Abstract
Background:
Tamoxifen is the most widely used drug for treating patients with estrogen receptor-sensitive breast cancer. There is evidence that breast cancer patients treated with tamoxifen exhibit cognitive dysfunction. However, the underlying neural mechanism remains unclear. The present study aimed to investigate the neural mechanisms underlying working memory deficits in combination with functional connectivity changes in premenopausal women with breast cancer who received long-term tamoxifen treatment.
Methods:
A total of 31 premenopausal women with breast cancer who received tamoxifen and 32 matched healthy control participants were included. The participants completed n-back tasks and underwent resting-state functional magnetic resonance imaging, which measure working memory performance and brain functional connectivity, respectively. A seed-based functional connectivity analysis within the whole brain was conducted, for which the dorsolateral prefrontal cortex was chosen as the seed region.
Results:
Our results indicated that the tamoxifen group had significant deficits in working memory and general executive function performance and significantly lower functional connectivity of the right dorsolateral prefrontal cortex with the right hippocampus compared with the healthy controls. There were no significant changes in functional connectivity in the left dorsolateral prefrontal cortex within the whole brain between the tamoxifen group and healthy controls. Moreover, significant correlations were found in the tamoxifen group between the functional connectivity strength of the dorsolateral prefrontal cortex with the right hippocampus and decreased working memory performance.
Conclusion:
This study demonstrates that the prefrontal cortex and hippocampus may be affected by tamoxifen treatment, supporting an antagonistic role of tamoxifen in the long-term treatment of breast cancer patients.
Keywords: breast cancer, endocrine therapy, working memory, functional connectivity, tamoxifen
Significance Statement
Previous studies have reported that the breast cancer patients treated with tamoxifen (TMX) had cognitive dysfunction. But the underlying neural mechanism remains unclear. We report that TMX users had deficits in working memory performance and significantly lower functional connectivity of the right DLPFC with the right hippocampus (DLPFC-HP). We also find that the functional connectivity strength of the DLPFC-HP is associated with decreased working memory performance for TMX users. This brain functional connectivity change may be underlying neural mechanism of working memory deficits. Additionally, TMX may play an antagonistic role in the brain, particularly in the DLPFC and hippocampus.
Introduction
Endocrine therapies are an important component of systematic treatment for breast cancer. Tamoxifen (TMX), one type of endocrine therapeutic agent, is the most widely used drug for treatment of patients with estrogen receptor-sensitive breast cancer. TMX is a selective estrogen receptor modulator. It exerts differential effects by binding to estrogen receptors throughout the body and is recognized as having a mixed estrogen agonist/antagonist effect. TMX acts as an estrogen antagonist in breasts and is used to prevent and treat breast cancer. By acting as an estrogen receptor agonist, it also increases the risk of adverse side effects, including uterine cancer, vision problems, cardiovascular disease, and venous thrombosis (Perez, 2007).
TMX readily crosses the blood brain barrier and binds to estrogen receptors in the nervous system (Lien et al., 1991; McEwen and Alves, 1999), but its effects on the human brain remain unclear. Estrogen receptors are present in neurons of forebrain regions, such as the basal forebrain, the hypothalamus, the prefrontal cortex, and the hippocampus (Toran-Allerand et al., 1999). Thus, the actions of TMX on brain structure and function would be associated with cognitive function. Interestingly, emerging data show that breast cancer patients exhibit cognitive impairments related to TMX treatment, including memory deficits (Shilling et al., 2003; Schilder et al., 2009; Boele et al., 2015), visuospatial ability (Ahles et al., 2010), and executive dysfunction (Shilling et al., 2003; Palmer et al., 2008; Schilder et al., 2010). These cognitive impairments have a significant influence on patients’ daily function and quality of life and have become an important area of research.
As a major component of cognitive functions, memory is involved in accumulating and preserving individual experiences and plays an important role in the entire brain and mental activities. Previous studies focusing on this topic have shown that breast cancer patients who receive TMX treatment have significant memory deficits, such as verbal memory (Palmer et al., 2008; Collins et al., 2009; Schilder et al., 2010; Breckenridge et al., 2012) and visuospatial memory (Bender et al., 2007; Ahles et al., 2010). However, several studies reported that no adverse effects of TMX were observed on memory performance in breast cancer patients (Fan et al., 2005; Hermelink et al., 2008; Le Rhun et al., 2015). On the contrary, TMX treatment exerts positive effects on the relative protection of verbal episodic memory function from cholinergic blockades in postmenopausal women (Newhouse et al., 2013). These inconsistent results may be due to different experimental designs, methodological discrepancies, and population heterogeneity (Bakoyiannis et al., 2016).
