Abstract
Progesterone (P4), acting through its receptor (PR), is essential for the maintenance of pregnancy. P4 acts by suppressing uterine contractility and the expression of contraction-associated proteins (CAP) such as connexin 43 (Cx43). P4 levels must be reduced or its actions blocked to allow the increased expression of CAP genes and the initiation of labor. Although the importance of progesterone in pregnancy has been known for about 80 yr, the fundamental mechanisms by which P4/PR maintains myometrial quiescence and by which this signaling is blocked at term labor remain to be determined. In this manuscript, we demonstrate that ligand-bound PR interacts with the Cx43 gene promoter through activator protein-1 transcription factors. We show that the ability of PR to repress Cx43 transcription is conferred through the recruitment of the PR coregulator, polypyrimidine tract binding protein-associated splicing factor (PSF), and the further recruitment of the yeast switch independent 3 homolog A/histone deacetylase corepressor complex. PSF expression is elevated during pregnancy but falls toward term as a result of increased mechanical stretch of the myometrium and a rise in the concentrations of circulating estrogen. These data together indicate that PSF is a critical regulator of P4/PR signaling and labor. We suggest that decreased PSF at term may result in a de-repression of PR transcriptional control of CAP genes and thereby contributes to a functional withdrawal of progesterone at term labor.
Preterm birth occurs in 5–10% of all pregnancies and is associated with 70% of all neonatal deaths and up to 75% of neonatal related disability (www.cdc.gov/features/prematurebirth/). Extensive research has failed to find an effective therapy to diagnose or prevent this disease. Recent studies provide some optimism that progesterone (P4) or its derivatives may reduce the incidence of preterm delivery in some women at high risk of preterm labor (1, 2). However, despite 80 yr since its discovery by Allen (3), we have limited understanding as to how P4 acts to maintain pregnancy and how progesterone receptor (PR) action is modulated at the end of pregnancy in women to allow the initiation of labor.
P4/PR signaling is critical for the maintenance of pregnancy through modulating gene transcription (4). In most animal species, circulating P4 levels fall near term, and this has been suggested to induce a transition within the myometrium from a state of relative quiescence and unresponsiveness to one in which the myometrium is excitable, highly responsive to uterotonic agonists, and exhibits the high degree of cell-cell communication that is required to generate the intense contractions of labor (5). Biochemically this phenotypic transition is characterized by the up-regulation of myometrial contraction-associated proteins (CAP) including connexin 43 (Cx43) (6), prostaglandin synthases such as prostaglandin H synthase enzyme 2/cyclooxygenase 2(Cox2) (7), oxytocin receptor (8), and prostaglandin receptors (9). Expression of these genes and labor is suppressed by exogenous P4, whereas the removal of P4 or antagonism of PR (e.g. after administration of RU486) leads to the premature up-regulation of CAP and preterm labor (10, 11). In addition, the growing fetus exerts mechanical tension on the myometrial wall, further promoting CAP gene expression (12). In contrast to animal species, there is no decrease in the level of circulating P4 before the onset of human labor. Moreover, even though levels of P4 fall in animal species at term, the levels within uterine are still sufficiently high to saturate and activate PR (13). Therefore, we (4) as well as others (14) have suggested that there is a functional withdrawal of P4 as a result of blockade of PR transcriptional activity. It is suggested that this blockade is mediated by the interaction of PR coregulators in both human and animal species (4, 14, 15).
PR is a ligand-dependent transcription factor, which is expressed in the human myometrium as two major isoforms: the full-length PR-B and an N-terminally 164-amino acid truncated PR-A isoform. PR-A is generally a weaker transcriptional activator than PR-B and can also act as a repressor of PR-B and other steroid receptors (16). Classically, ligand-activated PR binds the progesterone response element (PRE) in target gene promoters and recruits coactivators or corepressors to either positively or negatively modulate gene transcription (17). Recent emerging evidence suggests an indirect action of PR in controlling myometrium contractility, through inhibition of inflammatory signals and mechanical stretch during pregnancy (12, 18, 19). Several mechanisms have been proposed by which a functional withdrawal of P4 might be achieved: 1) increases in the ratio of PR-A-PR-B expression (20); 2) altered expression/function of PR coregulators (15, 21); and 3) myometrial stretch and inflammatory stimuli, which trigger signaling to compromise the action of PR and its coregulators (12, 18, 19). It appears that none of these mechanisms alone can explain fully the functional withdrawal of P4, suggesting that multiple mechanisms might be coordinated to regulate myometrium contractility.
We have previously identified polypyrimidine tract binding protein-associated splicing factor (PSF) as a corepressor of PR (22). PSF acts by recruiting the corepressor complex, Sin3 homolog A (Sin3A)/histone deacetylase (HDAC) to the receptor complex (23, 24). Based on these data, we hypothesized that PSF might also contribute to the functional blockade of PR within the myometrium at term. Therefore, in this study, we investigated the following: 1) the expression of PSF protein in the myometrium during pregnancy and with the onset of labor; 2) the regulation of PSF protein levels by mechanical stretch and steroid hormones; 3) the functional impact of PSF on PR regulation of CAP gene transcription; and 4) the molecular mechanisms by which PSF induces a functional withdrawal of P4.
Materials and Methods
Pregnancy rat model
Wistar rats (Charles River Co., St. Constance, Canada) were housed individually under standard environmental conditions (12 h light, 12 h dark cycle) and fed Purina Rat Chow (Ralston Purina, St. Louis, MO) and water ad libitum. Female virgin rats were mated with male Wistar rats. Day 1 of gestation was designated as the day a vaginal plug was observed. The average time of delivery was the morning of d 23. The institutional Animal Care Committee approved all animal experiments. Myometrial tissue samples were collected on gestational d 0 and d 6, 8, 10, 14, 15, 19, 21, and 23 and 1 d postpartum. Labor samples (d 23) were collected during active labor once the animals had delivered at least one pup. For each day of gestation, tissues were collected from four to five animals.
