Abstract
The pituitary transcription factor POU1F1 is required for the differentiation of lactotrope, thyrotrope, and somatotrope cells. Its expression is maintained in the adult and is crucial for the expression of prolactin, GH, and TSHβ-subunit. Different studies indicated that POU1F1 could also have other functions in these cells. The identification of new targets of this factor could be useful to obtain a better understanding of these functions. To address this question we combined data obtained from expression microarrays and from chromatin immunoprecipitation (ChIP)-chips. Gene expression microarray assays were used to detect genes that have their expression modified in somatolactotrope GH4C1 cells by the expression of a dominant-negative form of POU1F1, POU1F1(R271W), and led to the identification of 1346 such genes. ChIP-chip experiments were performed from mouse pituitaries and identified 1671 POU1F1-binding sites in gene-promoter regions. Intersecting the gene expression and the ChIP-chip data yielded 121 potential new direct targets. The initial set of 1346 genes identified using the microarrays, as well as the 121 potential new direct targets, were analyzed with DAVID bioinformatics resource for gene ontology term enrichment and cluster. This analysis revealed enrichment in different terms related to protein synthesis and transport, to apoptosis, and to cell division. The present study represents an integrative genome-wide approach to identify new target genes of POU1F1 and downstream networks controlled by this factor.
The anterior pituitary-specific transcription factor POU1F1 (also called PIT-1) was initially identified and cloned as a transactivator of prolactin (PRL), GH, and TSHβ-subunit genes (1, 2). Based on the study of natural mouse mutants bearing mutation of POU1F1, it was also found to be a major regulator of lactotrope, somatotrope, and thyrotrope lineage development and differentiation (3). Indeed, these mice, the Snell dwarf with the specific mutation W261C and the Jackson dwarf with a chromosomal rearrangement leading to a defect of POU1F1 expression, exhibit an anterior pituitary hypoplasia with PRL, GH, and TSH deficiency and lack of the corresponding lineages (for review see Ref. 4).
In humans, mutation in the POU1F1 gene has been shown to be responsible for combined pituitary hormone deficiency. At least 27 different mutations have been described for this factor, the most common being R271W. This mutant is able to bind DNA, and it appears to act as a dominant-negative factor (5, 6).
In a previous work we have shown that expression of the dominant-negative mutant POU1F1(R271W) in the somatolactotrope cell line GH4C1 led to the decrease of the expression of Prl and Gh genes and to reduced cell viability by decreasing growth rate and inducing apoptosis via a caspase-independent pathway (6). These results indicate that the function of POU1F1 is not limited to the regulation of the expression of the three hormones, PRL, GH, and TSHβ, but would also include regulation of proliferation and apoptosis in the corresponding cell lineages. POU1F1 has also been found to regulate receptors involved in growth control (3), c-Fos gene (7) and, more recently, synaptogamin (8). To have a better idea of the global function of POU1F1, identification of new genes regulated by this factor seems necessary.
In the present work we combine two approaches to obtain a list of new potential direct POU1F1 target genes. First, to obtain a global view of the genes modulated by POU1F1, we evaluated the consequence, on global gene expressions, of the expression of the dominant-negative mutant POU1F1(R271W) in the GH4C1 cell line. Indeed, these cells express POU1F1 that regulates, e.g. the Prl or Gh genes, and introducing a dominant-negative mutant of this factor will block the action of this endogenous POU1F1 on its targets and lead to a modification of their expression. Second, we performed a chromatin immunoprecipitation (ChIP)-Chip experiment in the mouse pituitary using anti-POU1F1 antibodies to identify promoters bound by POU1F1. The intersection of these two sets of complementary data resulted in the identification of 121 potential direct target genes of POU1F1.
Materials and Methods
Generation of lentiviral vectors and transduction
Lentiviral vectors were generated as described previously (6). Lentiviral vector particles were produced in 293T cells using a polyethylenimine (Aldrich, Milwaukee, WI)-mediated transfection procedure. 293T cells were transfected with 1) the packaging plasmid pCMVΔR8.91 expressing HIV-1 gag, pol, tat, and rev proteins; 2) the envelope plasmid pMD-G expressing the vesicular stomatitis virus envelope G glycoprotein (VSV-G); and 3) the lentiviral vector construct containing the transgene of interest under the control of the PGK promoter [pGK-POU1F1(R271W)-internal ribosome entry site-enhanced green fluorescent protein (EGFP) transducing POU1F1(R271W), or pGK-EGFP used as controls]. Conditioned medium was harvested 48 and 72 h after transfection, cleared of debris by low-speed centrifugation, filtered through 0.22-μm membrane, and concentrated by centrifugation at 10,000 × g for 1 h at 4 C in the presence of polyethylene glycol (molecular weight 8000). The viral pellets were then resuspended in 1/100 volume of PBS. Viral stocks were stored in aliquots at −80 C, and the titers were determined by transducing HelaT cells in a limiting dilution assay. No replication competent virus was detected in the concentrated lentiviral stocks. For infections, GH4C1 cell cultures plated in 12-well plates (10 × 104 cells per well) were supplemented with lentiviral particles containing medium in the presence of 2 μg/ml polybrene at a multiplicity of infection (MOI) of 5. The virus-containing medium was removed 16 h later, after which the cells were washed and grown in regular medium for an additional 4 d.
RNA preparation and microarray analyses
Total RNA from cells infected with the lentiviral vectors was isolated by using the RNeasy Kit (Qiagen, Hilden, Germany), including on-column DNase I digestion according to the manufacturer's recommendations. The quality of isolated RNA was controlled by the Lab-on-Chip-System Bioanalyser 2100 (Agilent Technologies, Inc., Palo Alto, CA).
Affymetrix microarrays (Rat Genome 230 2.0) were processed using the manufacturer's standard protocol at the Microarray Core Facility of the Institute of Research on Biotherapy, CHRU-INSERM-UM1 Montpellier (http://irb.chu-montpellier.fr/).
