Abstract
Decidualization is an ovarian steroid-induced remodeling/differentiation process of uterus essential for embryo implantation and placentation. Here, we investigated the possible involvement of enhanced Ca2+ dynamics in the decidualization process in human endometrial stromal cells (hESC) in its connection with a recently emerging nonvoltage-gated Ca2+ entry channel superfamily, the transient receptor potential (TRP) protein. Combined application of 17β-estradiol (E2) (10 nm) and progesterone (P4) (1 μm) for 7–14 d resulted in morphological changes of hESC characteristic of decidualization (i.e. cell size increase), whereas sole application of E2 exerted little effects. A 7- to 14-d E2/P4 treatment greatly increased the expression level of decidualization markers IGF binding protein-1 (IGFBP-1) and prolactin and also up-regulated the expression of TRPC1, a canonical TRP subfamily member that has been implicated in store-operated Ca2+ influx (SOC) in other cell types. In parallel with this up-regulation, SOC activity in hESC, the nuclear translocation of phosphorylated cAMP responsive element binding protein (p-CREB) and the expression of Forkhead box protein 01 were enhanced significantly. Small interfering RNA knockdown of TRPC1 counteracted the E2/P4-induced up-regulation of IGFBP-1 and prolactin and enhancement of SOC activity together with the inhibition of hESC size increase, p-CREB nuclear translocation, and FOXO1 up-regulation. Coadministration of SOC inhibitors SK&F96365 or Gd3+ with E2/P4 also suppressed the up-regulation of IGFBP-1 and hESC size increase. Similar inhibitory effects were observed with extracellularly applied TRPC1 extracellular loop 3-directed antibody, which is known to bind a near-pore domain of TRPC1 channel and block its Ca2+ transporting activity. These results strongly suggest that up-regulation of TRPC1 protein and consequent enhancement of SOC-mediated Ca2+ influx may serve as a crucial step for the decidualization process of hESC probably via p-CREB-dependent transcriptional activity associated with FOXO1 activation.
Decidualization is the postovulatory process of endometrial remodeling in preparation for pregnancy (1). Although the adult endometrium consists of many distinct types of cells (i.e. epithelial, stromal, vascular smooth muscle, and endothelial and infiltrating blood cells), endometrial stromal cells (ESC) likely play a central role in the decidualization process as the main site expressing the receptors for ovarian hormones, 17β-estradiol (E2) and progesterone (P4). In response to costimulation by E2 and P4, ESC start to change their morphology, thereby providing an environment suitable for embryo implantation and maintenance of pregnancy. It is well known that, during the secretory phase of the menstrual cycle, ESC differentiate into decidual cells, which are characterized by large and rounded appearance, and then acquire the ability to secrete a variety of phenotypic antigens [prolactin (PRL), IGF binding protein-1 (IGFBP-1), and tissue factor] (2, 3), growth factors (4), cytokines (5), proteinases for extracellular matrices (6, 7), peptide hormones (8), and prostaglandins (7, 9, 10). These substances are essential to promote the decidualization process in autocrine and paracrine manners with concomitant extensive reprogramming of cellular functions, as demonstrated by genome-wide microarray analyses (11, 12). However, elucidation of the intracellular mechanism(s) underlying the decidualization is still underway, and available information is limited to several signaling pathways [e.g. cAMP-protein kinase A (PKA)/cAMP responsive element binding protein (CREB)/ cAMP-responsive element modulator (CREM), c-Src tyrosine kinase, signal transducer and activator of transcription (STAT), Forkhead box protein O (FOXO), Stathmin, and CCAAT/enhancer-binding protein (C/EBP-β)] (1, 13–17).
The transient receptor potential (TRP) protein is a recently emerging nonvoltage-gated Ca2+-permeable cation channel superfamily activated by a variety of physicochemical stimuli (18). The human homologues of this superfamily are classified into six families, including TRP canonical (TRPC), TRP vanilloid (TRPV), TRP melastatin (TRPM), TRP polycystin (TRPP), TRP mucolipin, and TRP ankyrin 1. Among them, TRPC are regarded as the most plausible molecular entities of Ca2+ entry channels activated upon phospholipase C-coupled receptor stimulation, i.e. those activated via generation of diacylglycerol and those activated by store depletion [store-operated Ca2+ influx (SOC)]. TRP proteins are likely involved in a broad range of biological functions, including sensory signal transduction and vascular tone regulation, as well as in some hereditary diseases as the causative genes (e.g. polycystic kidney diseases) (19). There is also some evidence linking TRP proteins to reproductive physiology and pathophysiology and to the extrareproductive actions of sex hormones, including the involvement of TRPC3 and TRPC4 as a SOC in the end-term myometrial contractility, TRPV6 and TRPP2 in placental cation transport, and TRPC6 in human breast cancer (20–24).
In this study, we explored the potential role of TRP proteins for the ovarian steroid-induced decidualization of human ESC (hESC) by evaluating their expression profiles and functions. This is because accumulating evidence suggests that many TRP isoforms contribute to both physiological and pathological tissue remodeling processes, such as angiogenesis, cancer, cardiac hypertrophy, proliferative occlusive vascular diseases, and neuronal/muscular degenerations (25–28). To elucidate the role of TRP in hESC decidualization, we employed primary cultured hESC according to the method established previously [e.g. Arnold et al. (29)]. We then examined 1) the altered expression profile of TRP proteins in hESC before and after ovarian steroid treatment by using the conventional RT-PCR, real-time PCR, and Western blot analyses; 2) resultant changes in Ca2+ dynamics therein by using the digital fluorescent imaging technique; and 3) causal relationship among increased expression of a TRP isoform(s), altered Ca2+ dynamics and its associated downstream signaling by use of RNA silencing strategy. Part of the present results has been communicated to the 36th International Congress of International Union of Physiological Sciences (IUPS2009) (30).
Results
E2/P4 treatment enhances the expression of TRPC1 and SOC activity in hESC
Acutely dissociated hESC showed a spindle-shaped fibroblast-like appearance with expression of α-smooth muscle actin and vimentin and, upon combined application of E2 (10 nm) and P4 (1 μm) for 7–14 d, became enlarged and rounded, showing a characteristic appearance of decidual cells (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org).
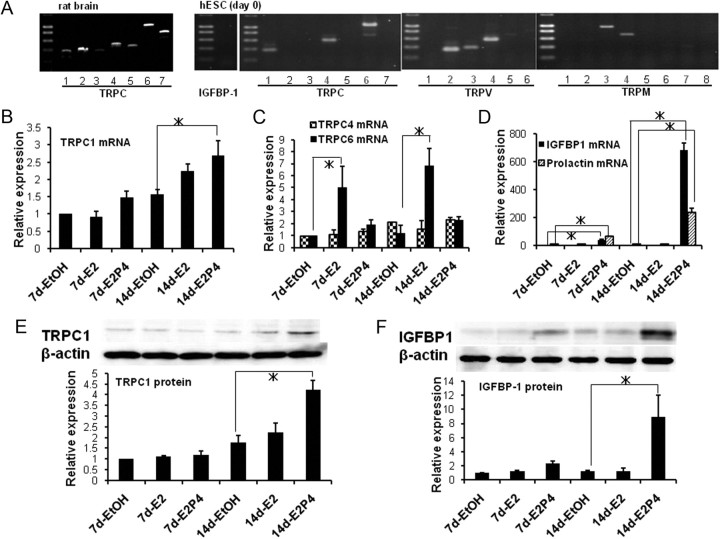
We next examined how E2/P4 treatment affects the expression of TRP isoforms by using the RT-PCR technique. As displayed in Fig. 1A, the mRNA of nine TRP isoforms, namely, TRPC1, TRPC4, TRPC6, TRPV2, TRPV3, TRPV4, TRPM3, TRPM4, and TRPM7, could be detected in freshly dissociated hESC by conventional RT-PCR. When these TRP isoforms were subjected to quantitative real-time PCR analysis, E2/P4 treatment for 14 d significantly enhanced the mRNA level of TRPC1, whereas that of TRPC6 was up-regulated solely by E2 treatment (Fig. 1, B and C). Importantly, the expression levels of decidualization markers PRL and IGFBP-1, which were marginally detected under unstimulated conditions by conventional RT-PCR and real-time PCR techniques (Fig. 1, A and D), were greatly enhanced only when E2 and P4 were administered simultaneously (Fig. 1F, rightmost columns). The extents of the mRNA up-regulation of TRPC1 and decidualization markers (PRL and IGFBP-1) were about 3-fold and more than 200-fold, respectively, as compared with unstimulated conditions (Fig. 1, B and D). Consistent with these results of real-time PCR, the expression of TRPC1 and IGFBP-1 proteins evaluated by immunoblotting was also increased time dependently in response to E2/P4 treatment. However, the effect of E2 alone was only marginal even after a 14-d treatment (Fig. 1, E and F).
Fig. 1.
