Abstract
The secretin receptor (SR), a G protein-coupled receptor, mediates the effects of the gastrointestinal hormone secretin on digestion and water homeostasis. Recently, high SR expression has been observed in pancreatic ductal adenocarcinomas, cholangiocellular carcinomas, gastrinomas, and bronchopulmonary carcinoid tumors. Receptor overexpression associates with enhanced secretin-mediated signaling, but whether this molecule plays an independent role in tumorigenesis is currently unknown. We recently discovered that pheochromocytomas developing in rats affected by the MENX (multiple endocrine neoplasia-like) syndrome express at very high-level Sctr, encoding SR. We here report that SR are also highly abundant on the membranes of rat adrenal and extraadrenal pheochromocytoma, starting from early stages of tumor development, and are functional. PC12 cells, the best characterized in vitro pheochromocytoma model, also express Sctr at high level. Thus, we used them as model to study the role of SR in neoplastic transformation. Small interfering RNA-mediated knockdown of Sctr decreases PC12 cells proliferation and increases p27 levels. The proproliferative effect of SR in PC12 cells is mediated, in part, by the phosphatidylinositol 3 kinase (PI3K)/serine-threonine protein kinase (AKT) pathway. Transfection of Sctr in Y1 adrenocortical carcinoma cells, expressing low endogenous levels of Sctr, stimulates cell proliferation also, in part, via the PI3K/AKT signaling cascade. Because of the link between SR and PI3K/AKT signaling, tumor cells expressing high levels of the receptor (MENX-associated primary pheochromocytoma and NCI-H727 human bronchopulmonary carcinoid cells) respond well and in a SR-dependent manner to PI3K inhibitors, such as NVP-BEZ235. The association between SR levels and response to PI3K inhibition might open new avenues for the treatment of tumors overexpressing this receptor.
The receptor of the secretin hormone is a glycoprotein belonging to the B family of the G protein-coupled receptors. The receptors of vasoactive intestinal polypeptide (VIP) and glucagon are additional members of this family of proteins (1). The secretin receptor (SR) has long been known for mediating secretin's important role in digestive functions and water homeostasis (2, 3). In physiological conditions, the human SR gene, SCTR, is highly expressed in secretin target organs, such as pancreas, kidney, and small intestine, and is expressed in the distal lung regions and liver, with trace levels in the brain, heart, and ovary (4). Upon binding to the secretin ligand, the SR activates intracellular adenylate cyclase, which leads to an increase in cAMP (5, 6) and to the subsequent activation of protein kinase A (PKA) (7, 8). Recently, deregulated expression of the SCTR gene and/or of the SR protein have been identified in pathological conditions, namely, in tumors arising from physiological secretin target tissues. Specifically, high SR expression has been reported in pancreatic ductal adenocarcinomas (PDAC) (9) and in cholangiocellular carcinomas (10, 11) and gastrinomas (12). Bronchopulmonary carcinoid tumor cells were also found to present SR at high density on their membranes (13). The functional consequences of SR overexpression in these tumors have yet to be fully explored.
MENX (multiple endocrine neoplasia-like) is a multitumor syndrome recently discovered in the rat, which is caused by a homozygous germline frameshift mutation in the Cdkn1b gene encoding the cell cycle inhibitor p27 (14). MENX-affected rats develop, among other endocrine tumors, bilateral pheochromocytoma with complete penetrance within their first year of life (15). We recently performed transcriptome analysis of hyperplastic and neoplastic (pheochromocytoma) adrenomedullary lesions from MENX mutant rats and identified the Sctr gene as the ninth most highly expressed gene in hyperplasia compared with normal rat adrenal tissue (16). Up-regulation of Sctr transcript is a very early genetic change in this model, being already evident in the adrenal medulla of 1-month-old mutant rats, before they show histopathological alterations in this tissue. Moreover, we found that rat PC12 cells, a well-established in vitro model of pheochromocytoma, also express the Sctr transcript at very high levels (16). Altogether, these data suggest that overexpression of Sctr might be involved in rat pheochromocytoma pathophysiology.
Peptide hormone receptors are heavily studied as therapeutic targets, because they are often overexpressed in endocrine tumor cells and regulate the growth and secretory functions of these tumor cells upon binding with specific ligands. Somatostatin receptor targeting is the clinically best established example: due to the high level of expression of somatostatin receptors in gastroenteropancreatic neuroendocrine tumors, these neoplasms can be visualized with radiolabeled somatostatin analogs, such as OctreoScan, and respond to targeted therapy with radiotoxic somatostatin analogs (17). As reported above, high expression of SR has been reported in several tumor entities, but the functional consequences of this genetic event are still unknown. Secretin, acting through its receptor, is known to stimulate the growth of nonmalignant human and mouse large cholangiocytes (11), but a possible direct role of SR in regulating cell proliferation has not been explored. Given both the potential of peptide hormone receptors as therapeutic targets and the high expression of SR in a subset of human tumors, a better understanding of the role that this molecule may play in tumorigenesis is highly relevant.
In the current study, we first demonstrate that the overexpression of the Sctr gene in MENX-associated adrenal and extraadrenal pheochromomocytoma translates into a high level of the functional receptor protein being present on the tumor cells, further supporting a potential role for this molecule in tumorigenesis. Then, we studied in more detail the effects of Sctr overexpression in adrenal-derived tumor cell lines. We found that SR plays a proproliferative role in adrenal tumor cells (PC12 and Y1), which is mediated, at least in part, by the phosphatidylinositol 3 kinase (PI3K)/serine-threonine protein kinase (AKT) pathway. Tumor cells expressing high levels of SR respond well to inhibitors of the PI3K signaling cascade, suggesting that SR levels may represent a potential predictor of response to PI3K/AKT inhibition.
Materials and Methods
Rat tissue samples
Rat adrenal, pituitary, thyroid, and pancreas tissues were snap frozen in liquid nitrogen and stored at −80 C. We analyzed by in vitro receptor autoradiography seven adrenal glands from mutant rats (ages 7–9 months) having pheochromocytoma and six adrenal glands of 2-month-old mutant rats having no detectable pathological changes in the adrenal medulla. In parallel, we analyzed adrenal glands of wild-type age-matched rats (see Table 1). We also analyzed three rat paragangliomas and five rat thyroid tumors (C-cell carcinomas) and three rat pituitary adenomas obtained from MENX-affected rats. Pancreas from mutant and wild-type rats was used as positive control.
Table 1.
SR density in the adrenal glands of wild-type and mutant rats at different ages
| No. rat | p27 genotype | Age (months) | SR density (mean ± sem) (dpm/mg tissue) |
|---|---|---|---|
| 09/1927 | wt/wt | 2 | Cortex, 0; medulla, 260 ± 260 |
| 06/883 | wt/wt | 3 | Cortex, 0; medulla, 446 ± 45 |
| 06/884 | wt/wt | 3 | Cortex, 0; medulla, 649 ± 72 |
| 09/1922 | wt/wt | 7 | Cortex, 0; medulla, 417 ± 206 |
| 08/822 | wt/wt | 7 | Cortex, 0 |
| 09/1636 | wt/wt | 7 | Cortex, 0 |
| 08/149 | wt/wt | 21 | Cortex, 0 |
| 09/1926 | mut/mut | 2 | Pheo, 2259 ± 539 |
| 10/147 | mut/mut | 2 | Pheo, 2511 ± 192 |
| 09/1925 | mut/mut | 2.5 | Pheo, 4898 ± 947 |
| 09/1635 | mut/mut | 7 | Cortex, 0; Pheo, 5178 ± 246 |
| 09/1923 | mut/mut | 7 | Pheo, 4086 ± 292 |
| 09/1924 | mut/mut | 7 | Pheo, 5557 ± 776 |
| 09/833 | mut/mut | 8 | Cortex, 0; Pheo, 4942 ± 136 |
| 09/858 | mut/mut | 9 | Cortex, 0; Pheo, 6211 ± 60 |
Density is reported for the adrenal cortex (cortex), the medulla, or the pheochromocytoma (Pheo) tissues of the rats ± sem.