Microarray analysis
Microarray analysis was done with ChipInspector (Genomatix) software using Affymetrix raw data files. ChipInspector carries out significance analysis on the level of single probes rather than predefined probe sets. This results in heavily reduced false-positive rates. Affymetrix CEL files were imported into the Genomatix ChipInspector software 1.4 and analyzed with a false discovery rate of 0.2% and three probes minimum coverage required. Microarray data are available in the GEO database, accession no. GSE33311.
Chromatin immunoprecipitation and ChIP-chip analysis
Pituitaries from pregnant female C57Bl/6J mice were collected at the end of the gestational period (d 17–d 18 of gestation, pregnant dams giving birth in our animal facility on average on embryonic d 19 to embryonic d 20). The pituitaries were gently homogenized in a buffer [250 mm sucrose, 25 mm KCl, 10 mm MgCl2, 0.1% Nonidet P-40, 10 mm Tris-HCl, pH 7.4, protease inhibitor cocktail (Sigma, St. Louis, MO)], nuclei were isolated by centrifugation (800 × g, 10 min), and resuspended, and cross-linking was performed for 10 min at 25 C in 1 ml of the same buffer without sucrose and containing 1% freshly prepared paraformaldehyde. After addition of glycine (0.25 m final concentration) to quench the cross-linking reaction and washing, the pellet was frozen until a sufficient number of pituitaries could be collected. Pellets corresponding to 12 pituitaries were resuspended in 1200 μl Tris-EDTA buffer containing protease inhibitors and 1% sodium dodecyl sulfate, and sonicated to obtain DNA fragments with an average size of 500 bp. ChIP was performed as described by Spencer et al. (9). using polyclonal antibodies directed against POU1F1 (sc-442, X7, from Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Three independent pools corresponding to three biological replicates were used. After elution of the DNA-protein complexes from protein A-Sepharose, decomplexation by heating overnight at 65 C, and digestion by RNAse A and proteinase K, DNA was purified using Nucleospin extract II (Macherey Nagel, Hoerdt, France) and amplified by ligation-mediated PCR according to the protocol given by NimbleGen (Madison, WI). Analysis was performed on three independent samples by NimbleGen using MM8 ChIP-chip promoter arrays for mouse. Results were analyzed using NimbleScan, and selected binding sites were inspected using SignalMap software, of NimbleGen. NimbleGen microarray data are available in the GEO database, accession no. GSE33318.
Quantitative PCR analysis
Reverse transcription was performed using a GoScript Reverse Transcription System kit (Promega Corp., Madison, WI). Quantitative PCR was performed using fast SYBR Green Mix (Applied Biosystems, Foster City, CA) on an Applied 7500 Fast cycler operated according to the manufacturer's recommendations. Primers used in this study were designed using AmplifX software (http://ifrjr.nord.univ-mrs.fr/AmplifX-Home-page) and are listed in Supplemental Table 9.
Cloning of the Crem P2 and Lmo4 promoters
The Crem P2 promoter was cloned by PCR from genomic DNA obtained from GH4C1 cells using the primers forward (F): ggggtaccccGCTGTAACTGGAGATGAAACTG and reverse (R): ccgctcgagcggTGAGGCAGAACGACCAGGATAAGA and the Kapa HiFi polymerase (Kapa Biosystems, Woburn, MA). The Lmo4 promoter was cloned by PCR from C57Bl/6N mouse genomic DNA using the primers F: actacggtaccAATGGGAGCAAGATGGGGTTAAGC and R: agttactcgagCCCCAACGTTTTAATCGCTCCGAA and Accuprime Pfx (Invitrogen). After digestion by KpnI and XhoI, the 2-kb Crem P2, and 2.4-kb Lmo4 promoter fragments were cloned into the pGL3 plasmid (Promega) and sequenced to verify the correctness of the amplification.
Cell culture, transfection, and luciferase assays
GH4C1 cells were grown in HamF10 medium supplemented with 15% horse serum and 2% fetal calf serum. Cells were transfected using Transfast transfection kit (Promega) in line following the manufacturer's instructions. Briefly, cells were plated at 125,000 cells per well in 24-well plates 24 h before transfection with 200 ng reporter plasmid and with various amounts of effector plasmid. Empty plasmid pcDNA3 were added to adjust the total mass of transfected DNA to 500 ng. Cells were incubated with the DNA-liposome complexes for 1 h and were then supplemented with 1.5 ml complete medium. GH4C1 cells were harvested 48 h after transfection and lysed in 200 μl Reporter Lysis Buffer (Promega). Luciferase activities were determined with the Luciferase Assay System (Promega). Each transfection done in triplicate was repeated at least three times. Cell viability was assayed using Cell Titer Glo (Promega) 48 h after the transfection to control that expression of POU1F1 or POU1F1 (R271W) has no effect on cell viability at this time.
Gene ontology and pathways analysis
Experimental data were analyzed by the ChipInspector and MatInspector softwares (Genomatix) and NimbleScan and SignalMap (NimbleGen). Further bioinformatic analysis of the data was performed using the DAVID software, i.e. the Database for Annotation, Visualization, and Integrated Discovery (http://david.abcc.ncifcrf.gov/) (10, 11).
Results
Identification of genes modulated by POU1F1 using the POU1F1 (R271W) dominant-negative mutant in GH4C1 cells
GH4C1 cells were transduced with pGK-EGFP or pGK-POU1F1(R271W)-internal ribosome entry site-EGFP. Cells were harvested 4 d after infection and total RNA was purified. This experiment was done in three biological replicates. The Affymetrix microarray Rat 230 2.0 used in this study analyzes the expression level of 30,000 transcripts including more than 28,000 well-substantiated rat genes.
The data obtained were analyzed using ChipInspector software (Genomatix). ChipInspector extracted, from the Affymetrix GeneChip microarrays, single probes corresponding to transcripts with significantly modified expression. We identified 1346 genes with an expression significantly modified by the expression of POU1F1(R271W) in the cells, as compared with control cells (see Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org), of which 822 were significantly down-regulated (with at least log2 −0.31-fold change) and 524 were significantly up-regulated (with at least log2 0.34-fold change).
