Abstract
The proglucagon gene is expressed not only in the pancreas and intestine but also in the hypothalamus. Proglucagon-derived peptides have emerged as potential regulators of energy homeostasis. Whether leptin, insulin, or cAMP activation controls proglucagon gene expression in the hypothalamus is not known. A key reason for this has been the inaccessibility of hypothalamic proglucagon-expressing neurons and the lack of suitable neuronal cell lines. Herein we describe the mechanisms involved in the direct regulation of the proglucagon gene by insulin, leptin, and cAMP in hypothalamic cell models. Insulin, through an Akt-dependent manner, significantly induced proglucagon mRNA expression by 70% in adult-derived mHypoA-2/10 neurons and significantly suppressed it by 45% in embryonic-derived mHypoE-39 neurons. Leptin, via the Janus kinase-2/ signal transducer and activator of transcription-3 pathway, caused an initial increase by 66 and 43% at 1 h followed by a decrease by 45 and 34% at 12 h in mHypoA-2/10 and mHypoE-39 cells, respectively. Furthermore, cAMP activation by forskolin up-regulated proglucagon expression by 87% in mHypoE-39 neurons and increased proglucagon mRNA, through Epac activation, in the mHypoE-20/2 neurons. Specific regions of the proglucagon promoter were regulated by cAMP signaling, as determined by transient transfections, whereas mRNA stability assays demonstrate that insulin and leptin increase proglucagon mRNA stability in the adult cells. These findings suggest that insulin, leptin, and cAMP act directly, but differentially, on specific hypothalamic neurons to regulate proglucagon gene expression. Because proglucagon-derived peptides are potential regulators of energy homeostasis, an understanding of hypothalamic proglucagon neurons is important to further expand our knowledge of alternative feeding circuits.
Among numerous appetite regulating neuropeptides, the proglucagon-derived peptides (PGDP), including glucagon, glucagon-like peptide (GLP)-1 and GLP-2, glicentin, and oxyntomodulin have emerged as potential regulators of feeding behavior (1). Proglucagon is encoded by a single proglucagon gene and is expressed in pancreatic α-cells, intestinal endocrine L cells, brain stem neurons, and the hypothalamus (2). PGDP are synthesized by posttranslational processing of precursor proglucagon in cell-specific manner by prohormone convertases (3). GLP-1, GLP-2, glicentin, and oxyntomodulin are synthesized in the intestinal endocrine L cells, which are located mainly in the distal ileum and colon, whereas glucagon is predominantly produced and secreted by pancreatic α-cells (3, 4). In the central nervous system, proglucagon is expressed mainly in the caudal brainstem and in selective hypothalamic neurons, and the processing of proglucagon in these neurons appears to mirror that of the intestine yielding GLP-1, GLP-2, glicentin, and oxyntomodulin as major products (2, 5, 6). Receptors for both GLP have been found in several areas of the brain that regulate appetite and energy homeostasis (4, 5).
The two key regulators of food intake and energy balance, insulin, and leptin are secreted in proportion to body fat mass. Both insulin and leptin cross the blood-brain barrier and interact with key neurons in the hypothalamus that express both insulin (7) and leptin receptors (8). Insulin is the main metabolic hormone that regulates glucose homeostasis and is secreted by pancreatic β-cells. Peripheral actions of insulin are anabolic as it increases energy storage, whereas central actions are catabolic as it reduces food intake and body weight (9). Neuron-specific insulin receptor knockout mice display an obese phenotype, indicating the importance of the central actions of insulin (10). Leptin is secreted by adipocytes and was cloned from the obese (ob) gene. Knockout mice with mutations in the ob gene or db gene (leptin receptor gene) are morbidly obese, and administration of leptin to ob/ob mice normalizes body weight and neuroendocrine status (11, 12), indicating the importance of leptin in energy homeostasis. It is well established that insulin and leptin receptors are located in the hypothalamus (7, 8, 13), and intracerebroventricular injection of insulin or leptin potently reduces food intake and body weight (14, 15). It is known that insulin and leptin regulate feeding and energy balance through interaction with complex neural circuits comprised of appetite-repressing anorexigenic and appetite-stimulating orexigenic neuropeptides. Similar to pathways activated by insulin and leptin, the activation of cAMP-dependent pathways also plays an important role in appetite regulation (16–18). It is well established that insulin, leptin, and cAMP act in the hypothalamus to regulate orexigenic neuropeptide Y (NPY)/agouti gene-related peptide and anorexigenic proopiomelanocortin/cocaine- and amphetamine-regulated transcript expression (16–22).
In contrast to the well-studied hypothalamic NPY/agouti gene-related peptide and proopiomelanocortin gene regulation, the regulation of hypothalamic proglucagon by insulin, leptin, or cAMP activators remains unknown. Few studies have examined changes in the local regulation of proglucagon and production of the GLP in the brain because proglucagon is expressed only in a small number of neurons in the brain and also because it is difficult to distinguish PGDP that have central or peripheral sources. Furthermore, due to the complexity of the in vivo architecture of the hypothalamus, these studies are very challenging to perform in intact brain/whole-animal models. Therefore, using embryonic- and adult-derived immortalized, clonal, hypothalamic cell models generated in our laboratory, we investigated how insulin, leptin and cAMP regulate hypothalamic proglucagon gene expression. We also studied the signal transduction and transcriptional mechanisms involved in this regulation.
Materials and Methods
Cell culture and reagents
Immortalized murine neuronal cells (mHypoA-2/10, mHypoE-39, and mHypoE-20/2) were grown in monolayer in DMEM (Sigma, Oakville, Canada), supplemented with 4.5 mg/ml glucose, 5% fetal bovine serum (Hyclone Laboratories, Logan, UT), and 1% penicillin/streptomycin (Gibco, Burlington, Canada), and maintained at 37 C in an atmosphere of 5% CO2 (23). For the mRNA expression study, cell culture medium was replaced with DMEM containing 0.5% fetal bovine serum and 1% penicillin/streptomycin for a minimum of 12 h before treatments. For the protein expression study, the cell culture medium was replaced with serum-free DMEM containing 1% penicillin/streptomycin for a minimum of 12 h before treatments. Insulin was a generous gift from Novo Nordisk Canada Inc. (Mississauga, Ontario, Canada). Recombinant murine leptin was obtained from Dr. A. F. Parlow (National Hormone and Peptide Program, Torrance, CA). Forskolin and 5,6-dichlorobenzimidazole 1-β-d-ribofuranoside (DRB), an inhibitor of transcription, were purchased from Sigma-Aldrich (Oakville, Canada). The protein kinase A (PKA) inhibitor H89 was the product of Calbiochem (EMD Biosciences, San Diego, CA). An exchange protein directly activated by cAMP (Epac) pathway-specific cAMP analog 8-(4-methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cAMP (8-pMeOPT-2′-O-Me-cAMP), defined in this study as ESCA, was provided by BioLog Life Science Institute (Bremen, Germany). Expression vectors pGL2 and pRL-CMV were purchased from Promega (Madison, WI). The G protein beta (Gβ) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against total Akt, phospho-Akt, total signal transducer and activator of transcription (STAT)-3, and phospho-STAT3 were obtained from Cell Signaling Technology Inc. (Danvers, MA). 3-Isobutyl-1-methylxanthine (IBMX), actinomycin D, wortmannin, LY 294002 hydrochloride, cucurbitacin I, and SD 1008 were obtained from Tocris Bioscience (Ellisville, MO).
One-step reverse RT-PCR
One-step RT-PCR was carried out to screen the cells for proglucagon, insulin receptor, and leptin receptor genes using a One-Step RT-PCR kit (QIAGEN, Mississauga, Ontario, Canada). The primer pairs used are as follows: insulin receptor forward, 5′-gtgataccagagcataggag-3′, insulin receptor reverse, 5′-ctgttcggaacctgatgac-3′; leptin receptor (Ob-Rb) forward, 5′-atgacgcagtgtactgctg-3′, leptin receptor (Ob-Rb) reverse, 5′-gtggcgagtcaagtgaacct-3′; NPY forward, 5′-taggtaacaagcgaatgggg-3′, NPY reverse, 5′-acatggaagggtcttcaagc-3′; γ-actin forward, 5′-gctccggcatgtgca-3′, γ-actin reverse, 5′-aggatcttcatgaggtagt-3′; and proglucagon forward, 5′-tgaagaccatttactttgtggct-3′, and proglucagon reverse, 5′-tggtggcaagattgtccagaat-3′ (24). Total RNA was isolated from the immortalized hypothalamic cells, 3T3, α-TC, and GluTag cells using the guanidinium thiocyanate phenol chloroform extraction method (25). One-step RT-PCR was performed using the One-Step RT-PCR kit (QIAGEN). Briefly, a total of 200 ng of RNA was used from all samples with 1× one step RT-PCR buffer, one-step enzyme mix, 0.4 mm deoxynucleotide triphosphates, 0.6 mm of sense primer, and 0.6 mm of antisense primer in a final volume of 25 μl. The reverse transcription protocol used for all genes was 50 C for 30 min, 95 C for 15 min, followed by amplification at 95 C for 15 sec, 60 C for 15 sec, and 72 C for 1 min for 40 cycles with final incubation for 7 min at 72 C. All PCR-amplified products were visualized on 1.2% agarose gel containing ethidium bromide under ultraviolet light. The PCR products were verified by purification and sequencing (The Centre for Applied Genomics, Toronto, Canada).
Quantitative RT-PCR (qRT-PCR)
The mHypoA-2/10, mHypoE-39, and mHypoE-20/2 cells were treated with vehicle insulin (10 nm), leptin (10 nm), forskolin/IBMX (10 μm), or ESCA (50 μm) and harvested at the indicated time points. For the inhibitor experiments, the cells were pretreated with either 25 μm LY294002, 1 μm wortmannin, 5 μm cucurbitacin I, 10 μm SD1008, 1 μm H89, or dimethylsulfoxide (DMSO) vehicle for 45 min to 1 h and then treated with 10 nm insulin or leptin or 10 μm forskolin/IBMX before RNA isolation at the indicated time points. Total RNA from the cells was isolated using the guanidinium thiocyanate-phenol-chloroform extraction method (25), and reverse transcription was performed with 2.0 μg of total RNA using SuperScript II and random primers as described in the Superscript II cDNA synthesis kit (Invitrogen, Toronto, Ontario, Canada). Real-time RT-PCR was performed with SYBR green PCR master mix according to the manufacturer's instructions (Applied Biosystems Inc., Streetsville, Ontario, Canada). Two hundred nanaograms of template were used in a total 10 μl reaction mixture containing 0.3× SYBR green dye, 1× carboxy-X-rhodamine, 1× buffer, 3 mm MgCl2, 0.2 mm deoxynucleotide triphosphate, and 0.5 U Platinum Taq (all from Invitrogen Life Technologies, Burlington, Ontario, Canada) and run on the Applied Biosystems Prism 7000 real-time PCR machine. The real-time RT-PCR conditions for all genes were as follows: 45 cycles for 15 sec at 95 C and 1 min at 65 C. The gene primer sequences were purchased from Integrated DNA Technologies (Coralville, IA) and are as follows: γ-actin-SYBR forward, 5′-cttccccacgccatcttg-3′, γ-actin-SYBR reverse, 5′-cccgttcagtcaggatcttcat-3′, and proglucagon-SYBR forward, 5′-gaggagaaccccagatcattcc-3′, proglucagon-SYBR reverse, 5′-gtggcgtttgtcttcattcatc-3′ (Primer Express software; Applied Biosystems). Data were represented as cycle threshold values, defined as the threshold cycle of PCR at which the amplified product was first detected and analyzed using an ABI Prism 7000 SDS software package (Applied Biosystems). Copy number of the amplified proglucagon mRNA was standardized to γ-actin using the standard curve method (ABI Prism 7000 users bulletin; Applied Biosystems).
