Abstract
The insula has been credited with a role in a number of functions, including speech production. Here, we recorded electrocorticography (ECoG) signals from the left insula during pseudoword articulation in two patients undergoing pre-surgical monitoring for the management of medically-intractable epilepsy. Event-related band power (ERBP) activity from electrodes implanted in the superior precentral gyrus of the insula (SPGI) was compared to that of other left hemisphere regions implicated in speech production. Results showed that SPGI contacts demonstrated significantly greater ERBP within the high-gamma frequency range (75–150 Hz) during articulation compared to a listening condition. However, frontal and post-central regions demonstrated significantly greater responses to the articulation task compared to the SPGI. Results suggest the SPGI is active during articulation, but frontal and post-central regions demonstrate significantly more robust responses. Given the small sample size, and number of electrodes implanted in the SPGI, further study is warranted to confirm these findings.
Keywords: Articulation, Electrocorticography, Insula, Speech production
1. Introduction
The insular cortex has been credited with a number of roles, ranging from communication (e.g., Ackermann & Riecker, 2004; Ackermann & Riecker, 2010; Ardila, 1999; Ardila, Benson, & Flynn, 1997; Baldo, Wilkins, Ogar, Willock, & Dronkers, 2011; Dronkers, 1996; Dronkers, Ogar, Willock, & Wilkins, 2004; Nagao, Takeda, Komori, Isozaki, & Hirai, 1999; Ogar et al., 2006), visceral functions (e.g., Augustine, 1985; Craig, 2002, 2009; Mayer, Naliboff, & Craig, 2006; Moisset et al., 2010), conscious awareness (Craig, 2009), addiction (Naqvi & Bechara, 2009, 2010), and psychiatric disorders (Klein, Ullsperger, & Danielmeier, 2013). The participation of the insula in visceral, emotional, and conscious processes is supported by theoretical models (e.g., Craig, 2002, 2009). However, the relationship between insular function and communication is less rooted in models of language production (e.g., Hickok, 2014; Tourville & Guenther, 2011), even though several studies have reported that the insula is implicated in communication disorders, such as apraxia of speech (AOS; a disorder of motor speech planning and programming that results in off-target articulation, speech sound distortions, and prosodic abnormalities) and aphasia.
To date, lesion-deficit studies of individuals with AOS have informed the study of the neuroanatomical correlates of speech production processes (e.g., Baldo et al., 2011; Basilakos, Rorden, Bonilha, Moser, & Fridriksson, 2015; Dronkers, 1996; Dronkers & Ogar, 2004; Dronkers et al., 2004; Graff-Radford et al., 2014; Hickok et al., 2014; Hillis et al., 2004; Itabashi et al., 2016; Richardson, Fillmore, Rorden, Lapointe, & Fridriksson, 2012). The earliest systematic, quantitative study that revealed a role of the insula in speech was conducted by Dronkers (1996). That study showed 100% lesion overlap in the superior precentral gyrus of the insula (SPGI) in patients with AOS, but 0% lesion overlap in the SPGI among patients without AOS. The relationship between the insula and speech was subsequently supported by functional imaging (Moser et al., 2009; Wise, Greene, Büchel, & Scott, 1999) and lesion (Nagao et al., 1999; Dronkers et al., 2004; Ogar et al., 2006) studies.
The insula as the primary region implicated in AOS has not been a unanimous finding. In a sample of acutely post-stroke patients, Hillis et al. (2004) found that insula damage was not a prerequisite for AOS. Instead, in their sample of patients with AOS (n=31) over half (n=19) did not demonstrate hypoperfusion to the insula; rather, hypoperfusion to the left inferior frontal gyrus pars opercularis (IFGpo) was more likely (n=26/31 patients). These findings were confirmed by an independent group of individuals at the chronic stage of stroke (≥6 months post-onset; Richardson et al., 2012). However, more recent studies suggest a role of the frontal motor and post-central areas in AOS. Collectively, these studies have suggested that pre- and post-central regions may instead be the areas crucially involved in planning, monitoring and executing the motor aspects of speech (Basilakos et al., 2015; Graff-Radford et al., 2014; Hickok et al., 2014; Hillis et al., 2004; Josephs & Duffy, 2008; Josephs et al., 2012; Whitwell et al., 2013).
1.2 Results from Intracranial EEG Studies
With its high temporal and anatomical resolution, electrocorticography (ECoG) has the advantage of providing rare data from brain activity within multiple target regions. Although several prior studies have used ECoG to investigate the role of auditory and motor cortices during speech production (e.g., Behroozmand et al., 2016; Chang, Niziolek, Knight, Nagarajan, & Houde, 2013; Greenlee et al., 2013; Kingyon et al., 2015), relatively fewer studies have investigated regions that are involved during overt production (e.g., see Bouchard, Mesgarani, Johnson, & Chang, 2013; Flinker et al., 2015).
