Abstract
Traumatic brain injury (TBI) is a major public health issue, producing significant patient mortality and poor long-term outcomes. Increasing evidence suggests an important, yet poorly defined, role for the immune system in the development of secondary neurological injury over the days and weeks following a TBI. Herein, we tested the hypothesis that peripheral macrophage infiltration initiates long-lasting adaptive immune responses after TBI. Using a murine controlled cortical impact model, we used adoptive transfer, transgenic, and bone marrow chimera approaches to show increased infiltration and pro-inflammatory (M1) polarization of macrophages for up to three weeks post-TBI. Monocytes purified from the injured brain stimulated the proliferation of naïve T lymphocytes, enhanced the polarization of T effector cells (Teff: TH1/TH17), and decreased the production of regulatory T cells (TREG) in a mixed lymphocyte reaction. Similarly, elevated Teff polarization within both blood and brain tissue was attenuated by myeloid cell depletion after TBI. Functionally, C3H/HeJ (TLR4 mutant) mice reversed both M1 macrophage and TH1/TH17 polarization after TBI, as compared to C3H/OuJ (wild-type) mice. Moreover, brain monocytes isolated from C3H/HeJ mice were less potent stimulators of T lymphocyte proliferation and TH1/TH17 polarization, as compared to C3H/OuJ monocytes. Taken together, our data implicate TLR4-dependent, M1 macrophage trafficking/polarization into the CNS as a key mechanistic link between acute TBI and long-term, adaptive immune responses.
INTRODUCTION
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide. Impact and/or coup-contrecoup injuries produce immediate mechanical injury, including cellular necrosis, diffuse axonal shearing, and tissue loss, that contribute toward poor neurological outcomes after TBI (1); however, one-third of hospitalized TBI patients die from secondary pathological processes that manifest in the days after the initial traumatic event. In particular, most axonal damage is not due to physical shearing at the time of TBI (2). Rather, delayed atrophy of white matter tracts develops for over one year post-TBI in rodents and progresses for decades in TBI patients to produce chronic cognitive, psychiatric, and motor deficits (1, 3).
Routine surveillance of the CNS is limited by the immunoprivileged status of the brain, yet a robust and sequential set of systemic immune responses develops after TBI (4, 5). Innate immune activation, which provides immediate, non-specific host responses to tissue injury, temporally correlates with widespread cellular necrosis after TBI (6). Surgical excision of necrotic brain tissue during the acute injury phase improved clinical outcomes at 6 months in TBI patients (7). Notably, damage associated molecular patterns (DAMPs), host molecules that are passively released after trauma, activate pattern-recognition receptors (PRR) to initiate innate immune responses (8). We reported that activation of myeloid toll-like receptor 4 (TLR4), a prototypical PRR, induced pro-inflammatory responses and exacerbated neurological injury after TBI (9); however, the mechanisms linking acute neurotrauma with the development of chronic inflammatory responses remain poorly defined.
The CNS was long believed to lack a classical lymphatic system; yet, recent studies documented the presence of specialized meningeal lymphatic vessels that preferentially drain cerebrospinal fluid (CSF) into the deep cervical lymph nodes (10, 11). These vessels also provide a conduit for the bidirectional movement of immune cells between the CNS and lymph nodes (10, 11). Activated macrophages, an important component of the mononuclear phagocyte system, exhibit antigen-presenting capability during a primary immune response and antigen-loaded macrophages travel to draining lymph nodes to initiate adaptive immune responses via an MHC-II-dependent process. Notably, T-lymphocytes are activated within deep cervical lymph nodes rather than at sites of nerve injury (12) and pharmacological inhibition of MHC Class II-dependent antigen processing reduced neurodegeneration after TBI (13). As macrophage activation temporally preceded brain T-lymphocyte infiltration and neurological deterioration after both experimental and clinical TBI (14, 15), early macrophage activation may initiate deleterious adaptive immune responses after TBI.
Macrophages polarize based on microenvironmental cues to generate divergent, context-specific functions. Along these lines, “classically activated” (M1) macrophages release pro-inflammatory cytokines and remove damaged cells from sites of injury; however, chronic M1 activation exacerbates secondary damage and impairs tissue repair (16). Conversely, “alternatively activated” (M2) macrophages release counter-inflammatory cytokines to dampen immune responses and to promote wound healing (17). A heterogeneous mixture of M1 and M2 phenotypes are observed after TBI, with an early, transient M2 phenotype yielding to a predominant M1 phenotype over the first days post-TBI (18, 19). Accumulation of M1 macrophages within both white and grey matter temporally preceded oligodendrocyte apoptosis and widespread myelin loss for weeks after experimental TBI (20). Similarly, a progressive increase in the ratio of M1:M2 macrophages, including the chronic accumulation of HLA-DR+ M1-like macrophages within the corpus callosum of >25% TBI patients, temporally correlated with white matter loss and neurological injury for two decades after TBI (19, 21–23). Moreover, conditioned media from M1 macrophages increased oligodendrocyte cell death after oxygen-glucose deprivation whereas conditioned media from M2 macrophages was protective (19). Thus, polarized macrophages may represent a critical determinant of outcomes after TBI.
The adaptive arm of the immune system is comprised of CD4+ helper T-lymphocytes (TH), CD8+ cytotoxic T-lymphocytes (CTL), and regulatory T-lymphocytes (TREG). Functionally, TH cells potentiate immune responses after antigen recognition by stimulating antibody production and by releasing cytokines to enhance the activation of CTL and macrophages. Conversely, counter-inflammatory TREG attenuate immune responses to prevent chronic inflammation and autoimmunity. Notably, release of context-specific cytokines by antigen presenting cells modulates adaptive immune responses to provide long-lasting, antigen-specific host protection (24). For instance, phenotype-specific cytokines released by activated macrophages differentiate naïve TH cells (TH0) into polarized TH subtypes, such as TH1, TH2, and TH17, which exhibit distinct cytokine repertoires and different functional phenotypes (24). Polarization toward a pro-inflammatory TH1 phenotype augments cell-mediated immunity, amplifies M1 macrophage activation, and perpetuates neurodegeneration (25, 26). Conversely, TH2 polarization is associated with humoral immunity, support of resting microglia, and delayed neurodegeneration (27). Whereas the TH1:TH2 ratio remained unchanged in pediatric TBI patients (28), the presence and activity of IL-17-producing TH17 cells is unexplored after TBI. Herein, we tested the hypothesis that activation of myeloid TLR4 is critical for TH polarization after TBI.
MATERIALS AND METHODS
Controlled cortical impact
The Institutional Animal Care and Use Committee (IACUC) at Augusta University approved all animal studies, in compliance with NIH guidelines. Adult male CD-1 (Charles River, Wilmington, MA), C57Bl/6 (Jackson Laboratories), C3H/OuJ (wild-type; Jackson Laboratories Stock #000635), or C3H/HeJ (TLR4 mutant; Jackson Laboratories Stock #000659), or CX3CR1-eGFP (Jackson Laboratories Stock #005582) mice were subjected to a sham injury or moderate controlled cortical impact (CCI), as detailed by our laboratory with minor modifications (29). Briefly, mice were anesthetized using 3% isoflurane, placed in a stereotaxic frame, and a craniotomy was made in the right parietal bone midway between bregma and lambda with the medial edge 1 mm lateral to the midline, leaving the dura intact. Mice were impacted at 3 m/s with a 100 ms dwell time and 3.0 mm depression using a 3 mm diameter convex tip (PinPoint PCI3000 Precision Cortical Impactor, Hatteras Instruments, Cary, NC). Sham-operated mice underwent the identical surgical procedures, but were not impacted. The skin incision was closed and mice were allowed to recover in a clean, warm cage. Body temperature was maintained at 37°C using a small animal temperature controller throughout all procedures (Kopf Instruments, Tujunga, CA, USA). Food and water were provided ad libitum.