SDS-PAGE and Western blot analysis
The mHypoA-2/10 and mHypoE-39 cells were grown to 80–90% confluency, serum starved overnight, and then treated with insulin (10 nm), leptin (10 nm), or vehicle. The cells were harvested at 5, 15, 30, and 60 min, as indicated, and total protein was isolated using a 1× lysis buffer supplemented with 1 mm phenylmethylsulfonyl fluoride as described previously (26). Protein concentration was determined using the bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL). Total protein (20 μg) was resolved on 8% SDS-PAGE gels and blotted onto Immun-Blot polyvinyl difluoride membrane (Bio-Rad Laboratories, Hercules, CA). The blots were blocked with 5% BSA in Tris-buffered saline with 0.1% Tween 20 for 1 h and then incubated overnight at 4 C with primary antibodies against phospho-Akt (Ser473, 1:1000), phospho-STAT3 (Tyr705, 1:1000), total Akt (1:1000), total STAT3 (1:1000), and Gβ (1:1000). After incubation with horseradish peroxidase-labeled secondary antirabbit IgG at a 1:5000 dilution for 1 h, enhanced chemiluminescence (ECL Advance kit; GE Healthcare, Princeton, NJ) was added and the immunoreactive bands were visualized using a Kodak IS2000 digital imaging system (Rochester, NY). For the inhibitor experiments, the cells were pretreated with 25 μm LY294002, 1 μm wortmannin, 5 μm cucurbitacin I, 10 μm SD1008, or DMSO for 1 h and then treated with 10 nm insulin or leptin for 15 min. For the analysis of the protein expression, phospho-Akt expression was normalized to total Akt, and phospo-STAT3 expression was normalized to total STAT3. Gβ was used as a loading control.
Reporter gene plasmids
Reporter constructs based on the pGL2 vector were constructed by transferring the inserts from the reporter gene plasmids based on the pGL3 vector containing 312 and 476 bases of the rat proglucagon promoter and human proglucagon promoter constructs containing 332 and 602 bases that have previously been described (27, 28). Human proglucagon promoter construct containing 829 bases of promoter was generated using a PCR based strategy as previously described (27), for which the following human proglucagon gene primers were used (the numbers in the names of primers identify the location of the 5′ end of each primer relative to the mRNA start site): −829 forward, 5′-GCGGGTACCGCCTGTGTGTCCAGTCACAAAAC-3′, and +58 reverse, 5′-GCAAGCTTAGAGCAAGCCCTCTTTGGGAAC-3′.
Transient transfections
The mouse hypothalamic mHypoE-39 and mhypoE-20/2 cell lines were grown in 24-well plates in DMEM without antibiotics and supplemented with 4.5 mg/ml glucose and 5% fetal bovine serum to 70–80% confluency. Before the transfection, the medium was changed to medium without serum (OptiMEM; Invitrogen Life Technologies). The luciferase reporter gene constructs were transfected into the cell lines using Lipofectamine 2000 (Invitrogen) as per the manufacturer's protocol, with 0.8 μg of DNA per well in a 24-well plate. The cells were incubated for 4–6 h with DNA-Lipofectamine 2000 complex and then washed and incubated in fresh media for 18 h before being treated with vehicle, insulin (10 nm), leptin (10 nm), or forskolin/IBMX (10 μm). Using three different passages of the cell lines, at least three separate transfections per plasmid were performed. For each transfection experiment, individual plasmids were transfected in three separate wells. The cells were harvested at 12–16 h after treatment, and a luciferase assay was performed to detect luciferase activity using a Firefly and Renilla luciferase assay kit (Biotum, Inc., Hayward, CA) and a Lumat LB 9501 luminometer (EG&G Berthold, Wellesley, MA). Protein concentrations were determined using the bicinchoninic acid protein assay kit (Thermo Scientific). Controls for transfections included the promoterless pGL2 basic vector (Promega) and the internal control reporter pRL-CMV vector (containing enhancer to express Renilla luciferase; Promega).
In silico analysis
MicroRNA (miRNA) binding sites on mouse proglucagon mRNA template were determined using a web tool MicroInspector (29). The mRNA-binding protein sites were searched by using a RNA-binding protein database (30).
Statistical analysis
Data are presented as the mean ± sem from at least three independent experiments. All luciferase assays were performed in triplicate. Data were analyzed using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA) and SigmaPlot (Systat Software Inc., Point Richmond, CA). Statistical analysis was performed using one-way or two-way ANOVA, and statistical significance was determined by post hoc analysis using Bonferroni test or Student's t test with P < 0.05.
Results
Characterization of the expression profile of the hypothalamic cell lines
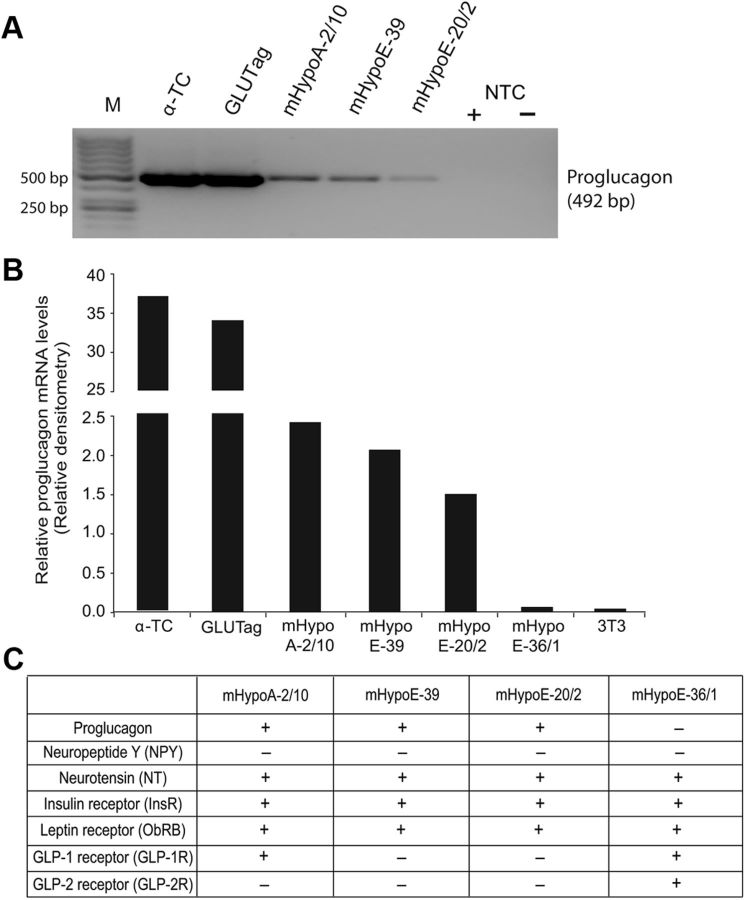
A series of hypothalamic cell lines has recently been developed, which display a variety of hypothalamic phenotypes, and a few of them were reported to express the proglucagon mRNA (23, 31). To confirm these reports, we conducted RT-PCR to show that mHypoA-2/10, mHypoE-39, and mHypoE-20/2 express both proglucagon mRNA (Fig. 1, A and B) and the insulin and leptin receptors (Fig. 1C). Another cell model mHypoE36/1 does not express the proglucagon gene. Proglucagon-positive pancreatic α-TC cells and intestinal GluTag cells served as positive controls, and fibroblasts 3T3 cells served as a negative control. We also analyzed the expression of other hypothalamic neuropeptides involved in appetite regulation and hormone receptors in these cell lines (Fig. 1C). All cell models express neurotensin, insulin receptor, and leptin receptor, whereas only mHypoE36/1 cell model expresses endogenous GLP-1 receptor and GLP-2 receptor. Currently there are no hypothalamic cell models reported with endogenous proglucagon and receptors for insulin or leptin; therefore, based on their unique neuropeptide and receptor profile, we selected mHypoA-2/10, mHypoE-39, and mHypoE-20/2 cell models to study insulin- and leptin-mediated regulation of proglucagon mRNA.
Fig. 1.
Characterization of the expression profile of the proglucagon-expressing hypothalamic cell lines. Expression of proglucagon mRNA transcripts in pancreatic α-TC cells; intestinal GLUTag cells; hypothalamic neuroendocrine cell lines mHypoA-2/10, mHypoE-39, mHypoE-20/2, and mhypoE-36/1; and fibroblast 3T3 cells. A, RT-PCR using specific primers for mouse proglucagon and γ-actin genes. Total RNA was isolated from the indicated cell lines and used as template for RT-PCR using the One-Step RT-PCR kit (QIAGEN). M, Markers; NTC, nontemplate control (± reverse transcriptase). B, Graphical representation of relative proglucagon mRNA transcripts levels quantified by densitometry. Proglucagon mRNA values were normalized to γ-actin levels. C, RT-PCR analysis results for the mRNA expression of neuropeptides and receptors in the indicated hypothalamic cells. + indicates that the gene is expressed, and − indicates that the gene is weakly expressed or not expressed.
Activation of signaling pathways by insulin and leptin in the hypothalamic neuronal cells
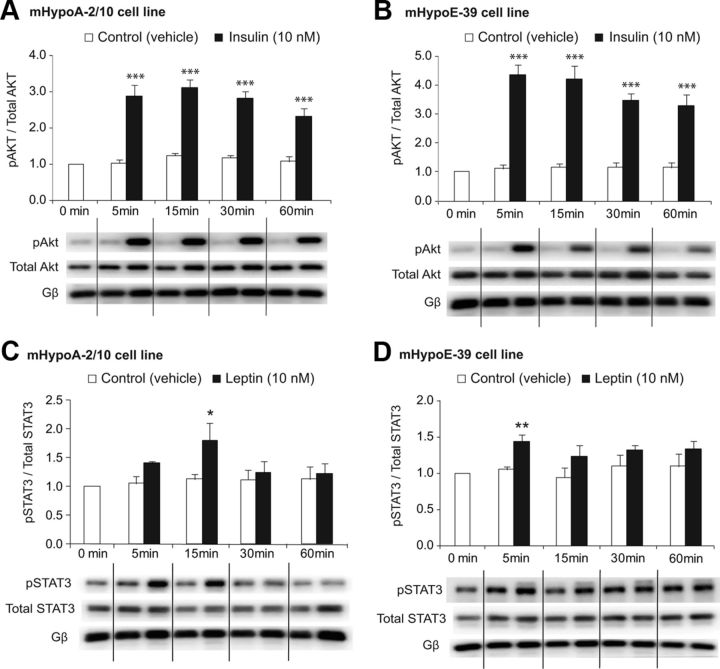
The key signaling pathway that insulin activates is the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. We next determined whether this signaling pathway is activated by insulin in our hypothalamic adult and embryonic cells. The neuronal cells were treated with 10 nm insulin, and activation of Akt was analyzed over 60 min. By Western blot analysis, we found that insulin induced phosphorylation of Akt at Ser473 in both adult mHypoA-2/10 and embryonic mHypoE-30 cell lines during the entire period of 60 min after treatment (Fig. 2). The maximum significant increase in Akt phosphorylation by 179% was observed at the 5-min time point in the mHypoA-2/10 cell model [pAkt/total Akt (5 min): vehicle (0.66 ± 0.05) vs. 10 nm insulin (1.83 ± 0.21), P < 0.001]. Similarly, in the mHypoE-39 cells, the maximum activation of Akt was by 390% at 5 min after treatment [pAkt/total Akt (5 min): vehicle (0.56 ± 0.06) vs. 10 nm insulin (2.74 ± 0.45), P < 0.001] (Fig. 2, A and B).
