the TSHR by TSH (41). This probably occurs, in part, through calcium entry and is thus hampered in shTRPC2 cells. Experiments done with chelation of extracellular calcium and TRPC2DN support our findings that calcium and TRPC2 are negatively regulating the phosphorylation of ERK1/2 and the TSHR expression. Moreover, all effects seen in shTRPC2 cells were reversed by transient transfection with GFP-TRPC2 and RTP1, which shows that the effects are specific for TRPC2.
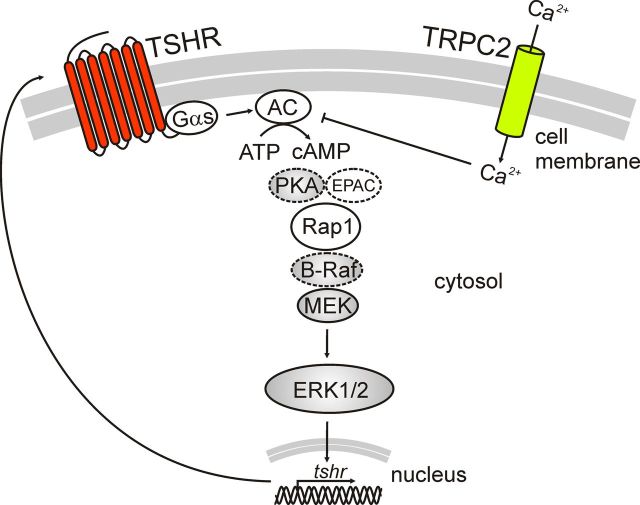
Fig. 8.
Schematic picture of proposed model. The calcium and the cAMP pathways regulate the phosphorylation of ERK1/2 and the expression of the TSHR. Calcium has an inhibitory action on ACs and thus inhibits cAMP production. Dashed lines indicate proteins in the pathway not studied here. B-Raf, B-rapidly accelerated fibrosarcoma; EPAC, exchange protein activated by cAMP; PKA, protein kinase A.
Furthermore, we investigated whether the aforementioned changes in signaling had any effects on Tg metabolism. We observed a perturbation in the posttranslational modification of Tg. Previous reports have shown that interfering with calcium signaling in FRTL-5 cells interferes with the glycosylation, dimerization, and secretion of Tg (39, 42). Several calcium-dependent chaperones are involved in the folding and maturation of Tg. Calnexin and calreticulin bind to Tg monomers in the ER in a manner dependent on the glycosylation status of Tg (21). ER calcium is apparently important in regulating this interaction, because inhibition of the ER Ca2+-ATPase with thapsigargin releases calnexin and calreticulin from Tg (21). Apparently due to retention of Tg in the ER, shTRPC2 cells secreted less Tg. We cannot exclude the possibility that other factors, e.g. perturbations in vesicular transport of Tg to the plasma membrane, could contribute to the diminished secretion. Interestingly, total cellular Tg content was not different between shC and shTRPC2 cells, although the expression of Tg has been reported to depend on calcium (22).
In conclusion, TRPC2 was the only member of the TRPC family expressed in FRTL-5 cells. Decreasing the expression of TRPC2 had major effects on calcium signaling, TSH signaling and Tg maturation and secretion. Because TRPC2 is expressed in primary rat thyroid cells, our observations suggest that TRPC2 is of major importance in regulating thyroid cell function. Our finding thus defines a novel role for the TRPC2 cation channel.
Acknowledgments
We thank Dr. Lutz Birnbaumer for providing invaluable comments during the preparation of the manuscript and Dr. Genevieve Bart (University of Eastern Finland, Kuopio, Finland), Dr. Wito Richter (University of California, San Francisco, CA), Dr. Jean de Gunzburg (Institut Curie, Paris, France), Dr. Debra Fadool (Florida State University, Tallahassee, FL), and Dr. Catherine Dulac (Harvard University, Cambridge, MA) for generously sharing plasmids.
This study was supported, in part, by the Academy of Finland, the Sigrid Jusélius Foundation, the Centre of Excellence in Cell Stress and Molecular Ageing (Åbo Akademi University), the Magnus Ehrnrooth Foundation, the University of Helsinki Research Funds, and the Receptor Research Program (University of Turku and Åbo Akademi University).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AC
- Adenylyl cyclase
- BAPTA-AM
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)
- Endo H
- endoglycosidase H
- ER
- endoplasmic reticulum
- FRTL-5
- Fischer rat thyroid low-serum 5%
- 5H
- five hormones
- 6H
- six hormones
- HBSS
- HEPES-buffered salt solution
- HRP
- horseradish peroxidase
- Hsc70
- heat-shock cognate protein 70
- MEK
- MAPK kinase
- NIS
- sodium-iodine symporter
- p
- phospho
- Pax8
- paired box gene 8
- PDE4D3
- phosphodiesterase 4D3
- qPCR
- quantitative PCR
- RaP
- Ras-related protein
- RTP1
- receptor-transporting protein 1
- shC
- control shRNA
- shRNA
- short hairpin RNA
- shTRPC2
- shRNA against TRPC2
- SOCE
- store-operated calcium entry
- Tg
- thyroglobulin
- TPO
- thyroid peroxidase
- TRPC
- transient receptor potential canonical
- TRPC2DN
- dominant-negative TRPC2
- TSHR
- TSH receptor
- TTF
- thyroid transcription factor
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene
- YFP
- yellow fluorescent protein.
