Abstract
Background:
Previous studies suggested that opiate withdrawal may increase anxiety and disrupt brain-derived neurotrophic factor function, but the effects of i.v. morphine self-administration on these measures remain unclear.
Methods:
Adult male Sprague-Dawley rats were implanted with a catheter in the jugular vein. After 1 week of recovery, the animals were allowed to self-administer either i.v. morphine (0.5 mg/kg per infusion, 4 h/d) or saline in the operant conditioning chambers. The acoustic startle reflex and prepulse inhibition were measured at a baseline and on self-administration days 1, 3, 5, and 7 (1- and 3-hour withdrawal). Blood samples were collected on self-administration days 3, 5, and 7 from separate cohorts of animals, and the levels of brain-derived neurotrophic factor and corticosterone were assayed using the enzyme-linked immunosorbent assay method.
Results:
Compared with the saline group, the morphine self-administration group showed hyper-locomotor activity and reduced defecation during the self-administration. The morphine self-administration increased acoustic startle reflex at 1-hour but not 3-hour withdrawal from morphine and disrupted prepulse inhibition at 3-hour but not 1-hour withdrawal. The blood brain-derived neurotrophic factor levels were decreased in the morphine self-administration group at self-administration days 3 and 5, while the corticosterone levels remained unchanged throughout the study.
Conclusions:
The current findings suggest that spontaneous withdrawal from i.v. morphine self-administration may have transient effects on acoustic startle, sensorimotor gating, and peripheral brain-derived neurotrophic factor levels, and these changes may contribute to the adverse effects of opiate withdrawal.
Keywords: i.v. morphine self-administration, acoustic startle reflex, prepulse inhibition, brain-derived neurotrophic factor, corticosterone, opiate withdrawal
Significance Statement
This study investigated the effects of acute withdrawal from i.v. morphine self-administration on acoustic startle reflex and sensorimotor gating in adult male rats. Because brain-derived neurotrophic factor and stress hormone corticosterone are implicated in substance abuse and stress-related disorders, we also measured their levels in blood following morphine self-administration. Our findings indicate that morphine self-administration increased acoustic startle reflex in 1-hour withdrawal and disrupted prepulse inhibition in 3-hour withdrawal. Peripheral brain-derived neurotrophic factor but not corticosterone levels were decreased in 2-hour withdrawal from morphine self-administration. The current results support previous studies that reported increased acoustic startle in morphine withdrawal and further suggest disrupted sensorimotor gating and reduced brain-derived neurotrophic factor function in morphine withdrawal.
Introduction
Morphine, a widely used potent analgesic opioid (Miyamoto and Patapoutian, 2011), enhances dopamine (DA) release in the nucleus accumbens of the mesolimbic system. This often results in reinforcing effects, which drive an individual into abuse and dependence (Vanderschuren et al., 2001). In addition, opiates tend to produce anxiety-like behaviors when discontinued (Harris and Gewirtz, 2004; Rothwell et al., 2010); this aspect of morphine has been studied extensively (Harris et al., 2006). Interestingly, although both acute and chronic administration of morphine are believed to increase anxiety, past acoustic startle and open field tests in rodents have shown that acute, rather than chronic, treatment produces greater anxiety (Cabral et al., 2009).
The acoustic startle reflex (ASR) has been accepted as a good measure of anxiety (Davis et al., 2010), particularly with morphine studies (Warren and Ison, 1982; Glover and Davis, 2008; Rothwell et al., 2009, 2010). Prepulse inhibition (PPI), a modified ASR and a measure of the sensory gating mechanism, has been used for the study of not only hearing impairment (Tziridis et al., 2012) but also psychiatric diseases such as schizophrenia (Geyer et al., 1993; Parwani et al., 2000; Ludewig et al., 2002). However, most previous studies that used ASR and PPI to investigate the effects of morphine on anxiety and brain information processing in rodents implemented an injection paradigm in which animals were given morphine passively. Consequently, although the results from these studies contributed to our current knowledge of the effects of morphine on anxiety, further investigation using different methodologies such as self-administration is necessary. This is because studies that use spontaneous self-administration have previously reported findings that are significantly different from those of passive administration studies (Jacobs et al., 2003; O’Connor et al., 2011). In addition, spontaneous self-administration is believed to have a better validity when translating the reported results to human studies (Mello and Negus, 1996). Recently, there has been a study that examined the effects of chronic morphine self-administration (MSA) on the ASR and PPI (Le et al., 2014). However, the study investigated the effects of MSA in longer withdrawal (1 day and 1 week), and it is still unknown whether acute withdrawal from MSA alters ASR and PPI following MSA. Therefore, the present study investigated the effects of morphine withdrawal (1 and 3 hours) on the ASR and PPI up to 7 days.
To further investigate the effects of daily i.v. MSA on anxiety-like behavior, we also analyzed the levels of blood brain-derived neurotropic factor (BDNF) during the course of MSA. BDNF is known to play an important role in the growth and differentiation of neurons (Acheson et al., 1995; Huang and Reichardt, 2001), and in particular the survival of DA neurons (Canudas et al., 2005). Also, due to the abundant presence of receptors for BDNF in DA neurons of the ventral tegmental area (VTA) (Seroogy et al., 1994), it has been implicated that BDNF is involved in a variety of neuropsychiatric conditions, including learning, memory, and drug addiction (Yamada and Nabeshima, 2003; Bolanos and Nestler, 2004; Kumar et al., 2005). Accordingly, a number of studies have investigated the role of BDNF on opiate addiction (Vargas-Perez et al., 2009; Mashayekhi et al., 2012; Lunden and Kirby, 2013; Geoffroy et al., 2015). However, these studies employed passive administration of morphine and the results were inconsistent. Therefore, we postulated that the effects of MSA on BDNF expression may be different from the previous studies. Furthermore, we investigated corticosterone (CORT) levels in blood, which is a well-known marker for stress responses. In previous studies, it has been demonstrated that morphine-withdrawn rats show elevated blood CORT levels, which may be caused by stress (Nunez et al., 2009; Ueno et al., 2011).
