Abstract
The NR4A orphan nuclear receptors Nur77, Nurr1, and Nor1 exert multiple cellular and metabolic functions. These transcriptional regulators are activated in response to extracellular stresses, including lipotoxic fatty acids (FA) and proinflammatory cytokines. The contribution of NR4As to β-cell pathophysiology is, however, unknown. We have therefore examined the role of NR4As as downstream contributors to FA-induced β-cell dysfunctions. Human pancreatic islets and insulinoma β-cells were used to determine transcriptional programs elicited by NR4A, which were compared to those triggered by palmitate treatment. Functional studies evaluated the consequence of an increased NR4A expression on insulin biosynthesis and secretion and cell viability in insulinoma β-cells. FA and cytokine treatment increased NR4A expression in pancreatic β-cells, with Nur77 being most highly inducible in murine β-cells. Nur77, Nurr1, or Nor1 modulated common and distinct clusters of genes involved notably in cation homeostasis and insulin gene transcription. By altering zinc homeostasis, insulin gene transcription, and secretion, Nur77 was found to be a major transcriptional mediator of part of FA-induced β-cell dysfunctions. The repressive role of Nur77 in insulin gene regulation was tracked down to protein-protein interaction with FoxO1, a pivotal integrator of the insulin gene regulatory network. The present study identifies a member of the NR4A nuclear receptor subclass, Nur77/NR4A1, as a modulator of pancreatic β-cell biology. Together with its previously documented role in liver and muscle, its role in β-cells establishes Nur77 as an important integrator of glucose metabolism.
The endocrine pancreas consists of islets that display a species-specific cellular architecture (1) harboring four cell types, of which the β-cell secretes insulin. It is now firmly established that type 2 diabetes (T2DM) does not appear without β-cell dysfunction (2). Insulin biosynthesis is controlled both at the translation level, for short-term responses (<20 min), and at the transcription level for long-term responses. This latter process is under the control of a transcription factor network in which mafA plays a critical role by binding to and activating the insulin gene promoter, in synergy with Pdx1 and NeuroD (3).
Glucose-stimulated insulin secretion (GSIS) occurs through the coupling of glucose metabolism to K+- and Ca++-dependent membrane polarization, which may also be affected by nervous inputs (4). Concomitant, acute exposure of β-cells to free fatty acids (FFA) synergistically induces insulin secretion. However, a chronic exposure to FFA in vitro is detrimental to β-cell functions (lipotoxicity), first preventing GSIS and then leading to apoptosis. High glucose concentrations also alter β-cell functions, and FFA-induced dysfunction is most prominent upon concomitant hyperglycemia (glucotoxicity) (5). Several mechanisms are involved in FFA-induced GSIS inhibition, including triglyceride storage, reactive oxygen species production through mitochondrial uncoupling, dysregulation of the glucose metabolic pathway (5), and GPR40 FFA membrane receptor activation (6). FFA derivatives, such as diacylglycerol and ceramide, also play an important role in the pathogenic process. In line with the view that chronic exposure to FFA is deleterious for pancreas function, obesity is established as a major risk factor for T2DM (7). Proinflammatory cytokines, such as IL-1β, TNF-α, and interferon(Ifn)-γ are prevailing deleterious signals in type 1 diabetes and contribute to T2DM progression by inhibiting β-cell metabolism and GSIS, ultimately causing apoptosis (8, 9).
Nuclear receptors (NR) are involved in pancreas development and in maintaining its functions during adulthood. All trans-retinoic acid, a ligand for the NR for retinoic acid (retinoic acid receptor), is required for pancreas formation (10, 11). The vitamin D receptor, thyroid hormone receptor, glucocorticoid receptor, orphan receptor hepatocyte nuclear factor-4 and oxysterol, bile acids and fatty acid (FA)-regulated liver X receptor, farnesoid X receptor, and peroxisome proliferator-activated receptor (PPAR) are also involved in β-cell pathophysiology (12, 13). The expression of NR4A nuclear orphan receptors [NR4A1 (Nur77/NGFI/TR3), NR4A2 (Nurr1/NOT), NR4A3 (Nor1, MINOR) hereafter called Nur77, Nurr1 and Nor1] is increased in response to several cellular stresses, including oxidative stress, growth factors, cytokines, and physical stimuli in a number of cell types including stimulated macrophages (14) and several inflamed tissues (15). We observed increased NR4A expression in isolated human pancreatic islets upon treatment with the saturated FA palmitate or with cytokines. Analogous results were obtained in rodent insulinoma β-cells, suggesting that NR4A regulate β-cell responses to cytotoxic signals. Transcriptome analysis showed that Nur77 overexpression altered clusters of genes, notably controlling ion homeostasis and insulin gene transcription. Functional analysis established that increased Nur77 expression is detrimental to β-cell function by decreasing intracellular insulin content and GSIS. The decreased insulin gene transcription could be attributed to direct protein-protein interaction of Nur77 with FoxO1 and subsequent inhibition of mafA expression.
Results
The NR4A orphan receptors are expressed in human and mouse pancreatic β-cells and induced by a lipotoxic stress
We first investigated the subtissular distribution of Nur77, Nurr1, and Nor1 in human pancreas. Immunohistochemical detection of NR4A receptors in this tissue from normal donors showed a localization in pancreatic islets and colocalization with chromogranin A (Fig. 1A), indicative of a β-cell-selective expression. Similarly, immunofluorescence studies using available antibodies, which allowed detection of only Nur77 and Nurr1 in mouse pancreas, demonstrated their localization in the cytoplasm of β-cells as shown by insulin costaining (Fig. 1B). Assaying the relative expression level of NR4A mRNA by reverse transcription-quantitative PCR (RT-QPCR) showed that purified human islets (HPI) expressed similar levels in Nur77 and Nurr1 at basal levels, whereas Nur77 was predominantly expressed in purified mouse islets (MPI). Nor1 always displayed the lowest expression (Fig. 1, C and D).
Fig. 1.