Enhanced expression of TRPC1 and IGFBP-1 in response to E2 and P4. A, mRNA expression pattern of TRPC isoforms in rat brain (left) and those of TRP isoforms and IGFBP-1 in hESC evaluated by conventional RT-PCR immediately after enzymatic dissociation (d 0; right). For the positive control of TRPM and TRPV isoforms, refer to Inoue et al. (56). B–D, Results of quantitative real-time PCR analysis for the mRNA levels of TRPC1 (B), TRPC4 and TRPC6 (C), and IGFBP-1 and PRL (D) after a 7- or 14-d treatment with EtOH (vehicle), E2 (10 nm), or E2 (10 nm) plus P4 (1 μm). Columns and bars indicate the mean ± sem obtained from three to four individual experiments for each condition. To reduce variability among different preparations, data are shown as the relative to those for EtOH at 7 d after normalization to the level of β-actin. E and F, Representative of immunoblot analysis (upper panel) and its summary (lower panel) on the expression of TRPC1 (E) and IGFBP-1 (F) proteins after a 7- or 14-d treatment with EtOH, E2 only or E2 plus P4. Columns and bars indicate the mean ± sem obtained from four individual experiments for each condition. *, P < 0.05 with Tukey's multiple comparison test.
It has been reported that stimulation of cAMP-dependent pathway also facilitates the decidualization (1). We therefore tested the effects of a membrane-permeable cAMP analog 8-bromo cAMP (8-bcAMP) on the expression of IGFBP-1 and TRPC1. 8-bcAMP itself induced the enlargement of hESC within 3 d at its extremely high concentration of 500 μm (data not shown). This effect was however only slight at 50 μm, which is also biologically supramaximal (note that stimulated cAMP level in living cells is known to be no more than several micromolar; see e.g. Ref. 31) (Supplemental Fig. 2A). However, when the same concentration of 8-bcAMP (50 μm) was applied together with E2/P4, a moderate enhancement compared with E2/P4 alone was observed in the expression level of IGFBP-1 and TRPC1 proteins (Supplemental Fig. 2B). These results suggest that there may be some cooperativity in inducing decidualization between the cAMP-dependent and ovarian steroid-dependent pathways, in which up-regulation of TRPC1 might also play a nontrivial role.
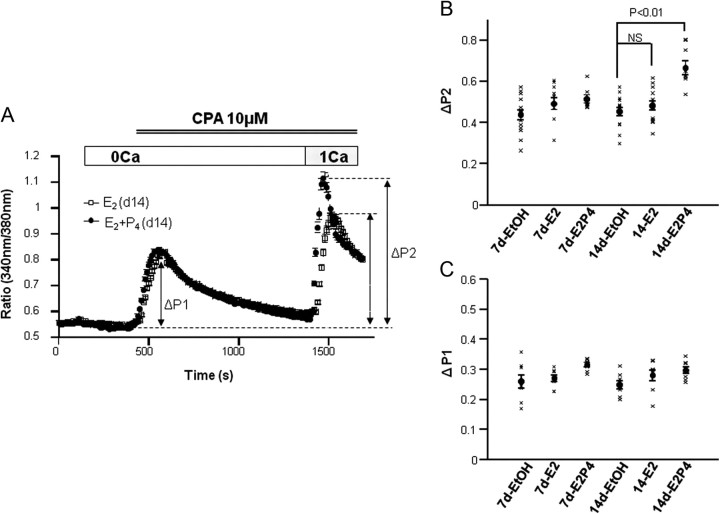
TRPC1 has been regarded as one of plausible SOC candidates in various cell types (32). Thus, it could be speculated that increased TRPC1 expression by E2/P4 treatment may cause the enhancement of Ca2+ influx into hESC somehow promoting the process of decidualization. In fact, SOC-mediated Ca2+ influx was significantly augmented by a 14-d E2/P4 treatment as compared with vehicle [ethanol (EtOH)] or E2 alone (Fig. 2A); the maximum magnitude of the influx (ΔP2) was increased by 30% on average (Fig. 2B, rightmost data). In contrast, there was no significant increase in the influx by the sole treatment with E2 (Fig. 2B, fifth vs. fourth data from the left), and the magnitude of Ca2+ release by store depletion (ΔP1) was little affected by either E2 or E2/P4 treatment (Fig. 2C).
Fig. 2.
Augmentation of SOC by E2 and P4. A, Typical Ca2+ responses of hESC evoked by store depletion after a 14-d treatment with E2 alone or E2 plus P4. hESC were first perfused with Ca2+-free external solution and stimulated by the endoplasmic reticulum Ca2+-ATPase inhibitor cyclopiazonic acid (CPA) (10 μm) to deplete internal stores and then subjected to 1 mm Ca2+ to evoke SOC-mediated influx. In contrast, excess K+ (75 mm) failed to increase [Ca2+]i, thus suggesting the lack of voltage-dependent Ca2+ channels in hESC (data not shown). B and C, Summary of the results such as shown in A for SOC-mediated Ca2+ influx (ΔP2; B) and Ca2+ release (ΔP1; C) after a 7- or 14-d treatment with EtOH, E2 only, or E2 plus P4. All data points from 8–14 independent experiments are displayed as black crosses, each of which represents the average from at least 100 cells, respectively. Filled circles and vertical bars indicate the mean ± sem calculated from data points. P < 0.01, evaluated by Tukey's multiple comparison test. NS, Statistically not significant.
These results raise the possibility that increased Ca2+ influx due to TRPC1 up-regulation may be causally related to the decidualization of hESC.
Knockdown of TRPC1 expression suppresses SOC and prevents the up-regulation of IGFBP-1 and PRL
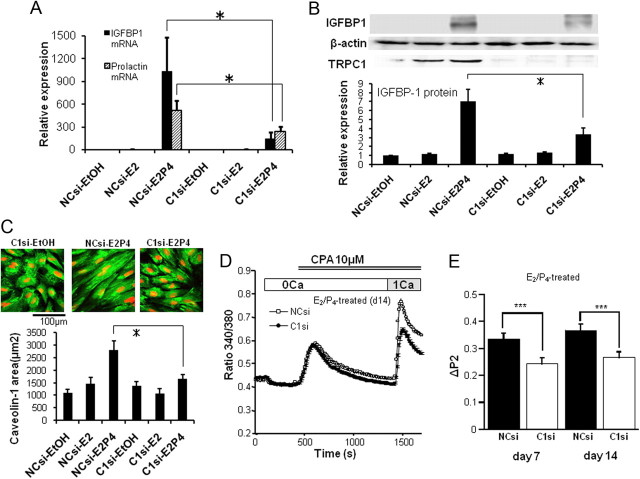
To more directly test the involvement of TRPC1 (and associated Ca2+ influx) in the decidualization process, we next employed the small interfering RNA (siRNA) strategy to knockdown TRPC1 expression and observed its consequences with immunoblotting, cell size measurement, and Ca2+ imaging technique. As demonstrated and summarized in Fig. 3, after significant reduction in TRPC1 protein expression by siRNA silencing, the up-regulation of IGFBP-1 mRNA and protein as well as that of PRL mRNA (Fig. 3, A and B), hESC size increase (Fig. 3C) and concomitant enhancement of SOC-mediated Ca2+ influx (Fig. 3, D and E) by E2/P4 treatment are all significantly reduced.
Fig. 3.
RNA silencing of TRPC1 inhibits the up-regulation of IGFBP-1 by E2/P4 treatment. A, Results of real-time PCR analysis for the mRNA levels of IGFBP-1 and PRL after a 14-d treatment with negative control (NCsi) or TRPC1-siRNA (C1si) under continued stimulation with EtOH, E2 only or E2 plus P4. B, Immunoblots of TRPC1 and IGFBP-1 proteins under the same conditions as in A. Representative immunoblots (upper) and relative expression of IGFBP-1 protein to β-actin protein (lower panel). C, Averaged 2D-area of caveolin-1-immunostained hESC (green) after a 14-d treatment with NCsi or C1si, with 4′,6-diamino-2-phenylindole nuclei staining (red). Columns and bars indicate the mean ± sem calculated from 20 cells. D, Typical Ca2+ response of hESC after TRPC1 knockdown under E2/P4 treatment for 14 d. The procedures employed were the same as in A–C. E, Summary of the effects of TRPC1 knockdown (C1si) on SOC-mediated Ca2+ influx (ΔP2) in comparison with negative control (NCsi) under E2/P4 stimulation for 7 or 14 d. Columns and bars in A, B, and E indicate the means ± sem obtained from five, four to five, and 9–15 individual experiments, respectively (in E, data are the average from at least 100 cells). * in A–C, P < 0.05 with Tukey's multiple comparison test; ***, P < 0.0005 with unpaired t test. CPA, Cyclopiazonic acid.