Until relatively recently, direct cortical recordings from the insula were less feasible due to the insula’s anatomical intricacies, being concealed from the lateral surface of the brain by the frontal and temporal opercula and covered by multiple branches of the middle cerebral artery. Advanced improvements in stereotaxic surgical techniques have resulted in successful ECoG electrode implantation in the insula (Isnard, Guénot, Sindou, & Mauguière, 2004) through stereoEEG (sEEG). To our knowledge, no published studies thus far have provided accounts of direct cortical recordings from the insula, or the SPGI specifically, during speech production.
Here, we report ECoG recordings from the SPGI in two patients without visible structural brain lesions who were undergoing pre-surgical monitoring of medically intractable epilepsy. The purpose of this study was to measure the SPGI’s response to speech production, compared to other grey matter regions of interest previously implicated in speech production based on the results of lesion studies (e.g., Dronkers, 1996; Baldo et al., 2011; Graff-Radford et al., 2014; Basilakos et al., 2015) and fMRI studies in non-brain damaged individuals (e.g., Wise et al., 1999) (see regions listed in Table 1). To this end, we aimed to test: 1) whether the SPGI would demonstrate greater response during articulation when compared to a non-articulation task, and 2) the relative magnitude and timing of cortical responses from the SPGI compared to other left hemisphere frontal and post-central regions during articulation.
Table 1.
Anatomical coordinates for each channel of interest
| Lobar Region | Anatomical Region | Patient | MNI Coordinate |
|---|---|---|---|
| Frontal 1 | IFGpt | 1 | −36, 27, 25 |
| Frontal 2 | SFG | 1 | −21, 44, 44 |
| Frontal 1 | SFG | 2 | −17, 20, 65 |
| Frontal 2 | MFG | 2 | −40, 54, 6 |
| Insular | SPGI | 1 | −29, 2, 4 |
| Insular 1 | SPGI | 2 | −30, 12, 10 |
| Insular 2 | Posterior insula | 2 | −31, −16, 15 |
| Post-Central | PoCG | 1 | −20, −30, 60 |
Abbreviations: IFGpt: inferior frontal gyrus pars triangularis; SFG: superior frontal gyrus; MFG: middle frontal gyrus; SPGI: superior precentral gyrus of the insula; PoCG: post-central gyrus
2. Method
2.1 Participants
Two patients with surgically implanted electrodes undergoing monitoring for intractable epilepsy were recruited for study. Both patients were female, right-handed, ages 33 (Patient 1) and 31 (Patient 2). Epilepsy onset was six years prior to testing for Patient 1, and 14 months for Patient 2. Neither patient reported premorbid speech and/or language difficulties or concomitant neurological impairment in addition to epilepsy. Clinical pre-surgical neuroimaging was unremarkable for any structural brain abnormalities. During pre-surgical evaluation of their epilepsies, both patients had poorly localized and poorly lateralized seizure onsets during ictal scalp EEG monitoring, leading to broad bilateral sEEG coverage to further elucidate seizure onset. Results from sEEG monitoring revealed that both patients had seizures localized to medial temporal lobes (MTL): Patient 1 had independent seizure onset on the right and left hippocampi, whereas Patient 2 had right hippocampal seizure onset. The insula was neither the location of ictal onset nor was it involved in seizure propagation in either one of the patients. None of the recorded seizures was poorly localized.
A WADA test was not indicated for Patient 1 since resective surgery was not possible and the patient subsequently underwent treatment with responsive neurostimulation implanted on the MTL bilaterally. Since Patient 1 was right handed and the recordings from the left frontal electrodes indicated neuronal activity related to speech articulation (as described below), the left hemisphere was considered dominant for language. Patient 2’s WADA testing revealed language processes were lateralized to the left hemisphere.
Patients consented to testing by signing an informed consent form approved by the Institutional Review Board at the Medical University of South Carolina.
