Abstract
Group B streptococci (GBS, Streptococcus agalactiae) are a major cause of invasive infections in newborn infants and in patients with type II diabetes. Both patient groups exhibit peripheral insulin resistance and alterations in polymorphonuclear leucocyte (PML) function. Here, we studied the PML response repertoire to GBS with a focus on TLR signaling and the modulation of this response by insulin in mice and humans. We found that GBS-induced, MyD88-dependent chemokine formation of PML was specifically down-modulated by insulin via insulin receptor mediated induction of PI3-kinase. PI3-kinase inhibited transcription of chemokine genes on the level of NFkB activation and binding. Insulin specifically modulated the chemokine response of PML to whole bacteria, but affected neither activation by purified TLR agonists nor antimicrobial properties, such as migration, phagocytosis, bacterial killing and formation of reactive oxygen species. The targeted modulation of bacteria-induced chemokine formation by insulin via PI3-kinase may form a basis for the development of novel targets of adjunctive sepsis therapy.
Introduction
The immune system and the endocrine system are interwoven on many levels. A prominent example for this is insulin, which has been assigned regulatory properties both for the antimicrobial and the inflammatory response in infection (1–3). Insulin resistance is associated with increased susceptibility to invasive bacterial infections (4–7).
Group B streptococcus (Streptococcus agalactiae, GBS) is particularly interesting in this context, since it causes invasive infection in two groups of patients with peripheral insulin resistance, newborn infants and patients with type II diabetes (8–11). GBS are mucocutaneous colonizers in 15 – 20% of all people, and 10 % of newborn infants. Yet, invasive infection with this organism is a comparatively rare event that affects only about 1% of all colonized infants (12–14). Containment of GBS at mucocutaneous surfaces is conceivable only if occasionally invading microorganisms are rapidly killed by phagocytes, in order to prevent bacterial dissemination and systemic inflammation. Polymorphonuclear granulocytes (PML) are key effector cells in several models of mucocutaneous bacterial infections (15–17). In response to both bacterial particles and specific TLR activation, PML initiate various pathogen eliminating strategies, such as phagocytosis, the generation of reactive oxygen species and prolonged survival (reviewed by Prince et al (18)). Multiple lines of evidence suggest that PML are targets of inflammatory control mediated by insulin. In patients with insulin resistance, inflammatory and antimicrobial PML functions have been found to be altered (1, 19). An immunomodulatory effect of insulin has been experimentally confirmed in mouse models (20). Furthermore, insulin therapy may modulate the outcome in sepsis, although this remains a controversial issue (21–24).
However, whereas an association of diabetes and susceptibility to bacterial infections is widely accepted, the underlying molecular mechanisms are poorly understood. It remains to be established whether immunological alterations in insulin resistance are mainly due to changes in glucose provision, or whether the insulin receptor (IR) has further, glucose metabolism independent, modulatory properties in cell autonomous inflammatory signaling.
Here, we found that in PML, GBS induced substantial amounts of chemokines in a phagocytosis-independent fashion. In contrast to monocytes and macrophages, inflammatory cytokines such as TNF and IL6 were poorly induced. Insulin specifically inhibited the chemokine response to GBS and other bacteria by modulating NFkB binding via activation of PI3-kinase. In contrast, insulin affected neither the PML response to purified TLR agonists, nor did it alter directly antibacterial properties e.g. formation of reactive oxygen species or chemotaxis.
Materials and Methods
Reagents were obtained from Sigma-Aldrich, unless stated otherwise. PBS, DMEM, and trypsin were purchased from Cambrex. Low endotoxin FBS was obtained from HyClone. LPS derived from Escherichia coli strain 0111:B4 was purchased from Sigma-Aldrich and extracted twice by phenol chloroform, as described in (25). C57BL/6 wild type mice were purchased from Jackson Laboratories, Bar Harbor, ME. MyD88-deficient mice (C57 BL/10) were generated as described in (26) and kindly provided by Shizuo Akira, Dept. of Biochemistry, Hyogo College of Medicine, Hyogo, Japan). Plasmids encoding for MyD88, IRAK1 and TRAF6 were a kind gift from Douglas Golenbock (Div. of Infectious Diseases and Immunology, University of Massachusetts Medical School, Worcester, USA).
Generation of heat-fixed GBS
GBS type III strain COH1, initially isolated from a newborn infant with sepsis, has been previously described (27). Bacteria were grown on blood agar plates (REMEL). Bacterial colonies were removed from the plates after overnight culture and washed three times in PBS. The resulting bacterial suspension was used to inoculate culture medium (DMEM plus 10% FBS) and grown to mid-log phase (adsorption650 = 0.27–0.30). Subsequently, bacteria were harvested, washed, and suspended in pyrogen-free water at a concentration of 20 mg/ml (corresponding to ~1 × 1010 organisms/ml as determined by CFU/ml). If indicated, GBS were employed live for stimulation. In all other cases, GBS were used as heat fixed, lyophilized preparations (fixation at 80°C over 1h). For opsonization fixed GBS were incubated with specific anti-GBS antibodies for 30 min on a rotating platform. The preparations were essentially free of endotoxin, as described previously (28).
PML isolation
Cells were isolated from heparinized venous blood (5 units per ml of blood) from healthy adult volunteers. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of the University Medical Center Freiburg (Protocol Permit Number: 282/11). Consent was documented for all participating volunteers. PML were separated by centrifugation employing Percoll (density of 1.076 g/ml). Contaminating erythrocytes were lysed in ice-cold medium containing 155 mmol/l NH4Cl, 10 mol/l KHCO3, and 0.1 mmol/l EDTA, pH 7.4. Cells were washed and suspended in either RPMI 1640 medium with 10% FBS and 10 μg of ciprofloxacin/ml, or Hepes-buffered saline solution (132 mmol/l NaCl, 6.0 mmol/l KCl, 1.0 mmol/l CaCl2, 1.0 mmol/L MgSO4, 1.2 mmol/l potassium phosphate, 20 mmol/l Hepes, 5.5 mmol/l glucose, and 0.5% (wt/vol) human serum albumin, pH 7.4). Both purity and viability of PML were typically > 95% as assessed by morphology and trypan blue staining.
Mouse peritoneal PML
This study was carried out in strict accordance with the recommendations of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS 123). The animal experimental protocol was approved by the ethics committee of the University Medical Center Freiburg (Protocol Permit Number: 35-9185.81/G-09/42). All animal experiments were planned and executed in order to minimize suffering. Eight-week-old mice (MyD88 or C57BL6/J wild type, Jackson Laboratories, Bar Harbor, ME) were injected intraperitoneally with 2.5 ml of a 3% thioglycollate solution (Remel, Lenexa, KS). After 5–8 h, peritoneal exsudate cells were harvested as described previously (27). For further purification peritoneal neutrophils were stained for CD11b (PE-Cy7-labeled anti-human CD11b antibody, eBioscience), Ly6G (FITC-labeled anti-human Ly6G antibody, BD Pharmigen) and CD115 (MCSF-R, primary biotin-labeled anti-human CD115 antibody, secondary Pacific blue-labeld Streptavidin linked antibody, eBioscience). Subsequently, cells were washed and subjected to FACS sorting. PMN were identified by gating on CD11b+ cells and subsequently on the clearly defined Ly6GhiCD115- population to obtain Ly6G+CCD115-CD11b+ neutrophils.
Cells were then washed and suspended in medium (RPMI 1640 medium containing 10% FBS and 10 μg of ciprofloxacin/ml), counted with a hemocytometer, plated and allowed to rest for at least 1h.
Inhibition of signaling cascades by chemical inhibitors, insulin and antibodies
Cells were treated with insulin, an insulin receptor specific antibody (directed against the α-subunit, Thermo Fischer Scientific, Freemond, CA) or isotype control (30μg/ml) and/or the chemical inhibitors Cytochalasin D, SB203580, Wortmannin and Ly294002 at indicated concentrations for 30 min prior to further treatment or stimulation. Cell activation was determined as indicated and described in the respective section.