References
- 1. Wes PD , Chevesich J , Jeromin A , Rosenberg C , Stetten G , Montell C. 1995. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA 92:9652–9656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu X , Chu PB , Peyton M , Birnbaumer L. 1995. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett 373:193–198 [DOI] [PubMed] [Google Scholar]
- 3. Zhu X , Jiang M , Peyton M , Boulay G , Hurst R , Stefani E , Birnbaumer L. 1996. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell 85:661–671 [DOI] [PubMed] [Google Scholar]
- 4. Salido GM , Sage SO , Rosado JA. 2009. TRPC channels and store-operated Ca(2+) entry. Biochim Biophys Acta 1793:223–230 [DOI] [PubMed] [Google Scholar]
- 5. Abramowitz J , Birnbaumer L. 2009. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J 23:297–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leypold BG , Yu CR , Leinders-Zufall T , Kim MM , Zufall F , Axel R. 2002. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA 99:6376–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liman ER , Corey DP , Dulac C. 1999. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA 96:5791–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stowers L , Holy TE , Meister M , Dulac C , Koentges G. 2002. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295:1493–1500 [DOI] [PubMed] [Google Scholar]
- 9. Kimchi T , Xu J , Dulac C. 2007. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448:1009–1014 [DOI] [PubMed] [Google Scholar]
- 10. Jungnickel MK , Marrero H , Birnbaumer L , Lémos JR , Florman HM. 2001. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol 3:499–502 [DOI] [PubMed] [Google Scholar]
- 11. Chu X , Cheung JY , Barber DL , Birnbaumer L , Rothblum LI , Conrad K , Abrasonis V , Chan YM , Stahl R , Carey DJ , Miller BA. 2002. Erythropoietin modulates calcium influx through TRPC2. J Biol Chem 277:34375–34382 [DOI] [PubMed] [Google Scholar]
- 12. Gailly P , Colson-Van Schoor M. 2001. Involvement of trp-2 protein in store-operated influx of calcium in fibroblasts. Cell Calcium 30:157–165 [DOI] [PubMed] [Google Scholar]
- 13. Lucas P , Ukhanov K , Leinders-Zufall T , Zufall F. 2003. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron 40:551–561 [DOI] [PubMed] [Google Scholar]
- 14. Vannier B , Peyton M , Boulay G , Brown D , Qin N , Jiang M , Zhu X , Birnbaumer L. 1999. Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion-activated capacitative Ca2+ entry channel. Proc Natl Acad Sci USA 96:2060–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dunn JT , Dunn AD. 2001. Update on intrathyroidal iodine metabolism. Thyroid 11:407–414 [DOI] [PubMed] [Google Scholar]
- 16. Weiss SJ , Philp NJ , Grollman EF. 1984. Iodide transport in a continuous line of cultured cells from rat thyroid. Endocrinology 114:1090–1098 [DOI] [PubMed] [Google Scholar]
- 17. Törnquist K , Ekokoski E , Dugué B. 1996. Purinergic agonist ATP is a comitogen in thyroid FRTL-5 cells. J Cell Physiol 166:241–248 [DOI] [PubMed] [Google Scholar]
- 18. Ekokoski E , Webb TE , Simon J , Törnquist K. 2001. Mechanisms of P2 receptor-evoked DNA synthesis in thyroid FRTL-5 cells. J Cell Physiol 187:166–175 [DOI] [PubMed] [Google Scholar]
- 19. Okajima F , Sho K , Kondo Y. 1988. Inhibition by islet-activating protein, pertussis toxin, of P2-purinergic receptor-mediated iodide efflux and phosphoinositide turnover in FRTL-5 cells. Endocrinology 123:1035–1043 [DOI] [PubMed] [Google Scholar]
- 20. Saji M , Ikuyama S , Akamizu T , Kohn LD. 1991. Increases in cytosolic Ca++ down regulate thyrotropin receptor gene expression by a mechanism different from the cAMP signal. Biochem Biophys Res Commun 176:94–101 [DOI] [PubMed] [Google Scholar]
- 21. Di Jeso B , Ulianich L , Pacifico F , Leonardi A , Vito P , Consiglio E , Formisano S , Arvan P. 2003. Folding of thyroglobulin in the calnexin/calreticulin pathway and its alteration by loss of Ca2+ from the endoplasmic reticulum. Biochem J 370:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rivas M , Mellström B , Naranjo JR , Santisteban P. 2004. Transcriptional repressor DREAM interacts with thyroid transcription factor-1 and regulates thyroglobulin gene expression. J Biol Chem 279:33114–33122 [DOI] [PubMed] [Google Scholar]
