Abstract
Pilocytic astrocytoma is a slowly growing neoplasia that represents the most frequent cerebral tumor in pediatric age. Malignant transformation is rare and it is usually related to previous radiotherapy. The authors describe a case of a spontaneous malignant transformation of a pilocytic astrocytoma. A 3-year-old boy was diagnosed with a cerebellar hemisphere tumor. He was submitted to a complete excision of the lesion, and histological findings were consistent with pilocytic astrocytoma. It was negative for p53. Twelve years later he presented with a local recurrence. Histopathological diagnosis was glioblastoma and it was positive for p53. Death from disease progression occurred 16 months after the diagnosis of glioblastoma. This case suggests that patients with pilocytic astrocytoma need closer follow-up and further genotypic studies in order to provide clues to clinical behavior. Such understanding can allow us to stratify treatment accordingly and to proceed to more aggressive treatment when necessary.
Keywords: low-grade gliomas, pilocytic astrocytoma, malignant transformation
Pilocytic astrocytoma accounts for about 25% of all pediatric brain tumors.1 It develops during the first 2 decades of life and its most common locations are the cerebellum and the hypothalamic/optical pathways. On magnetic resonance imaging, pilocytic astrocytoma is generally a well-demarcated lesion often with both cystic and nodular components that enhance contrast heterogeneously. Histologically, it has a characteristic pattern of dense fibrillary areas interspersed with microcysts (biphasic pattern). Rosenthal fibers are frequently encountered and are a useful pathologic hallmark.2 The standard initial approach is surgical resection. However, complete surgical resection is not always feasible, namely, in locations like the diencephalon or the brain stem. Chemotherapy has been proven to be the standard of care for such cases and preferable to radiotherapy that does not improve prognosis and has higher long-term morbidity with cognitive and endocrine effects.3 These patients have an excellent prognosis, with 10-year survival rate of up to 80%, even with incomplete tumor resection.4,5 Sometimes an unfavorable evolution can occur, such as malignant transformation, local recurrence, multicentric disease, or leptomeningeal dissemination.6–9 Malignant transformation occurs in less than 5% of cases and is more frequent in patients who have been previously irradiated.10 In this article, the authors describe a case of spontaneous malignant transformation of a pilocytic astrocytoma of the cerebellum after a period of 12 years without previous radiotherapy.
Case Summary
A 15-year-old boy presented initially to an outside hospital at the age of 3 years. He was the third child of nonconsanguineous parents. There was no known family history of neurological, oncological, or inherited diseases. His previous personal history was unremarkable. At the age of 3 years, he was diagnosed with a cystic tumor of the right cerebellar hemisphere by computerized tomography scan and he underwent a complete surgical excision of the lesion. Histological examination demonstrated findings consistent with the diagnosis of pilocytic astrocytoma. Although slight nuclear polymorphism and slight endothelial proliferation were observed, necrosis, mitosis, and microvascular proliferation were absent, there was a low Ki-67 proliferative index, and p53 was negative in immunostaining (less than 10% of cancer cells with positive nuclear staining; Figure 1A-C).
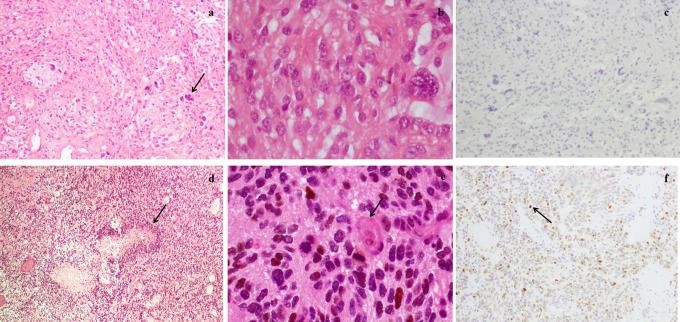
Figure 1.
Histopathology: (A) Surgical specimens from the first operation stained with hematoxylin-eosin (×100), demonstrating the fasciculate pattern, mucoid regions, and bipolar neoplastic cells and some giant cells (arrow). B, Stained with hematoxylin-eosin (×400), absence of mitosis and cells with nuclear anaplasia. C, Negative representative results of p53 immunohistochemical staining. D, Surgical specimens from the second operation stained with hematoxylin-eosin (×100), showing hypercellularity, with small and anaplastic cells and necrosis with a palisade pattern (arrow). E, Stained with hematoxylin-eosin (×400), with mitosis (arrow) and endothelial proliferation. F, Positive representative results of p53 immunohistochemical staining (arrow).