Transfection of RAW 264.7 macrophages, activation of luciferase reporter constructs and determination of transcriptional gene activation
RAW 264.7 cells were seeded into 96-well tissue culture plates at a density of 105 cells /well (DMEM with 10% FBS and ciprofloxacin at 10 μg/ml). The following day, cells were transfected with luciferase reporter constructs comprising minimal AP-1 and NFkB promoters (Stratagene, La Jolla, CA) or human wild-type and mutant IL8 promoters (provided by N. Mackman, The Scripps Research Institute, La Jolla, CA) with Fugene (Roche) per the manufacturer’s recommendations. In individual experiments, cells were co-transfected with a constitutively active Renilla-luciferase reporter gene (Promega) to normalize for transfection efficacy. The following day, the cells were stimulated as indicated for 4–6h or cotransfected with signaling protein expressing plasmids. Cells were lysed in passive lysis buffer (Promega), and reporter gene activity was measured in a luminometer (MicroLumat Plus; Berthold Detection Systems). In all cases, the data shown represent one of at least three separate, but similar experiments, and are presented as the mean values of arbitrary light units ± SD of triplicate samples, unless SD was <1%.
Measurement of inflammatory activity
For determination of cytokine formation, cells were seeded in 96-well dishes (105 cells/well) in RPMI 1640 with 10% FBS plus 10 μg of ciprofloxacin/ml and incubated for 16 h (37°C, 5% CO2). If indicated, cells were treated with insulin and/or the indicated chemical inhibitor for 30 min. Then, cells were stimulated as indicated over 5h. Supernatants were processed directly for determination of cytokines (ELISA; R&D Systems, Minneapolis, MN) or frozen until analysis. For determination of intracellular cytokines, cells were treated with 0.05% TritonX and subjected to ELISA measurement. Shown are representative results from three or more individual experiments in triplicate wells ±SD. For intracellular measurement of IL8 formation by FACS, freshly isolated PMN were incubated with the indicated antibody or a vehicle control for 30 min at 37°C. Subsequently, insulin (2000 ng/ml) was added where indicated. After another 30 min of incubation, PML were stimulated as indicated for 1h. Then, Brefeldin A (golgi stop) was added. 4h later cells were washed and fixed with 2% PFA, permeabilized and stained for intracellular IL-8 (anti-human IL8 PE, Biolegend).
Analysis of intracellular kinase activation
Phosphorylation of MAPK and IκB was evaluated according to standard protocols. Briefly, lysates of stimulated cells were separated by SDS page and analyzed by Western immunoblotting using nitrocellulose membranes (HyClone, Erembodegem, Belgium) and antibodies for phosphorylated p38 (Cell Signaling Technology, Beverly, MA), c-Jun (Cell Signaling Technology, Beverly, MA), phosphorylated Erk-1 (Cell Signaling Technology, Beverly, MA) and intracellular IκB (Santa Cruz Biotechnologies, Heidelberg, Germany). For analysis of cytosolic p65 phosphorylation human PMN were stimulated with GBS (108/ml) in the presence or absence of insulin (2000 ng/ml). After 20 min cells were fixed, permeabilized and intracellularly stained for phosphorylated p65 (primary antibody: rabbit anti-Phospho-NF-κB p65 (Ser536), Cell Signaling Technology, Beverly, MA); secondary antibody: donkey anti-rabbit Alexa Fluor 568 (Invitrogen, Carlsbad, CA, USA)). Cells were analyzed by FACS for intracellular phospho-p65.
Generation of nuclear extracts
Human PLM were stimulated as indicated and harvested (1500 rpm at 4°C) and strictly kept on ice. Cells were suspended in 100 μl hypotonic buffer, incubated for 15 min, suspended in 10% NP40 buffer. Nuclei were collected by centrifugation (13000 rpm, 5 min), and resuspended in 25 μl extraction buffer. After further incubation (15 min), nuclear lysates were collected (centrifugation at 21.200 rpm for 5 min) and stored at −20°C until further processing.
Analysis of NFkB binding by EMSA
For EMSA 5′biotinylated oligonucleotides were obtained from Biomers (Ulm, Germany) with the following sequence:
5′ AGCTCAGAGGGGACTTTCCGAGAG 3′
3′ GTCTCCCCTGAAAGGCTCTCTCGA 5′
The ElectroMobility Shift Assay (EMSA) was performed with the Chemiluminescent Nucleic Acid Detection Module Kit (#89880, Pierce, Rockford, IL, USA) according to the manufacturer with slight modifications. A 6% polyacrylamide gel was used (22,2 ml H20, 6 ml 30% Polyacrylamide, 1,5 ml 10x TBE buffer, 300 μl 10% APS and 30 μl TEMED). The binding reaction was performed in 1M Hepes plus 5M NaCl, 0,1M MgCl2 and DTT (5 mM). Nuclear extracts were incubated for 15 min on ice before a master mix containing the indicated oligonucleotide, dI:dC (1 μg/μl; Pierce) and 10% BSA were added. Finally, 5μl loading buffer (Pancoll + Bromphenole blue) was added to each sample. Subsequently the gel was loaded with 5 μg protein per lane and run at 80V for 1,5h, then 120V for an additional 1h in 0,5x TBE. The gel was blotted onto a nylon membrane. DNA crosslinking was performed for 15 min on UV table. The membrane was developed using 50 μl Streptavidin:HRP. The membrane was washed and equilibrated for 5 min in 30 ml equilibration buffer before activation in luminol with peroxide. Analysis was performed by means of luminescence measurement.
Analysis of nuclear translocation of NFkB by fluorescence microscopy
PML were seeded onto coverslips in 6 well plates (5×105/well) and rested for 2 h at 37°C. Cells were then treated with insulin (2000 ng/ml) or a vehicle control for 30 min. Subsequently cells were stimulated with GBS as indicated. Cells were then fixed in methanol solution and stored at 4°C over night. The next day, cells were stained for intracellular NFkB employing horse p65 antibody (Santa Cruz Biotechnology) and anti-horse Texas Red antibody (BioSciences). For the discrimination of cytosolic and nuclear areas cell, nuclei were additionally stained with DAPI. Cells were washed twice and fixed with DAKO anti-bleaching solution onto microscopic slides before being subjected to confocal analysis with a Zeiss LSM 710 microscope using the following settings: Magnification 100x, Plan-Apochromat 100x/1.40 Oil DIC objective lenses, 266 μm numerical aperture/pinhole, static sample with respect to temperature, DAKO mounting medium, DAPI & Alexa 546 fluorochromes, AxioCamHRm camera, Carl Zeiss Zen 2009 acquisition software. Software for image processing: Photoshop gamma: 0.45.
RNA isolation and quantitative reverse transcription-PCR
Neutrophils were seeded onto 24-well plates at a density of 5,000,000 cells per well. Cells were stimulated with heat-fixed GBS (107/ml) for 5 hours. Subsequently, cells were washed with ice-cold PBS and samples were frozen until RNA isolation. Total RNA was extracted from samples by using an RNeasy mini kit (Qiagen). For quantitative two-step reverse transcription-PCR, 2 μg of total RNA was reverse transcribed to first-strand cDNA with an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Aliquots of 20 ng of cDNA were subsequently used as a template for quantitative PCR with the following specific primers for human IL8: 5 ′-TGCTAGCCAGGATCCACAAG-3′ and 3′-TGCTTCCACATGTCCTCACA-5′. Human gapdh (glyceraldehyde-3-phosphate dehydrogenase) gene served as a control for constitutive gene expression. Primers employed: 5′-ACACCCACTCCTCCACCTTT-3′ and 3′-TACTCCTTGGAGGCCATGTG-5′. Amplifications were performed with 20 μl of SYBR green JumpStart TaqReadyMix (Sigma-Aldrich, Munich, Germany) and 350 nM oligonucleotides, using an Eppendorf RealPlex thermal cycler (Eppendorf, Hamburg, Germany). After an initial activation step at 95°C for 7 min, 40 cycles (94°C for 30 s, 60°C for 30 s, 72°C for 30 s, and 82°C for 15 s) were performed, and a single fluorescence reading was obtained after the 82°C step of each cycle. A melting curve was determined at the end of cycling to ensure amplification of only a single PCR product. Threshold cycle values were determined with the RealPlex version 1.5 software program, supplied with the instrument. Comparative expression levels (2-ΔΔCT) were calculated according to the method of Livak and Schmittgen (29). The expression levels are relative to the level of GAPDH expression, which was constant in all RNA samples used and was set to 1. The values shown are representative of six samples from two biological experiments performed using quantitative PCR in triplicates. Shown is one out of three representative experiments ± SD. In T-test analysis the two-tailed P value equals * p = 0.0043 and ** p = 0.031. By conventional criteria, this difference is considered to be statistically significant.
Adhesion
Human PML were labeled with the fluorophor calcein-AM (1 μM final concentration; Molecular Probes, Leiden, Netherlands), incubated with insulin or a vehicle control and stimulated as indicated for 30 min. Then, the plate was washed to remove non-adherent cells. Adherent cells were lyzed with Triton X and fluorescence was measured for each well. Depicted is one representative out of three or more experiments.