2.2 ECoG Contacts and Localization
Patients were surgically implanted with 10-channel sEEG depth electrodes (0.86 mm diameter, 5 mm spacing; Ad-Tech Corporation, Racine, WI) prior to testing. Exact electrode coordinates were determined using post-implant structural T1-MRI Coordinates for each electrode were obtained through the following procedures. First, using MRIcron software, electrodes were “masked” on each patient’s native T1-MRI This was completed for all contacts for each patient. Electrode masks were then used in the estimation of normalization parameters using the cost-function masking (Brett, Leff, Rorden, & Ashburner, 2001) features in the Clinical Toolbox (Rorden, Bonilha, Fridriksson, Bender, & Karnath, 2012) for SPM8 to ensure that tissue surrounding electrode locations would be normalized without interference from the electrode sites The normalized, skull-stripped T1 images were visually inspected for distortions and then used to identify specific coordinate locations for each individual channel in standard space. The central point of each sEEG electrode was manually localized and anatomical coordinates were recorded and compared with spatial coordinates from the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) and with the previous literature on the SPGI anatomical boundaries (Dronkers, 1996; Fedorenko, Fillmore, Smith, Bonilha, & Fridriksson, 2015). We selected channels of interest from the insular, frontal, and post-central cortices across the left hemisphere. For both patients, a measure of insular activity during speech production was recorded from the SPGI, in addition to a measure of posterior insula activity recorded from Patient 2. Prefrontal recordings were taken from superior frontal gyrus (SFG; Patients 1 and 2), the middle frontal gyrus (Patient 2) and the inferior frontal gyrus pars triangularis (IFGpt; Patient 1). Last, an electrode located in the post-central gyrus (PoCG; Patient 1) served as a measure of post-central cortical activity. Note that electrode locations differed slightly between the two patients due to individualized variations of sEEG electrode placement given cortical anatomy and pre-surgical planning. The anatomical coordinates of each chosen contact are presented in Table 1, and will be referred to by lobar region throughout the remaining text. Figure 2 displays T1 scans for each patient, with crosshairs at each channel of interest. Since sEEG depths are placed orthogonally to the pial surface, some electrodes are situated in the cortex, while other are in the cortical pial transition, gray and white matter transition, or in the white matter. In order to accurately sample from cortical sources, for each depth, we chose the electrodes whose placement was in the center of the cortical depth and not in the cortical inner or outer boundary zones.
Figure 2.
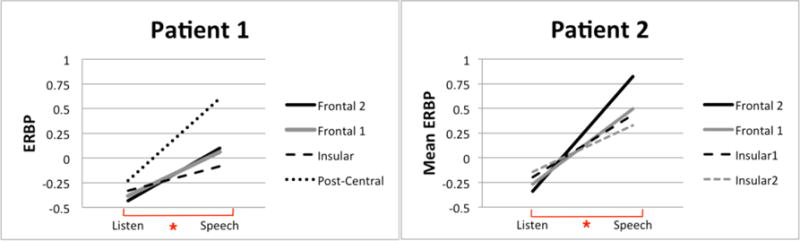
Mean ERBP for each channel for the Listen and Speech conditions. Both figures illustrate the condition (Listen v. Speech) by channel interactions for both patients. The asterisk at the bottom of each figure indicates a significant difference between the Listen and Speech conditions.
2.3 Design, Materials, and Procedure
The articulation task consisted of listening and repeating twelve different bisyllabic pseudowords presented over eight blocks (96 total trials). Pseudowords were chosen instead of real words to emphasize that the ECoG measurements reflected articulation as opposed to lexical processing. The pseudowords were recorded by a native English speaker in a neutral accent. The second syllable of each stimulus was always a consonant vowel (CV) syllable (e.g., __fo, __po), and the first syllable of each pseudoword was either a simple CV structure (e.g., pow__, bih__) or consonant cluster, compatible with the English language (e.g., tr__, fr__). Pseudowords were adapted from Fedorenko et al. (2015).
The PsychToolbox (Brainard, 1997) for Matlab (version 2012a, Mathwoks, Natic MA), was used for the presentation of the pseudowords and to record each patient’s responses. In each trial, a pseudoword was auditorily presented. Each contact’s response to this auditory presentation was recorded, herein referred to as the Listen condition. Following a 3000 ms delay, a visual cue appeared on the computer screen, and patients were instructed to repeat the pseudoword as soon as this cue appeared. 3000 ms were allowed for speech production. ECoG signal response during pseudoword production is herein referred to as the Speech condition. To avoid patient fatigue, total experimental testing did not exceed 15 minutes. Patient responses were audio recorded for the purpose of offline scoring.
2.4 Data Acquisition and Analysis
ECoG data were recorded using a XLTEK EEG system (Natus Medical, Inc.) at a sampling rate of 2 KHz. Each speech stimulus prompt and the visual cues for patient pseudoword production were marked separately in the EEG signal using a photodiode pulse synched to the onset and offset of each event. These pulses were used to identify the occurrence of experimental events in the recorded ECoG signals. Patients’ speech responses were recorded using the same experimental computer using Matlab and Psychtoolbox at 44.1 KHz.
ECoG data were converted using EEGLab and imported to Matlab for preprocessing and analysis using in-house developed Matlab scripts. The data were first band pass filtered at 1–300 Hz, and a notch filter was applied for the 58–63 Hz band. Channels with excess noise were identified and removed through the following process: the mean activity of all channels was calculated and we removed channels that demonstrated mean activity greater than one standard deviation higher or lower than the grand mean of all channels. The mean activity of the remaining channels was then used as an average reference for further analysis. Data were baseline corrected by subtracting the mean activity compared with the time window encompassing 1000 ms before the onset of the presented stimulus (listen condition) or the cued speech onset (speech condition).