Tissue collection
At designated time points, blood was collected by cardiac puncture from deeply anesthetized mice and placed into ice-cold heparinized tubes. Mice were next perfused with 30 mL of ice-cold phosphate buffered saline (PBS) and whole brains were carefully harvested. A 3-mm coronal brain section centered on the contusion was prepared using an acrylic brain matrix. Brain hemispheres were further subdivided into ipsilateral (injured) and contralateral (uninjured) cerebral cortices for analysis, as detailed below.
Quantitative RT-PCR (qRT-PCR)
Total RNA was isolated using a SV RNA Isolation kit (Promega, Madison, WI) and qRT-PCR was performed as detailed by our group (29). Primers utilized were as follows: CCL2: (FP 5′-AGGTCCCTGTCATGCTTCTG-3′; RP 5′-GCTGCTGGTGATCCTCTTGT-3′), CCL3: (FP 5′-ATGAAGGTCTCCACCACTGC-3′; RP 5′-CCCAGGTCTCTTTGGAGTCA-3′), CCL12: (FP 5′-GTCCTCAGGTATTGGCTGGA-3′; RP 5′-GGGTCAGCACAGATCTCCTT-3′), CXCL1: (FP 5′-GCTGGGATTCACCTCAAGAA-3′; RP 5′-TGGGGACACCTTTTAGCATC-3′), CXCL2: (FP 5′-AGTGAACTGCGCTGTCAATG-3′; RP 5′-TTCAGGGTCAAGGCAAACTT-3′), CXCL10: (FP 5′-AAGTGCTGCCGTCATTTTCT-3′; RP 5′-GTGGCAATGATCTCAACACG-3′). RPS3: (FP 5′-AATGAACCGAAGCACACCATA-3′; RP 5′-ATCAGAGAGTTGACCGCAGTT-3′). Product specificity was confirmed by melting curve analysis. Gene expression levels were quantified and data were normalized to RPS3, a housekeeping gene that was unaffected by TBI, as detailed by our group (29). Data are expressed as fold change versus placebo treatment.
Preparative and analytical flow cytometry
Freshly harvested brain tissue was sieved through a 100 μM cell strainer (BD Biosciences, San Diego, CA), followed by centrifugation (1,000 rpm, 10 min) to prepare single-cell suspensions. Blood (100 μL) was collected via cardiac puncture, as above. Cells were incubated with antibodies against cell surface markers CD3, CD4, CD8, CD45, CD25, CD71, CD11b, CD68, F4/80, CD206, and TLR4 (All antibodies purchased from eBioSciences, San Diego, CA). Following a PBS wash, cells were fixed and permeabilized using a Fixation/Permeabilization Concentrate (Affymatrix eBioscience), and then incubated with antibodies for intracellular labeling of TNFα, TGFβ, IL-17, IL-12, IL-10, and FoxP3 (BD BioSciences, Bedford, MA, USA). After a final wash, cells were analyzed using a 4-color flow cytometer (FACSCalibur, BD Biosciences, San Diego, CA, USA), and CellQuest software (BD Biosciences, San Jose, CA, USA), as we described previously (30, 31). Isotype-matched controls were analyzed to set the appropriate gates for each sample. For each marker, samples were analyzed in duplicate. To minimize false-positive events, the number of double-positive events detected with the isotype controls was subtracted from the number of double-positive cells stained with corresponding antibodies (not isotype control), respectively. Cells expressing a specific marker were reported as a percentage of the number of gated events. Mean channel fluorescence intensity (MFI) derived from fluorescence histogram was used to study the level of cell surface TLR expression. Delta MFI (ΔMFI) was calculated as the difference between MFI of the TBI group – MFI of the sham group.
Adoptive transfer of CFSE-labeled macrophages
Total splenocytes and bone marrow were collected, enriched, and CD11b+ CD68+ F4/80+ macrophages were consecutively sorted three times by magnetic bead isolation (Miltenyi Biotech) to achieve >95% purity. Purified macrophages were labeled with 5 μM CFSE (Molecular Probes, Eugene, OR), a green fluorescent cell staining dye, and resuspended in sterile PBS, as we described (32). A total of 6×105 cells/mouse were injected via the tail vein immediately after sham/TBI injury. Trafficking, distribution, and phenotype of adoptively transferred CFSE+ macrophages were analyzed by ex vivo fluorescence imaging, confocal imaging, and flow cytometry, respectively. To quantify the polarization and CNS infiltration of CFSE+ macrophages, 100 μL of blood was collected from deeply anesthetized mice via cardiac puncture and macrophage polarization was assessed by flow cytometry, as detailed above. Mice were then perfused with ice-cold PBS and brains were carefully harvested into a sterile petri dish. Brains then were imaged on a SPECTRAL Ami-X imager (Spectral Instruments Imaging, Tucson, AZ) using an excitation wavelength=430 nm, emission wavelength=570 nm, excitation power=20, exposure time=6, FOV 10). Fluorescence intensity, calculated as the mean intensity difference between ipsilateral and contralateral hemispheres, was quantified within regions of interest (ROI) in triplicate using Amiview software. Data were expressed as mean photons per unit area per group. To provide spatial analysis of macrophage trafficking into the brain, deeply anesthetized mice were perfused with PBS, followed by fixation with 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Brains were post-fixed overnight in paraformaldehyde followed by cryoprotection with 30% sucrose (pH 7.4) until brains permeated. Serial coronal sections (12 μm) were prepared using a cryostat microtome and directly mounted onto glass slides. Anti-fade mounting media was added and glass cover slips were placed atop the slide. CFSE immunofluorescence was imaged using a LSM780 Meta confocal laser microscope and vendor supplied software (Carl Zeiss).
Bone marrow chimera
C57BL/6 recipient mice were whole body irradiated with sub-lethal dose of 6 Gy (Cs137). After 24 hours, recipient mice were intravenously injected with 5x106 / 100 μL bone marrow cells collected from transgenic mice expressing monomeric red fluorescent protein (RFP) (mCherry) under the direction of the human ubiqutin C promoter [B6(Cg)-Tyrc-2J Tg(UBC-mCherry)1Phbs/J; Jackson Laboratories, Stock#017614], as detailed previously (33). Beginning at two weeks post-transplantation, 10 μL of blood was collected from the orbital sinus and RFP expression was measured in peripheral blood using flow cytometry. Blood from C57BL/6 mice without irradiation or RFP+ cell transplantation were used as a control. On day 30, following confirmation of efficient bone marrow engraftment, mice were subjected to sham/TBI. The presence of infiltrated RFP+ myeloid cells were analyzed in brain tissue by flow cytometry.
Mixed lymphocyte reactions (MLR)
MLR were performed, as we described, with minor modifications (31, 34). Responder T-lymphocytes were initially enriched using magnetic activated cell sorting, labeled with 5 μM CFSE for 10 minutes at 37 °C, and plated at 1×104 cells/well. Brain tissue was harvested at 24h post-sham/TBI and CD11b+ CD68+ F4/80+ brain macrophages were consecutively sorted three times by magnetic bead isolation (Miltenyi Biotech). Purified brain macrophages were used as stimulators following plating at 5×104 cells/well. Combinations of responders (naïve T-lymphocytes) and stimulators (brain macrophages) were prepared in triplicate wells. Cells were cultured in 200 μL/well of RPMI 1640 medium supplemented with fetal bovine serum, penicillin, streptomycin, L-glutamine, and 2-mercaptoethanol. After 72 – 96h of incubation at 37°C in a humidified, 5% CO2 incubator, cells were harvested into flow cytometry tubes. Following a PBS wash, samples were incubated at 4°C in for 20 minutes in the dark with anti-rat CD71-phycoerythrin-conjugated antibody to label activated and dividing T cells. Samples were then washed with PBS and T-cell proliferation and polarization phenotype was quantified in triplicate by flow cytometry.