Chemotaxis
PML migration was determined in a 2 chamber system containing Fluoroblok inserts (Falcon; Becton Dickinson, San Jose, CA). Human PML (5×106/ml) were labeled with calcein-AM for 30 minutes at 37°C, washed twice, and suspended in HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid) buffer at a concentration of 2 x106/ml. Chemoattractants (platelet activating factor [PAF], interleukin 8 [IL8], and complement 5a [C5a], all at 10 nM) in HEPES or HEPES alone were placed in the lower chamber of a 24-well plate (0.8 ml/well). Then, inserts (3 μm pore size) were placed on top and 0.3 ml PML suspension was pipetted into the inserts. Fluorescence in the lower compartment as a parameter of migrated PML was measured every 2.5-minutes for a total of 45 minutes. The maximal slope of migration was calculated over a 10-minute interval. Migrational direction and speed of PML was assessed with the ibidi μ-slide system (ibidi, GmbH, Munich, Germany). An fMLP gradient was created and PML, pretreated with insulin or a vehicle, were loaded through the cell inlet. Slides were incubated at 37°C for up to 1 h, and cell movement was assessed employing a Nikon BioStation IM (Nikon Instruments, New York, NY). For image analysis ImageJ software (Rasband W.S., ImageJ, NIH, Bethesda, Maryland, USA) and the following plugins were used: Manual Tracking plugin (Fabrice Cordelières, Institut Curie, Orsay, France), Chemotaxis and Migration Tool (ibidi, Munich, Germany). Data were analyzed for migrational speed and direction. Depicted is one representative experiment.
Phagocytosis
Human PML were plated on a 24 well plate (2×105 cells/well) and treated with insulin (2000 ng/ml) or a vehicle for 30 min. Subsequently, cells were incubated with or without FITC-labeled GBS (108/ml) for 7 min. Then, cells were harvested, washed and extracellular fluorescence was quenched with trypan blue. Cells were analyzed for intracellular fluorescence by FACS employing FlowJo software.
Formation of reactive oxygen species
Human PML were plated at a density of 105 cells per well into a 96-well plate and incubated with GBS (106 and 108/ml) as indicated. Lucingenin was used to measure extracellular O2− over 90 min. Depicted is the mean fluorescence intensity from triplicate wells ± SD of one out of at least three experiments.
Statistic analysis
Standard deviations were calculated by either Excel or Graphpad Prism. Statistical calculations for migrational capacity of live cell imaging data were performed with the ImageJ Chemotaxsis plugin. Unless stated otherwise, further statistical analysis was performed using one-way ANOVA test followed by Tukey’s Multiple Comparism Test in Graphpad Prim 5. P-values are depicted only for relevant data pairs (* p < 0.05, ** p < 0,01, *** p < 0,001).
Results
GBS induces chemokines in neutrophils in a MyD88-dependent, but phagocytosis-independent fashion
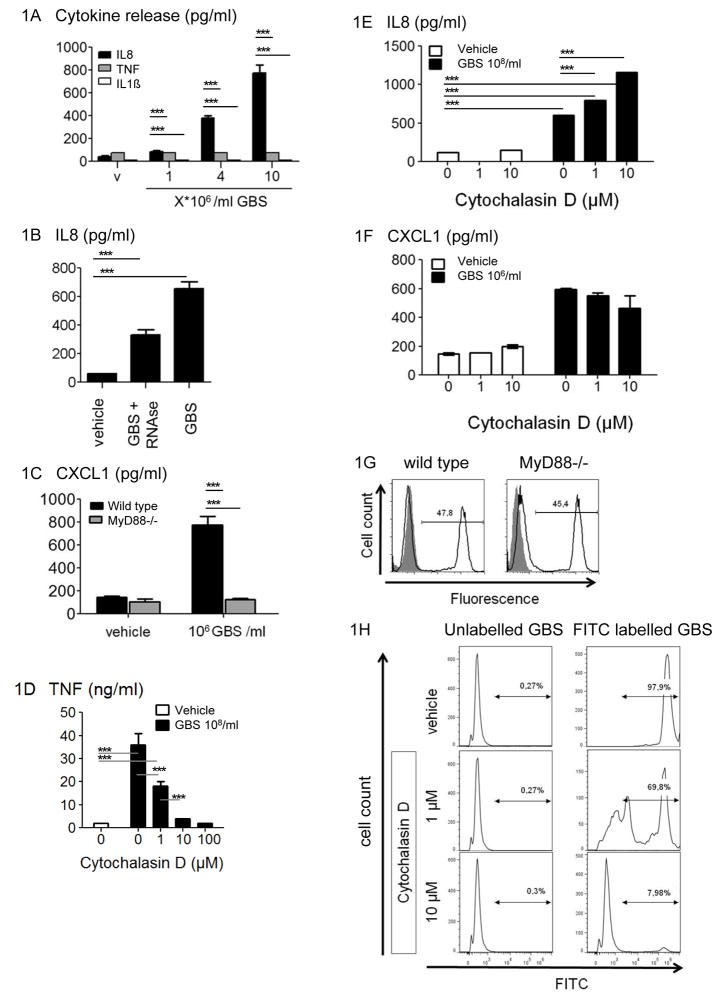
The transcriptional regulation of inflammatory genes in mononuclear phagocytes stimulated by GBS has been studied in detail by us and others (27, 30–32). In contrast, requirements and consequences of PML stimulation by streptococci are incompletely understood. Yet, alterations in PML function have been implicated in the specific susceptibility of infants and diabetic patients to invasive GBS infections (33). Accordingly, we first characterized the cytokine and chemokine response of human PML to GBS. We found, that – upon stimulation with GBS - PML produced high levels of IL8 (Figure 1A). In contrast, they did not form relevant amounts of IL1β or TNF, the latter of which is potently induced in monocytes and macrophages. However, similar to GBS recognition in macrophages (32), recognition of GBS ssRNA was important for the IL8 response by PML, since specific digestion of ssRNA by RNAse A, substantially decreased IL8 formation (Figure 1B). Moreover, and similar to the situation in macrophages, the chemokine response of PML was largely dependent on MyD88, as determined for CXCL1/KC formation in sorted peritoneal PML from MyD88-deficient and sufficient mice (Figure 1C).
Figure 1. GBS induces predominantly chemokines in PML in a phagocytosis independent fashion.
Human PML were stimulated in triplicates wells with escalating doses of GBS (A) and RNAse treated GBS (B) for 5 h. Supernatants were analyzed as indicated for IL8, TNF, and IL1β by ELISA. (C) FACS-sorted mouse peritoneal PML from wild type or MyD88−/− mice were stimulated with GBS organism as indicated for 5 h. Then, supernatants were analyzed for CXCL1 by ELISA. Depicted are means ± SD from triplicate wells. (D) Mouse bone-marrow derived macrophages were pretreated with the actin polymerization inhibitor Cytochalasin D or a vehicle control for 30 min. Then, cells were stimulated with GBS (108 /ml) for 5 h. Supernatants were analyzed for TNF by ELISA and depicted as mean values ±SD from triplicate wells. (E) Human PML were treated with Cytochalasin D or a vehicle control over 30 min. After stimulation with GBS as explained above, IL8 was determined by ELISA. In (A) to (E), means + SD from triplicate wells are depicted. The p-value for all of the depicted differences was < 0,001. (F) Peritoneal PML were elicited 5h after Thioglycollate injection and sorted for Ly-6Ghigh expressing cells. Cells were treated with Cytochalasin D or a vehicle control over 30 min. Then, GBS (108 /ml) was added for 5h. Finally, supernatants were analyzed for IL8 by ELISA. Depicted are means from triplicate wells ± SD. (G) Ly-6Ghigh peritoneal PML from WT or MyD88−/− mice were stimulated with FITC-labeled GBS 107/ml (black line) or a vehicle control (grey line) for 10 min. Then extracellular fluorescence was quenched with trypan blue and PML were analyzed for intracellular fluorescence by FACS. (H) Ly-6Ghigh mouse peritoneal PML were incubated with Cytochalasin D plus FITC-GBS 108 /ml for 10 min. Internalization of GBS was determined as indicated in (G). All experiments are representative of at least three independent experiments.
