Abstract
Background:
Polymorphisms in the CACNA1C gene are associated with human mood disorders. The rodent social defeat model of stress/mood-disorder susceptibility results in maladaptive consequences mediated by altered function of mesolimbic circuits.
Methods:
mRNA levels of Cacna1c in the nucleus accumbens of mice exposed to social defeat were assessed. Cacna1c was selectively deleted in the nucleus accumbens of floxed Cacna1c mice using viral Cre-recombinase to examine Cacna1c in social defeat susceptibility.
Results:
Reduced expression of Cacan1c in the nucleus accumbens is associated with increased susceptibility to social defeat stress, and a knockdown of Cacna1c in the nucleus accumbens significantly increases susceptibility measured by social interaction and female urine preference.
Conclusions:
Cacna1c reduction causally predisposes to the maladaptive outcomes of social stress. Normal Cacna1c function in the nucleus accumbens is crucial for resiliency to social stressors. Variations in expression of CACNA1C in the nucleus accumbens may mediate human risk for developing mood disorders and be a target for therapeutic intervention.
Keywords: CACNA1C, depression, bipolar disorder, genetics, nucleus accumbens
Significance Statement
Human genome wide genetic association studies have identified polymorphisms within the CACNA1C gene, which codes for the alpha1C subunit of the Cav1.2 L-type calcium channel, with the risk of developing bipolar and major depressive mood disorders. We assessed the role of Cacna1c in mediating susceptibility to maladaptive responses to chronic social stress, showing that in mice susceptible to chronic social defeat stress, Cacna1c levels in the nucleus accumbens are reduced. Reduced Cacna1c expression selectively in the nucleus accumbens did not itself directly induce maladaptive social and anhedonic behaviors. In contrast, following sub threshold social defeat, social interaction and hedonic responses are impaired in animals with reduced nucleus accumbens Cacna1c expression. This knowledge of how Cacna1c promotes maladaptive behaviors may implicate pharmacological target points, leading to improved treatments for mood disorders.
Introduction
Genome-wide association studies have identified polymorphisms within the CACNA1C gene, which codes for the α1C subunit of the Cav1.2 L-type calcium channel (LTCC), with the risk of developing schizophrenia, bipolar disorder, and major depressive disorder (Sklar et al., 2008; Green et al., 2010; Liu et al., 2011; Smoller et al., 2013; Ripke et al., 2014). In healthy volunteers, risk-associated single nucleotide polymorphisms in CACNA1C predict higher scores on measures of depression, anxiety, interpersonal sensitivity, neuroticism, increased anxiety, and negative mood (Erk et al., 2010; Roussos et al., 2011).
LTCC function has previously been associated with maladaptive responses to acute stress. In rodents, pharmacological blockade of LTCCs results in antidepressant effects in the learned helplessness paradigm as well as the forced swim and tail suspension tests (Mogilnicka et al., 1987; Cohen et al., 1997; Saade et al., 2003; Sinnegger-Brauns et al., 2004). Similarly, Cacna1c haploinsufficiency is associated with resilience in the learned helplessness paradigm and decreased immobility in the forced swim and tail suspension tests, but also increases the expression of anxiety phenotypes in conflict tests such as the elevated plus maze (EPM) (Dao et al., 2010). These previous rodent studies used short-term induction of physical stress to elicit behavioral responses, providing limited insight into the role that Cacna1c may have in modulating the maladaptive consequences of social stress, which is recognized as a model of a risk factor for human depression (Nestler et al., 2002).
A primary brain circuit associated with social stress-induced maladaptive behaviors is the mesolimbic dopamine system, where social defeat results in a series of molecular and physiological changes within the ventral tegmental area and nucleus accumbens (NAc) (Krishnan et al., 2007). Although there is substantial evidence indicating that the ventral tegmental area to NAc pathway (mesolimbic system) mediates a subset of stress-elicited behaviors, the role of Cacna1c in this region in mediating such behaviors remains essentially unknown. We assessed the role of Cacna1c in mediating susceptibility to maladaptive responses to social defeat stress by first quantifying levels of Cacna1c in mice susceptible and resilient to chronic stress, and secondly by assessing behavioral effects of reducing Cacna1c selectively in the NAc.