Unilateral pregnant rats
Under general anesthesia, virgin female rats underwent tubal ligation through a flank incision to ensure that they subsequently became pregnant in only one horn (25). Animals were allowed to recover at least 7 d from surgery before mating. Pregnant rat myometrial samples from nongravid (empty) and gravid horns were collected at noon on gestational d 17, 21, and 23 (Labor) with four animals per time point. For the cylinder insertion experiments, unilaterally ligated rats (on gestational d 19) were randomized into three groups as we reported (25). In the control group, no cylinders were inserted into the empty horn. In the cylinder group, a nonexpandable polyvinyl cylinder (1 mm in diameter and 3 cm in length) was inserted into the empty horn through midline abdominal incision. In the laminaria group, an expandable laminaria cylinder (2 mm in diameter and 3 cm in length) was inserted into the empty horn through midline abdominal incision.
Ovariectomized (OVX) rat
Ovariectomy was performed on virgin Wistar rats as described (26). Rats recovered for 7 d to deplete endogenous ovarian steroids and were then injected with 17β-estradiol (E2; 1 μg per 100 g body weight) or E2 plus progesterone (4 mg per 225 g body weight) for the indicated period of time. Animals were euthanized by CO2 inhalation and myometrial samples were collected for immunoblotting, real-time RT-PCR, and immunohistochemical analyses.
Immunoblotting, real-time RT-PCR, and immunohistochemistry
Myometrial tissues were collected by mechanical scraping as we described (25). The tissues were flash frozen in liquid nitrogen and stored at −80 C. Protein was extracted in buffer (50 mm Tris (pH 8.0), 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate), and immunoblotting assays were performed as described (25). Antibodies used in immunoblotting included the following: PSF (B92; Sigma, Oakville, Canada), Cx43 (Ab1728; Millipore, Bedford, MA), Flag tag (M5; Sigma), PR (H190; Santa Cruz Biotechnology, Santa Cruz, CA), and vinculin (H-10; Santa Cruz Biotechnology). Total RNA was extracted using Trizol (Life Technologies, Grand Island, NY), RNeasy minikit (QIAGEN, Valencia, CA) purified, and reverse transcribed by a TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed using the ABI PRISM 7900 HT sequence detection system (Applied Biosystems) (25). Relative quantitation of gene transcription was calculated by fold changes relative to the vehicle treatment. Immunohistochemistry assays used PSF antibody (1:100; Sigma) and followed the protocol described previously (27). For the assessment of staining intensity, tissue sections from each of the three sets were observed on a Leica DMRXE microscope (Leica Microsystems, Heidelberg, Germany). A minimum of five fields were examined for each myometrial sample and representative tissue sections were photographed by Sony DXC-970 MD 3CCD color video camera (Sony Ltd., Toronto, Canada).
Transfection and luciferase assay
Human embryonic kidney cell line 293T, human telomerase immortalized myometrial cells (hTERT-HM), and Syrian hamster myometrium (SHM) cells were maintained in DMEM. For experiments involving steroid exposure, the medium was substituted with phenol red-free DMEM plus 5% charcoal-treated fetal bovine serum (HyClone Laboratories, Logan, UT). Transfection was performed using Lipofectmine 2000 (Life Technologies) or FuGENE 6 (Roche Applied Science, Mannheim, Germany) according to the manufacturer's protocol. Luciferase activities were determined using the luciferin reagent (Promega, Madison, WI) according to the manufacturer's protocol. Transfection efficiency was normalized to Renilla luciferase activities.
Construction of stable cell lines by lentivirus
Lentiviral vector (pFUGWBW) was used as the backbone to construct expression vectors encoding Flag-PR-A and Flag-PR-B using a gateway system (Invitrogen, Carlsbad, CA) as described (28). Briefly, 3μg of each lentiviral vector (pFUGWBW mock vector, Flag-PR-A or Flag-PR-B) together with 9μg of the ViraPower packaging mix (Invitrogen) were transfected into 293T cells, using Lipofectamine 2000 reagent (Life Technologies) at 37 C and 5% CO2. Lentiviral particles were harvested by removing medium 48 h after transfection and used to infect hTERT-HM cells. After 10 ng/μl blasticidin selection for 3 wk, three stable cell lines were generated: hTERT(mock), hTERT(PR-A), and hTERT(PR-B).
Coimmunoprecipitation and chromatin immunoprecipitation (ChIP)
Cells were lysed in a buffer of 50 mm Tris 7.4, 150 mm NaCl, 1 mm EDTA, and 1% Triton plus a protease inhibitor cocktail. Precleared lysates were precipitated with Flag or PR antibody. The associated proteins were blotted by specific antibodies. ChIP and re-ChIP assays were performed as we previously reported (24). DNA templates retrieved from ChIP were analyzed by quantitative real-time PCR on ABI PRISM 7900 HT system (Applied Biosystems) using the FastStart Universal SYBR Green Master (Roche) according to the manufacture's instruction. Recruitment of PR and its cofactors were calculated as fold change over nonspecific antibodies (29). The sem was determined from three to five independent ChIP with triplicates in each DNA sample. Primers used for human Cx43 promoter are: 5′-tac aac ttt atc ctg atc cca ctg and 3′-gta act tgg agc aca gag ctt tta. Primers used for mouse Cx43 promoter are: 5′-ttt ctc cta gcc cct cct tc and 3′-tca aag tct gct gct gtt gg.
Statistics
Means ± sem were calculated from three or more different experiments and statistical differences tested using Student's unpaired t test or one-way ANOVA. Statistical analysis was carried out by GraphPad InStat 3 (GraphPad, San Diego, CA) with the level of significance for comparison set at P < 0.05.