Comparative genomic hybridization analysis is shown in Table 1. Adjuvant therapy was not administered. After surgery, clinical and image surveillance did not show evidence of relapse. He was lost to follow-up at the age of 10 years. Five years later, he presented with a 3-month history of intermittent headaches and vomiting. At the time of the diagnosis, he presented with an altered state of consciousness, ataxia, and dysmetria. Evaluation with a computed tomography scan revealed a large cystic lesion with a hemorrhagic component located in the right cerebellar hemisphere. This mass enhanced contrast and caused obstructive hydrocephalus (Figure 2A and B).
Table 1.
Comparison of Comparative Genomic Analysis Between the First Lesion, Pilocytic Astrocytoma and the Second, Glioblastoma.
| Comparative genomic analysis | |
|---|---|
| Pilocytic astrocytoma | Total gain of chromosomes 17 and 19; partial gain of the regions 1q21-q23, 2q34-q35; 7q11.2, 7q21.3-q22, 7q32-q34, 10p13-p12, 11p12-q13.3, 12p13-p12.3, 12q12-q13, 12q21.3-q24.2, 16p13.2-p12, 16q12.1-q12.2, 16q22, 20q11.2-q13.2 and partial loss of the regions 1p31.1-p22, 2q21, 9p21, 11q14 |
| Glioblastoma | Partial gain of the regions: 1p21, 1q24-32, 2p25-q35, 3p25-p12.3, 4p14-q34, 5p15.3-q33, 5p15.3-p13, 6p22, 6p21.1, 7p21-q34, 8p23.1-p12, 9p24-p22, 9p13.1, 9q21.3-q34.1, 10q11.2-q24.1, 10q24.3, 11p15.3-p11.2, 11p13.1, 11q13.3-q23.3, 12p13-q22, 13q12-q33, 14q12-q24, 17q21-q25, 18q12-q23, 20p12, 20q12-q13 and partial loss of the regions: 1p36.1, 1p33-p32.3, 4p15.3, 6q12-q27, 8q11.2-q24.3, 16q22.3-q23, 19q13.1, 22q11.2-q13.1 |
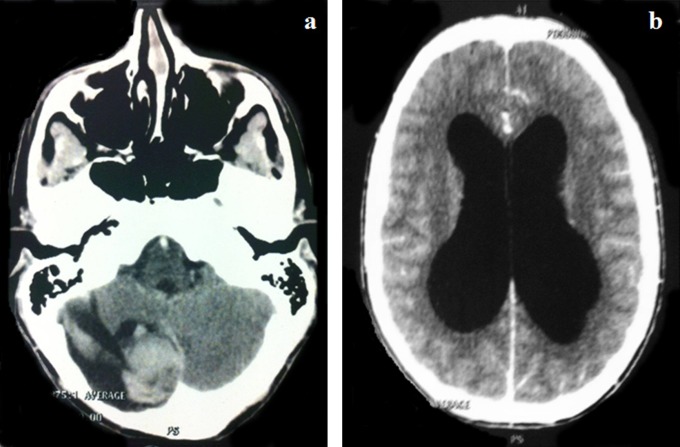
Figure 2.
Axial postcontrast computed tomography scan showing the local relapse, 12 years after the pilocytic astrocytoma diagnosis. A, Cystic right cerebellar tumor with a hemorrhagic component. B, Hydrocephalus caused by the lesion.
The patient underwent his second surgery with a gross total resection of the lesion. The surgery and the postoperative period were uneventfully, and the magnetic resonance imaging after this second surgery did not show any signs of residual lesion or medullar lesions. In contrast to the pathology, 12 years prior, histopathological findings were compatible with glioblastoma, showing anaplastic and pleomorphic cells, necrosis, endothelial proliferation, a high proliferative index with Ki-67 immunostaining, and positive p53 (more than 50% of cancer cells with positive nuclear staining; Figure 1D-F).
Comparative genomic hybridization analysis of the second lesion is shown in Table 1. BRAF gene exon 15 mutations were not found in either the first or the second tumor. He received adjuvant therapy with high doses of methotrexate, cisplatin, etoposide, ifosfamide, and vincristine plus focal radiotherapy (59.4 Gy). Two months after the end of this therapy, his magnetic resonance imaging showed dorsal leptomeningeal seeding (Figure 3).
Figure 3.

Axial postcontrast T1-weighted magnetic resonance imaging after adjuvant therapy showing abnormal leptomeningeal contrast enhancement.
He was then submitted to 5 courses of temozolomide plus focal radiotherapy on the symptomatic dorsal lesion. Two months after the fifth course of temozolomide, the patient presented a rapid neurologic deterioration. Neuroimaging showed progressive intracranial and spinal leptomeningeal seeding and hemorrhage along the ventricular system. Death from disease progression occurred 16 months after the diagnosis of glioblastoma and 14 years after the diagnosis of pilocytic astrocytoma.