Working memory is a key area of study of the memory system and is considered as a core component of many other cognitive functions, including language comprehension, learning, reasoning, and problem solving (Baddeley, 1992). There is an emerging consensus that working memory plays an important role in temporary information processing and guidance of complex cognitive behavior involving reentrant loops among the frontal and posterior cortical structures and subcortical areas (Eriksson et al., 2015). Evidence has suggested that brain areas in the dorsolateral prefrontal cortex (DLPFC) are involved in working memory functions (Owen et al., 2005; Barbey et al., 2013). Previous animal studies (Funahashi et al., 1989; Levy and Goldman-Rakic, 2000) and clinical brain lesion studies (D’Esposito and Postle, 1999) have demonstrated that the DLPFC is causally involved in normal working memory function. Human neuroimaging studies have suggested persistent neural activity in the DLPFC during working memory tasks (Curtis and D’Esposito, 2003; Feredoes et al., 2011; Brunoni and Vanderhasselt, 2014). Several meta-analyses have reported the fundamental role of the DLPFC based on activation in working memory operations (Nee et al., 2013; Brunoni and Vanderhasselt, 2014). Resting state functional connectivity, which is measured by resting state functional magnetic resonance imaging (fMRI), assesses the temporal correlations of intrinsic low-frequency fluctuations across individual blood oxygenation level-dependent (BOLD) time points during rest and has been used to explore the complex cognitive processes and brain networks and synchronous brain activity (van den Heuvel and Hulshoff Pol, 2010; Rosazza and Minati, 2011). Using this technique, researchers have found disrupted functional connectivity in various types of neurological impairment, including Alzheimer’s disease (Chase, 2014), Parkinson’s disease (Campbell et al., 2015), and psychiatric disorders (Schilbach et al., 2015). Neuroimaging studies have also shown that both structural and functional brain changes occur in females who undergo TMX treatment, such as inferior and dorsal lateral prefrontal lobe hypometabolism based on positron emission tomography and decreased hippocampal volume revealed by MRI (Eberling et al., 2004). Therefore, it is reasonable to presume that TMX has negative effects on the connectivity of the DLPFC and that these effects are associated with working memory performance.
Several neuroimaging methods can be used to examine the effects of TMX on brain structure and function to investigate the underlying mechanisms contributing to cognitive changes. Studies examining the effects of TMX for treatment of breast cancer on brain functional connectivity are limited, and the effects are confounded by menopausal symptoms, chemotherapy treatment, and age (Ahles and Saykin, 2007; Palmer et al., 2008). Thus, the aim of the present study was to investigate the neural mechanisms underlying working memory deficits in combination with functional connectivity changes in premenopausal women with breast cancer who were treated with TMX but not chemotherapy. Based on the data mentioned above, we chose the DLPFC as the seed region and calculated the functional connectivity within the whole brain via resting state fMRI. We hypothesized that breast cancer patients treated with TMX would have decreased working memory performance and altered functional connectivity of the DLPFC with whole brain, particularly with hippocampus, compared with healthy control participants. We also examined the relationship between working memory deficits and the functional connectivity strength of the DLPFC.
Materials and Methods
Participants and Clinical Diagnosis
The current study was approved by the Research Ethics Committee of the First Affiliated Hospital of Anhui Medical University. Following a complete description of the study objective, all participants provided written informed consent. All 33 patients with breast cancer (stages I-II, female) were recruited from the First Affiliated Hospital of Anhui Medical University, where they were treated with TMX (20 mg daily) for a mean of 40.45 ± 9.63 months, and TMX was administered for at least 24 months. All the participants were carefully screened by self-report screening questionnaires to ensure that they were premenopausal and had received no chemotherapy treatment; had not been diagnosed with dementia, brain injury, psychiatric treatment, or alcohol or drug abuse; and exhibited no MRI contraindications. Thirty-three age- and education-matched healthy controls recruited from the local community and from among the patients’ relatives also participated in this study. Particularly, these relatives were not first-degree relatives of the included breast cancer patients. All participants had no subtle or severe affective disorders (Hamilton Depression Rating Scale (HAMD) scores <8 and/or Hamilton Anxiety Rating Scale (HAMA) scores <8). The detailed information gathered from each participant is described in Table 1.
Table 1.
Participant Demographic Characteristics
| TMX group (n = 31) | HC group (n = 32) | t | P | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Age (y) | 44.97 (4.56) | 43.66 (4.66) | 1.126 | 0.264 |
| Education (y) | 10.74 (2.02) | 11.53 (2.26) | -1.462 | 0.149 |
| Breast cancer stage | ||||
| I | 25 | NA | NA | NA |
| II | 6 | NA | NA | NA |
| Received radiotherapy | 12 | NA | NA | NA |
| HAMA | 4.97 (1.30) | 4.47 (1.39) | 1.468 | 0.147 |
| HAMD | 5.03 (1.02) | 4.81 (1.35) | 0.727 | 0.470 |
| FACT-B | ||||
| PWB | 1.42 (1.09) | 1.03 (0.86) | 1.572 | 0.121 |
| SWB | 1.13 (0.76) | 1.06 (0.80) | 0.337 | 0.737 |
| FWB | 1.45 (1.23) | 0.97 (1.00) | 1.709 | 0.092 |
Abbreviations: FACT-B, Functional Assessment of Cancer Therapy-Breast questionnaire; FWB, functional well-being; HAMA, Hamilton Anxiety Rating Scale; HAMD, Hamilton Depression Rating Scale; HC, healthy control; NA, not applicable; PWB, physical well-being; SWB, social/ family well-being; TMX, tamoxifen.
Neuropsychological Background Tests
All participants were evaluated using neuropsychological background tests, which were conducted by skilled psychologists and required approximately 60 minutes to complete. The Beijing Version of the Montreal Cognitive Assessment Test (MoCA Test) was administered to assess general cognitive function. The digit span test was used to measure short-term memory. The Stroop test and Trail Making test were conducted to evaluate general executive function. The HAMD and HAMA tests were used to assess the participants’ symptoms of depression and anxiety, respectively. The Functional Assessment of Cancer Therapy-Breast questionnaire was used to assess quality of life; it included three subscales of physical, social, and functional well-being.