Results
Gestational profile of myometrial PSF protein during pregnancy
We measured PSF protein levels during pregnancy and labor in rats (30). Immunohistochemical analysis defined the temporal and spatial distribution of PSF protein in the myometrium across gestation (Fig. 1A). PSF was expressed at similar levels in myocytes within both the circular and longitudinal myometrial layers. The staining intensity and numbers of positively-stained cells were maintained at relatively high levels during early and midpregnancy. Starting from d 18, PSF-positive cell number and PSF staining intensity decreased and reached the lowest levels on d 21 and 23 (during labor). PSF expression subsequently increased 1 d postpartum. Immunoblotting assays confirmed high myometrial PSF protein levels during early and middle pregnancy. Starting from d 19, PSF began to decrease reaching the lowest level on d 21–23 (P < 0.01) (Fig. 1B). Thus, PSF protein expression in myometrium falls before the onset of labor.
Fig. 1.

Immunohistochemistry analysis of PSF in rat myometrium during pregnancy and labor. A, Immunostaining with PSF antibody was performed on sections of longitudinal and circular myometrial tissues from nonpregnant (NP) rats and rats on d 6, 10, 14, 18, 21, and 23 (labor) of pregnancy and 1 d postpartum (n = 4/time point). Nuclear PSF staining intensity and percentage of positive myometrial cell numbers decreased significantly at late gestational d 21–23. A tissue section incubated with nonspecific mouse IgG served as the negative control (Ng). Magnification was at ×40. Scale bar represents 50 um. B, Representative immunoblots showed protein expression of PSF and α-tubulin in rat myometrial tissues from different gestational ages. Densitometry analysis illustrated the relative PSF protein levels to tubulin (n = 4–5 rats/time point). Results were expressed as mean ± sem. *, P < 0.05; **, P < 0.01. ROD, Relative OD.
Mechanical stretch and estradiol down-regulate PSF protein expression
To study whether PSF down-regulation is regulated by stretch, we used a unilaterally pregnant rat model (25), in which both uterine horns are exposed to the same circulating hormonal environment, but only the gravid horn contains fetuses and is exposed to mechanical stretch. PSF protein levels were significantly decreased in the gravid compared with the empty uterine horns (Fig. 2A), consistent with a role for mechanical stretch in down-regulating PSF expression.
Fig. 2.
Stretch and estradiol regulate PSF protein expression. A, Representative immunoblots showed PSF and α-tubulin expression in rat myometrial tissues collected from empty (E) and gravid (G) horns from indicated gestational ages. Densitometry analysis illustrated the relative PSF protein levels to α-tubulin (n =4 rats/group) and presented as relative OD (ROD). B, Immunoblots showed PSF and actin protein levels in myometrial tissues collected from gravid and empty horns with control, nonexpandable, and expandable laminaria cylinder cylinders as described in Materials and Methods (n =4 rats/group). C, OVX rats were treated with vehicle (V), estradiol, or estradiol and P4 for 24 h (n =4 rats/group). Representative immunoblots showed PSF and α-tubulin expression. Densitometry analysis of PSF levels were normalized to α-tubulin. D, OVX rats were treated with estradiol for 0, 3, 6, and 24 h (n = 4 rats/group). PSF staining intensity was similar among these OVX-treated rat tissue sections. PSF immunoreactivity was calculated by counting PSF-positive myometrial cells from each of the four sets of samples (per gestational time point) with a minimum of five fields per samples. The proportion of PSF-positive cells per total cell number within circular myometrium is calculated and plotted against the time course of estradiol treatment. Magnification was at ×40. Scale bar represented 50 um. Results were expressed as mean ± sem. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To examine whether the difference in PSF expression between gravid and empty uterine horns was due to mechanical stretch or other factors, such as placental hormones or fetal-derived factors, we inserted cylinders of dry laminaria into the empty horns of the unilaterally pregnant rats. The cylinders of laminaria are hygroscopic and increase in size as they absorb water, inducing a gradual stretch on the myometrial wall. As control, we inserted 1 mm (nonexpandable) plastic cylinders into the empty horns of other unilaterally pregnant rats. The gravid horns of the unilaterally pregnant rats showed the decrease in PSF expression with the onset of labor (Fig. 2B). PSF expression in the empty horns or those with the plastic cylinders remained elevated and significantly higher than in the gravid horns. In contrast, the expression of PSF in empty horns that had been stretched by the laminaria cylinders decreased significantly and was similar to that in the gravid horns. These data confirm the role of mechanical stretch in down-regulating PSF protein expression in the myometrium.
To determine whether PSF expression was also regulated by steroid hormones, we used an OVX rat model (26). Immunoblotting assays indicated that E2 treatment reduced myometrial PSF protein levels by 50% when compared with vehicle controls (P < 0.01). The inhibitory action of E2 was antagonized by P4 (Fig. 2C). E2 significantly decreased PSF immunoreactivity (P < 0.01) in both circular (Fig. 2D) and longitudinal (Supplemental Fig. 1A, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org) myometrium in a time-dependent manner. These changes were correlated with an up-regulation of Cx43 protein expression (P < 0.01) (Supplemental Fig. 1B). These data indicate that E2 in addition to mechanical stretch can down-regulate PSF protein expression in myometrium. We suggest that a combination of increased stretch and rising E2 likely contribute to the low PSF protein levels during late pregnancy.
