Abstract
Glucocorticoids (GC) induce cell cycle arrest and apoptosis in different cell types and therefore are widely used to treat a variety of diseases including autoimmune disorders and cancer. This effect is mediated by the GC receptor (GR), a ligand-activated transcription factor that translocates into the nucleus where it modulates transcription of target genes in a promoter-specific manner. Glycogen synthase kinase-3 (GSK3) regulates GR response by genomic and nongenomic mechanisms, although the specific role of each isoform is not well defined. We used GSK3 pharmacological inhibitors and isoform-specific small interfering RNA to evaluate the role of GSK3 in the genomic regulation induced by GC. GSK3 inhibition resulted in the reduction of GC-induced mRNA expression of GC-induced genes such as BIM, HIAP1, and GILZ. Knockdown of GSK3β but not GSK3α reduced endogenous GILZ induction in response to dexamethasone and GR-dependent reporter gene activity. Chromatin immunoprecipitation experiments revealed that GSK3 inhibition impaired the dexamethasone-mediated binding of GR and RNA polymerase II to endogenous GILZ promoter. These results indicate that GSK3β is important for GR transactivation activity and that GSK3β inhibition suppresses GC-stimulated gene expression. Furthermore, we show that genomic regulation by the GR is independent of known GSK3β phosphorylation sites. We propose that GC-dependent transcriptional activation requires functional GSK3β signaling and that altered GSK3β activity influences cell response to GC.
Glucocorticoids (GC) are steroid hormones that regulate essential biological processes, including growth, development, metabolism, survival, differentiation, proliferation, and apoptosis in a large variety of cell types and are commonly used in the treatment of various inflammatory diseases and cancer. Specifically, GC are currently being used in the treatment of hematopoietic malignancies such as chronic lymphocytic leukemia (CLL), T-acute lymphoblastic leukemia, multiple myeloma, and non-Hodgkin's lymphoma due to their ability to induce intrinsic caspase-dependent apoptosis in these cell types (1).
Most of the actions of GC are mediated through the GC receptor (GR), a member of the steroid receptor superfamily (2). The unliganded GR resides primarily in the cytoplasm in an inactive state as part of a large heat-shock protein heterocomplex that includes various chaperone proteins, such as heat-shock protein 90 (3). Upon GC binding, the GR undergoes a conformational change that results in its dissociation from the cytoplasmic chaperone multiprotein complex and unmasking of the nuclear localization signal, leading to its translocation to the nucleus. Once in the nucleus, the dimerized GR binds GC response elements (GRE), usually located in the promoter of GR-regulated genes, resulting in gene transactivation or transrepression (4).
It has been shown that the modulation of the GR phosphorylation cycle by phosphatases maintains steady-state receptor phosphorylation at a low basal level in the absence of ligand, and GC-dependent GR phosphorylation affects GR target gene expression (5). Previous studies have highlighted the involvement of different protein kinases in GC-mediated effects (6). Recently, a protein kinase screening in lymphoid cells showed that glycogen synthase kinase-3 (GSK3) has a role in GC-induced apoptosis (7). Pharmacological inhibition of GSK3 blocked GC-induced apoptosis in different hematopoietic cell lines (7), and attenuated GC-induced up-regulation of BIM (8), a Bcl-2 homology domain-3-only protein involved in GC-induced apoptosis in leukemia cells (9–11).
GSK3 is a serine/threonine protein kinase highly conserved from yeast to mammals (12–14). It was initially identified as a key regulator of insulin-dependent glycogen synthesis, but it has been demonstrated that GSK3 is a multifunctional kinase involved in cellular metabolism, signaling transduction, growth, differentiation, and cell fate determination (13). There are two homologous mammalian GSK3 isoforms encoded by different genes, GSK3α and GSK3β. They share 98% identity within their catalytic domain, but N- and C-terminal sequences diverge, making them structurally similar but not functionally identical (13, 15).
GSK3 demonstrates a preference for prephosphorylated (primed) substrates by different priming kinases (12–14). GSK3β phosphorylates different substrates, including glycogen synthase, and transcription factors such as c-myc, β-catenin, and Tau-microtubule-associated protein (12). There is a hormone-dependent GR phosphorylation on human serine 404 (Ser404) by GSK3β, which plays an important role in GR protein stability and regulates GR-dependent gene expression (6). Additionally, GSK3β-mediated phosphorylation of rat GR threonine 171 (Thr171) has been described (16). Different interactions between GSK3 and the GR have been previously described. In the absence of a ligand, GSK3α is bound to the GR, and exposure to GC leads to its dissociation from the GR (7). Moreover, it has been described that the GR associates with GSK3β in the presence of dexamethasone but not with GSK3α (6). Thus, it seems that GSK3 isoforms regulate GR cellular response by using different mechanisms.
In the present study, we have used pharmacological inhibitors and GSK3 isoform-specific small interfering RNA (siRNA) to analyze the role of GSK3 isoforms in the regulation of GR-mediated transcriptional activation.
Materials and Methods
CLL samples and cell isolation
Blood samples from CLL patients were obtained from the Hospital de Bellvitge, L'Hospitalet de Llobregat, Spain. CLL was diagnosed according to standard clinical and laboratory criteria. Written informed consent was obtained from all patients in accordance with the Hospital de Bellvitge Ethical Committee. Peripheral blood mononuclear cells were isolated by centrifugation on a Ficoll-Hypaque (Seromed, Berlin, Germany) gradient. Human lymphocytes were cultured immediately after thawing or isolation at a concentration of 0.5–3 × 106 cells/ml in RPMI 1640 culture medium (Biological Industries) supplemented with 10% heat-inactivated fetal bovine serum (Biological Industries, Kibbutz Beit Haemek, Israel), 2 mm l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 C in a humidified atmosphere containing 5% carbon dioxide.
Cell culture
Jurkat GR wild-type (WT) cells, derived from a parental cell line of Jurkat T-acute lymphoblastic leukemia cells harboring a nonfunctional GR were generated by expressing the rat GR under control of the β-actin promoter (17, 18). These cells were kindly provided by Dr. Carme Caelles (Institute for Research in Biomedicine, Universitat de Barcelona, Barcelona, Spain). Parental Jurkat, Jurkat GR WT, and BxPC-3 cells were grown in RPMI 1640 medium (Biological Industries), 2 mm l-glutamine, 100 μg/ml penicillin, and 100 mg/ml streptomycin at 37 C in a humidified atmosphere at 5% carbon dioxide. WT, GSK3α−/−, and GSK3β−/− mouse embryonic fibroblast (MEF) cells were kindly given by Dr. J. Woodgett (Samuel Lunenfeld Research Institute, Toronto, Canada). HeLa, MC3T3, and MCF-7 cells and WT, GSK3α−/−, and GSK3β−/− MEF cells were maintained in DMEM (Biological Industries) containing 10% fetal bovine serum (Biological Industries).
Plasmids and reagents
Dexamethasone and SB216763 were purchased from Sigma-Aldrich (St. Louis, MO). Akt inhibitor VIII, SB203580, U0126, LY294002, bisindolylmaleimide I, and rapamycin were purchased from Calbiochem (La Jolla, CA). SP600125, GSK650394, and KU0063794 were from Tocris Bioscience (Bristol, UK). Lithium chloride (LiCl), PP242, and MG-132 were from Sigma-Aldrich, and LY333531 from Enzo Life Sciences. ABT-737 was purchased from Selleck Chemicals LLC (Houston, TX). Roscovitine was kindly provided by Dr. Jacint Boix (Universitat de Lleida, Lleida, Spain). Suberoylanilide hydroxamic acid (vorinostat, Zolinza) was obtained from Cayman Chemical (Ann Arbor, MI) and Kendine-92 (5-diaryl-1H-pyrrole-2-carboxamide derivates) was generously provided by Dr. Fernando Cossío (Universidad del Pais Vasco, Bilbao, Spain). Annexin V allophycocyanin was purchased from eBiosciences (San Diego, CA). MMTV-Luc reporter plasmid containing two consensus GRE was kindly provided by Dr. Carme Caelles (Institute for Research in Biomedicine, Universitat de Barcelona), and p-1940Luc (19) was a kind gift of Dr. Marc Pallardy (Institut National de la Santé et de la Recherche Médicale Unité 461, Université de Paris, Paris, France).
Analysis of apoptosis and cell viability by flow cytometry
Cell viability was determined by measuring phosphatidylserine exposure and membrane integrity. This was determined by annexin V APC staining and a flow cytometric analysis using the FACSCalibur and the CellQuest software (Becton Dickinson, San Jose, CA). Cell viability was measured as the percentage of annexin V APC-negative cell population, and it is expressed as the percentage of nonapoptotic cells. In total, 2.5 × 105 cells were incubated for 24 h with the indicated factors. Cells were washed and incubated with 150 μl annexin-binding buffer and 1.5 μl annexin V APC for 15 min in the dark. Cells were then analyzed by flow cytometry.
Western blot analysis and antibodies
Cells were lysed with Laemmli sample buffer, and Western blot analysis was performed as described previously (20) using the following antibodies: MCL-1 (Santa Cruz Biotechnology, Santa Cruz, CA), BIM (Cell Signaling Technology, Danvers, MA), GILZ (Santa Cruz), cleaved caspase-9 (Cell Signaling), pro-caspase-3 (BD Biosciences, San Jose, CA), β-catenin (BD Biosciences), GR (H-300) (Santa Cruz), GSK3α/β (StressGen Biotechnologies), cytochrome oxidase subunit II (Molecular Probes Inc., Eugene, OR), α-tubulin (Oncogene Research Products), and ERK2 (Upstate Biotechnology, Lake Placid, NY). Antibody binding was detected by using a secondary antibody conjugated to horseradish peroxidase and the enhanced chemiluminescence detection system (Amersham, Piscataway, NJ).