GBS phagocytosis and TLR recognition are tightly interrelated events synergizing in cytokine formation by macrophages (34, 35). However, we found that, in contrast to the situation in macrophages (Figure 1D), human and mouse PML formed chemokines despite abrogation of GBS uptake by cytochalasin D (Figure 1 E, F and H). Why abrogation of phagocytosis rather increased chemokine formation in human PML remains to be established. Moreover, deficiency in MyD88 did not affect GBS phagocytosis, in contrast to its role in GBS-mediated chemokine formation (Figure 1 C and G). In summary, GBS induced predominantly chemokines in PML, and this response was not inhibited when phagocytosis is blocked.
Insulin inhibits GBS-induced chemokine formation in PML in an insulin receptor dependent fashion
Next, we assessed the effect of insulin on the chemokine response of PML. We found that insulin dose-dependently inhibited the IL8 release of human PML stimulated with GBS (Figure 2A). This effect was observed for both heat fixed (Figure 2B left panel) and live (Figure 2B right panel) bacteria. In resting cells, IL8 is found in preformed vesicles (36). However, IL8 release by GBS-stimulated PML predominantly resulted from de novo synthesis, since it was blocked by actinomycin D (data not shown). In line with this notion, both intracellular and extracellular IL8 were reduced by insulin in GBS-treated granulocytes (Figure 2C). Furthermore, insulin inhibited transcription of IL8, as determined by quantitative PCR (Figure 2D). Similarly to human PML, mouse PML showed a decreased chemokine response. We analyzed CXCL1/KC, the mouse analogue of the human chemokine functional IL8 equivalent, since mice do not express a structural IL8 homologue (Figure 2E). Next we wondered, whether insulin exerted its effects via engagement of the insulin receptor (IR) or via binding to alternative sensors, such as the IGF receptors. First we confirmed insulin receptor expression on human PML by flow cytometry, and function by insulin induced glucose transport (data not shown). Blocking of insulin-IR interaction with a specific insulin receptor antibody blocked the inhibitory effect of insulin on IL8 formation (Figure 2F). Thus, insulin inhibited chemokine formation via direct interaction with the insulin receptor.
Figure 2. Insulin down-regulates TLR-mediated cytokine induction in response to GBS. (A).
Human PML incubated with insulin at indicated concentrations for 30 min and stimulated in triplicates with escalating doses of heat fixed GBS for 5 h. Supernatants were analyzed for IL8. Depicted are mean values from triplicate wells ±SD. (B) Human PML were treated with insulin and stimulated as indicated with either heat fixed or live GBS for 5 h. In conditions stimulated with live GBS, Ciprofloxacin was added after 2 hours at a final concentration of 10μg/ml. Depicted are mean values of triplicate wells ±SD as determined by ELISA. (C) Human PML were treated with insulin and stimulated as indicated. Supernatants were analyzed for extracellular cytokine release and cellular lysates were prepared to determine intracellular cytokine concentrations. Depicted are cumulative mean values from triplicate wells ±SD. (D) Human PML were incubated with insulin in triplicate wells and stimulated with escalating doses of heat fixed GBS for 5 h. Samples were analyzed for RNA content by qRT-PCR. (E) FACS sorted mouse PML were incubated with insulin and stimulated with GBS for 5h (triplicates) as indicated. Supernatants were analyzed for CXCL-1 by ELISA. Depicted are mean values from triplicate wells ±SD. (F) Human PML were incubated with 30 μg/ml of anti-IR-Ak or isotype control antibody. Subsequently, insulin or a vehicle control was added as indicated. Cells were then stimulated with heat fixed GBS for 5h and IL-8 formation was determined by FACS (expressed as geometric mean fluorescence intensity, gMFI).
Insulin targets NFkB for modulation of chemokine formation
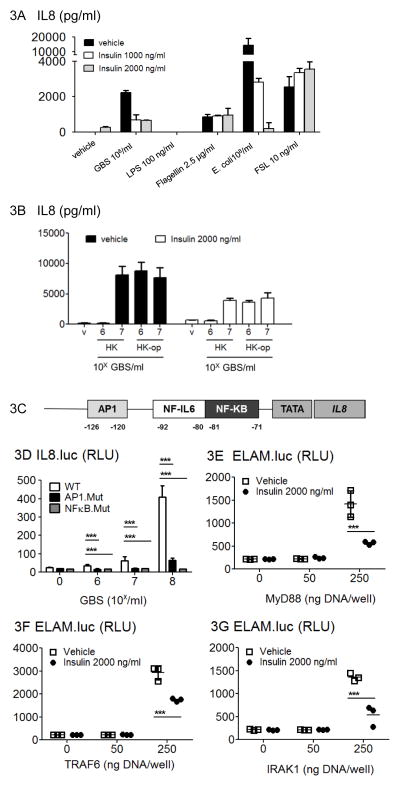
Next we asked, whether the insulin effect was specific for GBS, or whether it generally modulated cellular activation by microbial stimuli. We found that insulin inhibited only the chemokine response to bacterial particles, whereas the response to the purified TLR ligands FSL-1 (TLR2/6 agonist) and flagellin (TLR5) remained unaffected (Figure 3A). Furthermore, insulin exerted its effect under both opsonizing and non-opsonizing conditions. Addition of fresh complement and a GBS type III specific antiserum shifted the dose response to the left by about one log, as compared to non-opsonizing conditions. However, the magnitude of inhibition of the chemokine response was similar for opsonic and non-opsonic conditions (Figure 3B).
Figure 3. The insulin dependent down-regulation of inflammatory cytokines is particle dependent.
(A) Human PML were incubated with insulin in triplicate wells and stimulated with TLR ligands as indicated. Supernatants were analyzed for IL8. (B) Human PML were incubated with insulin and stimulated over 5 h with heat fixed GBS with or without opsonization by antibodies raised against GBS capsular polysaccharide. Supernatants were analyzed for IL8. Depicted are mean values from triplicate wells ±SD. (C) The human IL8 promoter contains transcription factor binding sites for NFkB, NF-IL6 and AP1. (D) RAW276.4 macrophages were transfected with a luciferase reporter plasmid comprising the IL8 wild type promoter sequence. reporter constructs carrying point mutations in either the NFkB (NFkB-Mut) or the AP-1 (AP1-Mut) binding side. Cells were stimulated with heat fixed GBS over 5h and cellular lysates were analyzed by luminometry for reporter activity. E, F and (G) RAW267.4 cells were transfected with the NFkB-dependent reporter Elam.luc-pCDNA, plus a constitutive Renilla luciferase reporter. For activation of TLR-specific signaling MyD88 (E), IRAK1 (F), TRAF 6 (G) or a parental plasmid (pcDNA) were coexpressed. After 16 h luciferase activity was determined. All experiments are performed in triplicates and representative of three or more independent experiments.
The transcription factors AP1, NF-IL6 and NFkB are critical for activation of the IL8 promoter (Figure 3C) (37). Here, we determined the roles of NFkB and AP1 in IL8 activation by GBS organism. A reporter assay comprising the IL8 promoter with established mutations for transcription factor binding revealed that NFkB and AP-1 were both essential for IL8 induction (Figure 3D). Next, we determined the level of functional interference between the chemokine-inducing and insulin-dependent pathways. We analyzed ligand independent activation of the ELAM promoter by heterologous expression of MyD88 and its downstream signaling intermediates (38). This ELAM-luciferase reporter construct comprises three NFkB- and one AP-1 binding sites. With this cellular model, we found that insulin inhibited NFkB- /AP-1 activation upon expression of MyD88 (Figure 3E), IRAK-1 (Figure 3F) and TRAF6 (Figure 3G). Therefore insulin interacted with NFkB- /AP-1 activation at the level of or downstream of TRAF6.