To correlate these changes with overall biological functions, the 1346 differentially expressed genes were submitted to GO classification using DAVID software (biological process, GOTERM_BP_FAT with Ease scores <0.05 and enrichment scores >1.5). A large number of annotation terms was found to be associated with this gene list: 65 genes are involved in protein transport, 26 in protein folding, 40 in protein catabolism, 71 in regulation of programmed cell death, 13 in the proteasome, 27 in the fatty acid metabolism, etc. (see Table 1 for summarized results and Supplemental Table 2 for complete results). Functional annotation of these 1346 genes with DAVID identified enriched annotation clusters. Thus, the major biological processes deregulated by the POU1F1(R271W) are: membrane-enclosed lumen, protein transport, vesicle, regulation of cell death, protein catabolism process, and nucleotide binding (see Supplemental Table 3).
Table 1.
List of selected entries identified by GO (Biological Process) as being associated with the 1346 genes modulated by the presence of POU1F1(R271W)
| GO Biological process | Gene no. | P value |
|---|---|---|
| Protein folding (GO:0006457) | 26 | 1.484 E-7 |
| Protein transport (GO:0015031) | 65 | 6.012 E-7 |
| Establishment of protein localization (GO:0045184) | 65 | 8.286 E-7 |
| Regulation of programmed cell death (GO:0043067) | 71 | 2.106 E-5 |
| ncRNA metabolic process (GO:0030163) | 27 | 3.041 E-5 |
| Protein catabolic process (GO:0030163) | 40 | 8.991 E-5 |
| Fatty acid metabolic process (GO:0006631) | 27 | 9.482 E-5 |
| Transcription (GO:0006350) | 68 | 1.079 E-4 |
| Cellular protein catabolic process (GO:0044257) | 38 | 1.117 E-4 |
| Nuclear import (GO:0051170) | 14 | 1.454 E-4 |
| Protein import (GO:0017038) | 17 | 1.499 E-4 |
| tRNA metabolic process (GO:0006399) | 25 | 1.616 E-4 |
Some of these genes were identified as related to significantly overrepresented specific molecular KEEG pathways (see Table 2 for summarized results and Supplemental Table 4 for complete results). Fatty acid metabolism, arginine and proline metabolism, and proteasome pathways contained the most numerous members of the list and were the most relevant.
Table 2.
List of the pathways identified by KEGG classification for the 1346 genes modulated by the presence of POU1F1(R271W)
| Term | Count | P value |
|---|---|---|
| Fatty acid metabolism | 14 | 4.17 E-7 |
| Arginine and proline metabolism | 12 | 1.95 E-4 |
| Proteasome | 11 | 5.27 E-4 |
| β-Alanine metabolism | 7 | 0.0013 |
| Lysine degradation | 8 | 0.0089 |
| Glycolysis/gluconeogenesis | 12 | 0.0094 |
| Propanoate metabolism | 7 | 0.011 |
| Pentose phosphate pathway | 6 | 0.011 |
| Amino sugar and nucleotide sugar metabolism | 8 | 0.011 |
| Ascorbate and aldarate metabolism | 5 | 0.015 |
| PPAR signaling pathway | 10 | 0.022 |
| Limonene and pinene degradation | 4 | 0.023 |
| Pyruvate metabolism | 7 | 0.024 |
PPAR, Peroxisome proliferator-activated receptor.
Identification of POU1F1 binding sites using ChIP-chip assay
ChIP-chip analysis was performed on three biological replicates from pregnant female mouse pituitaries using POU1F1 polyclonal antibodies and a NimbleGen mouse promoter microarray. Pregnant females were chosen to favor identification of POU1F1 target genes involved in regulation of cell proliferation, because cell division in the pituitary is known to be increased during later pregnancy (12). The microarray is based on 50–75 bp tiled DNA probes that cover the transcriptional start site of some 50,000 annotated transcripts, from 3250 kb upstream to 750 bp downstream.
The analysis of ChIP-chip experiment with NimbleScan identified 1671 peaks of POU1F1 fixation common to the three samples and with false discovery rate score <0.2, indicative of a binding site (Supplemental Table 5). These peaks correspond to 1669 genes with at least one binding site for POU1F1 in their promoters. These genes could be considered as new potential POU1F1 target genes.
Identification of potential new POU1F1 direct targets by combining microarray and ChIP-chip gene expression data
The two types of data obtained with the dominant negative R271W mutant and the ChIP-chip experiments provide complementary information about regulation of transcription by POU1F1. To approach better the identity of targets of POU1F1, we combined the results obtained with the microarray and the ChIP-chip studies. We thus identified 121 genes that bound POU1F1 in their promoter region and the expression of which was significantly changed in presence of POU1F1(R271W) (Supplemental Table 6). These genes can be considered as potential direct targets of POU1F1. POU1F1 is a positive regulator of 74 of these genes (they are down-regulated in the presence of the dominant-negative mutant) and a negative regulator for 47 of them.
To support these results, we searched for potential POU1F1 binding elements in the peak regions identified by ChIP-chip, using MatInspector (Genomatix) and setting core similarity to 0.75 and a matrix similarity to −0.02. In this software two matrices are defined, pit1–01 and pit1–02, corresponding to the matrix obtained, respectively, from 17 genomic binding sites defined from the literature and from a screening using oligonucleotides (13). Among the 121 promoters, we identified 25 (20.6%) with a pit1–01 consensus sequence motif and 32 (26.4%) with a pit1–02 consensus sequence motif present in the peak region. Intersection analysis of these two lists shows that 49 genes (40.5%) have at least one POU1F1 consensus sequence motif in the peak region. Because POU1F1 is a member of the POU-homeodomain transcription factor family, we searched also for the presence of binding sites for others members of this family [Octamer binding protein (Oct1), Brn-2, Brn-3, Brn-4, and Brn-5 POU domain factors] that could also bind POU1F1. In all the promoters not identified in the previous analysis but five, a potential binding sequence for a member of the POU transcription factor family was found. Thus, only five promoters of the 121 identified from the intersection of the microarray and ChIP-chip data contain no binding site for the POU transcription factor family (Supplemental Table 6).