Working Memory Performance
Working memory was evaluated via a letter n-back task, including a 0-back, a 1-back, and a 2-back task block. The 0-back task block was the primary task to measure attention. The advanced task including 1-back and 2-back task blocks was also carried out to assess the working memory performance. The letter stimuli were presented to the participants on a computer, and the responses were collected via two mouse buttons. During the 0-back task block, the letter was presented randomly at the center of the field, the participants were instructed to press the left mouse button if the letter that appeared on the screen was “X”, and otherwise to press the right mouse button. During the 1-back and 2-back task blocks, the participants were instructed to press the left mouse button if the letter that appeared on the screen was identical to the one presented either 1 or 2 letters earlier, respectively, and otherwise to press the right mouse button. Each task block consisted of 20 trials. Each letter stimulus was presented for 500 ms with an inter-stimulus interval of 2500 ms. “No Response” was recorded if the individual did not press the mouse button within 3000 ms. Before the experiment, the participants were verbally instructed and performed a practice block. Thereafter, the participants were guided to perform the tasks 0-back, 1-back, and 2-back in order. E-Prime 1.0 software (Psychology Software Tools, Pittsburgh, PA) was used to present the stimuli and to collect the accuracy and mean reaction time (RT).
Image Data Acquisition
The neuropsychological background tests and working memory task were performed in a quiet room outside the MRI scanner before performing magnetic resonance scanning for each participant. All the participants’ MRI images were collected by using the same GE 3.0 T magnetic resonance scanner (GE Medical Systems, Milwaukee, WI) equipped with a standard head coil. During the MRI scans, all participants were instructed to keep their eyes closed, think of nothing in particular, relax, move as little as possible, and not to fall asleep. High-resolution 3D T1-weighted brain volume MRI images were obtained with the following parameters: repetition time (TR)/echo time (TE) ratio = 8.676/3.184 milliseconds, inversion time = 800 milliseconds, flip angle = 8 degrees, field of view (FOV) = 256 × 256 mm2, matrix size = 256 × 256, slice thickness = 1 mm, voxel size = 1 × 1 × 1 mm3, and number of slices = 188. The acquisition time of the sequence was 5 minutes and 45 seconds. Subsequently, resting-state functional MRI images were collected with the following parameters: TR/TE ratio=2000/22.5 milliseconds, flip angle = 30 degrees, 33 slices, thickness/gap ratio = 4.0/0.6 mm, voxel size = 3.4×3.4×4.6 mm3, matrix size=64×64, and FOV =220×220 mm2. Resting state functional MRI images were continuously acquired across 240 scans within 8 minutes.
T2-weighted TSE (19 transversal slices, 240×240-mm2 FOV, 5-mm slice thickness, 5290 ms TR, and 120 ms TE) and a FLAIR (19 transversal slices; 240×240 mm2 FOV, 136.6 ms TE, 9000 ms TR, 5-mm slice thickness, and 256×256 matrix) were also acquired to search for primary brain pathology as an exclusion criterion. The participants were asked whether they had fallen asleep during and after the scanning to ensure that none of them had fallen asleep.
fMRI Preprocessing
The fMRI data preprocessing was completed by using the Analysis of Functional NeuroImages software tool (Medical College of Wisconsin, Milwaukee, WI). First, we discarded the first 10 volumes of data to allow the magnetization to reach the equilibrium. Then, the anatomical and functional images were reconstructed and realigned using a unified orientation. Next, we performed skull stripping and motion correction, followed by coregistration between functional and anatomical images and normalization to the standard Montreal Neurological Institute (MNI) 152 brain atlas and resampled with the voxel size of 3×3×3 mm3. We excluded from subsequent analyses participants with head motion >2 mm of maximal displacement or 0.2 mm of temporal differential displacement (in any direction: x, y, or z) or 2º maximum spin or 0.2º mm of relative spin in any angular dimension. All data were band-pass filtered (0.01–0.08 Hz) to remove low-frequency drift and high-frequency noise and spatially smoothed by using a 6-mm Gaussian kernel at full-width at half-maximum. Then, several sources of spurious covariance were removed from the data by linear regression, including the signals from the cerebrospinal fluid and white matter, and the 6 head motion parameters were obtained by rigid body correction. After preprocessing, the individual data were used for further functional connectivity analyses.
Functional Connectivity Analyses
For each participant, we calculated the functional connectivity within the whole brain based on 2 seed regions in the left and right DLPFC as the regions of interest (ROIs). The ROIs were defined as 2 spherical regions with a 6-mm radius centered at the MNI coordinate of left DLPFC (-42, 33, 33) and right DLPFC (42, 33, 33) according to a previous meta-analysis study (Rottschy et al., 2012). It should be noted that these MNI coordinates of DLPFC were calculated from the MNI coordinates of left and right caudal lateral prefrontal cortex as reported in the meta-analysis study. First, we computed the Pearson’s correlation coefficients between the average BOLD time series in the ROIs and those from each voxel in the brain. Then, the correlation coefficients were transformed to better fit a normal distribution using Fisher’s Z transformation. Thus, the values of whole brain functional connectivity with the DLPFC were obtained for each participant.
Statistical Analysis
We used the 2-sample t tests to assess the differences in the participants’ demographic characteristics between the TMX user group and the healthy control group. Voxel-wise 2-sample t tests with one covariate (age) were performed to assess the differences in the whole brain functional connectivity with the DLPFC among these 2 groups. The Monte Carlo method correction was performed using a whole brain mask of the MNI template. The voxel-wise threshold of statistical significance was set to P = .005, α = 0.005 and a minimum cluster size of 52 voxels. A Pearson’s correlation analysis was further performed to assess the association between the changes in functional connectivity and working memory performance.