Fig. 2.
Insulin activates Akt and leptin activates STAT3 in the hypothalamic neuronal cells. The mHypoA-2/10 and mHypoE-39 neurons were serum starved overnight and then treated with insulin (10 nm), leptin (10 nm), or vehicle. Protein was isolated over 60 min at the indicated time points, resolved on 10% SDS-PAGE, transferred to polyvinyl difluoride membrane, and immunoblotted with antisera for phospho-Akt, total Akt, phosphorylated STAT3 (pSTAT3), total STAT3, and Gβ (G protein β-subunit). Insulin increased phosphorylation of Akt in the mHypoA-2/10 cells (A) and mHypoE-39 cell line (B). Leptin induced phosphorylation of STAT3 in both mHypoA-2/10 (C) and mHypoE-39 (D) cell lines. Phosphorylation of Akt and STAT3 was normalized to total Akt and total STAT3, respectively. Gβ was used as a loading control. Representative Western blots are shown. All results shown in the bar graphs are expressed as mean ± SEM (n = 4–6 independent experiments). *, P < 0.05, **, P < 0.01, ***, P < 0.001 vs. vehicle control. Statistical significance was calculated by two-way ANOVA.
Furthermore, leptin was found to significantly increase phosphorylation of STAT3 at Tyr705 by 60% at 15 min in the adult neuronal cells [phosphorylated STAT3/total STAT3 (15 min): vehicle (0.71 ± 0.06) vs. 10 nm leptin (1.13 ± 0. 19), P < 0.05] (Fig. 2C). Similarly, leptin significantly increased phosphorylation of STAT3 at 5 min in the embryonic cells by 42% [phosphorylated STAT3/total STAT3 (5 min): vehicle (0.72 ± 0.03) vs. 10 nm leptin (0.99 ± 0. 08), P < 0.01] (Fig. 2D). Interestingly, our findings indicate a temporal difference in the activation of the two pathways because Akt is strongly and continuously activated by insulin over 60 min, whereas STAT3 is only activated by leptin for 5–15 min.
Akt can be activated by its phosphorylation in a PI3K-dependent or -independent manner. Activated Akt can then activate or deactivate its downstream substrates via its kinase activity such as mammalian target of rapamycin or other signaling pathways to regulate effector genes. Activated STAT3 forms homo- or heterodimers that translocate to the cell nucleus, in which they act as transcription activators and bind to STAT elements within the promoter region of downstream target genes to regulate their expression. Overall, activation of Akt and STAT3 in our neuronal cells suggests that insulin and leptin could potentially regulate downstream target genes endogenously expressed in these neuronal cells.
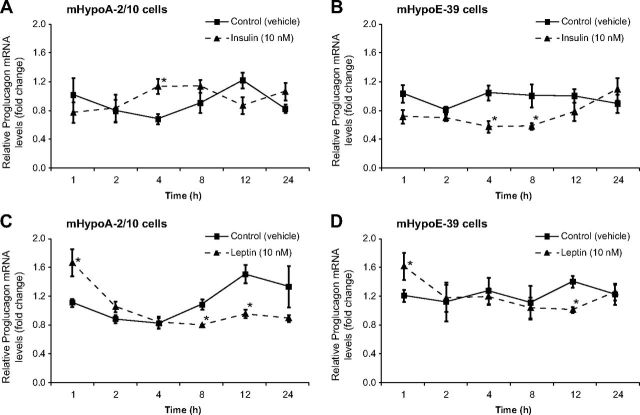
Regulation of proglucagon mRNA transcript expression by insulin and leptin
We determined whether insulin and leptin directly regulate proglucagon mRNA expression. The adult and embryonic hypothalamic neuronal cells were exposed to 10 nm insulin or leptin over a 24-h time course. Using real-time qRT-PCR, we found that in the adult mHypoA-2/10 neurons proglucagon mRNA levels were significantly up-regulated by 70% by insulin at 4 h after treatment [vehicle (0.66 ± 0.07) vs. 10 nm insulin (1.12 ± 0.11), P < 0.05] (Fig. 3A), whereas in the embryonic mHypoE-39 neurons, insulin significantly suppressed the mRNA expression by 45 and 43% at 4 and 8 h after treatment, respectively [4 h, vehicle (1.04 ± 0.10) vs. 10 nm insulin (0.57 ± 0.08), P < 0.05; 8 h, vehicle (1.00 ± 0.16) vs. 10 nm insulin (0.57 ± 0.05), P < 0.05] (Fig. 3B). With respect to the leptin action on the proglucagon mRNA expression in the adult mHypoA-2/30 cell line, we found that leptin caused a significant up-regulation of the mRNA levels by 66% at 1 h after treatment followed by a down-regulation by 45% at 12 h [1 h, vehicle (1.00 ± 0.07) vs. 10 nm leptin (1.66 ± 0.22), P < 0.05; 12 h, vehicle (1.47 ± 0.15) vs. 10 nm leptin (0.81 ± 0.07), P < 0.05] (Fig. 3C). Leptin induced a similar biphasic effect in the mHypoE-39 cell line by initially up-regulating proglucagon mRNA by 43% at 1 h, followed by its suppression by 34% at 12 h after treatment [1 h, vehicle (1.15 ± 0.10) vs. 10 nm leptin (1.64 ± 0.22), P < 0.05; 12 h, vehicle (1.38 ± 0.10) vs. 10 nm leptin (0.91 ± 0.05), P < 0.05] (Fig. 3D). These results clearly indicate that the proglucagon gene is regulated by insulin and leptin in the hypothalamic neuronal cell models.
Fig. 3.
Insulin and leptin regulate proglucagon mRNA expression in the hypothalamic neuronal cells. The mHypoA-2/10 (A and C) and mHypoE-39 (B and D) cells were exposed to either insulin (10 nm) (A and B), leptin (10 nm) (C and D), or vehicle over a 24-h time course; total RNA was extracted at the indicated time points and used as a template for real-time RT-PCR with primers specifically designed to amplify proglucagon mRNA. Proglucagon mRNA levels from both cell lines were quantified using the cycle threshold method and normalized to the internal control (γ-actin). All results shown are relative to corresponding control mRNA levels at each time point and are expressed as mean ± sem (n = 4–6 independent experiments). *, P < 0.05 vs. vehicle control). Statistical significance was calculated by two-way ANOVA.
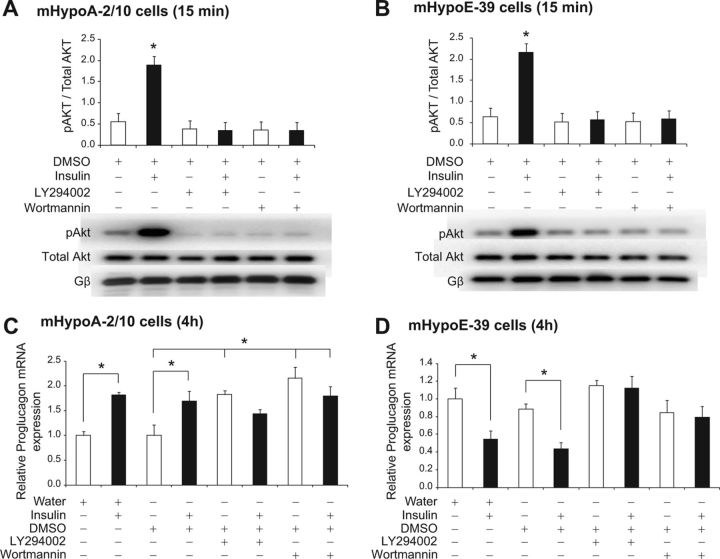
Reversal of insulin-mediated regulation of proglucagon mRNA expression by PI3K inhibitors
We then investigated the involvement of Akt, a PI3K-dependent protein kinase, in the regulation of proglucagon mRNA expression by insulin. We used pharmacological inhibitors of PI3K (LY294002 at 25 μm and wortmannin at 1 μm concentrations) (32). First, we determined the efficacy and specificity of each inhibitor, in which the adult mHypoA-2/10 and embryonic mHypoE-39 cells were pretreated for 1 h with either of the inhibitors before exposure to 10 nm insulin. Total protein was isolated at 15 min after the insulin treatment for analysis using phospho-specific antibody for Akt. DMSO was used as a vehicle control because the inhibitors were dissolved in DMSO. Using Western blot analysis, we found that wortmannin and LY294002 inhibited the phosphorylation of Akt by insulin in both cell models [mHypoA-2/10 cell line (15 min): DMSO (0.55 ± 0.13) vs. DMSO + 10 nm insulin (1.90 ± 0.31), P < 0.05; LY294002 (0.38 ± 0.18) vs. LY294002 + 10 nm insulin (0.34 ± 0.20), P > 0.05; wortmannin (0.36 ± 0.19) vs. wortmannin + 10 nm insulin (0.34 ± 0.20), P > 0.05; mHypoE-39 cell line (15 min): DMSO (0.64 ± 0.27) vs. DMSO + 10 nm insulin (2.16 ± 0.33), P < 0.05; LY294002 (0.37 ± 0.24) vs. LY294002 + 10 nm insulin (0.48 ± 0.23), P > 0.05; wortmannin (0.41 ± 0.26) vs. wortmannin + 10 nm insulin (0.53 ± 0.17), P > 0.05] (Fig. 4, A and B).
Fig. 4.
Regulation of proglucagon mRNA expression by insulin via activation of the PI3K/Akt pathway. mHypoA-2/10 (A and C) and mHypoE-39 (B and D) neuronal cells were pretreated with a PI3K inhibitor, either LY294002 (25 μm) or wortmannin (1 μm), for 1 h followed by either vehicle (water or DMSO) or insulin (10 nm) treatment. To determine the efficacy and specificity of the inhibitors, total protein was isolated at 15 min after insulin treatment and analyzed using Western blot analysis with phospho-specific antibodies against Akt (A and B). Phosphorylation of Akt was normalized to total Akt. Gβ was used as a loading control. Representative Western blots are shown. To investigate the involvement of the PI3K/Akt pathway in proglucagon mRNA regulation by insulin, cells were harvested for total RNA isolation at 4 h after insulin exposure, and proglucagon mRNA expression was determined by real-time RT-PCR (C and D). mRNA expression data are shown relative to water only-treated mRNA levels (set to 1.0). All results are expressed as mean ± sem (n = 4 independent experiments). *, P < 0.05. Statistical significance was calculated by two-way ANOVA. White bars, Control (water or DMSO with or without PI3K inhibitor); black bars, treatment (insulin with or without PI3K inhibitor).