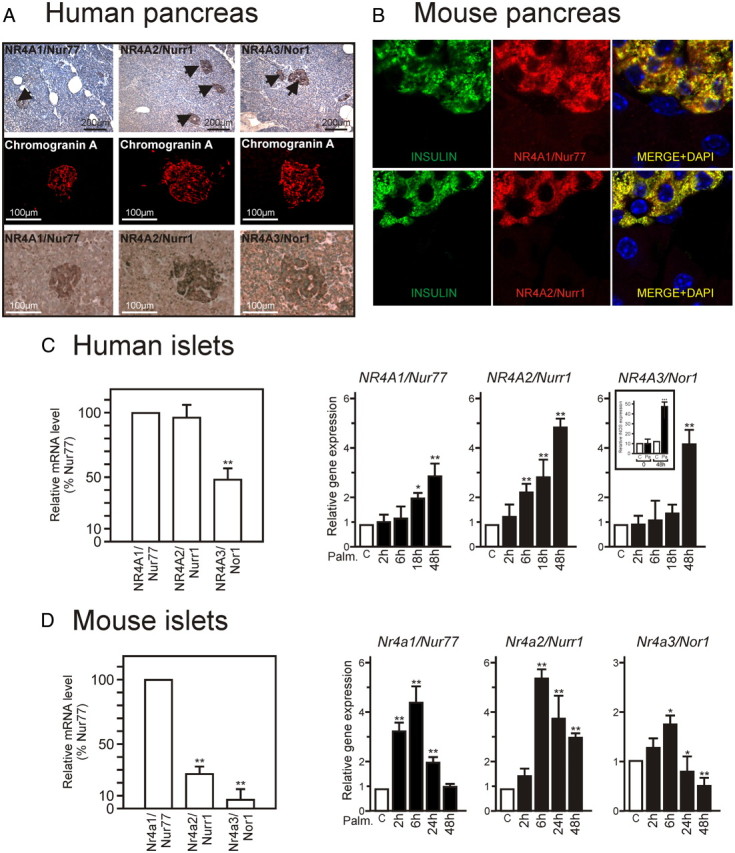
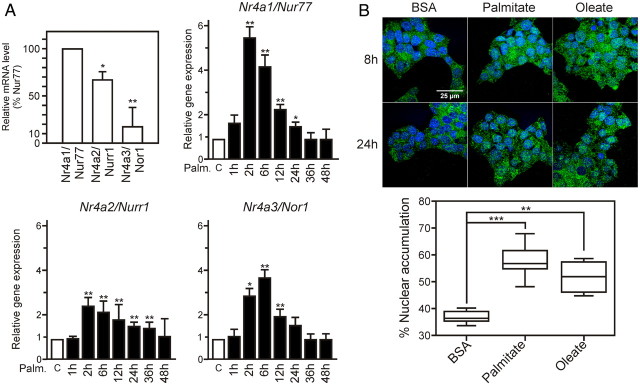
NR4A orphan receptors are expressed in pancreatic β-cells and induced upon palmitate treatment. A, Immunohistochemical staining of human pancreas. Human pancreata were processed as described in Materials and Methods. Every other section was stained for the selective β-cell marker chromogranin A or NR4A1/Nur77, NR4A2/Nurr1, or NR4A3/Nor1 (lower panels). Representative sections are shown at low (upper row) and higher (lower rows) magnification. B, Immunofluorescence staining of mouse pancreas. Mouse pancreas were processed as in panel A, and insulin (green) and NR4As (red) were detected by indirect immunofluorescence. Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI). C and D, The relative level of expression of NR4A was assayed by Taqman-based QPCR and expressed relative to that of Nur77 (set to 100%). Increased expression of NR4A-encoding genes upon 0.4 mm palmitate treatment in purified human pancreatic islets (C) and mouse pancreatic islets (D). mRNA coding for each NR4A were quantified by Taqman-based QPCR. Basal level in uninduced tissues (ranging form 23 to 26 Ct) was arbitrarily set to 1 (n = 3). Data are presented as the mean ± sem. Statistical significance was evaluated by one-way ANOVA followed by Dunnett's post hoc test, and is displayed as *, P < 0.05 or **, P < 0.01. C, (inset), Expression level of inducible NOS (iNOS) mRNA. iNOS mRNA was used as a quality control for palmitate efficiency (50).
HPI were challenged with palmitate, a stimulus known to induce many of the changes in β-cell gene expression observed in T2DM (16), or with time-matched vehicle control (BSA). BSA treatment alone did not induce any significant change in gene expression at any time in HPI or murine β-cells (Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). HPI mRNA were analyzed for NUR77, NURR1, and NOR1 gene expression, with Nurr1 displaying the earliest and highest expression (Fig. 1C). Palmitate also induced NR4A expression in MPI, in which the Nur77 gene was the most inducible and with faster kinetics than in HPI (Fig. 1D). Thus, challenging HPI and MPI with palmitate, one of the two most abundant circulating FA elevated in T2DM, triggers an increased expression of NR4A-encoding genes.
A lipotoxic stress promotes NR4A nuclear translocation
The β-insulinoma cell line Min6 was characterized for the response of NR4A-encoding genes to palmitate treatment (Fig. 2A). As noted for MPI, Nur77 mRNA was most abundantly expressed at basal level and strongly induced upon palmitate challenge, peaking at 2–6 h. Oleate was able to induce NR4A expression similarly to palmitate in HPI and in Min6 cells (Supplemental Fig. 1) as well as in rat β-insulinoma INS-1 cells (Carpentier, R., and P. Lefebvre, data not shown), suggesting that the effector pathway is distinct from the ceramide/ER stress and apoptotic pathways (17). Finally, a cytokine mix (IL1β, Ifnγ, and TNF) also promoted NR4A expression in HPI and Min6 cells, with a species-specific pattern similar to that elicited by palmitate (Supplemental Fig. 2). Thus up-regulation of NR4A expression in response to cellular stresses is common to human and rodent β-cells, and Min6 cells are a relevant model by which to study this phenomenon.
Fig. 2.
NR4A orphan receptors are translocated to the nucleus in lipotoxic conditions and regulate overlapping and distinct subsets of genes. A, Expression levels of NR4As at basal level (upper left panel) and after palmitate stimulation in Min6 cells. B, Intracellular localization of Nur77 in Min6 cells. Min6 cells were treated for 24 h with BSA, BSA-palmitate, or BSA-oleate, and Nur77 was detected by indirect immunofluorescence. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) and slides were examined using a confocal microscope (Zeiss LSM710) in a sequential mode of acquisition. Pictures were treated, analyzed, and quantified (lower panel) using ImageJ software. A representative Z section is shown. Data are presented as the mean ± sem. Statistical significance was evaluated by one-way ANOVA followed by Dunnett's post hoc test and is displayed as *, P < 0.05; **, P < 0.01; ***, P < 0.005.