Macrophage depletion
A clodronate macrophage depletion kit, containing control liposomes (encapsome) and clodronate liposomes (clodrosome) (Encapsala NanoSciences, Brentwoood, TN), was utilized to deplete endogenous myeloid cells. Intraperitoneal administration of 200 μL of placebo or clodronate liposomes (5 mg/mL) was performed once daily for three consecutive days. At 24h after the final injection, blood was collected via the retro-orbital sinus and myeloid cells (CD11b+, F4/80+) depletion was confirmed by flow cytometry. Sham or TBI was induced upon the confirmation of myeloid cell depletion.
Statistical analysis
Multi-group comparisons were made using a one-way analysis of variance (ANOVA) followed by Student-Newman-Keul’s post-hoc test. Two group comparisons were analyzed by t-test. Results are expressed as mean ± SEM. A p<0.05 was considered to be statistically significant.
RESULTS
Chronic M1 macrophage activation within the CNS after TBI
CX3CR1-eGFP mice were subjected to TBI. Scattered eGFP expression, which likely represented resident microglia, was noted throughout the cerebral cortex of sham-operated mice and in the contralateral cortex after TBI whereas intense fluorescence was observed throughout the peri-contusional cortex at 72h post-TBI (Fig 1A). While providing a valuable tool, interpretation of data using CX3CR1-eGFP mice are confounded by the expression of fluorescence in monocytes, as well as in dendritic cells, NK cells, and microglia. Thus, to more specifically determine whether peripheral macrophages traffic into the CNS, bone marrow-derived monocytes were fluorescently labeled with CFSE and intravenously administered to recipient mice immediately after sham or TBI. Ex vivo spectral imaging showed a 10-fold increase in brain macrophage infiltration after TBI, as compared to brains from sham-operated mice (Fig 1B). Histological analysis revealed increased fluorescence throughout the peri-contusional cortex at 72h post-TBI, whereas few fluorescent cells were observed in the contralateral hemisphere or in the brain of sham-operated mice (Fig 1C). In a parallel cohort, CFSE+ macrophages were recovered from peri-contusional brain tissue (or anatomically matched sham-operated brain tissue) or blood. FACS analysis of CFSE+ cells revealed an elevated ratio of M1 macrophages, as compared to M2 macrophages, in both brain and blood after TBI (Fig 1D, E).
Figure 1. Peripheral macrophage trafficking into the CNS after TBI.
(A.) Sham or TBI was induced in CX3CR1-eGFP reporter mice, which express green fluorescence in monocytes, dendritic cells, and microglia. Representative confocal images are depicted showing increased macrophage infiltration/activation within the injured cortex at 72h post-TBI, as compared to the contralateral cortex or to the cortex of sham-injured mice. Data are representative of n=5 mice/group (B–D.) CFSE-labeled macrophages (6 x 105 cells/mouse) were delivered via tail vein at 1h after sham/TBI. (B.) At 72h post-TBI, ex vivo spectral imaging of brains was performed and fluorescence was calculated within pre-defined ROI. Scatterplots depict mean ± SEM of CFSE Intensity, as represented by photons/s/cm2, from n=6 mice/group. Data were analyzed by t-test, ** p<0.01 vs. sham. (C.) Confocal imaging of infiltrated CFSE+ macrophages adjacent to the peri-contusional cortex. Note the strong increase in fluorescence after TBI. (D.) CFSE+ cells were recovered from blood or brain tissue after sham/TBI and subjected to flow cytometry to quantify M1 (CFSE+, CD11b+, F4/80+, TNF-α+) and M2 (CFSE+, CD11b+, CD206+, IL-10+) polarization. As shown, TBI strongly increased M1 polarization in both blood and brain tissue and blood at 72h post-injury. In contrast, there was no change in M2 polarization after TBI.
To ensure that the observed effect of TBI on macrophage polarization was neither an artifact of CFSE labeling nor due to exogenous administration, an irradiation bone marrow chimera approach was utilized. Transplantation of RFP+ bone marrow into irradiated wild-type mice achieved successful engraftment at day 28 post-transplantation. Mice then were subjected to sham or TBI and the CNS infiltration of RFP+ myeloid cells was analyzed by flow cytometry. In agreement with the CFSE experiment, TBI increased the infiltration of RFP+, CD11b+, CD45+, F4/80+ macrophages into the peri-contusional cortex, as compared to sham injured mice (Fig 2B).
Figure 2. Macrophage trafficking in bone marrow chimera mice.
Irradiated wild-type (C57BL/6) mice were transplanted with bone marrow from donor transgenic mice that express monomeric RFP under the direction of the human ubiquitin C promoter. At 72h post-sham/TBI, RFP+, CD11b+ macrophages were assessed in brain tissue by flow cytometry. Inset numbers represent the % of total cells. Over 99% of infiltrated RFP+ macrophages were also CD45+, F4/80+, supporting the infiltration of peripheral macrophages into the brain after TBI.
Macrophage infiltration and polarization was next analyzed in native (non-transgenic, non-labeled) cells. TBI prominently increased the number of CD11b+, F4/80+, CD45HIGH infiltrating macrophages in the injured cortex whereas a smaller elevation in the activation of CD11b+, F4/80+, CD45LOW resident microglia was noted (Fig 3A). M1 polarization was observed within blood at 24h post-injury, as compared to sham operated mice (p<0.001 vs. sham). These changes persisted at 72h (p<0.001 vs. sham) and returned to baseline by three weeks (Fig 3B). In contrast, no significant changes in M2 polarization were noted in blood at any time point within this study (Fig 3B). A similar increase in M1 polarization of peripheral macrophages was observed within peri-contusional brain tissue at 24h post-TBI (p<0.05 vs. sham). M1 polarization also was significantly elevated at 72h and at three weeks post-injury (p<0.05 vs. sham), albeit at a reduced magnitude as compared to the 24h time point (Fig 3B). M2 polarization was not significantly changed at 24h or 72h after TBI; however, a significant reduction in M2 polarization was observed within peri-contusional brain tissue at 3 weeks (Fig 3B). Overall, an increased ratio of M1:M2 polarized macrophages was observed in blood and brain over the first 72h post-TBI, with this effect persisting within the brain for 3 weeks after injury, indicative of a pro-inflammatory shift within the injured brain (Fig 3C).
Figure 3. Increased M1 polarization after TBI.
(A.) Quantification of macrophage polarization by flow cytometry in peri-contusional brain tissue or in anatomically matched sham brain tissue at 72h. Activated myeloid cells were selected using CD11b and F4/80 (boxes; top left panels), then analyzed using CD45 to differentiate infiltrating macrophages (CD11b+ CD45HI; HIGH arrows, shaded box) from resident microglia (CD11b+ CD45LOW; LOW arrows). Polarization of infiltrating macrophages was assessed using functional cytokines for M1 (TNF-α, IL-12) or M2 (TGF-β, IL-10) phenotypes. Representative scatterplots are provided. (B.) Quantification of macrophage polarization in blood and brain tissue at 24h, 72h, or 3 weeks after sham or TBI. Data are mean ± SEM of n=8–10 mice/group. *p<0.05, ***p<0.001. (C.) Graphic representation of the M1:M2 ratio in blood or brain after sham or TBI. *p<0.05, **p<0.01, ***p<0.001.