Results
Among the 66 participants, 2 patients and 1 healthy control were excluded from the analysis because of excessive head motion artifacts during data acquisition. Therefore, the final analytical sample size was 31 patients and 32 healthy controls. Participant demographic and clinical information are shown in Table 1. No significant differences in age, years of education, quality of life, depression, and anxiety score were observed between the TMX group and healthy controls.
Neuropsychological Background Tests
As shown in Table 2, there were no significant differences observed between the TMX group and the healthy control group with respect to general cognitive function (MoCA), short-term memory (digit span), or proceeding speed (the Stroop color and word tests, Trail Making Test A). The TMX group performed significantly worse in the tests that evaluated general executive function (the Stroop Interference Test and Trail Making Test B). Compared with the healthy control group, TMX users had statistically significant differences with respect to the accuracy and RT in the 1-back and 2-back tasks but not in the 0-back task.
Table 2.
Summary of Neuropsychological Test and Working Memory Performance
| TMX group (n = 31) | HC group (n = 32) | t | P | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| MoCA | 25.94 (1.21) | 26.50 (1.61) | -1.572 | 0.121 |
| Short term memory | ||||
| WAIS Digit Span (forward) | 5.84 (1.16) | 6.16 (1.30) | -1.024 | 0.310 |
| WAIS Digit Span (backward) | 5.00 (1.21) | 5.28 (1.20) | -0.927 | 0.358 |
| Processing speed | ||||
| Stroop Color test (sec) | 15.20 (2.60) | 14.37 (3.21) | 1.120 | 0.267 |
| Stroop Word test (sec) | 18.82 (2.84) | 17.90 (3.10) | 1.230 | 0.223 |
| Trailmaking A (sec) | 53.18 (11.64) | 51.52 (10.15) | 0.607 | 0.546 |
| Executive function | ||||
| Stroop Interference test (sec) | 34.35 (6.90) | 29.96 (6.32) | 2.628 | 0.011 |
| Trailmaking B (sec) | 102.41 (13.48) | 94.83 (10.73) | 2.475 | 0.016 |
| Working memory | ||||
| Primary task | ||||
| 0-back ACC (%) | 94.52 (6.75) | 96.56 (5.45) | -1.325 | 0.190 |
| 0-back RT (ms) | 666.97 (136.86) | 610.13 (128.13) | 1.702 | 0.094 |
| Advanced task | ||||
| 1-back ACC (%) | 76.77 (11.22) | 82.97 (9.41) | -2.378 | 0.021 |
| 1-back RT (ms) | 831.70 (186.03) | 737.98 (163.54) | 2.125 | 0.038 |
| 2-back ACC (%) | 59.52 (11.57) | 67.50 (10.08) | -2.923 | 0.005 |
| 2-back RT (ms) | 1129.1 (222.66) | 956.85 (254.45) | 2.861 | 0.006 |
Abbreviations: ACC, accuracy; HC, healthy control; MoCA, Montreal Cognitive Assessment Test; RT, reaction time; TMX, tamoxifen; WAIS, Wechsler Adult Intelligence Scale.
Functional Connectivity and Correlation
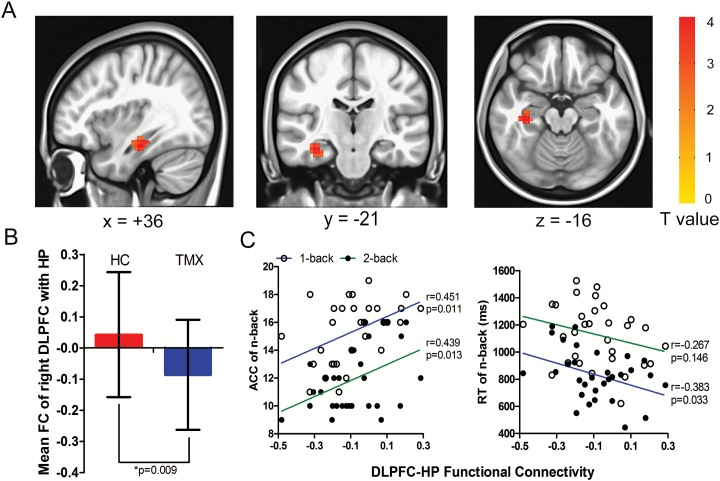
In this study, we explored the whole-brain functional connectivity differences in the DLPFC between the TMX group and healthy controls. We found that the TMX group had significantly lower functional connectivity of the right DLPFC with the right hippocampus (peak voxel MNI coordinate: x = 36, y = -21, z = -16; corrected P < .005; T = 3.599; cluster size = 79) compared with the healthy controls (Figure 1A-B). However, we observed that there were no significant functional connectivity changes in the left DLPFC with any other brain areas between the TMX group and healthy controls. To study the correlation between FC and working memory performance, a mean value of functional connectivity is calculated for each participant by computing the Pearson’s correlation coefficient between the averaged BOLD series within the seed regions of the DLPFC and hippocampus. The Pearson’s correlation analysis showed that there were significant correlations between the functional connectivity strength of the right DLPFC with the right hippocampus (DLPFC-HP) and the accuracy in the 1-back task (r = 0.451, P = .011) and 2-back task (r = 0.439, P = .013), and the RT in the 2-back task (r = -0.383, P = .033), but not in the 1-back task (r = -0.267, P = .146) in the TMX group (Figure 1C). No significant correlations were observed between the strengths of the functional connectivity of the DLPFC-HP and the ages of the participants, the MoCA, or duration of TMX treatment, HAMD, or HAMA scores, as well as between the working memory performance and demographic variables above (all P>.05). Within the healthy control group, no correlations were found demonstrated among these variables (all P>.05).
