Abstract
Runx2, a runt-related transcriptional factor family member, is involved in the regulation of osteoblast differentiation. Interestingly, it is abundant in growth-arrested 3T3-L1 preadipocytes and was dramatically down-regulated during adipocyte differentiation. Knockdown of Runx2 expression promoted 3T3-L1 adipocyte differentiation, whereas overexpression inhibited adipocyte differentiation and promoted the trans-differentiation of 3T3-L1 preadipocytes to bone cells. Runx2 was down-regulated specifically by dexamethasone (DEX). Only type I Runx2 was expressed in 3T3-L1 preadipocytes. Using luciferase assay and chromatin immunoprecipitation-quantitative PCR analysis, it was found that DEX repressed this type of Runx2 at the transcriptional level through direct binding of the glucocorticoid receptor (GR) to a GR-binding element in the Runx2 P2 promoter. Further studies indicated that GR recruited histone deacetylase 1 to the Runx2 P2 promoter which then mediated the deacetylation of histone H4 and down-regulated Runx2 expression. Runx2 might play its repressive role through the induction of p27 expression, which blocked 3T3-L1 adipocyte differentiation by inhibiting mitotic clonal expansion. Taken together, we identified Runx2 as a new downstream target of DEX and explored a new pathway between DEX, Runx2, and p27 which contributed to the mechanism of the 3T3-L1 adipocyte differentiation.
Runx2 is a master regulatory gene essential for osteoblast differentiation (1, 2). It belongs to the runt family of transcription factors, members of which are characterized by a DNA-binding domain homologous to Drosophila runt domain (3, 4). Targeted disruption of Runx2 resulted in a loss of bone formation both intramembranous and endochondral ossification, due to the failure of transcriptional activation of the principal osteoblastic-specific genes, including alkaline phosphatase, osteocalcin, type I collagen, osteopontin, and bone sialoprotein (1, 2). Runx2 is also required for chondrocytes hypertrophy (5), cell cycle regulation of osteoblast (6–9), and vascular invasion of developing skeletons (10). Previous studies indicate that Runx2-deficient chondrocytes and osteoblasts spontaneously undergo adipocyte differentiation, with the loss of chondrocyte characteristics and osteogenesis (11). In addition, induction of Runx2 by bone morphogenetic protein 2 treatment inhibits the late adipocyte maturation of human bone marrow precursor cells (12), overexpression of peroxisome proliferator-activated receptors γ (PPARγ), and introduction of PPARγ ligand inhibit Runx2 expression and osteoblast differentiation (13). These studies indicate that Runx2 is involved in inhibiting adipocyte differentiation and appears to be repressed or down-regulated during adipocyte development.
Adipocytes and osteoblasts are derived from the same progenitor cells: multipotential mesenchymal stem cells (14). Runx2 is expressed in the mesenchymal stem cells (15), and its expression increases when mesenchymal stem cells committed to osteoblasts during the osteogenesis (13). Because Runx2 is a transcription factor that promotes osteogenesis and inhibits the adipogenesis (10), its expression is expected to decrease when mesenchymal stem cells commit to preadipocytes. However, our present data indicated that Runx2 is highly expressed in preadipocytes such as 3T3-L1, which seems contradictory to the role of Runx2 as a master regulatory gene of osteogenesis.
3T3-L1 is the most commonly used cell line for the study of the terminal adipocyte differentiation. A combination of dexamethasone (DEX), methylisobutylxanthine (MIX), and insulin is used as the standard protocol for the in vitro differentiation of 3T3-L1 preadipocyes (16). After exposure to the inducers, postconfluent 3T3-L1 preadipocytes undergo several rounds of mitotic clonal expansion (MCE) before terminal differentiation (17, 18). After the induction CCAAT enhancer binding protein β (C/EBPβ) and C/EBPδ, induced immediately by MIX and DEX, respectively (19, 20), activate the expression of two master adipogenic genes, i.e. C/EBPα and PPARγ (21), which in turn activate the expression of adipocyte-specific genes producing the adipocyte phenotype. Previous studies have demonstrated that C/EBPδ is the major target of DEX in adipogenesis (22, 23). However, C/EBPδ alone did not activate C/EBPα, PPARγ, and 422/aP2 expression (23, 24), indicating that DEX plays a greater role than simply activating C/EBPδ expression.
In this paper, we tried to determine the regulation of Runx2 during 3T3-L1 adipocyte differentiation, including the upstream and the downstream regulation of Runx2, to illustrate the role of this gene in adipogenesis. We have found that DEX was the upstream regulator of type I Runx2 (the only type of Runx2 expressed in 3T3-L1 preadipocytes) gene expression during 3T3-L1 adipocyte differentiation, and it decreased Runx2 expression by direct binding of the glucocorticoid receptor (GR) to the GR consensus site in the Runx2 P2 promoter. Lowering endogenous Runx2 levels consistently reduced the requirement for DEX in the promotion of adipogenesis, supporting a model whereby the rapid decrease of Runx2 gene transcription upon DEX exposure might be a mechanism by which glucocorticoids promote adipocyte differentiation. GR could also recruit histone deacetylase (HDAC) 1 to Runx2 P2 promoter, which mediated the deacetylation of histone H4 in this promoter and decreased its expression. Finally, Runx2 inhibited adipogenesis through the induction of p27, which kept 3T3-L1 preadipocytes in a growth-arrested state and blocked the MCE and terminal differentiation. In conclusion, we have first shown that DEX promotes adipogenesis of 3T3-L1 preadipocytes, in part, by repressing the transcriptional level of type I Runx2, which then facilitated the process of MCE and terminal differentiation.
Materials and Methods
Cell culture and induction of differentiation
3T3-L1 preadipocytes were propagated and maintained in DMEM containing 10% (vol/vol) calf serum. Two-day postconfluent (designated d 0) cells were induced to differentiate with DMEM containing 10% (vol/vol) fetal bovine serum (FBS), 1 μg/ml insulin (I), 1 μm dexamethasone (DEX), and 0.5 mm MIX until d 2. Cells were fed with DMEM supplemented with 10% FBS and 1 μg/ml insulin for 2 d, after which they were fed every other day with DMEM containing 10% FBS. Expression of adipocyte genes and acquisition of adipocyte phenotype began on d 3 and was maximal by d 8.