Macrophages differentially stimulate TH polarization after TBI
Whereas considerable effort has been put forth to define the temporal and phenotype changes of macrophages after TBI, the functional consequences of macrophage polarization remain largely unstudied. To address this issue, infiltrated macrophages from the brains of sham- or TBI-injured mice were used as stimulators in a MLR with naïve T lymphocytes (Fig 4A). The proportion of CD3+ CD4+ cells relative to the total number of cells in the well was not statistically different between sham and TBI treated groups at study initiation (Fig 4B), suggesting any observed changes were due to T-lymphocyte proliferation rather than plating differences. Addition of brain macrophages from TBI-injured mice induced a 7.2 fold increase in the proliferation of CD3+ CD71+, naïve T lymphocytes, as compared to macrophages isolated from the brains of sham mice (p<0.001 vs. cultures treated with brain macrophages from sham mice) (Fig 4A,C). Phenotypic analysis of recovered T-lymphocytes revealed a significant increase in TH1 (p<0.001 vs. sham) and TH17 polarization (p<0.001 vs. sham) following treatment with TBI brain macrophages (Fig 4D). In contrast, no significant changes in TH2 polarization were observed whereas a statistically significant reduction in TREG production (p<0.01 vs. sham) was noted (Fig 4D).
Figure 4. Brain-derived monocytes stimulate T-cell proliferation and differentiation after TBI.
(A.) Purified brain monocytes were obtained from brain at 72h after sham or TBI and cells were added to naïve T lymphocytes labeled with CFSE in a MLR. Representative scatterplots depicted cellular proliferation after a 72h incubation. The percentage of CD3+, CD4+ cells within each well, prior to the addition of brain-derived monocytes, is provided. (B.) Quantification of proliferation data depicted in panel A. Data are expressed as the % Total CD3+, CD71+ T-lymphocytes. Data are mean ± SEM from n=6 mice/group, all experiments performed in triplicate wells. ***p<0.001 vs. sham. (C.) Assessment of T-lymphocyte polarization at the conclusion of the MLR. TH1 cells (CD3+ IFNγ+), TH2 cells (CD3+ IL-4+), TH17 cells (CD3+ IL-17A+) and TREG (CD3+ FoxP3+) were quantified as the % of total T-lymphocytes. Data for each phenotype was compared by t-test, **p<0.01, ***p<0.001 vs. sham.
To determine whether the MLR reflected changes in vivo, we next explored TH polarization in blood and brain tissue after TBI. A peak increase in TH1 polarization was observed in brain and blood at 24h (p<0.001 vs. sham), with persistent elevation at 72h (p<0.001 vs. sham in brain; p<0.01 vs. sham in blood) and 3 weeks (p<0.05 vs. sham in blood and brain) post-TBI (Fig 5). The pattern of TH17 polarization mirrored that of TH1 polarization, with peak TH17 polarization found at 24h post-TBI in both blood and brain (p<0.001 vs. sham), followed by increased expression at 72h post-injury (p<0.001 vs. sham in blood and brain). These changes were still observed at 3 weeks post-TBI (p<0.05 vs. sham in blood, p<0.001 in brain) (Fig 5). Conversely, TH2 polarization was not significantly different between sham and TBI groups at 24h in either blood or brain. At 72h post-injury, we found a suppression of TH2 polarization in brain (p<0.05 vs. sham) and blood (p<0.01 vs. sham) whereas only a reduction in brain was observed at 3 weeks (p<0.05 vs. sham) (Fig 5). Finally, a consistent and prolonged reduction in TREG production was seen between 24h – 3 weeks post-TBI in both blood (p<0.05, p<0.01, p<0.05 vs. sham at 24h, 72h, and 3 weeks, respectively) and brain (p<0.001, p<0.01, p<0.05 vs. sham at 24h, 72h and 3 weeks, respectively) (Fig 5). In line with a pro-inflammatory myeloid shift (Fig 4C), a pronounced, statistically significant increase in the TH17:TREG ratio was observed throughout the study in both blood and brain tissue after TBI, with a more dramatic change noted in the brain (Fig 5).
Figure 5. TBI promotes TH1/TH17 polarization.
Blood and brain tissue was collected at 24h, 72h or 3 weeks after sham/TBI. TH1, TH2, TH17, and TREG polarization was assessed by flow cytometry. Graphs depict % of Total T cells within the brain or Total T cells within the blood. Lower panels depict the ratio of TH17:TREG in brain and blood across time points. Data are mean ± SEM from n=6–8 mice/group. *p<0.05, **p<0.01, ***p<0.001 vs. sham.
We next used a myeloid cell depletion strategy to determine whether activated macrophages are critical for TH polarization after TBI. Administration of clodrosome significantly reduced the number of circulating CD11b+ F4/80+ myeloid cells (Fig 6A), as compared to encapsome treated mice. These changes in blood were similarly reflected by a ~97% reduction in CD11b+F4/80+ myeloid cells within the brain (Fig 6B), validating the functional depletion of macrophages. Clodrosome administration significantly reduced both TH1 (p<0.05 vs. placebo in blood, p<0.01 vs. encapsome in brain) and TH17 (p<0.01 vs. encapsome in blood and brain) polarization after TBI (Fig 6C). In contrast, clodrosome had no significant effect on TH2 polarization in blood or brain whereas TREG production was slightly, but significantly, increased in both blood and brain (p<0.05 vs. encapsome treatment) (Fig 6C).
Figure 6. Myeloid cell depletion attenuates TH1/TH17 polarization after TBI.
(A.) Control liposomes (Encapsome) or clodronate liposomes (Clodrosome) were administered i.p. for three consecutive days. (A.) Blood or (B.) brain tissue was obtained following the final injection and myeloid cells (CD11b+ F4/80+) were quantified by flow cytometry. Representative scatterplots are provided. (C.) Sham or TBI was induced at 24h following the third clodronate injection. At 72h post-TBI, blood and brain tissue were collected and T-lymphocyte polarization was assessed by flow cytometry. Data are mean ± SEM from n=6–8 mice/group. *p<0.05, **p<0.01, ***p<0.001, as indicated.
TLR4 activation is essential for pro-inflammatory activation after TBI
Elevated expression of TLR4+ macrophages was observed in CD11b+ F4/80+ CD45HI, infiltrated peripheral macrophages within the peri-contusional cortex after TBI (Fig 7A). C3H/HeJ mice, which contain an inactivating point mutation in the TLR4 signaling domain, reduced M1 macrophage polarization at 72h post-TBI in both blood (p<0.05 vs. wild type) and brain (p<0.001 vs. wild type), as compared to C3H/OuJ (wild-type) mice (Fig 7B). Similarly, M1 polarization was attenuated in the blood and brain of TLR4−/− mice (p<0.01), as compared to wild-type mice (p<0.01); however, M2 polarization was unchanged in both the blood and brain of TLR4−/−, as compared to wild-type mice (Fig 7C).
Figure 7. TLR4 mediates macrophage polarization after TBI.
(A.) Representative scatterplots showing increased expression of TLR4 in brain myeloid cells at 72h post-sham/TBI. TLR4 was expressed in both infiltrated peripheral macrophages (CD11b+ F4/80+ CD45 HI) and to a lesser extent, in resident microglia (CD11b+ F4/80+ CD45LO). Histogram of mean fluorescence intensity of TLR4 in CD45+ cells is provided after sham (MFI=207.21) or TBI (MFI=237.14). Macrophage polarization was quantified in (B.) C3H/OuJ (wild-type) or C3H/HeJ (TLR4 mutant) mice or in (C.) C57BL/6 (wild-type) or TLR4−/− mice at 72h post-sham/TBI. Data are mean ± SEM from n=6–8 mice/group. *p<0.05, **p<0.01, ***p<0.001 as indicated.