Methods
Animals
Mice were male wild-type (Jackson Laboratories, Bar Harbor, ME) or conditional Cacna1c knockout mice (Jeon et al., 2010) on a C57BL/6 background 8 and 14 weeks of age. CD-1 retired breeder mice were from Charles River (Raleigh, NC). Experimental procedures were approved by the UMB IACUC and conducted in full accordance with the NIH Guide for the Care and Use of Laboratory Animals.
qPCR for Cacna1c Expression
mRNA was extracted from the NAc of control mice and mice classified as resilient or susceptible following chronic social defeat. Primers: Cacna1c (5’-CACCATTGCCTCCGAACATTAC-3’, 5’-GGCTTTATTGGCTGTGTCTTGC-3’), beta-actin (5’-TGAGACCTT CAACACCCCAG-3’, 5’-GAGCATAGCCCTCGTAGATG-3’), GAPDH (5’-CCACTCACGGCAAATTCAAC-3’, 5’-AGACTCCACGAC ATACTCA-3’), and 18-S (5’-CCAGTAAGTGCGGGTCATAAGC-3’, 5’-CCATCCAATCGGTAGTAGCGAC-3’) were from Integrated DNA Technologies (Coralville, IA). The PCR reactions were run on a ViiA 7 Real-Time PCR System (Life Technologies) with a reaction volume of 15 µL and an annealing temperature of 60°C. ViiA 7 software (Life Technologies) was used to determine Ct values. Cacna1c levels were normalized to the geometric mean of beta-actin, GAPDH, and 18-S, and fold difference was determined using the 2-ΔΔCt method (Livak and Schmittgen, 2001).
Virus Injections
A total of 0.7 µL AAV-CMV-Cre-recombinase (Cre)-green fluorescent protein (GFP) or AAV-CMV-GFP (UNC Vector Core, Chapel Hill, NC) was injected bilaterally into the NAc (+1.6 anterior/posterior, +1.5 lateral, and -4.4 dorsal/ventral, 10° angle). Injections were performed at a rate of 0.1 uL/min, and the needle was left in place for 10 minutes prior to removal. A 2-week recovery period was given prior to experiments. Following experiments, mice were euthanized and the brain immediately sectioned into 1.0-mm slices using a matrix (ASI Instruments, Warren, MI) and placed in cold PBS. Brain slices were then visualized through a fluorescent microscope (Leica Microsystems GmbH, Wetzlar, Germany). Mice that did not show fluorescence bilaterally in the NAc were excluded from the results.
Microscopy
The 30-µm coronal sections of paraformaldehyde perfused brains were cut in a cryostat and placed in 1x PBS. Sections were then blocked in 20% Triton X-100 (Sigma-Aldrich, St. Louis, MO) for 30 minutes and incubated overnight with primary antibody (chicken anti-GFP, 1:4000, Aves Labs, Inc., Tigard, OR) at room temperature. Sections were washed and incubated in secondary antibody for 2 hours at room temperature (donkey anti-chicken Alexa-488 Green, 1:1000, Life Technologies, Grand Island, NY), mounted, and cover slipped. After drying, sections were visualized and photographed under a confocal microscope (Olympus Fluoview, Tokyo, Japan).
Chronic Social Defeat
Based on a previously described protocol, C57BL6 mice were exposed to a different aggressive retired CD-1 breeder for 10 min/d, after which they were housed in the same cage as the aggressor on the other side of a perforated divider for 24 hours, which was repeated for 10 days (Krishnan et al., 2007). Control mice were housed with one mouse on each side of a perforated divider and were rotated at the same intervals. On day 11, the time spent in the interaction zone during the first (target absent) and second (target present) trials was measured. The interaction ratio was calculated as 100 × (interaction time, target present)/(interaction time, target absent). Resilient and susceptible mice were defined as above and below 100, respectively (Krishnan et al., 2007).