Transfection analysis of Runx2 P2 promoter
−1219-+200 of Runx2 (NM_001145920) P2 promoter was amplified with the forward primer (CGGGGTACCGAAGGTCAGAGAGTG GCAACTGCGCTA) and reverse primer (GGAAGATCTCAAGGTGCCGGGAGGTA AGTGGGGGCGG) and was cloned into the KpnI and BglII sites of the pGL3-basic luciferase vector (Promega Corp., Madison, WI). The promoter was sequenced and the authenticity verified. The mutant Runx2 promoter with the GR binding element deleted was made with a KOD-Plus-mutagenesis Kit (TOYOBO, Japan) using upstream primer (GTTATATGTCTTGCCTAACCTATTATTTTA) and downstream primer (CGCTGAGAGGTGAGCCAGCCCGATATT). Transfection experiments were performed with the transfection kit Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's instruction, and luciferase activities were normalized to internal control Renilla luciferase activity.
Western blotting
Cells were lysed with lysis buffer containing 2% sodium dodecyl sulfate (SDS), 10 mm dithiothreitol, 50 mm Tris-HCl, pH 6.8, 10% glycerol, 0.002% bromphenol blue, 1 × protease inhibitor mixture. Equal amounts of protein were separated by SDS-PAGE, and transferred to polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA), immunoblotted with antibodies [anti-Flag and anti-actin monoclonal antibody were obtained from Sigma (St. Louis, MO), anti-p27 antibody was obtained from GeneTex (GTX100446), anti-GR and anti-Runx2 antibody were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA)], and visualized with horseradish peroxidase-coupled secondary antibodies.
RT-PCR and real-time quantitative PCR
Total RNA was isolated using the Trizol reagent (Invitrogen) according to the manufacturer's instruction. First-strand cDNA synthesis was performed using RevertAid First Strand cDNA Synthesis Kit (Fermentas, Inc., Glen Burnie, MD) with random primer. PCR were performed using the synthesized cDNA as the template in a 25-μl reaction mixture containing specific primers. The PCR was carried out following a cycling protocol: an initial denaturation step at 95 C for 2 min followed by 27 cycles each of a denaturation at 94 C (30 sec), annealing at 58 C (20 sec), and elongation at 72 C (15 sec). The reaction products were resolved by electrophoresis on a 1.5% agarose gel and visualized with ethidium bromide. Real-time quantitative PCR were performed with 2 × PCR Master Mix (Power SYBR Green, ABI, Carlsbad, CA) on a Bio-Rad Q5 instrument (Bio-Rad Laboratories, Inc., Hercules, CA). The threshold cycles (Ct) for the Runx2, p27 genes, and 18s rRNA control signals were determined in triplicate experiments, and the relative RNA quantity was calculated using the comparative Ct method.
Knockdown expression of Runx2, p27, HDAC1, and GR with small interfering RNA (siRNA)
Synthetic siRNA oligonucleotide specific for Runx2 (NM_001145920) (5′ to 3′: UAGCGGCAGAAUGGAUGAGUCUGUUtt), p27 (NM_009875) (5′ to 3′: GCUU GCCCGAGUUCUACUAtt), GR-1 (X13358) (5′ to 3′: UGACUGCCUUACUAAG AAATT), GR-2(X13358) (5′ to 3′: GCCAUUUCUGUUCAUGGCG UGAGUATT), HDAC1 (NM_008228.2) (5′ to 3′: GCAGCGUCUCUUUGAGAACTT) were designed and synthesized by Invitrogen. 3T3-L1 preadipocytes at 30–50% confluency were transfected with the siRNA oligonucleotide by using Lipofectamine RNAi MAX (Invitrogen). After cells reached confluency (30–40 h), they were subjected to the standard differentiation protocol as described above, and at various times thereafter cells were prepared for the test. Stealth siRNA Negative Control Duplexes (Invitrogen) were used as a negative control.
Overexpression of Runx2, p27 in 3T3-L1 preadipocytes
We cloned Runx2 from the plasmid, which was kindly given by Professor Kun-Liang Guan, and p27 was cloned from cDNA into MSCV vector. Retrovirus was packed in 293T cells, and the supernatant was harvested 48 h after the transfection of MSCV-Flag-Runx2 or MSCV-Flag-p27 and ecopack plasmids. 3T3-L1 preadipocytes were infected in approximately 20–30% confluence with the empty MSCV and MSCV-Flag-Runx2 or MSCV-Flag-p27 supernatant, respectively. Western blot was performed to detect the overexpression.
Oil Red O staining
Cells were washed three times with PBS and fixed for 2 min with 3.7% formaldehyde. Oil Red O (0.5% in isopropanol) was diluted with water (3:2), filtered through a 0.45-μm filter, and incubated with the fixed cells for 1 h at room temperature (RT). Cells were washed with water, and the stained fat droplets in the cells were visualized by light microscopy and photographed.
Alizarin red and alkaline phosphatase (ALP) staining
MSCV-Vector or MSCV-Runx2-infected 3T3-L1 cells were cultured for 2 wk in the presence of ascorbic acid (100 g/ml) and glycerophosphate (5 mm), rinsed twice with PBS, fixed in 90% ethanol, and stained with 1% alizarin red solution for 5 min. ALP was stained by alkaline phosphatase staining kit (Sigma, 069K4337). Cells were washed with water, and the stained mineralized nodes in the cells were visualized by light microscopy.
Fluorescence-activated cell sorting analysis
3T3-L1 preadipocytes were transfected with Stealth siRNA Negative Control Duplexes (Invitrogen) or Runx2 or p27 siRNA, as described above. After they were treated with differentiation inducer, 3T3-L1 cells were harvested every 4 h until 28 h. Cells were trypsinized, washed with 1 ×PBS and fixed with 2% (wt/vol) paraformaldehyde in PBS. They were treated with 0.5 mg/ml RNase A for 1 h at RT and incubated with 0.1 mg/ml propidium iodide (Sigma) for 45 min at 37 C. DNA content was determined by flow cytometry (Bio-Rad Laboratories).