In vitro SR autoradiography
Rat tissues were investigated for SR protein expression on the basis of specific binding of radioiodinated secretin using in vitro autoradiography. The procedure was carried out as previously described (13). Nonspecific radioligand binding was assessed by incubating tissue sections in the incubation solution containing 100 nm nonradiolabeled (cold) human secretin in addition to 125I-[Tyr10] rat secretin. At this concentration, cold secretin completely and specifically displaces 125I-[Tyr10] rat secretin at the receptors. To distinguish SR from other receptors of the same family, which bind secretin with low affinity, serial tissue sections were incubated with 125I-[Tyr10] rat secretin and increasing concentrations of one of the following cold peptides: human secretin, VIP (Bachem, Bubendorf, Switzerland), or glucagon (1-29) (Bachem). After incubation, the slides were washed five times in ice-cold HEPES containing 1% BSA and twice in ice-cold HEPES without BSA. The slides were dried for 15 min under a stream of cold air and then exposed to Kodak films Biomax MR for 7 d at 4 C (Kodak, Rochester, NY). The signals on the films were analyzed in correlation with morphology, using a corresponding hematoxylin and eosin-stained tissue section. The signal density was quantitatively assessed using tissue standards for iodinated compounds (Amersham, Aylesbury, UK) and using a computer-assisted image processing system (Analysis Imaging System, Interfocus, Mering, Germany). In all experiments, rat pancreas served as positive control.
Cell culture, transfections, and immunofluorescence
PC12 cells (purchased from LGC Promochem, Teddington, UK) were grown in F12 medium supplemented with 15% horse serum, 2.5% fetal calf serum; Y1 cells (kindly provided by Felix Beuschlein, Munich, Germany) were maintained in DMEM supplemented with 10% fetal bovine serum; and NCI-H727 cells (kind gift of Christoph J. Auernhammer, Munich, Germany) were grown as described (18). The human immortalized nonmalignant cholangiocyte cell line H-69 was kindly provided by G. J. Gores (Mayo Clinic, Rochester, MN) and cultures were as described (19). Ex vivo pheochromocytoma cells from MENX mutant rats were established following the previous report (20).
Transient transfections in each cell lines were carried out as already reported (14). The plasmid expressing the Myc-Sctr fusion protein was generated by cloning the full-length rat Sctr cDNA into the pCMV-Myc vector backbone (CLONTECH, Heidelberg, Germany).
cAMP assays
PC12 cells were transfected with plasmid or small interfering RNA (siRNA) and were then incubated with 100 nm rat secretin (Phoenix Pharmaceuticals, Karlsruhe, Germany) in complete medium for additional 24 h. The concentration of cAMP was determined in each sample using a Parameter cAMP Assay kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
RT-PCR analysis of SR and secretin transcripts
Receptor autoradiography results were confirmed by semiquantitative and quantitative TaqMan RT-PCR for SR (Sctr/SCTR) transcripts using RNA extracted from macrodissected adrenomedullary cells of the same tissue samples. Primers used for semiquantitative analysis were: rat Sctr forward (fw), atgctcagcaccatgagac; rat Sctr reverse (rev), ttgctgcagcctcagatgatac. Moreover, we analyzed Sctr expression in mouse Y1 cells by semiquantitative RT-PCR using primers: mouse Sctr fw, ggtggagggcctctatcttc; mouse Sctr rev, ccaggcgcttataatggtgt. We analyzed SCTR expression in human H-69 and NCI-H727 cells by TaqMan RT-PCR. Quantitative RT-PCR was performed using TaqMan inventoried primers and probes for Sctr/SCTR and for the rat β-2-microglobulin gene, mouse β-2-microglobulin gene or human TBP gene as internal control (Applied Biosystems, Foster City, CA). RNA was extracted, and TaqMan assays were set up as previously reported (16). For semiquantitative detection of secretin mRNA, we employed the following primers: rat Sct fw, cctacaggattggcttctgc and rat Sct rev, gcctggttgtttcagtccac; and mouse Sct fw, gcccttagaggaccagctct and mouse Sct rev, tgaacgatcaacagcagacc. Conditions for the RT-PCR reaction were as previously reported (16).
Protein extraction and Western blotting
For protein extraction, cells were collected after treatments, washed twice in PBS, and lysed in lysis buffer essentially as previously reported (21). Protein concentration was assessed by BCA assay (Pierce Chemical Co., Rockford, IL). Total extracts were subjected to polyacrylamide gel electrophoresis using Bis-Tris 4–12% NuPAGE gels, blotted, and probed with the following antibodies: monoclonal, against phosphorylated-Akt (P-Akt) (Ser473), P-p42/44 MAPK (ERK1/2), and polyclonal against total-Akt, total-p44/42 MAPK (Erk1/2) (all from Cell Signaling, Beverly, MA); monoclonal anti-p27 (BD Biosciences, Franklin Lakes, NJ); and monoclonal anti-α-tubulin (Sigma, Steinheim, Germany). Immunoreactive proteins were visualized using West Pico chemiluminescent substrates (Pierce Chemical Co.). The bands that we obtained by Western blotting were quantified using the Molecular Imager ChemiDoct XRS and analyzed with Quantity Ones software (both from Bio-Rad Laboratories, Hercules, CA). Western blot analyses from biological replicates showed similar expression data, attesting to the reproducibility of the results.
Drug treatment and cell proliferation assays
NVP-BKM120 and NVP-BEZ235 were kindly provided from Novartis Pharma (Basel, Switzerland). NVP-BKM120 and NVP-BEZ235 were dissolved in dimethylsulfoxide (DMSO) and stored at −20 C. Dilutions to the final concentration were made in the culture medium right before use. PC12, Y1, and NCI-H727 cells (transfected or native), as well as primary rat pheochromocytoma cells, were seeded in 96-well plates treated with PI3K inhibitors, and cell proliferation was measured as previously reported (21). In some experiments, rat secretin (100 nm) or human secretin (Tocris, Bristol, UK) was added to the culture medium 1 h before adding NVP-BEZ235, and cells were then incubates for additional 24 h before assessing cell proliferation. A reduction in proliferation of −20% was considered significant (21). The reported results represent the mean of six replicates ± sd.
Statistical analysis
Results of the cell viability assays are shown as the mean of values obtained in independent experiments ± sem. A paired two-tailed Student's t test was used to detect significance between two series of data, and P < 0.05 was considered statistically significant.
Results
SR expression in rat pheochromocytoma tissues
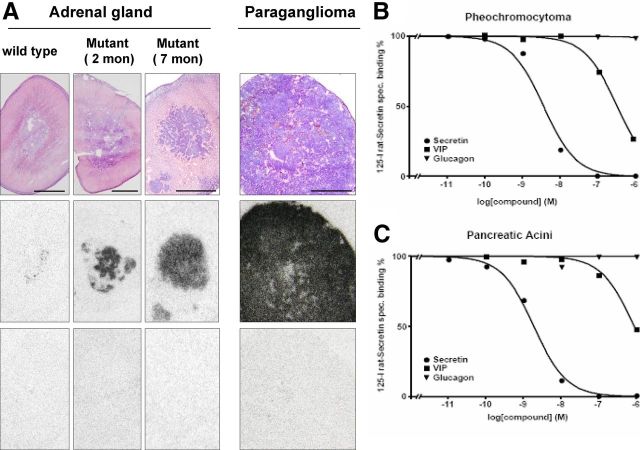
MENX mutant rats develop adrenomedullary hyperplasia starting at 3–4 months of age; by the age of 6 months, they all present with bilateral pheochromocytoma (14). We previously showed that the Sctr gene is highly overexpressed in both adrenomedullary hyperplasia and pheochromocytoma vs. normal rat adrenal medulla (by microarray and TaqMan analysis), and this overexpression is already detectable in the adrenal medulla of 1-month-old mutant rats (16). Although both expression arrays and TaqMan give an estimate of the amount of transcript, a correlation with protein levels does not automatically follow. Therefore, we set out to determine whether overexpression of the Sctr transcript translates into higher amount of the encoded peptide in rat tumor cell membranes. To this aim, we performed SR autoradiography with radioactive 125I-[Tyr10] rat secretin ligand on adrenal glands of MENX-affected rats (n = 7, age 2–9 months) and of control wild-type rats (n = 6) (Table 1). We observed a much higher binding of the secretin radioligand in the adrenal medulla of mutant rats compared with wild-type rats (Fig. 1A), in agreement with the Sctr mRNA expression results. In affected animals, radioligand binding increased with age, but it was already much higher in 2-month-old mutant rats than in age-matched wild-type littermates (Table 1). Because acini in the exocrine pancreas are known to express high levels of SR (22), we employed this tissue as positive control for the autoradiography experiments. Very strong binding of the 125I-[Tyr10] secretin radioligand was observed in pancreata from both wild-type and mutant rats, with no differences in SR density and distribution between the two animal groups (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org).