Activation of MAP-kinases is not inhibited by insulin
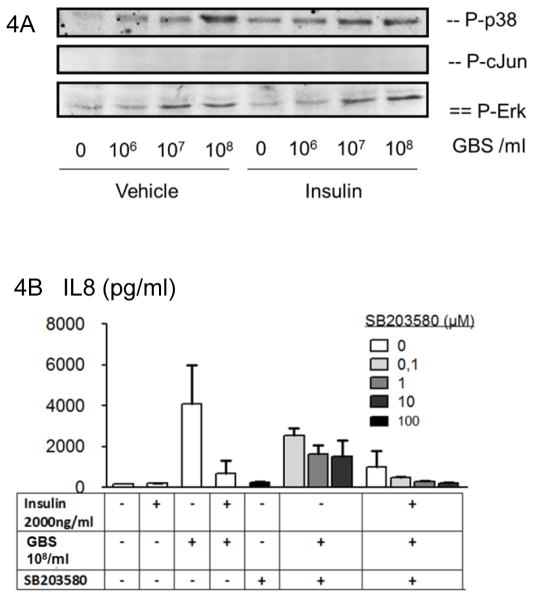
In earlier studies we found that MAP-kinases are important for in GBS-induced cytokine formation by mononuclear phagocytes (27, 28, 30). With respect to AP-1 activation, the MAP kinase p38 is essential (39). Accordingly, we analyzed the effect of insulin on MAP-kinase activation in human PML. We found that p38 phosphorylation was induced by insulin alone. This was further propagated by subsequent stimulation with GBS. In contrast, Erk, NFkB and cJun kinase (JNK) were not activated by insulin (Figure 4A, and Figure 6A). GBS induced phosphorylation of ERK (Figure 4A), but, in contrast to the situation in macrophages (27, 30), we did not detect GBS induced cJun phosphorylation in PML (Figure 4A). Since insulin alone induced p38 phosphorylation, we wondered whether p38 negatively regulated the chemokine response to GBS. Accordingly, PML were treated with insulin plus the p38 inhibitor SB203580. Subsequently cells were stimulated with GBS and analyzed for IL8 release. We found that inhibition of p38 phosphorylation plus insulin further inhibited GBS-induced IL8. Therefore, under the applied conditions, p38 plays a role in IL8 formation, however, does not appear to mediate the insulin-mediated inhibition of IL8 (Figure 4B).
Figure 4. GBS mediated MAP-kinase signaling is not altered by insulin.
(A) Human PML (5×106 per condition) were treated with insulin or a vehicle control and stimulated with GBS as indicated. Cellular lysates analyzed by Western blot for phosphorylated p38, cJun, or Erk. Data from one representative membrane out of three or more similar experiments are shown. (B) Human PML were treated with insulin as indicated and/or the p38 phosphorylation inhibitor SB203580. Cells were then stimulated with GBS (108/ ml) for 5h. Supernatants were analyzed for IL8. All experiments are representative of three or more independent experiments.
Figure 6. PI3-kinase plays a dual role in IL8 activation.
(A) Human PML (5×106 per condition) were treated with insulin or a vehicle control for 30 min. Cellular lysates were prepared and analyzed by Western blot for phosphorylation of Akt at the T306 and the S473 residue and of p38. Staining of total p38 was employed as loading control. (B, C, and D) Human PML were treated as indicated with insulin 2000 ng/ml, Wortmannin 1 nM (B.) or Ly294002 1 nM (C.), a combination of insulin plus chemical inhibitor or the PPARγ agonist Pioglitazone (μM as indicated) (D) for 30 min. Cells were then stimulated with GBS 108 /ml for 5h and supernatants were analyzed for IL8. All experiments are representatives of three or more independent experiments.
Insulin inhibits p65 phosphorylation and NFkB-DNA binding
Since MAP-kinases were not essentially involved in mediating the insulin effect, we speculated that NFkB activation was the target of insulin signaling. Accordingly, we first analyzed the influence of insulin on IkB degradation, the essential process in NFkB liberation. GBS induced IkB degradation in human PML; however this process was not modulated by insulin (Figure 5A). Next, we analyzed nuclear localization of the NFkB subunit p65 in human PML by confocal microscopy. As shown in Figure 5B, GBS-induced nuclear translocation of p65 was inhibited by concomitant insulin treatment. Accordingly, whereas degradation of IkB, and therefore NFkB liberation, was not altered by insulin, presence of NFkB in the nucleus was reduced by insulin. Since nuclear presence of NFkB depends on the on- and off-rate at its binding sites, we determined the binding of NFkB in PML treated with GBS plus insulin by electrophoretic mobility shift assays. We found that insulin indeed decreased DNA binding of NFkB (Figure 5C). DNA binding of NFkB depends on aminoterminal serine phosphorylation of p65 (Ser536), the so-called transactivation. Accordingly we tested whether insulin interfered with p65 phosphorylation induced by GBS. As shown in Figure 5D, p65 is phosphorylated in PML stimulated with GBS. However, concomitant insulin treatment inhibits p65 phosphorylation. In conclusion, GBS induces il8 transcription in PML via MyD88, and insulin negatively regulates this process at the level of p65 phosphorylation and NFkB binding.
Figure 5. TLR and IR pathways interact downstream of NFkB.
(A) Human PML (5×106 per condition) were treated with insulin or a vehicle control prior to stimulation with GBS (107/ ml) if indicated. Cellular lysates were analyzed by Western blot analysis for total IkB. Depicted is one representative Western blot membrane and results from quantitative densitometry. (B) Human PML were treated with insulin where indicated and stimulated with GBS over 4h. Cell were fixed and stained for intracellular p65 and nuclear DNA according to the protocol described in the section “Materials and Methods”. Cells were analyzed by confocal microscopy. (C) Human PML were treated with insulin (2000 ng/ml) or a vehicle control and stimulated with heat fixed GBS as indicated. Nuclear lysates were prepared and analyzed by EMSA for NFkB as described under “Material and Methods”. (D) Human PML were treated with insulin or a vehicle control, stimulated with GBS as indicated and analyzed for phosphorylation of p65 (Ser536) by FACS. Depicted are histogram plots including percentage of pp65 positive cells from one representative experiment. Geometric mean fluorescence intensities (gMFI) were 1.46 (vehicle), 5.56 (GBS), 1.12 (insulin) and 1.73 (insulin + GBS).
Insulin is well established as a potent activator of PI3-kinase, both via direct interaction of the insulin receptor with the regulatory subunit p85, and via the insulin receptor substrate-1 (IRS-1). PI3-kinase has furthermore modulating properties for TLR signaling, with the majority of studies implicating a role in signal propagation (40, 41). Accordingly, we wondered whether insulin induces Akt-phosphorylation as an endpoint of PI3-kinase in PML and, if so, how signaling intermediates of classic TLR pathways were modified. We found that insulin alone induced phosphorylation of the established PI3-kinase target Akt at residues Ser473 and Thr308 (Figure 6A). In order to better understand the role of PI3-kinase in inflammatory regulation of PML, we employed the inhibitors Wortmannin and Ly294002 to inhibit insulin-induced PI3-kinase. We found that both inhibitors efficiently reverted the insulin mediated decrease in IL8 (Figure 6B and 6C). Importantly, this effect of PI3-kinase inhibition occurred when inhibitors were used in nanoM concentrations, which did not interfere with transcriptional activation of IL8 per se. In line with a regulatory role of PI3-kinase in GBS-induced IL8 formation, we found that the PPARγ agonist pioglitazone, which has previously been shown to activate PI3-K, mimics the insulin effect (Figure 6D) (42, 43). Thus PI3-kinase appeared to be the essential intermediate between insulin receptor activation and inhibition of GBS-induced transcriptional activation of IL8.
Insulin does not alter antimicrobial properties of PML
The primary role of PML in the context of bacterial infections is the rapid recruitment to sites of microbial invasion and subsequent killing of bacteria. Accordingly, we wondered whether insulin affected these antimicrobial properties as well. First, we analyzed cellular adhesion to a variety of stimuli, as an initial step in PML recruitment from the blood to the site of infection, and found it not to be influenced by insulin concentrations as high as 2000 ng/ml (Supplementary Figure S1A). Similarly, PML migration in response to fMLP, C5a or IL8 was not affected in a qualitative fashion. To better quantify migration, live cell imaging was employed. Repetitive measurements over up to 50 min did not show any differences between insulin treated cells and the untreated control (Supplementary Figure S1B and C). Mean velocity was 2.48 units/sec (SD 37.96) for untreated and 3.55 units/sec (SD 0.6) for insulin treated cells. The mean accumulated difference was 144.03 (SD 37.96) units for untreated and 205.71 (SD 40.72) units for insulin-treated cells. Accordingly, there was no significant difference between treated (7.31×10−9) and untreated cells (6.05×10−9) with respect to the Raleigh test for vector data (Supplementary Figure S1B). Taken together, these results indicate that insulin does not interfere with chemotaxis. Next, we studied, whether insulin influenced bacterial phagocytosis. PML were stimulated with FITC-labeled GBS for 7 min and analyzed for bacterial internalization by FACS. We did not find any difference between insulin treated cells and vehicle controls (Supplementary Figure S1D). In addition, the formation of reactive oxygen species by human PML and inducible programmed cell death were not modulated by insulin (Supplementary Figure S1E and data not shown).