We analyzed whether the PI3K/Akt pathway is involved in the regulation of proglucagon by insulin. The adult mHypoA-2/10 and embryonic mHypoE-39 cells were pretreated with the inhibitors for 1 h, followed by insulin treatment. Total RNA was isolated at 4 h after insulin treatment and analyzed using real-time qRT-PCR. As compared with the vehicle treatment, insulin treatment alone significantly induced an increase in the proglucagon mRNA levels in mHypoA-2/10 neuronal cells, whereas pretreatment with wortmannin or LY294002 attenuated insulin-induced increase in proglucagon mRNA expression [mHypoA-2/10 cell line (4 h): water (0.60 ± 0.13) vs. 10 nm insulin (1.10 ± 0.15), P < 0.05; DMSO (0.60 ± 0.04) vs. DMSO + 10 nm insulin (1.02 ± 0.12), P < 0.05; LY294002 (1.10 ± 0.16) vs. LY294002 + 10 nm insulin (0.87 ± 0.08), P > 0.05; wortmannin (1.30 ± 0.23) vs. wortmannin + 10 nm insulin (1.09 ± 0.22), P > 0.05] (Fig. 4C). In the mHypoE-39 cells, pretreatment with PI3K inhibitors attenuated insulin-mediated repression of proglucagon mRNA expression [mHypoE-39 cell line (4 h): water (1.15 ± 0.14) vs. 10 nm insulin (0.63 ± 0.11), P < 0.05; DMSO (1.02 ± 0.07) vs. DMSO + 10 nm insulin (0.50 ± 0.08), P < 0.05; LY294002 (1.33 ± 0.07) vs. LY294002 + 10 nm insulin (1.29 ± 0.16), P > 0.05; wortmannin (0.98 ± 0.16) vs. wortmannin + 10 nm insulin (0.91 ± 0.15), P > 0.05] (Fig. 4D). These findings indicated that the PI3K/Akt pathway is involved in the regulation of hypothalamic proglucagon mRNA expression by insulin. We also found that PI3K inhibitors increase basal proglucagon mRNA levels in the adult cells, suggesting that the PI3K pathway may regulate basal proglucagon mRNA turnover (Fig. 4C).
Reversal of leptin-mediated regulation of proglucagon mRNA expression by Janus kinase (JAK)-2/STAT3 inhibitors
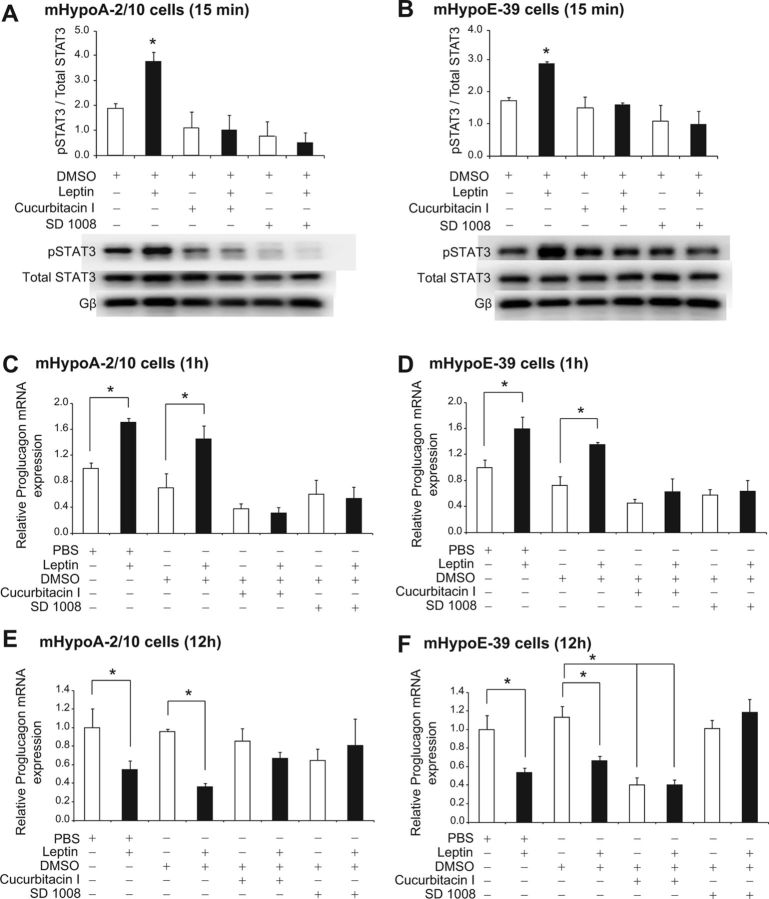
We further investigated whether activation of STAT3 by leptin is involved in the regulation of proglucagon mRNA expression. We used pharmacological inhibitors of JAK2/STAT3 (cucurbitacin I at 5 μm and SD1008 at 10 μm concentrations) (33, 34). To determine the efficacy and specificity of each inhibitor, we pretreated the adult mHypoA-2/10 and embryonic mHypoE-39 cells for 1 h with either of the inhibitors before treatment with 10 nm leptin. Total protein was isolated at 15 min after the leptin treatment and analyzed using a phospho-specific antibody for STAT3. Because the inhibitors were dissolved in DMSO, it was used as a vehicle control. We found that cucurbitacin I and SD1008 inhibited the phosphorylation of STAT3 by leptin in both cell models [mHypoA-2/10 cell line (15 min): DMSO (0.19 ± 0.02) vs. DMSO + 10 nm leptin (0.38 ± 0.04), P < 0.05; cucurbitacin I (0.14 ± 0.06) vs. cucurbitacin I + 10 nm leptin (0.13 ± 0.06), P > 0.05; SD1008 (0.11 ± 0.08) vs. SD1008 + 10 nm leptin (0.08 ± 0.05), P > 0.05; mHypoE-39 cell line (15 min): DMSO (0.17 ± 0.01) vs. DMSO + 10 nm leptin (0.29 ± 0.01), P < 0.05; cucurbitacin I (0.15 ± 0.03) vs. cucurbitacin I + 10 nm leptin (0.18 ± 0.01), P > 0.05; SD1008 (0.11 ± 0.05) vs. SD1008 + 10 nm leptin (0.10 ± 0.04), P > 0.05] (Fig. 5, A and B).
Fig. 5.
Regulation of proglucagon mRNA expression by leptin via activation of the JAK2/STAT3 pathway. mHypoA-2/10 (A, C, and E) and mHypoE-39 (B, D, and F) neuronal cells were pretreated with a JAK2/STAT3 inhibitor, either cucurbitacin I (5 μm) or SD1008 (10 μm), for 1 h followed by either vehicle (PBS or DMSO) or leptin (10 nm) treatment. To determine the efficacy and specificity of the inhibitors, total protein was isolated at 15 min after leptin treatment and analyzed using Western blot analysis with phospho-specific antibodies against STAT3 (A and B). Phosphorylation of STAT3 was normalized to total STAT3. Gβ was used as a loading control. Representative Western blots are shown. To investigate the involvement of the JAK2/STAT3 pathway in proglucagon mRNA regulation by leptin, cells were harvested for total RNA isolation at 1 h (C and D) and 12 h (E and F) after leptin treatment, and proglucagon mRNA expression was determined by real-time RT-PCR (C–F). mRNA expression data are shown relative to PBS only-treated mRNA levels (set to 1.0). All results are expressed as mean ± sem (n = 4 independent experiments). *, P < 0.05. Statistical significance was calculated by two-way ANOVA. White bars, Control (PBS or DMSO with or without JAK2/STAT3 inhibitor); black bars, treatment (leptin with or without JAK2/STAT3 inhibitor).
Next, we analyzed whether the JAK2/STAT3 pathway is involved in the regulation of proglucagon by leptin. The adult mHypoA-2/10 and embryonic mHypoE-39 cells were pretreated with the inhibitors for 1 h, followed by leptin treatment, and total RNA was isolated at 1 and 12 h. Using real-time qRT-PCR, we found that as compared with the vehicle treatment, leptin treatment alone significantly up-regulated proglucagon mRNA levels in both mHypoA-2/10 and mHypoE-39 neuronal cells, whereas pretreatment with cucurbitacin I or SD1008 attenuated the leptin-induced increase in proglucagon mRNA expression [mHypoA-2/10 cell line (1 h): PBS (1.26 ± 0.10) vs. 10 nm leptin (2.15 ± 0.07), P < 0.05; DMSO (0.88 ± 0.28) vs. DMSO + 10 nm leptin (1.83 ± 0.25), P < 0.05; cucurbitacin I (0.47 ± 0.10) vs. cucurbitacin I + 10 nm leptin (0.39 ± 0.11), P > 0.05; SD1008 (0.75 ± 0.28) vs. SD1008 + 10 nm leptin (0.67 ± 0.23), P > 0.05; mHypoE-39 cell line (1 h): PBS (1.27 ± 0.15) vs. 10 nm leptin (1.88 ± 0.36), P < 0.05; DMSO (0.91 ± 0.17) vs. DMSO + 10 nm leptin (1.61 ± 0.05), P < 0.05; cucurbitacin I (0.57 ± 0.08) vs. cucurbitacin I + 10 nm leptin (0.79 ± 0.26), P > 0.05; SD1008 (0.72 ± 0.11) vs. SD1008 + 10 nm leptin (0.80 ± 0.21), P > 0.05] (Fig. 5, C and D).
At 12 h after treatment, we found that the JAK2/STAT3 inhibitors reversed the down-regulation of proglucagon mRNA expression caused by leptin [mHypoA-2/10 cell line (12 h): PBS (1.30 ± 0.27) vs. 10 nm leptin (0.71 ± 0.11), P < 0.05; DMSO (0.88 ± 0.03) vs. DMSO + 10 nm leptin (0.47 ± 0.04), P < 0.05; cucurbitacin I (1.12 ± 0.15) vs. cucurbitacin I + 10 nm leptin (0.87 ± 0.08), P > 0.05; SD1008 (0.84 ± 0.14) vs. SD1008 + 10 nm leptin (1.06 ± 0.32), P > 0.05; mHypoE-39 cell line (12 h): PBS (1.27 ± 0.19) vs. 10 nm leptin (0.68 ± 0.05), P < 0.05; DMSO (1.27 ± 0.13) vs. DMSO + 10 nm leptin (0.84 ± 0.05), P < 0.05; cucurbitacin I (0.51 ± 0.09) vs. cucurbitacin I + 10 nm leptin (0.51 ± 0.06), P > 0.05; SD1008 (1.28 ± 0.10) vs. SD1008 + 10 nm leptin (1.50 ± 0.16), P > 0.05] (Fig. 5, E and F). These findings indicate that the JAK2/STAT3 pathway is involved in the regulation of hypothalamic proglucagon mRNA expression by leptin. Furthermore, we found that JAK2/STAT3 inhibitors decrease basal proglucagon mRNA levels, suggesting that the JAK2/STAT3 pathway may regulate basal proglucagon transcription (Fig. 5F).