The main goal of the current study was to investigate the effects of acute withdrawal from MSA on anxiety-like behavior, sensorimotor gating, BDNF and CORT levels in the blood of male Sprague-Dawley rats. Based on the previous literature, we hypothesized that MSA may increase the ASR and CORT levels and decrease BDNF levels in withdrawal. To our knowledge, this is the first study reporting the effects of i.v. morphine on peripheral BDNF and CORT levels following daily self-administration sessions in rats.
Materials and Methods
Animals
Male Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing 250 to 300 g at the beginning of the study were used. Rats were housed in an environment with a reversed 12-h-light/-dark cycle (lights off from 6:00 am to 6:00 pm), a room temperature of 22±2℃, and humidity of 60±2% with free access to food and water. The animals were tested during the dark cycle because they are nocturnal animals. All procedures were conducted in accordance with the Institutional Animal Care and Use Committee at the Uniformed Services University.
Surgery
After an adaptation period, rats were anesthetized with a cocktail of ketamine/xylazine (100 mg/kg and 10 mg/kg, i.p.), and a silastic catheter (Dow Corning, Midland, MI; 0.02″ ID × 0.037″ OD) was surgically implanted into the right jugular vein and fixed with a mersilene surgical mesh (Ethicon Inc., Somerville, NJ) to the surrounding tissue. The catheter was exposed to the outside through the back using a 22-gauge guide cannulae (Plastics One, Roanoke, VA) by skin incision. The silastic tubing and guide cannulae were fixed with dental cement and secured with Marlex surgical mesh (Davol Inc., Woburn, MA). The incision was closed with wound clips and antibiotic ointment was applied. Then 0.2 mL of saline containing heparin (30 U/mL) and gentamicin (5–8 mg/kg) was injected daily into the catheter during recovery to maintain catheter patency.
Apparatus
Animals self-administered morphine in operant conditioning chambers placed in sound-attenuated cubicles with ventilation (Med Associates, St. Albans, VT). Each operant conditioning chamber was equipped with a house light mounted on the wall and a cue light located above the active lever on the opposite wall. The house light turned on at the start of the session and was extinguished when the animal pressed the active lever. The cue light was illuminated for 5 seconds when the animal pressed the active lever. A “time-out” (TO) period followed, during which the cue light turned off and the rat spent 15 seconds in darkness. During the TO period, both active and inactive lever responses were recorded but had no programed consequences. When an animal pressed the active lever, a signal was delivered to the computer installed with the experiment program (Med PC, Med Associates) and a motor pump located beside the operant chamber pushed the syringe, delivering morphine solution to the animal’s jugular vein. The animal’s activity level in each 4-hour session was quantified using an automatic measurement system with infrared beams that detected the rat’s ambulation (Med Associates, St. Albans, VT).
MSA
In experiment 1 (Figures 1 and 3), animals were allowed to self-administer morphine sulfate solution or saline (SSA) with a fixed ratio 1 (FR1) schedule for 7 days (5 d/wk, 4 h/d). Sterile saline containing heparin and gentamicin (0.2 mL) was flushed into the catheter immediately before and after each session. If the rats pressed the active lever, 0.1 mL of either morphine solution (0.5 mg/kg per infusion) or saline was infused for 5 seconds. In experiment 2 (Figures 2 and 4), animals self-administered either saline or morphine (0.5 mg/kg per infusion) with an FR1 schedule for 7 days and were then allowed to self-administer with an FR3 schedule on day 8.
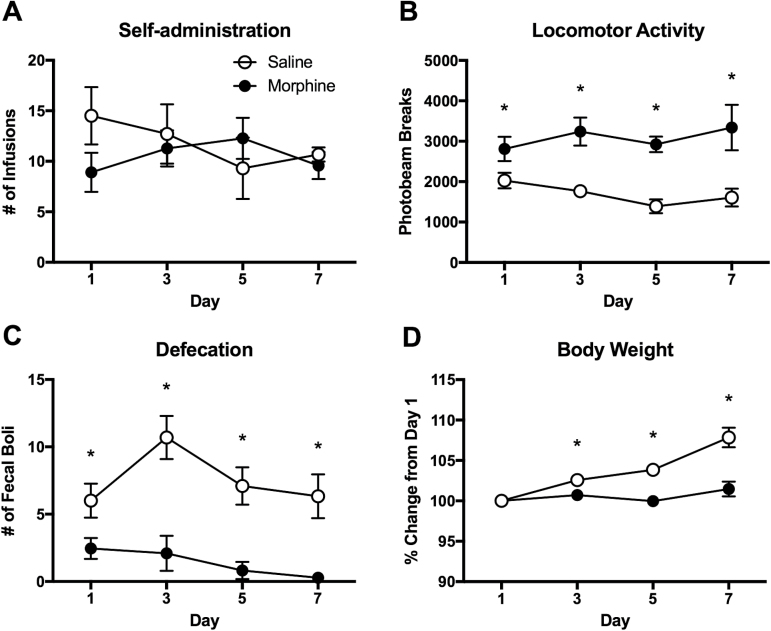
Figure 1.
Intravenous morphine self-administration in rats. (A) Number of infusions for morphine and saline self-administered animals in daily sessions (0.5 mg/kg/infusion, 4 h/d). (B) Locomotor activity during the 4-hour self-administration sessions. (C) Number of fecal boli produced by morphine (MSA) and saline self-administered (SSA) animals during the sessions. (D) Body weight change during the period of self-administration. Body weights are adjusted from those of self-administration day 1. Data are shown as mean ± SEM. 2-way ANOVA and post hoc tests. *Significant at P < .05, MSA vs SSA. n = 10/group.