Reverse transcriptase multiplex ligation-dependent probe amplification (RT-MLPA)
Total RNA was isolated from Jurkat GR WT cells using the RNeasy Micro Kit (QIAGEN, Hilden, Germany) according to the manufacturer's protocol. RNA content was analyzed by RT-MLPA using SALSA MLPA kit R011-C1 apoptosis mRNA from MRC-Holland (Amsterdam, Netherlands) for the simultaneous detection of 38 mRNA molecules (21). In brief, RNA samples (200 ng total RNA) were first reverse transcribed using a gene-specific probe mix. The resulting cDNA was annealed overnight at 60 C to the MLPA probe mix. Annealed oligonucleotides were ligated by adding Ligase-65 (MRC-Holland) and incubated at 54 C for 15 min. Ligation products were amplified by PCR (35 cycles of 30 sec at 95 C, 30 sec at 60 C, and 1 min at 72 C) with one unlabeled and one FAM-labeled primer. The final PCR fragments amplified were separated by capillary electrophoresis on a 48-capillary ABI-Prism 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA). Peak area and height were measured using GeneScan version 3.0 analysis software (Applied Biosystems). The sum of all peak data was set at 100% to normalize for fluctuations in total signal among samples, and individual peaks were calculated relative to the 100% value. The mRNA levels of all the genes were standardized to those of β-glucoronidase (GUS) for Jurkat cells and PARN for HeLa cells.
Reverse transcriptase quantitative PCR (RT-qPCR) analysis
Total RNA was isolated from cells using the RNeasy Micro Kit (QIAGEN) according to the manufacturer's protocol. Two micrograms of total RNA were reverse-transcribed using a Ready-To-Go You-Prime First-Strand Beads Kit (GE Healthcare, Buckinghamshire, UK) and Random Hexamers (Applied Biosystems). Quantitative PCR were carried out using ABI Prism 7900 HT Fast Real-Time PCR System, and designed human TaqMan assays (Applied Biosystems) were used to quantify gene expression of BIM (Hs00197982_m1), GILZ (Hs00608272_m1), GILZ (Mm00726417_s1), and HIAP1 (HS00154109_m1) according to the manufacturer's guidelines. The housekeeping gene GUS (Hs99999908_m1) or GAPDH (Mm99999915_g1) was used as a control for RNA quality and used for normalization. PCR data were captured and analyzed using the Sequence Detector software (SDS version 2.2.2; Applied Biosystems).
Transient transfection and reporter assays
Jurkat GR WT were transiently transfected using Neon transfection system (Invitrogen, Carlsbad, CA). Jurkat GR WT cells (1 × 106) were resuspended in 100 μl Neon resuspension buffer R. For each electroporation, cells and 10 μg plasmid DNA were aliquoted into a sterile microcentrifuge tube. A Neon tip was inserted into the Neon pipette and the cell-DNA mixture was aspirated into the tip avoiding air bubbles. The Neon pipette was then inserted into the Neon tube containing 3 ml Neon electrolytic buffer E in the Neon pipette station. Cells were pulsed three times with a voltage of 1350 V and a width of 10 msec. After the pulse, cells were quickly transferred into a culture plate containing complete medium. After 24 h, cells were split before reaching confluence and treated with dexamethasone and/or SB216763 for 4 h. HeLa cells were transiently transfected with 2 μg plasmid DNA using Lipofectamine 2000 (Invitrogen). Luciferase activity was quantified using the luciferase assay system (Promega, Madison, WI). Luciferase values were normalized by protein quantification for Jurkat GR WT and Jurkat parental cells and with the luminescent β-galactosidase kit II for HeLa cells.
Chromatin immunoprecipitation (ChIP) assays
Jurkat GR WT cells (20 × 106) were treated with 10 μm SB216763 and/or 10 nm dexamethasone for 2 h. ChIP assays were performed using the ChIP assay kit (Upstate) following the manufacturer's instructions. ChIP assays were performed using an antibody against rabbit IgG (Upstate) as a negative control. Recruitment of GR (H-300) (Santa Cruz) and RNA polymerase II (Upstate) are relative to the input signal. We used previously described GILZ-specific primers (22), which amplify a portion containing a GRE and another containing the transcription starting site. Densitometric scanning and quantification of the intensities in PCR bands were carried out using Image J version 1.44o software-based analysis (National Institute of Health, Bethesda, MD).
siRNA transfection
HeLa cells were transfected with commercially available scramble siRNA, anti-GSK3α, anti-GSK3β. or both siRNA (Invitrogen) at a concentration of 200 nm using Lipofectamine 2000 transfection reagent (Invitrogen). After 48 h, cell populations at a density of 50–60% in six-well plates were transfected with 1–2 μg MMTV-Luc plasmid DNA, after the formation of lipid-DNA complexes for 20 min at room temperature in OptiMEM I medium (GIBCO, Paisley, UK). Complexes were added directly to growing cells in DMEM and incubated for 4–6 h followed by washing with PBS buffer and addition of fresh DMEM. Cells were used in experiments 72 h after siRNA transfection.
Cellular fractionation
Jurkat GR WT cells (5 × 106) were harvested, washed once with ice-cold PBS, and gently lysed for 30 sec in 80 μl ice-cold lysis buffer [250 mm sucrose, 1 mm EDTA, 0.05% digitonin, 25 mm Tris (pH 6.8), 1 mm dithiothreitol, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml aprotinin, 1 mm benzamidine, and 0.1 mm phenylmethylsulfonyl fluoride]. Lysates were centrifuged at 12,000 × g for 3 min to obtain the supernatants (cytosolic extracts free of mitochondria) and the pellets (membrane fraction that contains nuclei and mitochondria), as described previously (23). Supernatants (50 μg) and pellet lysates (40 μg) were separated by SDS-PAGE.
Confocal laser scanning microscopy
Jurkat GR WT cells were collected after treatment with 10 μm SB216763 and/or 10 nm dexamethasone for 2 h, resuspended in PBS, and incubated at room temperature for 30–60 min over poly-l-lysine-coated coverslips (0.01% solution; Sigma-Aldrich). HeLa cells were grown on sterilized glass coverslips and then treated with 100 nm dexamethasone for 3 h. Coverslips containing attached cells were fixed with 4% paraformaldehyde, permeabilized with 0.25% Triton X-100 in PBS, and incubated for 1 h in 5% PBS-BSA to block nonspecific binding. Slides were incubated overnight at 4 C in a humidified chamber with rabbit polyclonal anti-GR (H-300) (1/100) primary antibody (Santa Cruz). Afterward, the slides were washed three times with PBS and further incubated with Alexa Fluor 647 antirabbit secondary antibody (1/500; Invitrogen) for 1 h. Nuclei were stained with YOYO-1 iodide (Invitrogen). To validate the specificity of the immunostaining, controls were performed by applying the same protocol but replacing primary antibody with 5% PBS-BSA. Images were then obtained with a spectral confocal microscope (TCS-SL; Leica Microsystems, Wetzlar, Germany) using a Plan-Apochromat ×63/1.4 numeric aperture immersion oil objective (Leica Microsystems). We used a HeNe laser at 633 nm (Lasos Inc., Jena, Germany) and pinhole of 114.54 μm for Alexa Fluor 647 GR staining and argon laser at 488 nm and pinhole of 114.54 μm for YOYO-1 nuclear staining. Images were captured using the accompanying image processing software from Cytovision (Leica Microsystems).
Reporter plasmids pSGF-T171A-Luc, pSGF-S424A-Luc, and double mutant
The mutants pSGF-T171A-Luc (with Thr171 mutated to Ala), pSGF-S424A-Luc (with Ser424 mutated to Ala), and double mutant (with Thr171 and Ser424 mutated to Ala) were generated by PCR using the rat GR DNA as a template and the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The primers used for generating the mutations were the following: for Thr171 mutation, forward, 5′-GCAACTGGGTGTGCTGCCCCGACAGAGAA-3′, and reverse, 5′-TTCTCTGTCGGGGCAGCACACCCAGTTGC-3′; and for Ser424, forward, 5′-CCAGATGTAAGCGCTCCTCCATCCAGCTC-3′, and reverse, 5′-GAGCTGGATGGAGGAGCGCTTACATCTGG-3′. The mutated nucleotide is underlined. All plasmids and mutagenesis products were verified by DNA sequencing with the following primers: forward, 5′-CCTACAGCTCCTGGGCAACGTGCTGGTTA-3′; reverse, 5′-CGAGTCAGTGAGCGAGGAAGCGGAAGAGT-3′; forward, 5′-TCTCAGCAGCAGGATCAGAA-3′; and reverse, 5′-GCTGGATGGAGGAGAGCTTA-3′.
Statistical analysis
Results are shown as the mean ± sem of values obtained in three or more independent experiments. Data were analyzed using SPSS version 11.0 software package. The paired Student's t test was used to compare the differences between paired samples. ANOVA-Tukey was used to compare the differences between treatments. Differences were considered significant at P values < 0.05.