Time–frequency analysis of the ECoG signals was performed on a trial-by-trial basis using a complex Morlet wavelet transform (Oya, Kawasaki, Howard, & Adolphs, 2002) with center frequencies ranging from 1 to 300 Hz with 1 Hz spectral resolution. The wavelet constant ratio was defined as fc/σf = 10, where fc is the center frequency of the wavelet and σf is its standard deviation in frequency domain defined as σf = 1/(2πσt). At 100 Hz, this leads to a wavelet width (2σt) of 31.8 ms and to a spectral bandwidth (2σf) of 20 Hz. The wavelet convoluted ECoG data corresponding to the auditory presentation of each pseudoword (Listen condition) and patient response (Speech condition) were calculated for each condition as an event-related band power (ERBP) response for each electrode according to the following formula:
The log transformation function was used to ensure that the data were normally distributed for statistical analysis. ERBP responses for the pseudoword presentation (Listen condition) were calculated by normalizing signal power P following presentation of the pseudoword stimulus, relative to the power at baseline (Pbasline; 1000 ms duration prior pseudoword presentation). ERBP for speech responses was computed similarly, with signal power P normalized following each patient’s production, relative to 1000 ms prior to the visual cue for speech onset.
ERBP for trials in each condition (Listen and Speech) were then averaged for each electrode location into 100 ms time bins from 500 ms prior to the cued response interval, and allowing 3000 ms for speech response. This resulted in 30 bins for further analysis (note that the last bin from each analysis was removed due to edge artifact). Finally, mean ERBP responses for each channel across the Listen and Speech conditions were compared with two separate 2×4 (condition × channel) within-subjects repeated measures ANOVAs (using SPSS, version 24). ANOVA results were followed-up with post-hoc pairwise comparisons for each channel’s mean ERBP response. All comparisons were Bonferroni corrected for multiple comparisons for channel (i.e., p=0.05 divided by six channel comparisons, yielding a significant p-value of p<0.008). Since mean ERBP responses were collapsed per channel across time, we did not correct the p value based on the number of time bins.
3. Results
3.1 Behavioral performance
Both patients completed the articulation task with high accuracy (Patient 1: 90%; Patient 2: 92%), with the infrequent errors consisting of sound substitutions, omissions, distortions and additions. Mean latency to speech onset was 827.11 ms (SD=302) for Patient 1 and 553.15 ms (SD=189.7) for Patient 2.
3.2 Mean ERBP responses by condition
All channels demonstrated significantly greater high-gamma (75–150 Hz) ERBP during Speech versus Listen. There were significant condition by channel interactions for both patients: Patient 1: F(3, 87)=28.99,p<0.001, partial η2=0.50; Patient 2: F(3, 87)=124.96, p<0.001, partial η2=.81. We did not observe significant increase in ERBP during Speech v. Listen for other frequencies (alpha: 8.5–13Hz, beta: 13.5 – 30Hz, low gamma: 30–75Hz). Accordingly, ERBP in the sections that follow refer to responses in the high gamma range.
3.3 Channel comparisons
Overall, ERBP was greater for the Speech conditions compared to the Listen conditions (mean difference between conditions for Patient 1: 0.51, p<0.0001; Patient 2: 0.76, p<0.0001). Mean ERBP for all channels for both conditions in presented in Figure 2. Bonferroni corrected pairwise comparisons for each channel of interest for each condition are as follows:
Inspection of ERBP responses for Patient 1 during the Listen condition revealed no significant differences in ERBP for the two frontal channels (p=0.014) or between the Insular and Frontal 1 channel (p=0.029). The Post-central channel had the greatest ERBP compared to all other channels (p<0.005 for all). The Frontal 1 channel had the smallest ERBP response, significantly different from the Insular [t(29)=4.93, p<0.001] and Post-central channels [t(29)=9.53, p<0.001]. For Patient 2, all channels were significantly different during Listen (Bonferroni corrected-p<0.008), except for the two frontal channels (p=0.009). Both insula channels (i.e., SPGI, posterior insula) demonstrated significantly greater ERBP than the frontal channels [(Insular 1 v. Frontal 1: t(29)=4.1, p<0.001; Insular 1 v. Frontal 2: t(29)=8.61, p<0.001); Insular 2 v. Frontal 2: t(29)=10.321, p<0.001; Insular 2 v. Frontal 1: t(29)=6.94, p<0.001], and Insular 2 channel was significantly greater than Insular 1 [t(29)=3.109, p<0.005].