Using GO classification, these 121 genes were classified in different functional categories (Table 3 and Supplemental Table 7), the most relevant categories being Wnt receptor signaling pathway, translation and protein transport, regulation of apoptosis, and regulation of cell proliferation.
Table 3.
List of the entities identified by GO (Biological Process) for the potential new 121 POU1F1 target genes
| GO Biological Process | Gene no. | P value | Genes |
|---|---|---|---|
| Wnt receptor signaling pathway | 5 | 3.66 E3 | Dixdc1 Sox4 Lrrfip2 Hbp1 Tax1bp3 |
| Translation | 10 | 4.83 E3 | Mrpl24, Tars, Mrpl13, Gspt1, Mrps18b, Eif4a2, Abtb1, Lars, Eif3s10, Eif3s9 |
| Protein transport | 11 | 8.10 E3 | Scamp3, Rab3c, Vcp, Cep57, Jun, Arf4, Arcn1, Bet1, Carl, Prl, Sec61a1 |
| Establishment of protein localization | 11 | 8.60 E3 | Scamp3, Rab3c, Vcp, Cep57, Jun, Arf4, Arcn1, Bet1, Carl, Prl, Sec61a1 |
| Regulation of cell proliferation | 12 | 1.10 E2 | Ptpn6, Myd88, Dbp, Cdkn2c, Jun, F3, Adk, Sox4, Tax1bp3, Carl, Prl, Sod2 |
| Protein ubiquitination | 4 | 2.38 E2 | Ube2d3, Gspt1, Cand1, Rbbp6 |
| Golgi vesicle transport | 11 | 2.50 E2 | Deaf1, Rnf4, Dbp, Jun, Lmo4, Crem, Cand1, Hbp1, Tfb1m, Tcf4, Lrpprc |
| Regulation of apoptosis | 11 | 3.05 E2 | Ptpn6, Myd88, Syvn1, Vcp, Gspt1, Cdkn2c, Jun, F3, Dad1, Sox4, Sod2 |
| Transcription | 11 | 3.24 E2 | Deaf1, Rnf4, Dbp, Jun, Lmo4, Crem, Cand1, Hbp1, Tfb1m, Tcf4, Lrpprc |
Annotations could also be aggregated in clusters (Supplemental Table 8), and the results show a great number of clusters related to protein synthesis, folding, transport, localization, and degradation but also cluster related to nucleotides binding, transcription, and apoptosis.
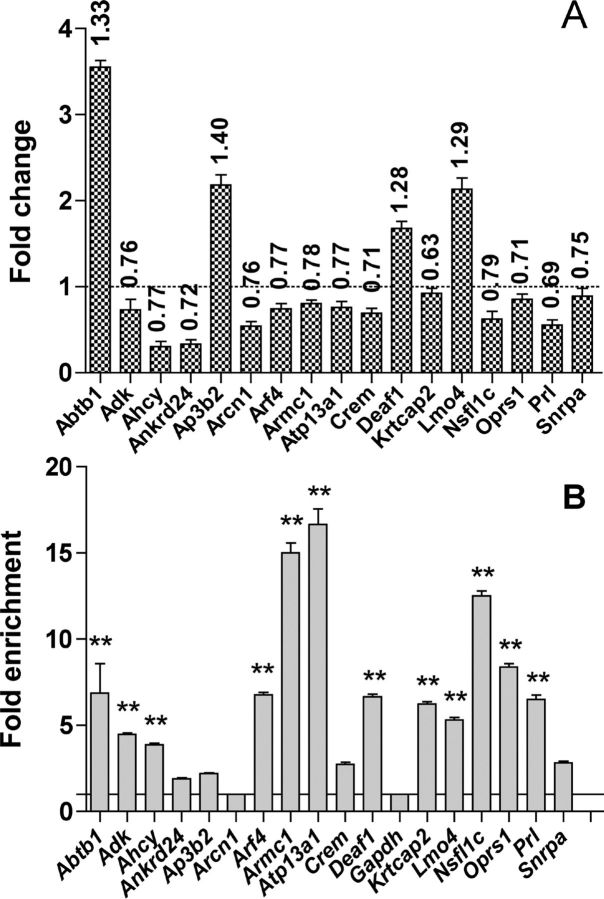
To validate the overall procedure for the set of 121 genes, 16 of them were analyzed by quantitative PCR (qPCR) to confirm the effect of POU1F1(R271W) on their expression in the GH4C1 cell line (Fig. 1A) and by ChIP-qPCR to confirm the results of ChIP-chip experiment (Fig. 1B). These 16 genes included the nine first genes of the list by alphabetical order (Abtb1, Adk, Ahcy, Ankrd24, Ap3b2, Arcn1, Arf4, Armc1, Atp13a1) of which five contain in their promoter a Pit-1 consensus binding site, the five genes that do not have binding sites defined for a POU transcription factor family (Deaf1, Krtcap2, Nsfl1c, Oprs1, Snrpa), and two genes chosen for their potential biological interest (see below), Lmo4 with a potential binding site for members of the POU transcription factors family, and Crem with a potential binding site for POU1F-1. Prl was also added to serve as a positive control. The effect of POU1F1 on the gene expression was confirmed for all the genes tested (Fig. 1A). In the ChIP-qPCR experiment Gapdh was used as a negative control and Prl as a positive control. Overall, for 16/17 of the candidate target genes an enrichment was observed, and for 12 of them this enrichment was statistically significant. The only exception was, surprisingly, Arcn1, which contains a potential POU1F1 binding site (Fig. 1B).
Fig. 1.