Macrophages isolated from the brain of C3H/HeJ or C3H/OuJ mice after TBI were next used to stimulate naïve T lymphocytes in a MLR. As observed in Fig 4, macrophages derived from the brain of sham-operated mice did not significantly enhance T-lymphocyte polarization (Fig 8A,B). In contrast, macrophages derived from either C3H/OuJ or C3H/HeJ mice subjected to TBI significantly increased T-lymphocyte proliferation (p<0.01 vs. genotype matched, sham-treated cultures); however, the stimulatory effect of C3H/HeJ macrophages after TBI was reduced, as compared to macrophages recovered from C3H/OuJ mice after TBI (p<0.001) (Fig 8A,B). T lymphocytes recovered after the MLR were used to assess whether deletion of myeloid TLR4 affected TH polarization. No differences in TH polarization were observed following stimulation by macrophages from either genotype after sham injury (Fig 8C). In contrast, macrophages recovered from either C3H/OuJ or C3H/HeJ mice after TBI significantly increased both TH1 and TH17 (p<0.001 vs. sham for both phenotypes in C3H/OuJ; p<0.01 vs. sham for TH1 and p<0.001 vs. sham for TH17 polarization in C3H/HeJ) (Fig 8C); however, the stimulatory effect of C3H/HeJ macrophages on TH1/TH17 polarization was significantly reduced, as compared to macrophages from C3H/OuJ (p<0.001 vs. C3H/OuJ for both phenotypes). TH2 polarization was not significantly affected by either injury or genotype. Conversely, TREG production was suppressed by macrophages obtained from C3H/OuJ mice (p<0.01 vs. sham) whereas macrophages derived from C3H/HeJ mice had no affect on TREG polarization (Fig 8C).
Figure 8. Myeloid TLR4 stimulates T-lymphocyte polarization after TBI.
(A.) Purified brain monocytes were isolated from C3H/OuJ (wild-type) or C3H/HeJ (TLR4 mutant) mice at 72h after sham/TBI and added to naïve T lymphocytes in a MLR. Representative scatterplots depicted cellular proliferation after a 72h incubation. The percentage of CD3+, CD4+ cells within each well, prior to the addition of brain-derived monocytes, is provided. (B.) Quantification of T-lymphocyte proliferation following stimulation with brain monocytes derived from C3H/OuJ and C3H/HeJ mice, as shown in panel A. Data are presented as the % Total CD3+ CD71+ T-lymphocytes. Data are mean ± SEM from n=6 mice/group, all experiments performed in triplicate wells. ***p<0.001. (C.) T-lymphocyte polarization was assessed in cells obtained at the termination of the MLR. TH1 cells (CD3+ IFNγ+), TH2 cells (CD3+ IL-4+), TH17 cells (CD3+ IL-17A+) and TREG (CD3+ FoxP3+) were quantified as the % of total T-lymphocytes. **p<0.01, ***p<0.001, as indicated. ns = not significant.
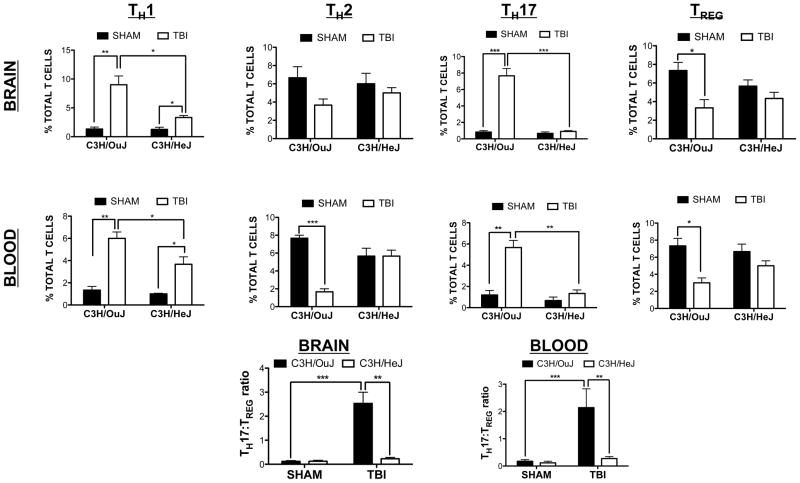
Finally, we determined whether the observed changes in the MLR reflected changes in TH polarization after TBI. In line with ex vivo data, TH1 (p<0.01 vs. sham) and TH17 polarization (p<0.01 in blood, p<0.001 in brain) were increased in C3H/OuJ mice after TBI (Fig 9). C3H/HeJ mice exhibited a comparatively smaller, yet significant increase in TH1 polarization (p<0.05 vs. sham). More importantly, C3H/HeJ mice displayed a reduction in TH1 polarization after TBI, as compared to C3H/OuJ mice (p<0.05 vs. C3H/OuJ mice). Notably, TH17 polarization was completely reversed to sham levels in C3H/HeJ mice (not significantly different from sham, p<0.01 vs. C3H/OuJ after TBI in blood, p<0.001 vs. C3H/OuJ after TBI in brain). A reduction in TH2 polarization was found in blood, but not the brain, of C3H/OuJ mice (p<0.001 vs. sham C3H/OuJ) whereas this effect was not observed in C3H/HeJ mice. Finally, C3H/OuJ mice displayed a consistent reduction in TREG in both blood and brain after TBI (p<0.05 vs. sham). In contrast, TREG were not significantly changed in C3H/HeJ mice after TBI. In keeping with a pro-inflammatory role for TLR4, the increased TH17:TREG ratio observed in the blood and brain of C3H/OuJ mice after TBI was completely reversed to sham levels in C3H/HeJ mice (Fig 9).
Figure 9. TLR4 mediates T cell polarization after TBI.
TH polarization was assessed by flow cytometry in blood and brain tissue from C3H/OuJ (wild-type) and C3H/OuJ (TLR4 mutant) mice at 72h after sham/TBI. Graphs depict % of Total T cells within the brain or blood. Lower panels depict the ratio of TH17:TREG in brain and blood of C3H/OuJ an C3H/HeJ at 72h post-sham/TBI across time points. Data are mean ± SEM from n=6–8 mice/group. *p<0.05, **p<0.01, ***p<0.001, as indicated.
DISCUSSION
In this report, we make three important mechanistic observations that implicate infiltrating macrophages as key initiators of adaptive immune responses after TBI. First, we demonstrate that peripheral macrophages polarize into a M1 phenotype, traffic into the CNS, and remain persistently elevated for weeks after TBI. Second, we show that CNS-infiltrated macrophages isolated early after TBI potently stimulate T-lymphocyte proliferation and polarization toward pro-inflammatory TH1 and TH17 phenotypes, with a concomitant reduction in counter-inflammatory FoxP3+ TREG production. These changes produced a persistently elevated TH17:TREG ratio for weeks after TBI. Finally, we provide evidence that activation of myeloid TLR4 mediates, at least in part, TH polarization after TBI, establishing a molecular link between acute trauma and long-term adaptive immune responses.
We and others determined that increased plasma or cerebrospinal fluid levels of the DAMP, high mobility group box protein 1 (HMGB1), were independent predictors of one-year mortality and unfavorable outcomes in severe TBI patients (9, 35, 36). We also reported acute neuronal release of HMGB1 induced pro-inflammatory responses and exacerbated neurological damage via activation of myeloid TLR4 after experimental TBI (9). Consistent with a report showing activation of TLR4 increased the polarization of bone marrow-derived macrophages toward a M1 phenotype and reprogrammed M2 macrophages toward a M1 phenotype (37), we observed that M1 polarization was attenuated in both TLR4 mutant and knockout mice after TBI. While acknowledging that macrophages likely exist along a dynamic continuum rather than as rigid, binary polarization phenotypes (38), activation of myeloid TLR4 may establish a deleterious, pro-inflammatory environment within the CNS after TBI.