During the Speech conditions, mean ERBP responses for Patient 1 showed that the Insular 1 channel (i.e., the SPGI) had the lowest mean ERBP when compared to both the frontal and the post-central channels (p<0.001 for all comparisons). For Patient 2, the Insular 1 and Frontal 1 channels did not differ in ERBP response (p=0.009), but all other channels differed significantly (p<0.001 for all). Inspection of channels with the greatest mean ERBP during the Speech conditions shows that for Patient 1, mean ERBP of the post-central region was significantly greater than that of all other regions (Post-central v. Frontal 1: t(29)=7.98, p<0.001; Post-central v. Frontal 2: t(29)=8.04, p<0.001; Post-central v. Insular: t(29)=14.84, p<0.001). For Patient 2, mean ERBP of the Frontal 2 channel was significantly greater than all other channels (Frontal 1 v. Frontal 2: t(29)=7.89, p<0.001; Frontal 1 v. Insular 1: t(29)=10.3, p<0.001; Frontal 1 v. Insular 2: t(29)=11.56, p<0.001).
We subsequently investigated the time point in which ERBP for Speech conditions differed significantly from the Listen conditions. For Patient 1, the Post-central channel was the first region to demonstrate a significantly higher ERBP in Speech when compared to Listen (at the 100 ms time bin), followed by Frontal 2 (800 ms time bin), Insular (1000 ms) and Frontal 1 (1200 ms). For Patient 2, differences emerged in all four channels at the 100 ms time bin following the cue to speak. Figure 3 (panels A and B) presents mean ERBP for each 100 ms time bin for the Speech conditions. The first time bin in which ERBP in the Speech condition significantly exceeded the Listening condition are marked with an asterisk.
Figure 3.
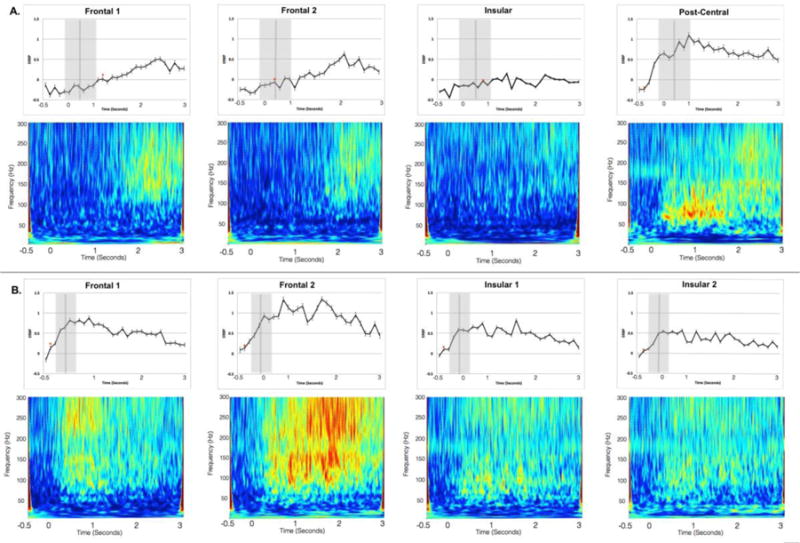
Channel responses to the Speech conditions at each 100 ms time bin. Patient 1 data are presented in panel A, and Patient 2 in panel B. The 0 second time corresponds to the onset of the cue to produce speech, and the vertical grey bars correspond to the mean articulation latency from the cue to produce each target pseudoword (standard deviation indicated by the surrounding grey interval). The asterisks indicate the first time point in which ERBP for the Speech condition significantly exceeded that of the Listen condition. Spectrograms for each channel for the Speech conditions are presented below each time course figure; warmer colors (red, yellow) indicate higher activity across the three-second-response interval. The frequency bands of interest in this analysis are 75–150 Hz high gamma range.
4. Discussion
Using ECoG recordings, the present study demonstrated a direct increase in high gamma ERBP activity in left hemisphere frontal and post-central regions during pseudoword articulation. In contrast, the insular cortex had a tendency toward eliciting significantly weaker high gamma activity compared with the frontal and post-central cortex during pseudoword articulation. This pattern was seen across both patients. Although there were slight differences in activity between the frontal channels for both mean high-gamma response and the ERBP response over time, it should be noted that the exact placement of the electrodes differed between patients, and therefore, ERBP activity in the selected channels is a broad representation of frontal lobe regions during speech production.