Inhibition of TRPC1-assoiated SOC also attenuates IGFPB-1 up-regulation
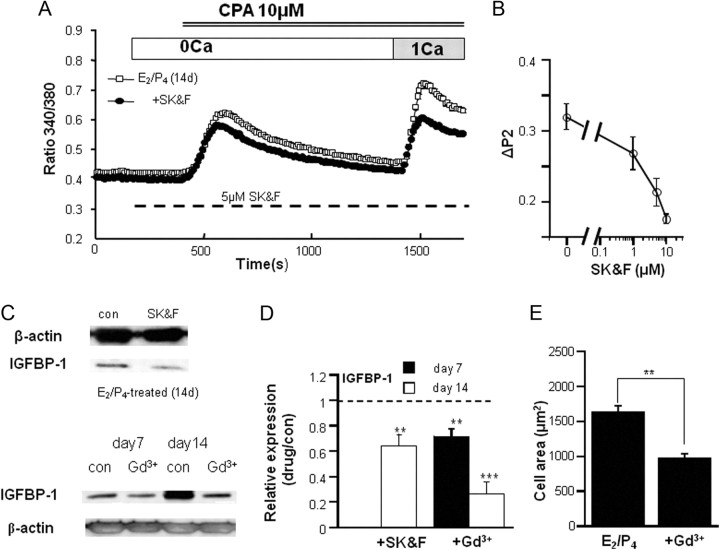
The results of the siRNA experiments support the idea that increased expression of TRPC1 underlies the up-regulation of IGFBP-1 as well as enhanced SOC activity. However, it is still uncertain whether the TRPC1-associated Ca2+-influx itself is responsible for the acceleration of decidualization process. To investigate this point further, we next tested the effects of a nonspecific SOC inhibitor SK&F96365 (33). SK&F96365 dose dependently reduced the SOC-mediated Ca2+ influx in hESC (Fig. 4, A and B) and, at its concentration effectively inhibiting SOC-mediated Ca2+ influx (5 μm) (Fig. 4B), significantly counteracted the up-regulation of IGFBP-1 by E2/P4 treatment (Fig. 4, C and D). A similar inhibition of the E2/P4-induced IGFBP-1 up-regulation and hESC size increase was observed with another SOC inhibitor Gd3+ (1 μm) (Fig. 4, C–E) (33).
Fig. 4.
Suppression of SOC-mediated Ca2+ influx attenuates the up-regulation of IGFBP-1 by E2/P4 treatment. A and B, Typical Ca2+ response to a SOC inhibitor SK&F96365 of hESC from donor 5 (A) and its concentration-dependent inhibitory effects (B) (obtained from 3–11 individual experiments; data are the average from at least 100 cells). The experimental conditions used were the same as in Fig. 2A except that the drug was applied at the dashed bar. C, Representative immunoblots of IGFBP-1 protein after a 7- and/or 14-d E2/P4 treatment in the absence and presence of 5 μm SK&F96365 (SK&F) or 1 μm Gd3+. Because higher concentrations of SK&F caused progressive death of hESC, its moderate concentration which inhibited SOC-mediated Ca2+ influx partially (5 μm) was chosen; 1 μm was enough high for Gd3+ to inhibit the Ca2+ influx (data not shown). D, Compromised IGFBP-1 protein expression in the presence of SOC inhibitors (either 5 μm SK&F or 1 μm Gd3+) under 7- or 14-d E2/P4 treatment (obtained from four and five individual experiments, respectively). The relative expression is defined as the ratio of normalized IGFBP-1 protein level by β-actin in the presence (drug) vs. absence (con) of drugs. Data were obtained as a paired set (drug vs. no drug) from the same donor. **, P < 0.01; ***, P < 0.005 with paired t test. E, Averaged 2D-area of hESC after a 14-d E2/P4 treatment in the absence and presence of 1 μm Gd3+ calculated from 40 cells for each drug. **, P < 0.01 with unpaired t test. CPA, Cyclopiazonic acid.
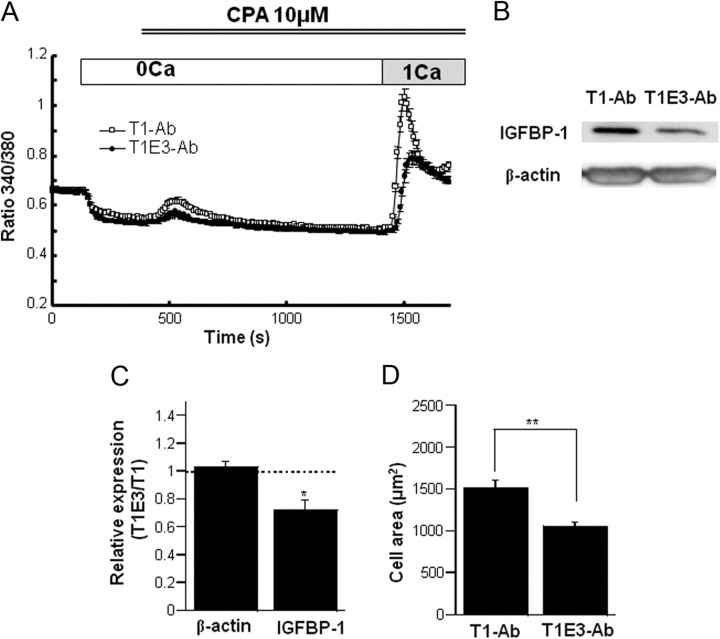
In the next series of experiments, we tested the effects of T1E3-Ab, which is known to selectively inhibit TRPC1 extracellular loop 3-directed antibody (TRPC1)-associated Ca2+ influx from the external side of the cell membrane (34). A 24-h incubation of hESC with 1:200 diluted T1E3-Ab significantly reduced the SOC-mediated Ca2+ entry by approximately 50%, as compared with control antibody, which only recognizes the intracellular domain of TRPC1 protein (T1-Ab) (Fig. 5A). Strikingly, T1E3-Ab at the same dilution significantly suppressed the E2/P4-induced up-regulation of IGFBP-1 protein as compared with T1-Ab (by ∼30% on average) (Fig. 5, B and C) and prevented the enlargement of hESC (Fig. 5D).
Fig. 5.
Specific inhibition of TRPC1-associated SOC prevents hESC decidualization. A, Externally applied T1E3-Ab inhibited the Ca2+ response of hESC induced by store depletion, as compared with another ineffective antibody (T1-Ab), which specifically recognizes the intracellular domain of TRPC1 protein; 100 cells were used for each condition from donor 6. B, T1E3-Ab inhibited the up-regulation of IGFBP-1 protein by E2/P4 treatment (14 d). Representative data. C, Summary of the inhibitory effect of T1E3-Ab on IGFBP-1 expression. Paired data (T1E3-Ab vs. T1-Ab) were obtained for β-actin or IGFBP-1 in hESC under 14-d E2/P4 stimulation. Relative expression is defined as the ratio of data with T1E3-Ab vs. T1-Ab for each donor. *, P < 0.05 with paired t test. D, Averaged 2D-area of hESC evaluated from phase-contrast images after a 14-d E2/P4 treatment in the presence of T1-Ab or T1E3-Ab. Evaluated from 40 cells for each condition. **, P < 0.01 with unpaired t test. CPA, Cyclopiazonic acid.
These results strongly suggest that Ca2+ influx mediated via TRPC1-associated Ca2+ entry pathway contributes to the up-regulation of IGFBP-1 and the decidualization of hESC.
CREB transcriptional pathway may be involved in TRPC1/SOC-mediated Ca2+ signaling
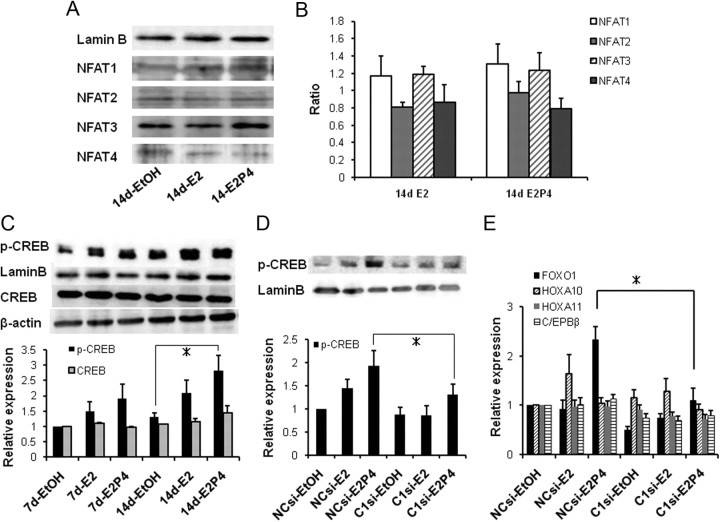
Finally, to gain more insight into the downstream signaling involved in TRPC1/SOC-mediated Ca2+ influx in hESC, we tested two representative Ca2+ transcriptional pathways, nuclear factor of activated T-cells (NFAT) and CREB. As summarized in Fig. 6, A and B, for four different NFAT isoforms, 14-d treatment of E2 alone, or combination of E2 and P4 did not significantly affect their nuclear translocation. In sharp contrast with this, 14-d treatment with E2/P4 produced a clear increase in the phosphorylated form of CREB (p-CREB) and its nuclear translocation (Fig. 6C). siRNA knockdown of TRPC1 expression significantly counteracted these changes (Fig. 6D). These results support the idea that increased Ca2+ influx through up-regulated TRPC1/SOC pathway may facilitate the phosphorylation of CREB and its consequent nuclear translocation.