The biological activity of NR4A receptors is dependent on their subcellular localization (18). Nur77 (Fig. 2B) and Nurr1 (Supplemental Fig. 3) proteins exhibited a predominant cytoplasmic localization in untreated Min6 cells, thus mimicking mouse primary β-cell subcellular repartition (Fig. 1B). After 8-h palmitate or oleate treatment, a 2-fold increase in the Nur77 content was observed in the nuclear compartment (Fig. 2B). Taken together, our results (Figs. 1 and 2) show that a lipotoxic insult promotes increased expression and nuclear translocation of Nur77 and Nurr1, suggesting that they may serve as molecular relays for at least part of FA-induced transcriptional responses.
NR4A regulate distinct but functionally overlapping subsets of genes in human and murine β-cells
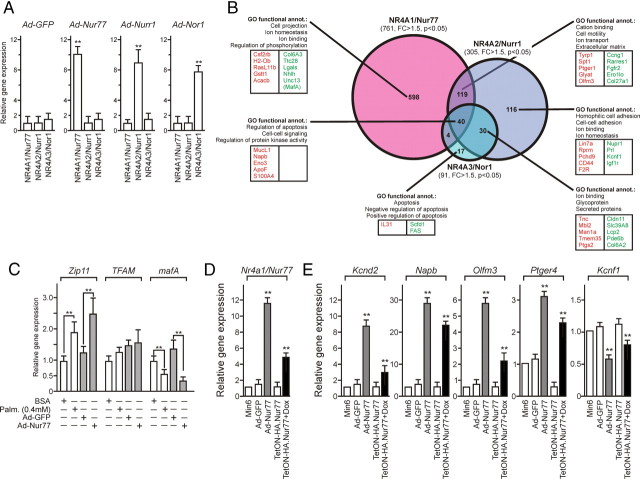
To compare the transcriptional program elicited by each NR4A in our system, Min6 cells were transduced with adenoviral particles coding for the wild-type form of each receptor, in conditions inducing a moderate 6- to 10-fold overexpression at the mRNA (Fig. 3A) and protein level (see Fig. 6E), which was accompanied by the nuclear localization of the overexpressed NR4A isotype (Fig. 6E and data not shown). The gene expression pattern induced by each receptor was compared with control condition [Ad-green fluorescent protein (GFP)] using DNA microarrays (Fig. 3B and Supplemental worksheet 1). NR4A1/Nur77 overexpression modulated the expression of 761 genes with a fold change above 1.5 (P < 0.05). NR4A2/Nurr1 modulated the expression of 305 genes, and Nor1 of 91 genes (Fig. 3B). Of note, only 40 genes were modulated by all three NR4A whereas 119 genes were regulated by both NR4A1/Nur77 and NR4A2/Nurr1. A gene ontology (GO) classification was used to identify functional clusters of genes and is depicted in Fig. 3B. The transcriptional program specific to NR4A1/Nur77 pertains mostly to ion binding and ion homeostasis and was thus similar, in this respect, to that specifically controlled by NR4A2/Nurr1. The set of genes regulated in common by NR4A1/Nur77 and NR4A2/Nurr1 defined regulatory pathways involved in cation binding and ion transport. Finally, the NR4A3/Nor1-controlled transcriptome consisted of a reduced list of genes with very moderate fold changes (<2-fold for 96% of genes, see Supplemental worksheet 1), with a putative role in apoptosis.
Fig. 3.
Nur77 is most efficient at regulating a transcriptional response in Min6 cells. A, Relative expression levels of NR4A after adenoviral transduction. Min6 cells were transduced at MOI 30 and mRNA were analyzed 24 h later by RT-QPCR. Basal levels were set to 1. Statistical significance was evaluated by a t test and is displayed as *, P < 0.05; **, P < 0.01. B, Venn diagram and GO analysis of NR4A target genes in Min6 cells. The total number of genes modulated by each receptor is indicated in brackets, and the Venn diagram indicates specific and overlapping subsets of target genes. The GO functional classification is indicated with the five most significant categories, and the five most up-regulated (red) and most down-regulated (green) genes are mentioned for each target gene subset. C, Comparative expression levels of Nur77-regulated genes after Nur77 adenoviral transduction and palmitate treatment. Min6 cells were either transduced with Ad-Nur77 or treated with 0.4 mm palmitate for 24 h. mRNA levels were quantified by RT-QPCR and expressed relative to the basal level arbitrarily set to 1. Statistical significance was evaluated by a t test and is displayed as *, P < 0.05; **, P < 0.01; ***, P < 0.005. D, Relative Nur77 expression levels after Ad-Nur77 transduction (Ad-Nur77) or doxycyclin induction in the Tet-ON system (TetON-HA.Nur77). Results are expressed as in panel C. E, Comparative expression levels of Nur77-regulated genes in adenovirally transduced or TetON-HA.Nur77 Min6 cells. Results are expressed as in panel C.
Fig. 6.
