Abstract
TSH is a major regulator of thyroid cell growth and endocrine function. It is known that cAMP and phosphatidylinositol 3-kinase (PI3K) are responsible for mediating the action of TSH. Activation of these signals results in the induction of a series of transcription factors and cell cycle regulating proteins, which induce cell proliferation. In addition to such canonical transcriptional regulation, it was recently shown that microRNA (miRNA or miR) constitutes another key mechanism for the regulation of gene expression. However, whether TSH action is mediated by miRNA in the thyroid is unknown. In this study, we have performed miRNA microarray analysis and demonstrated that TSH significantly decreases expression of 47 miRNA in thyroid cells. Among these, we have shown, using their specific agonists, that overexpression of miR-16 and miR-195 suppressed cell cycle progression and DNA synthesis that was induced by TSH. In silico analysis predicted that Mapk8, Ccne1, and Cdc6, the expression of which was up-regulated by TSH, are potential target genes for these miRNA, and overexpression of miR-16 and miR-195 suppressed expression of these target genes. The decrease of miR-16 and miR-195 expression by TSH was reproduced by forskolin and N6,2′-O-dibutyryladenosine cAMP and reversed by the protein kinase A inhibitor H89 and the PI3K inhibitor LY294002. These results suggest that TSH activates cAMP/protein kinase A and PI3K cascades to decrease miR-16 and miR-195, which induce Mapk8, Ccne1, and Cdc6 to activate cell proliferation.
TSH, produced by the anterior lobe of the pituitary, regulates thyroid cell growth and its endocrine function, such as synthesis and secretion of thyroid hormone. Binding of TSH to its receptor, a member of the G protein-coupled receptor superfamily (1), on the basolateral surface of the thyrocyte, is the first step in its action. Then, the associated G protein couples to adenylate cyclase to produce cAMP, which activates protein kinase A (PKA) and finally induces expression of various genes. Phosphatidylinositol 3-kinase (PI3K) is also activated by TSH, and its signaling induces cell proliferation (2, 3). The genes induced by cAMP and/or PI3K signals include cell cycle-related proteins, such as cyclin D1, cyclin E, cyclin-dependent kinase (CDK)2, and CDK4 (4, 5).
Over the past decade, the intimate links between microRNA (miRNA or miR) expression and cell proliferation and cancer development have been demonstrated. miRNA is a RNA that binds and suppresses the expression of its target genes. The roles of miRNA have been shown in cell proliferation, differentiation, and apoptosis (6–8), especially in relation to cancer (9). However, studies on the role of miRNA in endocrine tissues in physiological condition are limited. It was recently reported that glucose-induced insulin secretion was suppressed by miR-375 through inhibition of myotrophin in the islets (10) and that the release of progesterone, estradiol, and testosterone is regulated by multiple miRNA in cultured primary ovarian cells (11).
In thyroid tissue, the possible roles of miRNA have been reported only in carcinomas (12–14). It was shown that miR-26a and miR-125b were down-regulated in anaplastic carcinomas, which is related to cell proliferation (15). Other studies demonstrated that miR-197, miR-346, and miR-221 were up-regulated in follicular and papillary carcinomas and promoted cell proliferation (14, 16). It was also shown in thyroid carcinomas that miR-17–5p and miR-19a suppressed expression of retinoblastoma protein and phosphatase and tensin homolog, respectively (17) and that miR-221 and miR-222 suppressed p27kip1, which binds to several classes of cyclin and CDK molecules to suppress cell proliferation (18). Thus, although the roles of some miRNA in thyroid carcinomas have been investigated, it is still unknown whether the normal physiological actions of TSH are mediated by miRNA in the normal thyroid.
In this study, we have identified miRNA whose expression was suppressed by TSH and involved in thyroid cell growth in nonneoplastic cells. We have also determined the target genes and signaling cascades responsible for the expression and function of such miRNA.
Results
Identification of miRNA that mediate TSH action and increase cell proliferation
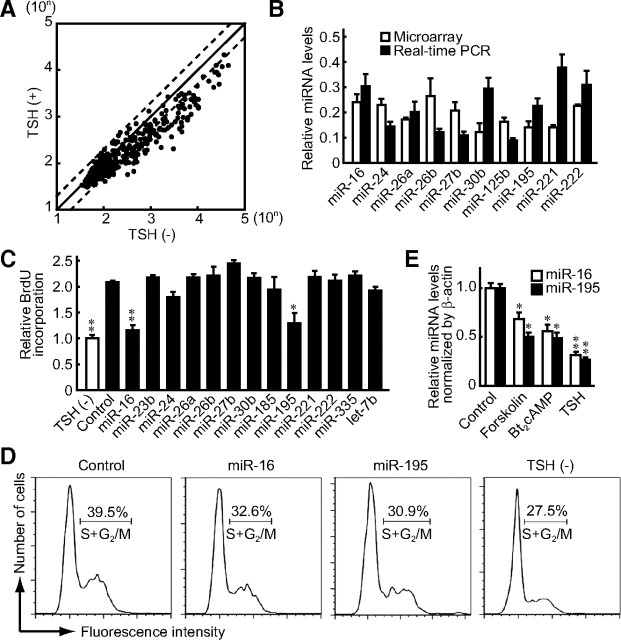
We investigated the miRNA expression profile of thyroid cells after TSH stimulation under physiologic conditions. Thus, we stimulated rat thyroid FRTL-5 cells with TSH and analyzed miRNA expression using microarray, on which 237 rat miRNA probes were mounted in triplicate. Differentiated endocrine function and growth activity of FRTL-5 cells are tightly regulated by TSH (19, 20). The quiescent FRTL-5 cells were cultured in the presence or absence of 1 mU/ml TSH for 24 h, and small RNA were isolated and labeled by Alexa Fluor dyes to hybridize to the microarray. As shown in Fig. 1A, the expression of most miRNA was decreased by TSH. Indeed, expression of 47 miRNA was reduced to less than 50% of their original levels (Table 1). TSH-mediated decreases in these miRNA were confirmed by real-time PCR analysis (Fig. 1B). The decrease was evident by 4 h and further decreased over the 24-h period after the initiation of TSH stimulation (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org).