Discussion
Maintaining homeostasis in both the endocrine and the immune system is critical for ensuring individual resistance against external stressors. It has long been appreciated that both systems are interdigitating, and that interference with one system, may –negatively or positively – affect the other. Here we report that insulin is involved in regulating the PML response to GBS, an important bacterial pathogen in states of insulin resistance. Insulin inhibits chemokine formation in a cell autonomous fashion at the level of NFkB activation. In contrast, antimicrobial properties of the same cell are left untouched.
When bacteria such as GBS break the mucocutaneous barrier, resident cells such as keratinocytes, dendritic cells and tissue macrophages are activated. Inflammatory signal activation upon physical contact between GBS and macrophages has been defined by us and others in considerable detail (reviewed in (44, 45)). Phagocytosis and phagolysosomal processing of the bacteria are an absolute requirement for recognition of bacterial particles via their ssRNA, and subsequent signal induction (32). The host signaling molecules MyD88, UNC-93B, the kinases JNK, p38 and ELK-1, and the transcription factors NFkB, AP-1 and EGR-1 are pivotal downstream intermediates that lead to the formation of TNF and IL6 (27, 30–32). Escalation of the response of resident phagocytes results in rapid recruitment of PML, which clearly dominate the cellular infiltrate of hematopoietic origin in the early stages of infection. We show here that, in contrast to macrophages, PML respond with a program, which is biased towards chemokine formation and antimicrobial properties, whereas classical inflammatory cytokines (TNF, IL-1beta, IL6) are not substantially activated. Our observation on a restricted monokine response in human PML and mouse peritoneal PML is backed up by other studies which found a similar response profile (45, 46). However, it contradicts a number of reports on TNF formation by PML in response to a variety of stimuli (e.g. (48)). It seems essential that in the assessment of cell autonomous PML functions, the avoidance of any contamination with monocytes is utterly important, since it will dramatically alter the inflammatory phenotype. Furthermore, the source from which mouse PML are isolated is essential. In contrast to PML isolated from peripheral blood or the peritoneal cavity, less committed bone-marrow derived PML, which are isolated by negative selection, are capable of forming TNF (M. Mergen, S. Kenzel, P. Henneke, unpublished observation).
Moreover, we found that particle processing is not necessary for chemokine formation by PML. This is in sharp contrast to macrophages, which respond to GBS only, if the bacteria are processed via the phagosomal route. This seems particularly notable, since recognition of GBS by both macrophages and PML requires the presence of bacterial ssRNA and MyD88. It remains to be established whether PML are capable of digesting GBS in the extracellular space through externalization of granular factors, thereby making ssRNA accessible for the PML recognition system.
The MyD88-dependent chemokine formation in response to GBS and other bacterial particles was subject to insulin-receptor-mediated modulation. Notably, cell autonomous transcriptional regulation by insulin was confined to the response to bacterial particles, whereas the response to purified TLR ligands was not altered under the same conditions. The inhibitory effect of insulin on chemokine formation involved PI3-kinase activation as a downstream event. PI3-kinase is one of several regulators of TLR signaling, such as IRAK-M, MyD88s, A20, the SOCS proteins and GSK3 (49–54). PI3-kinase was originally characterized as a positive TLR-regulator by Arbibe et al. (40), although its role in IL-1R1 signaling was already known at this stage (55). PI3-kinase has since been established as a putative regulator in signaling processes linked to the activation of TLR2 and TLR4 (40, 41, 56), TLR5 (57) and TLR9 (58) Furthermore, most recently, Fortin et al. demonstrated the involvement of PI3K as both ligand and cell type specific regulator in human proinflammatory cytokine production (59). In view of the exquisite role of PI3-kinase in insulin receptor signaling, a regulatory role of PI3-kinase in the MyD88-dependent response to bacteria seems plausible. Notably, regulatory properties of insulin may be both species and lineage specific. In a mouse model of endotoxemia, insulin down-modulated the global cytokine response (e.g. (20)). However, in full accordance with our data on the chemokine response to pure TLR ligands, insulin levels did not influence the cytokine release or endothelial activation in a model of human endotoxemia (60).
The predominance of an antibacterial and chemokine program in PML suggests a model, where PML are tailored to localize the infection through recruitment of further effector cells, rather than eliciting a systemic response. The specific conditions, under which negative PML regulation via the insulin receptor impacts on host defense against bacteria, remain to be established. Furthermore, we currently do not know, whether the repression of bacterial particle induced chemokine production is advantageous or disadvantageous to the host. Notably, insulin concentrations in several tissues are higher than in plasma (61), although detailed information on human tissues such as skin are not available. It is conceivable, that high tissue insulin concentrations may interfere with phagocytic host defense, thereby facilitating local bacterial infections. On the other hand, attenuation of chemoattractive PML properties by insulin may be beneficial under some circumstances, since PML recruitment may affect tissue homeostasis. Importantly, insulin does not interfere with motility of PML, hence PML remain chemokine responsive, while at the same time, their contribution to the chemotactic milieu is dampened. How the modulation of normal human and mouse PML by insulin relates to the dysregulation of innate immunity in diabetes, both with respect to altered cytokine levels and depressed neutrophil functions, is not clear at this stage (1, 19). In type II diabetes and neonatal insulin resistance, both hyperinsulinemia and hyperglycemia are found. Hyperglycemia itself may have a specific impact on clinical and cellular PML immunocompetence, e.g. via posttranslational effector molecule glycosylation (1, 62, 63). Accordingly, both direct effects of insulin and indirect effects of glucose levels may underlie the adjuvant therapeutic effects of insulin in sepsis (24, 64–66). The incomplete molecular understanding on how insulin modulates host immunity has in part promoted uncertainties about the feasibility of tight glucose control in the critically ill, given the associated risk of severe hypoglycemia (67–70). It is particularly noteworthy that PPARγ agonists such as pioglitazone, which are known as insulin sensitizers and activators of PI3-K (42, 43) modulate the chemokine response to GBS and therefore constitute putative immunomodulators in invasive infections.
In conclusion, insulin regulates the transcriptional response program to bacteria such as streptococci, which exist both as common colonizers and dangerous invasive organisms, on the single PML level. It remains an intriguing possibility, that the nutritional host state directly influences the single cell innate response program, and that modulating it may have clinical implications.
Supplementary Material
Acknowledgments
We are grateful for expert technical assistance by Anita Imm, Julia Kalnitski, Bernhard Kremer and Anton Tool.
Footnotes
This study was supported by grants from the Federal Ministry of Education and Research (BMBF 01 EO 0803) from the DFG (HE 3127/3-1 and HE 3127/5-1) and from the National Institutes of Health (ROI AI052455-06A1). J.W. is recipient of an ESPID fellowship.