Regulation of the human or rat proglucagon 5′ flanking promoter constructs by insulin and leptin
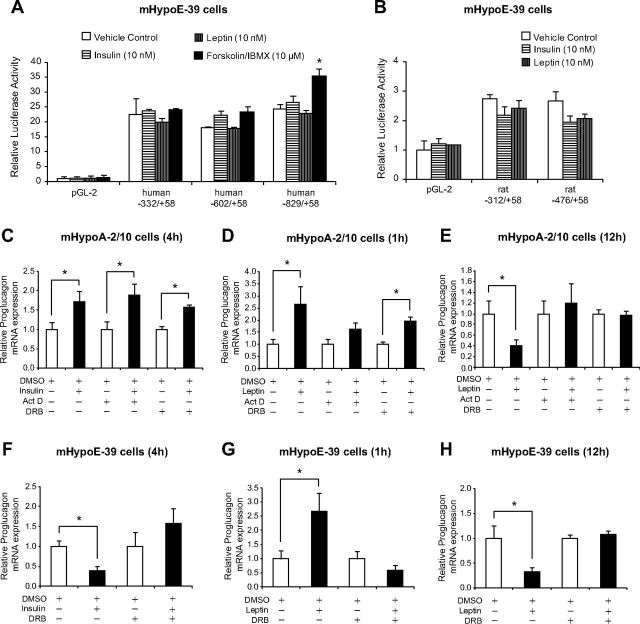
To determine whether insulin and leptin regulate proglucagon at the level of gene transcription, proglucagon promoter reporter genes were transiently transfected into the mHypoA-2/10 and mHypoE-39 cells. The human proglucagon constructs consisted of three lengths of human proglucagon 5′ flanking region, −332 to +58, −602 to +58, and −829 to +58, inserted into luciferase reporter vectors. The rat proglucagon constructs consisted of two lengths of rat proglucagon 5′ flanking region, −312 to +58, and −476 to +58, inserted into luciferase reporter vectors. The transfection efficiency in the adult hypothalamic cell model mHypoA-2/10 was less than 10% in contrast to more than 70% observed in the mHypoE-39 and mHypoE-20/2 cell lines. Therefore, we decided to use the embryonic cell models for the transient transfection analysis. Twenty-four hours after transfection, the cells were treated with 10 nm of insulin or leptin and harvested at 12 h after treatment. All three human proglucagon promoter constructs had basal luciferase expression 22.51-, 18.15-, and 24.35-fold higher than the promoterless pGL2 plasmid (Fig. 6A). However, the basal activity of rat proglucagon promoter constructs was much lower than the basal activity of the human proglucagon plasmids because it was only 2.74- and 2.66-fold higher than the promoterless pGL2 plasmid (Fig. 6B). Interestingly, insulin or leptin treatment did not affect the human plasmid transcription [human −332/+58: PBS (22.51 ± 5.42), 10 nm insulin (23.79 ± 0.49), 10 nm leptin (19.94 ± 1.43), P > 0.05 (PBS vs. leptin or insulin); human −602/+58: PBS (18.15 ± 0.33), 10 nm insulin (22.34 ± 1.32), 10 nm leptin (17.80 ± 0.41), P > 0.05 (PBS vs. leptin or insulin); human −829/+58: PBS (24.36 ± 1.64), 10 nm insulin (26.43 ± 2.28), 10 nm leptin (22.87 ± 1.02), P > 0.05 (PBS vs. leptin or insulin)] (Fig. 6A) or the rat plasmid transcription [rat −312/+58: PBS (2.74 ± 0.33), 10 nm insulin (2.20 ± 0.27), 10 nm leptin (2.42 ± 0.27), P > 0.05 (PBS vs. leptin or insulin); rat −476/+58: PBS (2.66 ± 0.21), 10 nm insulin (1.97 ± 0.19), 10 nm leptin (2.06 ± 0.16), P > 0.05 (PBS vs. leptin or insulin)] (Fig. 6B). These results indicated that insulin and leptin do not affect the transcription of the human or rat proglucagon 5′ flanking gene promoter regions in the mouse mHypoE-39 cell line.
Fig. 6.
Insulin and leptin do not affect the transcription of proglucagon promoter constructs but regulate mRNA stability. mHypoE-39 cells were transfected with proglucagon 5′ flanking plasmids or promoterless control plasmid pGL2 (A and B), incubated for 24 h, and then treated with vehicle, insulin (10 nm), leptin (10 nm), or forskolin/IBMX (10 μm). Cells were harvested 12–16 h after treatment and a luciferase assay was performed. Data were normalized to protein concentration. To investigate the regulation of mRNA stability by insulin (C and F) and leptin (D, E, G, and H), mHypoA-2/10 cells (C–E) and mHypoE-39 (F–H) were serum starved overnight, pretreated with a RNA polymerase II gene transcription inhibitor, either actinomycin D (Act D) (10 μg/ml) or DRB (60 μm), for 1 h followed by vehicle (DMSO), insulin (10 nm), or leptin (10 nm) treatment. Total RNA was isolated at 1 h (D and G), 4 h (C and F), and 12 h (E and H) after treatment, and proglucagon mRNA expression was determined by real-time RT-PCR. mRNA expression data are shown relative to DMSO with or without RNA polymerase II gene transcription inhibitor-treated mRNA levels (set to 1.0). All results are expressed as mean ± sem (n = 4 independent experiments). *, P < 0.05). Statistical significance was calculated by two-way ANOVA. C–H, White bars, Control (DMSO with or without RNA polymerase II gene transcription inhibitor); black bars, treatment (insulin or leptin with or without RNA polymerase II gene transcription inhibitor).
Regulation of mRNA stability by insulin and leptin in mHypoA-2/10 and mHypoE-39 neuronal cells
We further determined the stability of the proglucagon mRNA in the presence of insulin or leptin using RNA polymerase II gene transcription inhibitors (actinomycin D at 10 μg/ml and DRB at 60 μm concentrations). The adult mHypoA-2/10 and embryonic mHypoE-39 cells were pretreated with the inhibitors for 1 h, followed by insulin or leptin treatment. We observed that actinomycin D was not tolerated well by these cells because this RNA polymerase II gene transcription inhibitor caused significant cell death at the selected dose. Therefore, we pretreated the embryonic mHypoE-39 cells with DRB only because it was well tolerated by these cells. The mHypoA2/10 and mHypoE-39 cells were harvested for RNA isolation at 4 h after insulin treatment and at 1 and 12 h after leptin treatment. Total RNA was isolated and analyzed using real-time qRT-PCR. As compared with the vehicle DMSO treatment, insulin treatment alone significantly induced an increase in the proglucagon mRNA levels in mHypoA-2/10 neuronal cells that was not attenuated by the pretreatment with either actinomycin D or DRB [mHypoA-2/10 cell line (4 h): DMSO (0.23 ± 0.04) vs. DMSO + 10 nm insulin (0.39 ± 0.06), P < 0.05; actinomycin D (0.19 ± 0.04) vs. actinomycin D + 10 nm insulin (0.37 ± 0.06), P < 0.05; DRB (1.88 ± 0.13) vs. DRB + 10 nm insulin (2.95 ± 0.12), P < 0.05] (Fig. 6C). Similar effects were observed at 1 h after treatment with leptin because the pretreatment with either RNA polymerase II gene transcription inhibitor did not suppress the leptin-induced up-regulation of proglucagon mRNA expression; however, the effect was significant only with DRB pretreatment [mHypoA-2/10 cell line (1 h): DMSO (0.23 ± 0.05) vs. DMSO + 10 nm leptin (0.60 ± 0.17), P < 0.05; actinomycin D (0.33 ± 0.07) vs. actinomycin D + 10 nm leptin (0.53 ± 0.09), P > 0.05; DRB (1.45 ± 0.17) vs. DRB + 10 nm leptin (2.86 ± 0.26), P < 0.05] (Fig. 6D). In contrast, at 12 h after treatment with leptin, the pretreatment with the RNA polymerase II gene transcription inhibitors reversed the leptin-induced suppression of proglucagon mRNA expression [mHypoA-2/10 cell line (12 h): DMSO (0.55 ± 0.13) vs. DMSO + 10 nm leptin (0.23 ± 0.06), P < 0.05; actinomycin D (0.24 ± 0.05) vs. actinomycin D + 10 nm leptin (0.28 ± 0.09), P > 0.05; DRB (2.39 ± 0.17) vs. DRB + 10 nm leptin (2.32 ± 0.21), P > 0.05] (Fig. 6E). These findings suggest that insulin and leptin increase proglucagon mRNA stability in the adult cell line at 4 and 1 h after treatment and that the mRNA stability remains unaffected at a later period after leptin treatment. Our raw data indicate that DRB, but not actinomycin D, increases basal proglucagon mRNA levels in the adult cells, suggesting that DRB may inhibit the transcription of proglucagon gene suppressor factors that could regulate basal proglucagon mRNA levels.
As compared with the vehicle DMSO treatment, insulin treatment alone significantly decreased proglucagon mRNA levels in mHypoE-39 neuronal cells that was not reversed by the pretreatment with DRB [mHypoE-39 cell line (4 h): DMSO (0.26 ± 0.04) vs. DMSO + 10 nm insulin (0.10 ± 0.03), P < 0.05; DRB (1.32 ± 0.47) vs. DRB + 10 nm insulin (2.32 ± 0.51), P > 0.05] (Fig. 6F). At 1 h after treatment with leptin, the pretreatment with the RNA polymerase II gene transcription inhibitor suppressed the leptin-induced up-regulation of proglucagon mRNA expression [mHypoE-39 cell line (1 h): DMSO (0.49 ± 0.14) vs. DMSO + 10 nm leptin (1.31 ± 0.31), P < 0.05; DRB (1.38 ± 0.36) vs. DRB + 10 nm leptin (0.82 ± 0.27), P > 0.05] (Fig. 6G). Similarly, at 12 h after treatment with leptin, the pretreatment with DRB reversed the leptin-induced suppression of proglucagon mRNA expression [mHypoE-39 cell line (12 h): DMSO (0.15 ± 0.04) vs. DMSO + 10 nm leptin (0.04 ± 0.01), P < 0.05; DRB (1.82 ± 0.13) vs. DRB + 10 nm leptin (1.99 ± 0.15), P > 0.05] (Fig. 6H). These findings suggest that insulin and leptin do not regulate proglucagon mRNA stability in the embryonic cell line.
Regulation of proglucagon mRNA expression by a cAMP-activated pathway
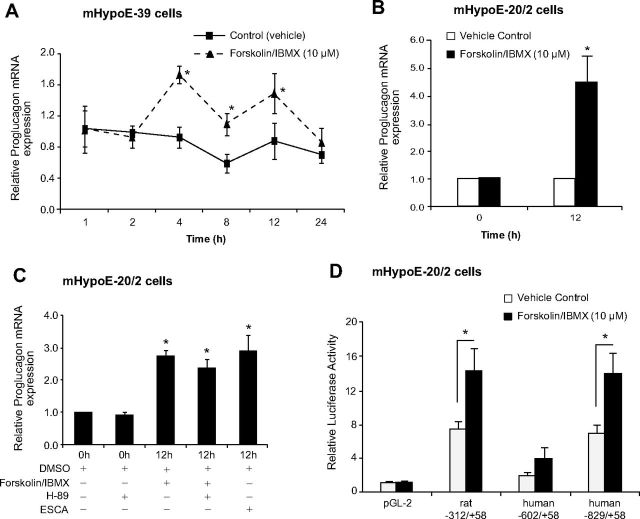
cAMP is a second messenger that is used as a second messenger by multiple cell surface receptors to regulate expression of genes and has been demonstrated to modulate expression of proglucagon expression in islet and intestinal cells (35). To determine whether proglucagon gene expression can be modulated by changes in cAMP, we treated the embryonic mHypoE-39 and mHypoE-20/2 cells over 24 h with forskolin and IBMX (10 μm). Using real-time qRT-PCR, we measured the levels of proglucagon mRNA and found that in the mHypoE-39 cells, proglucagon mRNA was significantly up-regulated by 87% at 4 h, 82% at 8 h, and 68% at 12 h after treatment [4 h, vehicle (0.93 ± 0.14) vs. 10 μm forskolin/IBMX (1.74 ± 0.11), P < 0.05; 8 h, vehicle (0.61 ± 0.12) vs. 10 μm forskolin/IBMX (1.11 ± 0.15), P < 0.05; 12 h, vehicle (0.89 ± 0.23) vs. 10 μm forskolin/IBMX (1.50 ± 0.26), P < 0.05] (Fig. 7A). In the mHypoE-20/2 cells, maximal changes in mouse prolgucagon mRNA levels, an approximate 4.5-fold increase, were observed at 12 h after forskolin/IBMX treatment (Fig. 7B). Thus, all subsequent experiments used this period of treatment in these cells. When mHypoE-36/1, a cell line that does not express endogenous proglucagon gene (Fig. 1A), was treated with forskolin/IBMX, no change in proglucagon mRNA could be detected because the proglucagon mRNA levels remained undetectable (data not shown). Thus, proglucagon gene expression in the hypothalamus, similar to results found in the islets and intestine, can be regulated by cAMP (36).
