Abstract
Background:
The integrity of the brain histaminergic system is necessary for the unfolding of homeostatic and cognitive processes through the recruitment of alternative circuits with distinct temporal patterns. We recently demonstrated that the fat-sensing lipid mediator oleoylethanolamide indirectly activates histaminergic neurons to exerts its hypophagic effects. The present experiments investigated whether histaminergic neurotransmission is necessary also for the modulation of emotional memory induced by oleoylethanolamide in a contextual fear conditioning paradigm.
Methods:
We examined the acute effect of i.p. administration of oleoylethanolamide immediately posttraining in the contextual fear conditioning test. Retention test was performed 72 hours after training. To test the participation of the brain histaminergic system in the cognitive effect of oleoylethanolamide, we depleted rats of brain histamine with an i.c.v. injection of alpha-fluoromethylhistidine (a suicide inhibitor of histidine decarboxylase) or bilateral intra-amygdala infusions of histamine H1 or H2 receptor antagonists. We also examined the effect of oleoylethanolamide on histamine release in the amygdala using in vivo microdialysis.
Results:
Posttraining administration of oleoylethanolamide enhanced freezing time at retention. This effect was blocked by both i.c.v. infusions of alpha-fluoromethylhistidine or by intra-amygdala infusions of either pyrilamine or zolantidine (H1 and H2 receptor antagonists, respectively). Microdialysis experiments showed that oleoylethanolamide increased histamine release from the amygdala of freely moving rats.
Conclusions:
Our results suggest that activation of the histaminergic system in the amygdala has a “permissive” role on the memory-enhancing effects of oleoylethanolamide. Hence, targeting the H1 and H2 receptors may modify the expression of emotional memory and reduce dysfunctional aversive memories as found in phobias and posttraumatic stress disorder.
Keywords: emotional memory, in vivo microdialysis, pyrilamine, zolantidine, alpha-fluoromethylhistidine
Significance Statement
The amygdala elaborates emotionally salient stimuli to provide the appropriate information to be memorized for adequate behavioral responses. Histaminergic neurons have important modulatory influences on memory formation; H1 and H2 histamine receptors in the basolateral amygdala (BLA) facilitate memory consolidation of aversive memories. This study provides evidence that the brain histaminergic system has a permissive role for the unfolding of the memory-enhancing effects of oleoylethanolamide (OEA). OEA, a lipid mediator that is released following the ingestion of fat, when administered posttraining in rats induced and exaggerated emotional response in the contextual fear conditioning paradigm that was abrogated by the inhibition of histaminergic neurotransmission or the local blockade of either H1 or H2 receptors in the BLA. Hence, targeting these receptors with classical, clinically approved antihistamines may modify the expression of emotional memory and reduce dysfunctional aversive memories as found in phobias and posttraumatic stress disorder.
Introduction
Good memory consolidation of fear tasks requires emotional arousal and the engagement of the basolateral amygdala (BLA) (Izquierdo et al., 2016). Emotionally relevant experiences activate the histaminergic system (Contreras et al., 2016) that is crucial for the consolidation, retrieval, and extinction of such memories with different anatomical and temporal patterns (Fiorenza et al., 2012; Benetti et al., 2015; Fabbri et al., 2016). In particular, activation of histamine H1 or H2 receptors in the BLA (but also in the hippocampus, infralimbic, and ventromedial prefrontal corteces) facilitates memory consolidation of inhibitory avoidance and contextual fear conditioning, whereas their specific antagonists have an opposite effect in the consolidation of different tasks (Cangioli et al., 2002; Giovannini et al., 2003; Silva et al., 2006; Benetti and Izquierdo 2013).
The tuberomamillary nucleus (TMN) in the posterior hypothalamus is the only source of histaminergic fibers that reaches virtually all brain regions (Panula et al., 1989); consequently, the brain histaminergic system regulates several brain functions, including alertness (Takahashi et al., 2006) and food intake (Provensi et al., 2015). In this respect, we recently showed that a small group of histaminergic neurons in the TMN respond to the peripheral administration of oleoylethanolamide (OEA, a dietary fat-induced satiety factor) (Rodriguez de Fonseca et al., 2001) with increased activity, as shown by enhanced expression of the early gene c-fos, and presumably these neurons mediate, at least in part, the hypophagic effect of OEA (Provensi et al., 2014). We found that in mice deficient of the histamine synthesizing enzyme histidine decarboxylase (HDC), or acutely depleted of histamine by i.c.v. infusions of the HDC blocker α-fluoromethylhistidine (α-FMHis) (Garbarg et al. 1980), the effect of exogenously administered OEA was significantly attenuated (Provensi et al., 2014).
Exogenous administration of OEA after behavioral training was found to improve memory retention in the inhibitory avoidance task, an effect that requires the activation of noradrenergic neurotransmission in the BLA (Campolongo et al., 2009). Rats’ cognitive performance produced by OEA is mimicked by the peripheral administration of agonists of the peroxisome-proliferator-activated receptor-alpha (PPAR-α; Campolongo et al., 2009), where OEA acts as an endogenous high-affinity agonist (Fu et al. 2003). Physiologically, newly formed OEA in enterocytes binds to PPAR-α that in turn activate sensory fibers of the vagus nerve with an as-yet-unknown mechanism (Rodríguez de Fonseca et al., 2001) to convey information to the nucleus tractus solitarius (NTS) in the hindbrain and from here to other brain regions (for review, see Piomelli, 2013). We therefore speculated that OEA might modulate emotional memory in another aversive learning paradigm, the contextual fear conditioning test, and that histaminergic neurotransmission in the BLA is necessary for the cognitive effect of OEA. In contextual fear conditioning, animals learn to associate the context (the training box) with a punishment (footshocks), and upon reexposure to the same context they display a freezing behavior, that is, a generalized immobility caused by a generalized tonic response. In inhibitory avoidance, on the other hand, animals learn to refrain from stepping into a compartment where they previously received a footshock, but they are not refrained from moving, nor do they behave in any way passively. Both tests induce emotional arousal and require activation of the BLA, but different circuits and/or cellular machineries may be engaged at different times during memorization (Phelps et al., 2014; Izquierdo et al., 2016). Here we adopted a contextual fear conditioning paradigm in which rats were administered OEA, received intra-BLA compounds to manipulate the histaminergic system immediately after training, and were then reexposed to the threatening context 72 hours later (Benetti et al., 2013) when all drugs presumably have been metabolized. We also measured histamine release from the BLA of freely moving rats following OEA administration, as a putative neurochemical correlate of the cognitive effects. Our findings revealed that the cognitive effects of OEA are blunted in rats with impaired histamine neurotransmission. We discuss our results in terms of a “permissive” role of brain histamine on the memory-enhancing effects of OEA that may bear relevance in the treatment of dysfunctional aversive memories as found in phobias and PTSD.