Subthreshold Social Defeat
The subthreshold social defeat was similar to the chronic social defeat procedure, except C57BL/6 mice were exposed to the CD-1 mouse for 2 minutes, then separated by the perforated divider for 15 minutes, which was repeated a total of 3 times, every 15 minutes, with a new CD-1 aggressor (Krishnan et al., 2007). At 24 hours following subthreshold defeat, mice were tested for social interaction in an apparatus with a small chamber connected to two 18-cm x 20-cm chambers (Stoelting Co.) illuminated at 20 lux as described (Zanos et al., 2016). A small wire cage, or an identical cage with a CD-1 mouse inside, was placed within each of the 2 chambers. The amount of time that mice spent sniffing each cage was assessed using CleverSys tracking software (CleverSys, Inc.).
Female Urine Sniffing Test (FUST)
Our FUST procedure (Malkesman et al., 2010) was previously described (Zanos et al., 2016). The amount of time mice spent interacting with a cotton-tipped applicator soaked in either fresh male or female mouse urine during a total of 3 minutes was recorded.
EPM
The EPM consisted of 2 closed arms and 2 open arms, each 39 cm in length x 5 cm in width, elevated 50 cm above the floor (Stoelting, Woodale, IL), and was illuminated at 3 to 5 lux. The amount of time spent in each arm and total distanced moved was determined using CleverSys tracking software (CleverSys, Inc., Reston VA).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism Version 6 (GraphPad Software, San Diego, CA) and Statistica (StatSoft, Tulsa, OK). The statistics used were 2-tailed t-test, 1-way ANOVA, or repeated-measures 3-way ANOVA, followed by Bonferroni posthoc tests. Data are reported as mean ± SEM and P < .05 was considered significant.
Results
Social Defeat Susceptibility Is Associated with a Reduction of NAc Cacna1c Expression
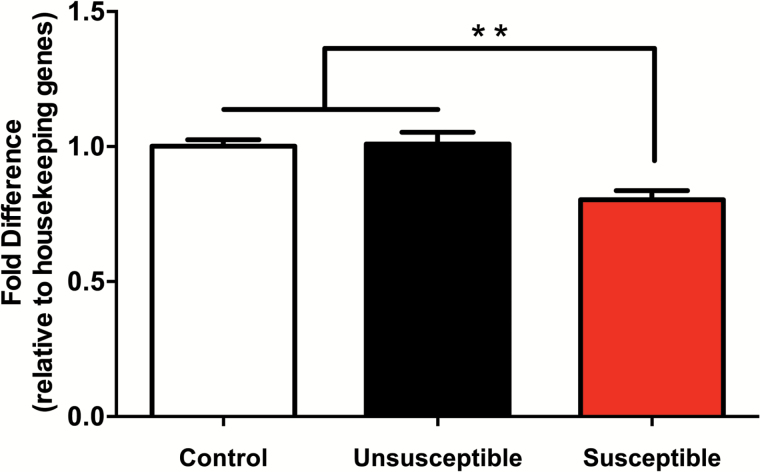
Mice exposed to social defeat stress segregate behaviorally into 2 outcomes: those susceptible to social defeat and those that are resilient. To determine if there is a relationship between Cacna1c mRNA levels in the NAc and susceptibility to chronic social defeat, we quantified Canca1c in mRNA from mice that manifested resilience and susceptibility following chronic social defeat. Control mice were animals that had not undergone a social defeat training paradigm. Our data (Figure 1) demonstrate that lower levels of Cacna1c in the NAc are present in mice that have manifested defeat susceptibility (F(2,15)= 0.002, P<.01). A Bonferroni posthoc test revealed that there is a significant difference between control and susceptible mice (t=3.702, P<.01) as well as between resilient and susceptible mice (t=3.989, P<.01) (Figure 1). The expression levels of Cacna1c in control animals that did not undergo social defeat exhibit expression levels equivalent to the resistant group. Susceptibility to defeat is thus likely not due to a preexisting difference in Cacna1c expression, but instead there is an interaction between induction of defeat and gene expression in a subset of animals.
Figure 1.