PSF regulates PR inhibition on CAP gene expression
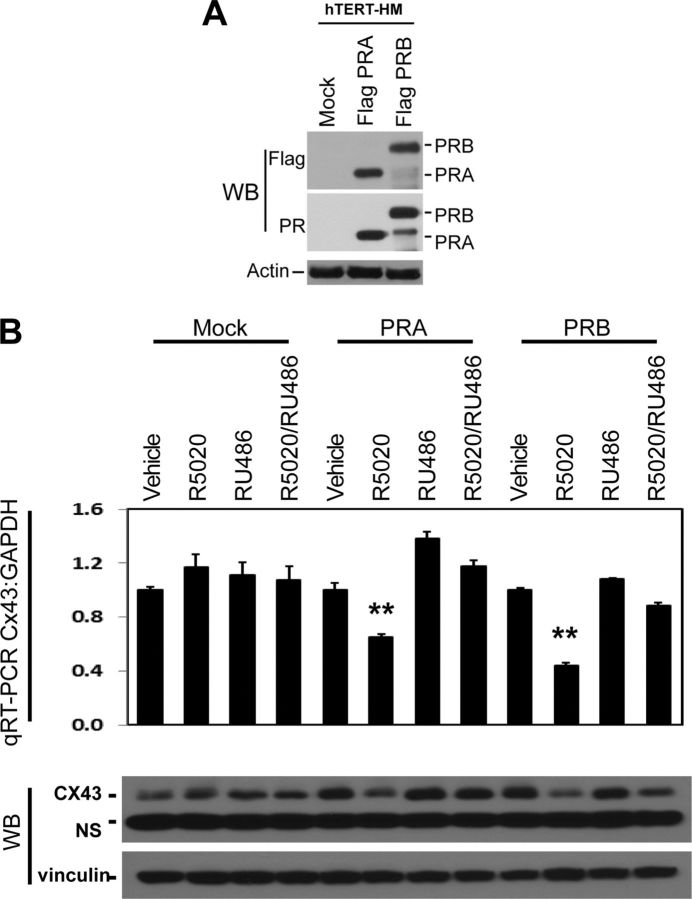
To study the role of PSF on PR transactivation of CAP genes, we used the human myometrial cell line, hTERT-HM, to construct stable lines overexpressing either Flag tagged PR-A or PR-B using lentiviral transduction. The following three stable lines were created: hTERT(mock), hTERT(PR-A), and hTERT(PR-B). PR expression from these lines is shown in Fig. 3A. Neither R5020 (the PR agonist) nor RU486 (the PR antagonist) altered Cx43 transcription in hTERT(mock) cells (Fig. 3B). However, R5020 reduced Cx43 mRNA levels by approximately 50% (P < 0.01) in both hTERT(PR-A) and hTERT(PR-B) cells. Although the PR antagonist, RU486, alone had no effect, it blocked the inhibitory effect of R5020 on Cx43 transcription, indicating that repression of Cx43 transcription was mediated through PR. PR agonists and antagonists induced similar changes in Cx43 protein. Therefore, progesterone inhibits Cx43 expression in human myometrial cell lines in a similar manner to that seen in vivo (31, 32).
Fig. 3.
Construction of hTERT-HM stable lines overexpressing PR isoforms. A, hTERT-HM cells were used to construct stable lines overexpressing mock or Flag-tagged PR-A or PR-B by lentivirus followed with blasticidin selection. Immunoblotting assays with PR and Flag antibodies confirmed PR expression levels. WB, Western blot. B, hTERT (mock), hTERT (PR-A), and hTERT (PR-B) cells were treated with vehicle, 1 nm R5020, and/or 25 μm RU486 for 24 h. Cx43 mRNA and protein levels were measure by real-time qRT-PCR and immunoblotting assays.
To determine whether PSF mediates PR inhibition of Cx43 expression, we used lentivirus encoding short hairpin RNA (shRNA) to knock down endogenous PSF expression (shPSF). shPSF induced a greater than 50% reduction in PSF expression (mRNA and protein) compared with scrambled RNA (Supplemental Fig. 2). In hTERT(mock) cells, no differences in Cx43 mRNA levels were observed between scrambled and shPSF transduced cells treated with R5020 (P = 0.218) (Fig. 4A). However, the ability of R5020 to inhibit Cx43 mRNA levels was abolished in hTERT (PR-A) cells treated with shPSF (P = 0.0083). A similar observation was obtained in the hTERT (PR-B) stable lines. Changes in Cx43 mRNA levels correlated with Cx43 protein expression, indicating that PSF mediated de-repression of PR function contributes to increased CAP gene expression during labor. We also overexpressed PSF in all three hTERT-HM stable lines. Although no changes were observed in Cx43 mRNA and protein in hTERT(mock) cells, PSF overexpression strengthened PR inhibition on Cx43 expression in both hTERT (PR-A) (P = 0.0134) and hTERT (PR-B) cells (P = 0.0246) (Fig. 4B). In both gain- and loss-of-function studies, the impact on PSF protein and mRNA levels were confirmed by quantitative RT-PCR (qRT-PCR) and immnunoblotting assays (Supplemental Figs. 2 and 3). Interestingly, alterations in expression of PSF occurred without any change in expression of the PSF heterodimer, p54nrb, at both mRNA and protein levels, suggesting that the regulatory role of PSF on PR action is independent of p54nrb. This set of experiments led us to conclude that PSF modulates PR inhibition on CAP genes including Cx43, an effect that is independent of p54nrb.
Fig. 4.

PSF regulates PR-inhibitory action on Cx43 transcription. hTERT (mock), hTERT (PR-A), and hTERT (PR-B) cells were transiently infected by lentivirus encoding scramble (scr) or shPSF RNA for 48 h (A) or overexpressing mock and PSF for 24 h (B). Cells were then treated with vehicle or 1 nm R5020 for another 24 h. Cx43 mRNA and protein levels were measure by real-time qRT-PCR and immunoblotting assays. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; WB, Western blot.