A, Effect of POU1F1(R271W) on the expression of 17 genes of the 121 potential direct targets in GH4C1 cell line. Total RNA was prepared from GH4C1 cells using a NucleoSpin RNAII kit (Macherey-Nagel), and reverse transcription was performed followed by qPCR. Results were calculated using the ΔΔCt method (29), in which Gapdh was chosen as normalizer because, as evidenced in the microarray data, its level was not affected by the expression of the POU1F1(R271W). The graph represents the fold change in gene expression (n = 3 biological replicates). Numbers above the bars represent the fold change obtained previously in the microarray experiment. Prl was used as positive control. The sequences of the primers used can be found in Supplemental Table 9. Note that the primers used for Crem on this figure detect the ICER isoform of this gene. B, Recruitment of POU1F1 to the promoter of the same 17 genes. ChIP-qPCR was performed using mouse pituitaries and POU1F1 antibody. Gapdh and Prl were used, respectively, as negative and positive controls (n = 3 biological replicates). The sequences of the primers used can be found in Supplemental Table 9. Results were calculated using the fold enrichment method (30). The ΔCt {ΔCt = Ct sample − Ct input} then the ΔΔCt were calculated [ΔΔCt = ΔCt (immune serum) − ΔCt (nonimmune serum)]. The fold difference between experimental sample (immune serum) and negative control (nonimmune serum) was obtained using 2(−ΔΔCt). Statistically significant differences were determined using Dunnett's multiple comparison tests, and data are expressed as means ± se (*, P < 0.05; **, P < 0.01).
POU1F1 modulates the transcription of Lmo4 and a Crem isoform, ICER
To test the validity of the strategy and show that it does identify direct targets of POU1F1, we chose two of the 121 potential target genes to test the effect of POU1F1 on their expression. Given their known functions, Crem and Lmo4 seemed interesting candidates. Indeed, the peak POU1F1 binding identified by ChIP-chip was localized within the promoter P2 of the Crem gene that controls the expression of an isoform of this gene named inducible cAMP early repressor (ICER) (14). ICER is identical to the C-terminal part of cAMP response element modulator (CREM) corresponding to the DNA-binding domain of the latter and is a powerful repressor of cAMP-mediated gene expressions (14). Lmo4 encodes a cysteine-rich protein that contains two LIM domains but lacks a DNA-binding homeodomain. It is a transcriptional adapter that has been found to have critical functions in mammalian development and oncogenesis.
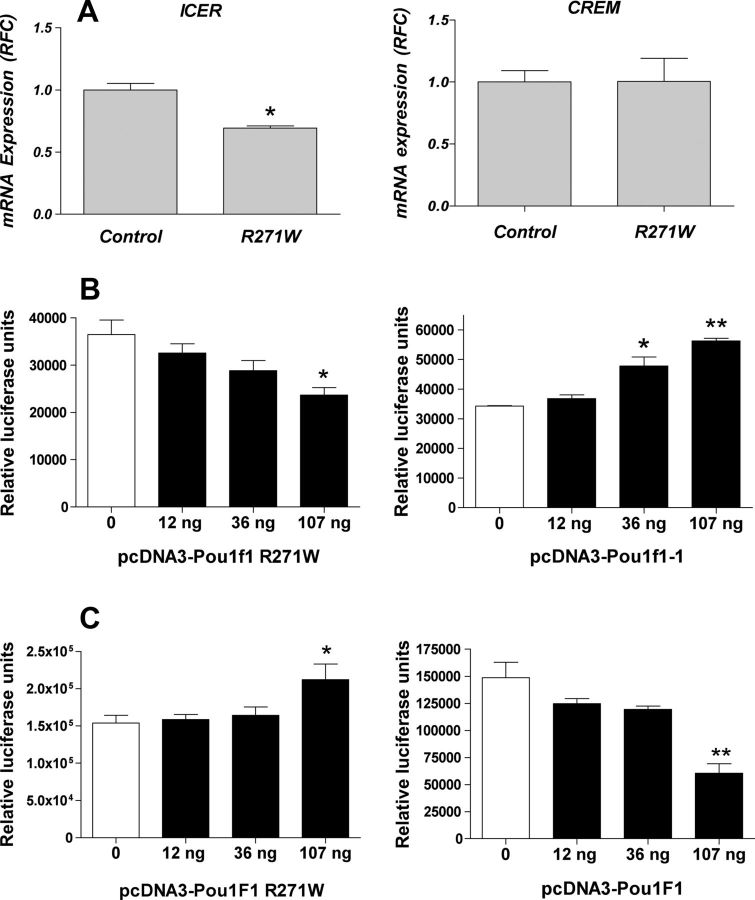
Binding of POU1F1 to the P2 promoter of Crem and to the promoter of Lmo4 were confirmed by ChIP-qPCR (Fig. 1B). We also confirmed by qPCR the microarray results for Lmo4 (Fig. 1A) and showed that the expression of ICER in GH4C1 was decreased by the expression of the dominant-negative POU1F1(R271W) (Figs. 1A and 2A). By contrast, the expression of the other isoform of Crem, regulated by an upstream P1 promoter devoid of POU1F1 binding site, was not affected by this mutant (Fig. 2A).
Fig. 2.
A, Effect of POU1F1 R271W on Icer and Crem mRNA expression in GH4C1 cells. Total RNA from cells infected with the lentiviral vectors (with or without the transgene) was isolated by using the RNeasy Kit (QIAGEN), and reverse transcription was performed followed by qPCR. The graph represents the relative fold change (RFC) in mRNA expression (n = 3 biological replicates). The sequences of the primers used can be found in Supplemental Table 9. B and C, Transcriptional activation of Crem P2 (B) and Lmo4 (C) promoters by wild-type and mutant POU1F1 in GH4C1 cells. GH4C1 cells were transfected with pGL3-Crem-P2 (B) or pGL3-Lmo4 (C) and varying amount of pcDNA3-POU1F1 or pcDNA3-POU1F1(R271W). Cells were harvested after 48 h and assayed for luciferase. Transfections were performed in triplicate. Statistically significant differences were determined using Dunnett's multiple comparison tests, and data are expressed as means ± se (*, P < 0.05; **, P < 0,01).