Figure 1.
Effect of tamoxifen (TMX) on the right dorsal lateral prefrontal lobe (DLPFC) with the right hippocampus (DLPFC-HP) functional connectivity and working memory performance. (A) An analysis of seed-based functional connectivity of the whole brain using the right DLPFC (seed voxel Montreal Neurological Institute [MNI] coordinate: x = 42, y = 33, z = 33) as the seed region revealed increased DLPFC connectivity with the right HP (peak voxel MNI coordinate: x = 36, y = -21, z = -16) in the TMX group compared with healthy controls (Monte Carlo method corrected P<.005). (B) The 2-sample t tests showed that the values of DLPFC-HP functional connectivity were different between the 2 groups. (C) The TMX users’ functional connectivities of the DLPFC-HP were positively correlated with the accuracy in the 1-back and 2-back tasks and negatively correlated with the reaction time in the 1-back task but not the 2-back task.
Discussion
Previous studies have reported evidence that breast cancer patients who received TMX treatment exhibit cognitive impairments (Palmer et al., 2008; Buwalda and Schagen, 2013). In the current study, we chose the n-back tasks and resting state functional connectivity to investigate the neural mechanisms underlying working memory deficits in long-term survival breast cancer patients treated with TMX. Our findings demonstrated that the TMX users had working memory impairments and lower functional connectivity of the right DLPFC-HP compared with healthy controls. In addition, significant correlations were found between the accuracy and RT in working memory tests and the DLPFC-HP connectivity for TMX users. Furthermore, consistent with previous studies results (Schilder et al., 2009, 2010; Breckenridge et al., 2012), we also found that the TMX users performed worse in some neuropsychological background tests (the Stroop Interference test and the TMTB) that evaluated general executive function compared with the healthy controls.
Previous studies have found marked effects of estrogen on the brain, suggesting its potential neuroprotective properties (Sherwin, 2003; Eberling et al., 2004; Krug et al., 2006). Basic studies have demonstrated that estrogen may exert neuroprotective effects by binding estrogen receptors to modulate molecules and by increasing the concentration of choline acetyltransferase (Sherwin, 2003). There is strong evidence that estrogen could enhance plasticity in neural network connectivity, neurogenesis, and synaptic transmission in the brain, particularly in the hippocampus and prefrontal cortex (Brinton, 2009). The majority of clinical studies have found beneficial effects of estrogen on the performance of several tasks, primarily including executive function tasks and verbal and spatial working memory tasks (Krug et al., 2006; Sherwin and Henry, 2008). Estrogen receptors are present in the prefrontal cortex and the hippocampus (Toran-Allerand et al., 1999). As we know, estrogen is necessary for the prefrontal cortex to control different brain functions, including attention, working memory, the inhibition of competing responses, and executive functions (Alvarez and Emory, 2006). Furthermore, many studies have suggested that patients with hippocampal lesions have visuospatial recognition memory impairments (Olson et al., 2006), given the crucial role of the hippocampus in tasks involving working memory (Baddeley et al., 2011). In this study, we report that TMX users exhibit worse performance in memory and executive function during n-back tasks and neuropsychological background tests. The altered performances in the neuropsychological executive function tests are consistent with previous reports that investigated executive function in breast cancer patients who received TMX treatment (Palmer et al., 2008; Schilder et al., 2010). As mentioned above, TMX, binding to estrogen receptors in the nervous system, affects cognitive function and brain structure, such as the hippocampus and the prefrontal cortex, suggesting a plausible antagonist influence. Based on these findings, it is possible to hypothesize that TMX may play an antagonistic role in brain structure and function.
Given the aforementioned evidence in the introduction, it can be inferred that the DLPFC plays an important role in working memory performance. Human and animal research has provided key insights into the neuronal basis of working memory, with the DLPFC playing a critical role by exerting top-down control over other working memory-related brain areas (Levy and Goldman-Rakic, 2000; Edin et al. 2009; Gazzaley and Nobre 2012). In the current study, to investigate the neural mechanisms underlying working memory deficits, we compared the differences in the functional connectivity across the whole brain with seed regions of DLPFC between TMX users and healthy controls. The results showed that TMX users had significantly lower functional connectivity of the right DLPFC with the right hippocampus, but not with other brain regions. Additionally, the functional connectivity strength of the DLPFC-HP was significantly associated with working memory performance. Recent neuroimaging studies have also provided evidence that dorsolateral prefrontal-hippocampal interactions are implicated in working memory. Liu et al. (2014), using the same resting-state functional connectivity technique, found that the DLPFC-HP connectivity of healthy controls affected working memory performance differently depending on the individual genotype. A task fMRI study showed that DLPFC-HP coupling may represent a systems-level mechanism specific to working memory, recommending its utility for modeling cognitive dysfunction in translational neuroscience (Bahner et al., 2015). Furthermore, these DLPFC-HP interactions existed in psychiatric conditions with cognitive dysfunction, such as schizophrenia (Meyer-Lindenberg et al., 2005). Hence, in light of our results and the aforementioned investigations, we believe that TMX may influence changes in DLPFC-HP functional connectivity, resulting in an impaired working memory performance, and that these deficits may be due to the antagonistic effect of TMX in patients with breast cancer.