Decreased Cacan1c expression in mice susceptible to chronic social defeat stress. Cacna1c mRNA levels from the nucleus accumbens (NAc) of control mice, and mice resilient or susceptible to social defeat was quantified. There is a significant effect of group on Cacna1c expression (P<.01). Bonferroni posthoc tests revealed a significant difference in Cacna1c expression in susceptible mice compared with control and unsusceptible mice (P<.01). Data are the mean ± SEM; n = 5 (control), 7 (unsusceptible), 6 (susceptible). **P<.01
Susceptibility to Subthreshold Social Defeat Is Enhanced following Cacna1c Knockdown in the NAc
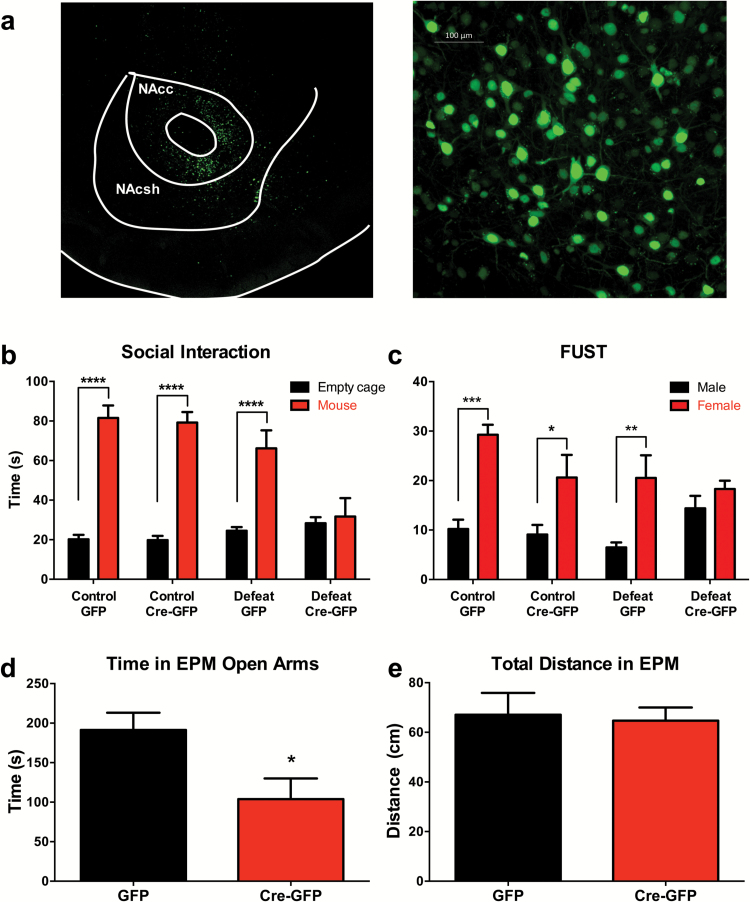
To test whether reduced Cacna1c in the NAc is causally linked to maladaptive behaviors or to susceptibility to social defeat stress, AAV-Cre-GFP was injected bilaterally in the NAc of mice containing 2 floxed Cacna1c alleles (Figure 2a). In mice that received cre-GFP containing virus, there was a significant reduction in the level of Cacna1c expression in whole NAc punches of 36% measured by qPCR compared with AAV-GFP (t=4.43, P<.01).
Figure 2.