Materials and Methods
Animals and Drugs
Male Wistar rats (3 months old, 300–330 g) purchased from Envigo (Bresso, Italy) were housed in the animal facility of Ce.S.A.L (Università di Firenze) in a temperature-controlled room (22±1°C) with a 12-h-light/dark cycle (light on 7:00 am to 7:00 pm), at a constant temperature and humidity with standard diet (4RF21; Mucedola s.r.l., Milan, Italy) and freely available water. All procedures were conducted in accordance with the Council Directive of the European Community (2010/63/EU) of the Decreto Legislativo Italiano 26 (13/03/2014) and National Institutes of Health guidelines on animal care and were approved by veterinarian supervision.
Alpha-FMHis was synthesized at Johnson & Johnson Laboratories (kind gift of Dr. Nicholas Carruthers), pyrilamine was purchased from Sigma-Aldrich (UK), and zolantadine and OEA from Tocris Bioscience (UK). OEA was dissolved in saline/polyethylene glycol/Tween80 (90/5/5, v/v), whereas zolantidine and pyrilamine were dissolved in saline. All other reagents and solvents were of HPLC grade or the highest grade available (Sigma).
Behavioral Experiments: Surgery
One week after arrival, rats were anaesthetized (75 mg/kg ketamine plus 10 mg/kg xylazine) and placed on a stereotaxic frame (Stellar; Stoeling, Co., Wood Dale, IL). A stainless-steel cannula (22 gauge) was implanted in the lateral ventricle and fixed to the skull by using dental cement according to the following coordinates (Paxinos and Watson, 1998) in mm: AP=−0.9; L=−1.5; DV=−2.6 and used for α-FMHis/saline administration. Rats were also implanted bilaterally with 22-gauge guide cannulae 1 mm above the BLA according to the following coordinates from bregma (Paxinos and Watson, 1989) in mm: AP = −2.8; L = ±4.9; DV = +7.6. Animals were allowed 7 days to recover from surgery before behavioral procedures and were handled once daily before the experimental day.
Infusion Procedure and Experimental Groups
At the time of drug microinfusions, the animals were gently restrained by hand, and the injection needle (30 gauge) was fitted tightly into the guides, extending 1 mm from the tip of the guide cannulae. The injection needle was connected to a 10-μL Hamilton microsyringe, and the infusions were performed at a rate of 0.5 μL/30 s. The infusion cannula was left in place for an additional 60 seconds to minimize backflow. The entire bilateral infusion procedure took approximately 90 seconds. Alpha-FMHis (5 mM, 1 µL) was infused i.c.v. 24 hours before contextual fear training, and controls received equal volumes of sterile saline. Zolantidine (0.1 μM, 0.5 µL side) or pyrilamine (0.9 μM, 0.5 µL side) were infused intra-BLA bilaterally immediately after training. OEA (10 mg/kg) was injected i.p. 10 minutes after fear conditioning, while controls received equivalent volumes of vehicle.
Contextual Fear Conditioning
Contextual fear conditioning was induced in a Skinner box module (29 × 31 × 26 cm, Modular Operant Cage; Coulbourn Instruments Inc.), equipped with a grid floor connected to a shock-delivery apparatus (Modular Operant Cage/Grid Floor Shocker E13-08; Coulbourn Instruments) and placed in an acoustically insulated room at 20±1°C. The number of the electric shocks and the inter-shock interval duration was predetermined by a stimulus programming device (Scatola di comando Arco 2340, Italy). Illumination inside the room was 60 lux. The rat was left undisturbed for 3 minutes and subsequently six, 1-seconds 0.8-mA electric footshocks were administered at 30-second intervals. The footshock intensity was chosen according to previous published data from our laboratory (Benetti et al., 2013). This is a strong enough footshock to guarantee retention at 72 hours postacquisition without inducing generalization (Baldi et al., 2004). The rat was removed 2 minutes after the end of the stimulation, therefore spending a total time of 8 minutes inside the conditioning apparatus.
Freezing Measurement
Seventy-two hours after conditioning, rats were again placed inside the conditioning apparatus in the soundproof room and left undisturbed for 6 minutes. The rats’ behavior was recorded by means of a closed-circuit television system by an experimenter unaware of the animal’s treatment. Freezing was defined as the complete absence of somatic motility, with the exception of respiratory movements. Measurements were performed with a stopwatch by personnel unaware of the experimental group each animal belonged to. Total cumulated freezing time (i.e., total seconds spent freezing during each 6-minute period) was calculated and results expressed in seconds of freezing time. All behavioral tests were performed between 10:00 am and 12:00 pm to avoid interference with the circadian rhythm (Kamin, 1957).
Microdialysis Experiments
Surgery
Rats were anesthetized with (75 mg/kg ketamine plus 10 mg/kg xylazine) and positioned in a stereotaxic frame (Stellar, Stoelting Co.). One guide cannula (MAB 4.15.IC, Microbiotech) was implanted according to the following coordinates from bregma (Paxinos and Watson, 1988, in mm): paraventricular nucleus (PVN) AP = -1.9, L = -0.5, DV = +7.5; ventromedial hypothalamus (VMH), AP=-2.6; L=-0.5; DV=+9.0; BLA, AP = -2.8, L = -4.8, DV = +7.8. A surgical screw served as an anchor, and the cannulae were fixed to the skull with acrylic dental cement. The animals were then housed one per cage and left to recover.