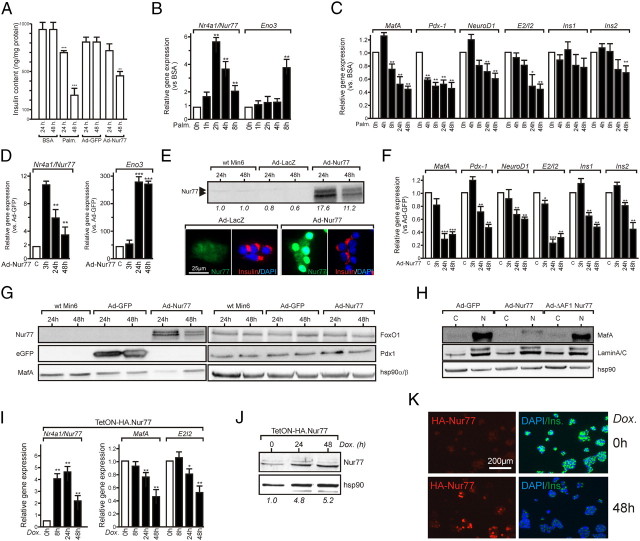
Nur77 regulates insulin synthesis. A, Min6 insulin intracellular content. After palmitate treatment or adenoviral transduction for the indicated times, cell layers were processed to extract and assay insulin. Data are presented as the mean ± sem (n = 3). Statistical significance was evaluated by one-way ANOVA followed by Dunnett's post hoc test and is displayed as *, P < 0.05; **, P < 0.01; ***, P < 0.005. B, Nur77 responsiveness to palmitate treatment. Min6 cells were treated with 0.4 mm palmitate for the indicated times, and gene expression levels were assayed by QPCR. Basal levels were arbitrarily set to 1. Data are presented as the mean ± sem (n = 3) and were analyzed as in panel A. C, Nur77 target genes expression after palmitate treatment. Min6 cells were treated for the indicated times, and gene expression was assayed and analyzed as in panel A. D, Adenoviral transduction of Nur77 elevates enolase3 expression. Min6 cells were transduced with Ad-Nur77, and gene expression was monitored and analyzed as in panel A. E, Nur77 protein expression level in transduced Min6 cells. Min6 cells were transduced with the indicated adenovirus, and the Nur77 polypeptide was detected by either Western blot (upper panel) or indirect immunofluorescence (lower panel). Data were quantified using the ImageQuant software. F, Nur77 target genes expression after adenoviral transduction. Min6 cells were transduced as in panel D, and data were collected and expressed as in panel A. G, Adenovirus-mediated expression of Nur77 decreases mafA expression. Whole-cell extracts were prepared 24 or 48 h after transduction with the indicated adenoviruses and analyzed by Western blotting. H, mafA is expressed in the nucleus. Cytosolic (C) and nuclear fractions (N) were prepared in conditions similar to panel G and analyzed by Western blotting using an anti-mafA, an anti-Nur77 (targeting the N terminus of Nur77), or anti-laminA/C and anti-hsp90, used as a marker for the nuclear fraction or for even loading, respectively. I–K, Nur77 equally represses insulin expression in the doxycyclin-dependent system TetON-HA.Nur77. I, Gene expression in TetON-Nur77 cells. Cells were treated by doxycyclin for the indicated time, after which total RNA was extracted and analyzed for its content in Nur77, mafA, and insulin pre-mRNA content (E2/I2). The abundance of each mRNA in the basal state was arbitrarily set to 1, and data are presented as the mean ± sem (n = 3). Data are presented as the mean ± sem (n = 3) and were analyzed as in panel A. J, Nur77 protein expression levels in the doxycyclin-dependent system TetON-HA.Nur77. Bands were quantified using ImageQuant software. K, Insulin intracellular content in the doxycyclin-dependent system TetON-HA.Nur77. Nur77 and insulin were detected by indirect immunofluorescence in noninduced (0 h) and after a 48-h doxycyclin treatment (48 h).
Nur77 appeared as the most active transcriptional modulator in Min6 cells and was the most efficiently induced receptor upon palmitate treatment in rodent β-cells. A particular attention was paid to individual gene regulation by Nur77, which revealed that mafA and Nkx6-1, both transcriptional regulators of insulin gene expression, were significantly down-regulated upon NR4A1/Nur77 overexpression. Palmitate exerts measurable effects on insulin secretion after a 24-h treatment, and Nur77 overexpression for the same duration mimicked in most instances palmitate-induced gene regulation of various genes involved in β-cell biology (Fig. 3C and Supplemental Figs. 4 and 7). Of note, mafA, Nkx6-1, Pdx1, and NeuroD1 expression was blunted upon Nur77 expression and palmitate treatment (Fig. 3C and Supplemental Fig. 5). Observed up- or down-regulations were dose dependent, because two distinct Nur77 expression systems (adenoviral transduction or TetOn system, see Fig. 6, E and J) induced gene expression alterations as a function of Nur77 expression level (Fig. 3, D and E). Thus, Nur77 represses insulin-regulatory genes in a manner similar to palmitate.
Because Nurr1 expression in HPI was the most sensitive to palmitate treatment, being in that respect analogous to Nur77 in MPI and Min6 cells, we wished to establish a functional parallel between Nurr1 in HPI and Nur77 in Min6 cells. Nurr1 was thus overexpressed in HPI by adenoviral transduction (∼6-fold), and its impact on HPI transcriptome was assessed by microarray analysis. Nurr1 modulated the expression of 1051 genes, which were clustered around GO functional categories (Supplemental Fig. 5). Although a relatively small fraction of identical genes (17%) were commonly regulated by Nurr1 in HPI and Nur77 in Min6 cells, this in silico analysis highlighted again cation homeostasis as a significantly altered gene cluster (Supplemental Fig. 5). To evaluate whether Nurr1 could participate significantly to palmitate-induced transcriptional reprogramming, we carried out a similar transcriptomic analysis of palmitate-treated HPI. Again, GO functional clustering of palmitate-regulated genes revealed functional gene sets similarly regulated by palmitate and Nurr1 in HPI, which are involved in response to molecules of bacterial origin, response nutrient, regulation of apoptosis, and activation of MAPK activity (compare Supplemental Fig. 5 with Supplemental Fig. 6). Quite strikingly, a cation homeostasis cluster was again identified, suggesting 1) that palmitate and Nurr1 control subsets of genes functionally similar in HPI, and 2) that mouse Nur77 and human Nurr1 share a common function (cation homeostasis regulation) in pancreatic β-cells.
To further validate this analysis pointing to a potential role of Nur77 in the regulation of cation homeostasis, we first monitored the expression of zinc transporters in Min6 cells. A preliminary characterization excluded several members of the Znt and Zip gene families because they are only weakly expressed in Min6 cells. Quantitative PCR (QPCR) quantification of significantly expressed (Ct<32) Znt and Zip transcripts showed that whereas Slc30a1/Znt1 and Slc39a8/Zip8 were down-regulated in Nur77-expressing cells, Slc30a6/Znt6 and Slc39a11/Zip11 were up-regulated in these conditions (Supplemental Fig. 7, A and B). Labile intracellular zinc labeling was performed using FluoZin3 to monitor whether these dysregulated gene expressions have functional consequences (Supplemental Fig. 7, C and D). Because Ad-lacZ and Ad-Nur77 transduction of Min6 cells did not induce significant variation in the intracellular localization of chelatable zinc (Supplemental Fig. 7C), variations in zinc staining are very likely to reflect true variations in labile zinc content. This parameter was assessed by fluorescence-activated cell sorting analysis of FluoZin3-stained Min6 cells (Supplemental Fig. 7D). Whereas Ad-lacZ or AΔ-DAF1-Nur77 overexpression did not increase intracellular zinc content, Ad-Nur77 significantly increased this parameter. Similar to Ad-Nur77, palmitate induced a strong increase in intracellular free zinc content.