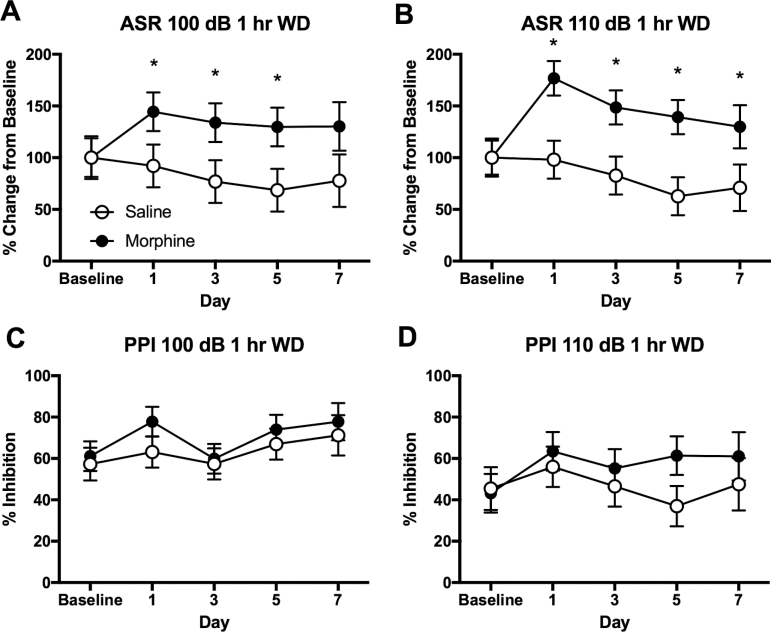
Figure 3.
Increased acoustic startle reflex (ASR) at 1-hour withdrawal from morphine self-administration (MSA) in rats. (A) Increased ASR of 100 dB at 1-hour withdrawal from MSA. (B) Increased ASR of 110 dB at 1-hour withdrawal from MSA. (C) No significant effects of MSA on prepulse inhibition (PPI) of 100 dB. (D) No significant effects of MSA on PPI of 110 dB. The ASR data are adjusted from the baseline of individual animals. Data are shown as mean ± SEM. 2-way ANOVA and post hoc Tukey tests. *Significant at P < .05, MSA vs SSA. n = 10/group.
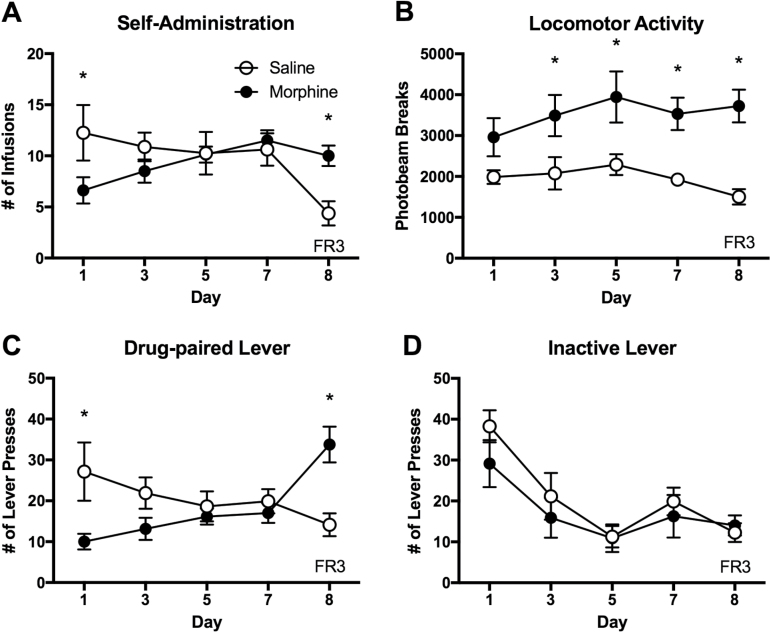
Figure 2.
Intravenous morphine self-administration with a FR3 schedule of reinforcement in rats. The animals were tested with a FR1 schedule for 7 days and with a FR3 schedule on day 8. (A) Number of morphine and saline infusions in daily 4-hour sessions. (B) Locomotor activity during the 4-hour self-administration sessions. (C) Number of drug- and saline-paired lever presses during the 4-hour sessions. (D) Number of inactive lever presses during the sessions. Data are shown as mean ± SEM. 2-way ANOVA and post hoc tests. *Significant at P < .05, MSA vs SSA. n= 8/group.
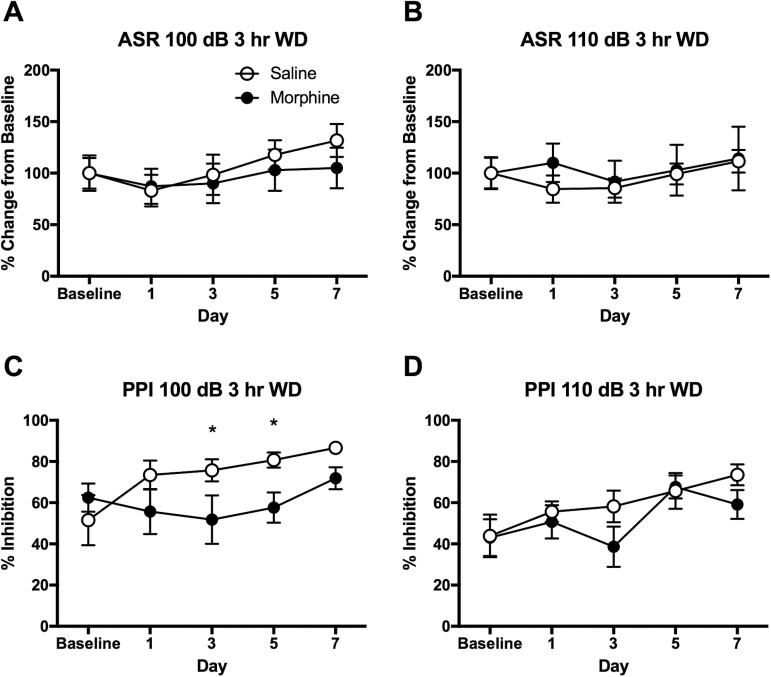
Figure 4.
Disrupted prepulse inhibition (PPI) at 3-hour withdrawal from morphine self-administration (MSA) in rats. (A) No changes in acoustic startle reflex (ASR) of 100 dB in 3-hour withdrawal from MSA. (B) No changes in ASR of 110 dB in 3-hour withdrawal from MSA. (C) Disrupted PPI of 100 dB in 3-hour withdrawal from MSA on days 3 and 5. (D) No significant changes in PPI of 110 dB in 3-hour withdrawal from MSA across 7 days of self-administration. ASR data are adjusted from the baseline of individual animals. Data are shown as mean ± SEM. 2-way ANOVA and post hoc tests. *Significant at P < .05, MSA vs SSA. n = 8/group.