To determine whether the effect of PSF on CAP gene expression is regulated at the level of gene transactivation, we assessed the effect of PSF on the promoter activity of two CAP genes, Cx43 and Cox2. Previously we showed that PR inhibited Cx43 promoter activity in hamster myometrial SHM (33). In the present study, we showed that both PRA and PRB inhibit Cx43 promoter activity in 293T cells in a dose-dependent fashion (Fig. 5, A and B). PSF knockdown by shRNA reversed PRA and PRB inhibition of Cx43 promoter activity. Similar results were observed in hTERT-HM cells (Fig. 5, C and D). Importantly, we found that PSF knockdown in both 293T (Supplemental Fig. 4A) and hTERT-HM (Supplemental Fig. 4B) cells also reversed PR inhibition of the Cox2 promoter. The efficiency of endogenous PSF knockdown in 293T cells is shown in Supplemental Fig. 4C. We concluded that reductions in PSF expression de-repress PR action on both Cx43 and Cox2 promoters.
Fig. 5.
PSF knockdown de-repressed PR action on Cx43 promoter in both 293T and hTERT-HM cells. Human embryonic kidney 293T cells (A and B) and hTERT-HM cells (C and D) were transfected with Cx43 luciferase reporter genes and treated with 1 nm R5020 for 48 h. Cells were also cotransfected with mock, PR-A, or PR-B as well as different doses of shRNA vector targeting PSF as indicated. Luciferase activities were measured and calibrated with Renilla luciferase activities. Values were shown as means ± sem from three independent experiments. *, P < 0.05; **, P < 0.01.
Mechanisms by which PSF regulates PR inhibition of CAP gene transcription
To investigate whether PSF regulates PR inhibition of CAP gene transcription through PSF-PR protein interactions, we performed coimmunoprecipitation assays. Protein lysates were prepared from nonpregnant, d 6–8 (early gestation) and d 13–16 (midgestation) pooled pregnant rat myometrial tissues. Lysates were precipitated with PR antibody, and the associated proteins were blotted with both PR and PSF antibodies, showing that PSF formed a protein complex with PR (Fig. 6A). We repeated coimmunoprecipitation assays using hTERT-HM stable lines with Flag tag antibody. Both PR-A and PR-B isoforms had similar ability to interact with PSF. In addition, both PR isoforms also coprecipitated PSF interacting proteins, Sin3A and HDAC1 (Fig. 6B). Interestingly, the activator protein-1 (AP-1) family member, c-Jun, was also in the protein complex. These data suggest that the transcriptional repressive function of PR is likely mediated through association with the Sin3A/HDAC1 repressor complex. PR may not interact directly with the target gene promoter through protein-DNA interaction but rather PR may be recruited to the target promoters through protein-protein interactions with other transcription factors such as AP-1 members.
Fig. 6.

PSF form protein complex with PR in myometrial cells. A, Protein lysates were collected from nonpregnant, d 6–8, and d 13–16 pooled pregnant rat myometrial tissues. Lysates were immunoprecipitated by either control IgG or PR antibody. The associated proteins were blotted with PSF and PR antibodies. B, Protein lysates collected from hTERT (mock), hTERT (PR-A), and hTERT (PR-B) cells were used to precipitated by either control IgG or Flag antibody. The associated proteins were blotted with PR, PSF, Sin3A, HDAC1, and c-Jun antibodies. IP, Immunoprecipitation; NP, nonpregnant; WB, Western blot.
We then performed ChIP assays to test whether PR is recruited to the AP-1 region of the Cx43 promoter. Flag antibody was used to precipitate cross-linked chromatins from hTERT-HM stable lines. Both PR isoforms can bind the proximal AP-1 region of the human Cx43 promoter (Fig. 7, A and B). Re-ChIP assay indicated that PR formed a complex with PSF and Sin3A that was recruited onto the AP-1 region through association with c-Jun (Fig. 7C). To demonstrate that PR mediated the recruitment of PSF and the Sin3A/HDAC complex to the AP-1 region, we treated hTERT-HM stable lines with either vehicle or R5020 and then performed ChIP assays (Fig. 7D). Eluted DNA fragments were used as templates to perform real-time PCR to quantify the intensity of PR, PSF, Sin3A, HDAC1, and c-Jun recruitments. In hTERT (mock) cells, ChIP assay signals with PR, PSF, Sin3A, and HDAC1 antibodies did not show any difference over control IgG, indicating these proteins were not present on the AP-1 region when PR was absent. The c-Jun signal was approximately 2-fold greater than the IgG signal and was not altered by R5020. Addition of R5020 induced 2- to 4-fold increase in the ChIP signals associated with PR, PSF, Sin3A, and HDAC1 antibodies in hTERT (PR-A) cells (P < 0.05). In contrast, c-Jun signals remained unchanged. These data demonstrate that the PSF/Sin3A/HDAC1 complex is recruited to the AP-1 region dependent on the presence of ligand-bound PR. Similar data showing recruitment of Sin3A/HDAC1 to the AP-1 region in hTERT (PR-B) cells further supports our data that both PR-A and PR-B exert a similar inhibitory impact on Cx43 transcription.
Fig. 7.