TLR4-dependent M1 polarization meets the current operational definition of macrophage polarization; however, changes in the expression of surface markers and cytokines do not necessarily reflect functional significance. Activation of TLR4, which accelerated the clearance of myelin debris (39), increased MHC Class II expression and enhanced the migration of MHC Class II+ antigen presenting cells to secondary lymphoid organs (40). HLA-DR, an MHC Class II cell surface receptor that initiates adaptive immune responses after binding myelin basic protein (41, 42), is expressed by chronically infiltrated, M1-like macrophages after TBI (21). Interestingly, HLA-DR increased the responsiveness to TLR4 in HEK293 cells whereas peritoneal macrophages from MHC Class II knockout mice exhibited impaired responsiveness to TLR4 activation (43). Consistent with these reports, macrophages obtained from the brains of wild-type mice potently stimulated naïve T-lymphocyte proliferation whereas macrophages derived from TLR4 mutant mice were ineffective. Coupled with the observation that myeloid TLR4 activation increased oligodendrocyte injury and exacerbated hypomyelination in immature rodents (44), acute activation of TLR4 on infiltrating macrophages may initiate adaptive immune responses after TBI.
Rag1−/− mice, which are devoid of mature lymphocytes, exhibited no differences in outcomes for up to one week after TBI (45). While questioning the role of adaptive immunity after TBI, T-lymphocytes comprise functionally diverse subsets that exert both protective and detrimental roles in the CNS (46–48). In addition to antigen presentation, macrophages release phenotype-specific cytokines that polarize naïve TH0 cells into distinct TH subtypes to provide long-lasting, context- and antigen-specific immunity (49). In keeping with a pro-inflammatory myeloid shift, we observed a TLR4-dependent increase in TH1 and TH17 polarization with a concomitant reduction in TREG in both blood and brain for 3 weeks after TBI. Notably, the exogenous TLR4 agonist, lipopolysaccharide (LPS), was the most potent stimulus for antigen-loaded macrophages to drive TH17 polarization in human autologous co-cultures (50). Although T-lymphocytes do not routinely cross the blood-brain barrier (BBB) (51), intraperitoneal LPS administration increased the differentiation and brain influx of TH17 cells after neonatal hypoxia-ischemia (52). TH17 cells also comprised a larger proportion of total T-lymphocytes within the brain, as compared to blood; suggesting TH17 cells may locally polarize and/or preferentially traffic into the CNS after TBI. Consistent with the former possibility, activation of TLR4 in primary microglia or in peritoneal macrophages increased the expression of IL-23, a cytokine required for expansion and survival of TH17 cells and for suppression of FoxP3 (37, 53, 54). In addition, astrocytes induced IFNγ and IL-17 expression in T-lymphocytes via a mechanism involving IL-23 (55). In addition to central regulation, TH17 cells migrated across the BBB to exacerbate CNS inflammation via activation of IL-17 and IL-22 receptors on BBB endothelial cells (56).
The mechanisms whereby myeloid TLR4 activation enhanced TH1/TH17 polarization and simultaneously reduced TREG production after TBI remain undefined. Notably, we observed a TLR4 dependent release of IL-6 from primary human macrophages after HMGB1 treatment or after experimental TBI (9). HMGB1 increased the TH17:TREG ratio in atherosclerotic patients (57) whereas HMGB1 increased TH1 polarization (58) and stimulated TH17 polarization via an IL-6 dependent mechanism during acute allograft rejection (59). IL-6 also inhibited TGF-β1 induced Foxp3+ TREG generation while simultaneously synergizing with TGFβ1 to stimulate the differentiation of pathogenic TH17 cells from naïve T cells (60). Although incompletely explored after TBI, an elevated ratio of IL-6:IL-10 was associated with unfavorable outcomes at six months in severe TBI patients (61). In addition, IL-12, another TLR4 regulated cytokine produced by activated macrophages that is necessary for TH1 polarization (62), was acutely elevated in the CSF of pediatric severe TBI patients (28).
IL-17 promotes pro-inflammatory macrophage activation and brain infiltration of TH17 cells coincided with microglial activation and clinical symptomology after experimental autoimmune encephalopathy (EAE) (63, 64). Likewise, FTY720, which prevents lymphocyte egress from lymphoid organs, reduced CNS trafficking of both leukocytes and CD4+, IL-17+ T-lymphocytes, while simultaneously reducing neuroinflammation and white matter loss after neonatal hypoxia-ischemia (52). FTY720 similarly attenuated the accumulation of MHC-II+ macrophages and microglia after TBI (65). In keeping with these findings, a reduction in circulating TREG, which suppress the pro-inflammatory effects of human TH17 cells (66), correlated with a higher incidence of white matter microstructural abnormalities in ischemic stroke patients (67). Coupled with the observation that myelin basic protein increased IL-17 production in CD4+ T-lymphocytes from MS patients (68), an increased TH17:TREG ratio may establish a pro-inflammatory feedback loop to perpetuate chronic neurodegeneration after TBI (23).
Although we postulate a detrimental role after TBI, TH17 cells are not pathogenic per se. Adoptive transfer of myelin-specific TH17 cells increased basal hippocampal neurogenesis via actions in peripheral lymphoid tissues whereas the same myelin-reactive TH17 cells impaired endogenous remyelination in cuprizone-fed mice (69, 70). An explanation for these paradoxical effects is unclear, but the activation status of human antigen-presenting macrophages may dictate the efficiency of TH17 responses (50). Adoptive transfer of TH17 cells derived after TGF-β and IL-6 treatment are not detrimental whereas TH17 cells generated by treatment with TGFβ, IL-6, and IL-23 induced symptoms of EAE (71). Whether similar effects occur after TBI remain unstudied; however, an IL-23-IL-17 signaling pathway increased neuronal apoptosis whereas IL-23 deletion protected against cerebral ischemia-reperfusion injury (72, 73). In addition to differential regulation, TH17 cells also may trans-differentiate into TREG in the presence of TGF-β during the resolution of inflammation (74). Thus, the repertoire of cytokines released from polarized macrophages may direct TH polarization and pathological inflammation after TBI.
Finally, we cannot exclude the possibility that other professional antigen presenting cells contribute to our observed effects. Of note, clodrosome selectively depletes macrophages, yet phagocytic dendritic cells also may be affected (75). Trauma reportedly favors the differentiation of monocytes to macrophages, rather than to dendritic cells (76); thus, our future work will investigate the importance of dendritic cells after TBI. We also will explore whether chronically elevated production of TH17 cells functionally mediates delayed white matter loss after TBI. Similarly, we will establish whether TH17 polarization represents a biomarker to prospectively identify patients that may be susceptible for chronic white matter injury after TBI.
Acknowledgments
This work was supported, in part, by NS095154 and NS084228 from the National Institutes of Health (to KMD) and by a VA Merit Review (BX001117) to DWB.