Chromatin immunoprecipitation analysis
3T3-L1 cells were fixed in 1% formaldehyde in a fume hood for 10 min, incubated with 1/20 volume of 2.5 m glycine, and gently mixed at RT for 5 min, and washed three times with ice-cold 1×PBS containing 1 mm phenylmethylsulfonyl fluoride (PMSF). Cells were scraped off and washed three times with 1 ml lysis buffer with fresh PMSF. They were lysed in lysis buffer [1% SDS, 10 mm EDTA, 50 mm Tris-HCl (pH 8), 1 mm PMSF]. Lysates were vortexed and sonicated with a Bioruptor (Cosmo Bio USA, Carlsbad, CA). The average length of DNA fragments ranged between 300 and 800 bp. The lysates were clarified by centrifugation and diluted 5-fold in chromatin immunoprecipitation buffer [15 mm Tris (pH 8), 1% TritonX-100, 0.01% SDS, 1 mm EDTA, 150 mm NaCl, 1 mm PMSF, 1×PIC]. The samples were precleared using protein A-Sepharose beads for 1 h at 4 C, and 10% of each sample was used for input control. The samples were immunoprecipitated with anti-GR antibody antiacetylhistone H4, anti-HDAC1, HDAC2, or control IgG, and the immune complexes were washed with low-salt buffer [0.1% SDS, 1% TritonX-100, 2 mm EDTA, 20 mm Tris-HCl (pH 8), 150 mm NaCl], followed by high-salt buffer [0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl (pH 8), 500 mm NaCl], washed with LiCl buffer [0.25 m LiCl, 1% NP-40, 1% deoxycholate, 1 mm EDTA, 10 mm Tris-HCl (pH 8), followed by TE buffer [10 mm Tris-HCl (pH 8), 1 mm EDTA], and finally eluted with elution buffer [50 mm Tris-HCl (pH 8), 10 mm EDTA, 1% SDS]. Immunoprecipitated DNA was reverse cross-linked at 65 C for 12 h and purified using DNA Purification Kit (Tiangen Biotech, Beijing, China). The same amount of purified DNA from IgG and specific antibodies was used to perform the quantitative PCR with the primer (sense primer, 5-GCATAGTTGTAAGTGGTGCA-3; antisense primer, 5-GACTGCTTGCTCTCTCACAG-3) and the relative enrichment of the GR, HDAC1, HDAC2, and acetylated-H4 in Runx2 P2 promoter were calculated, respectively, by dividing the CT value from these antibodies by the values from IgG antibody.
Results
Runx2 is widely expressed in mesenchymal derived cell lines and down-regulated during 3T3-L1 adipocyte differentiation
The expression of Runx2 was analyzed by real time RT-PCR and Western blotting in mesenchymal-derived cell lines, including C3H10T1/2 mesenchymal stem cells, 3T3-L1 preadipocytes, and 3T3-E1 preosteoblasts. Our results indicated that Runx2, the bone-specific gene, was not only expressed in 3T3-E1 preosteoblasts and C3H10T1/2 mesenchymal stem cells, but also in 3T3-L1 preadipocytes (Fig. 1, A and 1B), which attracted our interest in the role of Runx2 in the adipogenesis. 3T3-L1 preadipocytes were used for all further studies. RT-PCR and Western blotting analysis indicated that Runx2 can be dramatically down-regulated transcriptionally (Fig. 1C) and translationally (Fig. 1D) when postconfluent 3T3-L1 preadipocytes are exposed to inducers of differentiation, indicating that Runx2 may play an inhibitory role during adipocyte differentiation.
Fig. 1.
Runx2 is down-regulated during 3T3–L1 adipocyte differentiation. A and B, C3H10T1/2, 3T3–L1, 3T3–F442A, and 3T3–E1 cells were grown to postconfluency, Runx2 mRNA and protein level were detected by quantitative RT-PCR and Western blotting, respectively. C and D, Postconfluent 3T3–L1 preadipocytes were induced to differentiation as described, total RNA and protein were isolated at different time-points, and quantitative RT-PCR (C) and Western blotting (D) were used to analyze the expression of Runx2; and exogenously expressed Runx2 was used as a positive control (Pos Con).
Knockdown of the expression of Runx2 by stealth RNAi promoted 3T3-L1 adipocyte differentiation
To define the role of Runx2 during adipogenesis of 3T3-L1 preadipocytes, Runx2 expression was knocked down by a specific siRNA, which was confirmed by quantitative real-time PCR and Western blotting (Fig. 2, A and B). Knockdown of the Runx2 expression promoted the adipogenesis of 3T3-L1 preadipocytes as indicated by an earlier and larger appearance of lipid droplets accumulation (Fig. 2C), as well as adipocyte-specific gene expression (Fig. 2D). RT-PCR was also used to determine the mRNA level of some adipocyte-specific genes, which was consistent with the protein levels (Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). The specificity of the Runx2 siRNA was also confirmed in 3T3-E1 preosteoblasts. The osteogenic ability of 3T3-E1 cells was significantly reduced when cells were treated with this specific Runx2 siRNA oligo, as indicated by decreased alizarin red and ALP staining and a decrease of bone-specific gene expression (Supplemental Fig. 2).
Fig. 2.
Down-regulation of Runx2 promotes 3T3–L1 adipocyte differentiation. 3T3–L1 preadipocytes were transfected with Runx2 siRNA and 48 h after reaching confluence, cells were induced to differentiate as described. A and B, Quantitative RT-PCR and Western blotting were used to analyze the expression of Runx2, respectively. C, Cells stained with or without Oil Red O were visualized and photographed at the times indicated. D, Expression of C/EBPα, PPARγ, aP2, and actin were analyzed by Western blotting at the times indicated.
Overexpression of Runx2 inhibits 3T3-L1 adipocyte differentiation and promotes transdifferentiation of 3T3-L1 preadipocytes into bone cells
To confirm the inhibitory role of Runx2 during adipocyte differentiation, Runx2 was overexpressed in 3T3-L1 preadipocytes. Western blotting analysis with anti-flag antibody was used to confirm the expression of exogenous Runx2 (Fig. 3B). To some extent, forced expression of Runx2 inhibited adipogenesis of 3T3-L1 preadipocytes, as indicated by decreased Oil Red O staining (Fig. 3A) and the expression of adipocyte-specific proteins (Fig. 3B). In addition, the expression of bone-specific genes, mineralized nodes formation, as well as the positive ALP staining and alizarin red staining significantly increased in 3T3-L1 cells when Runx2 was overexpressed and bone inducers were used (Fig. 3, C and D). Runx2 therefore plays an inhibitory role during 3T3-L1 adipocyte differentiation.
Fig. 3.