ASR and PPI
The ASR and PPI tests were performed in acoustic startle boxes (Coulbourn Instrument, Columbus, OH) during 1- or 3-hour withdrawal from self-administration on days 1, 3, 5, and 7. Animals were kept individually in small cages and placed on weight-sensitive platforms in the acoustic startle boxes. Following an adaptation period of 3 minutes, the animals were tested with 6 types of acoustic startle pulses (100 and 110 decibel (dB) alone, 100 and 110 dB accompanied by prepulse of 84 dB, prepulse alone, and no stimulus). The prepulse occurred 100 milliseconds prior to the pulse. Background noise was 60 dB. To avoid order effects, each type of acoustic startle pulse was randomly tested 8 times. The baseline levels of the ASR and PPI were measured 3 days before the initiation of self-administration. The ASR after self-administration was converted to a percent compared to the baseline level. The amount of inhibition by prepulse was expressed as PPI.
BDNF ELISA Assay
Three batches of animals (MSA, n= 8 and SSA, n=8 per batch) were used for trunk blood collection on self-administration days 3, 5, and 7, respectively. Blood BDNF levels were assayed using the ELISA kit (Aviscera Bioscience, Santa Clara, CA). Serum samples were obtained from trunk blood (2 hours after the session) and were centrifuged at 2000 rpm for 20 minutes. Samples were diluted to 1/40 with dilution buffer, and then the plate was incubated for 2 hours with gentle shaking. The wells were washed 4 times and 100 μL of antibody working solution was added. After a second incubation on the shaker for 2 hours, the wells were washed 4 times. Then 100 μL of conjugate working solution was added followed by a 3rd incubation for 1 hour. After washing the wells 4 more times, 100 μL of substrate solution was added and the wells were incubated for 8 minutes on a shaker. A stop solution of 100 μL was added, and after incubation, the optical density was detected at 450 nm using an Infinite M200 Pro Microplate Reader (Tecan US, Morrisville, NC). To test for the possibility of intra-assay variation, each sample was tested in duplicate. This assay protocol followed the manufacturer’s instructions and a previous study (Polacchini et al., 2015).
CORT ELISA Assay
The same blood samples collected on self-administration days 3, 5, and 7 were used for a CORT assay using the ELISA kit (Arbor Assays, Ann Arbor, MI). The blood was centrifuged at 2000 rpm for 20 minutes and the plasma was diluted to 1/100 with assay buffer. Standard solutions were made with assay buffer and stock solution in decreasing concentration. Then 50 μL of each sample and standard were placed into a 96-well plate, and 25 μL of CORT conjugate and antibody were added to each well. After shaking for 1 hour at room temperature, the plate was aspirated and each well was washed 4 times with 300 μL of wash buffer. The TMB substrate (100 μL) was added to each well and the plate was incubated for 30 minutes at room temperature without shaking. Afterwards, 50 μL of stop solution was added. The optical density was read at 450 nm using an Infinite 200 Pro Microplate Reader (Tecan US). This protocol followed the method of a previous study (Larco et al., 2012).
Statistical Analysis
The behavioral data of self-administration, the ASR, and PPI, were analyzed using a 2-way ANOVA and post hoc Tukey test. The blood sample analyses for BDNF and CORT were performed using a 2-way ANOVA and post hoc tests. Statistical significance was regarded with P < .05.
Results
MSA
During the 7-day period of self-administration, the number of infusions was similar between the MSA and SSA groups (Figure 1A). The 2-way ANOVA revealed no significant interaction (F[3,68]= 1.195, P>.05) and main effect of drug (F[1,68]= 0.558, P>.05) and time (F[3,68]= 0.228, P>.05). However, locomotor activity in the self-administration chambers was significantly higher in the MSA compared with the SSA group from the first day of self-administration (Figure 1B). The 2-way ANOVA indicated a significant main effect of drug (F[1,68]= 46.83, P < .0001) on locomotor activity. I.v. MSA reduced defecation during the 4 hr self-administration sessions (Figure 1C), indicating a significant main effect of drug (F[1,68]= 49.41, P < .0001). The fecal boli counts of the morphine group corresponded to 40.82%, 19.52%, 11.55%, and 4.58% of those of the saline group on the 1st, 3rd, 5th, and 7th day, respectively, indicating a gradual increase in the constipation effect. Body weight gain was significantly reduced in the MSA group compared with the SSA group (Figure 1D). The 2-way ANOVA indicated a significant interaction (F[3,68]= 15.17, P < .001) and main effects of time (F[3,68]= 28.46, P < .0001) and drug (F[1,68]= 81.36, P < .0001). On the last day of self-administration, average body weight changes for the MSA and SSA groups were 101.47% and 107.84%, respectively, compared with their initial body weights.
To test the specificity of MSA, separate groups of animals were tested with a FR3 schedule of reinforcement on the 8th day of self-administration. The MSA animals maintained their daily morphine intake while the SSA animals reduced saline intake with the FR3 schedule (Figure 2A). The 2-way ANOVA indicated a significant interaction between drug and time (F[4,70]= 3.729, P < .01). Locomotor activity during the self-administration sessions was greater in the MSA group compared with that of the SSA group (Figure 2B). The 2-way ANOVA indicated a significant main effect of drug (F[1,70]= 41.68, P < .0001). The MSA group increased the number of drug-paired lever presses by 3 times, while the SSA group actually reduced theirs with the FR3 schedule (Figure 2C). There was a significant interaction between drug and time on lever presses (F[4,70]= 6.791, P < .0001). The number of inactive lever presses was not different between the MSA and SSA groups across the testing period (Figure 2D). The 2-way ANOVA indicated no significant interaction between drug and time (F[4,70]= 0.514, P>.05) and main effect of drug (F[1,70]= 1.581, P>.05). These data indicate that the MSA was voluntary and response contingent while the SSA was not.