In Vivo Microdialysis Experiments
The microdialysis experiments were performed as previously described (Munari et al., 2013). Briefly, 48 h after surgery, the stylet was removed from the guide cannula, and the microdialysis probes (1 mm, molecular weight cut-off = 6000 Da, MAB 4.15.1.PES Microbiotech) were inserted. Probes were perfused with Ringer’s solution (in mM: NaCl, 147; CaCl2, 1.2; and KCl, 4.0 at pH = 7.0) at a flow rate of 2 μL/min using a microperfusion pump (Carnegie Medicine; Mod CMA/100). After 120-min equilibration, the sample collection started at 15-minute intervals. Spontaneous release was defined as the average value of the five 15-minute fractions collected during 75 minutes of perfusion with Ringer’s solution prior to OEA 10 mg/kg systemic (i.p.) treatment. All subsequent fractions were expressed as percentage of this value. To prevent degradation of histamine, 1.5 mL of 5 mM HCl was added to each sample. The dialysates were kept at -80°C until analysis.
Determination of Histamine
Histamine contents in the dialysates were determined by HPLC-fluorometry (Munari et al., 2013). In brief, the column (Hypersil ODS, 3 µm, 2.1×100 mm; Thermo Fisher Scientific, Waltham, MA) was eluted with 0.25 M potassium dihydrogen phosphate containing 5% octanesulfonic acid (Sigma-Aldrich, St. Louis, MO) at a flow rate of 0.4 mL/min. The eluate from the column was mixed first with 0.1% o-phthalaldehyde solution at a flow rate of 0.1 mL/min and then to a solution containing 4 M sodium hydroxide and 0.2 M boric acid (flow rate, 0.137 mL/min) to adjust the reaction mixture to pH 12.5. The reaction took place at 45°C. Then 17% orthophosphoric acid was added to the solution (flow rate, 0.137 mL/min) to reach a final reaction mixture at pH 3. The fluorescent intensity was measured with a spectrofluorometer (series 1100; Agilent, Waldbronn, Germany) at 450 nm with excitation at 360 nm. The sensitivity limit was 10 fmol and the signal/noise ratio was >3. Histamine levels in the dialysate samples were calculated as fmol/30 min and were not corrected for probe recovery (about 40%).
Histology
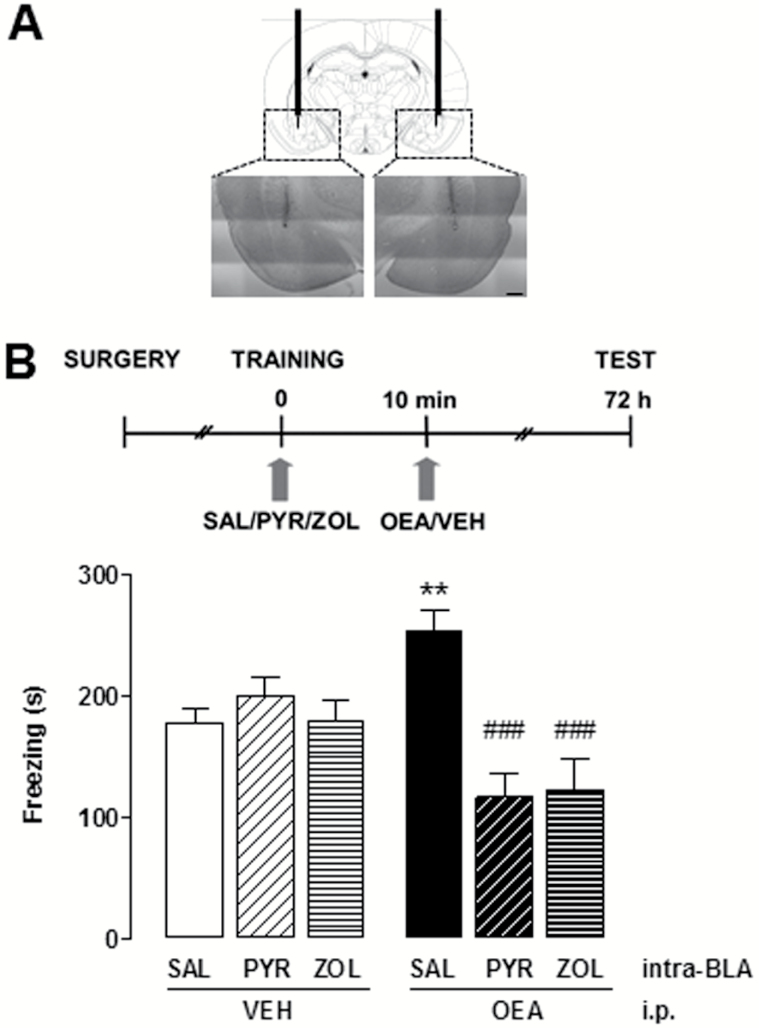
The placement of microdialysis membranes and infusion cannulae was verified postmortem (Figure 3A). Rats were overdosed with chloral hydrate and the brains removed and stored in 10% formalin for 10 days. Forty-μm sections were sliced on a cryostat, mounted on gelatine-coated slides, and then stained with cresyl violet for light microscopic observation. Data from rats in which the membranes were not correctly positioned were discarded (<5%).
Figure 3.
Effect of histamine receptor antagonism on oleoylethanolamide (OEA)-induced freezing enhancement. (A) Schematic diagram and photomicrographs showing the position of the cannulae. The injection needle protruded 1 mm below the tip of the cannulae. (B) Rats received bilateral intra-basolateral amygdala (BLA) infusions of zolantidine (ZOL), pyrilamine (PYR), or vehicle, and OEA or vehicle i.p. immediately after training. Data are expressed as means ± SEM of 9 to 14 animals for each group; ANOVA and Newman-Keuls’ posthoc test, **P < .01 vs SAL/VEH controls; ###P < .001 vs SAL/OEA.
Statistical Analysis
All values are expressed as means ± SEM, and the number of rats used in each experiment is also indicated. The presence of significant treatment effects was determined by a Student’s t test or a 1-way ANOVA followed by Newmann Keuls’ MCT test, as appropriate. For all statistical tests, P<.05 was considered significant. In the figures and figure legends of microdialysis experiments, we reported only the significant differences vs the last sample before drug treatment for clarity purposes.