Fig. 6.
A 14-d E2/P4 treatment up-regulates the nuclear expression level of p-CREB but not of NFAT. A, Representative immunoblots for NFAT extracted from hESC treated with EtOH, E2, or E2P4 for 14 d. To confirm the equal loading and specificity of samples, blots were reprobed for LaminB. B, Histograms show the levels of NFAT proteins relative to a nuclear marker LaminB. Among four isoforms tested, the expression of NFAT3 was most prominent. C, Representative immunoblots for p-CREB (nuclear fractions), CREB (total cell lysate) from EtOH, E2, or E2P4-stimulated hESC. To confirm the equal loading and specificity of samples, blots were reprobed for LaminB or β-actin, respectively. Histograms show the levels of p-CREB and CREB proteins relative to LaminB and β-actin, respectively. D, Nuclear p-CREB expression in hESC treated with TRPC1-siRNA just before E2/P4 treatment (14 d). E, Nuclear expression of transcription factors FOXO1, HOXA10, HOXA11, and C/EBP-β in hESC treated with TRPC1-siRNA and E2/P4 (14 d). Evaluated by quantitative real-time PCR. Columns and bars in B–E indicate the means ± sem obtained from three to four, four to six, five, and five individual experiments, respectively. * in C–E, P < 0.05 with Tukey's multiple comparison test.
It has been reported that p-CREB interacts with its binding protein CREB-binding protein (CBP) in the nucleus, thereby initiating many transcriptional activities including FOXO1 (35, 36). We therefore tested whether the expression of several transcription factors involved in the decidualization of hESC (1) can be affected by E2/P4 treatment in our experimental system by real-time PCR. As shown in Fig. 6E, 14-d treatment with E2/P4 most remarkably up-regulated the expression of FOXO1, which was almost completely abrogated by the siRNA knockdown of TRPC1 expression. This implies that TRPC1/SOC-mediated Ca2+ influx may be linked with the p-CREB-FOXO1-mediated transcriptional signaling.
Discussion
The present results have provided, to our knowledge, the first evidence that enhanced Ca2+ dynamics under ovarian steroid stimulation may contribute to the decidualization process of human stromal cell in vitro. Importantly, this process appears mediated by enhanced expression of TRPC1 protein, one of the most plausible candidates for SOC in many cell types (32, 33). The rationale behind this conclusion is recapitulated as follows: 1) E2/P4-induced decidualization was paralleled by up-regulation of TRPC1 and increased SOC activity, 2) siRNA knockdown of TRPC1 not only reduced SOC activity but also inhibited the up-regulation of decidualization markers IGFBP-1 and PRL in hESC, 3) in addition to nonspecific SOC blockers (SK&F96365 and Gd3+), the functionally effective TRPC1-specific antibody (T1E3) inhibited the SOC-mediated Ca2+ influx and simultaneously attenuated the E2/P4-induced IGFBP-1 up-regulation and decidualization of hESC. In aggregate, these results strongly suggest that up-regulation of SOC-mediated Ca2+ influx via TRPC1-associated pathway is a prerequisite for the decidual transformation of hESC in response to E2/P4. Furthermore, additional lines of evidence have suggested that this Ca2+-dependent signaling may involve Ca2+-dependent phosphorylation of CREB as a downstream event, which would then facilitate the transcription of genes responsible for hESC decidualization presumably in part via activation of a FOXO1-mediated pathway.
Recent evidence suggests that several TRP isoforms regulate the proliferative potential of a variety of cells, and disruption of these TRP genes or protein expression may lead to cancers, cardiac hypertrophy, muscle, and neuronal degenerations, and proliferative occlusive vascular diseases (25, 26, 37). Some TRP isoforms may also participate in the phenotypic changes of cells from differentiated to proliferative types (25, 38). In each case, Ca2+ influx through activated TRP channels appears to serve as a crucial step of triggering downstream Ca2+-dependent events that switch on transcriptional activities or death-programming processes. For example, in cardiac myocytes, the excessive activation of TRPC1, TRPC3, and TRPC6 channels has been shown to produce hypertrophic responses via activation of a Ca2+-dependent transcription factor, NFAT (26, 39, 40). In a rat vascular injury model and human vein grafts, increased expression of TRPC1 and consequent enhancement of SOC activity have been shown to stimulate the neointimal vascular smooth muscle cell hyperplasia (25, 41). The SOC activity mediated by TRPC1 has also been implicated in Ca2+-induced differentiation of human keracinocytes (42). There is also evidence that altered transmembrane Ca2+ influx via TRP channels may induce tumorigenicity (TRPV6 and TRPC6) (28) or trigger necrotic/apoptotic processes causing skeletal and cardiac muscle degeneration (TRPC1 and TRPV2) (37, 43) and neuronal death (TRPM2 and TRPM7) (44). It is thus reasonable to assume that some steps of E2/P4-induced decidualization of hESC may also be Ca2+ dependent.
Consultation with the literature suggests that Ca2+ may play nontrivial roles in the endometrial decidualization. In an artificial deciduogenic model of mice, the decidualization was shown to be facilitated by intrauterine administration of Ca2+, which was significantly inhibited by various Ca2+ channel blockers and inhibitors for Ca2+-ATPase and Ca2+/calmodulin (45). In the rat uterine, angiotensin II, which is essential for the maximal decidual response, was reported to increase the [Ca2+]i in ESC. Moreover, the effects of angiotensin II were mimicked by a Ca2+ ionophore A23187 when coadministered with prostaglandin E2 (PGE2), and interestingly, the production of PGE2 was also enhanced by angiotensin II (46).
It is known that in vitro decidualizing effects of ovarian steroids are so weak as to require 8–10 d to differentiate cultured ESC into a decidual phenotype (1). However, costimulation of cAMP-producing pathways, e.g. by PGE2, relaxin, corticotrophin-releasing hormone, or pituitary gonadotropins, greatly accelerates the decidualization initiated by ovarian steroids (1). Our present results confirmed that such a cross talk is present between ovarian steroid- and cAMP-dependent pathways (Supplemental Fig. 2). Further, it has been reported that E2 itself can stimulate PGE2 secretion via cyclooxgenase 2 (COX-2) up-regulation to promote cAMP production in the endometrium (47). The mechanisms for the decidualizing actions of cAMP likely involve multileveled regulations. In addition to the activation of nuclear transcriptional factors CREB/CREM-CBP/p300, altered recruitment of coactivators/corepressors for the P4 receptor (PR), induction of expression/activation of the transcription factors interacting with PR (specificity protein 1, FOXO1, STAT-3 or STAT-5, and C/EBP-β), and posttranslational modification of PR, e.g. by sumoylation, have been suggested (1).
It is presently unclear in which steps of the decidualization Ca2+ would be involved. However, our present results most likely support a pivotal role of CREB therein, because the CREB/CREM signaling has been implicated in endometrial decidualization (1), and our results show that the phosphorylation of CREB and its nuclear translocation in hESC upon E2/P4 treatment is significantly counteracted by the siRNA knockdown of TRPC1/SOC activity with paralleled inhibition of decidualization. The mechanism(s) whereby to initiate the TRPC1/SOC-dependent decidualization is unclear, but there are a few interesting possibilities accounting for the Ca2+-dependent activation of CREB-mediated transcriptional signaling cascades. It has been reported that a PKA-mediated phosphorylation site on CREB protein (Ser133) can also be phosphorylated by the nuclear calmodulin-dependent kinase (CaMK) IV in response to [Ca2+]i elevation (48). Further, in cultured vascular smooth muscle cells, Ca2+ influx associated with TRPC1 and STIM1 was found to cause CREB phosphorylation, thereby promoting cell growth (49). Although the involvement of CaMK remains to be determined, these facts collectively suggest that increased Ca2+ influx through the TRPC1-SOC pathway may contribute to the phosphorylation of CREB via Ca2+-dependent activation of CaMK and subsequent decidualization in hESC. It is well known that p-CREB specifically binds a nuclear transcriptional coactivator CBP/p300 (35, 48). This in turn facilitates the formation of a transcription initiation complex at the promoter sites with other signal-responsive transcription factors, including FOXO1 (36), to enhance transcriptional activities (48). If this would occur for hESC, the signaling pathway associated with TRPC1/SOC-dependent decidualization may involve FOXO1-mediated gene transcription via Ca2+-dependent phosphorylation of CREB and its binding to CBP. Indeed, in line with this possibility, the present results indicated that the expression of FOXO1 was greatly up-regulated by E2/P4 treatment, which was critically dependent on concomitant enhancement of TRPC1 expression.