Overexpression of Runx2 inhibits adipogenesis and promotes the trans-differentiation of 3T3–L1 preadipocytes to bone cells. 3T3–L1 preadipocytes were infected with retrovirus expressing flag-tagged Runx2 or with empty vector. Cells were exposed, 2 d after reaching confluency, to adipocyte differentiation inducers (A and B) or bone differentiation media (C and D). Expression of Runx2 (with anti-flag antibody), C/EBPα, PPARγ, and aP2 were analyzed by Western blotting (B), and Oil Red O staining (A) was performed on d 8. ALP and alizarin red staining were performed and photographed (C), and the expression of bone-related genes was analyzed by RT-PCR at 2 wk (D). OCN, Osteocalcin; OPN, osteopontin.
Runx2 is down-regulated by DEX through directly binding of GR to Runx2 P2 promoter
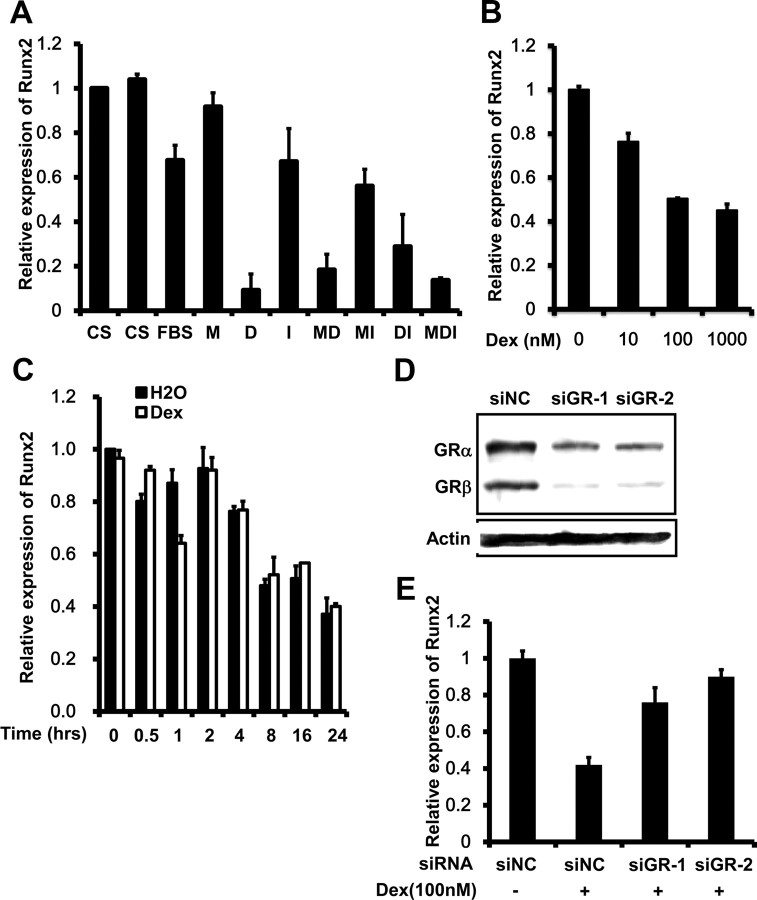
Different combinations of three adipogenic inducers (MIX, DEX, and insulin) were used to find out which inducer caused the down-regulation of Runx2 expression. Quantitative RT-PCR analysis indicated that DEX significantly repressed expression, and insulin and MIX had little effect (Fig. 4A). The repressive effect of DEX was dose dependent (Fig. 4B) with a maximum effect at approximately 100 nm.
Fig. 4.
Runx2 is down-regulated by DEX. A, 3T3-L1 preadipocytes were treated with inducing medium with different combination of the MDI inducers and quantitative real time RT-PCR was used to detect the Runx2 mRNA level. B, 3T3-L1 preadipocytes were treated with DEX at different concentrations and Runx2 mRNA levels were analyzed. C, Postconfluent 3T3-L1 cells were pretreated 5 μg/ml actinomycin D for 2 h, followed by treatment with DMEM containing 10% FBS together with 1 mm dexamethasone or water for 24 h. Runx2 mRNA levels were detected at different time-points. D, 3T3-L1 preadipocytes were transfected with 2 different GR siRNAoligos. E, Forty-eight hours after reaching confluence, cells were treated with or without DEX for 24 h and Runx2 mRNA levels were detected.
DEX might influence mRNA degradation rate or mRNA stability (25–27). However, the degradation rate of Runx2 mRNA treated with DEX was no different from the control (Fig. 4C), indicating that this was not the mechanism by which DEX down-regulated Runx2. Glucocorticoids act normally through the glucocorticoid receptor (GR) (28), which is retained in cytoplasm in the absence of its ligand and translocates to the nucleus after ligand activation, where it acts as a transcription factor or cofactor. To test whether the GR was involved, GR expression was knocked down by siRNA (Fig. 4D), resulting in significantly decreasing the repression of Runx2 by DEX (Fig. 4E).
The major Runx2 are types I and II, which are derived from two promoters and share the same C terminus with different N termini and play a similar role in osteogenesis (29–31). To determine which type of Runx2 was expressed in 3T3-L1 preadipocytes, RT-PCR was performed; two pairs of primers were designed, and 3T3-E1 preosteoblasts were used as positive control. Whereas 3T3-E1 preosteoblasts expressed both types, 3T3-L1 preadipocytes only expressed type I Runx2 (Fig. 5A). To investigate the mechanism by which DEX repressed Runx2, −1219 to +200 of the Runx2 P2 promoter with the GR-binding element (−891 to ∼−883) (Fig. 5B), as determined by the transcription element search system, was amplified and cloned into a pGL3.0 basic plasmid. Transient transfection showed that DEX could repress the activity of the Runx2 P2 promoter (Fig. 5C). It was reported that GR could repress the transcription by the direct binding to promoter (32). Chromatin immunoprecipitation (ChIP)-quantitative PCR was used to confirm that this GR element was enriched in this region of the promoter by a specific antibody (Fig. 5D). Deletion of this GR element in Runx2-P2-pGL3.0 plasmid almost completely abolished DEX repression (Fig. 5E). These results suggested that DEX-mediated repression of Runx2 P2 promoter activity is through the binding of GR to the GR element in this promoter.
Fig. 5.