Fig. 1.
Identification of miRNA responsible for TSH-induced DNA synthesis in thyroid cells. A, miRNA microarray analysis was performed using FRTL-5 cells treated with TSH. The signal intensities of the triplicate 237 rat miRNA were plotted. The linear fitted curve is y = 0.3439x + 40.627, R2 = 0.8594. B, The same RNA samples used for the microarray were subjected to real-time PCR analysis by TaqMan miRNA assays to evaluate expression of ten representative miRNA that showed various levels of expression in microarray analysis. The fold decrease of TSH-treated samples was illustrated as the mean ± sem (n = 3). C, After 1 wk of depletion of TSH from FRTL-5 cell culture medium, pre-miR miRNA agonists were transfected in the presence of TSH for 30 h, and BrdU labeling was carried out for 6 h at the end of incubation. Representative results are shown. The relative amounts of BrdU incorporated were normalized to control samples without TSH stimulation and were expressed as the mean ± sem (n = 3). *, P < 0.05; **, P < 0.01 by Student's t test. D, After 1 wk of depletion of TSH from FRTL-5 cell culture medium, pre-miR miRNA agonists were transfected in the presence of TSH for 24 h, and cells were analyzed with the FACSCalibur. The ratios corresponding to the S+G2/M phases of the cell cycle are indicated. E, After a 1 wk depletion of TSH, forskolin or Bt2cAMP was added for 24 h. miRNA levels were determined by quantitative real-time PCR analysis. Results were expressed as the mean ± sem (n = 3). *, P < 0.05; **, P < 0.01 relative to control by Student's t test.
Table 1.
miRNA decreased over twice by TSH
| miRNA | Signal intensity |
Fold change | |
|---|---|---|---|
| TSH (−) | TSH (+) | ||
| miR-30b | 2891 | 283 | −10.21 |
| miR-195 | 5251 | 653 | −8.05 |
| miR-221 | 3442 | 506 | −6.81 |
| miR-27b | 4244 | 755 | −5.62 |
| miR-26a | 20363 | 3632 | −5.61 |
| miR-125b | 8328 | 1496 | −5.57 |
| miR-10b | 290 | 55 | −5.30 |
| miR-22* | 4903 | 976 | −5.02 |
| miR-16 | 5765 | 1288 | −4.48 |
| miR-222 | 1100 | 254 | −4.33 |
| miR-24 | 7003 | 1680 | −4.17 |
| miR-26b | 5950 | 1476 | −4.03 |
| miR-23a | 2207 | 559 | −3.95 |
| miR-352 | 1470 | 404 | −3.64 |
| let-7a | 36209 | 10175 | −3.56 |
| miR-320 | 316 | 89 | −3.55 |
| miR-422b | 607 | 181 | −3.36 |
| miR-218 | 358 | 108 | −3.31 |
| miR-22 | 257 | 80 | −3.23 |
| miR-342 | 518 | 176 | −2.94 |
| miR-130a | 686 | 241 | −2.85 |
| let-7i | 5149 | 1873 | −2.75 |
| miR-191 | 3070 | 1127 | −2.73 |
| miR-185 | 1895 | 713 | −2.66 |
| miR-487b | 295 | 112 | −2.64 |
| miR-30c | 3816 | 1460 | −2.61 |
| miR-361 | 419 | 161 | −2.60 |
| miR-1* | 35055 | 14147 | −2.48 |
| miR-30d | 961 | 389 | −2.47 |
| let-7d | 12561 | 5106 | −2.46 |
| let-7d* | 12694 | 5173 | −2.45 |
| miR-152 | 248 | 101 | −2.44 |
| miR-29a | 1565 | 650 | −2.41 |
| let-7f | 19344 | 8066 | −2.40 |
| miR-103 | 1688 | 723 | −2.34 |
| let-7b | 13963 | 6032 | −2.31 |
| miR-27a | 517 | 228 | −2.27 |
| miR-374 | 306 | 137 | −2.24 |
| miR-539 | 304 | 141 | −2.15 |
| miR-106b | 831 | 390 | −2.13 |
| let-7c | 26498 | 12489 | −2.12 |
| miR-29b | 169 | 81 | −2.09 |
| miR-497 | 233 | 112 | −2.07 |
| miR-23b | 3834 | 1853 | −2.07 |
| miR-378 | 120 | 58 | −2.07 |
| miR-21 | 319 | 157 | −2.04 |
| let-7e | 5042 | 2527 | −2.00 |
We next evaluated the possible roles of these miRNA in thyroid cell growth. To do this, miRNA in FRTL-5 cells that were reduced by TSH stimulation were complemented by transfecting a series of pre-miR miRNA agonists. As a result, overexpression of miR-16 and miR-195 significantly reduced the bromodeoxyuridine (BrdU) incorporation that was originally up-regulated by TSH (Fig. 1C). To confirm the results, cell cycle analysis was performed using flow cytometry. Overexpression of miR-16 and miR-195 reduced the cells in S+G2/M phases of the cell cycle in the presence of TSH (Fig. 1D). Although miR-16 and miR-195 did not completely abolish the effect of TSH, the reduction of S+G2/M cells was comparable with the effects of the miRNA in reducing BrdU incorporation. Consistent with these results, the number of cells was decreased by overexpression of miR-16 and miR-195 in TSH-treated cells after 48 h (Supplemental Fig. 2). These results indicate that TSH induces cell proliferation, at least in part, by suppressing miR-16 and miR-195 expression in FRTL-5 cells. It was also demonstrated that forskolin and N6,2′-O-dibutyryladenosine cAMP (Bt2cAMP) significantly suppressed expression of miR-16 and miR-195 (Fig. 1E), suggesting that cAMP signaling is responsible for the regulation of these miRNA.