Another potential connection of Ca2+ to CREB-mediated signaling is the Ca2+-dependent activation of COX-2-PGE2-cAMP-PKA signaling pathway (49–51). A number of previous studies reported that expression of COX-2 is controlled in a Ca2+-dependent manner (50–53). Moreover, we have found that 7- to 14-d E2/P4 treatment up-regulates the COX-2 expression in hESC, which is counteracted by siRNA knockdown of TRPC1 with accompanying decrease in SOC-mediated Ca2+ influx (Kawarabayashi, Y., and R. Inoue, unpublished data). Thus, if this TRPC1-dependent COX-2 up-regulation would be mediated by the elevation of intracellular Ca2+, the Ca2+ influx through TRPC1/SOC channel could activate the CREB-mediated gene transcription (presumably via CBP/FOXO1) in both direct (via Ca2+) and indirect (via cAMP) fashions, thereby synergistically accelerating the decidualization process of hESC. Such cross talk between Ca2+ and cAMP may rather be a common mechanism to regulate a variety of cellular functions (54). Obviously, further studies will be needed to corroborate these possibilities.
Contribution of TRP proteins and associated Ca2+ mobilization does not seem restricted to the regulation of endometrial function. Recent data have shown that mechanical stretch increases the expression of TRPC3 and TRPC4 to enhance basal and SOC-mediated Ca2+ influxes in myometrial smooth muscle cells. This may have particular physiological significance in the modulation of uterine growth during pregnancy and contractility near term (20). For another instance, it has been shown that stimulation of myometrial smooth muscle cells with IL-1β, which initiates preterm labor, can induce [Ca2+]i oscillations and increase SOC-mediated Ca2+ influx that presumably reflects the up-regulation of TRPC1 (21). Other TRP isoforms, such as TRPV6 and TRPP2, have been implicated in placental cation transports (22, 23). Thus, the roles of TRP channels in reproductive physiology and pathophysiology are now rapidly emerging. It will be of great importance in future to elucidate how Ca2+ influx through these TRP isoforms can produce functional alterations/modifications of the uterus, particularly with respect to associated intracellular signaling pathways.
In summary, the present results have presented unequivocal evidence that application of ovarian hormones (E2/P4) can induce the decidual transformation of cultured hESC where up-regulation of TRPC1 and consequent enhancement of SOC-mediated Ca2+ influx play a crucial role probably via p-CREB-mediated transcription, to which the activation of FOXO1 might contribute. This novel finding may provide a new therapeutic strategy for proliferative endometrial disorders, such as endometriosis and endometrial cancers.
Materials and Methods
Tissue sampling and bioethical considerations
Human endometrial tissues were obtained by curettage under sterile conditions from nine female donors in the proliferative phase (5–7 d after the last menstruation) and one in the secretory phase of menstrual cycle. Except for one donor who took hysteroscopy for endometrial polyps, all others underwent hysterectomy for uterine fibroids. The range and mean of age of the ten donors were 36–45 and 42 yr old, respectively, all of which, except for one, had one to three labors in the past. All donors had regular menstrual cycles (28–30 wk), none of whom had received hormonal treatment. Immediately after collection, the obtained endometrial tissues were processed for primary cell culture.
Before the present study, its detailed proposal was submitted to the institutional bioethics review board of Fukuoka University and approved. Signed informed consent for the use of the endometrium was obtained from each donor. Any experimental procedures employed in the present study conform to the regulations of Fukuoka University for Human Rights Protection.
Dissociation and culture of hESC
Dissociation of hESC was performed according to the protocol modified from Arnold et al. (29). Briefly, the obtained endometrial tissues were minced into small pieces and incubated in DMEM/F-12 containing 0.2% type I collagenase (Worthington Biochemical, Lakewood, NJ) for 60 min at 37 C. Single cells were dispersed mechanically by triturating the digested pieces with a large-bored Pasteur pipette. The resultant single cells were separated by successive filtrations through a 75-μm (FALCON) and 40-μm nylon cell strainer (BD Falcon, San Diego, CA). hESC in the filtrate were collected and plated in a 92-mm culture dish coated with collagen type I, which was kept at 37 C in humidified 5% CO2/95%O2 air environment. hESC grew fast with little discernible morphological changes as reported previously (55). The purity of hESC in culture was more than 98%, as judged by positive immunostaining for vimentin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), the intermediate filament protein, and a marker for cells of the mesodermal origin. For each experiment, hESC obtained from a single donor, rather than a mixture of those derived from multiple donors, were used.
In vitro decidualization and siRNA procedure
After grown to about 80% confluence in a six-well culture plate filled with DMEM/F-12 supplemented with 10% fetal bovine serum (FBS) plus antibiotics (penicillin, streptomycin), hESC were rinsed and treated with 2.0% charcoal-stripped FBS in the presence of 10 nm E2 and/or 1 μm P4. Culture media were changed freshly every 3 or 4 d. After 7 or 14 d with E2/P4 treatment, hESC transformed to the decidual phenotype, which were used for subsequent experiments.
When necessary, in addition to E2/P4 treatment, hESC were simultaneously transfected with stealth TRPC1 siRNA or treated with a 1:200 diluted anti-TRPC1 antibody raised against the 20 amino acids 'CVGIFCEQQSNDTFHSFIGT' in the putative third extracellular loop of TRPC1 channel (T1E3-Ab) (34) or control antibody targeting the intracellular domain of TRPC1 protein 'QLYDKGYTSKEQKDC' (T1-Ab). Stealth siRNA for TRPC1 (HSS110982, AUAUCAAGACGAAACCUGGAAUGCCGGCAUUCCAGGUUUCGUCUUGAUAU; Invitrogen, Carlsbad, CA) was transfected to hESC with the aid of Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions just before E2/P4 treatment (7 or 14 d). Six hours after the transfection, the culture medium was replaced by a new one free of stealth siRNA. To avoid the reduced efficacy, siRNA was retransfected with the same protocol 7 d after the first transfection. The stealth RNA interference negative control duplexes supplied by the manufacturer (Invitrogen), which are designed to minimize sequence homology to any vertebrate transcript and contain medium GC contents, were used as negative controls of siRNA experiments.
RT-PCR and quantitative real-time PCR
Total RNA extracted from hESC (∼106 cells) or whole rat brain by using the RNeasy RNA extraction kit (QIAGEN, Munich, Germany) were subjected to conventional RT-PCR using the following protocol: preheating at 94 C for 1 min followed by 35 cycles (denaturation at 94 C for 10 sec, annealing at 57 to 65 C for 30 sec, and extension at 72 C for 1 min) and final extension at 72 C for 10 min. PCR amplicons were electrophoresed and visualized by ethidium bromide.
Primers pairs used for RT-PCR (forward/reverse, from 5′ to 3′) were:
TRPC1 GCG TAG ATG TGC TTG GGA GAA A/GCT CTC AGA ATT GGA TCC TCC TCT;
TRPC2 CCA GGT GGT CCT CTG CGG AA/CAT CCT CAC TGG CCA GCG AGA;
TRPC3 GCT GGCC CAA GCT GGC CAA/GAA CAC AAG CAG ACC CAG GAA GA;
TRPC4 CCT CTC AGC ACA TCG ACA GGT/CCA AAT ATT GAC CAA AAC AGG GA;
TRPC5 CAA GCT TCT AAC CTG CAT GAC CA/CCT AAG TGG GAG TTG GCT GTG AA;
TRPC6 CAT CCC AGT GGT GCG GAA GA/GCC TTC AAA TCT GTC AGC TGC A;
TRPC7 GAG GAG GAG CGC TTC CTG GAC T/GGC TCA GAC TTG GAC GGT GGT;
TRPM1 GGG GAT GCC TTG AAA GAC CA/GCC AAG CTC AGC TGA TCT GGA;
TRPM2 CTT CCG GGA AGG CAA GGA TGG T/GAG GCT CAC TCC CTG CAC GTT;
TRPM3 GAG GAG ACC ATG TCC CCA ACT T/GAG TAG CTG TTG GCG CGC T;
TRPM4 GTC ATC GTG AGC AAG ATG ATG AA/GTC CAC CTT CTG GGA CGT GC;
TRPM5 CAA GTG TGA CAT GGT GGC CAT CTT/GCT CAG GTG GCT GAG CAG GAT;
TRPM6 GAG GAG ATG GAT GGG GGC CT/GGT CCA GTG AGA GAA AGC CAA CAT;
TRPM7 CCA TAC CAT ATT CTC CAA GGT TCC/CAT TCC TCT TCA GAT CTG GAA GTT;
TRPM8 GAA GGC ACC CAG ATC AAC CAA A /GAG CCT TCC ACC ACC ACA CA;
TRPV1 GAA GAT CGG GGT CTT GGC CTA/CTC ACT GTA GCT GTC CAC AAA CAA A;
TRPV2 GAC GTG CCT GAT GAA GGC TGT/CTG GTG TGG GTT CTC CAG GA;
TRPV3 AGT GGC AAC TGG GAG CTG G/GGG TCA GGG TGA TGT TGT AGA AGA;
TRPV4 GTG CCT GGG CCC AAG AAA/GGG CAG CTC CCC AAA GTA GAA;
TRPV5 CTC ACC CCC TTC AAG CTG GCT/CCC AGC ATC TGG AAT CCT CG;
TRPV6 GCC GAG ATG AGC AGA ACC TGC T/GTC TGG TCC AGG ATC TGG CGA;
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ATC ACC ATC TTC CAG GAG CGA G/TGG CAT GGA CTG TGG TCA TG.