Increased susceptibility to subthreshold social defeat stress and anxiety behavior following reduced Cacna1c in the nucleus accumbens (NAc). Conditional Cacna1c knockout mice received an injection of AAV-CMV-Cre-GFP or AAV-CMV-GFP bilaterally in the NAc and underwent either no defeat stress (control conditions) or subthreshold social defeat stress. (a) Representative image of GFP fluorescence indicates injection region (left) and cell specificity (right) in the NAc. (b) Social interaction following subthreshold defeat. Bonferroni posthoc tests revealed a significant difference in time spent interacting with the target mouse vs an empty cage in control AAV-GFP, control AAV-Cre, and defeat AAV-GFP mice (P < .0001). There was no preference in mice that received AAV-Cre and underwent subthreshold defeat (P < 1.0). (c) FUST performance following subthreshold defeat. There was a significant difference in time spent sniffing the female vs male urine in control AAV-GFP (P < .001), control AAV-Cre mice (P < .05), and defeat AAV-GFP mice (P < .01). There was no difference between male and female sniffing time in AAV-Cre mice that underwent subthreshold defeat (P = 1.0). n = 8 to 9/group. (d) Injection of AAV-Cre to the NAc resulted in decreased open arm times in the EPM (P < .05). (e) There was no effect of injection type on total distance travelled measured in the EPM (P = .815). Data are the mean ± SEM (n = 11–12/group). ****P < .0001, ***P < .001, **P < .01, *P < .05. Abbreviations: NAcc, nucleus accumbens core; NAcsh, nucleus accumbens shell; Control, mice that did not undergo social defeat; Defeat, mice that underwent subthreshold social defeat; Cre, Cre-recombinase; GFP, green fluorescent protein; FUST, female uring sniffing test; EPM, elevated plus maze.
To test defeat sensitivity in animals with normal and reduced Cacna1c expression in the NAc, we used a subthreshold social defeat paradigm. This paradigm is a variation of the chronic social defeat paradigm that does not result in substantial behavioral changes in control mice (Krishnan et al., 2007). Control animals, which did not undergo subthreshold social defeat, had no differences in social interaction between normal (GFP) and reduced Cacna1c expression (Cre-GFP) groups. However, when a subthreshold social defeat paradigm was administered, mice with reduced Cacna1c expression in the NAc showed a profound deficit in social interaction (Figure 2b). A 3-way ANOVA indicated a significant interaction between target presence, Cacna1c NAc knockdown, and susceptibility to defeat (F(1,31) = 11.539, P<.001) (Figure 2b). Bonferroni posthoc tests revealed that all control mice prefer to spend time interacting with a target mouse compared with an empty container (P<.0001). In contrast, Cre-GFP injected mice that underwent defeat showed no difference in time spent with the target mouse or empty chamber (P=1.0) (Figure 2b).
The FUST is a measure of anhedonia that relies upon the strong preference of male mice to the smell of female mouse urine, which has previously been found to be reduced by various stressors, including social defeat (Malkesman et al., 2010; Wagner et al., 2012; Zanos et al., 2016). We hypothesized that animals with induced social defeat will also manifest impaired “interest” in investigation of socially relevant and pleasurable cues. While there was no significant 3-way interaction between sniffing time, injection, and defeat, there was a significant interaction between sniffing time and injection (F(1,31)=8.30, P<.01), as well as a trend for an interaction between sniffing time and defeat (F(1,31)=3.43, P=.074) (Figure 2c). Control animals that did not undergo social defeat induction showed manifested FUST interaction with female urine. However, the reduction in Cacna1c expression abolished the preference for male or female urine in Cre-GFP injected mice that underwent subthreshold social defeat (P=.99). Taken together, these results indicate that elimination of the Cacna1c gene in a subset of NAc neurons does not induce social or anhedonia deficits but dramatically enhances susceptibility to social defeat stress resulting in maladaptive behavioral responses.
Cacna1c Knockdown in the NAc Increases Anxiety
Increased anxiety behavior and emotionality has been associated with maladaptive stress response in mice (Veenema et al., 2003; Ducottet and Belzung, 2004, 2005). To evaluate if reduced Cacna1c in the NAc increases anxiety-related behavior, mice with a bilateral viral-mediated knockdown of Cacna1c in the NAc (Figure 2a) were tested in the EPM. Injection of Cre-GFP in the NAc led to significantly reduced time spent in the open arm compared with GFP injected mice (t(21)=2.541, P=.019) (Figure 2d), as well as a significantly reduced ratio of time in open arms, compared with time in open and closed arms combined (t(21)=2.547, P=.019). Reduction in open arm time could be the result of motor deficit, which would manifest as reduced distance traveled. However, Cre-GFP-injected mice showed no difference in locomotor activity in the EPM compared with GFP-injected mice (t(21)=0.237, P=.815) (Figure 2e). These data indicate that knockdown of Cacnalc has no impact of motor performance, but significantly increases anxiety behavior.