Results
Dexamethasone-induced apoptosis is reverted by GSK3 inhibition
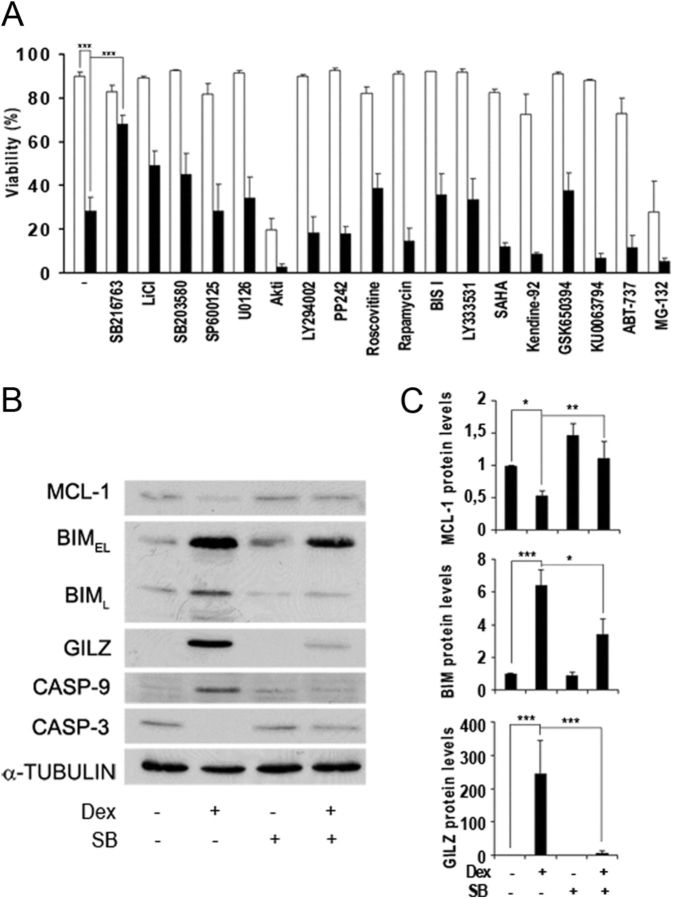
To study the protein kinases involved in GC-induced apoptosis, we examined cell viability upon treatment with dexamethasone in combination with different protein kinase inhibitors. We used the T cell leukemia-derived Jurkat cell line, which was stably transfected with a rat GR expression vector under the control of the β-actin promoter to ensure constant GR protein expression (Jurkat GR WT) (17). Exposure to dexamethasone resulted in a loss of viability of Jurkat GR WT cells at 24 h (Fig. 1A). Previous reports showed cell death induction upon GC treatment in primary CLL cells and other leukemic cells (7, 11, 24). Preincubation with the selective GSK3 inhibitor SB216763 significantly decreased this effect, in agreement with a recent observation where the pharmacological inhibition of GSK3 reduced GC-induced apoptosis in hematopoietic cell lines (7). Other protein kinase inhibitors, histone deacetylase inhibitors, Bcl-2 inhibitor, and proteasome inhibitor could not prevent apoptosis induction, including the less specific inhibitor of GSK3, lithium chloride (LiCl) (Fig. 1A). This suggests that GSK3 plays a major role in GC-mediated apoptotic signaling pathways in leukemic cells.
Fig. 1.
Effect of GSK3 inhibition on GC-induced apoptosis. A, Jurkat GR WT cells were preincubated for 30 min with different protein kinases, HDAC, and proteasome inhibitors (white bars) and treated with 10 nm dexamethasone for 24 h (black bars). Cell viability was analyzed by phosphatidylserine exposure. Data correspond to the mean ± sem of at least three representative experiments. Concentrations of inhibitors used were 10 μm SB216763, 10 mm LiCl, 10 μm SB203580, 10 μm SP600125, 10 μm U0126, 10 μm Akt inhibitor VIII (Akti), 20 μm LY294002, 50 nm PP242, 10 μm roscovitine, 10 nm rapamycin, 50 nm bisindolylmaleimide I (BIS I), 50 nm LY333531, 0.5 μm suberoylanilide hydroxamic acid (SAHA), 0.5 μm Kendine-92, 1 μm GSK650394, 5 μm KU0063794, 5 μm ABT-737, and 1 μm MG132. B, Jurkat GR WT cells were preincubated for 30 min with 10 μm SB216763 (SB) in the absence or presence of 10 nm dexamethasone (Dex) and harvested at 24 h. Analysis of MCL-1, BIMEL (extra large), BIML (large), GILZ, caspase-9 (CASP-9), and pro-caspase 3 (CASP-3) protein levels were analyzed by Western blot. α-Tubulin was used as loading control. C) MCL-1, BIMEL and GILZ were quantified by densitometric analysis and corrected by α-tubulin levels by using ImageJ software (National Institutes of Health). Mean ± sem of at least three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next examined the effect of SB216763 treatment on the expression of GC-induced proteins BIM and GILZ, two well-known targets of GC (9, 10, 25). Western blot analysis revealed that the expression of these proteins was induced by dexamethasone treatment, and preincubation with SB216763 reduced this up-regulation (Fig. 1, B and C). These data establish for the first time that GILZ, a GR direct target gene, requires GSK3 activity for its GC-dependent protein induction. Furthermore, prosurvival protein MCL-1 was down-regulated after 24 h of dexamethasone treatment, and this down-regulation was also diminished by GSK3 inhibition (Fig. 1, B and C). Next, we examined the activation of caspases. For this purpose, we analyzed caspase-9 activation, determined by the appearance of the intermediate cleavage product of 37 kDa and pro-caspase-3 disappearance as a parameter of caspase activation. We observed that SB216763 treatment prevented caspase-3 and caspase-9 activation (Fig. 1B), contributing to the blockade of dexamethasone-induced cell death (Fig. 1A).
GSK3 inhibition alters GR-mediated gene expression at the transcriptional level
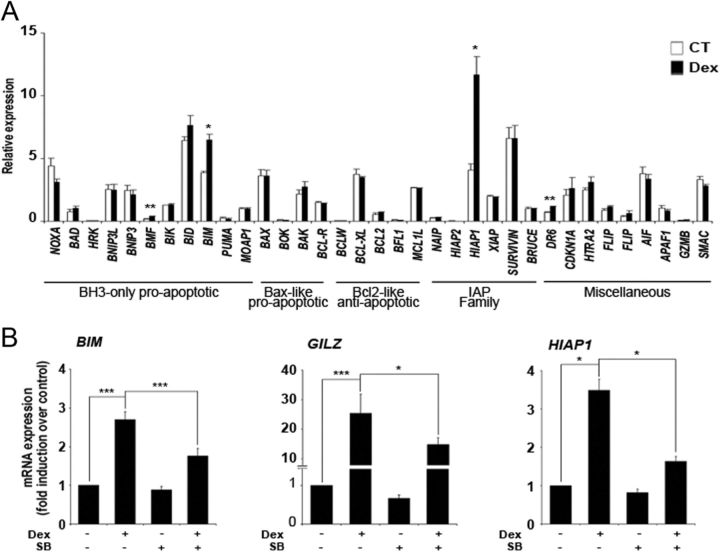
We first examined the effect of dexamethasone treatment for 90 min on the mRNA expression of the BCL-2 family members and other genes involved in the control of apoptosis by performing RT-MLPA. Dexamethasone significantly induced the expression of BIM (Fig. 2A), which has been suggested to be critical for regulating the switch from survival to apoptosis (9, 11). Additionally, an increase in mRNA levels was also observed for the antiapoptotic gene HIAP1, another GC-induced gene (26, 27). Other genes modulated by dexamethasone treatment were the proapoptotic gene BMF and DR6, a member of the TNF receptor family, but their expression levels were low compared with that of BIM and HIAP1. To evaluate the role of GSK3 in GC-induced transcriptional modulation, we examined the effect of SB216763 in the transcriptional induction of GC target genes after dexamethasone treatment. RT-qPCR showed that dexamethasone treatment significantly induced BIM, GILZ, and HIAP1 mRNA levels, and pretreatment with SB216763 significantly reduced their induction in response to dexamethasone (Fig. 2B). These results were confirmed by RT-MLPA analysis (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org).
Fig. 2.
GR ability to regulate GC-induced gene expression is affected by GSK3 inhibition. A, RT-MLPA gene expression profile induced by GC. Jurkat GR WT cells were untreated [control (CT)] or treated with 10 nm dexamethasone (Dex) for 90 min. Cells were lysed and the expression of apoptotic-related genes of the Bcl-2 family, IAP (inhibitors of apoptosis) family, and other genes implicated in apoptosis was analyzed by RT-MLPA as described in Materials and Methods. The mRNA levels of all the genes were normalized with respect to those of GUS. B, Jurkat GR WT cells were preincubated with 10 μM SB216763 (SB) in the absence or presence of 10 nm dexamethasone (Dex) and harvested at 90 min. BIM, GILZ, and HIAP1 mRNA were measured by RT-qPCR. The mRNA levels of all genes were normalized with respect to those of GUS. These results are shown as the mean ± sem of at least three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. untreated cells.