As is the case with most ECoG studies collecting invasive and valuable data, our results are limited by the small number of patients and by the fact that both patients suffered from uncontrolled epilepsy. Even though there were no visible structural lesions on the location of the electrodes, it is not possible to ascertain that the underlying cortex is similar to the general population. Nonetheless, both patients were able to complete the task with few errors1, and the studied electrodes were not placed at the location of seizure onset. Specifically, the seizure onsets were restricted to the MTL - bilateral independent MTL for Patient 1 and restricted to the right MTL for Patient 2. The insula was not implicated in seizure onset or in seizure propagation in both cases. None of the recorded seizures were poorly localized or succeeded clinical seizure manifestations. Although the MTL is implicated in some aspects of lexical production (e.g., semantic processes; Visser, Jefferies, Embleton, & Lambon Ralph, 2012), it is not implicated in motor speech production or articulation. Accordingly, we contend that seizure onset locus was unlikely to have influenced recording sites or performance for this task. Since direct cortical recordings from the insula are exceptional and no previous studies have reported SPGI recordings during an articulation task, we believe that this study provides unprecedented insights into the magnitude and timing of evoked cortical responses during speech production.
Based on these results, we suggest that the insula’s role in motor speech production is secondary to the activity in the pre- and post-central areas, but further study is warranted to confirm these findings. Here we found that the insula is active during articulation, but that it may not play a primary role in production (see also Fedorenko et al., 2015). From our sample, the earliest peak gamma activation was noted in the post-central region, which is in accordance with contemporary models of motor speech control such as the Hierarchical State Feedback Control (HSFC) model (Hickok, 2012; Houde & Nagarajan, 2011) and the Directions into the Velocities of Articulators (DIVA) (Guenther & Vladusich, 2012; Tourville & Guenther, 2011). According to these models, speech motor control depends on the sensorimotor transformation of stored auditory targets, accessed via post-central regions, into articulatory targets, which are planned and executed in frontal motor regions (Hickok, 2012). Although it has been argued that pseudoword stimuli “isolate” articulatory processes (e.g., Hickok & Poeppel, 2004), a caveat is that the computations required for pseudoword articulation may place greater processing demands upon the auditory-motor system (e.g., Baldo et al., 2012; Rogalsky et al., 2015). That is, results from two lesion-symptom mapping studies where post-stroke individuals completed pseudoword repetition tasks showed that the extent of lesion damage related to pseudoword repetition was spatially similar to that of real word repetition, but spatial representation was more extensive (Baldo et al., 2012; Rogalsky et al., 2015). In the context of this study, it may be that the pseudoword task elicited greater activation across all channels of interest; however, further work comparing the response of these regions to a real word task would be needed to confirm this inference.
In conclusion, our results support the contemporary models of motor speech (HSFC and DIVA) and demonstrate that the frontal and post-central cortices are strongly associated with motor speech control. Discrepancies regarding whether or not the insula is the cortical seat of speech production may be related to methodological issues across studies that either relied on lesion analyses in patients (for discussion, see Richardson et al., 2012) or functional magnetic resonance imaging (fMRI; Fedorenko et al., 2015; Moser et al., 2009; Wise et al., 1999). Due to natural divisions of the cerebral vasculature, the insula is highly susceptible to damage following a stroke affecting the middle cerebral artery, and it may be overrepresented in lesion analysis methods since it is often concurrently lesioned alongside crucial speech areas (Hillis et al., 2004; Kodumuri et al., 2016). Similarly, the sluggish temporal resolution of fMRI’s blood-oxygenation related signal limits the ability to adjudicate whether insula activation is essential for speech production or may be related to other factors such as respiration and/or oral motor movements (Ackermann & Riecker, 2004; Ackermann & Riecker, 2010; Fedorenko et al., 2015). The use of ECoG allowed for investigation of the insula during speech production, without the potential confound of these factors. Nevertheless, the specific nature of the supportive role of the insula in speech should be tested by future studies.
Figure 1.
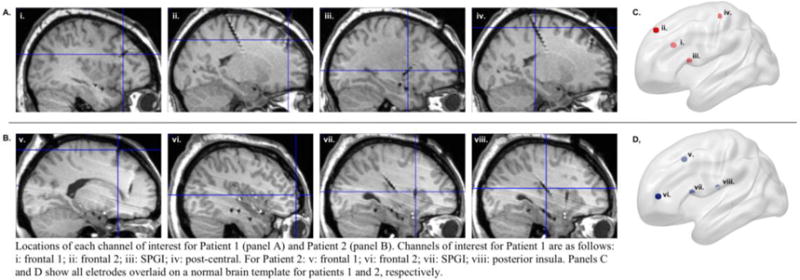
Locations of each channel of interest for Patient 1 (panel A) and Patient 2 (panel B). Channels of interest for Patient 1 are as follows: i: frontal 1; ii: frontal 2; iii: SPGI; iv: post-central. For Patient 2: v: frontal 1; vi: frontal 2; vii: SPGI; viii: posterior insula. Panels C and D show all electrodes overlaid on a normal brain template for Patients 1 and 2, respectively.
Highlights.