Discussion
In rodents, chronic social defeat is considered one of the most clinically relevant approaches to model components of human depression, with high face, construct, and predictive validity (Berton et al., 2006; Golden et al., 2011). Exposure to social defeat stress leads to a robust phenotype of maladaptive social behaviors, which includes increased social avoidance and anhedonia (Krishnan et al., 2007; Golden et al., 2011). We show that in mice susceptible to chronic social defeat stress, Cacna1c levels in the NAc are reduced (Figure 1). Expression of Canca1c is unchanged in resilient mice compared with control mice not exposed to social stress (Figure 1). Thus, we conclude that in susceptible mice, social defeat likely results in a reduction of Canca1c. However, reduced Cacna1c expression selectively in the NAc does not itself induce maladaptive social and anhedonic behavior directly (Figure 2). Instead, following subthreshold social defeat, social interaction and FUST preference are impaired in animals with reduced Cacna1c expression, thus similarly implicating an interaction between Cacna1c levels and the social defeat experience.
Previous studies suggest that decreased Cacna1c expression either globally (or in the prefrontal cortex) is associated with resilience to acute stress and that pharmacological inhibition of L-type calcium channels exerts antidepressant effects in rodent behavioral tests predictive of antidepressant efficacy (Mogilnicka et al., 1987; Cohen et al., 1997; Saade et al., 2003; Sinnegger-Brauns et al., 2004; Dao et al., 2010; Kabir et al., 2017). However, we found that region-specific knockdown of Cacna1c in the NAc promotes susceptibility. Biochemical and physiological findings in the NAc relevant to social stress susceptibility may be in the opposite direction compared with those findings in other brain regions, most notably in the hippocampus. For example, it is established that decreased BDNF in the hippocampus is associated with increased susceptibility to social defeat (Tsankova et al., 2006), but increased BDNF is associated with increased susceptibility in the NAc (Berton et al., 2006). It may be that Cacna1c is involved differently in acute vs chronic stress, or that Cacna1c exerts directionally different effects dependent upon the brain region and/or cell type in which it is expressed. This potentially has implications for human treatment, as the prevention vs reversal of depression phenotypes may respond differently to treatments targeted at Cav1.2.
CACNA1C gene expression is reproducibly associated with risk of developing neuropsychiatric disorders (Sklar et al., 2008; Smoller et al., 2013; Ripke et al., 2014). The results from our study demonstrate that when levels of Cacna1c are reduced in the NAc, a sensitivity to social stress, and an anxiety phenotype emerges. As dysregulation of the mesolimbic dopamine system is known to contribute to the etiology of mood disorders, the knowledge that Cacna1c is important for behaviors mediated in part by the mesolimbic dopamine system has considerable implications for our understanding of how Cacna1c may confer risk. Our data indicate Cacna1c changes are likely upstream of the maladaptive social behaviors, conferring elevated susceptibility to maladaptive outcomes following defeat stress. However, the NAc is a heterogeneous structure with distinct neuronal populations. Knowledge of how Cacna1c promotes maladaptive behaviors through expression in specific neuronal types, and thus pharmacological target points, may lead to improved treatments for psychiatric conditions, including depression.
Statement of Interest
Dr. Gould has received consulting fees from Sunovion Pharmaceuticals and Janssen Pharmaceuticals, and research funding from Janssen Pharmaceuticals and Roche Pharmaceuticals during the preceding 3 years. All other authors report no financial interests to disclose.
Acknowledgments
This study was supported by US NIH grant MH103847 and Brain & Behavior Research Foundation Independent Investigator Award 20230 to T.D.G. Cacna1c conditional knockout mice were provided by Jean-Pierre Kinet, Harvard University.