Positive controls for respective TRPC isoforms are shown in Fig. 1A, and those for TRPM and TRPV isoforms have been shown in Inoue et al. (56).
Gene accession numbers for the targeted genes are: NM_003304 for TRPC1, NM_001109897 for TRPC2, NM_001130698 for TRPC3, NM_001135958 for TRPC4, NM_012471 for TRPC5, NM_004621 for TRPC6, NM_020389 for TRPC7, NM_145068 for TRPV1, NM_016113 for TRPV2, NM_145068 for TRPV3, NM_001177433 for TRPV4, NM_019841 for TRPV5, NM_018646 for TRPV6, NM_002420 for TRPM1, NM_003307 for TRPM2, NM_020952 for TRPM3, AF497623 for TRPM4, NM_014555 for TRPM5, NM_017662 for TRPM6, NM_017672for TRPM7, NM_024080 for TRPM8, NM_000596 for IGFBP-1, NM_000948 for PRL, and NM_002046 for GAPDH.
To quantify the mRNA expression level, quantitative real-time PCR was performed by using the 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) according to the following protocol: initial denaturation of 20 sec at 95 C followed by 40 cycles consisting of denaturation of 3 sec at 95 C and annealing/extension of 30 sec at 62 C, in a 20-μl PCR reaction solution (TaqMan Fats Universal Master Mix) with a set of primers [TaqMan Gene Expression Assays; assay ID, Hs00608195_m1, Hs00211805_m1, Hs00989190_m1, Hs00426285_m1, and Hs00168730_m1 for TRPC1, TRPC4, TRPC6, IGFBP-1, and PRL, respectively]. Human 18S rRNA was used as an internal control. For quantitative evaluation of the mRNA of transcription factors [FOXO1, homeobox protein (HOX)A10, HOXA11, and C/EPBβ], a 20-μl Power SYBR Green PCR Master Mix (Applied Biosystems) was used with primer sets listed below. The amplification protocol used was: initial denaturation of 10 min at 95 C, 40 cycles consisting of 5-sec denaturation at 95 C and 1-min annealing/extension at 60 C. Relative gene expression was calculated and normalized to the expression level of the internal standard gene GAPDH. Specificity of achieved reaction products was assessed on 2% agarose gel and second derivative of melting curve for the PCR reaction.
Human C/EBP-β (product size, 188 bp),
(sense, S) CAC AGC GAC GAC TGC AAG ATC C/(antisense, AS) CTT GAA CAA GTT CCG CAG GGT G;
human FOXO1 (product size, 178 bp),
(S) GGG CCC TAA TTC GGT CAT GT/(AS) TTG GGT CAG GCG GTT CAT AC;
human HOXA10 (product size, 146 bp),
(S) CTG ACT GGG CTG GGT TTG/(AS) ACC TCA GGC CAG ACA CCT C;
human HOXA11 (product size, 136 bp),
(S) CTC AGT GTC TGG CTG CAG AG/(AS) GCT TCC AAG CTC AGT TCA AGA;
and human GAPDH (product size, 94 bp),
(S)TGC CAA ATA TGA TGA CAT CAA GAA/(AS) GGA GTG GGT GTC GCT GTT G.
The obtained data were analyzed by the Sequence Detection Software (version 1.4) and 7500 Fast System SDS software.
Immunoblot
Total cell lysates from hESC were prepared in a sample buffer. The total protein concentration of the sample was determined using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Just before electrophoresis, 5% (vol/vol) 2-mercaptoethanol and 1% (wt/vol) bromophenol blue were added to the sample, and proteins were separated by 10% (wt/vol) SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 5% (wt/vol) skim milk dissolved in Tween-PBS and then incubated with 1:200 diluted anti-NFAT1, anti-NFAT3, anti-NFAT4 (Cell Signaling, Beverly, MA), anti-NFAT3 (Sigma-Aldrich, St. Louis, MO), CREB, anti-p-CREB (Millipore, Bedford, MA), anti-β-actin (Abcam, Cambridge, MA), anti-LaminB, anti-IGFBP-1 (Santa Cruz Biotechnology, Inc.), and anti-TRPC1 (Alomone, Jerusalem, Israel) antibodies overnight. Protein expression was visualized by incubating the membrane with the secondary antibody linked to horseradish peroxidase.
Fractionation assay
Fractionation assay was performed to examine the nuclear protein level of p-CREB, NFAT1 (NFATC2), NFAT2 (NFATC1), NFAT3 (NFATC4), and NFAT4 (NFATC3) in hESC. Nuclear proteins were extracted from hESC by using the ProteoJET, a cytoplasmic and nuclear protein extraction kit (Fermentas, Glen Burnie, MD), according to the manufacturer's instructions. Protein concentrations of samples were determined using a bicinchoninic acid protein assay kit (Pierce). LaminB was used as an internal control of nuclear fraction.
Immunostaining
Cultured hESC adhering on round coverslips were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.2% Triton-X/Dulbecco's PBS for 15 min at room temperature. After rinsing in PBS several times, the coverslips were preincubated for 1 h with 10% normal goat serum (Jackson ImmunoResearch, West Grove, PA) to reduce nonspecific binding and then incubated successively with: anti-α-smooth muscle actin, antivimentin, anticytokeratin, and anticaveolin-1 antibodies (each diluted at 1:500 with PBS) overnight at 4 C; Alexa Fluor 568 antimouse IgG or Alexa Fluor 488 antirabbit IgG (diluted at 1:200 with 3% normal goat serum in PBS; Molecular Probes, Eugene, OR) for 1 h after washing with PBS. Then, nuclei of hESC were stained by 4′,6-diamino-2-phenylindole (dissolved in methanol at 1 μg/ml) for 15 min. The coverslips were sealed with PermaFluor Aqueous (Immunon; ThermoShandon) to prevent evaporation. Immunostained cells were observed under a confocal laser scanning microscope (LSM 710-Zen2008; Carl Zeiss, Oberkochen, Germany) equipped with an argon/krypton laser source. A single wavelength of 568 nm was used for excitation, and the emitted fluorescence at 603 nm (Alexa Fluor 488, emitted at 519 nm) was collected through an oil-immersion objective lens with a ×100 magnification.
[Ca2+]i measurement
The intracellular Ca2+ concentration ([Ca2+]i) of hESC was monitored with fura 2 digital fluorescence imaging. The cells were plated on a collagen type I-coated glass chamber and loaded with fura 2-AM (5 μm with 0.01% pluronic acid F127) in the dark at room temperature for 30 min. With excitation at 340 and 380 nm, the intensity of fura 2 fluorescence emitted at 510 nm (±10 nm) was measured by a digital fluorescent image analysis system, consisting of an inverted fluorescent microscope (DMI600B; Leica Microsystems, Tokyo, Japan) and a low noise, high-intensifying EMCCD camera (Cascade; Nippon Roper, Tokyo, Japan). The data acquisition and analysis were made by the software SlideBook 4.2 (Intelligent Imaging Innovation, Inc., Denver, CO). The obtained fluorescence was corrected for the background fluorescence and changes in [Ca2+]i were defined as the ratio of corrected fluorescences at 340 and 380 nm (F340/F380).
Cell size measurement
The two-dimensional (2D) images of hESC immunostained for caveolin-1 were obtained by confocal laser scanning microscopy (see above). The cell contour of each caveolin-1-immunostained image was outlined by a polygonal area selection tool, and the enclosed 2D-area by a polygon was measured by using a free Excel-based graphic analysis software lenaraf220. hESC showing obscure contours were excluded from the evaluation.
Chemicals and reagents
Stock solutions of E2 (10 μm) or P4 (1 mm) were made by dissolving their powders in 100% EtOH and diluted 1000 times in culture media just before use. Type I collagenase, antibiotics (a mixture of penicillin and streptomycin), and 8-bcAMP were purchased from Sigma (St. Louis, MO). DMEM/F-12 was from Invitrogen. Charcoal-stripped FBS was from Biological Industries (Beit Haemek, Israel). Collagen type I was from Research Institute for the Functional Peptides (Yamagata, Japan). E2 and P4 were from Wako (Osaka, Japan).