Direct cortical recordings of insula activity during speech production are reported
Compared to the insula, frontal and post-central regions had greater activity during speech
Results support contemporary models’ emphasis on sensorimotor areas in speech production
Acknowledgments
This study was supported by the National Institute on Deafness and Other Communication Disorders (NIDCD), grant numbers DC014021 (LB) and DC009571 (JF). The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None of the authors have any conflicts of interest to report, financial or otherwise
Seven control individuals with no history of speech/language impairment completed the same articulation task with 93.6% accuracy (SD=6). Accordingly, the patient error rates were within one standard deviation of this control sample.
References
- Ackermann H, Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89(2):320–328. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Riecker A. The contribution (s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Structure and Function. 2010;214(5–6):419–433. doi: 10.1007/s00429-010-0257-x. [DOI] [PubMed] [Google Scholar]
- Ardila A. The role of insula in language: an unsettled question. Aphasiology. 1999;13(1):79–87. [Google Scholar]
- Ardila A, Benson DF, Flynn FG. Participation of the insula in language. Aphasiology. 1997;11(12):1159–1169. [Google Scholar]
- Augustine J. The insular lobe in primates including humans. Neurological research. 1985;7(1):2–10. doi: 10.1080/01616412.1985.11739692. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Katseff S, Dronkers NF. Brain Regions Underlying Repetition and Auditory-Verbal Short-term Memory Deficits in Aphasia: Evidence from Voxel-based Lesion Symptom Mapping. Aphasiology. 2012;26(3–4):338–354. doi: 10.1080/02687038.2011.602391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Wilkins DP, Ogar J, Willock S, Dronkers N. Role of the precentral gyrus of the insula in complex articulation. Cortex. 2011;47(7):800–807. doi: 10.1016/j.cortex.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Basilakos A, Rorden C, Bonilha L, Moser D, Fridriksson J. Patterns of Poststroke Brain Damage That Predict Speech Production Errors in Apraxia of Speech and Aphasia Dissociate. Stroke. 2015;46(6):1561–1566. doi: 10.1161/STROKEAHA.115.009211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behroozmand R, Oya H, Nourski KV, Kawasaki H, Larson CR, Brugge JF, et al. Neural Correlates of Vocal Production and Motor Control in Human Heschl’s Gyrus. The Journal of Neuroscience. 2016;36(7):2302–2315. doi: 10.1523/JNEUROSCI.3305-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard KE, Mesgarani N, Johnson K, Chang EF. Functional organization of human sensorimotor cortex for speech articulation. Nature. 2013;495(7441):327–332. doi: 10.1038/nature11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14(2):486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Chang EF, Niziolek CA, Knight RT, Nagarajan SS, Houde JF. Human cortical sensorimotor network underlying feedback control of vocal pitch. Proc Natl Acad Sci U S A. 2013;110(7):2653–2658. doi: 10.1073/pnas.1216827110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. 2009. [DOI] [PubMed] [Google Scholar]
- Dronkers N. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dronkers N, Ogar J. Brain areas involved in speech production. Brain. 2004;127:1461–1462. doi: 10.1093/brain/awh233. [DOI] [PubMed] [Google Scholar]
- Dronkers N, Ogar J, Willock S, Wilkins DP. Confirming the role of the insula in coordinating complex but not simple articulatory movements. Brain and Language. 2004;91(1):23–24. [Google Scholar]
- Fedorenko E, Fillmore P, Smith K, Bonilha L, Fridriksson J. The superior precentral gyrus of the insula does not appear to be functionally specialized for articulation. J Neurophysiol. 2015;113(7):2376–2382. doi: 10.1152/jn.00214.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinker A, Korzeniewska A, Shestyuk AY, Franaszczuk PJ, Dronkers NF, Knight RT, et al. Redefining the role of Broca’s area in speech. Proceedings of the National Academy of Sciences. 2015;112(9):2871–2875. doi: 10.1073/pnas.1414491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J, Jones DT, Strand EA, Rabinstein AA, Duffy JR, Josephs KA. The neuroanatomy of pure apraxia of speech in stroke. Brain Lang. 2014;129:43–46. doi: 10.1016/j.bandl.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee JD, Behroozmand R, Larson CR, Jackson AW, Chen F, Hansen DR, et al. Sensory-motor interactions for vocal pitch monitoring in non-primary human auditory cortex. PLoS One. 2013;8(4):e60783. doi: 10.1371/journal.pone.0060783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH, Vladusich T. A Neural Theory of Speech Acquisition and Production. J Neurolinguistics. 2012;25(5):408–422. doi: 10.1016/j.jneuroling.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. Computational neuroanatomy of speech production. Nat Rev Neurosci. 2012;13(2):135–145. doi: 10.1038/nrn3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. Toward an Integrated Psycholinguistic, Neurolinguistic, Sensorimotor Framework for Speech Production. Lang Cogn Process. 