PR recruits PSF and Sin3A/HDAC1 complex to AP-1 proteins on the Cx43 promoter. A, Schematic diagram describes the structure of human Cx43 promoter. Two AP-1 binding sites were marked by their locations relative to the transcription initiation site. The locations of the primers used in the ChIP and re-ChIP assays were also marked. B, Cross-linked chromatin from hTERT (mock), hTERT (PR-A), and hTERT (PR-B) cells were used to perform ChIP assay with Flag antibody. C, Cross-linked chromatin from hTERT (PR-A) were used to first perform a ChIP assay with a Flag antibody and then re-ChIP assay with antibodies against control IgG, PSF, Sin3A, and c-Jun. The eluted DNA fragments were used as a template for PCR amplification of the proximal AP-1 region in Cx43 promoter. D, hTERT (mock), hTERT (PR-A), and hTERT (PR-B) cells were treated with vehicle or 1 nm R5020 for 24 h. ChIP assays were performed with antibodies against IgG, PR, PSF, Sin3A, HDAC1, and c-Jun. Real-time PCR was used to measure the ChIP signal, calculated as fold change over control IgG as described in Materials and Methods. E, Hamster myometrial cells (SHM) were transfected with the mouse Cx43wt promoter reporter or Cx43 promoter with AP-1 site point mutation (Cx43m), together with mock, PR-A, or PR-B expression vector. ChIP assays were performed with PR, PSF, and c-Jun antibodies. Proximal AP-1 regions were amplified with primers specifically to mouse Cx43 promoter deriving from transfected reporter vectors. Values are shown as means ± sem from three to five independent experiments. *, P < 0.05.
The AP-1 region within the Cx43 promoter also contains binding sites for other transcription factors with which PR and its PSF/Sin3A/HDAC complex might associate. To exclude this possibility, we transfected a luciferase reporter plasmid, containing mouse wild type Cx43 promoter or Cx43 promoter carrying a point mutation at the AP-1 site (Cx43m) in the Cx43 promoter into hamster SHM cells. Mock, PR-A, and PR-B expression vectors were cotransfected (Fig. 7E). ChIP assays were performed with PR, PSF, and c-Jun antibodies followed by quantitative PCR using primers that amplify the AP-1 region only from the transfected mouse Cx43 promoter. The c-Jun signal was 3-fold greater than the IgG signal in cells transfected with the Cx43 wild-type (Cx43wt) promoter but remained the same as the IgG signal in cells transfected with Cx43m promoter, indicating that c-Jun interacts with the AP-1 site. The ChIP signal with PR antibody was 3-fold, whereas with PSF antibody it was 2-fold greater than the IgG signal (P < 0.05) from cells transfected with the Cx43wt promoter and PR-A expression vector. PR-B showed a greater recruitment (10-fold) than PR-A, and this was associated with greater PSF recruitment (4-fold) to the AP-1 region within the Cx43wt promoter (P < 0.05). All of the PR and PSF signals diminished when the Cx43m promoter was cotransfected. These data confirm that the recruitment of PR/PSF to Cx43 promoter is dependent on the presence of the AP-1 site and is mediated through interaction with AP-1 family members. Together these data lead us to conclude that PR represses Cx43 transcription by recruiting PSF and the Sin3A/HDAC complex. PR binds to the Cx43 promoter through a protein-protein interaction with AP-1 members.
Discussion
Progesterone maintains the quiescence of the myometrium during pregnancy. But the precise mechanisms of this action have not been defined. The actions of P4 are mediated by its nuclear receptor (PR), and recent investigations have revealed that coregulators control PR function. In the present study, we demonstrated that the coregulator, PSF, mediates PR repression of CAP genes in the pregnant myometrium. Our data suggest that the decrease in PSF protein at term impairs the repressive function of PR on CAP gene transcription (Fig. 8), contributing to increased CAP gene expression and the initiation of labor.
Fig. 8.
A model for the regulation of CAP gene transcription by PSF/PR during pregnancy and labor (see text for details).
During pregnancy the myometrium is quiescent and unresponsive, enabling the developing fetus to develop and grow within the uterine cavity. With the approach of labor, the phenotype of the myometrium undergoes a dramatic switch to a state in which the myocytes become excitable, highly responsive to uterotonic agonists, and develop enhanced cell-cell communication (34). We have previously reported that this switch in phenotype of the myometrium is regulated by both mechanical stretch on the uterine wall (due to fetal growth) and changes in circulating hormones (primarily increased levels of estrogen and decreased levels of progesterone) (35). Here we show that PSF levels are high during the quiescent phase of pregnancy (in association with elevated P4 levels) and then dramatically decrease in late pregnancy with the approach of labor as myometrial stretch and estrogen levels increase. We suggest that at term the increased levels of mechanical stretch of the myometrium and of circulating estrogen contribute to the decreased expression of PSF and that the consequences of reduced PSF protein expression is to de-repress PR-mediated inhibition of CAP gene expression (Fig. 8). In these experiments, we examined effects of E2 and E2+P4, but not P4 in the absence of E2, because E2 is required for the expression of PR, and lack of effect of P4 alone might simply reflect lack of PR (36).
Our data provide further insight into the mechanisms by which PR represses CAP gene transcription in the myometrium. Rather than binding to putative PRE in the target gene promoters, our data indicate that PR is recruited to CAP gene promoters through protein interactions with transcription factors such as AP-1. In turn, PR recruits corepressors including PSF, Sin3A, and HDAC1 to inhibit CAP gene transcription. Although the artificial 3 × PRE or MMTV promoters have been used extensively to study PR function, this system does not fully represent the intrinsic modulation of myometrial CAP gene transcription by PR. Most importantly, ligand-activated PR enhances 3 × PRE or MMTV promoter activities, whereas CAP gene transcription is usually suppressed by ligand-bound PR. In addition, no functional consensus PRE had been identified to interact directly with PR in CAP gene promoters. Although some half-site PRE have been suggested to be the docking sites, PR cannot be stably recruited onto these sites until it associates with other transcription factors. In contrast, almost all of the CAP gene promoters possess binding sites for AP-1, specificity protein-1, or nuclear factor-κB.