Identification of Mapk8, Ccne1, and Cdc6 as target genes for TSH-regulated miRNA
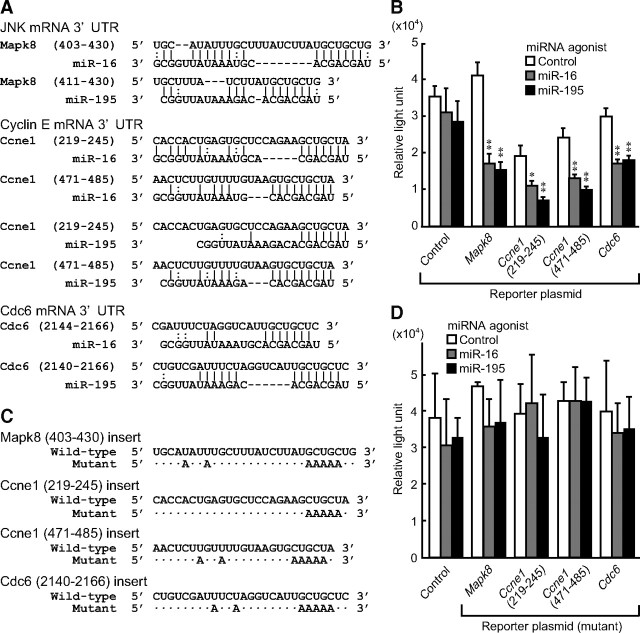
miRNA are thought to bind to their target genes and suppress expression. To identify possible target genes for miR-16 and miR-195, we first employed DNA microarray analysis to determine genes whose levels were increased by TSH in FRTL-5 thyroid cells and identified 3896 genes. These genes were subjected to computer-based prediction programs, TargetScan, miRDB, microT, and miRNA.org to predict potential target genes for miR-16 and miR-195. As a result, 157 genes were selected as candidates targeted by miR-16 and miR-195 in response to TSH (Supplemental Table 1). Of interest, miR-16 and miR-195 shared the same target genes with similar target sequences (Fig. 2A). These include genes related to cell proliferation, such as Mapk8, which encodes a MAPK c-Jun N-terminal kinase (JNK), Ccne1, whose product cyclin E regulates G1 to S phase transition of the cell cycle, and Cdc6, which is an essential component of the prereplication complex. As evident in Fig. 2A, miR-16 and miR-195 target almost the same region within the 3′-untranslated region (UTR) of each target gene, reflecting the fact that both miR-16 and miR-195 belong to the miR-15/107 family (21). Of note, these target sites were well conserved between rat and human homologues of Mapk8, Ccne1, and Cdc6 (Supplemental Table 2), suggesting the possibility that they play similar essential roles among species.
Fig. 2.
In silico prediction and functional characterization of target sequences of miR-16 and miR-195 within the 3′-UTR of Mapk8, Ccne1, and Cdc6. A, The predicted binding sites of miR-16 or miR-195 within the 3′-UTR of their target genes were analyzed by the microT and TargetScan 5.1 programs. A-U and G-C pair are shown as |, and G-U is shown as :. B, Synthesized oligo DNA were cloned into a luciferase reporter plasmid, pMIR-REPORT-luc, and transfected in FRTL-5 cells after miR-16 or miR-195 overexpression. Luciferase activity was measured as described in the Materials and Methods. Results were expressed as the means ± sem (n = 3). *, P < 0.05; **, P < 0.01 relative to control by Student's t test. C, The potential binding sites of miR-16 and miR-195 within their target 3′-UTR sequences were mutated to test the effect of miRNA. D, Synthesized oligo DNA for the mutants indicated in C were cloned into pMIR-REPORT-luc and transfected in FRTL-5 cells after miR-16 or miR-195 overexpression. Results of luciferase assay were expressed as the means ± sem (n = 3).
To study whether miR-16 and miR-195 actually bound to and cleaved these target sites, we created luciferase expression plasmids in which each 3′-UTR target site of Mapk8, Ccne1, and Cdc6 was inserted. These expression plasmids were transfected into FRTL-5 cells that overexpressed miR-16 or miR-195 by transfecting their agonists. All the expressed luciferase reporter activities were significantly suppressed by overexpression of miR-16 or miR-195, whereas control miRNA had no effect (Fig. 2B, open bars). Luciferase activity of the control expression plasmid was not affected by miR-16 or miR-195. It is known that the seed sequence formed by seven or eight nucleotides of a mature miRNA 5′-end is most crucial for interaction between the miRNA and its target genes (22). To further confirm the specificity of 3′-UTR sequences targeted by miR-16 and miR-195, luciferase reporter constructs containing mutant 3′-UTR (23) were constructed (Fig. 2C). Luciferase reporter gene activity of these mutants was not affected by overexpression of miR-16 or miR-195 (Fig. 2D). These data indicate that miR-16 and miR-195 directly associated with the 3′-UTR of Mapk8, Ccne1, and Cdc6 to repress their protein expression.
miR-16 and miR-195 regulate the expression of their target genes induced by TSH and cAMP
The RNA levels of Mapk8, Ccne1, and Cdc6 were increased by forskolin and Bt2cAMP as well as TSH (Fig. 3, A and B). To clarify whether miR-16 and miR-195 actually regulated the expression of these target genes, agonists for miR-16 and miR-195 were transfected into FRTL-5 cells. Overexpression of miR-16 and miR-195 suppressed RNA levels of Mapk8, Ccne1, and Cdc6 that were originally induced by TSH, whereas control and other miRNA agonists had no effects, as evidenced by the image of RT-PCR and the results of real-time PCR (Fig. 3, C and D). Similar to the suppression of RNA levels, the levels of their corresponding protein products, JNK, cyclin E, and cell division cycle (Cdc) 6, were increased by TSH but suppressed by miR-16 and miR-195, as shown by Western blot and densitometric analyses (Fig. 3, E and F). These results suggest that TSH suppresses miR-16 and miR-195, which results in an increase of JNK, cyclin E, and Cdc6 expression to induce cell proliferation.
Fig. 3.