We thus conclude that Nur77 is an efficient transcriptional modulator in murine insulinoma β-cells, regulating functional clusters of genes similar, in part, to those controlled by Nurr1 in HPI.
Both Nur77/NR4A1 overexpression and lipotoxicity decrease glucose-stimulated insulin secretion
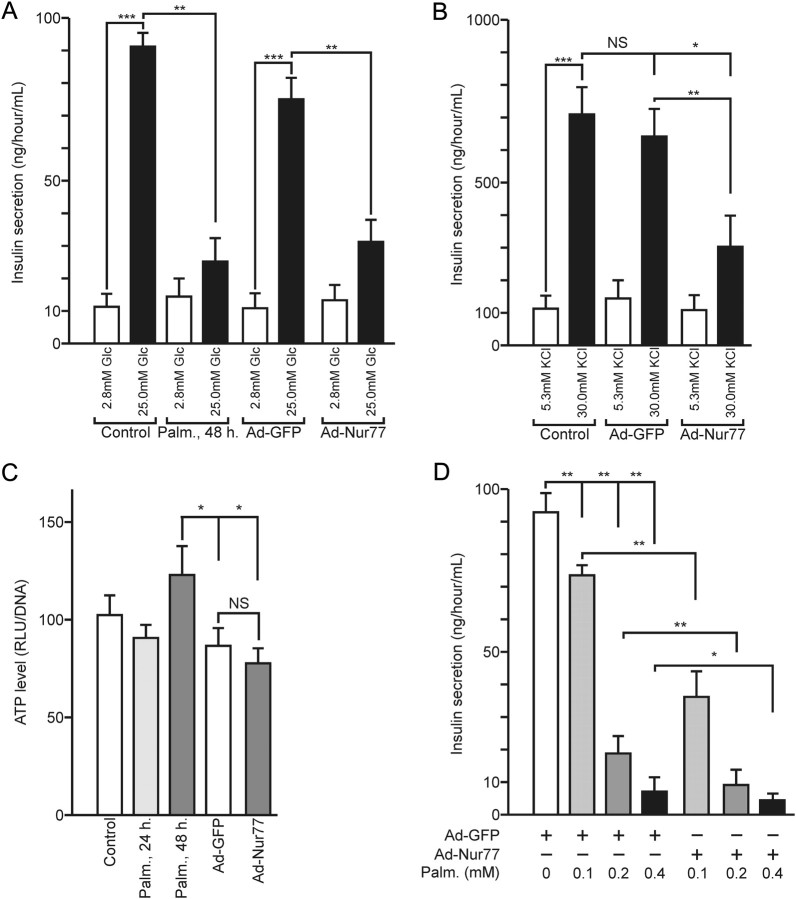
mRNA analysis described above also suggested a role for Nur77 in the control of insulin secretion and/or biosynthesis in Min6 cells. Glucose-induced secretion (GSIS) of naive cells was compared with that of Min6 cells transduced by either a control GFP- or a Nur77-encoding adenovirus (Fig. 4A). Min6 and GFP-transduced cells displayed an 8- to 9-fold increase in insulin secretion (IS) upon glucose challenge for 1 h. Palmitate treatment impaired GSIS in naive cells and GFP-expressing cells as expected, whereas overexpression of Nur77 significantly blunted GSIS, an effect also observed at earlier time points of glucose stimulation (Supplemental Fig. 8). When glucose was substituted for a depolarizing concentration of potassium (KSIS), a significant change in IS was also observed in naive and GFP-expressing cells. Again, Nur77 overexpression blunted the induced insulin secretion, indicating that Nur77 acts before insulin release per se (Fig. 4B). A possible interference could occur at any step of the ATP-generating glycolysis. However, the ATP content of Min6 cells did not vary significantly upon Nur77 overexpression, although a slightly decreased intracellular ATP concentration attributable to adenoviral transduction was detected (Fig. 4C). These results thus exclude major perturbation of the glycolytic oxidative pathway and of the metabolism-excitation coupling mechanism.
Fig. 4.
Nur77 regulates insulin secretion in Min6 cells. A, Glucose-induced insulin secretion. Min6 cells were treated with 0.4 mm palmitate or transduced for 48 h with a GFP- or a Nur77-encoding adenovirus. After a starvation period, cells were exposed to 2.8 mm or 25.0 mm glucose for 1 h. Secreted insulin was measured and expressed in ng/h/ml. Data are presented as the mean ± sem (n = 3). Statistical significance was evaluated by one-way ANOVA followed by Dunnett's post hoc test and is displayed as *, P < 0.05; **, P < 0.01; ***, P < 0.005. B, Potassium-induced insulin secretion. Cells were exposed to 5.3 mm or 30 mm KCl for 1 h. Secreted insulin was measured and expressed in ng/h/ml. Data are presented as the mean ± sem. Statistical significance was evaluated by one-way ANOVA followed by Dunnett's post hoc test and is displayed as *, P < 0.05; **, P < 0.01; ***, P < 0.005. C, ATP levels in Min6 cells. Min6 cells were treated as in panel A, and ATP levels were assayed by chemiluminescence. RLU, Relative light units. Data are presented as the mean ± sem. Statistical significance was evaluated by one-way ANOVA followed by Dunnett's post hoc test and is displayed as *, P < 0.05; **, P < 0.01; ***, P < 0.005. D, Nur77-expressing Min6 cells are more sensitive to palmitate. Min6 cells were transduced with Ad-GFP or Ad-Nur77 and submitted 24 h later to palmitate treatment. Insulin secretion was assayed, and data are presented as the mean ± sem (n = 2). Statistical significance was evaluated by one-way ANOVA followed by Dunnett's post hoc test and is displayed as *, P < 0.05; **, P < 0.01.