Anti-NFAT1, anti-NFAT3, anti-NFAT4 (Cell Signaling), anti-NFAT3 (Sigma-Aldrich) CREB, anti-p-CREB (Millipore), anti-β-actin (Abcam), anti-LaminB, anti-IGFBP-1 (Santa Cruz Biotechnology, Inc.), anti-TRPC1 (Alomone), and anticaveolin 1 (Thermo, Rockford, IL) antibodies were used for immunoblot and immunostaning analysis. Fura 2-AM (Wako), nifedipine (Calbiochem, San Diego, CA), and cyclopiazonic acid (Calbiochem) were used for Ca2+ imaging.
T1E3-Ab (see above) was a kind gift from D. J. Beech's at University of Leeds (Leeds, United Kingdom). T1-Ab (TRPC1-Ab) was purchased from Alomone and used for immunoblotting, Ca2+ imaging, and cell size measurement experiments.
Solutions
External solution used for Ca2+ imaging consisted of 140 mm NaCl, 5 mm KCl, 1 mm CaCl2, 1.2 mm MgCl2, 10 mm HEPES, and 10 mm glucose (pH 7.4, adjusted with Tris base).
Statistical analysis
The results are expressed as the mean ± sem. For the measurements of [Ca2+]i, representative data averaged from more than 100 cells in one experiment were plotted. Statistical significance in the difference between two groups was evaluated by paired and unpaired Student's t test. Multiple comparisons were made by using Tukey's method.
To minimize possible errors arising from variable conditions of experimental hardware (e.g. fluorescent imaging system) and materials (e.g. cell culture conditions), experiments aiming at a particular purpose were performed simultaneously by assigning hESC amplified from the same frozen stock to several different protocols, and this was repeated for hESC derived from several different donors for statistical evaluation.
Acknowledgments
We thank Professor T. Kawarabayashi and Professor S. Miyamoto (Department of Gynecology and Obstetrics, Faculty of Medicine, Fukuoka University) and Professor N. Wake (Department of Gynecology and Obstetrics, Graduate School of Medical Sciences, Kyushu University, Kyushu, Japan) for kind encouragement to Y.K. We also thank Dr. Y. Inoue (Department of Gynecology and Obstetrics, Fukuoka University), Dr. K. Kato, Dr. Y. Tanaka (Department of Gynecology and Obstetrics, Graduate School of Medical Sciences, Kyushu University), and our lab members (M. X. Mori, J. Hatae, and J. Ichikawa) for their pertinent advice and help during the course of the present study. We also thank Professor David Beech at the Institute of Membrane and Systems Biology (University of Leeds, Leeds, United Kingdom) for kindly proving us with T1E3 antibody.
This work was supported in part by the Japan Society for Promotion of Sciences, Tokyo Biochemical Research Foundation, Vehicle Racing Commemorative Foundation (R.I.), Grants-in-aid for Scientific Research on Innovative Areas (No. 22136008) and Scientific Research (C) (No. 21590246).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AS
- Antisense
- 8-bcAMP
- 8-bromo cAMP
- CaMK
- calmodulin-dependent kinase
- CBP
- CREB-binding protein
- C/EBPβ
- CCAAT/enhancer-binding protein
- COX-2
- cyclooxgenase 2
- CREB
- cAMP responsive element binding protein
- CREM
- cAMP-responsive element modulator
- 2D
- two dimensional
- E2
- 17β-estradiol
- ESC
- endometrial stromal cell
- EtOH
- ethanol
- FBS
- fetal bovine serum
- FOX
- Forkhead box protein
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- hESC
- human ESC
- HOX
- homeobox protein
- IGFBP-1
- IGF binding protein-1
- NFAT
- nuclear factor of activated T-cells
- P4
- progesterone
- p-CREB
- phosphorylated form of CREB
- PGE2
- prostaglandin E2
- PKA
- protein kinase A
- PR
- P4 receptor
- PRL
- prolactin
- S
- sense
- siRNA
- small interfering RNA
- SOC
- store-operated Ca2+ influx
- STAT
- signal transducer and activator of transcription
- T1E3-Ab
- TRPC1 extracellular loop 3-directed antibody
- TRP
- transient receptor potential
- TRPC
- TRP canonical
- TRPM
- TRP melastatin
- TRPP
- TRP polycystin
- TRPV
- TRP vanilloid.
References
- 1. Gellersen B , Brosens IA , Brosens JJ. 2007. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25:445–453 [DOI] [PubMed] [Google Scholar]
- 2. Tseng L , Gao JG , Chen R , Zhu HH , Mazella J , Powell DR. 1992. Effect of progestin, antiprogestin, and relaxin on the accumulation of prolactin and insulin-like growth factor-binding protein-1 messenger ribonucleic acid in human endometrial stromal cells. Biol Reprod 47:441–450 [DOI] [PubMed] [Google Scholar]
- 3. Lockwood CJ , Nemerson Y , Guller S , Krikun G , Alvarez M , Hausknecht V , Gurpide E , Schatz F. 1993. Progestational regulation of human endometrial stromal cell tissue factor expression during decidualization. J Clin Endocrinol Metab 76:231–236 [DOI] [PubMed] [Google Scholar]
- 4. Sakakibara H , Taga M , Saji M , Kida H , Minaguchi H. 1994. Gene expression of epidermal growth factor in human endometrium during decidualization. J Clin Endocrinol Metab 79:223–226 [DOI] [PubMed] [Google Scholar]
- 5. Dimitriadis E , White CA , Jones RL , Salamonsen LA. 2005. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update 11:613–630 [DOI] [PubMed] [Google Scholar]
- 6. Bany BM , Harvey MB , Schultz GA. 2000. Expression of matrix metalloproteinases 2 and 9 in the mouse uterus during implantation and oil-induced decidualization. J Reprod Fertil 120:125–134 [DOI] [PubMed] [Google Scholar]
- 7. Gamo T , Yamauchi N , Nishimura K , Watanabe R , Matsumoto K , Oozono S , Kubota K , He PJ , Soh T , Hattori MA. 2007. Effects of tumor necrosis factor-α on cell proliferation, prostaglandins and matrix-metalloproteinases production in rat endometrial stromal cells cultured in vitro. J Exp Zool A Ecol Genet Physiol 307:699–707 [DOI] [PubMed] [Google Scholar]
- 8. Tanaka K , Minoura H , Isobe T , Yonaha H , Kawato H , Wang DF , Yoshida T , Kojima M , Kangawa K , Toyoda N. 2003. Ghrelin is involved in the decidualization of human endometrial stromal cells. J Clin Endocrinol Metab 88:2335–2340 [DOI] [PubMed] [Google Scholar]
- 9. Yee GM , Kennedy TG. 1991. Role of cyclic adenosine 3′,5′-monophosphate in mediating the effect of prostaglandin E2 on decidualization in vitro. Biol Reprod 45:163–171 [DOI] [PubMed] [Google Scholar]
- 10. Chapdelaine P , Kang J , Boucher-Kovalik S , Caron N , Tremblay JP , Fortier MA. 2006. Decidualization and maintenance of a functional prostaglandin system in human endometrial cell lines following transformation with SV40 large T antigen. Mol Hum Reprod 12:309–319 [DOI] [PubMed] [Google Scholar]
- 11. Giudice LC. 2004. Microarray expression profiling reveals candidate genes for human uterine receptivity. Am J Pharmacogenomics 4:299–312 [DOI] [PubMed] [Google Scholar]
- 12. Giudice LC. 2006. Application of functional genomics to primate endometrium: insights into biological processes. Reprod Biol Endocrinol 4(Suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Telgmann R , Maronde E , Taskén K , Gellersen B. 1997. Activated protein kinase A is required for differentiation-dependent transcription of the decidual prolactin gene in human endometrial stromal cells. Endocrinology 138:929–937 [DOI] [PubMed] [Google Scholar]
- 14. Maruyama T , Yoshimura Y , Yodoi J , Sabe H. 1999. Activation of c-Src kinase is associated with in vitro decidualization of human endometrial stromal cells. Endocrinology 140:2632–2636 [DOI] [PubMed] [Google Scholar]
- 15. Mak IY , Brosens JJ , Christian M , Hills FA , Chamley L , Regan L , White JO. 2002. Regulated expression of signal transducer and activator of transcription, Stat5, and its enhancement of PRL expression in human endometrial stromal cells in vitro. J Clin Endocrinol Metab 87:2581–2588 [DOI] [PubMed] [Google Scholar]
- 16. Kajihara T , Jones M , Fusi L , Takano M , Feroze-Zaidi F , Pirianov G , Mehmet H , Ishihara O , Higham JM , Lam EW , Brosens JJ. 2006. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol Endocrinol 20:2444–2455 [DOI] [PubMed] [Google Scholar]
- 17. Tamura K , Yoshie M , Nishi H , Osakabe Y , Isaka K , Hara T , Kogo H. 2006. Expression of stathmin in human uterus and decidualizing endometrial stromal cells. Reproduction 132:625–636 [DOI] [PubMed] [Google Scholar]
- 18. Flockerzi V. 2007. An introduction on TRP channels. Handb Exp Pharmacol 179:1–19 [DOI] [PubMed] [Google Scholar]