- 23. Grasberger H , Van Sande J , Hag-Dahood Mahameed A , Tenenbaum-Rakover Y , Refetoff S. 2007. A familial thyrotropin (TSH) receptor mutation provides in vivo evidence that the inositol phosphates/Ca2+ cascade mediates TSH action on thyroid hormone synthesis. J Clin Endocrinol Metab 92:2816–2820 [DOI] [PubMed] [Google Scholar]
- 24. Raspé E , Laurent E , Andry G , Dumont JE. 1991. ATP, bradykinin, TRH and TSH activate the Ca(2+)-phosphatidylinositol cascade of human thyrocytes in primary culture. Mol Cell Endocrinol 81:175–183 [DOI] [PubMed] [Google Scholar]
- 25. Sho KM , Okajima F , Abdul Majid M , Kondo Y. 1991. Reciprocal modulation of thyrotropin actions by P1-purinergic agonists in FRTL-5 thyroid cells. Inhibition of cAMP pathway and stimulation of phospholipase C-Ca2+ pathway. J Biol Chem 266:12180–12184 [PubMed] [Google Scholar]
- 26. Törnquist K. 1992. Evidence for receptor-mediated calcium entry and refilling of intracellular calcium stores in FRTL-5 rat thyroid cells. J Cell Physiol 150:90–98 [DOI] [PubMed] [Google Scholar]
- 27. Törnquist K. 1993. Modulatory effect of protein kinase C on thapsigargin-induced calcium entry in thyroid FRTL-5 cells. Biochem J 290(Pt 2):443–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mansour SJ , Matten WT , Hermann AS , Candia JM , Rong S , Fukasawa K , Vande Woude GF , Ahn NG. 1994. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 265:966–970 [DOI] [PubMed] [Google Scholar]
- 29. Richter W , Jin SL , Conti M. 2005. Splice variants of the cyclic nucleotide phosphodiesterase PDE4D are differentially expressed and regulated in rat tissue. Biochem J 388:803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eggo MC , King WJ , Black EG , Sheppard MC. 1996. Functional human thyroid cells and their insulin-like growth factor-binding proteins: regulation by thyrotropin, cyclic 3′,5′ adenosine monophosphate, and growth factors. J Clin Endocrinol Metab 81:3056–3062 [DOI] [PubMed] [Google Scholar]
- 31. Babich LG , Ku CY , Young HW , Huang H , Blackburn MR , Sanborn BM. 2004. Expression of capacitative calcium TrpC proteins in rat myometrium during pregnancy. Biol Reprod 70:919–924 [DOI] [PubMed] [Google Scholar]
- 32. Barry PH. 1994. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 51:107–116 [DOI] [PubMed] [Google Scholar]
- 33. Hamill OP , Marty A , Neher E , Sakmann B , Sigworth FJ. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391:85–100 [DOI] [PubMed] [Google Scholar]
- 34. Abramoff MD , Magalhaes PJ , Ram SJ. 2004. Image processing with ImageJ. Biophoton Int 11:36–42 [Google Scholar]
- 35. Rivas M , Santisteban P. 2003. TSH-activated signaling pathways in thyroid tumorigenesis. Mol Cell Endocrinol 213:31–45 [DOI] [PubMed] [Google Scholar]
- 36. Hanoune J , Defer N. 2001. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 41:145–174 [DOI] [PubMed] [Google Scholar]
- 37. Iacovelli L , Capobianco L , Salvatore L , Sallese M , D'Ancona GM , De Blasi A. 2001. Thyrotropin activates mitogen-activated protein kinase pathway in FRTL-5 by a cAMP-dependent protein kinase A-independent mechanism. Mol Pharmacol 60:924–933 [DOI] [PubMed] [Google Scholar]
- 38. Ammoun S , Johansson L , Ekholm ME , Holmqvist T , Danis AS , Korhonen L , Sergeeva OA , Haas HL , Akerman KE , Kukkonen JP. 2006. OX1 orexin receptors activate extracellular signal-regulated kinase in Chinese hamster ovary cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol Endocrinol 20:80–99 [DOI] [PubMed] [Google Scholar]
- 39. Di Jeso B , Formisano S , Ulianich L. 1997. Perturbation of cellular calcium delays the secretion and alters the glycosylation of thyroglobulin in FRTL-5 cells. Biochem Biophys Res Commun 234:133–136 [DOI] [PubMed] [Google Scholar]
- 40. Mast TG , Brann JH , Fadool DA. 2010. The TRPC2 channel forms protein-protein interactions with Homer and RTP in the rat vomeronasal organ. BMC Neurosci 11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saji M , Akamizu T , Sanchez M , Obici S , Avvedimento E , Gottesman ME , Kohn LD. 1992. Regulation of thyrotropin receptor gene expression in rat FRTL-5 thyroid cells. Endocrinology 130:520–533 [DOI] [PubMed] [Google Scholar]
- 42. Di Jeso B , Pereira R , Consiglio E , Formisano S , Satrustegui J , Sandoval IV. 1998. Demonstration of a Ca2+ requirement for thyroglobulin dimerization and export to the golgi complex. Eur J Biochem 252:583–590 [DOI] [PubMed] [Google Scholar]