DEX decreases Runx2 expression at the transcriptional level. A, Total RNA was isolated from 3T3–L1 preadipocytes or 3T3–E1 preosteoblasts; RT-PCR was used to detect the expression of Runx2 with primers specific to type I or type II Runx2. B, The structure of the Runx2 P2 promoter region and the location and the sequence of glucocorticoid response element are presented. C, 3T3–L1 preadipocytes were transiently transfected with type I Runx2 P2 promoter reporter construct and pRL-TK plasmid, and treated with or without 1 μM DEX for 24 h. Cell extracts were prepared, and luciferase activities were analyzed and normalized to Renilla activity. D, Postconfluent 3T3–L1 preadipocytes were induced to differentiate as described, and quantitative ChIP-PCR was performed at 20 h after induction with anti-GR antibody and nonspecific IgG. Regions from PPARγ2 Insulin1 were used as positive and negative control, respectively. D, 3T3–L1 preadipocytes were transiently transfected with wt Runx2 P2 promoter reporter construct or Runx2 P2 promoter reporter with the GR element deleted (mut) together with pRL-TK plasmid; cells were then treated with or without 1 μm DEX for 24 h. Cell extracts were prepared, and luciferase activities were measured and normalized to Renilla activity. Data are presented as mean ± sd of at least three independent experiments. mut, Mutant; Rel, relative; wt, wild type.
GR recruits HDAC1 to Runx2 P2 promoter
Glucocorticoids stimulate adipogenesis through targeting of the HDAC1 complex (33). It was hypothesized that GR also helped recruit HDAC1 to the Runx2 P2 promoter, which deacetylated histone H4 and decreased the transcription of the Runx2 gene. ChIP-quantitative PCR was used to detect acetylated histone H4 by means of a specific antibody. Acetylation of Histone H4 on Runx2 P2 promoter significantly decreased after induction of differentiation in 3T3-L1 preadipocytes, with DEX playing the most important role in the histone H4 deacetylation (Fig. 6, A and B). DEX could significantly recruit HDAC1, instead of HDAC2, to the Runx2 P2 promoter (Fig. 6C). When HDAC1 was knocked down by the specific siRNA, even with the compensatory increase of HDAC2, Runx2 mRNA significantly increased (Fig. 6D). To further determine the role of GR in the recruitment of HDAC1 to Runx2 P2 promoter, we knocked down GR expression and detected the binding of HDAC1 to Runx2 P2 promoter. As shown in Fig. 6, D–F, the binding of GR and HDAC1 to Runx2 P2 promoter significantly decreased (Fig. 6, E and F) whereas the acetylation of histone H4 increased (Fig. 6G) when GR was knocked down by specific siRNA.
Fig. 6.

DEX recruits HDAC1 to the Runx2 P2 promoter. A, Postconfluent 3T3–L1 preadipocytes were induced to differentiation as described in the text. ChIP-quantitative PCR was performed from d 0 to d 6 after induction with antiacetylhistone H4 antibody and nonspecific IgG. B, 3T3–L1 preadipocytes were treated with inducing medium with different combination of the MDI inducers, and ChIP-quantitative PCR was performed at 24 h after induction with antiacetylhistone H4 antibody, nonspecific IgG being used as negative control. C, Postconfluent 3T3–L1 preadipocytes were induced with MI or MDI, and ChIP-quantitative PCR was performed at 24 h after induction with anti-HDAC1 or anti-HDAC2 antibodies, nonspecific IgG being used as the negative control. D, 3T3–L1 preadipocytes were transfected with HDAC1 siRNA, and 48 h after reaching confluence, cells were induced to differentiate as described in the text. Cells were treated with MI or MDI for 24 h, and Runx2 mRNA levels were measured. The knockdown effect was detected by Western blotting. E–G, 3T3–L1 preadipocytes were transfected with GR siRNA, and 48 h after reaching confluence, cells were induced with MI or MDI for 24 h. ChIP-quantitative PCR was performed at 24 h after induction with anti-GR, anti-HDAC1, antiacetylhistone H4 antibodies and nonspecific IgG. Rel, Relative.
Decreasing endogenous Runx2 expression reduces the requirement of DEX in the induction of 3T3-L1 adipocyte differentiation
Because Runx2 plays an inhibitory role during 3T3-L1 adipocyte differentiation, and DEX is the key adipogenic inducer that down-regulates the expression of Runx2, we asked whether down-regulation of Runx2 by siRNA decreases the amount of DEX required for the full differentiation of 3T3-L1 preadipocytes. Cells were transfected with siRunx2 as described, and then treated with standard insulin, MIX, and DEX at 0, 10, 100, and 1000 nm. Oil Red O staining and Western blotting analysis indicated that 3T3-L1 preadipocytes treated with siRunx2 required significantly less DEX (from 1000 nm to 10 nm) for full adipocyte differentiation (Fig. 7, A and B).
Fig. 7.
Down-regulation of Runx2 by siRNA reduces the amount of DEX required for full differentiation of 3T3–L1 preadipocyte.3T3–L1 preadipocytes were transfected with Runx2 siRNA as described, and 48 h after reaching confluence, cells were induced to differentiate with standard MIX and insulin, together with DEX at 0, 10, 100, or 1000 nm. A, Cells were stained with Oil Red O at d 7 and photographed. B, Expression of C/EBPα, PPARγ, aP2, and actin at d 8 were analyzed by Western blotting.
Runx2 represses adipogenesis of 3T3-L1 preadipocytes through inhibition of the MCE
It has been reported (34, 35) that MCE is required for adipocyte differentiation of 3T3-L1 preadipocytes, and down-regulation of the cyclin-dependent kinase inhibitor, p27, is required for the initiation of MCE. P27 plays an inhibitory role in adipogenesis (35, 36), as indicated by the fact that p27 knockout mice are fatter than the wild type (37). Interestingly, p27 appears to be the downstream target of Runx2 (38). We hypothesized that Runx2 might repress 3T3-L1 adipocyte differentiation by inhibiting MCE via the induction of p27, and DEX promoted adipogenesis by relieving this repression. The most frequently used concentration of DEX is 1 μm, which is the overdose for the study of the downstream. To test our hypothesis, control negative control siRNA (siNC)-treated 3T3-L1 cells were incubated with standard MIX/insulin together with 1 μm DEX or 10 nm DEX, respectively; and the siRunx2-treated 3T3-L1 cells were induced with standard MIX/insulin with 10 nm DEX only. Down-regulation of p27 was significantly delayed when the control siNC-treated 3T3-L1 cells were treated with 10 nm DEX compared with cells normally treated with 1 μm DEX (Fig. 8A); however, down-regulation of p27 was almost the same in siRunx2-treated 3T3-L1 cells when treated with 10 nm DEX as in siNC-treated control 3T3-L1 cells were treated with 1 μm DEX (Fig. 8A). There was no difference in the expression of cyclin-dependent kinase 4 among these three groups. Consistent with the difference in the down-regulation of p27, many more siRunx2-treated 3T3-L1 preadipocytes underwent MCE than control siNC-treated 3T3-L1 cells when they were given standard MIX/insulin and 10 nm DEX, which was almost the same as the control siNC-treated 3T3-L1 cells treated with standard MIX/insulin and 1 μm DEX (Fig. 8B). Consistently, overexpression of Runx2 in 3T3-L1 preadipocytes increased the expression of p27 and inhibited the MCE (Fig. 8, C and D).