Fig. 1.
Assessment of SR expression in serial rat adrenal gland tissue sections by receptor autoradiography. A, Hematoxyliln and eosin-stained tissue sections showing in the upper panels, A normal rat adrenal gland from a wild-type animal, the gland of a 2-month-old mutant rat, the gland of a 7-month-old mutant rat, and a paraganglioma from an 8-month-old mutant rat. The middle panels are autoradiograms showing total binding of 125I-[Tyr10] rat secretin to the tissues shown above. Very strong and homogeneous binding to the adrenomedullary tumor cells and paraganglioma cells in older mutant rats is observed, whereas glands of young mutant rats show heterogeneous binding. The lower panels represent autoradiograms showing nonspecific binding of 125I-[Tyr10] rat secretin in the presence of 100 nm cold human secretin. Complete displacement of 125I-[Tyr10] rat secretin by cold secretin provides proof of specific receptor binding in all four tissues. B and C, Representative pharmacological competition experiments showing displacement of 125I-[Tyr10] rat secretin by cold secretin, VIP, and glucagon (1-29) in (B) a pheochromocytoma and (C) pancreatic acini (control).
RNA extracted from some of the adrenomedullary tissue samples used for in vitro receptor binding experiments (two wild type and two mutant) was used for semiquantitative and quantitative real-time TaqMan analysis of the Sctr gene to confirm the relationship between mRNA and protein levels. Both methods showed a much higher level of Sctr mRNA in rat adrenomedullary lesions when compared with normal adrenal tissue (Supplemental Fig. 2, A and B). Ex vivo primary cultures of rat pheochromocytoma were also established, and we observed that these cells retain high Sctr expression (Supplemental Fig. 2B). While performing Sctr transcript analysis, we also discovered that the pheochromocytoma cells in MENX rats only express the full-length Sctr transcript, and no variants produced by alternative splicing were detected (Supplemental Fig. 2A). These variants have been identified in various human tumor entities and often associate with nonfunctional receptors (9, 23).
In addition to adrenal pheochromocytoma, MENX mutant rats develop extraadrenal pheochromocytoma (paraganglioma) with a frequency of approximately 20%. At the mRNA level, rat paragangliomas show an expression of the Sctr gene even higher than the pheochromocytomas (several thousand-fold increase vs. normal adrenal medulla) (16). Three paragangliomas were analyzed using in vitro SR autoradiography: they showed a homogeneous and very high expression level of SR protein (Fig. 1A).
MENX-affected rats develop hyperplastic and neoplastic lesions also in thyroid and pituitary glands (14). Thus, we tested whether rat medullary thyroid tumor cell and pituitary adenoma cells express SR at high level too, but we could not observe differences in the amount of 125I-[Tyr10] secretin radioligand binding between wild-type (normal) and mutant (tumor) rat tissues (Supplemental Fig. 1).
Our in vitro receptor autoradiography studies show that, in MENX mutant rats, chromaffin cell-derived neuroendocrine tumors, but not other tumor entities, present with high amount of functional SR molecules.
Pharmacological characterization of the SR in rat pheochromocytoma
Secretin binds with high affinity SR but also binds with lower affinity other receptors of the family, such as VIP and glucagon receptors (24). Therefore, it had to be proven that in the investigated adrenomedullary tissues, the radioligand 125I-[Tyr10] rat secretin was bound solely by SR. For this purpose, pharmacological competition experiments were performed to assess the rank order of potencies of secretin, VIP, and glucagon binding at the identified receptors. In all secretin-binding pheochromocytoma, 125I-[Tyr10] rat secretin was displaced by cold secretin with high affinity in the nanomolar concentration range and by VIP and glucagon with low affinity in the micromolar concentration range, as shown by two representative displacement curves for pheochromocytoma and pancreas control (Fig. 1B). This rank order of potencies provides strong pharmacological evidence that using receptor autoradiography, we specifically detected the SR in the mutant rat tumor tissues.
SR overexpression has a promitogenic role in adrenal chromaffin cells
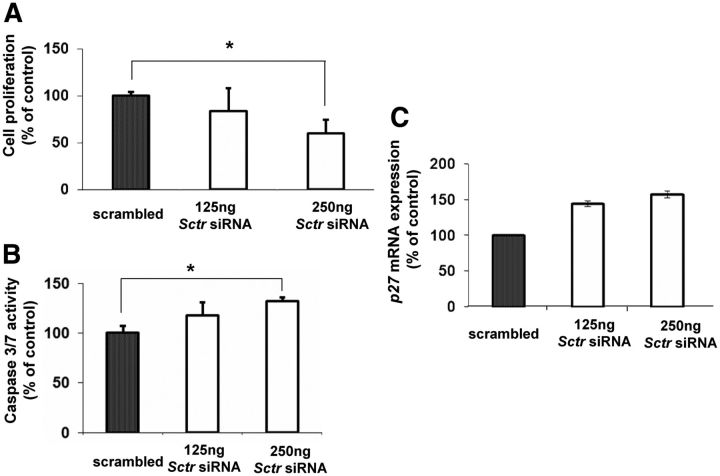
Measurable levels of the SR proteins are detected in the adrenal medulla of 2-month-old MENX mutant rats (Table 1), before they show any gross histopathological changes in this organ, making this molecular change a very early one in pheochromocytoma development. We previously showed that PC12 rat pheochromocytoma cells also express very high levels of the Sctr mRNA (average fold change, +200 vs. normal adrenal medulla) (16). To study whether the overexpression of SR may indeed play a role in tumorigenesis, we knocked down the expression of the endogenous Sctr gene in PC12 cells and checked for effects on cell proliferation. Reduction of Sctr expression significantly inhibited cell proliferation (up to −40% at the 24-h posttransfection time point) (Fig. 2A). To verify the efficiency of siRNA-mediated Sctr down-regulation, we could not rely on immunoblotting, because we had previously tested all commercially available antibodies against SR but failed to get a specific and reliable signal for the rat protein. Thus, we employed TaqMan RT-PCR, as shown in Supplemental Fig. 3A, and observed a reduction of Sctr mRNA in siSctr-transfected PC12 cells (−85%). In addition, we set up a functional assay to measure intracellular cAMP levels. It has been shown that after treatment of PC12 cells with secretin, there is an activation of the cAMP pathway through stimulation of the SR (25). Therefore, we measured intracellular cAMP levels as a readout of Sctr gene knockdown in PC12 cells. As expected, treatment with secretin increased the baseline cAMP level in untransfected PC12 cells (control) (Supplemental Fig. 3B). In the presence of Sctr overexpression (transfection of a plasmid expressing Myc-Sctr), addition of secretin caused an even greater increase in the intracellular amount of cAMP. In contrast, transfection with anti-Sctr siRNA oligos abrogated secretin-mediated increase in cAMP (Supplemental Fig. 3B), providing indirect evidence of successful siRNA-mediated Sctr knockdown.
Fig. 2.
Down-regulation of Sctr inhibits proliferation and induces apoptosis of PC12 cells. A, PC12 cells were transfected with scrambled (negative control) or 125 ng/250 ng of anti-Sctr siRNA oligos. Cell proliferation was assessed 24 h later by measuring ATP levels. B, In parallel to A, Caspase 3/7 activity was measured to monitor apoptosis. C, In parallel to A, RNA from the transfected PC12 cells was extracted, and TaqMan RT-PCR was performed to monitor the expression level of p27. For A and B, data were analyzed independently with six replicates each and were expressed as the mean ± sem; *, P < 0.05, vs. scrambled control.