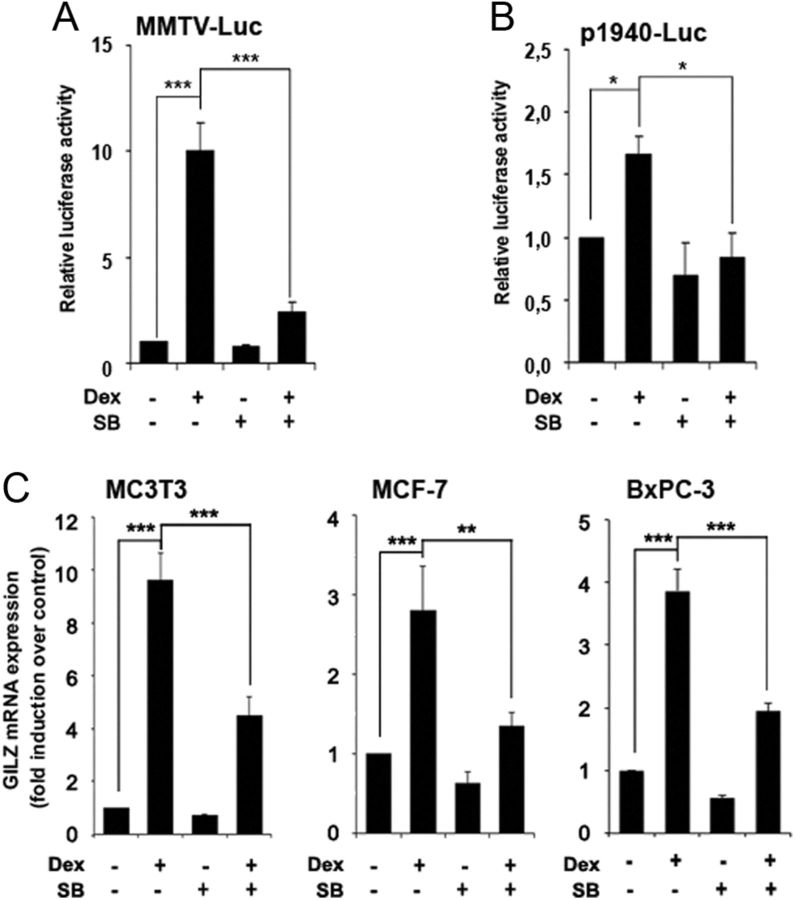
To further characterize the involvement of GSK3 in GC-induced gene expression, we examined the ability of the GR to regulate artificial GC-responsive gene promoter constructs in SB216763-treated Jurkat cells. As expected, cells treated with dexamethasone showed a significant increment in luciferase expression of a synthetic GRE promoter construct composed of two GRE (MMTV-Luc) (Fig. 3A) (28) and the p1940-Luc GILZ reporter construct (Fig. 3B) (29). Pretreatment of cells with SB216763 significantly decreased the response to GC as compared with cells treated with dexamethasone alone, especially in the case of MMTV-Luc, confirming the role of GSK3 on dexamethasone-induced GR transcriptional activity.
Fig. 3.
GSK3 is important for GR transcriptional function. A and B, Jurkat GR WT cells were transfected with MMTV-Luc (A) or p1940-Luc (B) vectors. At 24 h after transfection, cells were preincubated with 10 μm SB216763 (SB) for 30 min, followed by treatment with 10 nm dexamethasone (Dex) for another 4 h. Luciferase activity was measured and expressed relative to basal activity of untreated cells. C, MC3T3, MCF-7, and BxPC-3 cells were preincubated with 10 μM SB216763 (SB) in the absence or presence of 10 nm, 8 nm, and 4 μm dexamethasone (Dex), respectively, and harvested at 90 min. GILZ mRNA levels were measured by RT-qPCR. mRNA levels were normalized with respect to those of GUS in human cell lines or GAPDH in MC3T3. Mean ± sem of at least three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Moreover, we analyzed GILZ mRNA levels in different cell types. GILZ expression was also induced by GC treatment in MC3T3 mouse preosteoblast, MCF-7 human breast adenocarcinoma, and BxPC-3 human pancreatic adenocarcinoma cell lines and significantly reverted by SB216763 pretreatment (Fig. 3C). These results indicate that GSK3 is an important determinant in the GR transcriptional response to GC in a species- and cell type-independent manner.
GSK3 inhibition affects GC-induced apoptosis and GC-dependent gene induction in CLL cells
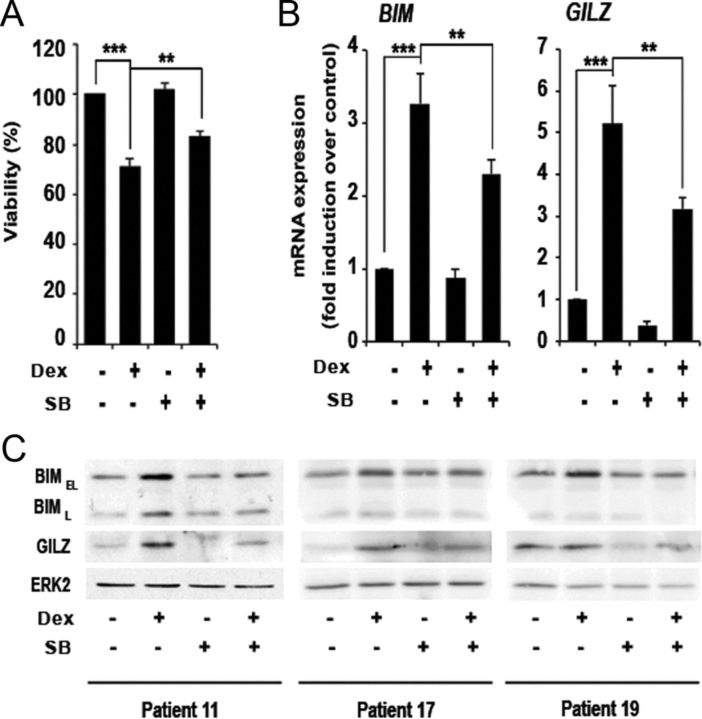
GC are used in the therapy of lymphoid malignancies because of their remarkable ability to induce apoptosis (30, 31). To study the effect of GSK3 inhibition over GC-induced apoptosis in a primary leukemia model, we employed B lymphocytes from patients with CLL. As we described previously, dexamethasone significantly induced cell death of CLL cells (24). Moreover, pretreatment with GSK3 inhibitor SB216763 significantly decreased dexamethasone-induced apoptosis by 11.87% in 19 of the 29 patients analyzed (Fig. 4A). Supplemental Table 1 shows the viability of the full cohort of patients analyzed. We next analyzed the effect of GSK3 inhibition on GC-dependent GILZ and BIM induction. mRNA levels were induced in response to dexamethasone treatment and reverted by GSK3 inhibition in the six patients analyzed (Fig. 4B). GILZ and BIM protein levels were also induced in response to GC and reverted by GSK3 inhibition in nine of 12 samples for GILZ and 11 of 13 samples for BIM (Fig. 4C). Collectively, these data indicate that GSK3 inhibition reduces sensitivity to GC-induced apoptosis in CLL cells and impairs GC-dependent gene and protein induction.
Fig. 4.
GSK3 inhibition affects GC-induced apoptosis and gene expression in CLL cells. Cells from CLL patients were preincubated for 30 min with 10 μm SB216763 (SB) and treated with 10 μm dexamethasone (Dex) for 24 h. A, Cell viability of the 19 patient samples in which SB216763 decreased GC-induced apoptosis was analyzed by phosphatidylserine exposure. Viability is expressed as the percentage of annexin APC-negative treated cells relative to untreated cells. B, BIM and GILZ mRNA levels of six patients were measured by RT-qPCR. The mRNA levels of both genes were normalized with respect to those of GUS. Data are shown as the mean value ± sem. *, P < 0.05; **, P < 0.01; ***P < 0.001. C, BIMEL, BIML, and GILZ protein levels were analyzed by Western blot. These are three representative patients of at least 12 that were analyzed. ERK2 was used to normalize protein levels.
GSK3 inhibition affects GR and RNA polymerase II recruitment to the GILZ gene promoter
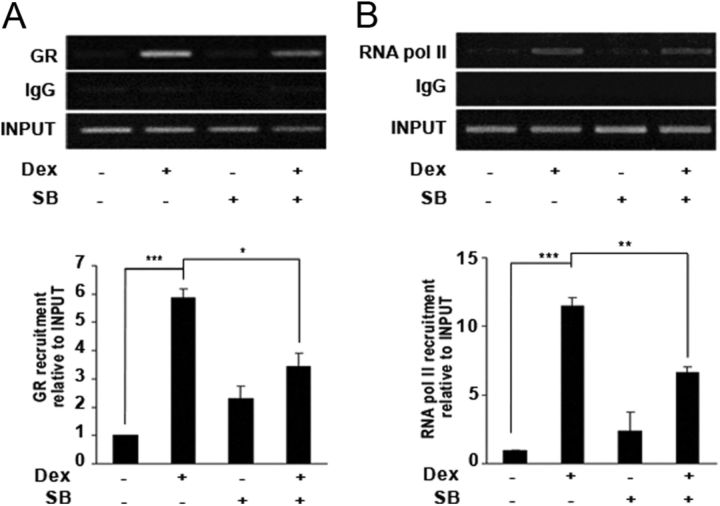
To establish the mechanism for GSK3-mediated GR transcriptional regulation, Jurkat GR WT cells were stimulated with dexamethasone for 2 h, and GILZ promoter occupancy was evaluated by ChIP analysis. Using previously described primers (22), we observed a significant increase in binding of the GR to the GILZ promoter (Fig. 5A) as well as increased binding of RNA polymerase II to the GILZ transcription start site (Fig. 5B) in dexamethasone-treated cells. Pretreatment with SB216763 significantly reduced GR and RNA polymerase II binding to the GILZ promoter induced by dexamethasone. Taken together, our results show that GC-dependent transcriptional activation requires a functional GSK3 signaling.
Fig. 5.