Fig. 7.
cAMP regulates proglucagon gene expression in the embryonic hypothalamic neuronal cells via activation of the Epac pathway. After overnight serum starvation, the mHypoE-39 (A) and mHypoE-20/2 (B and C) cells were exposed to vehicle, forskolin/IBMX (10 μm), or Epac pathway-specific cAMP analog ESCA (50 μm) over a 24-h time course. To determine the involvement of the PKA pathway in proglucagon mRNA regulation by cAMP, the mHypoE-20/2 neuronal cells were pretreated with a PKA inhibitor, H89 (1 μm), for 45 min followed by forskolin/IBMX (10 μm) treatment. Total RNA was extracted at the indicated time points and used as a template for real-time RT-PCR with primers specifically designed to amplify proglucagon mRNA. Proglucagon mRNA levels were quantified using the cycle threshold method and normalized to the internal control (γ-actin). All results shown are relative to corresponding control mRNA levels at each time point. For the transient transfection analysis, mHypoE-20/2 cells were transfected with proglucagon 5′ flanking plasmids or promoterless control plasmid pGL2 (D), incubated for 24 h, and then treated with vehicle or forskolin/IBMX (10 μm). Cells were harvested 12 h after treatment and a luciferase assay was performed. Data from the luciferase assay were normalized to protein concentration. All results are expressed as mean ± sem (n = 4 independent experiments). *, P < 0.05. Statistical significance was calculated by one- or two-way ANOVA.
Regulation of proglucagon expression by cAMP can be achieved by either a PKA-dependent pathway or an Epac pathway (37, 38). To determine which of these two pathways predominates in hypothalamic cells, we inhibited the PKA pathway by pretreating the mHypoE-20/2 cells with 1.0 μm H89, a PKA inhibitor, 45 min before stimulation with 10 μm forskolin/IBMX, as previously reported (39), and investigated whether PKA inhibition attenuates cAMP-induced increase in proglucagon mRNA expression. Next, we treated the cells with 50 μm 8-pMeOPT-2′-O-Me-cAMP (8-CPT), an Epac-specific cAMP analog to investigate its action on proglucagon mRNA levels. We found that H89 failed to prevent the cAMP-induced expression of the endogenous proglucagon gene (Fig. 7C) and observed that 8-CPT increased prolgucagon expression to an extent equal to that observed with the forskolin/IBMX treatment (Fig. 7C). In the proglucagon-negative mHypoE-36/1 cell line, H89 and 8-CPT treatment did not result in a change in the level of expression of proglucagon, it remained undetectable (data not shown). These observations suggest that similar to the intestinal endocrine proglucagon-expressing cells, in the hypothalamic mHypoE-20/2 neuronal cell line, the Epac pathway also plays a positive regulatory role in proglucagon expression.
Regulation of transiently transfected rat and human proglucagon 5′ flanking promoter constructs by cAMP
Because the endogenous proglucagon gene can be regulated by cAMP (Fig. 7, A–C), we tested whether transfected proglucagon promoter reporter gene constructs could also be regulated by cAMP. To test the activity of cAMP on the proglucagon promoter, we transfected embryonic cell models with differing lengths of rat and human proglucagon promoter constructs. Initially, we transfected human proglucagon promoter constructs containing −332, −602, and −829 bases of the 5′ flanking sequence into the embryonic mHypoE-39 cells as described earlier. Twenty-four hours after transfection, the cells were treated with 10 μm of forskolin/IBMX and harvested at 12 h after treatment. Forskolin/IBMX treatment affected transcription of only one plasmid that contained 829 bases of the human proglucagon promoter [human −332/+58: PBS (22.51 ± 5.42), 10 μm forskolin/IBMX (24.10 ± 0.43), P > 0.05; human −602/+58: PBS (18.15 ± 0.33), 10 μm forskolin/IBMX (23.34 ± 1.71), P > 0.05; human −829/+58: PBS (24.36 ± 1.63), 10 μm forskolin/IBMX (35.35 ± 2.49), P < 0.05] (Fig. 6A). These results indicate that a region between −602 and −828 bases is required for the transcription of the human proglucagon 5′ flanking promoter region by forskolin/IBMX in the mouse mHypoE-39 cell line.
Next, we used mHypoE-20/2 cells to test the rat proglucagon promoter construct containing 312 bases of 5′ flanking sequence and human proglucagon promoter constructs containing up to −602 and −829 bases of the 5′flanking sequence. Interestingly, all of the constructs showed a nearly 2-fold increase in the level of expression after treatment with forskolin and IBMX, including the short −602 bp human construct, but this increase did not reach statistical significance (Fig. 7D). These results indicate that −312 bases of the rat promoter and −829 bases of human promoter both contain a sequence that is responsive to cAMP in hypothalamic neurons. Again, we identified a novel area, between bases −602 and −829 in the human proglucagon promoter, which is required for the promoter-specific expression by cAMP in the hypothalamic cell lines.
Discussion
It is unknown whether insulin, leptin, or cAMP activators have any direct action on the hypothalamic proglucagon neurons. The lack of knowledge in this area is mostly due to inaccessibility to the hypothalamic proglucagon-expressing neurons. The previous studies on the regulation of hypothalamic PGDP were conducted using fetal rat hypothalamic primary cell cultures (6); however, these cultures are quite challenging to generate and maintain. To circumvent this issue, we used novel cell lines generated from the embryonic and adult mouse hypothalamii that endogenously express proglucagon mRNA, insulin, and leptin receptors and second messengers (23, 31).
Insulin-mediated proglucagon gene regulation in the pancreas has been extensively studied (35). This is the first study to demonstrate a direct role for insulin in the control of hypothalamic proglucagon gene expression. We showed that insulin up-regulated proglucagon mRNA expression in the adult hypothalamic cell models in contrast to its down-regulating action in the embryonic cell model. This variable regulation of proglucagon gene expression can be explained by the differences in the expression profile of PGDP in the adult and embryonic hypothalamus from which these cell models have been generated and immortalized. The developmental studies on the hypothalamic PGDP found that fetal rat hypothalamus predominantly expresses glicentin, oxyntomodulin, and glucagon, whereas adult rat hypothalamus expresses glicentin and oxyntomodulin in greater amounts than glucagon and GLP-1, suggesting that processing of proglucagon in the hypothalamus is age dependent and changes with development (6, 40). Thus, the differential profile of PGDP may underlie the differential control of proglucagon gene expression by insulin in these neuronal cells, and further investigations are required to confirm this hypothesis. Furthermore, tissue-specific variation in the regulation of proglucagon is quite possible because insulin was demonstrated to inhibit islet proglucagon gene expression via the regulation of gene transcription (41), whereas in GLUTag cells, a murine enteroendocrine cell line, insulin has been reported to stimulate proglucagon gene expression (42).
Among multiple signaling pathways activated by insulin, the PI3K-Akt pathway remains the main focus in the studies from the central nervous system. Several investigations have demonstrated that insulin activates PI3K in neurons (43, 44) and that PI3K inhibitors can block the ability of insulin to regulate food intake (44). In the present study, we confirm that in our proglucagon-expressing neuronal cell models insulin activates the PI3K/Akt pathway, as evidenced by phosphorylation of Akt. Interestingly, treatment with the PI3K inhibitors reversed the insulin-induced changes in proglucagon gene expression in both neuronal cell models. This indicates that similar to peripheral tissues, insulin mediates its action via Akt activation in the selective hypothalamic proglucagon neurons.
A number of reports suggest that leptin interacts with proglucagon-expressing neurons in mice and increases hypothalamic GLP-1 content as well as proglucagon mRNA levels in brainstem neurons through the STAT signaling pathway (45–49). Similar to these findings, we detected that leptin regulated proglucagon mRNA in a STAT3-dependent manner in the hypothalamic neuronal cell models. We found that leptin induced a rapid increase at 1 h in the proglucagon mRNA followed by a decrease at later time points. The leptin-mediated early proglucagon mRNA up-regulation is consistent with the action of ciliary neurotrophic factor, a cytokine that induced proglucagon mRNA in mHypoA-2/10 cells and simultaneously activated the JAK2/STAT3 pathway (31). In contrast, leptin-mediated delayed down-regulation is in accordance with the suppressive effect of other proinflammatory cytokines on proglucagon gene expression (50). The effect of leptin detected in our study is, however, difficult to interpret due to the biphasic regulation of proglucagon mRNA. This action also warrants further investigation on the synthesis and secretion of PGDP in the hypothalamic neuronal cells that seems to be challenging at present because the quantity of PGDP synthesized or secreted by our hypothalamic neuronal cells is quite low, most probably in the picomolar or femtomolar range, and current detection assays available are not sensitive enough for these studies.
Expression of proglucagon is regulated by many external factors, such as blood glucose, nutrient levels in the gut, and neurotransmitters, several of which use cAMP as a second messenger. Expression of the rat proglucagon gene in both islet and intestinal cells has been shown to be up-regulated by increased levels of cAMP, cAMP response element-binding protein, and other transcription factors (37, 51, 52). Central regulation of satiety is mediated by activation of the hypothalamic cAMP pathway by negatively regulating NPY gene expression and up-regulating transcription of cocaine- and amphetamine-regulated transcript, a potent appetite-suppressing peptide (16–18). In the present study, we show that both the endogenous proglucagon gene (Fig. 7, A–C) and transfected proglucagon promoters (Figs. 6A and 7D) are up-regulated in hypothalamic cell lines in response to cAMP activation by forskolin/IBMX treatment. This is not only the first demonstration that cAMP regulates proglucagon gene expression in hypothalamic cells but also that cAMP regulates the expression of the human proglucagon gene promoter.
Regulation of gene expression by cAMP can be mediated by at least two signaling pathways, the PKA or the Epac pathways (37, 38). A role for the PKA pathway in regulating proglucagon expression in islet α-cells has been demonstrated (53), whereas the Epac pathway appears to be a major contributor to regulation in islet α-cells and intestinal L cells (39, 54). To determine which of these pathways may predominate in hypothalamic cells, we examined changes in expression of the endogenous proglucagon gene when the PKA pathway was blocked or when the Epac pathway was specifically activated. As shown in Fig. 7C, blockage of the PKA pathway did not inhibit cAMP-induced proglucagon gene expression, suggesting that the PKA pathway is not the major mediator of cAMP signaling in these cells. Likewise, specific activation of the Epac generated a robust increase in proglucagon gene expression, suggesting that much of the cAMP-induced gene expression may be mediated through the Epac pathway.