We then assessed whether reduced Sctr expression induced apoptosis by measuring the amount of cleaved Caspase 3/7. We observed a slight induction of apoptosis (+30%) after siRNA-mediated knockdown of Sctr in PC12 cells (Fig. 2B). Then, we checked whether reduced proliferation of PC12 cells after Sctr knockdown was also associated to the induction of cell cycle inhibitors. To this aim, we determined whether the level of expression of the G1 phase cyclin-dependent kinase inhibitors Cdkn2c (p18), Cdkn1a (p21), and Cdkn1b (p27) changed after knockdown of Sctr. Among these genes, Cdkn1b (p27) showed the most pronounced increase in expression in siSctr-transfected PC12 cells (+32%) (Fig. 2C and data not shown). To confirm this finding, we performed Western blot analysis and observed an increase in the amount of the p27 protein in PC12 cells with knockdown of Sctr (see Fig. 3C). In conclusion, a reduction in the expression of Sctr decreases the proliferation of pheochromocytoma cells and leads to an increase in Cdkn1b/p27 levels.
Fig. 3.
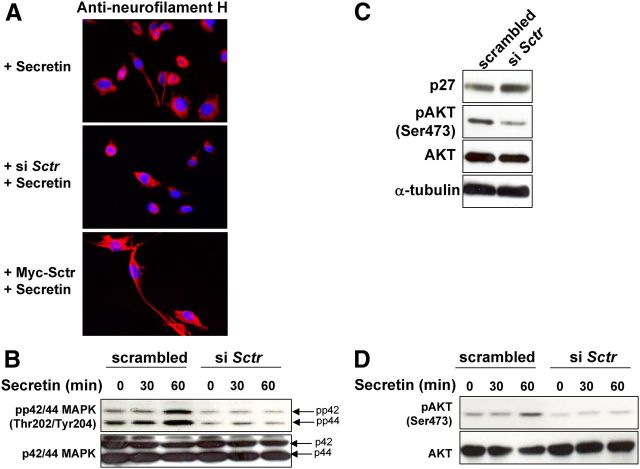
Role of SR in neurite outgrowth in PC12 cells and activation of downstream pathways. A, PC12 cells were plated on coverslips in 24-well plates, transfected with 250 ng of anti-Sctr siRNA oligos or Myc-Sctr plasmid or left untransfected. The next day, cells were incubated for additional 24 h with 100 nm secretin, fixed, and processed for immunofluorescent staining with antineurofilament high molecular weight protein (H) antibody. B and D, PC12 cells were transfected as in A and 24 h later incubated with 100 nm secretin. At the indicated time points, proteins were extracted, and Western blotting was performed to monitor P-p24/p44 MAPK (Thr202/Tyr204)/total-p42/p44 MAPK (B) and P-AKT (Ser473)/total-AKT (D) expression. C, PC12 cells were transfected as in A, and 24 h later, we assessed p27, P-AKT (Ser473), AKT, and α-tubulin expression levels by Western blotting. B–D, The ratios of the band intensities for phosphorylated proteins vs. total proteins, or for p27 vs. α-tubulin, normalized against the ratio in the untreated, scrambled-transfected control (ratio = 1), are reported below the panels.
The level of SR modulates the neurotrophic effects of secretin in PC12 cells
Secretin treatment induces neurite formation in PC12 cells (26). This phenomenon is mediated by the stimulation of SR, followed by the activation of the MAPK p42/p44 (also known as Erk2 and Erk1) (27). We decided to determine whether the amount of SR affects neuritogenesis in PC12 cells treated with secretin. To this aim, PC12 cells were transfected with anti-Sctr siRNA, exposed to secretin, and subsequently analyzed for neurite formation, by staining for neurofilament H (28), and for the expression of activated (phosphorylated) p42/44 MAPK. Knockdown of Sctr expression abrogates both secretin-mediated neurite outgrow (Fig. 3A) and activation of P-p42/44 MAPK in PC12 cells (Fig. 3B). These data demonstrate that reducing the amount of SR impairs the neurotrophic effect of secretin on PC12 cells.
SR inhibits cell viability of rodent adrenal cells through the PI3K/AKT pathway
As illustrated above, after siRNA-mediated knockdown of Sctr expression in PC12 cells, we observed an increase in Cdkn1b transcript and p27 protein (Fig. 3C). p27 lies downstream of several signaling cascades involved in cell proliferation, apoptosis, and migration, including the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway. Indeed, activation of AKT is associated to a decrease in p27 levels in a variety of normal and tumor cell types (29). To determine whether alterations in AKT signaling caused the increase of p27 in PC12 cells, we transfected them with anti-Sctr siRNA oligos and then assessed the amount of P-AKT. We observed that the level of P-AKT (Ser473), but not that of total-AKT, decreases in cells with reduced Sctr expression, concomitantly with an increase in p27 amount (Fig. 3C). This effect is also observed when siSctr-transfected PC12 cells are exposed to the secretin ligand to activate receptor signaling (Fig. 3D). Therefore, down-regulation of Sctr in PC12 cells decreases AKT activity in basal and secretin-stimulated conditions, and this in turn increases the amount of p27.
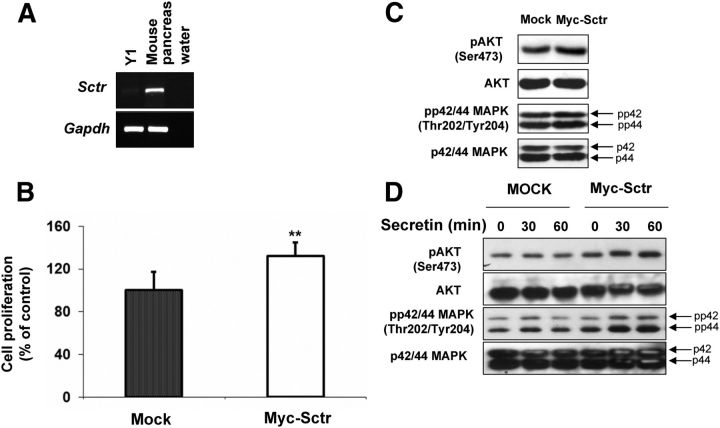
Altogether, the results that we reported so far support a proproliferative role of SR in PC12 adrenomedullary tumor cells mediated, in part, by activation of the PI3K/AKT pathway. To determine whether this receptor plays a similar role also in other endocrine cells, we employed the Y1 cell line derived from an adrenocortical carcinoma. These cells have low levels of endogenous Sctr, as demonstrated by RT-PCR (Fig. 4A), and have not been reported to respond to secretin treatment. Thus, we ectopically introduced the SR in Y1 cells by transfecting a plasmid expressing this molecule as Myc-tagged fusion protein. Control cells were transfected with the mock vector. Immunofluorescent staining using an anti-Myc antibody confirmed the expression of the exogenous SR (Supplemental Fig. 4). Overexpression of SR increased the proliferation of Y1 cells compared with mock-transfected cells (+30%) (Fig. 4B). Concordantly, high SR amount augmented the levels of P-p42/44 MAPK and P-AKT (Fig. 4C). The increase in the phosphorylated form of these kinases is less pronounced in Y1 cells than in PC12 cells, probably because the basal level of expression of P-AKT and P-MAPK is higher in the former than in the latter cell line (data not shown). We observed an increase in proliferation also of Sctr-transfected Y1 cells stimulated with secretin (Fig. 4D). In conclusion, the modulation of the levels of Sctr (up- or down-regulation) in two rodent cell lines derived from adrenal gland tumors supports a proproliferative role for this molecule in basal and secretin-stimulated conditions. To assess whether the effect of SR on cell proliferation in the absence of externally provided secretin could be due to an autocrine mechanism of stimulation, we assessed the expression of the secretin transcript in PC12 and Y1 cells and also in ex vivo primary rat pheochromocytoma cells. As shown in Supplemental Fig. 5, no endogenous secretin mRNA was detected in Y1 and rat primary pheochromocytoma cells, whereas PC12 cells show expression of Sctr, albeit at reduced level compared with duodonenum control tissue.
Fig. 4.
Overexpression of Sctr promotes cell growth and activates downstream pathways in Y1 cells. A, Semiquantitative RT-PCR was performed using primer specific to the mouse Sctr. The level of mRNA was normalized against Gadph. B, Y1 cells were incubated with 1 μg of mock vector or Myc-Sctr plasmid. Cell proliferation was assessed 24 h later by measuring ATP levels. C, In parallel to B, we monitored the P-AKT, total-AKT, P-p42/p44 MAPK(Thr202/Tyr204) and total-p42/p44 MAPK, and α-tubulin expression levels by Western blotting. D, Y1 cells were transfected as in B and 24 h later incubated with 100 nm secretin. At indicated time points, proteins were extracted, and Western blotting was performed to monitor P-p24/p44 MAPK (Thr202/Tyr204)/total-p42/p44 MAPK and P-AKT (Ser473)/total-AKT expression. C and D, The ratios of the band intensities for P-AKT vs. total-AKT, normalized against the ratio in the mock-transfected control (ratio = 1), are reported below the panels. **, P < 0.01.