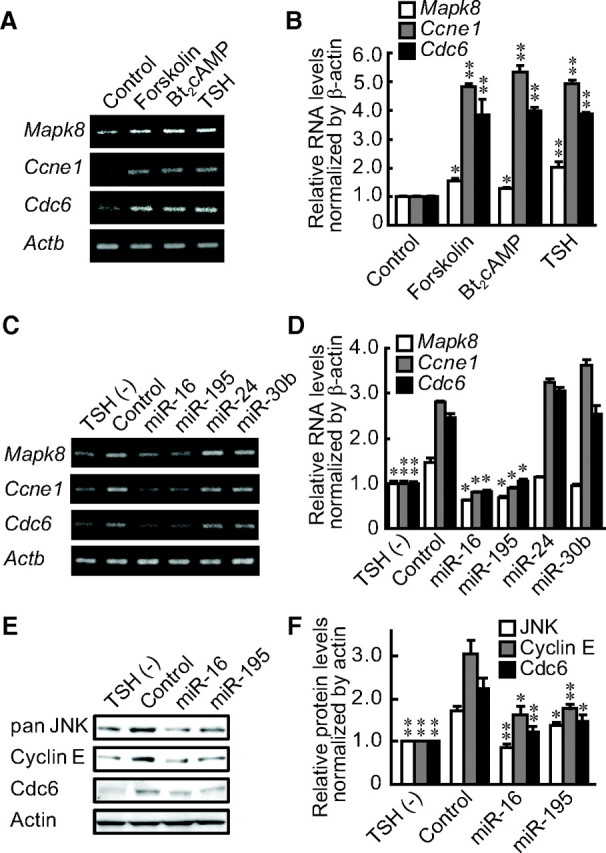
miR-16 and miR-195 regulate the expression of their target genes induced by TSH and cAMP. A and B, After a 1 wk of depletion of TSH, forskolin or Bt2cAMP was added for 24 h. Expression of miRNA target genes was evaluated by RT-PCR (A) and quantitative real-time PCR analysis (B). C and D, After a 1-wk depletion of TSH, the indicated pre-miR miRNA agonists were transfected. Expression of miRNA target genes was evaluated by RT-PCR (C) and quantitative real-time PCR analysis (D). E and F, Cells were treated the same condition as in B and C, and protein levels were evaluated by Western blotting (E) with densitometric analysis as described in Materials and Methods (F). Results were expressed as the mean ± sem (n = 3). *, P < 0.05; **, P < 0.01 relative to control by Student's t test.
To further examine the role of miR-16 and miR-195 on the regulation of Mapk8, Ccne1, and Cdc6 expression, we have suppressed expression of miR-16 and miR-195 by transfecting their corresponding antagonists (anti-miR miRNA Inhibitor; Applied Biosystems, Foster City, CA). We observed that suppression of miR-16 and miR-195 clearly increased expression of Mapk8, Ccne1, and Cdc6 (Supplemental Fig. 3A), although its effect on BrdU incorporation was not clear (Supplemental Fig. 3B). Further studies are needed to demonstrate whether or not Mapk8, Ccne1, and Cdc6 are sufficient targets of miR-16 and miR-195 to induce cell proliferation by mediating TSH action.
PKA and PI3K but not ERK-MAPK signaling regulates miRNA and their target genes
To explore possible signaling cascades that might regulate miR-16 and miR-195, we treated FRTL-5 cells with the PKA inhibitor H89, the MAPK/ERK kinase (MEK)1 inhibitor PD98059, or with the PI3K inhibitor LY294002 in the presence of TSH. We observed that H89 and LY294002 reversed TSH's decrease of miR-16 and miR-195 levels. The effect of PD98059 was less evident despite the fact that it might also have inhibited MEK5 at the concentration that we used (Fig. 4A) (24). Whether TSH/cAMP directly regulate miR-16 and miR-195 is not clear. However, it was indirectly corroborated by our finding that multiple potential cAMP response element (CRE) sites were identified within the 5′-flanking regions of rat miR-16 and miR-195 sites (Supplemental Table 3).
Fig. 4.
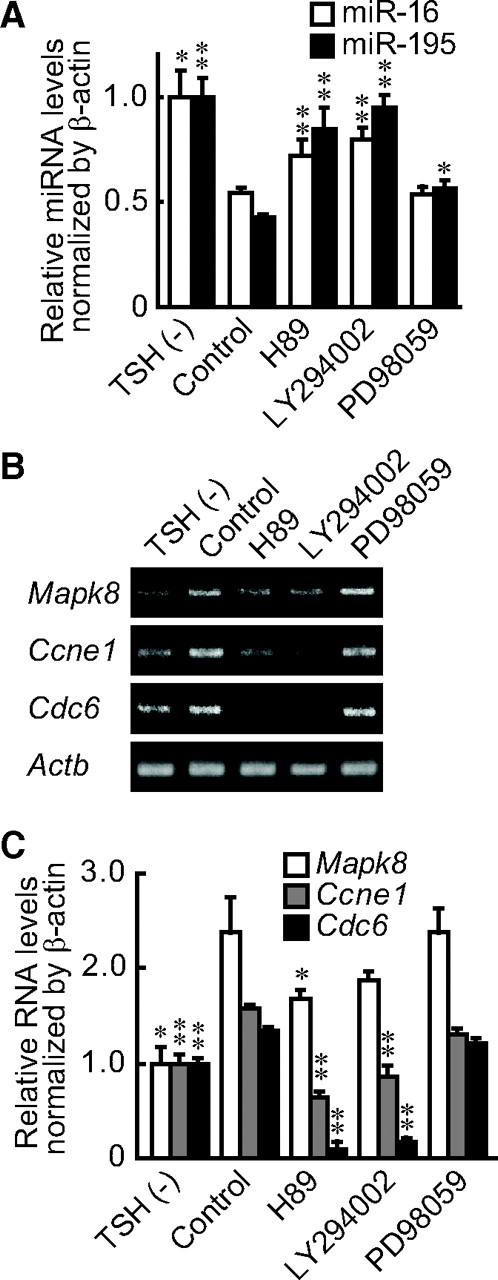
PKA and PI3K are involved in the TSH-mediated decrease of miRNA and the increased expression of their target genes. A, After a 1-wk depletion of TSH, PKA inhibitor H89, PI3K inhibitor LY294002, or MEK inhibitor PD98059 was added with TSH. miRNA levels were assessed by real-time PCR, and the results are expressed as the means ± sem (n = 3). B and C, After a 1-wk depletion of TSH, H89, LY294002, or PD98059 was added with TSH. Expression of miRNA target genes was evaluated by RT-PCR (B) and quantitative real-time PCR analysis (C). Results are expressed as the means ± sem (n = 3). *, P < 0.05; **, P < 0.01 relative to control by Student's t test.