We found no differences in the inhibitory actions of PR-A and PR-B isoforms on Cx43 transcription. These data contrast with evidence showing that an increased PR-A to PR-B ratio at term reduces progesterone responses and contributes to the functional withdrawal of P4. Those studies were largely based on the PR/PRE-luciferase functional assays, and it is possible that this assay system may not fully reflect the PR regulation of native CAP genes such as Cx43 (31, 32). Studies using transgenic mice indicate that animals with specific PR-B knockout and with normal PR-A expression go through regular pregnancy and parturition, whereas PR-A-specific knockout mice are infertile due to defects of ovary folliculogenesis, implantation, and decidualization (37). These studies suggest that PR-A, rather than PR-B, plays a role in parturition. Furthermore, microarray studies in other cell contexts such as breast cancer indicate that PR-A and PR-B have distinct target genes with some overlap. In human myometrial hTERT-HM cells, both PR-A and PR-B repress Cx43 transcription, indicating that Cx43 is one of the common target genes regulated by both PR isoforms.
PSF protein level reaches the lowest level near term and is subsequently up-regulated in the postpartum period. This expression pattern is consistent with PSF mRNA profile as we reported (22) but with a 2-d period of delay. This time gap between up-regulation of PSF mRNA (d 22) and protein levels (1 d postpartum) may possibly reflect the time required for PSF mRNA to be translated. But it is more likely that differential mechanisms regulate PSF mRNA and protein levels. The latter case is supported by our data that PSF mRNA and protein levels are not consistently regulated by E2 or stretch (data not shown). Nevertheless, it is the PSF in protein form that regulates PR signaling in myometrium.
In summary, our study suggests new mechanisms by which progesterone maintains myometrial quiescence during pregnancy and by which this action is blocked to allow the initiation of labor at term. First, we show that ligand-bound PR interacts with CAP gene promoters through AP-1 transcription factors. Second, the recruitment of PSF and its associated repressor complexes enables PR to repress CAP gene transcription. Third, we show that PSF expression decreases at the end of pregnancy as a result of increased mechanical stretch of the myometrium and increased circulating levels of estrogen. We suggest that the decrease in PSF protein expression at term de-represses PR inhibition on CAP gene transcription and thereby may contribute to a functional withdrawal of progesterone at term labor.
Acknowledgments
We thank Dr. Philip Bennett for sharing the Cox2 luciferase reporter and Dr. Jennifer Condon for the hTERT-HM cell line.
This work was supported by Operating Grants MOP-111148 (to S.Ly., J.R.G.C., and X.D.) and MOP-97934 (to X.D.) from the Canadian Institutes of Health Research.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP-1
- Activator protein-1
- CAP
- contraction-associated protein
- ChIP
- chromatin immunoprecipitation
- Cox-2
- cyclooxygenase-2
- Cx43
- connexin 43
- Cx43m
- Cx43 mutant
- Cx43wt
- Cx43 wild type
- E2
- 17β-estradiol
- HDAC
- histone deacetylase
- hTERT-HM
- human telomerase immortalized myometrial cells
- OVX
- ovariectomized
- P4
- progesterone
- PR
- progesterone receptor
- PRE
- progesterone response element
- PSF
- polypyrimidine tract binding protein-associated splicing factor
- SHM
- Syrian hamster myometrium
- shRNA
- short hairpin RNA
- shPSF
- short hairpin PSF expression
- qRT-PCR
- quantitative RT-PCR
- Sin3A
- Sin3 homolog A.
References
- 1. Mackenzie R , Walker M , Armson A , Hannah ME. 2006. Progesterone for the prevention of preterm birth among women at increased risk: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol 194:1234–1242 [DOI] [PubMed] [Google Scholar]
- 2. Norwitz ER , Caughey AB. 2011. Progesterone supplementation and the prevention of preterm birth. Rev Obstet Gynecol 4:60–72 [PMC free article] [PubMed] [Google Scholar]
- 3. Allen WM. 1935. The isolation of crystalline progestin. Science 82:89–93 [DOI] [PubMed] [Google Scholar]
- 4. Challis JRG , Matthews SG , Gibb W , Lye SJ. 2000. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 21:514–550 [DOI] [PubMed] [Google Scholar]
- 5. Challis JR , Lye SJ. 1986. Parturition. Oxf Rev Reprod Biol 8:61–129 [PubMed] [Google Scholar]
- 6. Garfield RE , Hertzberg EL. 1990. Cell-to-cell coupling in the myometrium: Emil Bozler's prediction. Prog Clin Biol Res 327:673–681 [PubMed] [Google Scholar]
- 7. Tsuboi K , Sugimoto Y , Iwane A , Yamamoto K , Yamamoto S , Ichikawa A. 2000. Uterine expression of prostaglandin H2 synthase in late pregnancy and during parturition in prostaglandin F receptor-deficient mice. Endocrinology 141:315–324 [DOI] [PubMed] [Google Scholar]
- 8. Arthur P , Taggart MJ , Mitchell BF. 2007. Oxytocin and parturition: a role for increased myometrial calcium and calcium sensitization? Front Biosci 12:619–633 [DOI] [PubMed] [Google Scholar]
- 9. Negishi M , Sugimoto Y , Ichikawa A. 1995. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim Biophys Acta 1259:109–119 [DOI] [PubMed] [Google Scholar]
- 10. Garfield RE , Gasc JM , Baulieu EE. 1987. Effects of the antiprogesterone RU 486 on preterm birth in the rat. Am J Obstet Gynecol 157:1281–1285 [DOI] [PubMed] [Google Scholar]
- 11. Stenlund PM , Ekman G , Aedo AR , Bygdeman M. 1999. Induction of labor with mifepristone—a randomized, double-blind study versus placebo. Acta Obstet Gynecol Scand 78:793–798 [PubMed] [Google Scholar]