Fig. 8.

Runx2 plays the inhibitory role in adipogenesis through the regulation of p27 and the following progression of MCE. A and B, 3T3–L1 preadipocytes were transfected with siRunx2 or control siNC, and 48 h after reaching confluence, siNC-treated cells were induced to differentiate with standard MIX, insulin, together with DEX at 10 nm or 1 μm, whereas siRunx2-treated cells were induced to differentiate with standard MIX, Insulin, together with DEX at 10 nm only. A, Expression of p27, CDK2, CDK4, and actin were analyzed by Western blotting. B, Cells at different time points were harvested for fluorescence-activated cell sorting, and the percentage of cells in S phase was analyzed and plotted. C and D, 3T3–L1 preadipocytes were infected with retrovirus expressing flag-tagged Runx2 or with empty vector. Cells were exposed, 2 d after reaching confluency, to adipocyte differentiation inducers. C, Expression of p27 and actin were analyzed by Western blotting. D, Cells at different time points were harvested for fluorescence-activated cell sorting, and the percentage of cells in S phase was analyzed and plotted. CDK, Cyclin D kinase.
Discussion
Adipocyte differentiation is the combined result of both the down-regulation of adipogenic inhibitors and the induction of the pro-adipogenic factors. Induction of 3T3-L1 adipocyte differentiation involves the integral down-regulation of inhibitory factors, including C/EBP homologous protein (CHOP 10) (39), pref1 (40), Wnt10b/catenin (41), TGFβ (42), and p27 (35, 36); and the increase of early-expressed genes including cAMP response element-binding protein, C/EBPδ, C/EBPβ, and Differentiation-dependent Factor 1 (ADD1)/sterol regulatory element binding protein-1c, and late-expressed genes such as C/EBPα and PPARγ during adipogenesis. We found that Runx2 was down-regulated by DEX during the induction of 3T3-L1 adipocyte differentiation, and this is required for adipogenesis. DEX plays its role through the binding with its GR, which then enters the nucleus, binds to the Runx2 P2 promoter, recruits the HDAC1, deacetylates the Histone H4, and represses the transcription of Runx2. Down-regulation of Runx2 promotes the MCE and finally 3T3-L1 adipocyte differentiation (Fig. 9). Evidence shows that: 1) Runx2 is highly expressed in 3T3-L1 preadipocytes and down-regulated by DEX, one component of the standard adipocyte differentiation inducers (Figs. 1, 4, 5, and 6); 2) Runx2 inhibits adipogenesis when it is overexpressed (Fig. 3), whereas Runx2 knockdown promotes adipocyte differentiation (Fig. 2); and 3) Runx2 might act through the inhibition of MCE (Figs. 7 and 8).
Fig. 9.
Model of the role of DEX in the regulation of Runx2 and 3T3–L1 adipocyte differentiation.
Runx2's inhibitory role in adipogenesis in vivo has been indicated elsewhere. Due to the complete absence of mineralized bone tissue, Runx2-deficient mice die before adipose tissue begins to expand and fat pads can be observed (1). However, chondrocytes from the rib cartilage of Runx2-null mice spontaneously underwent adipocyte differentiation, accompanied by the loss of their chondrocyte phenotype (11); calvaria-derived cells from Runx2-null mice lost the ability to differentiate to bone cells and also spontaneously differentiated into adipocytes (43). In addition, expression of PPARγ significantly increased in type II Runx2-deficient mice compared with wide type (30).
Both Runx2 and p27 are highly expressed in growth-arrested 3T3-L1 preadipocytes and play inhibitory roles during 3T3-L1 adipocyte differentiation. Runx2 has also been reported to play a growth-suppressive function in mesenchymal bone cell progenitors, which is important in the control of osteoblast maturation by functionally supporting exit from the cell cycle and activating genes that promote bone cell phenotype formation (6, 7, 44). Previous studies (24) indicated that Runx2 suppressed the cell cycle by inducting p27, which in turn inhibited the phosphorylation activity of cyclinA/ cyclin-dependent kinase 2 to pRb (45). In addition, p27 itself appears involved in the inhibition of adipogenesis and promotion of osteogenesis (35, 38, 46). We found strong evidence that Runx2 acts together with p27 to keep 3T3-L1 preadipocytes in growth-arrested state and inhibits reentry into MCE and terminal differentiation.
The role of Runx2 is primarily due to type I and type II that play similar roles during osteogenesis (29–31). Both types are expressed in 3T3-E1 preosteoblasts and C3H10T1/2 mesenchymal stem cells, whereas only type I Runx2 is expressed in 3T3-L1 preadipocytes, which is consistent with the previous finding that type I Runx2 is mainly expressed in nonosseous mesenchymal stem cells (31). Whereas mesenchymal stem cells have the potential to develop into adipocytes, muscle cells, and chondrocytes as well as osteoblasts (47), other studies have already indicated that osteoblasts and adipocytes are more closely related in their developmental pathway (48, 49). The data showing that both preadipocytes and preosteoblasts express Runx2 strengthens this conclusion. Because preadipocytes only express type I Runx2 indicates that the loss of type II may be involved in the commitment of mesenchymal stem cells to preadipocytes instead of osteoblasts, and the mechanisms involved in the regulation of these two different Runx2 promoters may determine the fate of mesenchymal stem cells to fat or bone cells.
Acknowledgments
We thank Dr .Chad Slawson (Assistant Professor of the Department of Biochemistry and Molecular Biology at University of Kansas Medical Center, Lawrence, KS) for critical reading and Dr. Kun-Liang Guan (Professor of Pharmacology, Purdue University, Lafayette, IN) for the Runx2 plasmid.