To confirm the regulation of the P2 promoter of Crem and the promoter of Lmo4 by POU1F1, these promoters were cloned into a reporter plasmid (pGL3). Increasing amounts of an expression vector encoding POU1F1 or POU1F1(R271W) were cotransfected together with the pGL3-Crem P2 or pGL3-Lmo4 luciferase reporter construct (Fig. 2B). Concerning the Crem-P2 promoter, POU1F1 increased luciferase activity in a dose-dependent fashion, whereas POU1F1(R271W) decreased it in a dose-dependent fashion (Fig. 2B). As expected on the basis of the previous microarray data, inverse results were obtained using Lmo4 promoter (Fig. 2C).
Discussion
In this study we describe a genome-wide study of the regulatory network governed by POU1F1 using a combination of microarray-based gene expression profiling and of ChIP-chip experiments.
The results obtained in GH4C1 cells in the presence of the dominant-negative POU1F1(R271W) provide a snapshot of the transcriptional activity governed by POU1F1. The level of expression of 1346 genes was modified when this dominant-negative mutant was expressed in the cells to antagonize the physiological action of endogenous POU1F1. The analysis of theses 1346 genes by GO biological process (Fig. 1 and Supplemental Table 2) or by clusters (Supplemental Table 3) showed that a great number of these genes are involved in transcription and in protein folding, transport, import, and catabolism. This indicates that POU1F1 plays an important role in the capacity of the pituitary cells to produce and transport proteins, in accordance with its known role in the regulation of pituitary hormone production. Another interesting observation is the large number of genes involved in regulation of programmed cell death. This is in good correlation with our previous results (6). A ChIP-chip approach was then used as the first promoter localization analysis of POU1F1 binding sites. We thus mapped 1671 binding peaks representing 1669 unique promoters containing at least one fixation site for POU1F1.
Although these two approaches give good indications concerning potential targets of POU1F1, they both present some drawbacks. On the other hand, the information they provide are, to some extent, complementary. Thus, microarray experiments provide a list of genes the expression of which is modified by blockade of endogenous POU1F1's action. However, part of these genes are certainly not direct targets of POU1F1 but are simply included, at some downstream level, in the gene cascades influenced by this factor, and the modification of their activity by the dominant-negative mutant is a second- or third-order effect. There exists also a remote possibility that the activity change of some of the genes identified is related to an effect specific to POU1F1(R271W) itself, linked to its interaction with some transcriptional complexes and independent of its interaction with POU1F1. Note, however, that the activity of some bona fide POU1F1 targets may not be modified by POU1F1(R271W), and these might have been missed in our screen. Indeed, it is known that, depending of the DNA sequence, POU1F1 binds to its specific sites as a monomer or a dimer (15), whereas POU1F1(R271W) binds to DNA only as a monomer (15). POU1F1 dimerization is required to attract cAMP response element binding protein (CREB)-binding protein to the transcriptional complex (16). Nevertheless, POU1F1 monomers can also exert an action through recruitment to some composite elements together with some other factors such as estrogen receptor or ETS-1 (15, 17, 18). Thus, the dominant-negative mutant may selectively inhibit transcriptional regulation mediated through POU1F1 dimeric binding and CREB-binding protein interaction and influence less those mediated by monomeric POU1F1 binding. Conversely, ChIP-chip studies give information concerning promoters bound by POU1F1 that therefore necessarily include direct targets. However, it is also known that the presence of a binding site for a given transcription factor in a promoter, as given by ChIP-chip studies, does not necessary correlate with effective modulation of transcription of the corresponding gene by the same factor (19). We can thus assume that the intersection of these two lists provides a list of potential direct targets modulated by POU1F1.
It appeared that 121 genes overlapped between these two genes lists. Thus, these genes can be considered as good candidates for being new direct targets of POU1F1. The validity of the results was tested using 16 of these potential new targets by independent qPCR to confirm the effect of POU1F1(R271W) and by ChIP-qPCR experiments. Only one of these candidate targets (Arcn1) was not confirmed with the ChIP-qPCR experiment.
When comparing the sequence of the peak of POU1F1 binding, as given by ChIP-chip, in the promoters of these 121 genes with the two known POU1F1 consensus motifs defined in Genomatix, POU1F1 binding sites was detected only in 49 of them. There are different potential explanations for the absence of this site in the other promoters. First, all POU-domain factors bind to AT-rich regions, and at the same time exhibit some flexibility in their DNA binding. Thus, POU1F1 may bind to other POU-domain factor-binding elements, similar but not identical to the POU1F1 element, within these other promoters. We have indeed found that 116 genes of the 121 have at least one POU domain factor-binding element within the ChIP peak. Thus, all in all, only five genes do not have a POU factor-specific element present in the peak regions identified by ChIP. Other possibilities explaining the absence of known POU1F1 binding elements, especially in these latter five promoters, are the existence of other undefined position weight matrix for POU1F1 binding, the existence of low-affinity binding sites requiring binding of another factor present in vivo to increase affinity toward POU1F1, or indirect binding of POU1F1 through association with other transcription factors that bind themselves to DNA.
Among the known targets of POU1F1, only two (Prl and Jun) were recovered in this 121-gene list. It has to be noted, however, that most of them (Prl, Gh, Ghrhr, Tshb, Jun) were included in the gene list given by the ChIP-chip study conducted on pituitary, and their absence in the intersectional list is related to their absence in the microarray results. Due to the stringency of analysis the change in Gh mRNA level after expression of POU1F1(R271W) has remained under the threshold required to be identified as statistically significant potential target. On the other hand, this microarray study was conducted on GH4C1 somatolactotrope cells, a differentiated in vitro cell line. Thus, part of the potential targets, such as Tshb, may be silenced in this line, explaining the lack of action of POU1F1.
Therefore, these 121 genes do not represent an exhaustive list of direct targets of POU1F1, and should be considered rather as corresponding to genes expressed in a somatolactotrope lineage. Concerning Syt1, for which a binding site of POU1F1 has been recently localized in an intron located near a new transcription start site (8), its absence in the ChIP-chip list is not surprising, because this intron was not, until now, considered as a promoter and was therefore certainly not present on the NimbleGen chip used. However, its mRNA level was increased in the presence of the POU1F1(R271W) and might have to be added to the list of the 121 potential direct targets.