References
- 1.Marhoffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15:256–260. doi: 10.2337/diacare.15.2.256. [DOI] [PubMed] [Google Scholar]
- 2.Martins JO, Ferracini M, Anger DB, Martins DO, Ribeiro LF, Sannomiya P, Jancar S. Signaling pathways and mediators in LPS-induced lung inflammation in diabetic rats: role of insulin. Shock. 2010;33:76–82. doi: 10.1097/SHK.0b013e3181a85ec4. [DOI] [PubMed] [Google Scholar]
- 3.Martins JO, Zanoni FL, Martins DO, Coimbra R, Krieger JE, Jancar S, Sannomiya P. Insulin regulates cytokines and intercellular adhesion molecule-1 gene expression through nuclear factor-kappaB activation in LPS-induced acute lung injury in rats. Shock. 2009;31:404–409. doi: 10.1097/SHK.0b013e318186275e. [DOI] [PubMed] [Google Scholar]
- 4.Lin J, Chang L, Lai C, Lin H, Chen Y. Clinical and Molecular Characteristics of Invasive and Noninvasive Skin and Soft Tissue Infections Caused by Group A Streptococcus. Society. 2011;49:3632–3637. doi: 10.1128/JCM.00531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mook P, Brien SJO, Gillespie IA. Concurrent Conditions and Human. Emerging Infectious Diseases. 2011;17:38–39. doi: 10.3201/eid1701.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wernich R, Hammerich A, Kjeldsen S, Carl H. Impact of diabetes and poor glycaemic control on risk of bacteraemia with haemolytic streptococci groups A, B, and G. Journal of Infection. 2011;63:8–16. doi: 10.1016/j.jinf.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Yanai H, Hamasaki H, Tsuda N, Adachi H, Yoshikawa R, Moriyama S, Masui Y, Mishima S. Group B streptococcus infection and diabetes: A review. Journal of Microbiology. 2012;4:1–5. [Google Scholar]
- 8.Matsubara K, Yamamoto G. Invasive group B streptococcal infections in a tertiary care hospital between 1998 and 2007 in Japan. Int J Infect Dis. 2009;13:679–684. doi: 10.1016/j.ijid.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sánchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, Watterberg KL, Saha S, Das A, Higgins RD, Neonatal HD RNEKSNI of CH. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, Bizzarro MJ, Goldberg RN, Frantz ID, Hale EC, Shankaran S, Kennedy K, Carlo WA, Watterberg KL, Bell EF, Walsh MC, Schibler K, Laptook AR, Shane AL, Schrag SJ, Das A, Higgins RD for the E. K. S. N. I. of C. H. and H. D. N. R. N. Network. Early Onset Neonatal Sepsis: The Burden of Group B Streptococcal and E. coli Disease Continues. Pediatrics. 2011;127:817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skoff TH, Farley MM, Petit S, Craig AS, Schaffner W, Gershman K, Harrison LH, Lynfield R, Mohle-Boetani J, Zansky S, Albanese Ba, Stefonek K, Zell ER, Jackson D, Thompson T, Schrag SJ. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;49:85–92. doi: 10.1086/599369. [DOI] [PubMed] [Google Scholar]
- 12.Trijbels-Smeulders MA, Kimpen JL, Kollée LA, Bakkers J, Melchers W, Spanjaard L, Wannet WJ, Hoogkamp-Korstanje MA. Serotypes, genotypes, and antibiotic susceptibility profiles of group B streptococci causing neonatal sepsis and meningitis before and after introduction of antibiotic prophylaxis. Pediatr Infect Dis J. 2006;25:945–948. doi: 10.1097/01.inf.0000237821.65559.08. [DOI] [PubMed] [Google Scholar]
- 13.Fluegge K, Siedler A, Heinrich B, Schulte-Moenting J, Moennig MJ, Bartels DB, Dammann O, von Kries R, Berner R GPSUS Group. Incidence and clinical presentation of invasive neonatal group B streptococcal infections in Germany. Pediatrics. 2006;117:e1139–1145. doi: 10.1542/peds.2005-2481. [DOI] [PubMed] [Google Scholar]
- 14.Kunze M, Ziegler A, Fluegge K, Hentschel R, Proempeler H, Berner R. Colonization, serotypes and transmission rates of group B streptococci in pregnant women and their infants born at a single University Center in Germany. J Perinat Med. 2011;39:417–422. doi: 10.1515/jpm.2011.037. [DOI] [PubMed] [Google Scholar]
- 15.Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Calum H, Moser C, Jensen P, Christophersen L, Maling DS, van Gennip M, Bjarnsholt T, Hougen HP, Givskov M, Jacobsen GK, Høiby N. Thermal injury induces impaired function in polymorphonuclear neutrophil granulocytes and reduced control of burn wound infection. Clin Exp Immunol. 2009;156:102–110. doi: 10.1111/j.1365-2249.2008.03861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spörri R, Joller N, Hilbi H, Oxenius A. A novel role for neutrophils as critical activators of NK cells. J Immunol. 2008;181:7121–7130. doi: 10.4049/jimmunol.181.10.7121. [DOI] [PubMed] [Google Scholar]
- 18.Prince LR, Whyte MK, Sabroe I, Parker LC. The role of TLRs in neutrophil activation. Curr Opin Pharmacol. 2011;11:397–403. doi: 10.1016/j.coph.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Mazade MA, Edwards MS. Impairment of type III group B Streptococcus-stimulated superoxide production and opsonophagocytosis by neutrophils in diabetes. Mol Genet Metab. 2001;73:259–267. doi: 10.1006/mgme.2001.3185. [DOI] [PubMed] [Google Scholar]
- 20.Kidd LB, Schabbauer GA, Luyendyk JP, Holscher TD, Tilley RE, Tencati M, Mackman N. Insulin activation of the phosphatidylinositol 3-kinase/protein kinase B (Akt) pathway reduces lipopolysaccharide-induced inflammation in mice. J Pharmacol Exp Ther. 2008;326:348–353. doi: 10.1124/jpet.108.138891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31:359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 22.Jeschke MG, Kulp GA, Kraft R, Finnerty CC, Mlcak R, Lee JO, Herndon DN. Intensive insulin therapy in severely burned pediatric patients: a prospective randomized trial. Am J Respir Crit Care Med. 2010;182:351–359. doi: 10.1164/rccm.201002-0190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373:547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 24.Ellger B, Debaveye Y, Vanhorebeek I, Langouche L, Giulietti A, Van Etten E, Herijgers P, Mathieu C, Van den Berghe G. Survival benefits of intensive insulin therapy in critical illness: impact of maintaining normoglycemia versus glycemia-independent actions of insulin. Diabetes. 2006;55:1096–1105. doi: 10.2337/diabetes.55.04.06.db05-1434. [DOI] [PubMed] [Google Scholar]
- 25.McIntire FC, Sievert HW, Barlow GH, Finley RA, Lee AY. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967;6:2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- 26.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted Disruption of the MyD88 Gene Results in Loss of IL-1- and IL-18-Mediated Function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 27.Kenzel S, Mancuso G, Malley R, Teti G, Golenbock DT, Henneke P. c-Jun kinase is a critical signaling molecule in a neonatal model of group B streptococcal sepsis. J Immunol. 2006;176:3181–3188. doi: 10.4049/jimmunol.176.5.3181. [DOI] [PubMed] [Google Scholar]
- 28.Henneke P, Takeuchi O, van Strijp JA, Guttormsen HK, Smith JA, Schromm AB, Espevik TA, Akira S, Nizet V, Kasper DL, Golenbock DT. Novel engagement of CD14 and multiple toll-like receptors by group B streptococci. J Immunol. 2001;167:7069–7706. doi: 10.4049/jimmunol.167.12.7069. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Kenzel S, Santos-Sierra S, Deshmukh SD, Moeller I, Ergin B, Fitzgerald KA, Lien E, Akira S, Golenbock DT, Henneke P. Role of p38 and early growth response factor 1 in the macrophage response to group B streptococcus. Infect Immun. 2009;77:2474–2481. doi: 10.1128/IAI.01343-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancuso G, Midiri A, Beninati C, Biondo C, Galbo R, Akira S, Henneke P, Golenbock D, Teti G. Dual role of TLR2 and myeloid differentiation factor 88 in a mouse model of invasive group B streptococcal disease. J Immunol. 2004;172:6324–6329. doi: 10.4049/jimmunol.172.10.6324. [DOI] [PubMed] [Google Scholar]