This research was supported, I part, by National Key Basic Research Project Grants 2011CB910201 and 2009CB825604, the State Key Program of National Natural Science Foundation 31030048C120114, and Shanghai Key Science and Technology Research Project 10JC1401000 (tr Q.Q.T.); Program for New Century Excellent Talents in UniversityNCET-08–0130 and Shanghai Rising Star Program 08QA14012 (to X.L.). The Department is supported by Shanghai Leading Academic Discipline Project, Project Number:B110 and 985 Project 985III-YFX0302.
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Nuclear Receptors: GR;
Ligands: Dexamethasone.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- ALP
- Alkaline phosphatase
- C/EBPα
- CCAAT enhancer binding protein α
- C/EBPβ
- CCAAT enhancer binding protein β
- DEX
- dexamethasone
- FBS
- fetal bovine serum
- GR
- glucocorticoid receptor
- HDAC
- histone deacetylase
- MCE
- mitotic clonal expansion
- MIX
- methylisobutylxanthine
- PMSF
- phenylmethylsulfonyl fluoride
- PPARγ
- peroxisome proliferator-activated receptor γ
- RT
- room temperature
- SDS
- sodium dodecyl sulfate
- siNC
- negative control siRNA
- siRNA
- small interfering RNA.
References
- 1. Otto F , Thornell AP , Crompton T , Denzel A , Gilmour KC , Rosewell IR , Stamp GW , Beddington RS , Mundlos S , Olsen BR , Selby PB , Owen MJ. 1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89:765–771 [DOI] [PubMed] [Google Scholar]
- 2. Ducy P , Zhang R , Geoffroy V , Ridall AL , Karsenty G. 1997. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754 [DOI] [PubMed] [Google Scholar]
- 3. Ogawa E , Maruyama M , Kagoshima H , Inuzuka M , Lu J , Satake M , Shigesada K , Ito Y. 1993. PEBP2/PEA2 represents a family of transcription factors homologous to the products of the Drosophila runt gene and the human AML1 gene. Proc Natl Acad Sci USA 90:6859–6863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kania MA , Bonner AS , Duffy JB , Gergen JP. 1990. The Drosophila segmentation gene runt encodes a novel nuclear regulatory protein that is also expressed in the developing nervous system. Genes Dev 4:1701–1713 [DOI] [PubMed] [Google Scholar]
- 5. Stricker S , Fundele R , Vortkamp A , Mundlos S. 2002. Role of Runx genes in chondrocyte differentiation. Dev Biol 245:95–108 [DOI] [PubMed] [Google Scholar]
- 6. Pratap J , Galindo M , Zaidi SK , Vradii D , Bhat BM , Robinson JA , Choi JY , Komori T , Stein JL , Lian JB , Stein GS , van Wijnen AJ. 2003. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res 63:5357–5362 [PubMed] [Google Scholar]
- 7. Coffman JA. 2003. Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell Biol Int 27:315–324 [DOI] [PubMed] [Google Scholar]
- 8. Teplyuk NM , Galindo M , Teplyuk VI , Pratap J , Young DW , Lapointe D , Javed A , Stein JL , Lian JB , Stein GS , van Wijnen AJ. 2008. Runx2 regulates G protein-coupled signaling pathways to control growth of osteoblast progenitors. J Biol Chem 283:27585–27597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galindo M , Pratap J , Young DW , Hovhannisyan H , Im HJ , Choi JY , Lian JB , Stein JL , Stein GS , van Wijnen AJ. 2005. The bone-specific expression of Runx2 oscillates during the cell cycle to support a G1-related antiproliferative function in osteoblasts. J Biol Chem 280:20274–20285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Komori T. 2003. Requisite roles of Runx2 and Cbfb in skeletal development. J Bone Miner Metab 21:193–197 [DOI] [PubMed] [Google Scholar]
- 11. Enomoto H , Furuichi T , Zanma A , Yamana K , Yoshida C , Sumitani S , Yamamoto H , Enomoto-Iwamoto M , Iwamoto M , Komori T. 2004. Runx2 deficiency in chondrocytes causes adipogenic changes in vitro. J Cell Sci 117:417–425 [DOI] [PubMed] [Google Scholar]
- 12. Gori F , Thomas T , Hicok KC , Spelsberg TC , Riggs BL. 1999. Differentiation of human marrow stromal precursor cells: bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J Bone Miner Res 14:1522–1535 [DOI] [PubMed] [Google Scholar]
- 13. Jeon MJ , Kim JA , Kwon SH , Kim SW , Park KS , Park SW , Kim SY , Shin CS. 2003. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem 278:23270–23277 [DOI] [PubMed] [Google Scholar]
- 14. Muruganandan S , Roman AA , Sinal CJ. 2009. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci 66:236–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao ZS , Hinson TK , Quarles LD. 1999. Cbfa1 isoform overexpression upregulates osteocalcin gene expression in non-osteoblastic and pre-osteoblastic cells. J Cell Biochem 74:596–605 [PubMed] [Google Scholar]
- 16. Rubin CS , Hirsch A , Fung C , Rosen OM. 1978. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3–L1 cells. J Biol Chem 253:7570–7578 [PubMed] [Google Scholar]
- 17. MacDougald OA , Lane MD. 1995. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem 64:345–373 [DOI] [PubMed] [Google Scholar]
- 18. Rosen ED , Spiegelman BM. 2000. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 16:145–171 [DOI] [PubMed] [Google Scholar]
- 19. Cao Z , Umek RM , McKnight SL. 1991. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3–L1 cells. Genes Dev 5:1538–1552 [DOI] [PubMed] [Google Scholar]
- 20. Yeh WC , Cao Z , Classon M , McKnight SL. 1995. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev 9:168–181 [DOI] [PubMed] [Google Scholar]
- 21. Tang QQ , Zhang JW , Daniel Lane M. 2004. Sequential gene promoter interactions by C/EBPβ, C/EBPα, and PPARγ during adipogenesis. Biochem Biophys Res Commun 318:213–218 [DOI] [PubMed] [Google Scholar]
- 22. Rosen ED , Walkey CJ , Puigserver P , Spiegelman BM. 2000. Transcriptional regulation of adipogenesis. Genes Dev 14:1293–1307 [PubMed] [Google Scholar]