Discussion
This case illustrates the potential risk of evolution of low-grade gliomas into malignant gliomas. Its underlying mechanism is still unknown. In the majority of published case reports, patients had received previous radiotherapy.4,9,11,12 Therefore, this therapy has been considered a key factor for malignant changes, despite there being no available information about cumulative incidence, risk factors, and molecular abnormalities.7 Malignant transformation without previous radiotherapy has also been reported but occasionally in a pediatric age and in locations other than the cerebellum.13–18
The factors that have been consistently associated with tumor recurrence or malignant degeneration are preoperative contrast enhancement imaging that often suggests a high-grade glioma, but it can also be present in 15% to 40% of patients with low-grade gliomas; tumor size, since the risk of recurrence increases 1.3 times for each centimeter increase in size; and subtotal resection and molecular abnormalities.4
Magnetic resonance imaging is an important diagnostic tool in detecting gliomas in early stages of their natural history once the degree of contrast enhancement has been used as an indicator of malignancy, and the absence of contrast enhancement may be suggestive of low-grade gliomas. However, some nonenhancing lesions can be malignant.19 Thus, radiological features are one component of a combination of criteria used to predict a response to therapy and outcome. Other criteria include clinical findings (age of the patient, neurologic performance status, and tumor location), extent of surgical resection, histological features, proliferation indices, and molecular characterization.
Regarding the molecular characterization of low-grade gliomas, many numerical and structural abnormalities were identified, with chromosomes 7, 8, and 17 being the most frequently affected.20 However, traditional karyotype analysis has been unrevealing in multiple studies, with chromosome 7 gain the only consistent finding.3 The deletion of 17p (p53 site) is associated with rapid recurrence of the disease, since p53 regulates cell cycle arrest, DNA repair, apoptosis, autophagy, senescence, suppression of pluripotency, and inhibition of stem cell self-renewal.21,22 The p53 tumor suppressor gene is frequently mutated or deleted in the early stages of the formation of gliomas. One hypothesis is that high-grade gliomas could arise and recur because of malignant transformation of neural stem cells residing in protected niche areas.22 TP53 mutations in children are restricted to 5% to 10% of low-grade gliomas that undergo malignant transformation.7 More recently, multiple groups have reported a small nonrandom duplication in the 7q34 region containing a BRAF-KIAA1549 gene fusion.23,24 This gene is present in 60% to 80% of patients with sporadic pilocytic astrocytoma and is associated with supratentorial location, recurrence, and incomplete resection.25,26
The present case report is of interest for the following reasons. First, this is the first description of a spontaneous malignant transformation of a cerebellar low-grade glioma in children. Second, p53 was expressed in the secondary tumor but not in the predecessor tumor, which confirms the role of p53 in the transformation process. The comparative genomic hybridization analysis of the pilocytic astrocytoma showed the most consistent finding on this type of tumor, which is gain of chromosome 7. None of the other molecular abnormalities previously described were identified. Since the features of low-grade gliomas appear to be closely related to the molecular and cellular biologic characteristics, large series of children with malignant transformation of low-grade gliomas involving clinical, molecular, and outcome analyses are warranted to elucidate the pathophysiologic mechanisms.
In conclusion, review of the literature and the current case support the need for long-term follow-up in patients diagnosed with low-grade gliomas. Molecular and biological variability can exist, therefore further genotypic studies are needed in order to predict which low-grade gliomas will progress to high-grade gliomas and can benefit from more aggressive adjuvant therapy.
Acknowledgments
All work occurred in Lisbon, Portugal. The authors acknowledge Lúcia Roque, MD, from the Department of Genetics and Manuela Mafra, MD, from the Department of Neuropathology of Portuguese Oncology Institute of Lisbon for helpful comments on the manuscript.
Footnotes
Author Contributions: JC wrote the first draft of the article and made further editions and editing. SN was the treating pediatric neurologist and provided patient information. DS edited the work and mentored the authors. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: The authors certify that the procedures followed in the description of this case were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. The authors also certify that informed consent was obtained from the legal representative of the child included in the study.