The level of mRNA in a cell is determined by either changes in mRNA stability or the direct regulation of gene transcription at the 5′ regulatory region. The regulation of mRNA half-life, and the sequences and trans-acting factors necessary for the expression and regulation of the proglucagon gene have been determined in islet α-cells and intestinal L cells (4, 35, 36, 55–58). Most studies on the proglucagon promoter have focused on the rat gene, although some studies with promoters from other species, including a few studies using the human gene promoter have been conducted (27, 28, 59). To complement studies with cell lines, transgenic mice have been generated, which have largely been concordant with results based on the cell lines (27, 28, 60). Yet little is known about the sequences or factors required for the proglucagon gene expression in select neurons of the hypothalamus (4, 55, 56). A key reason for this limited knowledge has been the lack of a suitable neuronal cell line. Because the embryonic and adult hypothalamic cell lines express endogenous proglucagon gene, we decided to study the regulation of proglucagon gene expression in these neuronal models by transfecting available rat and human proglucagon promoter reporter genes. However, we found that the transfection efficiency in the adult hypothalamic cell model mHypoA-2/10 was very low when compared with more than 70% in the mHypoE-39 and mHypoE-20/2 cell lines; thus, we continued with the embryonic cell models for the transient transfection analysis. Although it would have been optimal to use the mouse promoter in our mouse cell lines, these constructs are not yet available. The use of human or rat proglucagon promoter constructs in our study can be justified by the fact that the proximal promoter elements are highly conserved in the rodent and human proximal proglucagon promoter sequences (28). The homology between human and rat proglucagon promoters shows that they are highly related; however, there can be several differences in the nucleotide sequence (28). The G1 promoter is highly conserved as compared with the less well conserved G2-G4 enhancer sequences. These elements have binding sites for several transcription factors including isl-1, cdx-2/3, Brn-4, pax-6, cAMP response element-binding protein, and activator protein-1; however, putative STAT3 binding sites are not detected (28, 35). The use of nonspecies-specific promoters can confound the analysis, and the lack of insulin or leptin transcriptional effects using the rat or human promoter in the mouse hypothalamic cell lines does not conclusively exclude transcriptional regulation of the mouse proglucagon gene. Generation of mouse proglucagon promoter plasmids is required to conduct further studies.
Transfection of the reporter constructs containing 5′ flanking regions of the human proglucagon gene demonstrated that a short 312-base rat proglucagon promoter region is sufficient for activation by cAMP. By deletion analysis, we identified a novel area, between bases −602 and −829 in human proglucagon promoter, which is required for the promoter-driven induction by cAMP in the hypothalamic cell lines. Unlike cAMP, insulin and leptin did not directly affect proglucagon gene transcription at the level of the promoter. There could be two possible reasons for the lack of an effect. First, there could be low-sequence similarity between the human, rat, and mouse proglucagon 5′ regulatory regions. However, it is unlikely because human proglucagon promoter transgenic mice show that the human proglucagon promoters are expressed in mouse neurons (27, 28). Second, the 5′ flanking sequences used for the proglucagon constructs were only 829 bp long, and thus, cis-acting insulin or leptin responsive elements could be located upstream of these regions. Although using transcription factor search programs, we did not find STAT3 binding sequences within 829 bp upstream of the transcription initiation site, further search and investigation into distal promoter regions is required to detect STAT binding sequences and cis-elements for downstream transcription factors activated by Akt. Because of the lack of changes in the activity of proglucagon reporter gene constructs, we analyzed the stability of the proglucagon mRNA in the presence of insulin and leptin. Using RNA polymerase II gene transcription inhibitors, actinomycin D and DRB, in the hypothalamic neuronal cells, we detected that the changes in mRNA expression at early time points induced by both insulin and leptin were due to increased mRNA stability, whereas leptin-induced changes in the mRNA expression at the later time point do not seem to be controlled by changes in the rate of mRNA decay in the adult neuronal cells. The decrease in mRNA expression caused by leptin treatment at 12 h may occur due to suppressed transcription, for which further investigation of distal promoter regions for putative cis- and trans-elements is required. Mechanisms involved in proglucagon mRNA stability in islet α-cells and intestinal L cells are known to some extent (36, 57); however, at the present time, no information is available on regulatory mechanisms for proglucagon mRNA stability in the hypothalamus. Because we detected that insulin and leptin affected mRNA stability, we searched for putative miRNA binding sites on proglucagon mRNA template (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). We found binding sites for several miRNA including miRNA128, a brain-enriched miRNA, that regulates reelin and double-cortin gene expression in neuroblastoma cells (61), and miRNA494 that down-regulates phosphatase and tensin homolog in the bronchial epithelial cells (62). Because insulin and leptin have been shown to regulate miRNA expression (63, 64), it is possible that they may regulate proglucagon mRNA stability as well via miRNA activation or suppression. Next, using the RNA-binding protein database program, we scanned proglucagon mRNA sequence for putative mRNA-binding protein sites that may regulate proglucagon mRNA stability. We detected several RNA-binding proteins, including nervous system-specific RNA-binding protein embryonic lethal, abnormal vision, Drosophila-like 2 protein or quaking homolog, KH domain RNA binding protein, which have been found to regulate gene expression in the nervous system (65–67). Further studies to determine role of insulin, leptin, and RNA-binding proteins in proglucagon mRNA regulation are warranted.
Taken together, these experiments indicate that insulin, leptin, and cAMP activators can act directly on proglucagon neuronal cells to regulate neuropeptide gene expression. Insulin, through an Akt-dependent mechanism, and leptin, through JAK2/STAT3 activation, regulate proglucagon mRNA expression, although transfections with human proglucagon promoter reporter gene constructs indicate that insulin or leptin may not act directly at the level of transcription but may instead act to increase the stability of proglucagon mRNA. cAMP regulates proglucagon expression via the Epac pathway and also regulates the expression of rat and human 5′ regulatory regions. The PGDP are key regulators of feeding behavior; thus, a better understanding of the mechanisms through which insulin, leptin, and cAMP regulate hypothalamic proglucagon neurons is important to further expand and challenge our current knowledge of feeding circuits.
Acknowledgments
We express our gratitude to Golnaz Madadi, Jennifer Chalmers, and Margaret Koletar for technical assistance.
This work was supported by the Canadian Institutes for Health Research, the Canadian Diabetes Association, the Canada Foundation for Innovation, and the Canada Research Chairs Program (to D.D.B.). P.S.D. was supported by a Banting and Best Diabetes Research Centre Graduate Studentship, an Natural Sciences and Engineering Research Council of Canada Studentship, and an Ontario Graduate Scholarship.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 8-CPT
- 8-pMeOPT-2′-O-Me-cAMP
- DMSO
- dimethylsulfoxide
- DRB
- 5,6-dichlorobenzimidazole 1-β-d-ribofuranoside
- Epac
- exchange protein directly activated by cAMP
- ESCA
- 8-(4-methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cAMP (8-pMeOPT-2′-O-Me-cAMP)
- Gβ
- G protein β
- GLP
- glucagon-like peptide
- IBMX
- 3-isobutyl-1-methylxanthine
- JAK
- Janus kinase
- miRNA
- microRNA
- NPY
- neuropeptide Y
- PGDP
- proglucagon-derived peptide
- PI3K
- phosphatidylinositol 3-kinase
- PKA
- protein kinase A
- qRT-PCR
- quantitative RT-PCR
- STAT
- signal transducer and activator of transcription.
References
- 1. Ahima RS , Osei SY. 2001. Molecular regulation of eating behavior: new insights and prospects for therapeutic strategies. Trends Mol Med 7:205–213 [DOI] [PubMed] [Google Scholar]
- 2. Drucker DJ , Asa S. 1988. Glucagon gene expression in vertebrate brain. J Biol Chem 263:13475–13478 [PubMed] [Google Scholar]
- 3. Dhanvantari S , Seidah NG , Brubaker PL. 1996. Role of prohormone convertases in the tissue-specific processing of proglucagon. Mol Endocrinol 10:342–355 [DOI] [PubMed] [Google Scholar]
- 4. Kieffer TJ , Habener JF. 1999. The glucagon-like peptides. Endocr Rev 20:876–913 [DOI] [PubMed] [Google Scholar]
- 5. Vrang N , Larsen PJ. 2010. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Prog Neurobiol 92:442–462 [DOI] [PubMed] [Google Scholar]
- 6. Lui EY , Asa SL , Drucker DJ , Lee YC , Brubaker PL. 1990. Glucagon and related peptides in fetal rat hypothalamus in vivo and in vitro. Endocrinology 126:110–117 [DOI] [PubMed] [Google Scholar]
- 7. Corp ES , Woods SC , Porte D , Dorsa DM , Figlewicz DP , Baskin DG. 1986. Localization of 125I-insulin binding sites in the rat hypothalamus by quantitative autoradiography. Neurosci Lett 70:17–22 [DOI] [PubMed] [Google Scholar]
- 8. Schwartz MW , Seeley RJ , Campfield LA , Burn P , Baskin DG. 1996. Identification of targets of leptin action in rat hypothalamus. J Clin Invest 98:1101–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Niswender KD , Baskin DG , Schwartz MW. 2004. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab 15:362–369 [DOI] [PubMed] [Google Scholar]
- 10. Brüüning JC , Gautam D , Burks DJ , Gillette J , Schubert M , Orban PC , Klein R , Krone W , Müller-Wieland D , Kahn CR. 2000. Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- 11. Elmquist JK , Elias CF , Saper CB. 1999. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22:221–232 [DOI] [PubMed] [Google Scholar]
- 12. Halaas JL , Gajiwala KS , Maffei M , Cohen SL , Chait BT , Rabinowitz D , Lallone RL , Burley SK , Friedman JM. 1995. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546 [DOI] [PubMed] [Google Scholar]
- 13. Marks JL , Porte D , Stahl WL , Baskin DG. 1990. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology 127:3234–3236 [DOI] [PubMed] [Google Scholar]
- 14. Air EL , Benoit SC , Blake Smith KA , Clegg DJ , Woods SC. 2002. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav 72:423–429 [DOI] [PubMed] [Google Scholar]
- 15. Tang-Christensen M , Havel PJ , Jacobs RR , Larsen PJ , Cameron JL. 1999. Central administration of leptin inhibits food intake and activates the sympathetic nervous system in rhesus macaques. J Clin Endocrinol Metab 84:711–717 [DOI] [PubMed] [Google Scholar]
- 16. Dominguez G , Kuhar MJ. 2004. Transcriptional regulation of the CART promoter in CATH.a cells. Brain Res Mol Brain Res 126:22–29 [DOI] [PubMed] [Google Scholar]
- 17. Hsieh YS , Yang SF , Kuo DY. 2007. Intracerebral administration of protein kinase A or cAMP response element-binding protein antisense oligonucleotide can modulate amphetamine-mediated appetite suppression in free-moving rats. Am J Physiol Endocrinol Metab 292:E123–E131 [DOI] [PubMed] [Google Scholar]