- 23. Wu Z , Bucher NL , Farmer SR. 1996. Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBP β, C/EBP δ, and glucocorticoids. Mol Cell Biol 16:4128–4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu Z , Xie Y , Bucher NL , Farmer SR. 1995. Conditional ectopic expression of C/EBP β in NIH-3T3 cells induces PPAR γ and stimulates adipogenesis. Genes Dev 9:2350–2363 [DOI] [PubMed] [Google Scholar]
- 25. Lasa M , Brook M , Saklatvala J , Clark AR. 2001. Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Mol Cell Biol 21:771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henderson BR , Kefford RF. 1993. Dexamethasone decreases urokinase plasminogen activator mRNA stability in MAT 13762 rat mammary carcinoma cells. Br J Cancer 67:99–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu ZX , Rooney SA. 1997. Glucocorticoids increase fatty-acid synthase mRNA stability in fetal rat lung. Am J Physiol 272:L860–L864 [DOI] [PubMed] [Google Scholar]
- 28. Barnes PJ. 1998. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 94:557–572 [DOI] [PubMed] [Google Scholar]
- 29. Hata K , Nishimura R , Ueda M , Ikeda F , Matsubara T , Ichida F , Hisada K , Nokubi T , Yamaguchi A , Yoneda T. 2005. A CCAAT/enhancer binding protein beta isoform, liver-enriched inhibitory protein, regulates commitment of osteoblasts and adipocytes. Mol Cell Biol 25:1971–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiao ZS , Hjelmeland AB , Quarles LD. 2004. Selective deficiency of the “bone-related” Runx2-II unexpectedly preserves osteoblast-mediated skeletogenesis. J Biol Chem 279:20307–20313 [DOI] [PubMed] [Google Scholar]
- 31. Schroeder TM , Jensen ED , Westendorf JJ. 2005. Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today 75:213–225 [DOI] [PubMed] [Google Scholar]
- 32. Surjit M , Ganti KP , Mukherji A , Ye T , Hua G , Metzger D , Li M , Chambon P. 2011. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell 145:224–241 [DOI] [PubMed] [Google Scholar]
- 33. Wiper-Bergeron N , Wu D , Pope L , Schild-Poulter C , Haché RJ. 2003. Stimulation of preadipocyte differentiation by steroid through targeting of an HDAC1 complex. EMBO J 22:2135–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang QQ , Otto TC , Lane MD. 2003. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA 100:44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel YM , Lane MD. 2000. Mitotic clonal expansion during preadipocyte differentiation: calpain-mediated turnover of p27. J Biol Chem 275:17653–17660 [DOI] [PubMed] [Google Scholar]
- 36. Auld CA , Morrison RF. 2006. Evidence for cytosolic p27(Kip1) ubliquitylation and degradation during adipocyte hyperplasia. Obesity 14:2136–2144 [DOI] [PubMed] [Google Scholar]
- 37. Lin J , Della-Fera MA , Li C , Page K , Choi YH , Hartzell DL , Baile CA. 2003. P27 knockout mice: reduced myostatin in muscle and altered adipogenesis. Biochem Biophys Res Commun 300:938–942 [DOI] [PubMed] [Google Scholar]
- 38. Thomas DM , Johnson SA , Sims NA , Trivett MK , Slavin JL , Rubin BP , Waring P , McArthur GA , Walkley CR , Holloway AJ , Diyagama D , Grim JE , Clurman BE , Bowtell DD , Lee JS , Gutierrez GM , Piscopo DM , Carty SA , Hinds PW. 2004. Terminal osteoblast differentiation, mediated by runx2 and p27(KIP1), is disrupted in osteosarcoma. J Cell Biol 167:925–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang H , Lane MD , Tang QQ. 2005. Effect of serum on the down-regulation of CHOP-10 during differentiation of 3T3–L1 preadipocytes. Biochem Biophys Res Commun 338:1185–1188 [DOI] [PubMed] [Google Scholar]
- 40. Wang Y , Kim KA , Kim JH , Sul HS. 2006. Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J Nutr 136:2953–2956 [DOI] [PubMed] [Google Scholar]
- 41. Prestwich TC , Macdougald OA. 2007. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol 19:612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Choy L , Skillington J , Derynck R. 2000. Roles of autocrine TGF-β receptor and Smad signaling in adipocyte differentiation. J Cell Biol 149:667–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kobayashi H , Gao Y , Ueta C , Yamaguchi A , Komori T. 2000. Multilineage differentiation of Cbfa1-deficient calvarial cells in vitro. Biochem Biophys Res Commun 273:630–636 [DOI] [PubMed] [Google Scholar]
- 44. Rajgopal A , Young DW , Mujeeb KA , Stein JL , Lian JB , van Wijnen AJ , Stein GS. 2007. Mitotic control of RUNX2 phosphorylation by both CDK1/cyclin B kinase and PP1/PP2A phosphatase in osteoblastic cells. J Cell Biochem 100:1509–1517 [DOI] [PubMed] [Google Scholar]
- 45. Zavitz KH , Zipursky SL. 1997. Controlling cell proliferation in differentiating tissues: genetic analysis of negative regulators of G(1)->S-phase progression. Curr Opin Cell Biol 9:773–781 [DOI] [PubMed] [Google Scholar]
- 46. Drissi H , Hushka D , Aslam F , Nguyen Q , Buffone E , Koff A , van Wijnen A , Lian JB , Stein JL , Stein GS. 1999. The cell cycle regulator p27(kip1) contributes to growth and differentiation of osteoblasts. Cancer Res 59:3705–3711 [PubMed] [Google Scholar]
- 47. Pittenger MF , Mackay AM , Beck SC , Jaiswal RK , Douglas R , Mosca JD , Moorman MA , Simonetti DW , Craig S , Marshak DR. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- 48. Thompson DL , Lum KD , Nygaard SC , Kuestner RE , Kelly KA , Gimble JM , Moore EE. 1998. The derivation and characterization of stromal cell lines from the bone marrow of p53(−/−) mice: new insights into osteoblast and adipocyte differentiation. J Bone Miner Res 13:195–204 [DOI] [PubMed] [Google Scholar]
- 49. Yoshiko Y , Oizumi K , Hasegawa T , Minamizaki T , Tanne K , Maeda N , Aubin JE. 2010. A subset of osteoblasts expressing high endogenous levels of PPAR γ switches fate to adipocytes in the rat calvaria cell culture model. PLOS ONE 5. [DOI] [PMC free article] [PubMed] [Google Scholar]