However, many nonhuman studies have indicated neuroprotective effects of TMX via inhibiting excitotoxicity, boosting antiapoptotic cell death, and attenuating microglial inflammatory responses (Liu et al., 2010; Kuo et al., 2012; Tsai et al., 2014). Recently, several studies have reported that TMX improved allocentric memory performance and increased pyramidal neuronal dendritic density (Velazquez-Zamora et al., 2012; Zabihi et al., 2014). It has also been shown that TMX could enhance memory function by reducing dopamine metabolism and increasing acetylcholine levels in an amyloidosis mouse model (Pandey et al., 2015). Human studies have shown the beneficial effect of TMX on reversing cholinergic impairment in postmenopausal women (Ernst et al., 2002; Newhouse et al., 2013). All of the above data suggest that TMX may exert an effect similar to that of estrogen if administered in the absence of estradiol (Newhouse and Dumas, 2015). There seem to be 2 reasons accounting for these inconsistent results in our findings. The low estrogen level in vivo is a principal reason. In these animal studies, researchers used ovariectomized rodents for estrogen-deprived models to explore the relationship between the effect of TMX and cognitive performance. The participants who received TMX treatment were postmenopausal women, which was also the case in the above-mentioned human studies. However, there is evidence that TMX treatment is related to reduced memory performance in intact animal models (Chen et al., 2002; Esmaeili et al., 2009) and in human studies for which the participants were premenopausal women, where these studies indicated an antagonistic role of TMX (Palmer et al., 2008). Our findings seem to support the latter case. Another reason is the different durations of TMX treatments considered in these studies. The short duration treatments, from 2 to 12 weeks, were considered in the aforementioned studies, supporting the similar effects of TMX and estrogen. However, human studies that considered more than 1-year duration of TMX treatment suggested the antagonistic effect of TMX (Eberling et al., 2004; Palmer et al., 2008; Boele et al., 2015). Interestingly, Legault et al. (2009) reported significant changes in verbal memory through the course of their study; they found that the memory performance increased during the first year but significantly decreased after 2 years. Considering all data, including our findings, a plausible explanation of these results is that TMX, by binding to estrogen receptors in the brain, initially has estrogen-like effects. As time progresses, the ERs in the brain cannot bind more estrogen in vivo because of the high binding of TMX leads to estrogen-blocking. TMX was then shown to play an antagonistic role of estrogen at the late stage of treatment. Therefore, it is likely that the effect of TMX, acting as an estrogen agonist or antagonist in the human brain, corresponds to an inverted U-shaped curve on the memory performance, with an optimum mid-duration level being related to the best performance. It is worth noting that it is necessary to carry out longitudinal studies to identify this direct effect of TMX on memory performance in future studies.
Although we have excluded confounding factors, including menopausal symptoms and chemotherapy treatment, to better investigate the potential mechanism contributing to working memory changes in breast cancer patients treated with TMX, several limitations should be noted. First, the aim of this study was to show that patients with breast cancer treated with TMX have working memory deficits from the perspective of functional connectivity and to investigate the mechanism of this decline in performance. We did not completely rule out the factor of cancer diagnosis or explore the direct effects of TMX on working memory. However, for the TMX users in the current study, the average duration of the disease was more than 3 years, and the scores of quality of life, depression, and anxiety did not differ from those of healthy controls, suggesting that the TMX users had possibly restarted cancer-free lifestyles resembling those of the healthy controls. Future studies should recruit healthy participants treated with TMX but free of other diseases to assess the effects of TMX on working memory and functional connectivity. Second, this study is a cross-sectional study. None of the performance tasks and functional connectivity data were obtained from the patients prior to the TMX treatment. As our current findings showed that the premenopausal women with breast cancer who were treated with TMX exhibited changes in working memory and DLPFC-HP functional connectivity, it is necessary for further longitudinal studies to determine whether these changes are a consequence of the effect of TMX. Third, 12 of 31 patients had received radiotherapy in this study. There are reports suggesting that radiotherapy may impair cognition (Phillips et al., 2012; Stouten-Kemperman et al., 2015), although others showed no significant differences in cognitive function between patients with and without radiotherapy (Boele et al., 2015). We did not find significant cognitive changes between 12 patients with radiotherapy and 19 patients without radiotherapy, but it is difficult to conclude that the observed effects are due to TMX alone. We need to rule out the influence of radiotherapy to explore the effects of TMX on working memory in the future study.
In conclusion, this study represents a step toward understanding working memory deficits in the premenopausal women with breast cancer who are treated with TMX. Our results showed that TMX users have lower functional connectivity of the DLPFC-HP, which is associated with decreased working memory performance. Furthermore, these findings suggest that the prefrontal cortex and the hippocampus may be affected by TMX treatment, supporting an antagonistic role of TMX for long-term treatment in breast cancer patients.
Statement of Interest
None.
Acknowledgments
The authors are grateful to the patients of the Department of Breast Surgery of The First Hospital of Anhui Medical University for their participation in and assistance with the study.
This research was supported by the National Natural Science Foundation of China (nos. 31571149, 91432301, 81301176, and 81171273) and the National Basic Research Program of China (nos. 973 Program 2012CB720704 and 2015CB856405).