The corollary of these observations could be that Nur77 intracellular level impacts on the β-cell response to palmitate treatment. This prediction was tested by submitting naive, GFP- or Nur77-overexpressing Min6 cells to palmitate treatment and by monitoring GSIS. Palmitate caused indeed a more pronounced decrease in GSIS in Nur77-overexpressing cells (Fig. 4D). Nur77 thus appears as contributing significantly to FA-induced dysfunction in Min6 cells.
A transcriptionally-defective Nur77 mutant blunts palmitate inhibition of GSIS
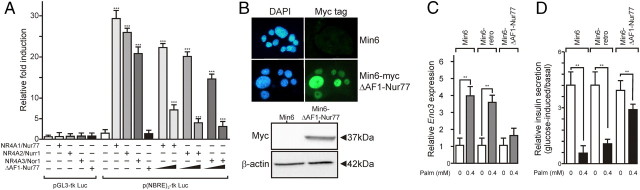
Gain of function experiments thus suggested that Nur77 is a causative factor in palmitate-induced GSIS impairment. Loss of function experiments were thus carried out using a transcriptionally dominant-negative Nur77 mutant (ΔAF1-Nur77), because short hairpin RNA-mediated knockdown of individual NR4A led to compensatory overexpression of other NR4A members. As shown by transient transfection experiments, this AF1-deleted Nur77 mutant was transcriptionally inactive by itself but able to oppose transcriptional activation of a Nur77-binding response element (NBRE)-driven luciferase reporter gene by full-length Nur77, Nurr1, or Nor1 when overexpressed at a 20-fold ratio over wild-type NR4A (Fig. 5A). A Min6 clone overexpressing ΔAF1-Nur77 at a 32-fold ratio over Nur77 was isolated (Fig. 5B), in which palmitate was unable to significantly induce enolase 3, a direct Nur77 target gene driven by an NBRE (19) (Fig. 5C)]. The ability of palmitate to blunt GSIS was assessed in this background. Expression of the dominant-negative ΔAF1-Nur77 mutant clearly protected Min6 cells from palmitate-induced lipotoxicity (Fig. 5C), in agreement with overexpression studies of full-length Nur77, which evidenced a sensitizing effect of Nur77 (Fig. 4D).
Fig. 5.
A transcriptionally defective Nur77 mutant partially blunts lipotoxic effects of palmitate. A, The ΔAF1-Nur77 mutant behaves as a dominant-negative transcription factor in Min6 cells. Min6 cells were transfected either with a control reporter gene (pGL3-tk Luc) or a NBRE-driven reporter gene [p(NBRE)3-tk Luc], with a fixed amount of an expression vector coding either for Nur77, Nurr1, or Nor1. A 5- or 20-fold stoichiometric excess of ΔAF1-Nur77 expression was also used where indicated. Luciferase activity was assayed 48 h after transfection and expressed relative to the basal luciferase activity detected in Min6 cells transfected with p(NBRE)3-tk Luc, which was set to 1. Data are presented as the mean ± sem. Statistical significance was evaluated by one-way ANOVA followed by Dunnett's post hoc test and is displayed as *, P < 0.05; **, P < 0.01; ***, P < 0.005. B, Characterization of Min6 cells overexpressing a myc-tagged version of the dominant-negative, transcriptionally inactive Nur77 mutant. Naive or retrovirally transduced Min6 cells were stained for Myc-tag detection (upper panel), and whole-cell extracts were analyzed by Western blot to assess the molecular mass of the N-terminally truncated Nur77 protein (lower panel). C, Palmitate induction of enolase 3. Min6 or Min6 ΔAF1-Nur77 was treated with palmitate, and enolase 3 expression was monitored by RT-QPCR. Data are presented as the mean ± sem (n = 2) with basal level set to 1. Statistical significance was evaluated by a t test and is displayed as *, P < 0.05; **, P < 0.01. D, Insulin secretion of Min6/ΔAF1-Nur77 cells. Min6 or Min6 ΔAF1-Nur77 cells were treated for 48 h with the indicated concentration of palmitate, and the insulin secretion was assayed as in panel A. Results are expressed as the ratio of the low-glucose to high-glucose insulin secretion. Data are presented as the mean ± sem (n = 2). Statistical significance was evaluated by a t test and is displayed as *, P < 0.05; **, P < 0.01.
Nur77/NR4A1 and the lipotoxic FA palmitate regulate similar subsets of genes controlling the expression of the mouse insulin genes
The Min6 intracellular insulin content was assayed after palmitate treatment or Nur77 overexpression. Both treatments reduced significantly this parameter (Fig. 6A). This suggested that the observed down-regulation of MafA, Pdx-1, and NeuroD1 (Fig. 3C and Supplemental Fig. 5), which are central regulators of the insulin 1 and 2 genes (Ins1 and Ins2), correlates with decreased insulin content. Their expression, as well as that of insulin mRNA and insulin pre-mRNA [noted E2/I2, (20)], was assayed in palmitate-treated or Nur77-expressing cells as a function of time. Palmitate treatment rapidly induced Nur77, which was followed by induction of enolase 3 (Eno3) mRNA (Fig. 6B). A significantly time-dependent reduced expression of mafA, Pdx-1, and NeuroD mRNA preceded the decrease of insulin pre-mRNA (Fig. 6C). In agreement with its stability, insulin2 mRNA decrease was detectable only after a 48-h treatment. Similarly, adenoviral-mediated overexpression of Nur77 induced Eno3 expression (Fig. 6D). The approximately 17-fold increase in Nur77 protein induced a clear decrease in insulin intracellular content as assayed by immunofluorescence (Fig. 6E), confirming mRNA and ELISA assays (Figs. 3A and 6A, respectively). MafA, Pdx-1, and NeuroD expression was strongly repressed upon Nur77 overexpression, concomitantly to E2/I2 and Ins2 RNA down-regulation (Fig. 6F).
Western blot analysis of Min6 whole-cell extracts showed that the highest expression level of Nur77 protein correlated with the most pronounced decrease of mafA polypeptide content (Fig. 6G), whereas Pdx1 and FoxO1 protein expression was not detectably altered. Importantly, the transcriptionally inactive ΔAF1-Nur77 did not trigger nuclear mafA down-regulation (Fig. 6H). A similar mafA and E2/I2 mRNA decrease was observed in a doxycyclin-inducible hemagglutinin (HA)-tagged Nur77 expression system (Fig. 6, I and J), which was also concomitant to a decreased intracellular insulin content (Fig. 6K). Doxycyclin alone had no impact either on NR4As expression or on GSIS in Min6 cells (Supplemental Fig. 9). Thus, irrespective of the overexpression system used, Nur77 exerts a strong repressive activity on insulin gene expression, which may stem from its ability to repress the expression of the mafA polypeptide.