ASR and PPI
MSA animals showed greater ASR than the saline controls when tested at 1-hour withdrawal from the self-administration. On day 1, the morphine group showed 144.49 ± 18.77% for 100 dB and 176.77 ± 16.64% for 110 dB compared with the baseline levels, while the saline group showed similar levels compared with the baseline levels. These increases in ASR at 1-hour withdrawal from MSA were maintained up to 5 days of testing. The 2-way ANOVA revealed significant main effects of morphine on ASR for both 100 (F[1,83]= 11.493, P < .001) and 110 dB (F[1,83]= 23.039, P < .001), as shown in Figure 3A and 3B, respectively. As expected, the prepulse inhibited ASR for both 100 and 110 dB in morphine and saline animals. There was no significant difference between the MSA and SSA groups in either PPI of 100 dB (Figure 3C) or PPI of 110 dB (Figure 3D).
However, when the animals were tested in 3-hour withdrawal from self-administration, the ASR was no longer elevated in the MSA group compared with that of saline controls. The 2-way ANOVA indicated no interaction (F[4,70]= 0.246, P>.05) or main effect of time (F[4,70]= 1.128, P>.05) or drug (F[1,70]= 0.676, P>.05) on ASR of 100 dB (Figure 4A). The 2-way ANOVA indicated no interaction (F[4,70]= 0.159, P>.05) or main effect of time (F[4,70]= 0.46, P>.05) or drug (F[1,70]= 0.438, P>.05) on ASR of 110 dB (Figure 4B). On the contrary, the PPI was disrupted at 3-hour withdrawal from MSA on days 3 and 5 of self-administration (Figure 4C). The 2-way ANOVA revealed a significant main effect of drug on PPI of 100 dB (F[1,70]= 7.417, P < .01). The PPI of 110 dB was not significantly different between the MSA and SSA groups (Figure 4D). The 2-way ANOVA indicated a significant effect of time (F[4,70]= 3.603, P < .01) but not drug (F[1,70]= 2.343, P>.05) on the PPI of 110 dB.
BDNF
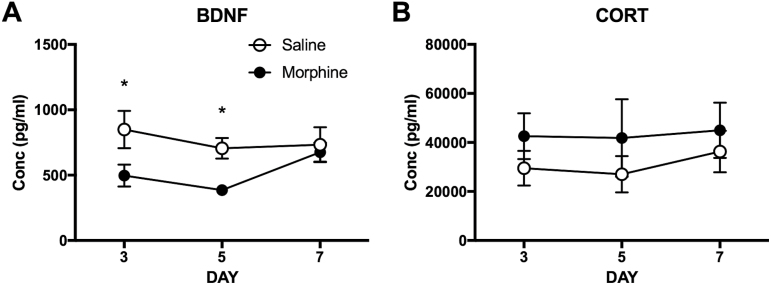
The BDNF levels in blood were measured on self-administration days 3, 5, and 7. The 2-way ANOVA indicated a significant main effect of drug (F[1, 42]= 9.011, P < .01) on BDNF levels. Posthoc tests revealed significant differences between the MSA and SSA groups on day 3 and 5 (P < .05) as shown in Figure 5A. The BDNF levels on self-administration day 7 were not different between the MSA and SSA groups. Results indicated that the BDNF levels following 5 days of MSA were lower than those of the SSA group by 58%.
Figure 5.
Reduced brain-derived neurotrophic factor (BDNF) levels in blood in 2-hour withdrawal from morphine self-administration (MSA) in rats. (A) Reduced BDNF levels in MSA compared with those of the saline controls on days 3 and 5. (B) No significant effects of MSA on corticosterone (CORT) levels during the period of self-administration. Data are shown as mean ± SEM. 2-way ANOVA and post hoc tests. *Significant at P < .05, MSA vs SSA. n = 8/group.
CORT
The CORT levels in blood were also measured on self-administration days 3, 5, and 7. The 2-way ANOVA indicated no significant effect of drug (F[1, 42]= 2.074, P>.05) on CORT levels (Figure 5B). The CORT levels in the MSA (41.85 ± 15.89 ng/mL) and SSA (27.88 ± 7.4 ng/mL) groups on self-administration day 5 were comparable with the levels collected at baseline, indicating that the animals were not overtly stressed due to the daily self-administration procedure.
Discussion
The current study found that i.v. MSA (4 h/d) increased ASR and disrupted PPI in rats in a time-dependent manner. From the first day of self-administration, morphine animals exhibited significantly higher locomotor activity and reduced defecation compared with those of saline animals. These results are consistent with previous studies that demonstrated the psychomotor activation effects of morphine (Jing et al., 2011; Liu et al., 2012; Wang et al., 2014; Wei et al., 2016). This is important to note, because the number of infusions was similar between the MSA and SSA groups with a FR1 schedule of reinforcement. The similar number of infusions between the MSA and SSA groups is due to the increased activity of animals that were tested in their dark cycle. With a unit dose of 0.5 mg/kg, animals did not self-administer morphine too frequently, because the plasma half-life of morphine is approximately 2 hours in rodents (Iwamoto and Klaassen, 1977). Moreover, when the reinforcement schedule was changed from FR1 to FR3, morphine animals increased the number of their lever presses to maintain stable morphine intake, while saline animals actually reduced their lever presses for saline intake. Thus, morphine animals showed response contingent self-administration behavior, whereas the saline animals did not. It is well described that morphine produces constipation (Hoot et al., 2013; Webster et al., 2014) and reduces body weight gain, which are side effects of opiate use (Sparber and Meyer, 1978). From the first day of self-administration, the morphine animals exhibited significantly less excretion, and there was no sign of tolerance to the constipation caused by morphine. Thus, these data indicate that the animals self-administered a significant amount of morphine and exhibited the adverse effects of morphine during the period.