References
- 1. Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17 (9):503–511. [DOI] [PubMed] [Google Scholar]
- 2. Kleinman GM, Schoene WC, Walshe TM, Richardson EP. Malignant transformation in benign cerebellar astrocytoma. J Neurosurg. 1978;49 (1):111–118. [DOI] [PubMed] [Google Scholar]
- 3. Sievert AJ, Fisher MJ. Pediatric low grade gliomas. J Child Neurol. 2009;24 (11):1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rotariu D, Gaivas S, Faiyad Z, et al. Malignant transformation of low grade gliomas into gliobastoma a series of 10 cases and review of the literature. Romanian Neurosurg. 2010;4(17):403–412. [Google Scholar]
- 5. Armstrong GT, Conklin HM, Huang S, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13 (2):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez AO, Herrero RS, Tejeiro MG, Martinez TZ. Spontaneous malignant transformation of a supratentorial pilocytic astrocytoma. Neurocirurgia. 2010;21 (3):245–252. [DOI] [PubMed] [Google Scholar]
- 7. Broniscer A, Baker SJ, West AN, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2006;25 (6):682–689. [DOI] [PubMed] [Google Scholar]
- 8. Gajjar A, Bhargava R, Jenkins JJ, et al. Low-grade astrocytoma with neuraxis dissemination at diagnosis. J Neurosurg. 1995;83 (1):67–71. [DOI] [PubMed] [Google Scholar]
- 9. Bowers DC, Krause TP, Aronson LJ, et al. Second surgery for recurrent pilocytic astrocytoma in children. Pediatr Neurosurg. 2001;34 (5):229–234. [DOI] [PubMed] [Google Scholar]
- 10. Dirks PB, Jay V, Becker LE, et al. Development of anaplastic changes in low-grade astrocytoma in childhood. Neurosurgery. 1994;34 (1):68–78. [PubMed] [Google Scholar]
- 11. Van der Wal EJ, Azzarelli B, Edwards-Brown M. Malignant transformation of a chiasmatic pilocytic astrocytoma in a patient with diencephalic syndrome. Pediatr Radiol. 2003;33 (3):207–210. [DOI] [PubMed] [Google Scholar]
- 12. Pruthi SK, Chakraborti S, Naik R, Ballal CK. Pilomyxoid astrocytoma with high proliferation index. J Pediatr Neurosci. 2013;8 (3):243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winograd E, Pencovich N, Yalon M, et al. Malignant transformation in pediatric spinal intramedullary tumors: case-based update. Childs Nerv Syst. 2012;28 (10):1679–1686. [DOI] [PubMed] [Google Scholar]
- 14. Unal E, Koksal Y, Cimen O, Paksoy Y, Tavli L. Malignant glioblastomatous transformation of a low-grade glioma in a child. Childs Nerv Syst. 2008;24 (12):1385–1389. [DOI] [PubMed] [Google Scholar]
- 15. Zoeller GK, Brathwaite CD, Sandberg DI. Malignant transformation of an optic pathway glioma without prior radiation therapy. J Neurosurg Pediatr. 2010;5 (5):507–510. [DOI] [PubMed] [Google Scholar]
- 16. Privett BJ, Liubinas SV, Tsui A, Gonzales M, Lo P. Pilocytic astrocytoma with neoplastic gemistocytes undergoing spontaneous transformation to glioblastoma multiforme without prior radiotherapy. J Clin Neurosci. 2011;18 (5):705–707. [DOI] [PubMed] [Google Scholar]
- 17. Peters KB, Cummings TJ, Gururangan S. Transformation of juvenile pilocytic astrocytoma to anaplastic pilocytic astrocytoma in patients with neurofibromatosis type I. J Pediatr Hematol Oncol. 2011;33 (5):198–201. [DOI] [PubMed] [Google Scholar]
- 18. Paraskevopoulos D, Patsalas I, Karkavelas G, Foroglou N, Magras I, Selviaridis P. Pilomyxoid astrocytoma of the cervical spinal cord in a child with rapid progression into glioblastoma: case report and literature review. Childs Nerv Syst. 2011;27 (2):313–321. [DOI] [PubMed] [Google Scholar]
- 19. Frazier JL, Johnson MW, Burger PC, Weingart JD, Quinones-Hinojosa A. Rapid malignant transformation of low-grade astrocytomas: report of 2 cases and review of the literature. World Neurosurg. 2010;73 (1):53–62. [DOI] [PubMed] [Google Scholar]
- 20. Qaddoumi I, Sultan I, Broniscer A. Review of Pediatric low-grade gliomas and the need for new options for therapy - Why and how? Cancer Biol Ther. 2009;8 (1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Willert JR, Daneshvar L, Sheffield VC, Cogen PH. Deletion of chromosome arm 17p DNA sequences in pediatric high-grade and juvenile pilocytic astrocytomas. Genes Chromosomes Cancer. 1995;12 (3):165–172. [DOI] [PubMed] [Google Scholar]
- 22. Hede S, Nazarenko I, Nist´er M, Lindström MS. Novel perspectives on p53 function in neural stem cells and brain tumors. J Oncol. 2011;2011:852970 doi:10.1155/2011/852970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sievert AJ, Jackson EM, Gai X, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19(3):449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sadighi Z, Slopis J. Pilocytic astrocytoma: a disease with evolving molecular heterogeneity. J Child Neurol. 2013;28(5):625–632. [DOI] [PubMed] [Google Scholar]
- 25. Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118(5):1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacob K, Albrecht S, Sollier C, et al. Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. Br J Cancer. 2009;101(4):722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]