The effects of these inhibitors were further confirmed by evaluating the expression of miRNA target genes. H89 and LY294002 strongly suppressed Mapk8, Ccne1, and Cdc6 mRNA levels, whereas PD98059 had no effect (Fig. 4, B and C) in accordance with the changes of miR-16 and miR-195 levels. These results indicate that TSH-induced DNA synthesis in thyroid cells is mediated, at least in part, by suppression of miR-16 and miR-195 expression through cAMP, PKA, and PI3K signaling, which in turn induces the expression of cell cycle-related genes.
Discussion
TSH is the primary regulator of thyrocytes and induces cell proliferation and gene expression necessary for endocrine function of the thyroid. In this study, 47 miRNA were initially found to be TSH responsive by miRNA microarray analysis. Using miRNA agonists, we identified miR-16 and miR-195 as the mediators of TSH's capacity to induce cell proliferation. We have also determined their target genes, Mapk8, Ccne1, and Cdc6, whose expression is regulated by miR-16 and miR-195 in response to TSH/cAMP/PKA/PI3K signals.
Ccne1 encodes cyclin E, which is a G1 cyclin needed for cell cycle progression from G0/G1 to S phase (25). Increases of cyclin E by TSH stimulation were previously reported in FRTL-5 cells (4, 5). In addition, Mapk8, encoding JNK, and Cdc6, the expression of which was increased by TSH stimulation, were also identified as target genes of miR-16 and miR-195. JNK is one of the kinases of the MAPK cascade that phosphorylates c-Jun to form the activator protein-1 complex and induces cell growth (26). Cdc6 is an essential component for the organization of the initiation complex of DNA replication (27). Thus, all of the genes identified as targets for miR-16 and miR-195 are involved in cell cycle regulation in the thyroid.
Ccne1 was reported to be a target of miR-16 and miR-195, mediating cell growth in various other nonthyroid cells, such as A549 lung epithelial cells, H1299 small cell lung carcinoma cells, U2OS osteosarcoma cells, MCF-7 breast adenocarcinoma cells, and mouse primary hepatic stellate cells (28–31). These reports support the present results, which demonstrated in FRTL-5 thyroid cells that Ccne1 is a target for miR-16 and miR-195, regulating cell proliferation. Whether Mapk8 and Cdc6 are targeted by miR-16 or miR-195 in other cell types must be addressed in the future. Several miRNA were reported to be associated with thyroid carcinomas. Although expression of miR-26a, miR-125b, and let-7c is reduced in thyroid cancers (15) (and all were decreased by TSH in our study), overexpression of miR-26a did not affect TSH-induced BrdU incorporation (Fig. 1C). Similarly, previous reports demonstrated that miR-221 and miR-222 were increased in thyroid carcinoma and induced cell proliferation (14, 32–34). However, although the expression of miR-221 and miR-222 was decreased by TSH, their agonists had no effect on DNA synthesis (Fig. 1C). To the contrary, there is no report showing the possible role of miR-16 and miR-195 in thyroid carcinogenesis, whereas both were identified as TSH mediators in the present report. These differences suggest that the physiological regulation of cell proliferation and neoplastic transformation are different at the miRNA level.
It is thought that each miRNA has hundreds of target genes (35). It was shown that miR-16 targets fibroblast growth factor receptor 1 and vascular endothelial growth factor and its receptor to regulate angiogenesis (36). miR-16 suppresses both zyxin, a component of a focal adhesion complex, and the serotonin receptor in noradrenergic neurons (37, 38). miR-16 is induced by DNA damage and suppresses wild-type p53-induced phosphatase 1 to repair the damage (39). Therefore, suppression of miR-16 by TSH in thyroid cells may also have other functions in addition to the cell cycle promotion. The signaling cascade that regulates the transcription of miRNA is largely unknown. We have shown that miR-16 and miR-195 mediate TSH/cAMP/PKA signaling that leads to thyroid cell growth. The cAMP cascade regulates expression of several miRNA during neonatal morphogenesis and neuronal differentiation (40, 41). However, miR-16 and miR-195 were not identified in those systems. Therefore, miR-16 and miR-195 might be specifically regulated by TSH/cAMP/PKA in mature thyroid cells. PKA is a representative kinase downstream of cAMP, which phosphorylates multiple substrates, including a transcription factor CRE-binding protein. It was recently reported that TSH suppresses several other miRNA through interaction of CRE-binding protein 1 with the CRE within the promoter regions of miRNA (42). Although we have shown potential CRE within 5′-flanking regions of miR-16 and miR-195, functional characterization is needed in FRTL-5 cells.
In addition to the cAMP cascade, we have also shown that PI3K is responsible for TSH-induced suppression of miR-16 and miR-195. PI3K is activated by the cAMP cascade (43) and by the βγ-subunits of G proteins that couple the cytoplasmic tail of TSH receptor (2). An inhibitor of PI3K, LY294002 not only reversed the effect of TSH but actually increased miR-16 and miR-195 expression above their original levels. It is possible that the PI3K activities were derived from TSH as well as from insulin and serum IGF-I that were suppressed by LY294002.
In conclusion, we have demonstrated in thyroid cells that TSH decreases miR-16 and miR-195, which in turn increases the expression of their cell cycle-related target genes to induce cell proliferation. The role of miR-16 and miR-195 seems to be different from other miRNA detected in thyroid carcinomas. These data will shed light on the physiological regulation of thyroid cell growth and function.