- 32.Deshmukh SD, Kremer B, Freudenberg M, Bauer S, Golenbock DT, Henneke P. Macrophages recognize streptococci through bacterial single-stranded RNA. EMBO Rep. 2011;12:71–76. doi: 10.1038/embor.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henneke P, Berner R. Interaction of neonatal phagocytes with group B streptococcus: recognition and response. Infection and immunity. 2006;74:3085–3095. doi: 10.1128/IAI.01551-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ip WK, Sokolovska A, Charriere GM, Boyer L, Dejardin S, Cappillino MP, Yantosca LM, Takahashi K, Moore KJ, Lacy-Hulbert A, Stuart LM. Phagocytosis and phagosome acidification are required for pathogen processing and MyD88-dependent responses to Staphylococcus aureus. J Immunol. 2010;184:7071–7081. doi: 10.4049/jimmunol.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Y, Kawamura I, Nomura T, Tsuchiya K, Hara H, Dewamitta SR, Sakai S, Qu H, Daim S, Yamamoto T, Mitsuyama M. Toll-like receptor 2- and MyD88-dependent phosphatidylinositol 3-kinase and Rac1 activation facilitates the phagocytosis of Listeria monocytogenes by murine macrophages. Infect Immun. 2010;78:2857–2867. doi: 10.1128/IAI.01138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schröder AK, Uciechowski P, Fleischer D, Rink L. Crosslinking of CD66B on peripheral blood neutrophils mediates the release of interleukin-8 from intracellular storage. Hum Immunol. 2006;67:676–682. doi: 10.1016/j.humimm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Kunsch C, Lang R, Rosen C, Shannon M. Synergistic transcriptional activation of the IL-8 gene by NF-kappa B p65 (RelA) and NF-IL-6. J Immunol. 1994;153:153–164. [PubMed] [Google Scholar]
- 38.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting Edge: Recognition of Gram-Positive Bacterial Cell Wall Components by the Innate Immune System Occurs Via Toll-Like Receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 39.Venza I, Cucinotta M, Caristi S, Mancuso G, Teti D. Transcriptional regulation of IL-8 by Staphylococcus aureus in human conjunctival cells involves activation of AP-1. Investigative ophthalmology & visual science. 2007;48:270–276. doi: 10.1167/iovs.06-0081. [DOI] [PubMed] [Google Scholar]
- 40.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 41.Santos-Sierra S, Deshmukh SD, Kalnitski J, Küenzi P, Wymann MP, Golenbock DT, Henneke P. Mal connects TLR2 to PI3Kinase activation and phagocyte polarization. EMBO J. 2009;28:2018–2027. doi: 10.1038/emboj.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wynne AM, Mocanu MM, Yellon DM. Pioglitazone mimics preconditioning in the isolated perfused rat heart: a role for the prosurvival kinases PI3K and P42/44MAPK. Journal of cardiovascular pharmacology. 2005;46:817–822. doi: 10.1097/01.fjc.0000188365.07635.57. [DOI] [PubMed] [Google Scholar]
- 43.Pereira RI, Leitner JW, Erickson C, Draznin B. Pioglitazone acutely stimulates adiponectin secretion from mouse and human adipocytes via activation of the phosphatidylinositol 3′-kinase. Life sciences. 2008;83:638–643. doi: 10.1016/j.lfs.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Kenzel S, Henneke P. The innate immune system and its relevance to neonatal sepsis. Curr Opin Infect Dis. 2006;19:264–270. doi: 10.1097/01.qco.0000224821.27482.bd. [DOI] [PubMed] [Google Scholar]
- 45.Wennekamp J, Henneke P. Induction and termination of inflammatory signaling in group B streptococcal sepsis. Immunol Rev. 2008;225:114–127. doi: 10.1111/j.1600-065X.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kessenbrock K, Dau T, Jenne DE. Tailor-made inflammation: how neutrophil serine proteases modulate the inflammatory response. J Mol Med. 2011;89:23–28. doi: 10.1007/s00109-010-0677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.József L, Khreiss T, El Kebir D, Filep JG. Activation of TLR-9 induces IL-8 secretion through peroxynitrite signaling in human neutrophils. J Immunol. 2006;176:1195–1202. doi: 10.4049/jimmunol.176.2.1195. [DOI] [PubMed] [Google Scholar]
- 48.Esmann L, Idel C, Sarkar A, Hellberg L, Behnen M, Möller S, van Zandbergen G, Klinger M, Köhl J, Bussmeyer U, Solbach W, Laskay T. Phagocytosis of apoptotic cells by neutrophil granulocytes: diminished proinflammatory neutrophil functions in the presence of apoptotic cells. J Immunol. 2010;184:391–400. doi: 10.4049/jimmunol.0900564. [DOI] [PubMed] [Google Scholar]
- 49.Kulkarni S, Sitaru C, Jakus Z, Anderson KE, Davidson K, Hirose M, Juss J, Oxley D, Tamara A, Chessa M, Ramadani F, Guillou H, Segonds-Pichon A, Fritsch A, Jarvis GE, Okkenhaug K, Ludwig R, Zillikens D, Mocsai A, Vanhaesebroeck B, Stephens LR, April PTH, Damoulakis G, Chessa TAM, Hawkins PT. PI3K{beta} Plays a Critical Role in Neutrophil Activation by Immune Complexes. Sci Signal. 2011;4:ra23. doi: 10.1126/scisignal.2001617. [DOI] [PubMed] [Google Scholar]
- 50.Tsukamoto K, Hazeki K, Hoshi M, Nigorikawa K, Inoue N, Sasaki T, Hazeki O. Critical roles of the p110 beta subtype of phosphoinositide 3-kinase in lipopolysaccharide-induced Akt activation and negative regulation of nitrite production in RAW 264.7 cells. J Immunol. 2008;180:2054–2061. doi: 10.4049/jimmunol.180.4.2054. [DOI] [PubMed] [Google Scholar]
- 51.Yu Y, Nagai S, Wu H, Neish AS, Koyasu S, Gewirtz AT. TLR5-mediated phosphoinositide 3-kinase activation negatively regulates flagellin-induced proinflammatory gene expression. J Immunol. 2006;176:6194–6201. doi: 10.4049/jimmunol.176.10.6194. [DOI] [PubMed] [Google Scholar]
- 52.Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull. 2007;30:1617–1623. doi: 10.1248/bpb.30.1617. [DOI] [PubMed] [Google Scholar]
- 53.Wang AB, Li HL, Zhang R, She ZG, Chen HZ, Huang Y, Liu DP, Liang CC. A20 attenuates vascular smooth muscle cell proliferation and migration through blocking PI3k/Akt singling in vitro and in vivo. J Biomed Sci. 2007;14:357–371. doi: 10.1007/s11373-007-9150-x. [DOI] [PubMed] [Google Scholar]
- 54.Park SH, Park-Min KH, Chen J, Hu X, Ivashkiv LB. Tumor necrosis factor induces GSK3 kinase-mediated cross-tolerance to endotoxin in macrophages. Nat Immunol. 2011;12:607–615. doi: 10.1038/ni.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brint EK, Fitzgerald KA, Smith P, Coyle AJ, Gutierrez-Ramos JC, Fallon PG, O’Neill LA. Characterization of signaling pathways activated by the interleukin 1 (IL-1) receptor homologue T1/ST2. A role for Jun N-terminal kinase in IL-4 induction. J Biol Chem. 2002;277:49205–49211. doi: 10.1074/jbc.M209685200. [DOI] [PubMed] [Google Scholar]
- 56.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 57.Ivison SM, Himmel ME, Hardenberg G, Wark PA, Kifayet A, Levings MK, Steiner TS. TLR5 is not required for flagellin-mediated exacerbation of DSS colitis. Inflamm Bowel Dis. 2010;16:401–409. doi: 10.1002/ibd.21097. [DOI] [PubMed] [Google Scholar]
- 58.Dil N, Marshall AJ. Role of phosphoinositide 3-kinase p110 delta in TLR4- and TLR9-mediated B cell cytokine production and differentiation. Mol Immunol. 2009;46:1970–1978. doi: 10.1016/j.molimm.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Fortin CF, Cloutier A, Ear T, Sylvain-Prévost S, Mayer TZ, Bouchelaghem R, McDonald PP. A class IA PI3K controls inflammatory cytokine production in human neutrophils. European journal of immunology. 2011;41:1709–1719. doi: 10.1002/eji.201040945. [DOI] [PubMed] [Google Scholar]
- 60.Stegenga ME, van der Crabben SN, Blümer RM, Levi M, Meijers JC, Serlie MJ, Tanck MW, Sauerwein HP, van der Poll T. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood. 2008;112:82–89. doi: 10.1182/blood-2007-11-121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agardh CD, Lesniak MA, Gerritsen GC, Roth J. The influence of plasma insulin concentrations on tissue insulin levels in rodents: a study of the diabetic Chinese hamster and the ob/ob mouse. Metabolism. 1986;35:244–249. doi: 10.1016/0026-0495(86)90208-8. [DOI] [PubMed] [Google Scholar]
- 62.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 63.Kado S, Nagase T, Nagata N. Circulating levels of interleukin-6, its soluble receptor and interleukin-6/interleukin-6 receptor complexes in patients with type 2 diabetes mellitus. Acta Diabetol. 1999;36:67–72. doi: 10.1007/s005920050147. [DOI] [PubMed] [Google Scholar]
- 64.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 65.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 66.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536–55. doi: 10.1007/s00134-004-2210-z. [DOI] [PubMed] [Google Scholar]
- 67.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 68.Preiser JC, Devos P. Clinical experience with tight glucose control by intensive insulin therapy. Crit Care Med. 2007;35:S503–507. doi: 10.1097/01.CCM.0000278046.24345.C7. [DOI] [PubMed] [Google Scholar]
- 69.Green DM, O’Phelan KH, Bassin SL, Chang CW, Stern TS, Asai SM. Intensive versus conventional insulin therapy in critically ill neurologic patients. Neurocrit Care. 2010;13:299–306. doi: 10.1007/s12028-010-9417-3. [DOI] [PubMed] [Google Scholar]
- 70.Ulate KP. A critical appraisal of Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G. Intensive insulin therapy for patients in paediat. Pediatr Crit Care Med. 2011;12:455–458. doi: 10.1097/PCC.0b013e318207097e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.