References
- 1.Su E, Bell M. Diffuse Axonal Injury. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. Boca Raton (FL): 2016. [Google Scholar]
- 2.Buki A, Povlishock JT. All roads lead to disconnection?--Traumatic axonal injury revisited. Acta neurochirurgica. 2006;148:181–193. doi: 10.1007/s00701-005-0674-4. discussion 193–184. [DOI] [PubMed] [Google Scholar]
- 3.Green RE, Colella B, Maller JJ, Bayley M, Glazer J, Mikulis DJ. Scale and pattern of atrophy in the chronic stages of moderate-severe TBI. Frontiers in human neuroscience. 2014;8:67. doi: 10.3389/fnhum.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nature reviews Immunology. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 5.Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Czigner A, Mihaly A, Farkas O, Buki A, Krisztin-Peva B, Dobo E, Barzo P. Kinetics of the cellular immune response following closed head injury. Acta neurochirurgica. 2007;149:281–289. doi: 10.1007/s00701-006-1095-8. [DOI] [PubMed] [Google Scholar]
- 7.Kawamata T, Katayama Y. Surgical management of early massive edema caused by cerebral contusion in head trauma patients. Acta neurochirurgica Supplement. 2006;96:3–6. doi: 10.1007/3-211-30714-1_1. [DOI] [PubMed] [Google Scholar]
- 8.Flohe SB, Flohe S, Schade FU. Invited review: deterioration of the immune system after trauma: signals and cellular mechanisms. Innate immunity. 2008;14:333–344. doi: 10.1177/1753425908100016. [DOI] [PubMed] [Google Scholar]
- 9.Laird MD, Shields JS, Sukumari-Ramesh S, Kimbler DE, Fessler RD, Shakir B, Youssef P, Yanasak N, Vender JR, Dhandapani KM. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia. 2014;62:26–38. doi: 10.1002/glia.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. The Journal of experimental medicine. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh JT, Zheng J, Smirnov I, Lorenz U, Tung K, Kipnis J. Regulatory T cells in central nervous system injury: a double-edged sword. J Immunol. 2014;193:5013–5022. doi: 10.4049/jimmunol.1302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobin RP, Mukherjee S, Kain JM, Rogers SK, Henderson SK, Motal HL, Rogers MK, Shapiro LA. Traumatic brain injury causes selective, CD74-dependent peripheral lymphocyte activation that exacerbates neurodegeneration. Acta neuropathologica communications. 2014;2:143. doi: 10.1186/s40478-014-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmin S, Soderlund J, Biberfeld P, Mathiesen T. Intracerebral inflammation after human brain contusion. Neurosurgery. 1998;42:291–298. doi: 10.1097/00006123-199802000-00047. discussion 298–299. [DOI] [PubMed] [Google Scholar]
- 15.Holmin S, Mathiesen T, Shetye J, Biberfeld P. Intracerebral inflammatory response to experimental brain contusion. Acta neurochirurgica. 1995;132:110–119. doi: 10.1007/BF01404857. [DOI] [PubMed] [Google Scholar]
- 16.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh CL, Kim CC, Ryba BE, Niemi EC, Bando JK, Locksley RM, Liu J, Nakamura MC, Seaman WE. Traumatic brain injury induces macrophage subsets in the brain. European journal of immunology. 2013;43:2010–2022. doi: 10.1002/eji.201243084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, Liou AK, Leak RK, Gao Y, Chen J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flygt J, Djupsjo A, Lenne F, Marklund N. Myelin loss and oligodendrocyte pathology in white matter tracts following traumatic brain injury in the rat. The European journal of neuroscience. 2013;38:2153–2165. doi: 10.1111/ejn.12179. [DOI] [PubMed] [Google Scholar]
- 21.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain : a journal of neurology. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiology of aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: microglial activation and traumatic brain injury. Annals of neurology. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 24.Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. The Journal of clinical investigation. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prajeeth CK, Lohr K, Floess S, Zimmermann J, Ulrich R, Gudi V, Beineke A, Baumgartner W, Muller M, Huehn J, Stangel M. Effector molecules released by Th1 but not Th17 cells drive an M1 response in microglia. Brain, behavior, and immunity. 2014;37:248–259. doi: 10.1016/j.bbi.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez H, Pacheco R. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. Journal of neuroinflammation. 2014;11:201. doi: 10.1186/s12974-014-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gimsa U, Wolf SA, Haas D, Bechmann I, Nitsch R. Th2 cells support intrinsic anti-inflammatory properties of the brain. Journal of neuroimmunology. 2001;119:73–80. doi: 10.1016/s0165-5728(01)00343-5. [DOI] [PubMed] [Google Scholar]
- 28.Amick JE, Yandora KA, Bell MJ, Wisniewski SR, Adelson PD, Carcillo JA, Janesko KL, DeKosky ST, Carlos TM, Clark RS, Kochanek PM. The Th1 versus Th2 cytokine profile in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2001;2:260–264. doi: 10.1097/00130478-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Laird MD, Sukumari-Ramesh S, Swift AE, Meiler SE, Vender JR, Dhandapani KM. Curcumin attenuates cerebral edema following traumatic brain injury in mice: a possible role for aquaporin-4? Journal of neurochemistry. 2010;113:637–648. doi: 10.1111/j.1471-4159.2010.06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baban B, Liu JY, Abdelsayed R, Mozaffari MS. Reciprocal relation between GADD153 and Del-1 in regulation of salivary gland inflammation in Sjogren syndrome. Experimental and molecular pathology. 2013;95:288–297. doi: 10.1016/j.yexmp.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Baban B, Hansen AM, Chandler PR, Manlapat A, Bingaman A, Kahler DJ, Munn DH, Mellor AL. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. International immunology. 2005;17:909–919. doi: 10.1093/intimm/dxh271. [DOI] [PubMed] [Google Scholar]
- 32.Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, Blazar BR, Mellor AL, Munn DH. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33:942–954. doi: 10.1016/j.immuni.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achyut BR, Shankar A, Iskander AS, Ara R, Knight RA, Scicli AG, Arbab AS. Chimeric Mouse model to track the migration of bone marrow derived cells in glioblastoma following anti-angiogenic treatments. Cancer biology & therapy. 2016;17:280–290. doi: 10.1080/15384047.2016.1139243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang N, Baban B, Isales CM, Shi XM. Crosstalk between bone marrow-derived mesenchymal stem cells and regulatory T cells through a glucocorticoid-induced leucine zipper/developmental endothelial locus-1-dependent mechanism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:3954–3963. doi: 10.1096/fj.15-273664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang KY, Yu GF, Zhang ZY, Huang Q, Dong XQ. Plasma high-mobility group box 1 levels and prediction of outcome in patients with traumatic brain injury. Clinica chimica acta; international journal of clinical chemistry. 2012;413:1737–1741. doi: 10.1016/j.cca.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 36.King MD, Laird MD, Ramesh SS, Youssef P, Shakir B, Vender JR, Alleyne CH, Dhandapani KM. Elucidating novel mechanisms of brain injury following subarachnoid hemorrhage: an emerging role for neuroproteomics. Neurosurgical focus. 2010;28:E10. doi: 10.3171/2009.10.FOCUS09223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189:3439–3448. doi: 10.4049/jimmunol.1201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boivin A, Pineau I, Barrette B, Filali M, Vallieres N, Rivest S, Lacroix S. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12565–12576. doi: 10.1523/JNEUROSCI.3027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kabelitz D, Medzhitov R. Innate immunity--cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Current opinion in immunology. 2007;19:1–3. doi: 10.1016/j.coi.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 41.Pizzolla A, Gelderman KA, Hultqvist M, Vestberg M, Gustafsson K, Mattsson R, Holmdahl R. CD68-expressing cells can prime T cells and initiate autoimmune arthritis in the absence of reactive oxygen species. European journal of immunology. 2011;41:403–412. doi: 10.1002/eji.201040598. [DOI] [PubMed] [Google Scholar]