To gain further insight into the potential biological roles of these newly identified POU1F1 targets, we classified the 121 genes by biological function (Table 3 and Supplemental Table 6). The most significant term was “Wnt receptor signaling pathway.” The five genes classified in this process (Dixdc1, Sox4, Lrrfip2, Hbp1, and Tax1bp3) were regulators of this pathway (20–23). Lrrfip2 was positively regulated by POU1F1 whereas the four others were negatively regulated by this factor (Supplemental Table 1). Further studies will be necessary to determine more precisely the importance of POU1F1 in the Wnt pathway regulation. Note that Sox4 was also described as a crucial developmental factor and is implicated in cell proliferation. The other most significant annotations concern proteins synthesis and transport: transcription, translation, protein maturation, transport, and ubiquitination, Golgi vesicle transport, all important processes for cells specialized, as are the somato-, lacto-, and thyrotrope cells, in the production of hormones. A further category concerns regulation of cell proliferation and regulation of apoptosis, supporting suggestions by earlier functional studies, including ours, concerning the role of POU1F1 to regulate survival and proliferation of its target cells (6, 24–26). Finally, POU1F1 appears also to regulate transcription of other different transcription factors or cofactors, such as the cAMP response element modulator Crem, Jun, and the circadian factor Dbp.
To further validate our strategy, we have characterized the transcriptional effect of POU1F1 on two of these 121 genes. Transient transfections of GH4C1 cells were performed with a reporter construct in which the luciferase gene was placed under the control of the proximal P2 promoter of Crem or of the promoter of Lmo4, containing the sequence corresponding to the ChIP-chip peak. The results obtained confirm the positive effect of POU1F1 and a negative effect of POU1F1(R271W) on P2 Crem promoter whereas the effects of these two factors were inverted for the Lmo4 promoter. Thus, our strategy does reveal bona fide direct targets of POU1F1.
A further validation should involve the identification of the physiological meaning of the regulation of these genes by POU1F1. Although this goes beyond the scope of the present work, we can note, concerning Crem, that the isoform regulated by POU1F1 is the inducible cAMP early repressor (ICER) that represses cAMP-dependent trancription by competing with CREB or CREM. It is known that the pituitary has a high basal expression of ICER (27), and that cAMP, through CREB is, on the other hand, involved in the regulation of targets of POU1F1, such as Prl. Thus, POU1F1 seems to play a role in the balance between phosphorylated CREB or CREM and ICER, adding a further complexity to the regulation of known targets, such as Prl, by POU1F1. The importance of the level of ICER in the balance between proliferation and differentiation could be important. It should also be noticed that the regulation of Crem P2 promoter by POU1F1 is not observed in CV1 or Hela cells, indicating that one or several specific factor present in GH4C1 cells could be necessary for the regulation of this factor by POU1F1. Concerning Lmo4, little is known on the role of this factor in the pituitary gland. Repression of Lmo4 expression by small interfering RNA in the pituitary-derived cell line LβT2 leads to a decrease of the glycoprotein hormone α-subunit promoter activity (28), and the overexpression of this factor leads to a repression of the Prl promoter activity (J.L. Franc, unpublished data). Thus Lmo4 could act in the pituitary as a repressor of the expression of at least two hormones, and the repression of the expression of this factor by POU1F1 would increase the expression of glycoprotein hormone α-subunit and reinforce the direct stimulating action of POU1F1 on the expression of Prl.
Overall, the data obtained in the present study provide new insights into the role played by the pituitary transcription factor POU1F1 in somatolactotroph cells and reveal 121 new potential direct target genes of POU1F1.
Acknowledgments
This work was supported by grants from the National Research Agency (ANR-08-GENO-026-01) and Pfizer.
Disclosure summary: The authors have nothing to disclose.
Footnotes
- ChIP
- Chromatin immunoprecipitation
- CREB
- cAMP response element binding protein
- CREM
- cAMP response element modulator
- EGFP
- enhanced green fluorescent protein
- ICER
- inducible cAMP early repressor
- PRL
- prolactin
- qPCR
- quantitative PCR.