2014;29(1):52–59. doi: 10.1080/01690965.2013.852907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Rogalsky C, Chen R, Herskovits EH, Townsley S, Hillis AE. Partially overlapping sensorimotor networks underlie speech praxis and verbal short-term memory: evidence from apraxia of speech following acute stroke. Front Hum Neurosci. 2014;8:649. doi: 10.3389/fnhum.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127(Pt 7):1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Houde JF, Nagarajan SS. Speech production as state feedback control. Frontiers in Human Neuroscience. 2011;5 doi: 10.3389/fnhum.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnard J, Guénot M, Sindou M, Mauguière F. Clinical Manifestations of Insular Lobe Seizures: A Stereo- electroencephalographic Study. Epilepsia. 2004;45(9):1079–1090. doi: 10.1111/j.0013-9580.2004.68903.x. [DOI] [PubMed] [Google Scholar]
- Itabashi R, Nishio Y, Kataoka Y, Yazawa Y, Furui E, Matsuda M, et al. Damage to the Left Precentral Gyrus Is Associated With Apraxia of Speech in Acute Stroke. Stroke. 2016;47(1):31–36. doi: 10.1161/STROKEAHA.115.010402. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR. Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr Opin Neurol. 2008;21(6):688–692. doi: 10.1097/WCO.0b013e3283168ddd. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(Pt 5):1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingyon J, Behroozmand R, Kelley R, Oya H, Kawasaki H, Narayanan N, et al. High-gamma band fronto-temporal coherence as a measure of functional connectivity in speech motor control. Neuroscience. 2015;305:15–25. doi: 10.1016/j.neuroscience.2015.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Ullsperger M, Danielmeier C. Error awareness and the insula: links to neurological and psychiatric diseases. Frontiers in human neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodumuri N, Sebastian R, Davis C, Posner J, Kim EH, Tippett DC, et al. The association of insular stroke with lesion volume. NeuroImage: Clinical. 2016 doi: 10.1016/j.nicl.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlab. Natick, MA: The MathWorks, Inc; 2012a. [Google Scholar]
- Mayer EA, Naliboff BD, Craig AB. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131(6):1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Moisset X, Bouhassira D, Ducreux D, Glutron D, Coffin B, Sabaté JM. Anatomical connections between brain areas activated during rectal distension in healthy volunteers: a visceral pain network. European Journal of Pain. 2010;14(2):142–148. doi: 10.1016/j.ejpain.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Moser D, Fridriksson J, Bonilha L, Healy EW, Baylis G, Baker JM, et al. Neural recruitment for the production of native and novel speech sounds. Neuroimage. 2009;46(2):549–557. doi: 10.1016/j.neuroimage.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Takeda K, Komori T, Isozaki E, Hirai S. Apraxia of speech associated with an infarct in the precentral gyrus of the insula. Neuroradiology. 1999;41(5):356–357. doi: 10.1007/s002340050764. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in neurosciences. 2009;32(1):56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Structure and Function. 2010;214(5–6):435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N. Clinical and anatomical correlates of apraxia of speech. Brain Lang. 2006;97(3):343–350. doi: 10.1016/j.bandl.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Oya H, Kawasaki H, Howard MA, Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. The journal of neuroscience. 2002;22(21):9502–9512. doi: 10.1523/JNEUROSCI.22-21-09502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JD, Fillmore P, Rorden C, Lapointe LL, Fridriksson J. Re-establishing Broca’s initial findings. Brain Lang. 2012;123(2):125–130. doi: 10.1016/j.bandl.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Poppa T, Chen KH, Anderson SW, Damasio H, Love T, Hickok G. Speech repetition as a window on the neurobiology of auditory–motor integration for speech: A voxel-based lesion symptom mapping study. Neuropsychologia. 2015;71:18–27. doi: 10.1016/j.neuropsychologia.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61(4):957–965. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourville JA, Guenther FH. The DIVA model: A neural theory of speech acquisition and production. Lang Cogn Process. 2011;26(7):952–981. doi: 10.1080/01690960903498424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Xia R, Mandrekar J, Machulda MM, et al. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang. 2013;125(3):245–252. doi: 10.1016/j.bandl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Embleton KV, Ralph MaL. Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. Journal of Cognitive Neuroscience. 2012;24(8):1766–1778. doi: 10.1162/jocn_a_00244. [DOI] [PubMed] [Google Scholar]
- Wise R, Greene J, Büchel C, Scott SK. Brain regions involved in articulation. The Lancet. 1999;353(9158):1057–1061. doi: 10.1016/s0140-6736(98)07491-1. [DOI] [PubMed] [Google Scholar]