SR expression sensitizes endocrine tumor cells to inhibitors of the PI3K/AKT pathway
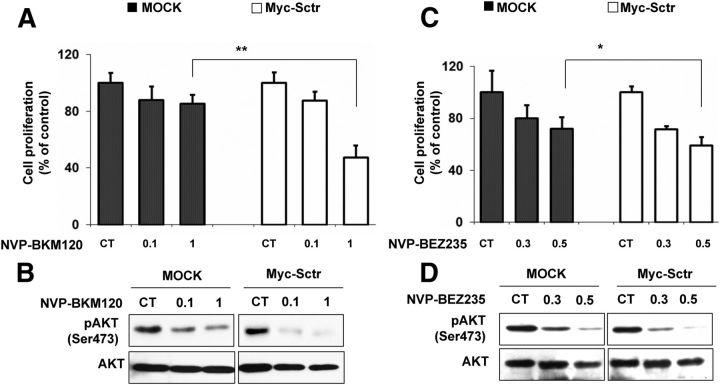
To obtain additional evidence supporting a role for the PI3K/AKT pathway in mediating SR-dependent effects on cell proliferation, we employed two inhibitors of this signaling cascade and checked their effect on cell growth in the presence of varying amounts of the receptor. Specifically, we used NVP-BKM120, a PI3K inhibitor, and NVP-BEZ235, a dual PI3K/mTOR inhibitor. Y1 cells transfected with Myc-Sctr vector (high SR level) or with the mock vector (low SR level) were treated with different concentrations of NVP-BKM120 or of NVP-BEZ235, and cell proliferation was assessed. After drug treatment, Y1 cells overexpressing the SR showed a stronger reduction in cell proliferation when compared with mock-transfected cells (Fig. 5, A and C). Specifically, we observed a reduction in cell viability of −60% in Myc-Sctr-transfected cells treated with 1 μm NVP-BKM120, and of −42% in transfected cells treated with 0.5 μm NVP-BEZ235, whereas untransfected cells had a much lower reduction at the same drug concentrations, especially upon NVP-BKM120 treatment. In agreement with the cell proliferation results, immunoblotting revealed a lower amount of activated P-AKT and P-MAPK in drug-treated, SR-transfected Y1 cells compared with drug-treated, mock-transfected cells (Fig. 5, B and D). Altogether, these data suggest that the response of Y1 cells to inhibitors of the PI3K pathway is, at least in part, dependent on the amount of SR.
Fig. 5.
Effect of high SR expression on the response of Y1 cells to PI3K inhibitors. Y1 cells were transfected with mock vector or Myc-Sctr plasmid and 24 h later treated with the NVP-BKM120 (A), NVP-BEZ235 (C), or DMSO. Cell proliferation was assessed 24 h later by measuring ATP levels. *, P < 0.05; **, P < 0.01 (Myc vs. SR). B and D, In parallel samples with A and C, we assessed the p-AKT (Ser473) and total-AKT expression levels by Western blotting. The ratios of the band intensities for P-AKT vs. total-AKT, normalized against the ratio in the untreated control (CT) (ratio = 1), are reported below the panels.
Based on these results, we formulated the hypothesis that tumor cells expressing high SR levels should respond well to PI3K inhibitors. As mentioned earlier, ex vivo rat primary pheochromocytoma cultures express high levels of Sctr (Supplemental Fig. 2B). Thus, we checked the response of these cells to NVP-BEZ235. We incubated eight independent ex vivo rat primary pheochromocytoma cultures with this drug and then assessed cell survival. NVP-BEZ235 treatment significantly reduced cell viability (−22%) already at the concentration of 10 nm (Supplemental Fig. 6A). Also, PC12 cells express SR at high levels. When incubated with NVP-BEZ235, PC12 cells showed a significant decrease in cell proliferation (−50%) when compared with untreated cells (Supplemental Fig. 6B). Addition of secretin to the culture media 1 h before drug treatment lead to an even greater inhibition of cell proliferation, suggesting that SR activation further sensitizes PC12 cells to the effects of the drug.
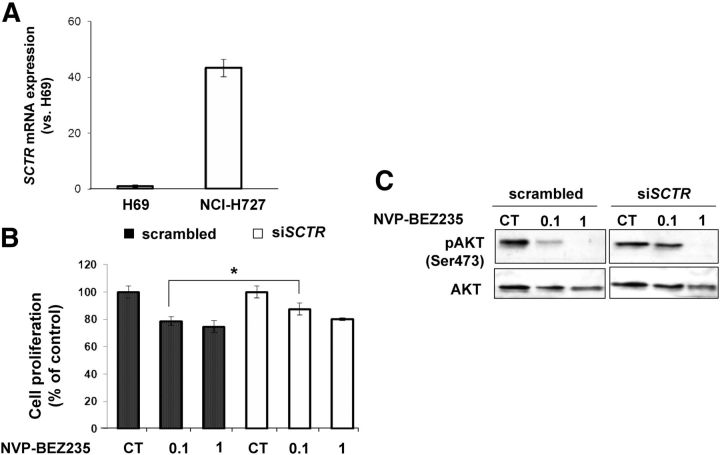
SR autoradiography has shown that pulmonary carcinoid tumor cells have a very high SR density on their membranes (13). Thus, we hypothesized that cells derived from this tumor entity might respond well, and possibly in a SR-dependent manner, to the growth inhibitory effects of PI3K inhibitors. We employed the NCI-H727 cell line derived from a human pulmonary carcinoid tumor as experimental model to test our hypothesis. We first determined the expression level of SCTR in NCI-H727 cells by TaqMan RT-PCR and compared it with that of the human immortalized cholangiocyte cells line H-69, previously reported to have high endogenous levels of the SCTR transcript (11). The results show that NCI-H727 cells express higher levels of SCTR (+40-fold) when compared with H-69 cells (Fig. 6A). Similarly to what we observed in PC12 cells, treatment of NCI-H727 cells with NVP-BEZ235 significantly reduced their proliferation (−30%) when compared with untreated cells, and the presence of secretin to stimulate receptor signaling SR lead to a further decrease in proliferation (Supplemental Fig. 6C).
Fig. 6.
Effect of SCTR down-regulation of on the response of NCI-H727 cells to PI3K inhibitors. A, Quantitative RT-PCR (TaqMan assay) for SCTR on H69 and NCI-H727 cells was performed. B, NCI-H727 cells were transfected with 250 ng of scrambled or anti-SCTR siRNA oligos and 24 h later treated with NVP-BEZ235 or DMSO. Cell proliferation was assessed 24 h later by measuring ATP levels. C, In parallel, we monitored the p-AKT (Ser473) and total-AKT expression levels by Western blotting. The ratios of the band intensities for P-AKT vs. total-AKT, normalized against the ratio in the untreated control (CT) (ratio = 1), are reported below the panels. *, P < 0.05.
NCI-H727 cells were then transfected with anti-SCTR siRNA oligos, or with scrambled oligos, and then treated with NVP-BEZ235 (concentrations 0.1 and 1 μm). Cell proliferation was assessed 24 h later. At the dose of 0.1 μm, we observed a significant reduction (−22%) in the proliferation of NCI-H727 cells transfected with scrambled siRNA oligos (Fig. 6B). Concomitantly, we observed a strong reduction of P-AKT already at the 0.1 μm concentration in scrambled siRNA-transfected cells. In contrast, after siRNA-mediated SCTR gene knockdown, the response of NCI-H727 cells to NVP-BEZ235 is reduced when compared with cells transfected with scrambled-siRNA both at the cellular (cell proliferation) and molecular (amount of P-AKT) level (Fig. 6, B and C). Indeed, siSCTR-transfected carcinoid cells showed less prominent decrease in cell proliferation compared with scrambled-transfected cells and displayed appreciable reduction of P-AKT only after exposure to the highest drug concentration (1 μm), whereas no change was observed at the 0.1 μm concentration (Fig. 6C). Thus, high SR levels sensitize human bronchopulmonary carcinoid cells to PI3K inhibition.