Results
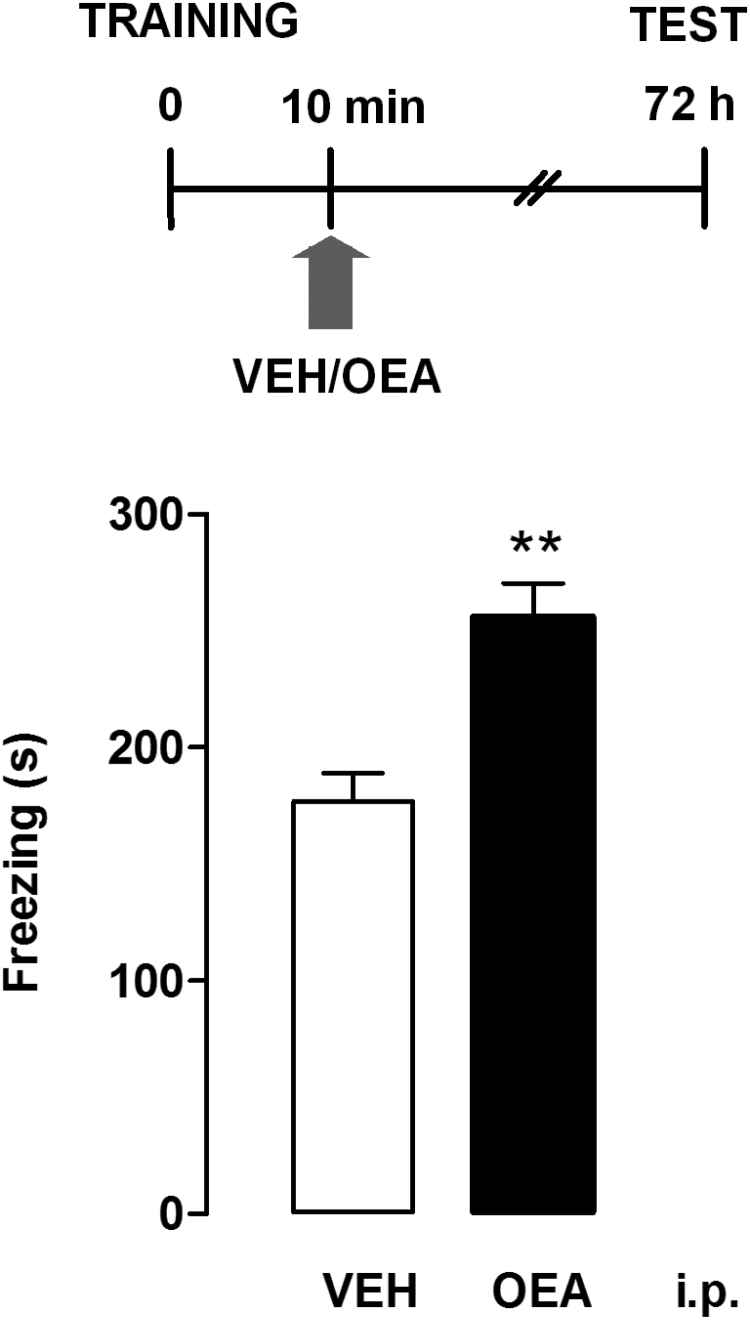
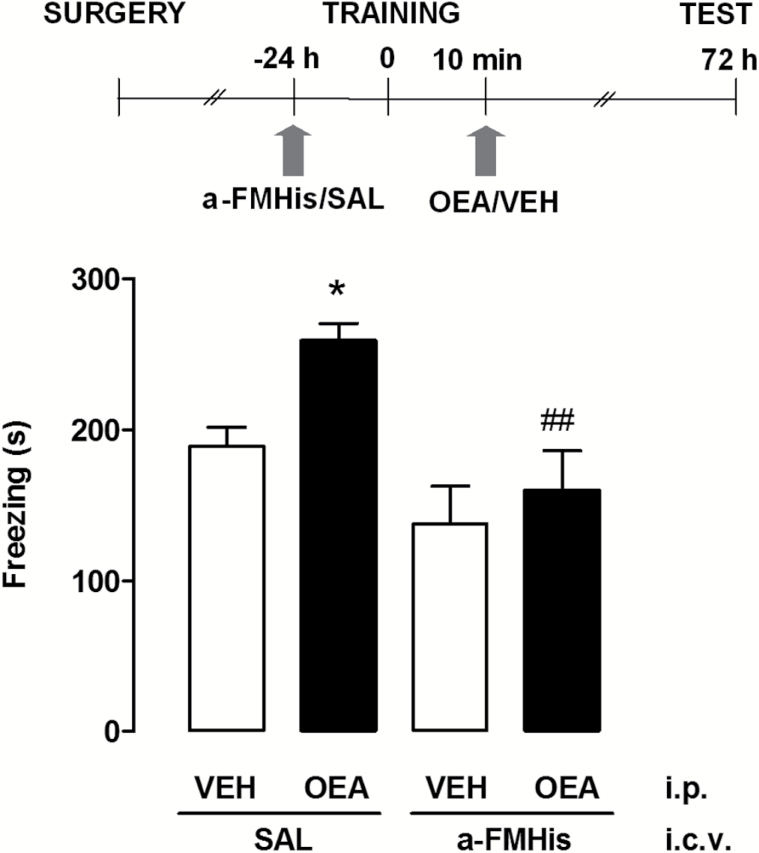
In a first set of experiments, we evaluated the effect of OEA (10 mg/kg i.p., a dose that does not change motility in the open field, nor anxiety-like responses) (Campolongo et al., 2009) administered within 10 minutes of contextual fear conditioning in satiated rats. Controls received an equivalent volume of vehicle. Retention test was carried out 72 hours after training. As shown in Figure 1, an unpaired Student’s t test showed that animals given OEA displayed a significant increase of time spent freezing compared with vehicle-treated rats (P<.01). To evaluate the role of the central histaminergic system in the cognitive effect of OEA, we infused the HDC inhibitor α-FMHis i.c.v. 24 hours prior to fear conditioning. We previously showed that administration of α-FMHis quickly suppressed baseline and histamine H3 receptor antagonist-evoked release of histamine from the TMN of freely moving rats (Benetti et al., 2015), as 180 minutes after injection, histamine release values decreased below the sensitivity of the method. OEA (10 mg/kg i.p.) was injected within 10 minutes after fear conditioning. Controls received saline i.c.v. and vehicle i.p. One-way ANOVA revealed a statistical difference across experimental groups (F3,45=6.756; P<.001) (Figure 2). Posthoc analysis with Newman-Keuls MCT showed that OEA increased the freezing time at retention test with respect to vehicle-treated rats receiving i.c.v. infusion of saline (P<.05). However, the OEA-elicited potentiation of freezing was abolished in brain histamine-depleted animals (P<.01) (Figure 2). Hence, the treatment with α-FMHis 24 hours prior to the test prevented the effect of OEA, indicating that the integrity of the central histaminergic system is necessary for the effects of OEA on memory consolidation. The freezing time of rats given vehicle i.p. and of those receiving α-FMHis or saline i.c.v. did not differ significantly at retention, thus indicating that all animals formed a memory trace of the training experience. The fact that histamine release is restored to control levels about 3 days after treatment with α-FMHis (Benetti et al., 2015) may explain this result.