Recruitment of GR and RNA polymerase II to the GILZ gene is affected by GSK3 inhibition. Jurkat GR WT cells were preincubated with 10 μm SB216763 (SB) and treated with 10 nm dexamethasone (Dex) for 2 h. ChIP analysis was performed by incubating DNA-protein complexes with antibodies against GR (A) or RNA polymerase II (RNA pol II) (B) and IgG as a negative control relative to the input signal. Primers specific for the GILZ promoter used for PCR analysis were described in Materials and Methods. Bars represent average values from densitometric analysis of the bands obtained in four separate experiments using ImageJ software (National Institutes of Health). Mean ± sem of at least three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
GSK3β gene silencing suppresses GC-stimulated gene expression
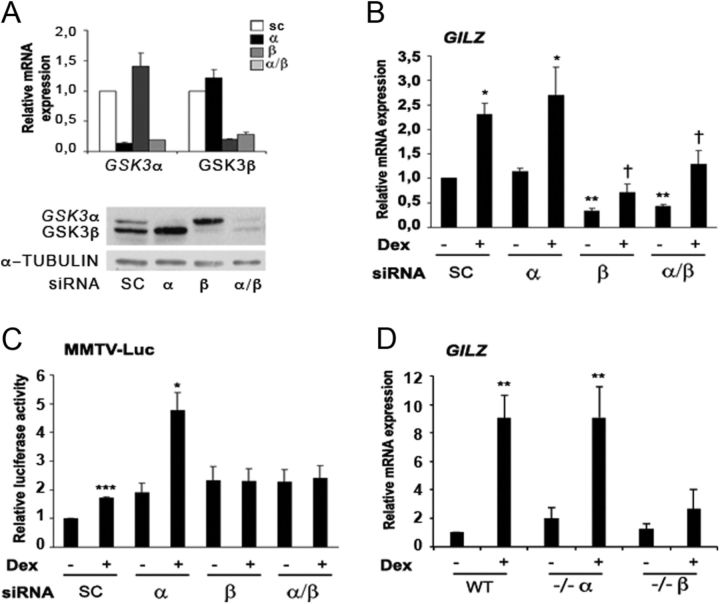
GSK3 inhibitors do not distinguish between the two GSK3 isoforms, so we next evaluated the individual roles of GSK3α and GSK3β on GR-mediated transcriptional activation by gene silencing analysis. Because we were unable to completely silence GSK3 gene expression in Jurkat cells, we performed GSK3α and GSK3β knockdown in HeLa cells. Relative mRNA levels and protein expression levels of GSK3α and GSK3β were determined after 72 h of siRNA by performing RT-qPCR and Western blot, respectively. Transfection of HeLa cells with siRNA for either GSK3α or GSK3β resulted in knockdown of their respective transcripts and proteins, whereas mRNA and protein levels of the GSK3 isoforms were unaffected after transfection with scramble siRNA (Fig. 6A and Supplemental Fig. 2).
Fig. 6.
Genetic knockdown of GSK3β by siRNA results in disruption of GC-stimulated gene expression. A, HeLa cells were transfected with GSK3 isoform-specific siRNA. mRNA and protein expression levels are shown of GSK3α and GSK3β isoforms after their knockdown of the specific gene. The mRNA levels of both genes were normalized with respect to those of GUS. B, Effect of genetic disruption of GSK3 isoforms α, β, or both on basal and dexamethasone (Dex)-induced GILZ mRNA levels in HeLa cells. Mean ± sem of four independent experiments. C, Effect of genetic disruption of GSK3 on basal and dexamethasone (Dex)-induced GR activity measured by MMTV-Luc luciferase reporter assay in HeLa cells. Cells were cotransfected with MMTV-Luc and β-galactosidase (internal control) constructs. The cells were then exposed to 100 nm dexamethasone for 4 h. The normalized values are relative to the scrambled siRNA untreated control. Mean ± sem of five independent experiments. D, WT and GSK3α- and GSK3β-null MEF cells were treated with 100 nm dexamethasone for 4 h. GILZ mRNA was measured by RT-qPCR and normalized with respect to GAPDH. Mean ± sem of four independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 of dexamethasone-treated cells compared with untreated cells; †, P < 0.05 compared with treated cells.
Next, we analyzed the effect of silencing GSK3α, GSK3β, or both isoforms over GILZ mRNA levels in HeLa cells. Depletion of GSK3α had no effect on basal or GC-induced mRNA expression levels of GILZ (Fig. 6B). Interestingly, GSK3β and GSK3α/β silencing resulted in a significant decrease in basal GILZ mRNA levels and attenuated the effects of dexamethasone treatment. We next investigated the effect of GSK3α/β silencing on GR-mediated transcription using the GC-inducible promoter MMTV-Luc. Luciferase activity was determined 4 h after dexamethasone addition (Fig. 6C). As it was previously described, basal transcription of GRE reporter was induced by GSK3 silencing (32). The degree of basal activation of MMTV-Luc was similar between GSK3α and GSK3β siRNA. Interestingly, GSK3β but not the GSK3α knockdown significantly decreased dexamethasone-stimulated MMTV-Luc promoter activity. The down-regulation of both GSK3 isoforms decreased dexamethasone-stimulated MMTV-Luc luciferase activity to the same extent as GSK3β silencing alone. As expected, the scramble siRNA had no effect over the MMTV-Luc reporter luciferase induction in response to dexamethasone.
We also used RT-MLPA to analyze changes in response to GSK3α/β silencing in HeLa cells (Supplemental Fig. 3). Apoptosis mRNA expression profile was quite different from Jurkat GR WT cells, because HeLa cells do not undergo apoptosis in response to dexamethasone treatment. Only BMF, MCL-1, BCL-XL, and HIAP1 were significantly induced by dexamethasone. GSK3β or GSK3α/β silencing resulted in down-regulation of GC-dependent BMF, BCL-XL, and MCL-1 mRNA induction, whereas HIAP1 was still induced by dexamethasone treatment.
To further confirm our results obtained in GSK3 knockdown experiments in HeLa cells, we used WT and GSK3α- or GSK3β-null MEF cells to analyze endogenous GILZ mRNA induction (Fig. 6D) in response to GC treatment. Moreover, we found that MEF cells deficient for GSK3β expression did not induce GILZ mRNA levels in response to dexamethasone, whereas WT and GSK3α-null MEF cells were able to significantly induce GILZ mRNA upon GC treatment. Additionally, we showed that MEF cells deficient for GSK3β did not exhibit dexamethasone-induced luciferase activity, whereas WT and GSK3α-null MEF cells significantly induced MMTV-Luc promoter activity in response to dexamethasone (Supplemental Fig. 4). On the other hand, RT-MLPA analysis showed no significant changes in MEF cells gene expression profile in response to GC, even though there were differences in basal gene expression between cell lines (Supplemental Fig. 5). Together, these results show that GSK3β activity, but not GSK3α, is required for the transcriptional GR-mediated activity.
GSK3 inhibition affects GR cellular distribution in response to GC
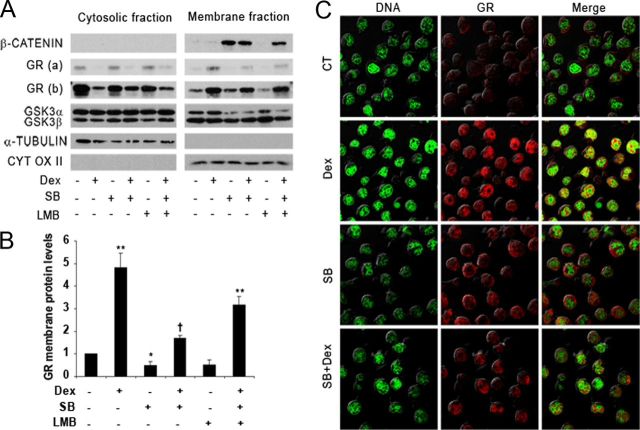
To examine the role of GSK3 in the regulation of GR subcellular localization, we analyzed cytosolic and membrane (which contains nuclei and mitochondria) fractions from Jurkat GR WT cells. In nonstimulated cells, the GR was detected mostly in the cytosolic fractions, whereas dexamethasone treatment induced its translocation to the membrane fractions (Fig. 7A). Dexamethasone-dependent GR nuclear protein localization was significantly reduced by SB216763 (Fig. 7, A and B). GSK3α and GSK3β levels were mainly observed in the cytoplasm and the nucleus, respectively. Under SB216763 treatment, GSK3α levels were reduced in the nucleus, and there was an increase in β-catenin protein levels in the membrane fraction, confirming GSK3 inhibition.
Fig. 7.
Subcellular localization of GR in Jurkat GR WT cells treated with dexamethasone, SB216763, and LMB. A, Jurkat GR WT cells were treated or not with 1 nm LMB. Thirty minutes later, cells were treated or not with 10 μm SB216763 (SB) before treatment with 10 nm dexamethasone (Dex). Three hours later, we lysed the cells to obtain cytoplasmic and membrane fractions as described in Materials and Methods. GSK3α/β, GR [(a), less exposed; (b), more exposed] and β-catenin were detected by Western blotting. α-Tubulin and cytochrome oxidase II (CYT OX II) were analyzed as a control for cytosolic and membrane extracts, respectively. B, Values obtained from membrane fractions were subjected to band densitometry using ImageJ software. GR protein nuclear levels were quantified and normalized by the cytochrome oxidase II protein levels. The graph shows the mean value ± sem of four experiments expressed as the fold induction compared with untreated cells. *, P < 0.05; **, P < 0.01 of dexamethasone (Dex)-treated cells compared with untreated cells; †, P < 0.05 compared with treated cells. C, Immunofluorescent staining of the GR in Jurkat GR WT cells. Cells were untreated (CT) or pretreated with 10 μm SB216763 (SB) and treated with 10 nm dexamethasone (Dex) for 2 h. YOYO-1 iodide (green) stains nuclear DNA, and Alexa Fluor 647 (red) stains GR. Merged images are shown for comparison. This is a representative experiment of three that were performed.