SR gene and protein in human pheochromocytoma
After our discovery that MENX-associated adrenomedullary lesions express Sctr at high levels and possess high amount of functional SR molecules on their membranes, we checked whether human pheochromocytomas are also characterized by an up-regulation of the SCTR gene. We performed real-time quantitative RT-PCR for SCTR on a panel of 30 sporadic and 13 familial human pheochromocytoma samples that we previously described (16). These samples include adrenal and extraadrenal pheochromocytomas (16). The results show that SCTR is overexpressed in only two out of 43 adrenal tumors (Table 2). The two tumors having higher SCTR expression do not possess distinctive clinical parameters. Both were noradrenergic (in total seven out of 43 were noradrenergic). The infrequent involvement of SCTR in human pheochromocytoma was also confirmed by in vitro receptor binding using radioactive 125I-[Tyr10] secretin as ligand: none of the 13 human pheochromocytoma samples that we analyzed showed detectable expression of the receptor (Supplemental Fig. 7).
Table 2.
Clinical characteristics of the two human pheochromocytoma patients having high levels of SCTR
| Sample ID | Sex | Age | Tumor size (cm) | Tumor biology | SCTR | Secreted hormone |
|---|---|---|---|---|---|---|
| PGL 295 | M | 74 | 3 | Benign | 6.97 | Noradrenalin |
| FI 326 | F | 37 | 5 | Benign | 12.01 | Noradrenalin |
SCTR values represent relative expression values normalized against normal adrenal medulla. M, Male; F, female.
Discussion
Our study shows that SR are overexpressed in various endocrine tumor cells and play a proproliferative role in pheochromocytoma and adrenocortical carcinoma cells. Moreover, we also demonstrate that SR signal, in part, through the PI3K/AKT pathway and their high expression, can sensitize cells to inhibitors of this signaling cascade.
After our previous observation of a very high expression of the Sctr gene in MENX-associated pheochromocytoma and paraganglioma (16), we here demonstrate that SR is highly expressed in these tumors also at the protein level. Importantly, in vitro receptor autoradiography shows that the SR is not only present on chromaffin tumor cells of MENX-affected rats, but it is indeed functional, being able to bind its natural ligand. We observed that the adrenomedullary cells of 2-month-old MENX mutant rats already possess a much higher density of SR on their membranes when compared with age-matched wild-type rats, in agreement with previous mRNA-based data on Sctr expression. SR density increases with time in affected rats, mirroring tumor progression. These observations support the hypothesis of a possible involvement of SR overexpression in rat pheochromocytoma tumorigenesis. We further addressed this issue by using PC12 pheochromocytoma cells as experimental model. Indeed, we previously reported that PC12 cells, similarly to MENX-associated pheochromocytoma (16), express Sctr at high level. To determine whether SR indeed plays a role in pheochromocytoma development, we evaluated the effect of modulating Sctr expression levels on PC12 cell proliferation. We observed that siRNA-mediated knockdown of Sctr decreased PC12 cell proliferation (−40%), supporting a proproliferative role for SR in this model. Importantly, this proproliferative effect occurred in basal conditions, in the absence of addition of the secretin ligand to the culture medium. Because PC12 cells are the only available pheochromocytoma cell line, we extended our analysis to another adrenal tumor-derived cell line, Y1 cells, derived from a murine adrenocortical carcinoma. We observed that also in these cells high expression of Sctr promotes proliferation.
Our results are in agreement with recent data showing that SR is important for regulating the basal proliferation of large cholangiocytes in mice. In the study by Onori et al. (11), Sctr expression was stably silenced by RNA interference in immortalized large mouse cholangiocyte cells. After Sctr knockdown, the authors observed a reduction in the proliferative capacity of the siRNA-transfected cells compared with mock-transfected cells. The authors interpreted their findings as being the results of a possible secretin-mediated autocrine regulation of basal proliferation of large murine cholangiocytes. We have not detected secretin mRNA expression in Y1 cells nor in MENX-associated pheochromocytoma cells, so an autocrine stimulatory loop is not likely to occur in these cells. In contrast, PC12 cells do express secretin transcript at low level, so the possibility of an autocrine receptor stimulation cannot be completely ruled out. However, the effect of SR on tumor cell proliferation is not dependent on secretin stimulation, because it is takes place in Y1 cells that do not produce the hormone.
While analyzing the Sctr transcript in MENX-associated pheochromocytoma tissues and primary tumor cells, we observed that they express only the full-length transcript of the gene. In human tissues (normal and neoplastic), splice variants of the SCTR gene have been identified and, in some cases, functionally characterized. For example, the splice variant with deletion of exon 3, observed in gastrinomas and PDAC, is nonfunctional and may also silence the wild-type receptor (9, 23). The finding that MENX rat pheochromocytoma cells do not express splice variants of Sctr is in agreement with the in vitro autoradiography data showing high levels of the functional SR on the membrane of these cells.
Data obtained several years ago concerning the response of PC12 cells to several peptide hormones can now be reinterpreted in light of the very high expression of Sctr that we found in these cells. In 1989, Roskoski et al. (30) reported that PC12 cells respond to neuropeptides members of the secretin family (namely, secretin, VIP, peptide histidine isoleucine, and glucagon) by increasing the activity of the enzyme tyrosine hydroxylase. Secretin showed a greater potency in activating tyrosine hydroxylase and in increasing cAMP levels when compared with the other tested peptides. The authors postulated the existence of secretin-preferring receptors in PC12 cells. These results could also be the consequence of the very high amount of Sctr expression in PC12 cells.
PC12 cells are particularly sensitive to treatment with growth factors, especially nerve growth factor, which can promote their differentiation into neuron-like cells able to form neurites (31). Secretin is among the factors able to induce neurite outgrowth in PC12 cells, thanks to the presence of SR (26). Down-regulation of Sctr attenuates secretin-dependent neurite formation, indicating that the receptor is essential to relay the differentiation signals triggered by the hormone. Although this might be intuitive, to our knowledge, it had not been demonstrated before.
At the molecular level, incubation of PC12 cells with secretin has been found to stimulate the classical signal transduction pathway: cAMP→PKA→ERK1/2 (ERK1/2 are also named p42/p44 MAPK). Secretin-dependent stimulation of this signaling cascade in PC12 cells has been shown to mediate the transcriptional activation of the endogenous tyrosine hydroxylase (Th) gene (32), the transcription of the chromogranin A (Cga) gene, the major vesicular core protein (33), and neurite formation (26). Consistent with these findings, we found that silencing of the Sctr gene in PC12 cells causes inhibition of p42/p44 MAPK (ERK1/2) phosphorylation. The observation that p27 levels increase in PC12 cells after knockdown of Sctr prompted us to extend our analysis to the PI3K/AKT pathway in these cells. Indeed, p27 is a direct target of the AKT kinase and, in many cell types, activation of the PI3K pathway results in down-regulation of p27 and increased proliferation (29, 34). We observed that knockdown of Sctr inhibits the phosphorylation of AKT in pheochromocytoma cells, suggesting that the receptor can also signal through this signaling cascade.
Encouraged by these initial findings, we decided to verify whether SR signal through the PI3K/AKT also in the adrenal-derived Y1 cell line. In contrast to the PC12 cells, Y1 cells express low endogenous levels of Sctr and no detectable secretin transcript. Thus, we transfected and ectopically overexpressed SR into these cells. High amount of the receptor enhances the phosphorylation of both AKT and p42/p44 MAPK and consequently stimulates Y1 cell proliferation, as mentioned above. These results further support the existence of a relationship between SR and PI3K/AKT signaling. This discovery is not entirely surprising, because other peptide receptors of the SR family signal through cAMP/PKA/ERK1–2 but also through PI3K/AKT, including VIP (35), glucagon-like peptide 1 (36, 37), and pituitary adenylate cyclase-activating polypeptide (38). However, the SR had not been previously associated to PI3K/AKT signaling.