- 19. Nilius B. 2007. TRP channels in disease. Biochim Biophys Acta 1772:805–812 [DOI] [PubMed] [Google Scholar]
- 20. Dalrymple A , Mahn K , Poston L , Songu-Mize E , Tribe RM. 2007. Mechanical stretch regulates TRPC expression and calcium entry in human myometrial smooth muscle cells. Mol Hum Reprod 13:171–179 [DOI] [PubMed] [Google Scholar]
- 21. Dalrymple A , Slater DM , Poston L , Tribe RM. 2004. Physiological induction of transient receptor potential canonical proteins, calcium entry channels, in human myometrium: influence of pregnancy, labor, and interleukin-1β. J Clin Endocrinol Metab 89:1291–1300 [DOI] [PubMed] [Google Scholar]
- 22. Montalbetti N , Li Q , Wu Y , Chen XZ , Cantiello HF. 2007. Polycystin-2 cation channel function in the human syncytiotrophoblast is regulated by microtubular structures. J Physiol 579:717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stumpf T , Zhang Q , Hirnet D , Lewandrowski U , Sickmann A , Wissenbach U , Dörr J , Lohr C , Deitmer JW , Fecher-Trost C. 2008. The human TRPV6 channel protein is associated with cyclophilin B in human placenta. J Biol Chem 283:18086–18098 [DOI] [PubMed] [Google Scholar]
- 24. Guilbert A , Dhennin-Duthille I , Hiani YE , Haren N , Khorsi H , Sevestre H , Ahidouch A , Ouadid-Ahidouch H. 2008. Expression of TRPC6 channels in human epithelial breast cancer cells. BMC Cancer 8:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beech DJ. 2007. Ion channel switching and activation in smooth-muscle cells of occlusive vascular diseases. Biochem Soc Trans 35:890–894 [DOI] [PubMed] [Google Scholar]
- 26. Nishida M , Kurose H. 2008. Roles of TRP channels in the development of cardiac hypertrophy. Naunyn Schmiedebergs Arch Pharmacol 378:395–406 [DOI] [PubMed] [Google Scholar]
- 27. Inoue R , Jian Z , Kawarabayashi Y. 2009. Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Ther 123:371–385 [DOI] [PubMed] [Google Scholar]
- 28. Gkika D , Prevarskaya N. 2009. Molecular mechanisms of TRP regulation in tumor growth and metastasis. Biochim Biophys Acta 1793:953–958 [DOI] [PubMed] [Google Scholar]
- 29. Arnold JT , Kaufman DG , Seppälä M , Lessey BA. 2001. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod 16:836–845 [DOI] [PubMed] [Google Scholar]
- 30. Kawarabayashi Y , Hai L , Honda A , Mori MX , Hatae J , Inoue R. 2009. Possible role of TRPC1-mediated Ca2+ entry in decidualization of human endometrial stromal cells. J Physiol Sci 59:229P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alberts B , Bray D , Lewis J , Raff M , Roberts K , Watson JD. 1994. Cell signalling. In: The molecular biology of the cell. 3rd ed Chap 15 New York: Garland Publishing, Inc.; 736 [Google Scholar]
- 32. Ambudkar IS , Ong HL , Liu X , Bandyopadhyay BC , Bandyopadhyay B , Cheng KT. 2007. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium 42:213–223 [DOI] [PubMed] [Google Scholar]
- 33. Parekh AB , Putney JW. 2005. Store-operated calcium channels. Physiol Rev 85:757–810 [DOI] [PubMed] [Google Scholar]
- 34. Xu SZ , Beech DJ. 2001. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res 88:84–87 [DOI] [PubMed] [Google Scholar]
- 35. Chrivia JC , Kwok RP , Lamb N , Hagiwara M , Montminy MR , Goodman RH. 1993. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 365:855–859 [DOI] [PubMed] [Google Scholar]
- 36. van der Heide LP , Smidt MP. 2005. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trend Biochem Sci 30:81–86 [DOI] [PubMed] [Google Scholar]
- 37. Allen DG , Whitehead NP , Yeung EW. 2005. Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: role of ionic changes. J Physiol 567:723–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. House SJ , Potier M , Bisaillon J , Singer HA , Trebak M. 2008. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch 456:769–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuwahara K , Wang Y , McAnally J , Richardson JA , Bassel-Duby R , Hill JA , Olson EN. 2006. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 116:3114–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Onohara N , Nishida M , Inoue R , Kobayashi H , Sumimoto H , Sato Y , Mori Y , Nagao T , Kurose H. 2006. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J 25:5305–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumar B , Dreja K , Shah SS , Cheong A , Xu SZ , Sukumar P , Naylor J , Forte A , Cipollaro M , McHugh D , Kingston PA , Heagerty AM , Munsch CM , Bergdahl A , Hultgårdh-Nilsson A , Gomez MF , Porter KE , Hellstrand P , Beech DJ. 2006. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res 98:557–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cai S , Fatherazi S , Presland RB , Belton CM , Roberts FA , Goodwin PC , Schubert MM , Izutsu KT. 2006. Evidence that TRPC1 contributes to calcium-induced diffrenetiation of human keracinocytes. Pflugers Arch Eur J Physiol 452:43–52 [DOI] [PubMed] [Google Scholar]
- 43. Iwata Y , Katanosaka Y , Arai Y , Komamura K , Miyatake K , Shigekawa M. 2003. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol 161:957–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aarts MM , Tymianski M. 2005. TRPMs and neuronal cell death. Pflugers Arch 451:243–249 [DOI] [PubMed] [Google Scholar]
- 45. Sakoff JA , Murdoch RN. 1996. The role of calcium in the artificially induced decidual cell reaction in pseudopregnant mice. Biochem Mol Med 57:81–90 [DOI] [PubMed] [Google Scholar]
- 46. Squires PM , Dixon SJ , Kennedy TG. 1993. Requirement for Ca2+ mobilization and increased prostaglandin production for maximal decidualization in rats and the involvement of angiotensin II. J Reprod Fertil 98:423–429 [DOI] [PubMed] [Google Scholar]
- 47. Waclawik A , Jabbour HN , Blitek A , Ziecik AJ. 2009. Estradiol-17β, prostaglandin E2 (PGE2), and the PGE2 receptor are involved in PGE2 positive feedback loop in the porcine endometrium. Endocrinology 150:3823–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. De Cesare D , Fimia GM , Sassone-Corsi P. 1999. Signaling routes to CREM and CREB: plasticity in transcriptional activation. Trends Biochem Sci 24:281–285 [DOI] [PubMed] [Google Scholar]
- 49. Takahashi Y , Watanabe H , Murakami M , Ono K , Munehisa Y , Koyama T , Nobori K , Iijima T , Ito H. 2007. Functional role of stromal interaction molecule 1 (STIM1) in vascular smooth muscle cells. Biochem Biophys Res Commun 361:934–940 [DOI] [PubMed] [Google Scholar]
- 50. Zonta M , Sebelin A , Gobbo S , Fellin T , Pozzan T , Carmignoto G. 2003. Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. J Physiol 553:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vega A , Chacón P , Monteseirín J , El Bekay R , Alba G , Martín-Nieto J , Sobrino F. 2007. Expression of the transcription factor NFAT2 in human neutrophils: IgE-dependent, Ca2+- and calcineurin-mediated NFAT2 activation. J Cell Sci 120:2328–2337 [DOI] [PubMed] [Google Scholar]
- 52. Si J , Fu X , Behar J , Wands J , Beer DG , Souza RF , Spechler SJ , Lambeth D , Cao W. 2007. NADPH oxidase NOX5-S mediates acid-induced cyclooxygenase-2 expression via activation of NF-κB in Barrett's esophageal adenocarcinoma cells. J Biol Chem 282:16244–16255 [DOI] [PubMed] [Google Scholar]
- 53. Celil Aydemir AB , Minematsu H , Gardner TR , Kim KO , Ahn JM , Lee FY. 2010. Nuclear factor of activated T cells mediates fluid shear stress- and tensile strain-induced Cox2 in human and murine bone cells. Bone 46:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bruce JI , Straub SV , Yule DI. 2003. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium 34:431–444 [DOI] [PubMed] [Google Scholar]
- 55. Rinehart CA , Haskill JS , Morris JS , Butler TD , Kaufman DG. 1991. Extended life span of human endometrial stromal cells transfected with cloned origin-defective, temperature-sensitive simian virus 40. J Virol 65:1458–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Inoue R , Jensen LJ , Shi J , Morita H , Nishida M , Honda A , Ito Y. 2006. Transient receptor potential channels in cardiovascular function and disease. Circ Res 99:119–131 [DOI] [PubMed] [Google Scholar]