Taking into account that chromosome region maintenance 1 (CRM1) exportin has been suggested to play a pivotal role in the early nuclear export of the GR (33), we wanted to determine whether the partial reduction of the GR nuclear translocation by SB216763 in dexamethasone-treated cells is a result of a CRM1-dependent export. For this purpose, nuclear translocation experiments were performed in the presence of leptomycin B (LMB), which blocks specifically the CRM1-dependent nuclear export. A significant increase in nuclear GR localization was observed when cells were treated with LMB, suggesting that there might be a CRM1-dependent nuclear export of the GR in response to GSK3 inhibition (Fig. 7, A and B).
We further confirmed the subcellular localization of the GR through immunofluorescent staining visualized by confocal microscopy. In control Jurkat GR WT cells, specific staining of GR was mainly observed in the cytoplasmic compartment (Fig. 7C). As expected, treatment with dexamethasone for 2 h induced GR translocation to the nuclear compartment predominantly to regions where euchromatin was present. Interestingly, when cells were preincubated with SB216763 in combination with dexamethasone, a decrease in the GR protein levels in the nucleus was observed compared with dexamethasone treatment alone. Altogether, these results indicate that GSK3 inhibition is able to affect GR protein localization and decreases early GR nuclear levels in dexamethasone-treated Jurkat GR WT cells, affecting GC-stimulated gene expression.
Next, we analyzed the contribution of GSK3α and GSK3β to GR subcellular distribution induced by dexamethasone using isoform-specific knockdown in HeLa cells. Treatment with dexamethasone always resulted in GR translocation to the nucleus even when GSK3β or GSK3α/β was silenced. Moreover, depletion of GSK3β or GSK3α/β proteins by siRNA led to an increase in general GR expression levels (Supplemental Fig. 6). These results are also in agreement with a previous report where mutation of human Ser404 (a residue phosphorylated by GSK3β) leads to the inability of the GR to exit the nucleus, making it inaccessible to the proteasome degradation machinery (6).
GSK3 inhibition alters transcriptional activity of GR phosphorylation mutants
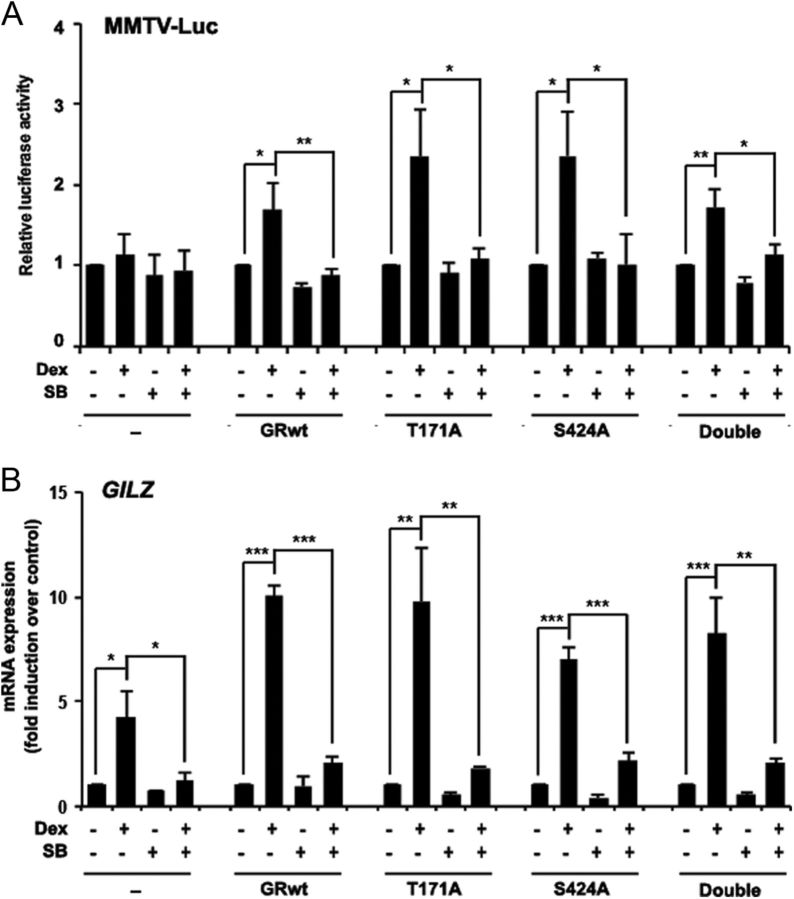
Besides phosphorylation of human GR at Ser404, GSK3β has been described to phosphorylate rat GR at Thr171 (16). Because Jurkat GR WT cells harbor a rat GR, we mutated both Thr171 and Ser424 (corresponding to human Ser404) (34) residues in the rat GR to analyze mutant transactivation capacity under GSK3 inhibition (Supplemental Fig. 7). WT and GR mutants were expressed in the Jurkat parental cell line, which harbor a function-impairing point mutation (R477H) in one of their GR alleles that causes GC resistance (18). We used the GC-inducible promoter MMTV-Luc to evaluate the effect of SB216763 over the dexamethasone-induced transactivation activity of single and double mutants. According to previous results in Jurkat GR WT cells, pretreatment of Jurkat cells with SB216763 significantly inhibits GR WT hormone-dependent transcriptional activity (Fig. 8A). Interestingly, the GR mutants (T171A, S424A, and double mutant) responded to dexamethasone to the same extent as GR WT, and SB216763 reverted dexamethasone-induced transactivation activity of all mutants (Fig. 8A). These results were also observed at longer incubation times (Supplemental Fig. 8).
Fig. 8.
GSK3 inhibition affects GC-induced transcriptional activity of GR mutants. A, Jurkat parental cells were transfected with MMTV-Luc alone or in combination with GR WT, T171A, S424A, or double mutant of the rat GR and preincubated with 10 μm SB216763 (SB) for 30 min followed by treatment with 10 nm dexamethasone (Dex) for another 4 h. Luciferase activity was measured and expressed relative to basal activity of untreated cells. B, Jurkat parental cells were nontransfected or transfected with GR WT, T171A, S424A, and double mutant, preincubated with 10 μM SB216763 in the absence or presence of dexamethasone, and harvested at 4 h. GILZ mRNA levels were measured by RT-qPCR. mRNA levels were normalized with respect to those of GUS. Mean ± sem of at least three independent experiments. *, P < 0.05; ** P < 0.01; ***, P < 0.001.
RT-qPCR showed that GR WT and GR mutants were equally able to induce endogenous GILZ mRNA levels in response to dexamethasone treatment. Pretreatment with SB216763 significantly reduced GC-dependent GILZ mRNA induction by all the GR constructions (Fig. 8B). Note that parental Jurkat cells slightly induced GILZ mRNA expression (Fig. 8B) but not MMTV-Luc luciferase activity (Fig. 8A) as previously described (18). This mRNA induction was also reverted by SB216763 treatment. These results suggest that there is an additional mechanism, not involving phosphorylation of these residues, by which GSK3 regulates GR transcriptional activity.
Discussion
GC induce apoptosis in different cell types including leukemia cells in a complex process regulated by multiple signaling pathways that alter gene expression profiles through GR-mediated transactivation and transrepression (1). In this report, we demonstrate that GSK3 regulates GR transcriptional activity by affecting GR protein localization, recruitment to target gene promoters, and changes in target gene expression in different cell types. We observed a critical role for GSK3 in GC-dependent cell death because inhibition of GSK3 by the specific GSK3 inhibitor SB216763 reverted GC-induced apoptosis, consistent with previous studies (7, 8, 35). The antiapoptotic Bcl-2 family member protein MCL-1 contains a conserved consensus site for GSK3 phosphorylation, which targets it for ubiquitin-dependent degradation (36). We observed that GC-dependent down-regulation of MCL-1 protein was prevented by GSK3 inhibition. This was accompanied by the reduction of dexamethasone-induced up-regulation of BIM (8) and GILZ protein levels. To the best of our knowledge, there are no previous studies demonstrating the involvement of GSK3 in the GC-dependent up-regulation of GILZ. We also evaluated the effect of GSK3 inhibition over GC-induced apoptosis in primary CLL cells. SB216763 pretreatment reverted GC-induced cell death in 19 of 29 patients analyzed. It is important to note that CLL patients have different genetic alterations that determine response to treatment. Additionally, GC-dependent BIM and GILZ mRNA and protein induction was reverted by GSK3 inhibition. The effect over GILZ mRNA induction was confirmed in other cell types, ruling out cell-type-specific effects.
Pharmacological inhibition of GSK3 resulted in a reduced induction of BIM, HIAP1, and GILZ endogenous mRNA levels and GC-responsive promoter constructs in response to GC. These results suggested that the inhibitory effect of SB216763 on GC-stimulated promoter activity was due to an impairment of GR DNA binding. Interestingly, ChIP assays showed that GSK3 inhibition reduced GR and RNA polymerase II recruitment to the GILZ promoter after dexamethasone treatment. These results indicate that GC-dependent transcriptional activation requires functional GSK3 signaling and show for the first time that GSK3 could be required by the GR for its activation as a transcription factor and for RNA polymerase II recruitment. These results are in the line with previous studies in which GSK3 inhibition represses other steroid receptor transcriptional activity in various cell types (37–40).