The PI3K/AKT/mTOR pathway sustains proliferation and survival and is hyperactivated in a great variety of human tumor cells. For this reason, drugs that inhibit this signaling cascade at various levels have been generated, and some of them are being evaluated in clinical trials for the therapy of various solid tumors (39, 40). To better estimate the contribution of PI3K/AKT signaling in mediating the proproliferative effect of the SR, we modulated the amount of the receptor in Y1 cells in the presence or absence of NVP-BKM120, a PI3K inhibitor, or NVP-BEZ235, a dual PI3K/mTOR inhibitor. Y1 cells overexpressing ectopic SR and incubated with these drugs showed a more pronounced reduction in cell proliferation and a stronger inhibition of AKT phosphorylation when compared with untransfected cells. These results provided indirect evidence that SR may stimulate cell proliferation in part by activating PI3K/AKT signaling. In agreement with these data, we could also show that exposure of PC12 cells or of ex vivo cultures of primary pheochromocytoma cells from MENX mutant rats to NVP-BEZ235 significantly reduced their viability, demonstrating that cells possessing high levels of SR respond well to the antiproliferative effects of PI3K inhibitors. It should be noted that although MENX-affected rats have low levels of p27 protein in their cells, upstream signaling through the PI3K/AKT pathway is not affected (21). Incubation of PC12 cells with secretin enhanced the antiproliferative effect of NVP-BEZ235, thereby suggesting that high SR levels or its stimulation by the ligand sensitizes these cells to PI3K inhibition. In line with our results showing that tumor cells expressing Sctr at high levels respond well to compounds blocking the PI3K/AKT signaling cascade are studies showing a substantial decrease in PC12 cell proliferation upon treatment with LY294002, a PI3K inhibitor (41). Our observation that the SR can stimulate the proliferation of specific tumor cells and does so, at least in part, by activating the PI3K/AKT pathway opens novel possibilities for the treatment of tumors expressing this receptor at high level.
In human tumors, SR does not seem to be involved in pheochromocytoma development, because these tumors (both sporadic and familial) rarely show overexpression of the SCTR gene (2/43, 4.6%) or of the receptor protein, as demonstrated by in vitro autoradiography. Conversely, SR may play a role in human bronchopulmonary carcinoid tumors. Indeed, it has been shown that the majority of these carcinoid tumor cells possess high SR density on their membrane by in vitro autoradiography (13). Patients suffering from bronchopulmonary carcinoid tumors may present with multiple lesions and also with distant metastases leading to an unfavorable outcome. These tumors poorly respond to conventional chemotherapy, therefore surgical resection is the only therapeutic option. However, surgery can be problematic in cases with multiple carcinoids or metastatic spread. As such, there is a need for new treatment modalities. Based on our results showing that high SR levels sensitize to the antiproliferative effects of PI3K inhibitors, we predicted that lung carcinoid tumor cells would respond well to such compounds and employed the human bronchopulmonary carcinoid tumor cell line NCI-H727 to verify our hypothesis. Indeed, these cells express high levels of endogenous SCTR. Exposure of NCI-H727 cells to NVP-BEZ235 strongly inhibits their proliferation. After knockdown of SCTR gene expression, these cells become less sensitive to the antitumor potential of the drug, whereas upon stimulation of the receptor by secretin, their sensitivity to NVP-BEZ235 increases. Molecular analysis of downstream effector proteins confirm our hypothesis that SR stimulates cell proliferation in part by activating PI3K/AKT signaling also in human tumor cells.
Our data raise the intriguing possibility that the level of SR may represent a predictor of response to compounds targeting the PI3K pathway. Further studies, analyzing additional tumor entities expressing the receptor at high level are required to validate our hypothesis. In any case, the association between SR expression and response to PI3K inhibition provides the rationale for a novel therapeutic approach for tumors expressing the receptor, including, in addition to lung carcinoids, also PDAC and cholangiocarcinomas. Noteworthy, a recent preclinical study reported that NVP-BEZ235 displays antitumor activity against experimental PDAC in vitro and in vivo (42). Concerning cholangiocarcinomas, in vitro data have demonstrated that combination treatment of the chemotherapeutic agent oxalipatin with LY294002 (PI3K inhibitor) arrests cell proliferation (43), but the effect of PI3K/AKT inhibition alone has not yet been assessed in these tumor cells. It will be interesting to evaluate whether the good response to NVP-BEZ235 in these tumor entities is also in part dependent on the expression of the SR.
Given the newly discovered proproliferative role of SR in pheochromocytoma, the MENX syndrome, having high amount of functional receptor molecules on the membranes of adrenal and extraadrenal chromaffin tumor cells, represents a useful model for further investigating the functions of the receptor in tumorigenesis and for testing agents targeting this receptor in vivo.
Acknowledgments
We thank Elenore Samson and David Mörzl for excellent technical assistance and Novartis Oncology for providing us with the drugs.
This work was supported by the Deutsche Forschungsgemeinschaft Grant SFB824-A04 and the Deutsche Krebshilfe Grant 107973 (to N.S.P.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AKT
- Serine-threonine protein kinase
- DMSO
- dimethylsulfoxide
- fw
- forward
- MENX
- multiple endocrine neoplasia-like
- mTOR
- mammalian target of rapamycin
- P-Akt
- phosphorylated-Akt
- PDAC
- pancreatic ductal adenocarcinoma
- PI3K
- phosphatidylinositol 3 kinase
- PKA
- protein kinase A
- rev
- reverse
- siRNA
- small interfering RNA
- SR
- secretin receptor
- VIP
- vasoactive intestinal polypeptide.
References
- 1. Segre GV , Goldring SR. 1993. Receptors for secretin, calcitonin, parathyroid hormone (PTH)/PTH-related peptide, vasoactive intestinal peptide, glucagonlike peptide 1, growth hormone-releasing hormone, and glucagon belong to a newly discovered G-protein-linked receptor family. Trends Endocrinol Metab 4:309–314 [DOI] [PubMed] [Google Scholar]
- 2. Chey WY , Chang TM. 2003. Secretin, 100 years later. J Gastroenterol 38:1025–1035 [DOI] [PubMed] [Google Scholar]
- 3. Chu JY , Lee LT , Lai CH , Vaudry H , Chan YS , Yung WH , Chow BK. 2009. Secretin as a neurohypophysial factor regulating body water homeostasis. Proc Natl Acad Sci USA 106:15961–15966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis RJ , Page KJ , Dos Santos Cruz GJ , Harmer DW , Munday PW , Williams SJ , Picot J , Evans TJ , Sheldrick RL , Coleman RA , Clark KL. 2004. Expression and functions of the duodenal peptide secretin and its receptor in human lung. Am J Respir Cell Mol Biol 31:302–308 [DOI] [PubMed] [Google Scholar]
- 5. Kato A , Gores GJ , LaRusso NF. 1992. Secretin stimulates exocytosis in isolated bile duct epithelial cells by a cyclic AMP-mediated mechanism. J Biol Chem 267:15523–15529 [PubMed] [Google Scholar]
- 6. Lenzen R , Alpini G , Tavoloni N. 1992. Secretin stimulates bile ductular secretory activity through the cAMP system. Am J Physiol 263:G527–G532 [DOI] [PubMed] [Google Scholar]
- 7. Alpini G , Glaser SS , Ueno Y , Pham L , Podila PV , Caligiuri A , LeSage G , LaRusso NF. 1998. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol 274:G767–G775 [DOI] [PubMed] [Google Scholar]
- 8. Das R , Esposito V , Abu-Abed M , Anand GS , Taylor SS , Melacini G. 2007. cAMP activation of PKA defines an ancient signaling mechanism. Proc Natl Acad Sci USA 104:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Körner M , Hayes GM , Rehmann R , Zimmermann A , Friess H , Miller LJ , Reubi JC. 2005. Secretin receptors in normal and diseased human pancreas: marked reduction of receptor binding in ductal neoplasia. Am J Pathol 167:959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Körner M , Hayes GM , Rehmann R , Zimmermann A , Scholz A , Wiedenmann B , Miller LJ , Reubi JC. 2006. Secretin receptors in the human liver: expression in biliary tract and cholangiocarcinoma, but not in hepatocytes or hepatocellular carcinoma. J Hepatol 45:825–835 [DOI] [PubMed] [Google Scholar]