References
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Sanger DJ. (1997) Assessment of the antidepressant-like effects of L-type voltage-dependent channel modulators. Behav Pharmacol 8:629–638. [DOI] [PubMed] [Google Scholar]
- Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, Shi J, Zandi PP, O’Donnell P, Knowles JA, Weissman MM, Coryell W, Scheftner WA, Lawson WB, Levinson DF, Thompson SM, Potash JB, Gould TD. (2010) Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry 68:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducottet C, Belzung C. (2004) Behaviour in the elevated plus-maze predicts coping after subchronic mild stress in mice. Physiol Behav 81:417–426. [DOI] [PubMed] [Google Scholar]
- Ducottet C, Belzung C. (2005) Correlations between behaviours in the elevated plus-maze and sensitivity to unpredictable subchronic mild stress: evidence from inbred strains of mice. Behav Brain Res 156:153–162. [DOI] [PubMed] [Google Scholar]
- Erk S, Meyer-Lindenberg A, Schnell K, Opitz von Boberfeld C, Esslinger C, Kirsch P, Grimm O, Arnold C, Haddad L, Witt SH, Cichon S, Nothen MM, Rietschel M, Walter H. (2010) Brain function in carriers of a genome-wide supported bipolar disorder variant. Arch Gen Psychiatry 67:803–811. [DOI] [PubMed] [Google Scholar]
- Golden SA, Covington HE, 3rd, Berton O, Russo SJ. (2011) A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, Gordon-Smith K, Fraser C, Forty L, Russell E, Hamshere ML, Moskvina V, Nikolov I, Farmer A, McGuffin P, Holmans PA, Owen MJ, O’Donovan MC, Craddock N. (2010) The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry 15:1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. (2010) Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci 13:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir ZD, Lee AS, Burgdorf CE, Fischer D, Rajadhyaksha AM, Mok E, Rizzo B, Rice RC, Singh K, Ota KT, Gerhard DM, Schierberl KC, Glass M, Duman RS, Rajadhyaksha AM (2017) Cacna1c in the prefrontal cortex regulates depression-related behaviors via REDD1. Neuropsychopharmacology. doi:10.1038/npp.2016.271. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, et al. (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. (2011) Meta-analysis of genome-wide association data of bipolar disorder and major depressive disorder. Mol Psychiatry 16:2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, Chen G, Crawley JN, Manji HK. (2010) The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol Psychiatry 67:864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilnicka E, Czyrak A, Maj J. (1987) Dihydropyridine calcium channel antagonists reduce immobility in the mouse behavioral despair test; antidepressants facilitate nifedipine action. Eur J Pharmacol 138:413–416. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. (2002) Preclinical models: status of basic research in depression. Biol Psychiatry 52:503–528. [DOI] [PubMed] [Google Scholar]
- Ripke S, et al. (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Georgakopoulos A, Robakis NK, Bitsios P. (2011) The CACNA1C and ANK3 risk alleles impact on affective personality traits and startle reactivity but not on cognition or gating in healthy males. Bipolar Disord 13:250–259. [DOI] [PubMed] [Google Scholar]
- Saade S, Balleine BW, Minor TR. (2003) The L-type calcium channel blocker nimodipine mitigates “learned helplessness” in rats. Pharmacol Biochem Behav 74:269–278. [DOI] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renstrom E, Wietzorrek G, Berjukov S, Cavalli M, Walter D, Koschak A, Waldschutz R, Hering S, Bova S, Rorsman P, Pongs O, Singewald N, Striessnig J. (2004) Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca 2+ channels. J Clin Invest 113:1430–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, et al. (2008) Whole-genome association study of bipolar disorder. Mol Psychiatry 13:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, et al. (2013) Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. (2006) Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9:519–525. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Meijer OC, de Kloet ER, Koolhaas JM. (2003) Genetic selection for coping style predicts stressor susceptibility. J Neuroendocrinol 15:256–267. [DOI] [PubMed] [Google Scholar]
- Wagner KV, Marinescu D, Hartmann J, Wang XD, Labermaier C, Scharf SH, Liebl C, Uhr M, Holsboer F, Muller MB, Schmidt MV. (2012) Differences in FKBP51 regulation following chronic social defeat stress correlate with individual stress sensitivity: influence of paroxetine treatment. Neuropsychopharmacology 37:2797–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Jr, Gould TD. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]