It has been suggested that the elevation of the ASR in morphine withdrawal may be a sign of anxiety in rodents (Harris and Gewirtz, 2004; Rothwell et al., 2009). In particular, withdrawal from a single injection of morphine (10 mg/kg) produced an increase in the ASR lasting up to 4 hours in rats (Harris and Gewirtz, 2004). It is not known whether acute withdrawal from MSA elevates ASR in rodents. The current study found that the ASR was elevated at 1-hour but not 3-hour withdrawal from daily MSA compared with the ASR of the saline controls. This potentiation of ASR at 1-hour withdrawal from MSA was persistent across several days of testing. The discrepancy between the current and previous studies may have been due to different routes of morphine administration, doses of morphine, and metabolism of morphine in animals. For instance, the animals self-administered i.v. morphine (approximately 5 mg/kg over a 4-h period), whereas the previous study injected 10 mg/kg morphine in rats (Harris and Gewirtz, 2004). Although the exact time course of ASR elevation is different between the studies, these findings are in line with the notion that withdrawal from morphine increases anxiety-like behaviors in rodents.
In addition to the ASR, the PPI was measured in the same animals. As expected, a prepulse presentation inhibited the ASR in both the morphine and saline groups. Interestingly, PPI was disrupted at 3-hour but not 1-hour withdrawal from morphine on self-administration days 3 and 5. This is similar to a study that reported disrupted PPI following repeated morphine injections (Meng et al., 2010). Disrupted PPI during spontaneous withdrawal from morphine may contribute to the adverse effects of opiate use. Taken together, the current results suggest that spontaneous withdrawal from i.v. MSA may affect anxiety and sensorimotor gating in a time-dependent manner.
Previous studies on the effects of morphine on BDNF levels are inconsistent. For example, decreased BDNF signaling in the VTA may underlie morphine’s reinforcing effect (Koo et al., 2012), whereas BDNF has been shown to promote opiate reward via GABAA receptor function with a single infusion into the VTA (Vargas-Perez et al., 2009). On the other hand, both chronic morphine administration and subsequent withdrawal produced no change in VTA BDNF levels (Numan et al., 1998). Other studies reported that the levels of mRNA (Lunden and Kirby, 2013) and histone methylation (Mashayekhi et al., 2012) of brain BDNF were decreased by 7 days of withdrawal from chronic morphine. Very little is known of the effects of morphine on blood BDNF levels. A study reported that 1-day withdrawal from repeated morphine injections did not change plasma BDNF levels, while 14 days of withdrawal increased BDNF levels (Geoffroy et al., 2015). In the current study, BDNF levels in the blood were decreased in 2-hour withdrawal from MSA compared with those from SSA. These effects were evident on self-administration days 3 and 5. To our knowledge, this is the first study to report a transient reduction of blood BDNF levels during the daily MSA in rats.
In addition, previous studies reported a contradictory role for BDNF in anxiety-like behavior. Social isolation-induced anxiety resulted in elevated BDNF expression in the cerebral cortex of mice (Kumari et al., 2016), whereas offspring of dams exposed to gestational stress showed reduced BDNF levels in blood (Zheng et al., 2016). Also, anxiety-like behaviors induced by monosodium glutamate (Rosa et al., 2016) and amnestic effects by MK-801 (Hill et al., 2015) reduced BDNF expression in the hippocampus. Therefore, given that BDNF levels in the brain and peripheral tissue are parallel (Karege et al., 2002; Klein et al., 2011), our study suggests a possibility that decreased blood BDNF levels may be associated with anxiety-like behavior in morphine withdrawal. Interestingly, a recent study demonstrated that the BDNF met allele, which is considered as a risk factor for anxiety, is related with reduced ASR (Armbruster et al., 2016). That study is in parallel with the current findings in that BDNF levels showed an inverse relationship with the ASR. Very little is known about the role of BDNF on sensorimotor gating in the brain. The current study found disrupted PPI in 3-hour withdrawal from MSA on self-administration days 3 and 5, which may be linked with reduced BDNF levels on the same days. A further study is warranted to investigate the functional significance of BDNF on sensorimotor gating mechanism in the brain.
Contrary to the BDNF levels, the CORT levels in blood were not altered following MSA in rats. Also, the CORT levels in both MSA and SSA groups were comparable with the basal CORT levels measured in home cage controls (data not shown). Given that CORT levels reflect stress responses in rats (Jia et al., 2015), it appears that the daily handling and testing of the animals did not increase stress responses. This is supported by the similar CORT levels of our data and of normal control groups of other studies (Gomez et al., 2000; Nunez et al., 2009). According to the previous studies (Nunez et al., 2009; Ueno et al., 2011), there is evidence of CORT levels rising in rats withdrawn from morphine. However, those studies used passive administration of a high dose of morphine, followed by opioid antagonist-precipitated withdrawal, which can be stressful to animals. In the current study, the animals self-regulated their own morphine intake and experienced spontaneous withdrawal, which is more relevant to human opiate addiction. Thus, differences in morphine doses, routes of administration, and types of withdrawal may have contributed to the discrepancy between the current and previous studies. It is important to characterize different withdrawal time points following chronic MSA to better understand the relationship between opiate abuse and the stress system in the body.
In conclusion, the current study found that spontaneous withdrawal from i.v. MSA increased the ASR, disrupted PPI, and reduced BDNF levels in a time-dependent manner. These results suggest that repeated opiate use may increase anxiety and impair sensory gating mechanism, and reduced peripheral BDNF may be a biological substrate for opiate abuse. A further study is necessary to investigate the functional significance of BDNF in opiate abuse and withdrawal to enhance our understanding of the biological basis of opiate use disorders.
Statement of Interest
None.
Acknowledgments
The authors thank Daegu Haany University for the Kylin Sabbatical of Dr. B. H. Lee.