The present study demonstrates that GSK3β is the isoform involved in the regulation of GR transcriptional activation in response to GC. GSK3α and GSK3β silencing in HeLa cells, resulted in the activation of a GRE-promoter construct at a basal level, but only GSK3β silencing was effective in reverting its GC-dependent transcriptional activation. Inhibition of GSK3β or double knockdown of α- and β-isoforms reduced both basal and GC-induced mRNA expression of endogenous GILZ. On the other hand, GSK3α silencing was unable to reduce any of them, indicating that GSK3β affects basal and GC-induced gene transcription. RT-MLPA analysis revealed differences in apoptosis expression profile when compared with Jurkat GR WT cells, because HeLa cells do not undergo apoptosis in response to dexamethasone (41). GSK3β and GSK3α/β silencing down-regulated GC-dependent gene induction. Nevertheless, HIAP1 was induced by dexamethasone even when GSK3β was silenced, suggesting that it might be regulated by an indirect mechanism. Moreover, GSK3β-null MEF cells were significantly unable to induce GILZ mRNA levels and MMTV-Luc promoter construct luciferase activity in response to dexamethasone treatment.
Although the mechanism responsible for the nuclear import of steroid receptors is well documented, the mechanisms of GR export remain largely unknown (42). It has been suggested that CRM1 plays a pivotal role in the early nuclear export of the GR (33). However, contradictory results have been reported on whether GR nuclear export is CRM1 dependent (43) or CRM1 independent (44, 45). We demonstrate that the pharmacological inhibition of GSK3 activity in combination with dexamethasone treatment in Jurkat GR WT cells targets the activated GR for a rapid export from the nucleus, thereby down-regulating early GR transcriptional activity. A similar effect was described for the androgen receptor (AR) in prostate cancer cell lines, where GSK3 inhibitors attenuated AR-dependent transcriptional activity and caused a rapid nuclear export of endogenous AR (46, 47). SB216763-induced nuclear export was partially inhibited by LMB, suggesting there might be a CRM1-dependent nuclear export of the GR in response to GSK3 inhibition. However, other participating export mechanisms like Ca2+-dependent calreticulin-based mechanism cannot be completely ruled out (4, 48, 49).
We demonstrate that GSK3β and GSK3α/β silencing in HeLa cells resulted in the reduction of GC-dependent induction of BMF, BCL-XL, and MCL-1, previously described GC-regulated genes (50–53). Nevertheless, we observed higher basal GR expression levels and presence of the GR in the nucleus in response to GC treatment in these cells. The increase in GR expression levels in GSK3β-silenced cells is in agreement with previously described phosphorylation of Ser404 by GSK3β, which favors GR protein down-regulation by proteasome degradation (6).
GSK3 also phosphorylates the rat GR at Thr171 (16). This phosphorylation site is not present in the human GR sequence, indicating that GSK3-mediated regulation of this residue is likely species specific (34). Our results with rat GR mutants of the residues that are phosphorylated by GSK3 show that these mutations do not abrogate the ability of the GR to translocate to the nucleus and transactivate a GRE-containing reporter construct. In the same line, all the mutants were able to induce endogenous GILZ mRNA levels. GSK3 inhibition reverted the induction of luciferase activity of MMTV-Luc reporter construct and endogenous GILZ mRNA of all mutants. These results indicate that there could be an additional mechanism, not involving the phosphorylation of these residues, by which GSK3 regulates GR transcriptional activity. The Jurkat parental cell line was able to slightly induce GILZ mRNA expression levels, whereas it was unable to induce MMTV-Luc reporter construct. These cells harbor a function-impairing point mutation (R477H) in one of their GR alleles (18). This mutation might cause GC resistance by impairing transactivation and transrepression without affecting GR ligand-dependent nuclear import. These results are unclear, even though it was previously described (18) and might be reflecting promoter-specific differences or nongenomic effects of GC over the GILZ promoter (1).
It has been previously described that in the absence of a ligand, GSK3α is bound to the GR, and exposure to GC or GSK3 inhibitor leads to the disruption of this interaction (7). It has also been described that the GR associates with GSK3β in the presence of dexamethasone but not with GSK3α (6). In the same line, our results indicate that GSK3 isoforms regulate GR cellular response by using different mechanisms besides GSK3-mediated phosphorylation of the GR.
In summary, the current study demonstrates the involvement of GSK3β on GC-dependent gene transcriptional induction through the regulation of GR and RNA polymerase II recruitment to target gene sequences and by affecting GR protein subcellular localization. Our results suggest that GSK3 plays an important role in regulating GC mechanism of action, suggesting that keeping GSK3 in an active state could improve GC therapy. Therefore, additional analyses of the involvement of GSK3 activity in GC treatment of lymphoma and leukemia malignancies may help gain insight into the molecular basis of these disorders.
Acknowledgments
We thank Dr. Jose Luis Rosa, Edgardo Rodríguez-Carballo, and Miguel Peña-Rico for helpful discussions and suggestions. We also thank Adriana Forero for English language editing. Moreover, we thank the Scientific-Technical Services of the Unitat de Bellvitge at the Universitat de Barcelona for their technical support. We thank Dr. Carme Caelles for kindly providing Jurkat GR WT cells and MMTV-Luc construct and Dr. Marc Pallardy for providing p-1940-Luc construct. We also thank Dr. J. Woodgett for providing WT, GSK3α−/−, and GSK3β−/− MEF cells.
This study was supported by grants from the Ministerio de Economía y Competitividad and FEDER (SAF2010-20519), the Instituto de Salud Carlos III (RTICC RD06/0020/0097), and the AGAUR-Generalitat de Catalunya (AGAUR- 2009SGR395). C.R.-P., A.P.-P., C.M.-M., and D.M.G.-G. are recipients of research fellowships from the Ministerio de Economía y Competitividad. D.I.-S. has a postdoctoral contract from Fundació Bosch i Gimpera.
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Nuclear Receptors: GR.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- AR
- Androgen receptor
- ChIP
- chromatin immunoprecipitation
- CLL
- chronic lymphocytic leukemia
- CRM1
- chromosome region maintenance 1
- GC
- glucocorticoids
- GR
- GC receptor
- GRE
- GC response elements
- GSK3
- glycogen synthase kinase-3
- LMB
- leptomycin B
- MEF
- mouse embryonic fibroblast
- RT-MLPA
- reverse transcriptase multiplex ligation-dependent probe amplification
- RT-qPCR
- reverse transcriptase quantitative PCR
- siRNA
- small interfering RNA
- WT
- wild type.
References
- 1. Kfir-Erenfeld S , Sionov RV , Spokoini R , Cohen O , Yefenof E. 2010. Protein kinase networks regulating glucocorticoid-induced apoptosis of hematopoietic cancer cells: fundamental aspects and practical considerations. Leuk Lymphoma 51:1968–2005 [DOI] [PubMed] [Google Scholar]
- 2. Zhou J , Cidlowski JA. 2005. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids 70:407–417 [DOI] [PubMed] [Google Scholar]
- 3. Oakley RH , Cidlowski JA. 2011. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem 286:3177–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beck IM , De Bosscher K , Haegeman G. 2011. Glucocorticoid receptor mutants: man-made tools for functional research. Trends Endocrinol Metab 22:295–310 [DOI] [PubMed] [Google Scholar]
- 5. Wang Z , Chen W , Kono E , Dang T , Garabedian MJ. 2007. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol Endocrinol 21:625–634 [DOI] [PubMed] [Google Scholar]
- 6. Galliher-Beckley AJ , Williams JG , Collins JB , Cidlowski JA. 2008. Glycogen synthase kinase 3β-mediated serine phosphorylation of the human glucocorticoid receptor redirects gene expression profiles. Mol Cell Biol 28:7309–7322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spokoini R , Kfir-Erenfeld S , Yefenof E , Sionov RV. 2010. Glycogen synthase kinase-3 plays a central role in mediating glucocorticoid-induced apoptosis. Mol Endocrinol 24:1136–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nuutinen U , Ropponen A , Suoranta S , Eeva J , Eray M , Pellinen R , Wahlfors J , Pelkonen J. 2009. Dexamethasone-induced apoptosis and up-regulation of Bim is dependent on glycogen synthase kinase-3. Leuk Res 33:1714–1717 [DOI] [PubMed] [Google Scholar]
- 9. Wang Z , Malone MH , He H , McColl KS , Distelhorst CW. 2003. Microarray analysis uncovers the induction of the proapoptotic BH3-only protein Bim in multiple models of glucocorticoid-induced apoptosis. J Biol Chem 278:23861–23867 [DOI] [PubMed] [Google Scholar]
- 10. Zhang L , Insel PA. 2004. The pro-apoptotic protein Bim is a convergence point for cAMP/protein kinase A- and glucocorticoid-promoted apoptosis of lymphoid cells. J Biol Chem 279:20858–20865 [DOI] [PubMed] [Google Scholar]
- 11. Iglesias-Serret D , de Frias M , Santidrián AF , Coll-Mulet L , Cosialls AM , Barragán M , Domingo A , Gil J , Pons G. 2007. Regulation of the proapoptotic BH3-only protein BIM by glucocorticoids, survival signals and proteasome in chronic lymphocytic leukemia cells. Leukemia 21:281–287 [DOI] [PubMed] [Google Scholar]