References
- Ahles TA, Saykin AJ. (2007) Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer 7:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. (2010) Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol 28:4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. (2006) Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev 16:17–42. [DOI] [PubMed] [Google Scholar]
- Baddeley A. (1992) Working memory. Science 255:556–559. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Jarrold C, Vargha-Khadem F. (2011) Working memory and the hippocampus. J Cogn Neurosci 23:3855–3861. [DOI] [PubMed] [Google Scholar]
- Bahner F, Demanuele C, Schweiger J, Gerchen MF, Zamoscik V, Ueltzhoffer K, Hahn T, Meyer P, Flor H, Durstewitz D, Tost H, Kirsch P, Plichta MM, Meyer-Lindenberg A. (2015) Hippocampal-dorsolateral prefrontal coupling as a species-conserved cognitive mechanism: a human translational imaging study. Neuropsychopharmacology 40:1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakoyiannis I, Tsigka EA, Perrea D, Pergialiotis V. (2016) The impact of endocrine therapy on cognitive functions of breast cancer patients: a systematic review. Clin Drug Investig 36:109–118. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. (2013) Dorsolateral prefrontal contributions to human working memory. Cortex 49:1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender CM, Sereika SM, Brufsky AM, Ryan CM, Vogel VG, Rastogi P, Cohen SM, Casillo FE, Berga SL. (2007) Memory impairments with adjuvant anastrozole versus tamoxifen in women with early-stage breast cancer. Menopause 14:995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boele FW, Schilder CM, de Roode ML, Deijen JB, Schagen SB. (2015) Cognitive functioning during long-term tamoxifen treatment in postmenopausal women with breast cancer. Menopause 22:17–25. [DOI] [PubMed] [Google Scholar]
- Breckenridge LM, Bruns GL, Todd BL, Feuerstein M. (2012) Cognitive limitations associated with tamoxifen and aromatase inhibitors in employed breast cancer survivors. Psychooncology 21:43–53. [DOI] [PubMed] [Google Scholar]
- Brinton RD. (2009) Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci 30:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Vanderhasselt MA. (2014) Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn 86:1–9. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Schagen SB. (2013) Is basic research providing answers if adjuvant anti-estrogen treatment of breast cancer can induce cognitive impairment? Life Sci 93:581–588. [DOI] [PubMed] [Google Scholar]
- Campbell MC, Koller JM, Snyder AZ, Buddhala C, Kotzbauer PT, Perlmutter JS. (2015) CSF proteins and resting–state functional connectivity in Parkinson disease. Neurology 84:2413–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase A. (2014) Alzheimer disease: altered functional connectivity in preclinical dementia. Nat Rev Neurol 10:609. [DOI] [PubMed] [Google Scholar]
- Chen D, Wu CF, Shi B, Xu YM. (2002) Tamoxifen and toremifene impair retrieval, but not acquisition, of spatial information processing in mice. Pharmacol Biochem Behav 72:417–421. [DOI] [PubMed] [Google Scholar]
- Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. (2009) Cognitive effects of hormonal therapy in early stage breast cancer patients: a prospective study. Psychooncology 18:811–821. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. (2003) Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7:415–423. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR. (1999) The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia 37:1303–1315. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. (2004) Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage 21:364–71. [DOI] [PubMed] [Google Scholar]
- Edin F, Klingberg T, Johansson P, McNab F, Tegnér J, Compte A. (2009) Mechanism for top-down control of working memory capacity. Proc Natl Acad Sci 106:6802–6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J, Vogel EK, Lansner A, Bergstrom F, Nyberg L. (2015) Neurocognitive architecture of working memory. Neuron 88:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Cooray D, Salvador C, Jovicich J, Walot I, Boone K, Chlebowski R. (2002) The effects of tamoxifen and estrogen on brain metabolism in elderly women. J Natl Cancer Inst 94:592–597. [DOI] [PubMed] [Google Scholar]
- Esmaeili B, Basseda Z, Gholizadeh S, Javadi Paydar M, Dehpour AR. (2009) Tamoxifen disrupts consolidation and retrieval of morphine-associated contextual memory in male mice: interaction with estradiol. Psychopharmacology (Berl) 204:191–201. [DOI] [PubMed] [Google Scholar]
- Fan HG, Houede-Tchen N, Yi QL, Chemerynsky I, Downie FP, Sabate K, Tannock IF. (2005) Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol 23:8025–8032. [DOI] [PubMed] [Google Scholar]
- Feredoes E, Heinen K, Weiskopf N, Ruff C, Driver J. (2011) Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc Natl Acad Sci U S A 108:17510–17515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. (1989) Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol 61:331–349. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC. (2012) Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci 16:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermelink K, Henschel V, Untch M, Bauerfeind I, Lux MP, Munzel K. (2008) Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: results of a multicenter, prospective, longitudinal study. Cancer 113:2431–2439. [DOI] [PubMed] [Google Scholar]
- Krug R, Born J, Rasch B. (2006) A 3-day estrogen treatment improves prefrontal cortex-dependent cognitive function in postmenopausal women. Psychoneuroendocrinology 31:965–975. [DOI] [PubMed] [Google Scholar]
- Kuo JR, Wang CC, Huang SK, Wang SJ. (2012) Tamoxifen depresses glutamate release through inhibition of voltage-dependent Ca2+ entry and protein kinase Calpha in rat cerebral cortex nerve terminals. Neurochem Int 60:105–114. [DOI] [PubMed] [Google Scholar]