Nur77/NR4A1 interacts with FoxO1 to inhibit insulin gene expression
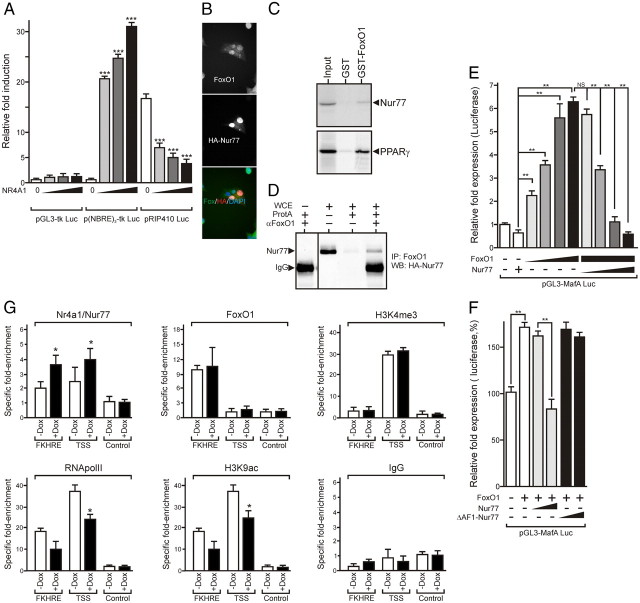
Insulin gene expression is mainly controlled through a highly conserved 400-bp enhancer region containing binding sites for mafA, Pdx-1, and NeuroD [A3, C1, and E1 response elements (21) and Fig. 8]. Increasing amounts of a Nur77 expression vector induced a dose-dependent activation of a NBRE-driven reporter gene [p(NBRE)3-tk Luc]. In similar conditions, Nur77 blunted, in a dose-dependent manner, the activity of pRIP410 Luc, a reporter gene driven by the rat insulin promoter containing the A3, C1, and E1 regions (Fig. 7A).
Fig. 8.
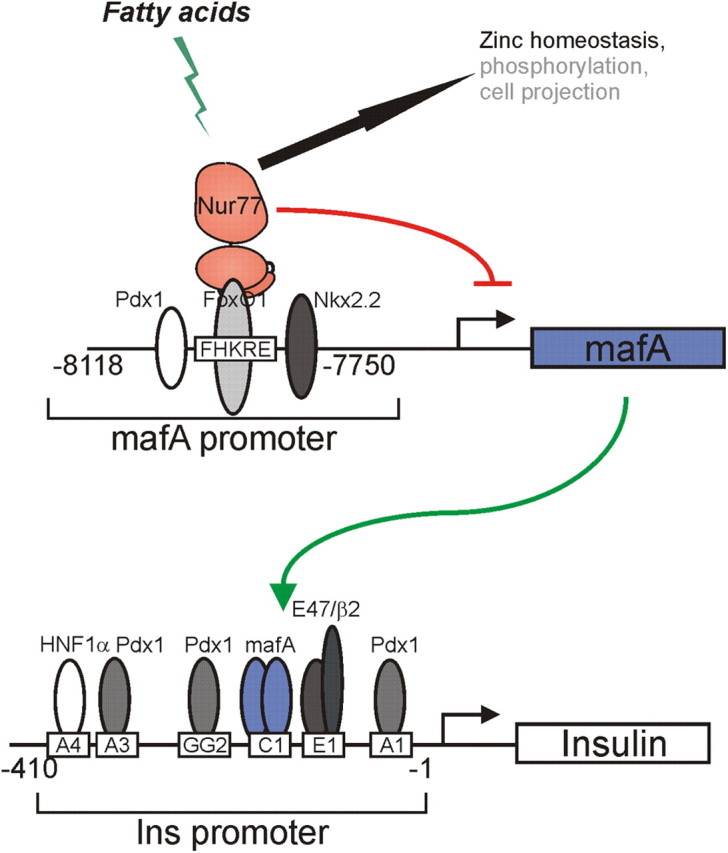
Mechanisms linking Nur77 activation by FA and insulin gene control in Min6 cells. Upon activation and nuclear translocation of Nur77, this orphan NR engages physical interaction with the FoxO1 transcriptional regulator bound to its upstream DNA binding sequence located in the 5′-mafA gene-regulatory sequence. This interaction leads to a decreased intracellular concentration of the mafA protein. This, in turn, prevents activation of the insulin gene, which is critically regulated by mafA, causing a decreased intracellular insulin concentration and impaired insulin secretion. Other mechanisms potentially interfering with insulin maturation and release (Zinc homeostasis) or signaling pathways affecting β-cell responses or architecture are indicated, based on GO annotation of Nur77-regulated genes in Min6 cells (Fig. 3B).
Fig. 7.
Nur77 inhibits mafA expression by interfering with FoxO1-mediated transcription. A, Nur77 inhibits insulin gene promoter activity in a dose-dependent manner. Min6 cells were transfected with the indicated combination of reporter and expression vectors. Luciferase activity was assayed 24 h after transfection, and data are presented as the mean ± sem (n = 3–5). Statistical significance was evaluated by one-way ANOVA followed by Dunnett's post hoc test and is displayed as *, P < 0.05; **, P < 0.01; ***, P < 0.005. The luciferase basal level detected in pGL3-tk Luc-transfected cells was arbitrarily set to 1. B, FoxO1 colocalizes with Nur77. TetON-HA.Nur77 cells were treated for 24 h with doxycyclin and costained for FoxO1 and Nur77. C and D, FoxO1 interacts physically with Nur77. GST pull-down experiments were carried out with GST or GST fused N-terminally to FoxO1 and 35S-labeled Nur77 or PPARγ as a positive control (C). TetON-HA.Nur77 cells were treated with doxycyclin and palmitate, and FoxO1 was immunoprecipitated from whole-cell extracts. Immunoprecipitates were analyzed by Western blot using an anti-HA antibody (D). WCE, Whole-cell extract. E, Nur77 prevents the FoxO1-mediated activation of the mafA promoter. Min6 cells were transfected with the indicated combination of plasmids (3, 6, 10, or 100 ng expression vectors), and the luciferase activity was assayed and analyzed as in panel A. Data are presented as the mean ± sem (n = 3–5). Statistical significance is displayed as *, P < 0.05; **, P < 0.01; ***, P < 0.005. The luciferase basal level detected in pGL3-mafA Luc-transfected cells was arbitrarily set to 1. F, The transcriptionally inactive Nur77 mutant does not repress FoxO1-mediated activation of the mafA promoter. Min6 cells were transfected as in panel E with FoxO1 expression vector (10 ng), and luciferase activity was assayed and graphed as in panel A. G, Chromatin immunoprecipitation assay at the FoxO1 response element (FKHRE) of the mouse mafA promoter. Min6 TetON-Nur77 cells were induced for 24 h by doxycyclin and chemically cross-linked with formaldehyde. Chromatin was extracted, fragmented, and immunoprecipitated with the indicated antibodies. FHKRE and TSS regions of the mafA promoter were amplified by QPCR, and results were normalized to myoglobin. Results are plotted as the fold enrichment over background. Control: myoglobin gene region.