Materials and Methods
Culture and treatment of cells
Rat FRTL-5 thyroid cells (ATCC CRL8305) were provided by the Interthyr Research Foundation (Athens, OH) and maintained as reported previously (19, 20). Seven days before each experiment, cells were shifted to control medium, which lacked TSH (quiescent FRTL-5 cells). One or more of the following was added for the indicated period of time: bovine pituitary TSH (1 mU/ml; Sigma, St. Louis, MO), forskolin (100 μm; Sigma), Bt2cAMP (1 mm; Sigma), H89 (30 μm; Sigma), PD98059 (20 μm; Cell Signaling Technology, Danvers, MA), or LY294002 (50 μm; Sigma); 50 nm pre-miR miRNA precursor molecule for miRNA transfection, anti-miR miRNA Inhibitor for miRNA suppression, or control nonspecific miRNA (Applied Biosystems) was transfected into quiescent cells overnight using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) in control medium lacking serum and TSH.
miRNA microarray
Quiescent FRTL-5 cells were cultured with or without TSH for 24 h, and total RNA was extracted and subjected to NCode multispecies miRNA V2 microarray (Invitrogen) after Alexa Fluor labeling as described in Supplemental Materials and Methods.
RT-PCR and real-time PCR
RNA was reverse transcribed using High Capacity cDNA Reverse Transcription kits (Applied Biosystems) or TaqMan MicroRNA Reverse Transcription kits (Applied Biosystems). The primer design for target genes and RT-PCR was carried out as described previously (44). Real-time PCR was carried out as described previously (44) with TaqMan MicroRNA Assays (Applied Biosystems) and TaqMan Universal PCR Master Mix (Applied Biosystems) for miRNA, and with SYBR Green PCR Master Mix (Applied Biosystems) and the same primer sets for RT-PCR to assay Mapk8, Ccne1, and Cdc6. Expression levels were based on the amount of the target message relative to that of the actin transcript as a control. Primer sets are listed in Supplemental Materials and Methods.
BrdU cell proliferation assay
Quiescent cells cultured in 96-well plates were transfected with pre-miR miRNA precursor molecule using 0.3 μl of Lipofectamine RNAiMAX. Then, TSH treatment was carried out for 30 h, with BrdU added for the last 6 h. BrdU incorporation was determined with the 5′-Bromo-2′-Deoxyuridine Labeling and Detection kit III (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's recommendations.
Flow cytometry
Quiescent cells cultured in six-well plates were transfected with pre-miR miRNA precursor molecules using 5 μl of Lipofectamine RNAiMAX. Then, TSH treatment was carried out for 24 h, and the cells were fixed and stained with a BrdU Flow kit (BD, Franklin Lakes, NJ). Flow cytometric analyses of propidium iodide-labeled cells were performed using the FACSCalibur (BD).
Rat gene expression microarray
Quiescent FRTL-5 cells were treated with or without TSH, and total RNA was isolated and subjected to rat expression 12×135k array (Roche Diagnostics) after Cy3 labeling as described in Supplemental Materials and Methods.
Prediction of miRNA target genes and sequences
The lists of all the target genes for each miRNA were obtained from the following prediction programs: TargetScan 5.1 (http://www.targetscan.org/) (45), miRDB (http://mirdb.org/miRDB/) (46), microT 3.0 (http://diana.cslab.ece.ntua.gr/microT/) (47), and miRNA.org (http://www.microrna.org/microrna/home.do) (48). Identified candidate genes were compared with the genes increased over 1.5-fold by TSH, which were separately determined by rat expression microarray. The sequence of the miRNA binding site on the 3′-UTR of the target gene was obtained by microT and confirmed by miRDB and TargetScan.
Protein preparation and Western blot analysis
Cellular protein was extracted and analyzed based on a previous report (49) as described in Supplemental Materials and Methods.
Construction of luciferase expression reporter plasmids and luciferase assays
Target sites of miR-16 and miR-195 were predicted in the 3′-UTR of Mapk8, Ccne1, and Cdc6 by microT 3.0 and TargetScan 5.1 programs (Fig. 2A). Synthetic oligodeoxynucleotides of wild type or mutant of these sites, 403–430 for the Mapk8 3′-UTR, 219–245 and 471–485 for the Ccne1 3′-UTR, and 2140–2166 of the Cdc6 3′-UTR, were cloned into miRNA expression reporter vector pMIR-REPORT Luciferase (Applied Biosystems) using a MluI site (5′-end) and a HindIII site (3′-end). FRTL-5 cells cultured in six-well plates were transfected with pre-miR miRNA precursor molecule using 1.5 μl of Lipofectamine RNAiMAX. The next day, 50 ng of each luciferase expression plasmid were transfected with 0.15 μl of FuGENE HD (Roche Diagnostics). After the treatment with TSH for 12 h, cells were lysed with 100 μl of Glo lysis buffer (Promega, Madison, WI). After addition of 100 μl of Bright-Glo luciferase assay reagent (Promega), luciferase activity was measured using FLUOstar Galaxy (BMG Labtechnologies, Offenburg, Germany) as described (49).
Statistical analysis
All experiments were repeated at least three times. Student's t test was used for statistical analyses.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research 15390296 and 21591187 (to K.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- Bromodeoxyuridine
- Bt2cAMP
- N6,2′-O-dibutyryladenosine cAMP
- Cdc
- cell division cycle
- CDK
- cyclin-dependent kinase
- CRE
- cAMP response element
- JNK
- c-Jun N-terminal kinase
- MEK
- MAPK/ERK kinase
- miRNA (or miR)
- microRNA
- PI3K
- phosphatidylinositol 3-kinase
- PKA
- protein kinase A
- UTR
- untranslated region.