Figure 1.
Postacquisition injections of oleoylethanolamide (OEA) affect contextual fear memory consolidation. The schematic drawing shows the sequence of procedures and treatment administrations. OEA (10 mg/kg) or vehicle (VEH) were injected i.p. 10 minutes after contextual fear conditioning. All animals were tested for fear retention 72 hours after conditioning. Bars represent mean values ± SEM of 8 to 10 rats/group; **P < .01; unpaired t test.
Figure 2.
Effect of histamine neurotransmission on oleoylethanolamide (OEA)-induced freezing enhancement. The schematic drawing shows the sequence of procedures and treatment administrations. Latencies of α-FMHis groups did not significantly differ from controls. Data are expressed as means ± SEM of 10 to 14 animals for each group; ANOVA and Newman-Keuls posthoc test, *P<.05 vs saline (SAL) controls. ##P<.01 vs OEA/SAL.
We then tested the hypothesis that histamine release and histamine receptor activation in the BLA might be relevant for the cognitive effect of OEA. We blocked H1 or H2 receptors in the BLA with local, bilateral infusions (0.5 µL) of the selective antagonists pyrilamine (0.9 μM) or zolantidine (0.1 μM), respectively, immediately prior to the administration of OEA. The concentrations used were those employed in previous studies (Benetti et al., 2013). Controls received comparable volumes of saline intra-BLA and of vehicle i.p. The results are shown in Figure 3B. One-way ANOVA showed significant differences among experimental groups (F5,64 = 8.436; P<.0001). Rats that received OEA i.p. and saline intra-BLA froze for a significantly longer time at retention compared with saline-/vehicle-treated rats (P<.05) in a comparable manner to the control groups in Figures 1 and 2. However, when either H1 or H2 receptors were blocked, OEA administration did not increase the freezing time (P<.001). Freezing time of rats that received vehicle i.p. and pyrilamine or zolantidine in the BLA was not significantly different from the freezing time of saline-/vehicle-treated rats (Figure 3B).
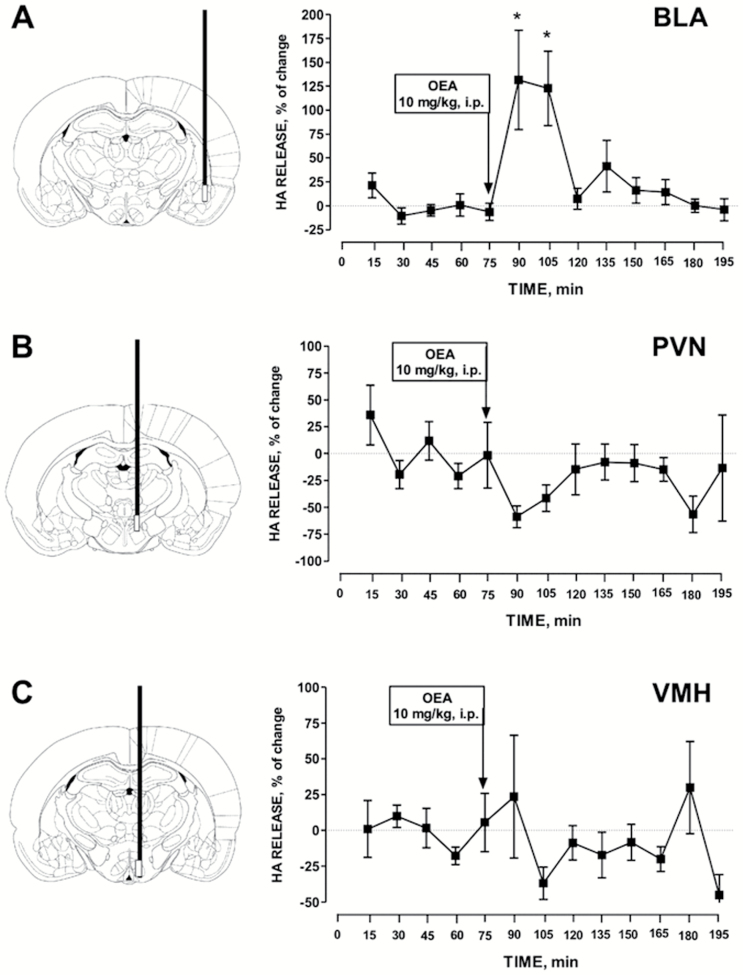
Given that OEA requires the integrity of the histaminergic neurotransmission in the BLA to exert its cognitive effects, we then investigated if OEA changes histamine release from the BLA by means of the microdialysis technique in freely moving rats. As shown in Figure 4A, i.p. injections of 10 mg/kg OEA induced a transient, but significant increase of histamine release from the BLA that reached a peak value approximately 30 minutes after the administration of OEA (F12,61 = 5.336; P<.001); it was sustained for the following 30 minutes and then returned to baseline level. To understand if the histaminergic system responds to OEA with a generalized increased activity throughout the brain, we also measured histamine levels in the VMH and PVN. We chose these 2 brain areas as they are innervated by histamine fibers and express high density of H1 receptors that regulate feeding behavior and obesity in rodents (Masaki and Yoshimatsu, 2006). Another reason is that we previously showed that systemic administration of OEA in hungry mice activates oxytocic neurons in the PVN by engaging histaminergic neurotransmission in this hypothalamic nucleus (Provensi et al., 2014). However, we found that histamine release was not changed significantly after OEA i.p. injection in either nucleus (Figure 4B-C). Rather, a trend towards decreased histamine release was observed in the PVN, although it did not reach significance.