- Le Rhun E, Delbeuck X, Lefeuvre-Plesse C, Kramar A, Skrobala E, Pasquier F, Bonneterre J. (2015) A phase III randomized multicenter trial evaluating cognition in post-menopausal breast cancer patients receiving adjuvant hormonotherapy. Breast Cancer Res Treat 152:569–580. [DOI] [PubMed] [Google Scholar]
- Legault C, Maki PM, Resnick SM, Coker L, Hogan P, Bevers TB, Shumaker SA. (2009) Effects of tamoxifen and raloxifene on memory and other cognitive abilities: cognition in the study of tamoxifen and raloxifene. J Clin Oncol 27:5144–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. (2000) Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res 133:23–32. [DOI] [PubMed] [Google Scholar]
- Lien EA, Solheim E, Ueland PM. (1991) Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res 51:4837–4844. [PubMed] [Google Scholar]
- Liu B, Zhang X, Hou B, Li J, Qiu C, Qin W, Yu C, Jiang T. (2014) The impact of MIR137 on dorsolateral prefrontal-hippocampal functional connectivity in healthy subjects. Neuropsychopharmacology 39:2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Tian DS, Li ZW, Qu WS, Zhan Y, Xie MJ, Yu ZY, Wang W, Wu G. (2010) Tamoxifen alleviates irradiation-induced brain injury by attenuating microglial inflammatory response in vitro and in vivo. Brain Res 1316:101–111. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. (1999) Estrogen actions in the central nervous system. Endocr Rev 20:279–307. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. (2005) Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62:379–386. [DOI] [PubMed] [Google Scholar]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, Jonides J. (2013) A meta-analysis of executive components of working memory. Cereb Cortex 23:264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse P, Albert K, Astur R, Johnson J, Naylor M, Dumas J. (2013) Tamoxifen improves cholinergically modulated cognitive performance in postmenopausal women. Neuropsychopharmacology 38:2632–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse P, Dumas J. (2015) Estrogen–cholinergic interactions: implications for cognitive aging. Horm Behav 74:173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. (2006) Working memory for conjunctions relies on the medial temporal lobe. J Neurosci 26:4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. (2005) N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JL, Trotter T, Joy AA, Carlson LE. (2008) Cognitive effects of tamoxifen in pre-menopausal women with breast cancer compared to healthy controls. J Cancer Surviv 2:275–282. [DOI] [PubMed] [Google Scholar]
- Pandey D, Banerjee S, Basu M, Mishra N. (2015) Memory enhancement by tamoxifen on amyloidosis mouse model. Horm Behav 79:70–73. [DOI] [PubMed] [Google Scholar]
- Perez EA. (2007) Safety profiles of tamoxifen and the aromatase inhibitors in adjuvant therapy of hormone-responsive early breast cancer. Ann Oncol 18 Suppl8:viii26–35. [DOI] [PubMed] [Google Scholar]
- Phillips KM, Jim HS, Small BJ, Laronga C, Andrykowski MA, Jacobsen PB. (2012) Cognitive functioning after cancer treatment: a 3-year longitudinal comparison of breast cancer survivors treated with chemotherapy or radiation and noncancer controls. Cancer 118: 1925–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosazza C, Minati L. (2011) Resting-state brain networks: literature review and clinical applications. Neurol Sci 32: 773–785. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. (2012) Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage 60:830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Hoffstaedter F, Muller V, Cieslik EC, Goya-Maldonado R, Trost S, Sorg C, Riedl V, Jardri R, Sommer I, Kogler L, Derntl B, Gruber O, Eickhoff SB. (2015) Transdiagnostic commonalities and differences in resting state functional connectivity of the default mode network in schizophrenia and major depression. Neuroimage Clin 10:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder CM, Eggens PC, Seynaeve C, Linn SC, Boogerd W, Gundy CM, Beex LV, Van Dam FS, Schagen SB. (2009) Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncol 48:76–85. [DOI] [PubMed] [Google Scholar]
- Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Huizenga HM, Nortier JW, van de Velde CJ, van Dam FS, Schagen SB. (2010) Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol 28:1294–1300. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. (2003) Estrogen and cognitive functioning in women. Endocr Rev 24:133–151. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. (2008) Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol 29:88–113. [DOI] [PubMed] [Google Scholar]
- Shilling V, Jenkins V, Fallowfield L, Howell T. (2003) The effects of hormone therapy on cognition in breast cancer. J Steroid Biochem Mol Biol 86:405–412. [DOI] [PubMed] [Google Scholar]
- Stouten-Kemperman MM, de Ruiter MB, Boogerd W, Veltman DJ, Reneman L, Schagen SB. (2015) Very late treatment-related alterations in brain function of breast cancer survivors. J Int Neuropsychol Soc 21: 50–61. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Singh M, Setalo G., Jr (1999) Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol 20:97–121. [DOI] [PubMed] [Google Scholar]
- Tsai YT, Wang CC, Leung PO, Lin KC, Chio CC, Hu CY, Kuo JR. (2014) Extracellular signal-regulated kinase 1/2 is involved in a tamoxifen neuroprotective effect in a lateral fluid percussion injury rat model. J Surg Res 189:106–116. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. (2010) Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 20:519–534. [DOI] [PubMed] [Google Scholar]
- Velazquez-Zamora DA, Garcia-Segura LM, Gonzalez-Burgos I. (2012) Effects of selective estrogen receptor modulators on allocentric working memory performance and on dendritic spines in medial prefrontal cortex pyramidal neurons of ovariectomized rats. Horm Behav 61:512–517. [DOI] [PubMed] [Google Scholar]
- Zabihi H, Hosseini M, Pourganji M, Oryan S, Soukhtanloo M, Niazmand S. (2014) The effects of tamoxifen on learning, memory and brain tissues oxidative damage in ovariectomized and naive female rats. Adv Biomed Res 3:219. [DOI] [PMC free article] [PubMed] [Google Scholar]