- 12. Beurel E , Jope RS. 2006. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol 79:173–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forde JE , Dale TC. 2007. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci 64:1930–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rayasam GV , Tulasi VK , Sodhi R , Davis JA , Ray A. 2009. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol 156:885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woodgett JR. 1990. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J 9:2431–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rogatsky I , Waase CL , Garabedian MJ. 1998. Phosphorylation and inhibition of rat glucocorticoid receptor transcriptional activation by glycogen synthase kinase-3 (GSK-3). Species-specific differences between human and rat glucocorticoid receptor signaling as revealed through GSK-3 phosphorylation. J Biol Chem 273:14315–14321 [DOI] [PubMed] [Google Scholar]
- 17. Helmberg A , Auphan N , Caelles C , Karin M. 1995. Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J 14:452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riml S , Schmidt S , Ausserlechner MJ , Geley S , Kofler R. 2004. Glucocorticoid receptor heterozygosity combined with lack of receptor auto-induction causes glucocorticoid resistance in Jurkat acute lymphoblastic leukemia cells. Cell Death Differ 11(Suppl 1):S65–S72 [DOI] [PubMed] [Google Scholar]
- 19. Asselin-Labat ML , David M , Biola-Vidamment A , Lecoeuche D , Zennaro MC , Bertoglio J , Pallardy M. 2004. GILZ, a new target for the transcription factor FoxO3, protects T lymphocytes from interleukin-2 withdrawal-induced apoptosis. Blood 104:215–223 [DOI] [PubMed] [Google Scholar]
- 20. Barragán M , Bellosillo B , Campàs C , Colomer D , Pons G , Gil J. 2002. Involvement of protein kinase C and phosphatidylinositol 3-kinase pathways in the survival of B-cell chronic lymphocytic leukemia cells. Blood 99:2969–2976 [DOI] [PubMed] [Google Scholar]
- 21. Eldering E , Spek CA , Aberson HL , Grummels A , Derks IA , de Vos AF , McElgunn CJ , Schouten JP. 2003. Expression profiling via novel multiplex assay allows rapid assessment of gene regulation in defined signalling pathways. Nucleic Acids Res 31:e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen W , Rogatsky I , Garabedian MJ. 2006. MED14 and MED1 differentially regulate target-specific gene activation by the glucocorticoid receptor. Mol Endocrinol 20:560–572 [DOI] [PubMed] [Google Scholar]
- 23. Piqué M , Barragán M , Dalmau M , Bellosillo B , Pons G , Gil J. 2000. Aspirin induces apoptosis through mitochondrial cytochrome c release. FEBS Lett 480:193–196 [DOI] [PubMed] [Google Scholar]
- 24. Bellosillo B , Dalmau M , Colomer D , Gil J. 1997. Involvement of CED-3/ICE proteases in the apoptosis of B-chronic lymphocytic leukemia cells. Blood 89:3378–3384 [PubMed] [Google Scholar]
- 25. D'Adamio F , Zollo O , Moraca R , Ayroldi E , Bruscoli S , Bartoli A , Cannarile L , Migliorati G , Riccardi C. 1997. A new dexamethasone-induced gene of the leucine zipper family protects T lymphocytes from TCR/CD3-activated cell death. Immunity 7:803–812 [DOI] [PubMed] [Google Scholar]
- 26. Webster JC , Huber RM , Hanson RL , Collier PM , Haws TF , Mills JK , Burn TC , Allegretto EA. 2002. Dexamethasone and tumor necrosis factor-α act together to induce the cellular inhibitor of apoptosis-2 gene and prevent apoptosis in a variety of cell types. Endocrinology 143:3866–3874 [DOI] [PubMed] [Google Scholar]
- 27. Rogatsky I , Wang JC , Derynck MK , Nonaka DF , Khodabakhsh DB , Haqq CM , Darimont BD , Garabedian MJ , Yamamoto KR. 2003. Target-specific utilization of transcriptional regulatory surfaces by the glucocorticoid receptor. Proc Natl Acad Sci USA 100:13845–13850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Drouin J , Sun YL , Chamberland M , Gauthier Y , De Léan A , Nemer M , Schmidt TJ. 1993. Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. EMBO J 12:145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asselin-Labat ML , Biola-Vidamment A , Kerbrat S , Lombès M , Bertoglio J , Pallardy M. 2005. FoxO3 mediates antagonistic effects of glucocorticoids and interleukin-2 on glucocorticoid-induced leucine zipper expression. Mol Endocrinol 19:1752–1764 [DOI] [PubMed] [Google Scholar]
- 30. Gokbuget N , Hoelzer D. 2006. Treatment of adult acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program 2006:133–141 [DOI] [PubMed] [Google Scholar]
- 31. Pui CH , Evans WE. 2006. Treatment of acute lymphoblastic leukemia. N Engl J Med 354:166–178 [DOI] [PubMed] [Google Scholar]
- 32. Liang MH , Chuang DM. 2006. Differential roles of glycogen synthase kinase-3 isoforms in the regulation of transcriptional activation. J Biol Chem 281:30479–30484 [DOI] [PubMed] [Google Scholar]
- 33. Itoh M , Adachi M , Yasui H , Takekawa M , Tanaka H , Imai K. 2002. Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol Endocrinol 16:2382–2392 [DOI] [PubMed] [Google Scholar]
- 34. Galliher-Beckley AJ , Cidlowski JA. 2009. Emerging roles of glucocorticoid receptor phosphorylation in modulating glucocorticoid hormone action in health and disease. IUBMB Life 61:979–986 [DOI] [PubMed] [Google Scholar]
- 35. Sun M , Meares G , Song L , Jope RS. 2009. XIAP associates with GSK3 and inhibits the promotion of intrinsic apoptotic signaling by GSK3. Cell Signal 21:1857–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maurer U , Charvet C , Wagman AS , Dejardin E , Green DR. 2006. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell 21:749–760 [DOI] [PubMed] [Google Scholar]
- 37. Liao X , Thrasher JB , Holzbeierlein J , Stanley S , Li B. 2004. Glycogen synthase kinase-3β activity is required for androgen-stimulated gene expression in prostate cancer. Endocrinology 145:2941–2949 [DOI] [PubMed] [Google Scholar]
- 38. Mazor M , Kawano Y , Zhu H , Waxman J , Kypta RM. 2004. Inhibition of glycogen synthase kinase-3 represses androgen receptor activity and prostate cancer cell growth. Oncogene 23:7882–7892 [DOI] [PubMed] [Google Scholar]
- 39. Medunjanin S , Hermani A , De Servi B , Grisouard J , Rincke G , Mayer D. 2005. Glycogen synthase kinase-3 interacts with and phosphorylates estrogen receptor α and is involved in the regulation of receptor activity. J Biol Chem 280:33006–33014 [DOI] [PubMed] [Google Scholar]
- 40. Grisouard J , Mayer D. 2009. Specific involvement of glycogen synthase kinase-3 in the function and activity of sex steroid hormone receptors reveals the complexity of their regulation. J Steroid Biochem Mol Biol 117:87–92 [DOI] [PubMed] [Google Scholar]
- 41. Mann CL , Cidlowski JA. 2001. Glucocorticoids regulate plasma membrane potential during rat thymocyte apoptosis in vivo and in vitro. Endocrinology 142:421–429 [DOI] [PubMed] [Google Scholar]
- 42. Vandevyver S , Dejager L , Libert C. 2012. On the trail of the glucocorticoid receptor: into the nucleus and back. Traffic 13:364–374 [DOI] [PubMed] [Google Scholar]
- 43. Carrigan A , Walther RF , Salem HA , Wu D , Atlas E , Lefebvre YA , Haché RJ. 2007. An active nuclear retention signal in the glucocorticoid receptor functions as a strong inducer of transcriptional activation. J Biol Chem 282:10963–10971 [DOI] [PubMed] [Google Scholar]
- 44. Liu J , DeFranco DB. 2000. Protracted nuclear export of glucocorticoid receptor limits its turnover and does not require the exportin 1/CRM1-directed nuclear export pathway. Mol Endocrinol 14:40–51 [DOI] [PubMed] [Google Scholar]
- 45. Kumar S , Saradhi M , Chaturvedi NK , Tyagi RK. 2006. Intracellular localization and nucleocytoplasmic trafficking of steroid receptors: an overview. Mol Cell Endocrinol 246:147–156 [DOI] [PubMed] [Google Scholar]
- 46. Rinnab L , Schütz SV , Diesch J , Schmid E , Küfer R , Hautmann RE , Spindler KD , Cronauer MV. 2008. Inhibition of glycogen synthase kinase-3 in androgen-responsive prostate cancer cell lines: are GSK inhibitors therapeutically useful? Neoplasia 10:624–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schütz SV , Cronauer MV , Rinnab L. 2010. Inhibition of glycogen synthase kinase-3β promotes nuclear export of the androgen receptor through a CRM1-dependent mechanism in prostate cancer cell lines. J Cell Biochem 109:1192–1200 [DOI] [PubMed] [Google Scholar]
- 48. Holaska JM , Black BE , Rastinejad F , Paschal BM. 2002. Ca2+-dependent nuclear export mediated by calreticulin. Mol Cell Biol 22:6286–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kumar S , Chaturvedi NK , Nishi M , Kawata M , Tyagi RK. 2004. Shuttling components of nuclear import machinery involved in nuclear translocation of steroid receptors exit nucleus via exportin-1/CRM-1* independent pathway. Biochim Biophys Acta 1691:73–77 [DOI] [PubMed] [Google Scholar]
- 50. Ploner C , Rainer J , Niederegger H , Eduardoff M , Villunger A , Geley S , Kofler R. 2008. The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia 22:370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scoltock AB , Heimlich G , Cidlowski JA. 2007. Glucocorticoids inhibit the apoptotic actions of UV-C but not Fas ligand in hepatoma cells: direct evidence for a critical role of Bcl-xL. Cell Death Differ 14:840–850 [DOI] [PubMed] [Google Scholar]
- 52. Xu B , Strom J , Chen QM. 2011. Dexamethasone induces transcriptional activation of Bcl-xL gene and inhibits cardiac injury by myocardial ischemia. Eur J Pharmacol 668:194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lynch JT , Rajendran R , Xenaki G , Berrou I , Demonacos C , Krstic-Demonacos M. 2010. The role of glucocorticoid receptor phosphorylation in Mcl-1 and NOXA gene expression. Mol Cancer 9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]