- 12. Ou CW , Orsino A , Lye SJ. 1997. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology 138:5398–5407 [DOI] [PubMed] [Google Scholar]
- 13. Pointis G , Rao B , Latreille MT , Mignot TM , Cedard L. 1981. Progesterone levels in the circulating blood of the ovarian and uterine veins during gestation in the mouse. Biol Reprod 24:801–805 [DOI] [PubMed] [Google Scholar]
- 14. Mendelson CR. 2009. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol 23:947–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Condon JC , Jeyasuria P , Faust JM , Wilson JW , Mendelson CR. 2003. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA 100:9518–9523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giangrande PH , Pollio G , McDonnell DP. 1997. Mapping and characterization of the functional domains responsible for the differential activity of the A and B isoforms of the human progesterone receptor. J Biol Chem 272:32889–32900 [DOI] [PubMed] [Google Scholar]
- 17. Smith CL , O'Malley BW. 2004. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 25:45–71 [DOI] [PubMed] [Google Scholar]
- 18. Hardy DB , Janowski BA , Corey DR , Mendelson CR. 2006. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-κB activation of cyclooxygenase 2 expression. Mol Endocrinol 20:2724–2733 [DOI] [PubMed] [Google Scholar]
- 19. Allport VC , Pieber D , Slater DM , Newton R , White JO , Bennett PR. 2001. Human labour is associated with nuclear factor-κB activity which mediates cyclo-oxygenase-2 expression and is involved with the 'functional progesterone withdrawal.' Mol Hum Reprod 7:581–586 [DOI] [PubMed] [Google Scholar]
- 20. Merlino AA , Welsh TN , Tan H , Yi LJ , Cannon V , Mercer BM , Mesiano S. 2007. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab 92:1927–1933 [DOI] [PubMed] [Google Scholar]
- 21. Leite RS , Brown AG , Strauss JF. 2004. Tumor necrosis factor-α suppresses the expression of steroid receptor coactivator-1 and -2: a possible mechanism contributing to changes in steroid hormone responsiveness. FASEB J 18:1418–1420 [DOI] [PubMed] [Google Scholar]
- 22. Dong X , Shylnova O , Challis JR , Lye SJ. 2005. Identification and characterization of the protein-associated splicing factor as a negative co-regulator of the progesterone receptor. J Biol Chem 280:13329–13340 [DOI] [PubMed] [Google Scholar]
- 23. Mathur M , Tucker PW , Samuels HH. 2001. PSF is a novel corepressor that mediates its effect through Sin3A and the DNA binding domain of nuclear hormone receptors. Mol Cell Biol 21:2298–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dong X , Sweet J , Challis JR , Brown T , Lye SJ. 2007. Transcriptional activity of androgen receptor is modulated by two RNA splicing factors, PSF and p54nrb. Mol Cell Biol 27:4863–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shynlova O , Tsui P , Dorogin A , Langille BL , Lye SJ. 2007. Insulin-like growth factors and their binding proteins define specific phases of myometrial differentiation during pregnancy in the rat. Biol Reprod 76:571–578 [DOI] [PubMed] [Google Scholar]
- 26. Jaffer S , Shynlova O , Lye S. 2009. Mammalian target of rapamycin is activated in association with myometrial proliferation during pregnancy. Endocrinology 150:4672–4680 [DOI] [PubMed] [Google Scholar]
- 27. Shynlova O , Tsui P , Dorogin A , Chow M , Lye SJ. 2005. Expression and localization of α-smooth muscle and γ-actins in the pregnant rat myometrium. Biol Reprod 73:773–780 [DOI] [PubMed] [Google Scholar]
- 28. Peacock JW , Palmer J , Fink D , Ip S , Pietras EM , Mui AL , Chung SW , Gleave ME , Cox ME , Parsons R , Peter ME , Ong CJ. 2009. PTEN loss promotes mitochondrially dependent type II Fas-induced apoptosis via PEA-15. Mol Cell Biol 29:1222–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Listerman I , Sapra AK , Neugebauer KM. 2006. Cotranscriptional coupling of splicing factor recruitment and precursor messenger RNA splicing in mammalian cells. Nat Struct Mol Biol 13:815–822 [DOI] [PubMed] [Google Scholar]
- 30. Shynlova O , Tsui P , Dorogin A , Lye SJ. 2008. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol 181:1470–1479 [DOI] [PubMed] [Google Scholar]
- 31. Petrocelli T , Lye SJ. 1993. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology 133:284–290 [DOI] [PubMed] [Google Scholar]
- 32. Zhao K , Kuperman L , Geimonen E , Andersen J. 1996. Progestin represses human connexin43 gene expression similarly in primary cultures of myometrial and uterine leiomyoma cells. Biol Reprod 54:607–615 [DOI] [PubMed] [Google Scholar]
- 33. Dong X , Yu C , Shynlova O , Challis JR , Rennie PS , Lye SJ. 2009. p54nrb is a transcriptional corepressor of the progesterone receptor that modulates transcription of the labor-associated gene, connexin 43 (Gja1). Mol Endocrinol 23:1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lye SJ , Tsui P , Dong X , Mitchell J , Dorogin A , MacPhee D , Oldenhoff A , Langille BL , Challis JR , Shynlova O. 2007. Myometrial programming: a new concept underlying the regulation of myometrial function during pregnancy. In: Preterm Birth. , Felice P , Strauss JF , Gabbe SG , Weiss G, eds. London: Informa Healthcare; 1–18 [Google Scholar]
- 35. Shynlova O , Tsui P , Jaffer S , Lye SJ. 2009. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol 144(Suppl 1):S2–S10 [DOI] [PubMed] [Google Scholar]
- 36. Milgrom E , Thi L , Atger M , Baulieu EE. 1973. Mechanisms regulating the concentration and the conformation of progesterone receptor(s) in the uterus. J Biol Chem 248:6366–6374 [PubMed] [Google Scholar]
- 37. Conneely OM , Mulac-Jericevic B , Lydon JP. 2003. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids 68:771–778 [DOI] [PubMed] [Google Scholar]