Figure 4.
Histamine extraneuronal levels were measured in fractions collected every 15 minutes. Baseline values were determined from the average of 5 samples preceding oleoylethanolamide (OEA) i.p. injection. OEA significantly increased the extraneuronal levels of histamine in the basolateral amygdala (BLA), but not in the paraventricular nucleus (PVN) nor the ventromedial hypothalamus (VMH). The mean basal extraneuronal level of histamine was (fmol/15 min) 22.8 ± 23.1 in the BLA (n = 5), 118.5 ± 74.2 in the PVN (n = 6), and 47.4 ± 24.4 in the VMH (n = 6). ANOVA and Newman-Keuls’ posthoc test, *P < .05 vs baseline levels.
Discussion
Emotional arousal enhances consolidation of memory traces, a homeostatic response of our organism that is modulated by stress hormones (McGaugh and Roozendaal, 2002). There is extensive evidence derived from observations in both experimental animals and humans that the amygdala is indispensable to enable the acquisition and retention of lasting memories of emotional experiences. The compelling evidence led McGaugh and his collaborators (McGaugh et al., 2004) to propose that the activation of the BLA is fundamental in the establishment of an arousal state triggered by fear (as unconsciously occurs in humans, LeDoux, 2014) and that arousal is a major component in the establishment of posttraining memory consolidation. The elegant work of Campolongo and coauthors (2009) demonstrated that there are unsuspected players in addition to stress hormones (i.e., adrenaline and corticosteroids) that affect memory consolidation of fearful stimuli, namely the satiety factor OEA, normally secreted by the intestine after a fatty meal. Exogenous OEA at the concentration used here does not readily pass the blood brain barrier (Campolongo et al., 2009). By activating peripheral PPAR-α it engages the vagus nerve and activates the NTS that in turn provides the amygdala, together with the locus coeruleus (LC), with a dense supply of norepinephrine. Stimulation of NTS (Clayton et al., 2000) or LC (Sara, 2009; Rosa et al., 2013) contributes to memory processing by influencing noradrenergic neurotransmission in the amygdala. OEA enhances memory consolidation of the inhibitory avoidance task by inducing noradrenergic activation of BLA neurons (Campolongo et al., 2009). The cognitive effects of OEA are lost in PPAR-α mice and in rats that received intra-NTS infusion of lidocaine, further substantiating the hypothesis that vagal afferents contribute to the cognitive effects of OEA (Campolongo et al., 2009). Our present results show that OEA increases memory expression of another aversively motivated task, contextual fear conditioning, by eliciting histaminergic neurotransmission in the BLA. Accordingly, both depletion of releasable histamine in the brain with α-FMHis that blocks HDC, and intra-BLA infusion of histaminergic antagonists prevent the freezing-enhancing effects of OEA. The concept of several neurotransmitter systems contributing to emotional memory consolidation within the same brain region is indisputable (Izquierdo et al., 1992; McGaugh 2004; Izquierdo et al., 2016). We think that the emotional arousal that is generally considered indispensable for good memory consolidation of fear tasks is provided by both the noradrenergic and histaminergic transmission in the BLA. We recently observed that OEA elicits NTS activation in HDC-KO mice (hence unable to synthesize histamine) as well as in wild-type littermates (Umehara et al., 2016). Therefore, the regulatory action of histamine on the cerebral pathways triggered by OEA is most likely downstream of the NTS. A prudent interpretation of our results is that histamine released in the amygdala or in the LC (Korotkova et al., 2005; Lee et al., 2005) gates the effects of noradrenaline on BLA neurons, hence blocking either neurotransmitter within the BLA would lead to similar behavioral outcomes.
We previously documented that histamine neurotransmission in diverse brain areas is necessary for the consolidation of contextual fear memory, as infusions of H3 receptors antagonists in the BLA decrease the freezing time of contextual fear-conditioned rats and decrease acetylcholine release in the same region (Passani et al., 2001). Also, in the nucleus basalis magnocellularis memory improvement for contextual fear conditioning requires the activation of H2 receptors (Benetti et al., 2013), presumably by controlling the activity of the amygdalopetal cholinergic pathway (Power and McGaugh 2002). It is relatively surprising that both H1 and H2 receptor antagonists produce the same results. The arrangement of these receptors on BLA neurons is not known, and explanations may only be speculative. Jiang et al. (2005) recorded intracellular excitatory postsynaptic potentials from BLA neurons ex vivo in the presence of histamine and described the opposite responses of 2 neuronal populations with decreased or increased excitatory postsynaptic potential amplitude. The authors fell short of demonstrating what postsynaptic histamine receptors may partake in these effects; hence, it is not known if H1 and H2 receptors are on different cell populations. In the vascular system, H1 and H2 receptors may work synergistically via activation of common intracellular pathways (Panula et al., 2015), which is a mechanism that may also occur in neurons. It is plausible that when strong aversive stimuli are used, as in our paradigm, all histaminergic inputs to the BLA need to be blocked to prevent freezing-enhancing compounds such as OEA to exert their effect.
Of interest, BLA infusion of the H1 or H2 receptor antagonists did not affect the expression of baseline fear memory tested 72 hours after training; rather these treatments prevented the freezing enhancement induced by OEA. These results are in good agreement with our previous observations using the same behavioral protocol that neither pyrilamine nor zolantidine changed freezing responses when administered in the NBM (Benetti et al., 2013) but prevented the increased expression of fear memory induced by an H3 receptor antagonist.