References
- 1. Bodner M , Castrillo JL , Theill LE , Deerinck T , Ellisman M , Karin M. 1988. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell 55:505–518 [DOI] [PubMed] [Google Scholar]
- 2. Ingraham HA , Chen RP , Mangalam HJ , Elsholtz HP , Flynn SE , Lin CR , Simmons DM , Swanson L , Rosenfeld MG. 1988. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell 55:519–529 [DOI] [PubMed] [Google Scholar]
- 3. Li S , Crenshaw EB , Rawson EJ , Simmons DM , Swanson LW , Rosenfeld MG. 1990. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347:528–533 [DOI] [PubMed] [Google Scholar]
- 4. Kelberman D , Rizzoti K , Lovell-Badge R , Robinson IC , Dattani MT. 2009. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev 30:790–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Radovick S , Nations M , Du Y , Berg LA , Weintraub BD , Wondisford FE. 1992. A mutation in the POU-homeodomain of Pit-1 responsible for combined pituitary hormone deficiency. Science 257:1115–1118 [DOI] [PubMed] [Google Scholar]
- 6. Pellegrini I , Roche C , Quentien MH , Ferrand M , Gunz G , Thirion S , Bagnis C , Enjalbert A , Franc JL. 2006. Involvement of the pituitary-specific transcription factor pit-1 in somatolactotrope cell growth and death: an approach using dominant-negative pit-1 mutants. Mol Endocrinol 20:3212–3227 [DOI] [PubMed] [Google Scholar]
- 7. Gaiddon C , de Tapia M , Loeffler JP. 1999. The tissue-specific transcription factor Pit-1/GHF-1 binds to the c-fos serum response element and activates c-fos transcription. Mol Endocrinol 13:742–751 [DOI] [PubMed] [Google Scholar]
- 8. Howard PW , Jue SF , Maurer RA. 2009. Expression of the synaptotagmin I gene is enhanced by binding of the pituitary-specific transcription factor, POU1F1. Mol Endocrinol 23:1563–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spencer VA , Sun JM , Li L , Davie JR. 2003. Chromatin immunoprecipitation: a tool for studying histone acetylation and transcription factor binding. Methods 31:67–75 [DOI] [PubMed] [Google Scholar]
- 10. Huang da W , Sherman BT , Tan Q , Collins JR , Alvord WG , Roayaei J , Stephens R , Baseler MW , Lane HC , Lempicki RA. 2007. The DAVID Gene Functional Classification Tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol 8:R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang da W , Sherman BT , Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57 [DOI] [PubMed] [Google Scholar]
- 12. Yin P , Arita J. 2000. Differential regulation of prolactin release and lactotrope proliferation during pregnancy, lactation and the estrous cycle. Neuroendocrinology 72:72–79 [DOI] [PubMed] [Google Scholar]
- 13. Berger MF , Badis G , Gehrke AR , Talukder S , Philippakis AA , Peña-Castillo L , Alleyne TM , Mnaimneh S , Botvinnik OB , Chan ET , Khalid F , Zhang W , Newburger D , Jaeger SA , Morris QD , Bulyk ML , Hughes TR. 2008. Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell 133:1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Cesare D , Sassone-Corsi P. 2000. Transcriptional regulation by cyclic AMP-responsive factors. Prog Nucleic Acid Res Mol Biol 64:343–369 [DOI] [PubMed] [Google Scholar]
- 15. Holloway JM , Szeto DP , Scully KM , Glass CK , Rosenfeld MG. 1995. Pit-1 binding to specific sites as a monomer or dimer determines gene-specific use of a tyrosine-dependent synergy domain. Genes Dev 9:1992–2006 [DOI] [PubMed] [Google Scholar]
- 16. Cohen RN , Brue T , Naik K , Houlihan CA , Wondisford FE , Radovick S. 2006. The role of CBP/p300 interactions and Pit-1 dimerization in the pathophysiological mechanism of combined pituitary hormone deficiency. J Clin Endocrinol Metab 91:239–247 [DOI] [PubMed] [Google Scholar]
- 17. Duval DL , Jonsen MD , Diamond SE , Murapa P , Jean A , Gutierrez-Hartmann A. 2007. Differential utilization of transcription activation subdomains by distinct coactivators regulates Pit-1 basal and Ras responsiveness. Mol Endocrinol 21:172–185 [DOI] [PubMed] [Google Scholar]
- 18. Jean A , Gutierrez-Hartmann A , Duval DL. A 2010 Pit-1 threonine 220 phosphomimic reduces binding to monomeric DNA sites to inhibit Ras and estrogen stimulation of the prolactin gene promoter. Mol Endocrinol 24:91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. MacQuarrie KL , Fong AP , Morse RH , Tapscott SJ. 2011. Genome-wide transcription factor binding: beyond direct target regulation. Trends Genet 27:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sinner D , Kordich JJ , Spence JR , Opoka R , Rankin S , Lin SC , Jonatan D , Zorn AM , Wells JM. 2007. Sox17 and Sox4 differentially regulate β-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol 27:7802–7815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh KK , Ge X , Mao Y , Drane L , Meletis K , Samuels BA , Tsai LH. 2010. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron 67:33–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J , Bang AG , Kintner C , Orth AP , Chanda SK , Ding S , Schultz PG. 2005. Identification of the Wnt signaling activator leucine-rich repeat in Flightless interaction protein 2 by a genome-wide functional analysis. Proc Natl Acad Sci USA 102:1927–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim J , Zhang X , Rieger-Christ KM , Summerhayes IC , Wazer DE , Paulson KE , Yee AS. 2006. Suppression of Wnt signaling by the green tea compound (-)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J Biol Chem 281:10865–10875 [DOI] [PubMed] [Google Scholar]
- 24. Castrillo JL , Theill LE , Karin M. 1991. Function of the homeodomain protein GHF1 in pituitary cell proliferation. Science 253:197–199 [DOI] [PubMed] [Google Scholar]
- 25. Cañibano C , Rodriguez NL , Saez C , Tovar S , Garcia-Lavandeira M , Borrello MG , Vidal A , Costantini F , Japon M , Dieguez C , Alvarez CV. 2007. The dependence receptor Ret induces apoptosis in somatotrophs through a Pit-1/p53 pathway, preventing tumor growth. EMBO J 26:2015–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ben-Batalla I , Seoane S , Garcia-Caballero T , Gallego R , Macia M , Gonzalez LO , Vizoso F , Perez-Fernandez R. 2010. Deregulation of the Pit-1 transcription factor in human breast cancer cells promotes tumor growth and metastasis. J Clin Invest 120:4289–4302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kell CA , Dehghani F , Wicht H , Molina CA , Korf HW , Stehle JH. 2004. Distribution of transcription factor inducible cyclicAMP early repressor (ICER) in rodent brain and pituitary. J Comp Neurol 478:379–394 [DOI] [PubMed] [Google Scholar]
- 28. Susa T , Ishikawa A , Cai LY , Kato T , Matsumoto K , Kitahara K , Kurokawa R , Ono T , Kato Y. 2010. The highly related LIM factors, LMO1, LMO3 and LMO4, play different roles in the regulation of the pituitary glycoprotein hormone α-subunit (α GSU) gene. Biosci Rep 30:51–58 [DOI] [PubMed] [Google Scholar]
- 29. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mukhopadhyay A , Deplancke B , Walhout AJ , Tissenbaum HA. 2008. Chromatin immunoprecipitation (ChIP) coupled to detection by quantitative real-time PCR to study transcription factor binding to DNA in Caenorhabditis elegans. Nat Protoc 3:698–709 [DOI] [PMC free article] [PubMed] [Google Scholar]