References
- 1. Brunetti A , Chiefari E , Filetti S , Russo D. 2000. The 3′,5′-cyclic adenosine monophosphate response element binding protein (CREB) is functionally reduced in human toxic thyroid adenomas. Endocrinology 141:722–730 [DOI] [PubMed] [Google Scholar]
- 2. Suh JM , Song JH , Kim DW , Kim H , Chung HK , Hwang JH , Kim JM , Hwang ES , Chung J , Han JH , Cho BY , Ro HK , Shong M. 2003. Regulation of the phosphatidylinositol 3-kinase, Akt/protein kinase B, FRAP/mammalian target of rapamycin, and ribosomal S6 kinase 1 signaling pathways by thyroid-stimulating hormone (TSH) and stimulating type TSH receptor antibodies in the thyroid gland. J Biol Chem 278:21960–21971 [DOI] [PubMed] [Google Scholar]
- 3. Nedachi T , Akahori M , Ariga M , Sakamoto H , Suzuki N , Umesaki K , Hakuno F , Takahashi SI. 2000. Tyrosine kinase and phosphatidylinositol 3-kinase activation are required for cyclic adenosine 3′,5′-monophosphate-dependent potentiation of deoxyribonucleic acid synthesis induced by insulin-like growth factor-I in FRTL-5 cells. Endocrinology 141:2429–2438 [DOI] [PubMed] [Google Scholar]
- 4. Fukushima T , Nedachi T , Akizawa H , Akahori M , Hakuno F , Takahashi S. 2008. Distinct modes of activation of phosphatidylinositol 3-kinase in response to cyclic adenosine 3′, 5′-monophosphate or insulin-like growth factor I play different roles in regulation of cyclin D1 and p27Kip1 in FRTL-5 cells. Endocrinology 149:3729–3742 [DOI] [PubMed] [Google Scholar]
- 5. Takahashi SI , Nedachi T , Fukushima T , Umesaki K , Ito Y , Hakuno F , Van Wyk JJ , Conti M. 2001. Long-term hormonal regulation of the cAMP-specific phosphodiesterases in cultured FRTL-5 thyroid cells. Biochim Biophys Acta 1540:68–81 [DOI] [PubMed] [Google Scholar]
- 6. Alvarez-Garcia I , Miska EA. 2005. MicroRNA functions in animal development and human disease. Development 132:4653–4662 [DOI] [PubMed] [Google Scholar]
- 7. Cuellar TL , McManus MT. 2005. MicroRNAs and endocrine biology. J Endocrinol 187:327–332 [DOI] [PubMed] [Google Scholar]
- 8. Gantier MP , Sadler AJ , Williams BR. 2007. Fine-tuning of the innate immune response by microRNAs. Immunol Cell Biol 85:458–462 [DOI] [PubMed] [Google Scholar]
- 9. Tomaru Y , Hayashizaki Y. 2006. Cancer research with non-coding RNA. Cancer Sci 97:1285–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poy MN , Eliasson L , Krutzfeldt J , Kuwajima S , Ma X , Macdonald PE , Pfeffer S , Tuschl T , Rajewsky N , Rorsman P , Stoffel M. 2004. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 432:226–230 [DOI] [PubMed] [Google Scholar]
- 11. Sirotkin AV , Ovcharenko D , Grossmann R , Lauková M , Mlyncek M. 2009. Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J Cell Physiol 219:415–420 [DOI] [PubMed] [Google Scholar]
- 12. Nikiforova MN , Chiosea SI , Nikiforov YE. 2009. MicroRNA expression profiles in thyroid tumors. Endocr Pathol 20:85–91 [DOI] [PubMed] [Google Scholar]
- 13. Menon MP , Khan A. 2009. Micro-RNAs in thyroid neoplasms: molecular, diagnostic and therapeutic implications. J Clin Pathol 62:978–985 [DOI] [PubMed] [Google Scholar]
- 14. Pallante P , Visone R , Ferracin M , Ferraro A , Berlingieri MT , Troncone G , Chiappetta G , Liu CG , Santoro M , Negrini M , Croce CM , Fusco A. 2006. MicroRNA deregulation in human thyroid papillary carcinomas. Endocr Relat Cancer 13:497–508 [DOI] [PubMed] [Google Scholar]
- 15. Visone R , Pallante P , Vecchione A , Cirombella R , Ferracin M , Ferraro A , Volinia S , Coluzzi S , Leone V , Borbone E , Liu CG , Petrocca F , Troncone G , Calin GA , Scarpa A , Colato C , Tallini G , Santoro M , Croce CM , Fusco A. 2007. Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. Oncogene 26:7590–7595 [DOI] [PubMed] [Google Scholar]
- 16. Weber F , Teresi RE , Broelsch CE , Frilling A , Eng C. 2006. A limited set of human microRNA is deregulated in follicular thyroid carcinoma. J Clin Endocrinol Metab 91:3584–3591 [DOI] [PubMed] [Google Scholar]
- 17. Takakura S , Mitsutake N , Nakashima M , Namba H , Saenko VA , Rogounovitch TI , Nakazawa Y , Hayashi T , Ohtsuru A , Yamashita S. 2008. Oncogenic role of miR-17-92 cluster in anaplastic thyroid cancer cells. Cancer Sci 99:1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Visone R , Russo L , Pallante P , De Martino I , Ferraro A , Leone V , Borbone E , Petrocca F , Alder H , Croce CM , Fusco A. 2007. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer 14:791–798 [DOI] [PubMed] [Google Scholar]
- 19. Suzuki K , Kohn LD. 2006. Differential regulation of apical and basal iodide transporters in the thyroid by thyroglobulin. J Endocrinol 189:247–255 [DOI] [PubMed] [Google Scholar]
- 20. Suzuki K , Lavaroni S , Mori A , Ohta M , Saito J , Pietrarelli M , Singer DS , Kimura S , Katoh R , Kawaoi A , Kohn LD. 1998. Autoregulation of thyroid-specific gene transcription by thyroglobulin. Proc Natl Acad Sci USA 95:8251–8256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Finnerty JR , Wang WX , Hébert SS , Wilfred BR , Mao G , Nelson PT. 2010. The miR-15/107 group of microRNA genes: evolutionary biology, cellular functions, and roles in human diseases. J Mol Biol 402:491–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Doench JG , Sharp PA. 2004. Specificity of microRNA target selection in translational repression. Genes Dev 18:504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun Q , Zhang Y , Yang G , Chen X , Zhang Y , Cao G , Wang J , Sun Y , Zhang P , Fan M , Shao N , Yang X. 2008. Transforming growth factor-β-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Res 36:2690–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mody N , Leitch J , Armstrong C , Dixon J , Cohen P. 2001. Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett 502:21–24 [DOI] [PubMed] [Google Scholar]