Rats depleted of histamine with i.c.v. injections of α-FMHis had a behavior comparable with controls treated with saline, but α-FMHis prevented the effect of OEA on freezing time. We explain this considering that the effect of α-FMHis lasts approximately 3 days (Benetti et al., 2015); therefore at the time of the retention test (4 days after α-FMHis infusion) histamine neurotransmission should be restored to normal levels, which presumably allows the expression of the memory trace. These results are in agreement with what we observed using another behavioral paradigm, inhibitory avoidance, as the memory impairment induced by pretraining injections of α-FMHis lasted 48 hours; the step-down latency returned to control levels after 1 week (Benetti et al., 2015). Despite the well-documented differences between inhibitory avoidance, a procedure that requires a discriminative response, and fear conditioning, a Pavlovian procedure that does not require decision making (Maren, 2003; Tinsley et al., 2004), OEA appears to enhance the expression of BLA-dependent emotional memory regardless of the type of behavioral response and adverse situation. The microdialysis experiments offered one further piece of data in favor of the hypothesis that OEA requires the histamine neurotransmission in the BLA to modulate emotional responses. OEA administered at the same dose that increased freezing time augmented significantly histamine release from the BLA, but not from hypothalamic nuclei where histaminergic and OEA signalling converge to regulate appetite (Provensi et al., 2014). This could be a quite puzzling observation, but we believe that the histaminergic system responds to various stimuli, in this case OEA administered systemically, by engaging different pathways according to the required behavioral response. As an example, in food-restricted mice OEA increases c-fos expression in the PVN and supraoptic nucleus but not in other hypothalamic nuclei that receive histaminergic afferents and control eating behavior such as the VMH (Provensi et al., 2014) or dorsomedial hypothalamus (Umehara et al., 2016). In the present study, during dialysate collection satiated rats are quiet or sleeping, and it is plausible that in this circumstance OEA does not engage histaminergic afferents to the PVN to regulate their activity; rather it recruits the histaminergic afferents to the BLA. Indeed, we believe that the TMN serves as a neuronal gateway that integrates hormonal, interoceptive, and environmental signals to coordinate the adequate behavioral response. This hypothesis implies that HA neurons in the TMN are organized in distinct subpopulations differently regulated and innervating specific brain areas. There is much experimental evidence supporting this view (Blandina et al., 2012; Munari et al., 2013).
What is the physiological significance of exaggerated emotional response induced by OEA? The few human studies published so far showed that blood concentrations of N-acyl-ethanolamides like OEA and other endocannabinoids increase in response to acute stress in healthy human volunteers (Dlugos et al., 2012), and subjects affected by PTSD have significantly higher plasma concentrations of OEA (Hauer et al. 2013; Schaefer et al., 2014). Despite the fact that these results are based on small and dis-homogeneous study groups, they may have pathophysiological and/or diagnostic relevance. In this respect, our results suggest that activation of the histaminergic system in the BLA has a “permissive” role on the memory enhancing effects of OEA. In particular, targeting the H1 or H2 receptor with classical clinically approved antihistamine compounds may modify the expression of emotional memory and reduce dysfunctional aversive memories as found in phobias and PTSD.
Statement of Interest
None.
Acknowledgments
We thank Dr. Nicholas Carruthers, Johnson & Johnson Laboratories for the kind gift of α-FMHis.
This work was supported by Programmi di Ricerca di Rilevante Interesse Nazionale Grant 2009 2009ESX7T3_003 E 55.921 and Universitá di Firenze funds (M.B.P. and P.B.), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Brazil, process 201511/2014-2), and Fondazione Umberto Veronesi funds.
References
- Baldi E, Lorenzini CA, Bucherelli C. (2004) Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem 81:162–166. [DOI] [PubMed] [Google Scholar]
- Benetti F, Baldi E, Bucherelli C, Blandina P, Passani MB. (2013) Histaminergic ligands injected into the nucleus basalis magnocellularis differentially affect fear conditioning consolidation. Int J Neuropsychopharmacol. 16:575–582. [DOI] [PubMed] [Google Scholar]
- Benetti F, Furini CR, de Carvalho Myskiw J, Provensi G, Passani MB, Baldi E, Bucherelli C, Munari L, Izquierdo I, Blandina P. (2015) Histamine in the basolateral amygdala promotes inhibitory avoidance learning independently of hippocampus. Proc Natl Acad Sci 112:E2536–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti F, Izquierdo I. (2013) Histamine infused into basolateral amygdala enhances memory consolidation of inhibitory avoidance Int J Neuropsychopharmacol 16:1539–1545 [DOI] [PubMed] [Google Scholar]
- Blandina P, Munari L, Provensi G, Passani MB. (2012) Histamine neurons in the tuberomamillary nucleus: a whole center or distinct subpopulations? Front Syst Neurosci 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Cuomo V, Astarita G, Fu J, McGaugh JL, Piomelli D. (2009) Fat-induced satiety factor oleoylethanolamide enhances memory consolidation. Proc Natl Acad Sci 106:8027–8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangioli I, Baldi E, Mannaioni PF, Bucherelli C, Blandina P, Passani MB. (2002) Activation of histaminergic H3 receptors in the rat basolateral amygdala improves expression of fear memory and enhances acetylcholine release. Eur J Neurosci 16:521–528. [DOI] [PubMed] [Google Scholar]
- Clayton EC, Williams CL. (2000) Adrenergic activation of the nucleus tractus solitarius potentiates amygdala norepinephrine release and enhances retention performance in emotionally arousing and spatial memory tasks. Behav Brain Res 112:151–158. [DOI] [PubMed] [Google Scholar]
- Contreras M, Riveros ME, Quispe M, Sánchez C, Perdomo G, Torrealba F, Valdés JL. (2016) The histaminergic tuberomamillary nucleus is involved in appetite for sex, water and amphetamine. PLoS One 11:e0148484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos A, Childs E, Stuhr KL, Hillard CJ, de Wit H. (2012) Acute stress increases circulating anandamide and other n-acylethanolamines in healthy humans. Neuropsychopharmacology 37: 2416–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri R, Furini CR, Passani MB, Provensi G, Baldi E, Bucherelli C, Izquierdo I, de Carvalho Myskiw J, Blandina P. (2016) Memory retrieval of inhibitory avoidance requires histamine H1 receptor activation in the hippocampus. Proc Natl Acad Sci 113:E2714–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenza NG, Rosa J, Izquierdo I, Myskiw JC. (2012) Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behav Brain Res 232:210–216. [DOI] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodríguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D. (2003) Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 425:90–93. [DOI] [PubMed] [Google Scholar]