- 18. Sheriff S , Chance WT , Iqbal S , Rizvi TA , Xiao C , Kasckow JW , Balasubramaniam A. 2003. Hypothalamic administration of cAMP agonist/PKA activator inhibits both schedule feeding and NPY-induced feeding in rats. Peptides 24:245–254 [DOI] [PubMed] [Google Scholar]
- 19. Benoit SC , Air EL , Coolen LM , Strauss R , Jackman A , Clegg DJ , Seeley RJ , Woods SC. 2002. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci 22:9048–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz MW , Seeley RJ , Woods SC , Weigle DS , Campfield LA , Burn P , Baskin DG. 1997. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46:2119–2123 [DOI] [PubMed] [Google Scholar]
- 21. Schwartz MW , Baskin DG , Bukowski TR , Kuijper JL , Foster D , Lasser G , Prunkard DE , Porte D , Woods SC , Seeley RJ , Weigle DS. 1996. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 45:531–535 [DOI] [PubMed] [Google Scholar]
- 22. Schwartz MW , Marks JL , Sipols AJ , Baskin DG , Woods SC , Kahn SE , Porte D. 1991. Central insulin administration reduces neuropeptide Y mRNA expression in the arcuate nucleus of food-deprived lean (Fa/Fa) but not obese (fa/fa) Zucker rats. Endocrinology 128:2645–2647 [DOI] [PubMed] [Google Scholar]
- 23. Belsham DD , Cai F , Cui H , Smukler SR , Salapatek AM , Shkreta L. 2004. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology 145:393–400 [DOI] [PubMed] [Google Scholar]
- 24. Shin ED , Estall JL , Izzo A , Drucker DJ , Brubaker PL. 2005. Mucosal adaptation to enteral nutrients is dependent on the physiologic actions of glucagon-like peptide-2 in mice. Gastroenterology 128:1340–1353 [DOI] [PubMed] [Google Scholar]
- 25. Chomczynski P , Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- 26. Cui H , Cai F , Belsham DD. 2005. Anorexigenic hormones leptin, insulin, and α-melanocyte-stimulating hormone directly induce neurotensin (NT) gene expression in novel NT-expressing cell models. J Neurosci 25:9497–9506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou L , Nian M , Gu J , Irwin DM. 2006. Intron 1 sequences are required for pancreatic expression of the human proglucagon gene. Am J Physiol Regul Integr Comp Physiol 290:R634–R641 [DOI] [PubMed] [Google Scholar]
- 28. Nian M , Drucker DJ , Irwin D. 1999. Divergent regulation of human and rat proglucagon gene promoters in vivo. Am J Physiol 277:G829–G837 [DOI] [PubMed] [Google Scholar]
- 29. Rusinov V , Baev V , Minkov IN , Tabler M. 2005. MicroInspector: a web tool for detection of miRNA binding sites in an RNA sequence. Nucleic Acids Res 33:W696–W700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cook KB , Kazan H , Zuberi K , Morris Q , Hughes TR. 2011. RBPDB: a database of RNA-binding specificities. Nucleic Acids Res 39:D301–D308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Belsham DD , Fick LJ , Dalvi PS , Centeno ML , Chalmers JA , Lee PK , Wang Y , Drucker DJ , Koletar MM. 2009. Ciliary neurotrophic factor recruitment of glucagon-like peptide-1 mediates neurogenesis, allowing immortalization of adult murine hypothalamic neurons. FASEB J 23:4256–4265 [DOI] [PubMed] [Google Scholar]
- 32. Mayer CM , Belsham DD. 2010. Central insulin signaling is attenuated by long-term insulin exposure via insulin receptor substrate-1 serine phosphorylation, proteasomal degradation, and lysosomal insulin receptor degradation. Endocrinology 151:75–84 [DOI] [PubMed] [Google Scholar]
- 33. Duan Z , Bradner J , Greenberg E , Mazitschek R , Foster R , Mahoney J , Seiden MV. 2007. 8-Benzyl-4-oxo-8-azabicyclo[3.2.1]oct-2-ene-6,7-dicarboxylic acid (SD-1008), a novel Janus kinase 2 inhibitor, increases chemotherapy sensitivity in human ovarian cancer cells. Mol Pharmacol 72:1137–1145 [DOI] [PubMed] [Google Scholar]
- 34. Blaskovich MA , Sun J , Cantor A , Turkson J , Jove R , Sebti SM. 2003. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res 63:1270–1279 [PubMed] [Google Scholar]
- 35. Jin T. 2008. Mechanisms underlying proglucagon gene expression. J Endocrinol 198:17–28 [DOI] [PubMed] [Google Scholar]
- 36. Drucker DJ , Jin T , Asa SL , Young TA , Brubaker PL. 1994. Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Mol Endocrinol 8:1646–1655 [DOI] [PubMed] [Google Scholar]
- 37. Knepel W , Chafitz J , Habener JF. 1990. Transcriptional activation of the rat glucagon gene by the cyclic AMP-responsive element in pancreatic islet cells. Mol Cell Biol 10:6799–6804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sands WA , Palmer TM. 2008. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal 20:460–466 [DOI] [PubMed] [Google Scholar]
- 39. Lotfi S , Li Z , Sun J , Zuo Y , Lam PP , Kang Y , Rahimi M , Islam D , Wang P , Gaisano HY , Jin T. 2006. Role of the exchange protein directly activated by cyclic adenosine 5′-monophosphate (Epac) pathway in regulating proglucagon gene expression in intestinal endocrine L cells. Endocrinology 147:3727–3736 [DOI] [PubMed] [Google Scholar]
- 40. Lee YC , Brubaker PL , Drucker DJ. 1990. Developmental and tissue-specific regulation of proglucagon gene expression. Endocrinology 127:2217–2222 [DOI] [PubMed] [Google Scholar]
- 41. Philippe J. 1989. Glucagon gene transcription is negatively regulated by insulin in a hamster islet cell line. J Clin Invest 84:672–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yi F , Sun J , Lim GE , Fantus IG , Brubaker PL , Jin T. 2008. Cross talk between the insulin and Wnt signaling pathways: evidence from intestinal endocrine L cells. Endocrinology 149:2341–2351 [DOI] [PubMed] [Google Scholar]
- 43. Mirshamsi S , Laidlaw HA , Ning K , Anderson E , Burgess LA , Gray A , Sutherland C , Ashford ML. 2004. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci 5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niswender KD , Morrison CD , Clegg DJ , Olson R , Baskin DG , Myers MG , Seeley RJ , Schwartz MW. 2003. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus. Diabetes 52:227–231 [DOI] [PubMed] [Google Scholar]
- 45. Goldstone AP , Mercer JG , Gunn I , Moar KM , Edwards CM , Rossi M , Howard JK , Rasheed S , Turton MD , Small C , Heath MM , O'Shea D , Steere J , Meeran K , Ghatei MA , Hoggard N , Bloom SR. 1997. Leptin interacts with glucagon-like peptide-1 neurons to reduce food intake and body weight in rodents. FEBS Lett 415:134–138 [DOI] [PubMed] [Google Scholar]
- 46. Mercer JG , Moar KM , Findlay PA , Hoggard N , Adam CL. 1998. Association of leptin receptor (OB-Rb), NPY and GLP-1 gene expression in the ovine and murine brainstem. Regul Pept 75–76:271–278 [DOI] [PubMed] [Google Scholar]
- 47. Goldstone AP , Morgan I , Mercer JG , Morgan DG , Moar KM , Ghatei MA , Bloom SR. 2000. Effect of leptin on hypothalamic GLP-1 peptide and brain-stem pre-proglucagon mRNA. Biochem Biophys Res Commun 269:331–335 [DOI] [PubMed] [Google Scholar]
- 48. Vrang N , Larsen PJ , Jensen PB , Lykkegaard K , Artmann A , Larsen LK , Tang-Christensen M. 2008. Upregulation of the brainstem preproglucagon system in the obese Zucker rat. Brain Res 1187:116–124 [DOI] [PubMed] [Google Scholar]
- 49. Huo L , Gamber KM , Grill HJ , Bjørbaek C. 2008. Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology 149:492–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. González M , Böer U , Dickel C , Quentin T , Cierny I , Oetjen E , Knepel W. 2008. Loss of insulin-induced inhibition of glucagon gene transcription in hamster pancreatic islet α cells by long-term insulin exposure. Diabetologia 51:2012–2021 [DOI] [PubMed] [Google Scholar]
- 51. Drucker DJ , Brubaker PL. 1989. Proglucagon gene expression is regulated by a cyclic AMP-dependent pathway in rat intestine. Proc Natl Acad Sci USA 86:3953–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang J , Cao Y , Steiner DF. 2003. Regulation of proglucagon transcription by activated transcription factor (ATF) 3 and a novel isoform, ATF3b, through the cAMP-response element/ATF site of the proglucagon gene promoter. J Biol Chem 278:32899–32904 [DOI] [PubMed] [Google Scholar]
- 53. Gajic D , Drucker DJ. 1993. Multiple cis-acting domains mediate basal and adenosine 3′,5′-monophosphate-dependent glucagon gene transcription in a mouse neuroendocrine cell line. Endocrinology 132:1055–1062 [DOI] [PubMed] [Google Scholar]
- 54. Islam D , Zhang N , Wang P , Li H , Brubaker PL , Gaisano HY , Wang Q , Jin T. 2009. Epac is involved in cAMP-stimulated proglucagon expression and hormone production but not hormone secretion in pancreatic α- and intestinal L-cell lines. Am J Physiol Endocrinol Metab 296:E174–E181 [DOI] [PubMed] [Google Scholar]
- 55. Philippe J. 1991. Structure and pancreatic expression of the insulin and glucagon genes. Endocr Rev 12:252–271 [DOI] [PubMed] [Google Scholar]
- 56. Drucker DJ. 2003. Glucagon gene expression. In: , Henry HL , Norman AW, eds. Encyclopedia of hormones. San Diego: Academic Press; 47–55 [Google Scholar]
- 57. Philippe J , Drucker DJ , Habener JF. 1987. Glucagon gene transcription in an islet cell line is regulated via a protein kinase C-activated pathway. J Biol Chem 262:1823–1828 [PubMed] [Google Scholar]
- 58. Brubaker PL , Schloos J , Drucker DJ. 1998. Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag enteroendocrine cell line. Endocrinology 139:4108–4114 [DOI] [PubMed] [Google Scholar]
- 59. Tsai B , Yue S , Irwin DM. 2007. A novel element regulates expression of the proximal human proglucagon promoter in islet cells. Gen Comp Endocrinol 151:230–239 [DOI] [PubMed] [Google Scholar]
- 60. Efrat S , Teitelman G , Anwar M , Ruggiero D , Hanahan D. 1988. Glucagon gene regulatory region directs oncoprotein expression to neurons and pancreatic α cells. Neuron 1:605–613 [DOI] [PubMed] [Google Scholar]
- 61. Evangelisti C , Florian MC , Massimi I , Dominici C , Giannini G , Galardi S , Buè MC , Massalini S , McDowell HP , Messi E , Gulino A , Farace MG , Ciafrè SA. 2009. MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. FASEB J 23:4276–4287 [DOI] [PubMed] [Google Scholar]
- 62. Liu L , Jiang Y , Zhang H , Greenlee AR , Han Z. 2010. Overexpressed miR-494 down-regulates PTEN gene expression in cells transformed by anti-benzo(a)pyrene-trans-7,8-dihydrodiol-9,10-epoxide. Life Sci 86:192–198 [DOI] [PubMed] [Google Scholar]
- 63. Granjon A , Gustin MP , Rieusset J , Lefai E , Meugnier E , Güller I , Cerutti C , Paultre C , Disse E , Rabasa-Lhoret R , Laville M , Vidal H , Rome S. 2009. The microRNA signature in response to insulin reveals its implication in the transcriptional action of insulin in human skeletal muscle and the role of a sterol regulatory element-binding protein-1c/myocyte enhancer factor 2C pathway. Diabetes 58:2555–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hamrick MW , Herberg S , Arounleut P , He HZ , Shiver A , Qi RQ , Zhou L , Isales CM , Mi QS. 2010. The adipokine leptin increases skeletal muscle mass and significantly alters skeletal muscle miRNA expression profile in aged mice. Biochem Biophys Res Commun 400:379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aberg K , Saetre P , Jareborg N , Jazin E. 2006. Human QKI, a potential regulator of mRNA expression of human oligodendrocyte-related genes involved in schizophrenia. Proc Natl Acad Sci USA 103:7482–7487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yano M , Okano HJ , Okano H. 2005. Involvement of Hu and heterogeneous nuclear ribonucleoprotein K in neuronal differentiation through p21 mRNA post-transcriptional regulation. J Biol Chem 280:12690–12699 [DOI] [PubMed] [Google Scholar]
- 67. King PH , Levine TD , Fremeau RT , Keene JD. 1994. Mammalian homologs of Drosophila ELAV localized to a neuronal subset can bind in vitro to the 3′ UTR of mRNA encoding the Id transcriptional repressor. J Neurosci 14:1943–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]