This study was supported by the USUHS Intramural Grant (R088305316) and Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2010-0025821). The opinions or assertions contained herein are solely those of the authors and are not to be construed as official or reflective of the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
References
- Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. (1995) A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 374:450–453. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Ricci V, Pomponi M, Conte G, Mathe AA, Attilio Tonali P, Bria P. (2007) Chronic heroin and cocaine abuse is associated with decreased serum concentrations of the nerve growth factor and brain-derived neurotrophic factor. J Psychopharmacol 21:820–825. [DOI] [PubMed] [Google Scholar]
- Armbruster D, Muller-Alcazar A, Strobel A, Lesch KP, Kirschbaum C, Brocke B. (2016) BDNF val(66)met genotype shows distinct associations with the acoustic startle reflex and the cortisol stress response in young adults and children. Psychoneuroendocrinology 66:39–46. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Nestler EJ. (2004) Neurotrophic mechanisms in drug addiction. Neuromol Med 5:69–83. [DOI] [PubMed] [Google Scholar]
- Cabral A, Ruggiero RN, Nobre MJ, Brandao ML, Castilho VM. (2009) GABA and opioid mechanisms of the central amygdala underlie the withdrawal-potentiated startle from acute morphine. Prog Neuropsychopharmacol Biol Psychiatry 33:334–344. [DOI] [PubMed] [Google Scholar]
- Canudas AM, Pezzi S, Canals JM, Pallas M, Alberch J. (2005) Endogenous brain-derived neurotrophic factor protects dopaminergic nigral neurons against transneuronal degeneration induced by striatal excitotoxic injury. Brain Res Mol Brain Res 134:147–154. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. (2010) Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35:105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Fan X, Li Y, Guo J, Xia D, Ding L, Zheng Q, Wang W, Xue F, Chen R, Liu S, Hu L, Gong Y. (2016) Blunted inflammation mediated by NF-kappaB activation in hippocampus alleviates chronic normobaric hypoxia-induced anxiety-like behavior in rats. Brain Res Bull 122:54–61. [DOI] [PubMed] [Google Scholar]
- Fodor A, Kovacs KB, Balazsfi D, Klausz B, Pinter O, Demeter K, Daviu N, Rabasa C, Rotllant D, Nadal R, Zelena D. (2016) Depressive- and anxiety-like behaviors and stress-related neuronal activation in vasopressin-deficient female Brattleboro rats. Physiol Behav 158:100–111. [DOI] [PubMed] [Google Scholar]
- Geoffroy HA, Puig S, Benturquia N, Noble F. (2015) Temporal regulation of peripheral BDNF levels during cocaine and morphine withdrawal: comparison with a natural reward. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW. (1993) Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry 34:361–372. [DOI] [PubMed] [Google Scholar]
- Glover EM, Davis M. (2008) Anxiolytic-like effects of morphine and buprenorphine in the rat model of fear-potentiated startle: tolerance, cross-tolerance, and blockade by naloxone. Psychopharmacology (Berl) 198:167–180. [DOI] [PubMed] [Google Scholar]
- Gomez F, Leo NA, Grigson PS. (2000) Morphine-induced suppression of saccharin intake is correlated with elevated corticosterone levels. Brain Res 863:52–58. [DOI] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC. (2004) Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. Psychopharmacology (Berl) 171:140–147. [DOI] [PubMed] [Google Scholar]
- Harris AC, Atkinson DM, Aase DM, Gewirtz JC. (2006) Double dissociation in the neural substrates of acute opiate dependence as measured by withdrawal-potentiated startle. Neuroscience 139:1201–1210. [DOI] [PubMed] [Google Scholar]
- Heberlein A, Dursteler-MacFarland KM, Lenz B, Frieling H, Grosch M, Bonsch D, Kornhuber J, Wiesbeck GA, Bleich S, Hillemacher T. (2011) Serum levels of BDNF are associated with craving in opiate-dependent patients. J Psychopharmacol 25:1480–1484. [DOI] [PubMed] [Google Scholar]
- Hill XL, Richeri A, Scorza C. (2015) Measure of anxiety-related behaviors and hippocampal BDNF levels associated to the amnesic effect induced by MK-801 evaluated in the modified elevated plus-maze in rats. Physiol Behav 147:359–363. [DOI] [PubMed] [Google Scholar]
- Hoot MR, Sypek EI, Reilley KJ, Carey AN, Bidlack JM, McLaughlin JP. (2013) Inhibition of Gbetagamma-subunit signaling potentiates morphine-induced antinociception but not respiratory depression, constipation, locomotion, and reward. Behav Pharmacol 24:144–152. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Klaassen CD. (1977) First-pass effect of morphine in rats. J Pharmacol Exp Ther 200:236–244. [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. (2003) Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci 24:566–573. [DOI] [PubMed] [Google Scholar]
- Jia M, Smerin SE, Zhang L, Xing G, Li X, Benedek D, Ursano R, Li H. (2015) Corticosterone mitigates the stress response in an animal model of PTSD. J Psychiatr Res 60:29–39. [DOI] [PubMed] [Google Scholar]
- Jing L, Luo J, Zhang M, Qin WJ, Li YL, Liu Q, Wang YT, Lawrence AJ, Liang JH. (2011) Effect of the histone deacetylase inhibitors on behavioural sensitization to a single morphine exposure in mice. Neurosci Lett 494:169–173. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M. (2002) Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett 328:261–264. [DOI] [PubMed] [Google Scholar]
- Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S. (2011) Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol 14:347–353. [DOI] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno D, Crumiller M, Ohnishi YN, Ohnishi YH, Mouzon E, Dietz DM, Lobo MK, Neve RL, Russo SJ, Han MH, Nestler EJ. (2012) BDNF is a negative modulator of morphine action. Science 338: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. (2005) Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48:303–314. [DOI] [PubMed] [Google Scholar]
- Kumari A, Singh P, Baghel MS, Thakur MK. (2016) Social isolation mediated anxiety like behavior is associated with enhanced expression and regulation of BDNF in the female mouse brain. Physiol Behav 158:34–42. [DOI] [PubMed] [Google Scholar]
- Larco DO, Cruthirds DF, Weiser MJ, Handa RJ, Wu TJ. (2012) The effect of chronic immobilization stress on leptin signaling in the ovariectomized (OVX) rat. Endocrine 42:717–725. [DOI] [PubMed] [Google Scholar]