- Garbarg M, Barbin G, Rodergras E, Schwartz JC. (1980) Inhibition of histamine synthesis in brain by a-fluoromethylhistidine, a new irreversible inhibitor: in vitro and in vivo studies. J Neurochem 35:1045–1052. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Efoudebe M, Passani MB, Baldi E, Bucherelli C, Giachi F, Corradetti R, Blandina P. (2013) Improvement in fear memory by histamine-elicited ERK2 activation in hippocampal CA3 cells. J Neurosci 23:9016–9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer D, Schelling G, Gola H, Campolongo P, Morath J, Roozendaal B, Hamuni G, Karabatsiakis A, Atsak P, Vogeser M, Kolassa IT. (2013) Plasma concentrations of endocannabinoids and related primary fatty acid amides in patients with post-traumatic stress disorder. PLoS One 8:e62741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, da Cunha C, Rosat R, Jerusalinsky D, Ferreira MB, Medina JH. (1992) Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav Neural Biol 58:16–26. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Furini CR, Myskiw JC. (2016) Fear memory. Physiol Rev 96:695–750. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. (1957) The retention of an incomplete learned avoidance response. J Comp Physiol Psychol 50:457–460. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Ponomarenko AA, Haas HL. (2005) Histamine excites noradrenergic neurons in locus coeruleus in rats. Neuropharmacology 49:129–134. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. (2014) Coming to terms with fear. Proc Natl Acad Sci 111:2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Lee BY, Waterhouse BD. (2005) Retrograde study of projections from the tuberomammillary nucleus to the dorsal raphe and the locus coeruleus in the rat. Brain Res 1043:65–75. [DOI] [PubMed] [Google Scholar]
- Maren S. (2003) What the amygdala does and doesn’t do in aversive learning. Learn Mem 10:306–308. [DOI] [PubMed] [Google Scholar]
- Masaki T, Yoshimatsu H. (2006) The hypothalamic H1 receptor: a novel therapeutic target for disrupting diurnal feeding rhythm and obesity. Trends Pharmacol Sci 27:279–284. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. (2004) The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27:1–28. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. (2002) Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol 12:205–210. [DOI] [PubMed] [Google Scholar]
- Munari L, Provensi G, Passani MB, Blandina P. (2013) Selective brain region activation by histamine H3 receptor antagonist/inverse agonist ABT-239 enhances acetylcholine and histamine release and increases c-Fos expression. Neuropharmacology 70:131–140. [DOI] [PubMed] [Google Scholar]
- Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WL, Stark H, Thurmond RL, Haas HL. (2015) International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol Rev 67:601–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Pirvola U, Auvinen S, Airaksinen MS. (1989) Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience 28:585–610. [DOI] [PubMed] [Google Scholar]
- Passani MB, Cangioli I, Baldi E, Bucherelli C, Mannaioni PF, Blandina P. (2001) Histamine H3 receptor-mediated impairment of contextual fear conditioning, and in-vivo inhibition of cholinergic transmission in the rat basolateral amygdala. Eur J Neurosci 14:1522–1532 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1998) The rat brain in stereotaxic coordinates. New York: Academic Press. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Lempert KM, Sokol-Hessner P. (2014) Emotion and decision making: multiple modulatory neural circuits. Annu Rev Neurosci 37:263–287. [DOI] [PubMed] [Google Scholar]
- Provensi G, Blandina P, Passani MB. (2015) The histaminergic system as a target for the prevention of obesity and metabolic syndrome. Neuropharmacology doi: 0.1016/j.neuropharm.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Provensi G, Coccurello R, Umehara H, Munari L, Giacovazzo G, Galeotti N, Nosi D, Gaetani S, Romano A, Moles A, Blandina P, Passani MB. (2014) Satiety factor oleoylethanolamide recruits the brain histaminergic system to inhibit food intake. Proc Natl Acad Sci 111:11527–11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AE, McGaugh JL. (2002) Cholinergic activation of the basolateral amygdala regulates unlearned freezing behavior in rats. Behav Brain Res 134:307–315. [DOI] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Navarro M, Gómez R, Escuredo L, Nava F, Fu J, Murillo-Rodríguez E, Giuffrida A, LoVerme J, Gaetani S, Kathuria S, Gall C, Piomelli D. (2001) An anorexic lipid mediator regulated by feeding. Nature 414:209–212. [DOI] [PubMed] [Google Scholar]
- Rosa J, Myskiw JC, Furini CR, Sapiras GG, Izquierdo I. (2014) Fear extinction can be made state-dependent on peripheral epinephrine: role of norepinephrine in the nucleus tractus solitarius. Neurobiol Learn Mem 113:55–61. [DOI] [PubMed] [Google Scholar]
- Sara SJ. (2009) The locus coeruleus and noradrenergic modulation of cognition. Nature Rev Neurosci 10:211–223. [DOI] [PubMed] [Google Scholar]
- Schaefer C, Enning F, Mueller JK, Bumb JM, Rohleder C, Odorfer TM, Klosterkötter J, Hellmich M, Koethe D, Schmahl C, Bohus M, Leweke FM. (2014). Fatty acid ethanolamide levels are altered in borderline personality and complex posttraumatic stress disorders. Eur Arch Psychiatry Clin Neurosci 264:459–463. [DOI] [PubMed] [Google Scholar]
- Silva WC da, Bonini JS, Bevilaqua LR, Izquierdo I, Cammarota M. (2006) Histamine enhances inhibitory avoidance memory consolidation through a H2 receptor-dependent mechanism. Neurobiol Learn Mem 86: 100–106. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. (2006) Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 26:10292–10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley MR, Quinn JJ, Fanselow MS. (2004) The role of muscarinic and nicotinic cholinergic neurotransmission in aversive conditioning: comparing Pavlovian fear conditioning and inhibitory avoidance. Learn Mem 11:35–42. [DOI] [PubMed] [Google Scholar]
- Umehara H, Fabbri R, Provensi G, Passani MB. (2016) The hypophagic factor oleoylethanolamide differentially increases c-fos expression in appetite regulating centres in the brain of wild type and histamine deficient mice. Pharmacol Res 113:100–107. [DOI] [PubMed] [Google Scholar]