- 11. Onori P , Wise C , Gaudio E , Franchitto A , Francis H , Carpino G , Lee V , Lam I , Miller T , Dostal DE , Glaser SS. 2010. Secretin inhibits cholangiocarcinoma growth via dysregulation of the cAMP-dependent signaling mechanisms of secretin receptor. Int J Cancer 127:43–54 [DOI] [PubMed] [Google Scholar]
- 12. Ding WQ , Kuntz S , Böhmig M , Wiedenmann B , Miller LJ. 2002. Dominant negative action of an abnormal secretin receptor arising from mRNA missplicing in a gastrinoma. Gastroenterology 122:500–511 [DOI] [PubMed] [Google Scholar]
- 13. Körner MU , Hayes GM , Carrigan PE , Rehmann R , Miller LJ , Reubi JC. 2008. Wild-type and splice-variant secretin receptors in lung cancer: overexpression in carcinoid tumors and peritumoral lung tissue. Mod Pathol 21:387–395 [DOI] [PubMed] [Google Scholar]
- 14. Pellegata NS , Quintanilla-Martinez L , Siggelkow H , Samson E , Bink K , Höfler H , Fend F , Graw J , Atkinson MJ. 2006. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci USA 103:15558–15563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Molatore S , Pellegata NS. 2010. The MENX syndrome and p27: relationships with multiple endocrine neoplasia. Prog Brain Res 182:295–320 [DOI] [PubMed] [Google Scholar]
- 16. Molatore S , Liyanarachchi S , Irmler M , Perren A , Mannelli M , Ercolino T , Beuschlein F , Jarzab B , Wloch J , Ziaja J , Zoubaa S , Neff F , Beckers J , Höfler H , Atkinson MJ , Pellegata NS. 2010. Pheochromocytoma in rats with multiple endocrine neoplasia (MENX) shares gene expression patterns with human pheochromocytoma. Proc Natl Acad Sci USA 107:18493–18498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Virgolini I , Traub T , Novotny C , Leimer M , Fuger B , Li SR , Patri P , Pangerl T , Angelberger P , Raderer M , Burggasser G , Andreae F , Kurtaran A , Dudczak R. 2002. Experience with indium-111 and yttrium-90-labeled somatostatin analogs. Curr Pharm Design 8:1781–1807 [DOI] [PubMed] [Google Scholar]
- 18. Zitzmann K , de Toni E , von Rüden J , Brand S , Göke B , Laubender RP , Auernhammer CJ. 2011. The novel Raf inhibitor Raf265 decreases Bcl-2 levels and confers TRAIL-sensitivity to neuroendocrine tumour cells. Endocr-Relat Cancer 18:277–285 [DOI] [PubMed] [Google Scholar]
- 19. Grubman SA , Perrone RD , Lee DW , Murray SL , Rogers LC , Wolkoff LI , Mulberg AE , Cherington V , Jefferson DM. 1994. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol 266:G1060–G1070 [DOI] [PubMed] [Google Scholar]
- 20. Lichtenauer UD , Shapiro I , Geiger K , Quinkler M , Fassnacht M , Nitschke R , Rückauer KD , Beuschlein F. 2008. Side population does not define stem cell-like cancer cells in the adrenocortical carcinoma cell line NCI h295R. Endocrinology 149:1314–1322 [DOI] [PubMed] [Google Scholar]
- 21. Lee M , Theodoropoulou M , Graw J , Roncaroli F , Zatelli MC , Pellegata NS. 2011. Levels of p27 Sensitize to Dual PI3K/mTOR Inhibition. Mol Cancer Ther 10:1450–1459 [DOI] [PubMed] [Google Scholar]
- 22. Ulrich CD , Wood P , Hadac EM , Kopras E , Whitcomb DC , Miller LJ. 1998. Cellular distribution of secretin receptor expression in rat pancreas. Am J Physiol 275:G1437–G1444 [DOI] [PubMed] [Google Scholar]
- 23. Long SH , Berna MJ , Thill M , Pace A , Pradhan TK , Hoffmann KM , Serrano J , Jensen RT. 2007. Secretin-receptor and secretin-receptor-variant expression in gastrinomas: correlation with clinical and tumoral features and secretin and calcium provocative test results. J Clin Endocrinol Metab 92:4394–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ulrich CD , Holtmann M , Miller LJ. 1998. Secretin and vasoactive intestinal peptide receptors: members of a unique family of G protein-coupled receptors. Gastroenterology 114:382–397 [DOI] [PubMed] [Google Scholar]
- 25. Ip NY , Baldwin C , Zigmond RE. 1985. Regulation of the concentration of adenosine 3′,5′-cyclic monophosphate and the activity of tyrosine hydroxylase in the rat superior cervical ganglion by three neuropeptides of the secretin family. J Neurosci 5:1947–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim HS , Yumkham S , Kim SH , Yea K , Shin YC , Ryu SH , Suh PG. 2006. Secretin induces neurite outgrowth of PC12 through cAMP-mitogen-activated protein kinase pathway. Exp Mol Med 38:85–93 [DOI] [PubMed] [Google Scholar]
- 27. Fukuda M , Gotoh Y , Tachibana T , Dell K , Hattori S , Yoneda Y , Nishida E. 1995. Induction of neurite outgrowth by MAP kinase in PC12 cells. Oncogene 11:239–244 [PubMed] [Google Scholar]
- 28. Takeda S , Okabe S , Funakoshi T , Hirokawa N. 1994. Differential dynamics of neurofilament-H protein and neurofilament-L protein in neurons. J Cell Biol 127:173–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liang J , Zubovitz J , Petrocelli T , Kotchetkov R , Connor MK , Han K , Lee JH , Ciarallo S , Catzavelos C , Beniston R , Franssen E , Slingerland JM. 2002. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 8:1153–1160 [DOI] [PubMed] [Google Scholar]
- 30. Roskoski R , White L , Knowlton R , Roskoski LM. 1989. Regulation of tyrosine hydroxylase activity in rat PC12 cells by neuropeptides of the secretin family. Mol Pharmacol 36:925–931 [PubMed] [Google Scholar]
- 31. Greene LA , Tischler AS. 1976. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73:2424–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahata M , Zhang K , Gayen JR , Nandi S , Brar BK , Ghosh S , Mahapatra NR , Taupenot L , O'Connor DT , Mahata SK. 2011. Catecholamine biosynthesis and secretion: physiological and pharmacological effects of secretin. Cell Tissue Res 345:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahapatra NR , Mahata M , O'Connor DT , Mahata SK. 2003. Secretin activation of chromogranin A gene transcription. Identification of the signaling pathways in cis and in trans. J Biol Chem 278:19986–19994 [DOI] [PubMed] [Google Scholar]
- 34. Shin I , Yakes FM , Rojo F , Shin NY , Bakin AV , Baselga J , Arteaga CL. 2002. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med 8:1145–1152 [DOI] [PubMed] [Google Scholar]
- 35. Anderson P , Gonzalez-Rey E. 2010. Vasoactive intestinal peptide induces cell cycle arrest and regulatory functions in human T cells at multiple levels. Mol Cell Biol 30:2537–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buteau J , Roduit R , Susini S , Prentki M. 1999. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in β (INS-1)-cells. Diabetologia 42:856–864 [DOI] [PubMed] [Google Scholar]
- 37. Hui H , Nourparvar A , Zhao X , Perfetti R. 2003. Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5′-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology 144:1444–1455 [DOI] [PubMed] [Google Scholar]
- 38. May V , Lutz E , MacKenzie C , Schutz KC , Dozark K , Braas KM. 2010. Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase γ and vesicle endocytosis for neuronal survival. J Biol Chem 285:9749–9761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Engelman JA. 2009. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9:550–562 [DOI] [PubMed] [Google Scholar]
- 40. Guertin DA , Sabatini DM. 2007. Defining the role of mTOR in cancer. Cancer Cell 12:9–22 [DOI] [PubMed] [Google Scholar]
- 41. Adler JT , Hottinger DG , Kunnimalaiyaan M , Chen H. 2009. Inhibition of the PI3K pathway suppresses hormonal secretion and limits growth in pheochromocytoma cells. World J Surg 33:2452–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Awasthi N , Yen PL , Schwarz MA , Schwarz RE. 2012. The efficacy of a novel, dual PI3K/mTOR inhibitor NVP-BEZ235 to enhance chemotherapy and antiangiogenic response in pancreatic cancer. J Cell Biochem 113:784–791 [DOI] [PubMed] [Google Scholar]
- 43. Leelawat K , Narong S , Udomchaiprasertkul W , Leelawat S , Tungpradubkul S. 2009. Inhibition of PI3K increases oxaliplatin sensitivity in cholangiocarcinoma cells. Cancer Cell Int 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]