- Le T, Xia M, Jia M, Sarkar N, Chen J, Li H, Wynn GH, Ursano RJ, Choi KH. (2014) Association between initial morphine intake and body weight change, acoustic startle reflex and drug seeking in rats. Psychopharmacology 231:4569–4577. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang M, Qin WJ, Wang YT, Li YL, Jing L, Li JX, Lawrence AJ, Liang JH. (2012) Septal nuclei critically mediate the development of behavioral sensitization to a single morphine injection in rats. Brain Res 1454:90–99. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Etzensberger M, Vollenweider FX. (2002) Stability of the acoustic startle reflex, prepulse inhibition, and habituation in schizophrenia. Schizophr Res 55:129–137. [DOI] [PubMed] [Google Scholar]
- Lunden JW, Kirby LG. (2013) Opiate exposure and withdrawal dynamically regulate mRNA expression in the serotonergic dorsal raphe nucleus. Neuroscience 254:160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekhi FJ, Rasti M, Rahvar M, Mokarram P, Namavar MR, Owji AA. (2012) Expression levels of the BDNF gene and histone modifications around its promoters in the ventral tegmental area and locus ceruleus of rats during forced abstinence from morphine. Neurochem Res 37:1517–1523. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14:375–424. [DOI] [PubMed] [Google Scholar]
- Meng Z, Zhou D, Wang J, Ma Y. (2010) Chronic morphine treatment decreases acoustic startle response and prepulse inhibition in rats. Sci China Life Sci 53:1356–1360. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Patapoutian A. (2011) Why does morphine make you itch? Cell 147:261–262. [DOI] [PubMed] [Google Scholar]
- Numan S, Lane-Ladd SB, Zhang L, Lundgren KH, Russell DS, Seroogy KB, Nestler EJ. (1998) Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci 18:10700–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez C, Foldes A, Perez-Flores D, Garcia-Borron JC, Laorden ML, Kovacs KJ, Milanes MV. (2009) Elevated glucocorticoid levels are responsible for induction of tyrosine hydroxylase mRNA expression, phosphorylation, and enzyme activity in the nucleus of the solitary tract during morphine withdrawal. Endocrinology 150:3118–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EC, Chapman K, Butler P, Mead AN. (2011) The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev 35:912–938. [DOI] [PubMed] [Google Scholar]
- O’Neill CE, Newsom RJ, Stafford J, Scott T, Archuleta S, Levis SC, Spencer RL, Campeau S, Bachtell RK. (2016) Adolescent caffeine consumption increases adulthood anxiety-related behavior and modifies neuroendocrine signaling. Psychoneuroendocrinology 67:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, Sanfilipo M, Chappell PB, Chakravorty S, Gonzenbach S, Ko GN, Rotrosen JP. (2000) Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry 47:662–669. [DOI] [PubMed] [Google Scholar]
- Polacchini A, Metelli G, Francavilla R, Baj G, Florean M, Mascaretti LG, Tongiorgi E. (2015) A method for reproducible measurements of serum BDNF: comparison of the performance of six commercial assays. Sci Rep 5:17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa SG, Quines CB, Stangherlin EC, Nogueira CW. (2016) Diphenyl diselenide ameliorates monosodium glutamate induced anxiety-like behavior in rats by modulating hippocampal BDNF-Akt pathway and uptake of GABA and serotonin neurotransmitters. Physiol Behav 155:1–8. [DOI] [PubMed] [Google Scholar]
- Rothwell PE, Thomas MJ, Gewirtz JC. (2009) Distinct profiles of anxiety and dysphoria during spontaneous withdrawal from acute morphine exposure. Neuropsychopharmacology 34:2285–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PE, Gewirtz JC, Thomas MJ. (2010) Episodic withdrawal promotes psychomotor sensitization to morphine. Neuropsychopharmacology 35:2579–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM. (1994) Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol 342:321–334. [DOI] [PubMed] [Google Scholar]
- Sparber SB, Meyer DR. (1978) Clonidine antagonizes naloxone-induced suppression of conditioned behavior and body weight loss in morphine-dependent rats. Pharmacol Biochem Behav 9:319–325. [DOI] [PubMed] [Google Scholar]
- Tziridis K, Ahlf S, Schulze H. (2012) A low cost setup for behavioral audiometry in rodents. J Vis Exp 68:4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Maeda T, Kiguchi N, Kobayashi Y, Ozaki M, Kishioka S. (2011) Availability of serum corticosterone level for quantitative evaluation of morphine withdrawal in mice. Drug Discov Ther 5:71–75. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, De Vries TJ, Wardeh G, Hogenboom FA, Schoffelmeer AN. (2001) A single exposure to morphine induces long-lasting behavioural and neurochemical sensitization in rats. The European journal of neuroscience 14:1533–1538. [DOI] [PubMed] [Google Scholar]
- Vargas-Perez H, Ting AKR, Walton CH, Hansen DM, Razavi R, Clarke L, Bufalino MR, Allison DW, Steffensen SC, van der Kooy D. (2009) Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science 324:1732–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Qin WJ, Liu Q, Li YL, Liang H, Chen F, Lawrence AJ, Zhang XL, Liang JH. (2014) Chaperone heat shock protein 70 in nucleus accumbens core: a novel biological target of behavioural sensitization to morphine in rats. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 17:469–484. [DOI] [PubMed] [Google Scholar]
- Warren PH, Ison JR. (1982) Selective action of morphine on reflex expression to nociceptive stimulation in the rat: a contribution to the assessment of analgesia. Pharmacol Biochem Behav 16:869–874. [DOI] [PubMed] [Google Scholar]
- Webster L, Chey WD, Tack J, Lappalainen J, Diva U, Sostek M. (2014) Randomised clinical trial: the long-term safety and tolerability of naloxegol in patients with pain and opioid-induced constipation. Aliment Pharmacol Ther 40: 771–779. [DOI] [PubMed] [Google Scholar]
- Wei L, Zhu YM, Zhang YX, Liang F, Li T, Gao HY, Huo FQ, Yan CX. (2016) The alpha1 adrenoceptors in ventrolateral orbital cortex contribute to the expression of morphine-induced behavioral sensitization in rats. Neurosci Lett 610:30–35. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. (2003) Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci 91:267–270. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang X, Su H, Tao J, Xie Y, Han B, Lu Y, Wei Y, Sun H, Wang Y, Wu W, Zou S, Liang H, Zoghbi AW, Tang W, He J. (2014) Increased serum brain-derived neurotrophic factor levels during opiate withdrawal. Neurosci Lett 571:61–65. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Fan W, Zhang X, Dong E. (2016) Gestational stress induces depressive-like and anxiety-like phenotypes through epigenetic regulation of BDNF expression in offspring hippocampus. Epigenetics 11:150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]