- 42.Vergelli M, Pinet V, Vogt AB, Kalbus M, Malnati M, Riccio P, Long EO, Martin R. HLA-DR-restricted presentation of purified myelin basic protein is independent of intracellular processing. European journal of immunology. 1997;27:941–951. doi: 10.1002/eji.1830270421. [DOI] [PubMed] [Google Scholar]
- 43.Frei R, Steinle J, Birchler T, Loeliger S, Roduit C, Steinhoff D, Seibl R, Buchner K, Seger R, Reith W, Lauener RP. MHC class II molecules enhance Toll-like receptor mediated innate immune responses. PloS one. 2010;5:e8808. doi: 10.1371/journal.pone.0008808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weckbach S, Neher M, Losacco JT, Bolden AL, Kulik L, Flierl MA, Bell SE, Holers VM, Stahel PF. Challenging the role of adaptive immunity in neurotrauma: Rag1(−/−) mice lacking mature B and T cells do not show neuroprotection after closed head injury. Journal of neurotrauma. 2012;29:1233–1242. doi: 10.1089/neu.2011.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh JT, Watson N, Kipnis J. T cells in the central nervous system: messengers of destruction or purveyors of protection? Immunology. 2014;141:340–344. doi: 10.1111/imm.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raposo C, Graubardt N, Cohen M, Eitan C, London A, Berkutzki T, Schwartz M. CNS repair requires both effector and regulatory T cells with distinct temporal and spatial profiles. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:10141–10155. doi: 10.1523/JNEUROSCI.0076-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf SA, Fisher J, Bechmann I, Steiner B, Kwidzinski E, Nitsch R. Neuroprotection by T-cells depends on their subtype and activation state. Journal of neuroimmunology. 2002;133:72–80. doi: 10.1016/s0165-5728(02)00367-3. [DOI] [PubMed] [Google Scholar]
- 49.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 50.Arnold CE, Gordon P, Barker RN, Wilson HM. The activation status of human macrophages presenting antigen determines the efficiency of Th17 responses. Immunobiology. 2015;220:10–19. doi: 10.1016/j.imbio.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 51.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. Journal of neuroscience research. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 52.Yang D, Sun YY, Bhaumik SK, Li Y, Baumann JM, Lin X, Zhang Y, Lin SH, Dunn RS, Liu CY, Shie FS, Lee YH, Wills-Karp M, Chougnet CA, Kallapur SG, Lewkowich IP, Lindquist DM, Murali-Krishna K, Kuan CY. Blocking lymphocyte trafficking with FTY720 prevents inflammation-sensitized hypoxic-ischemic brain injury in newborns. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:16467–16481. doi: 10.1523/JNEUROSCI.2582-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mus AM, Cornelissen F, Asmawidjaja PS, van Hamburg JP, Boon L, Hendriks RW, Lubberts E. Interleukin-23 promotes Th17 differentiation by inhibiting T-bet and FoxP3 and is required for elevation of interleukin-22, but not interleukin-21, in autoimmune experimental arthritis. Arthritis and rheumatism. 2010;62:1043–1050. doi: 10.1002/art.27336. [DOI] [PubMed] [Google Scholar]
- 54.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. The Journal of biological chemistry. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 55.Miljkovic D, Momcilovic M, Stojanovic I, Stosic-Grujicic S, Ramic Z, Mostarica-Stojkovic M. Astrocytes stimulate interleukin-17 and interferon-gamma production in vitro. Journal of neuroscience research. 2007;85:3598–3606. doi: 10.1002/jnr.21453. [DOI] [PubMed] [Google Scholar]
- 56.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding JW, Zheng XX, Zhou T, Tong XH, Luo CY, Wang XA. HMGB1Modulates the Treg/Th17 Ratio in Atherosclerotic Patients. Journal of atherosclerosis and thrombosis. 2016;23:737–745. doi: 10.5551/jat.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173:307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 59.Duan L, Wang CY, Chen J, Gong Q, Zhu P, Zheng F, Tan Z, Gong F, Fang M. High-mobility group box 1 promotes early acute allograft rejection by enhancing IL-6-dependent Th17 alloreactive response. Laboratory investigation; a journal of technical methods and pathology. 2011;91:43–53. doi: 10.1038/labinvest.2010.141. [DOI] [PubMed] [Google Scholar]
- 60.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 61.Kumar RG, Boles JA, Wagner AK. Chronic Inflammation After Severe Traumatic Brain Injury: Characterization and Associations With Outcome at 6 and 12 Months Postinjury. The Journal of head trauma rehabilitation. 2015;30:369–381. doi: 10.1097/HTR.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 62.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. The Journal of experimental medicine. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain, behavior, and immunity. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 64.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 65.Zhang Z, Zhang Z, Fauser U, Artelt M, Burnet M, Schluesener HJ. FTY720 attenuates accumulation of EMAP-II+ and MHC-II+ monocytes in early lesions of rat traumatic brain injury. Journal of cellular and molecular medicine. 2007;11:307–314. doi: 10.1111/j.1582-4934.2007.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crome SQ, Clive B, Wang AY, Kang CY, Chow V, Yu J, Lai A, Ghahary A, Broady R, Levings MK. Inflammatory effects of ex vivo human Th17 cells are suppressed by regulatory T cells. J Immunol. 2010;185:3199–3208. doi: 10.4049/jimmunol.1000557. [DOI] [PubMed] [Google Scholar]
- 67.Yasuno F, Taguchi A, Yamamoto A, Kajimoto K, Kazui H, Kudo T, Kikuchi-Taura A, Sekiyama A, Kishimoto T, Iida H, Nagatsuka K. Microstructural abnormality in white matter, regulatory T lymphocytes, and depressive symptoms after stroke. Psychogeriatrics : the official journal of the Japanese Psychogeriatric Society. 2014;14:213–221. doi: 10.1111/psyg.12084. [DOI] [PubMed] [Google Scholar]
- 68.Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 2008;125:161–169. doi: 10.1111/j.1365-2567.2008.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niebling J, ERA, Schallenberg S, Kretschmer K, Kempermann G. Myelin-specific T helper 17 cells promote adult hippocampal neurogenesis through indirect mechanisms. F1000Research. 2014;3:169. doi: 10.12688/f1000research.4439.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baxi EG, DeBruin J, Tosi DM, Grishkan IV, Smith MD, Kirby LA, Strasburger HJ, Fairchild AN, Calabresi PA, Gocke AR. Transfer of myelin-reactive th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:8626–8639. doi: 10.1523/JNEUROSCI.3817-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. The Journal of experimental medicine. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lv M, Liu Y, Zhang J, Sun L, Liu Z, Zhang S, Wang B, Su D, Su Z. Roles of inflammation response in microglia cell through Toll-like receptors 2/interleukin-23/interleukin-17 pathway in cerebral ischemia/reperfusion injury. Neuroscience. 2011;176:162–172. doi: 10.1016/j.neuroscience.2010.11.066. [DOI] [PubMed] [Google Scholar]
- 73.Konoeda F, Shichita T, Yoshida H, Sugiyama Y, Muto G, Hasegawa E, Morita R, Suzuki N, Yoshimura A. Therapeutic effect of IL-12/23 and their signaling pathway blockade on brain ischemia model. Biochemical and biophysical research communications. 2010;402:500–506. doi: 10.1016/j.bbrc.2010.10.058. [DOI] [PubMed] [Google Scholar]
- 74.Gagliani N, Vesely MCA, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS, Ching T, Weaver C, Zi X, Pan X, Fan R, Garmire LX, Cotton MJ, Drier Y, Bernstein B, Geginat J, Stockinger B, Esplugues E, Huber S, Flavell RA. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nature reviews Immunology. 2011;11:788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 76.Miller-Graziano CL, De AK, Kodys K. Trauma Mediators Favor Differentiation of Monocytes to Macrophages Rather Than to Dendritic Cells. In: Marshall JC, Cohen J, editors. Immune Responses in the Critically Ill. Springer; Berlin Heidelberg: 2002. pp. 247–263. [Google Scholar]