- 25. Lazzereschi D , Sambuco L , Carnovale Scalzo C , Ranieri A , Mincione G , Nardi F , Colletta G. 1998. Cyclin D1 and cyclin E expression in malignant thyroid cells and in human thyroid carcinomas. Int J Cancer 76:806–811 [DOI] [PubMed] [Google Scholar]
- 26. Hara T , Namba H , Takamura N , Yang TT , Nagayama Y , Fukata S , Kuma K , Ishikawa N , Ito K , Yamashita S. 1999. Thyrotropin regulates c-Jun N-terminal kinase (JNK) activity through two distinct signal pathways in human thyroid cells. Endocrinology 140:1724–1730 [DOI] [PubMed] [Google Scholar]
- 27. Blow JJ , Dutta A. 2005. Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol 6:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sekiya Y , Ogawa T , Iizuka M , Yoshizato K , Ikeda K , Kawada N. 2011. Down-regulation of cyclin E1 expression by microrna-195 accounts for interferon-β-induced inhibition of hepatic stellate cell proliferation. J Cell Physiol 226:2535–2542 [DOI] [PubMed] [Google Scholar]
- 29. Wang F , Fu XD , Zhou Y , Zhang Y. 2009. Down-regulation of the cyclin E1 oncogene expression by microRNA-16-1 induces cell cycle arrest in human cancer cells. BMB Rep 42:725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ofir M , Hacohen D , Ginsberg D. 2011. miR-15 and miR-16 are direct transcriptional targets of E2F1 that limit E2F-induced proliferation by targeting cyclin E. Mol Cancer Res 9:440–447 [DOI] [PubMed] [Google Scholar]
- 31. Liu Q , Fu H , Sun F , Zhang H , Tie Y , Zhu J , Xing R , Sun Z , Zheng X. 2008. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res 36:5391–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He H , Jazdzewski K , Li W , Liyanarachchi S , Nagy R , Volinia S , Calin GA , Liu CG , Franssila K , Suster S , Kloos RT , Croce CM , de la Chapelle A. 2005. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA 102:19075–19080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen YT , Kitabayashi N , Zhou XK , Fahey TJ , Scognamiglio T. 2008. MicroRNA analysis as a potential diagnostic tool for papillary thyroid carcinoma. Mod Pathol 21:1139–1146 [DOI] [PubMed] [Google Scholar]
- 34. Mitomo S , Maesawa C , Ogasawara S , Iwaya T , Shibazaki M , Yashima-Abo A , Kotani K , Oikawa H , Sakurai E , Izutsu N , Kato K , Komatsu H , Ikeda K , Wakabayashi G , Masuda T. 2008. Downregulation of miR-138 is associated with overexpression of human telomerase reverse transcriptase protein in human anaplastic thyroid carcinoma cell lines. Cancer Sci 99:280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bagnyukova TV , Pogribny IP , Chekhun VF. 2006. MicroRNAs in normal and cancer cells: a new class of gene expression regulators. Exp Oncol 28:263–269 [PubMed] [Google Scholar]
- 36. Chamorro-Jorganes A , Araldi E , Penalva LO , Sandhu D , Fernández-Hernando C , Suárez Y. 2011. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler Thromb Vasc Biol 31:2595–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu H , Liu T , Wang R , Tian S , Liu M , Li X , Tang H. 2011. MicroRNA-16 targets zyxin and promotes cell motility in human laryngeal carcinoma cell line HEp-2. IUBMB Life 63:101–108 [DOI] [PubMed] [Google Scholar]
- 38. Baudry A , Mouillet-Richard S , Schneider B , Launay JM , Kellermann O. 2010. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science 329:1537–1541 [DOI] [PubMed] [Google Scholar]
- 39. Zhang X , Wan G , Mlotshwa S , Vance V , Berger FG , Chen H , Lu X. 2010. Oncogenic Wip1 phosphatase is inhibited by miR-16 in the DNA damage signaling pathway. Cancer Res 70:7176–7186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laneve P , Gioia U , Andriotto A , Moretti F , Bozzoni I , Caffarelli E. 2010. A minicircuitry involving REST and CREB controls miR-9-2 expression during human neuronal differentiation. Nucleic Acids Res 38:6895–6905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vo N , Klein ME , Varlamova O , Keller DM , Yamamoto T , Goodman RH , Impey S. 2005. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci USA 102:16426–16431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leone V , D'Angelo D , Ferraro A , Pallante P , Rubio I , Santoro M , Croce CM , Fusco A. 2011. A TSH-CREB1-microRNA loop is required for thyroid cell growth. Mol Endocrinol 25:1819–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Gregorio G , Coppa A , Cosentino C , Ucci S , Messina S , Nicolussi A , D'Inzeo S , Di Pardo A , Avvedimento EV , Porcellini A. 2007. The p85 regulatory subunit of PI3K mediates TSH-cAMP-PKA growth and survival signals. Oncogene 26:2039–2047 [DOI] [PubMed] [Google Scholar]
- 44. Akama T , Suzuki K , Tanigawa K , Kawashima A , Wu H , Nakata N , Osana Y , Sakakibara Y , Ishii N. 2009. Whole-genome tiling array analysis of Mycobacterium leprae RNA reveals high expression of pseudogenes and noncoding regions. J Bacteriol 191:3321–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Friedman RC , Farh KK , Burge CB , Bartel DP. 2009. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang X , El Naqa IM. 2008. Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics 24:325–332 [DOI] [PubMed] [Google Scholar]
- 47. Maragkakis M , Alexiou P , Papadopoulos GL , Reczko M , Dalamagas T , Giannopoulos G , Goumas G , Koukis E , Kourtis K , Simossis VA , Sethupathy P , Vergoulis T , Koziris N , Sellis T , Tsanakas P , Hatzigeorgiou AG. 2009. Accurate microRNA target prediction correlates with protein repression levels. BMC Bioinformatics 10:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Betel D , Wilson M , Gabow A , Marks DS , Sander C. 2008. The microRNA.org resource: targets and expression. Nucleic Acids Res 36:D149–D153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tanigawa K , Suzuki K , Nakamura K , Akama T , Kawashima A , Wu H , Hayashi M , Takahashi S , Ikuyama S , Ito T , Ishii N. 2008. Expression of adipose differentiation-related protein (ADRP) and perilipin in macrophages infected with Mycobacterium leprae. FEMS Microbiol Lett 289:72–79 [DOI] [PubMed] [Google Scholar]