FoxO1 regulates mafA expression and interacts with NR hepatocyte nuclear factor-4α (22) and PPARγ (23). Because FoxO1 is mostly nuclear in Min6 cells and thus colocalizes with Nur77 (Fig. 7B), we investigated a possible physical interaction between FoxO1 and Nur77 by glutathione-S-transferase (GST) pull-down experiments. Nur77 was indeed able to interact with FoxO1 (Fig. 7C). This interaction was confirmed in a coimmunoprecipitation assay (Fig. 7D). The functional significance of this interaction was tested in a transactivation assay in which the activity of the FoxO1-regulated mafA enhancer was measured (pGL3-mafA Luc, Fig. 7E). Increased FoxO1 expression activated this reporter in a dose-dependent manner, whereas increased Nur77 expression blunted FoxO1-mediated transcriptional activation. The ΔAF1-Nur77 mutant was inactive in this assay (Fig. 7F).
The interaction of Nur77 with the mafA enhancer was further characterized in the doxycycline-dependent HA-Nur77 expression system. Nur77 binding to the FoxO1-dependent enhancer (FKHRE) and transcriptional start site (TSS) of the mafA gene was assessed by chromatin immunoprecipitation (Fig. 7G). As expected, FoxO1 associated to FKHRE but was not detected at the mafA TSS. In agreement with the reduced mafA gene expression upon Nur77 expression, Nur77 bound to mafA FKHRE in a doxycycline-dependent manner and was detected at the TSS, suggesting DNA looping between these two regions. This increased occupancy correlated to a decreased RNA polymerase II and acetylated H3 (at K9) density at the TSS, and at the FKHRE, demonstrating a decreased transcriptional activity of the mafA promoter upon Nur77 loading. Finally, trimethylation of histone H3 lysine 4, a marker of active enhancer, was not altered upon Nur77 expression.
Discussion
The NR4A orphan receptors are involved in metabolic regulation via actions in the liver, skeletal muscle, and adipose tissues (24). No evidence has been provided so far concerning a role of NR4A in pancreatic physiology, although Nur77 up-regulation is assayed as a marker of cellular stress in cultured insulinoma β-cells (25, 26). Glucolipotoxicity in vitro, which mimics many of pancreatic β-cell functional defects observed in T2DM (27) is a good model by which to identify intracellular molecular relays for β-cell dysfunction and degeneration. In this respect, oxidative and endoplasmic reticulum stress, as well as activation of protein kinase C and inflammatory pathways, including nuclear factor-κB and activator protein 1, have been shown to play a role in β-cell functional dysregulations in response to a lipotoxic insult (28). More recently, cholesterol has been proposed as being an additional contributor to β-cell dysfunction (29). A role for NR4A in mediating the lipotoxic signal to the nucleus of pancreatic β-cells has been hitherto unsuspected. We studied the effect of Nur77 and Nurr1 on the transcriptional program of mouse insulinoma β-cells and purified human pancreatic islets, respectively, because both genes were strongly up-regulated upon FA treatment, in opposition to Nor1, which was minimally induced. Whereas NR4A predominantly regulate glucose transport, glycolysis, or gluconeogenesis in other tissues such as skeletal muscle and liver, NR4A affected genes controlling insulin biosynthesis and secretion in pancreatic β-cells. The direct interaction of Nur77 with FoxO1 provides a mechanistic basis for the Nur77-mediated inhibition of insulin biosynthesis through impaired mafA expression (Fig. 8), a master gene controlling insulin transcription and GSIS (30). In line with the reported ability of FoxO1 to protect β-cells from a lipotoxic shock (31), an increased expression of Nur77 led to an exacerbated sensitivity of Min6 cells to palmitate, identifying Nur77 as an important regulator of the β-cell response to lipotoxicity. Nur77 promotes, on the contrary, FoxO1-like responses in the liver, because both transcription factors act positively on hepatic glucose production (19, 32), suggesting that either the FoxO1/Nur77 interaction does not occur in hepatocytes, or that the net result of this interaction is a synergistic activation of gluconeogenic genes. Finally, we note that Nur77 also perturbs zinc homeostasis, suggesting that Nur77 impinges on β-cell biology by combinatorial mechanisms.
The three highly related NR4A displayed only limited overlapping transcriptional activities in mouse insulinoma cells in conditions of moderate overexpression. Because most of the amino acid sequence divergence occurs in the N-terminal domain of these receptors, it is likely that the N terminus harbors structural determinants conditioning the specific transcriptional property of each NR4A. It is worth noting that the ΔAF1-Nur77 mutant displays on its own virtually no transcriptional activity as assayed using DNA microarrays (Ploton, M., and P. Lefebvre, data not shown), indicating that the N terminus of Nur77 is the main transcriptionally active domain. How the isotype-selective transcriptional regulation is achieved is unknown at present but likely reflects the differential ability of NR4A to recruit distinct coactivators onto this domain. Another unanswered question is how FA induce NR4A expression. It has been long known that the Nur77 promoter is under the control of the cAMP-protein kinase A-CREB (cAMP response element binding protein) and hypoxia-inducible 1α pathways (33, 34) through specific cis-acting regulatory elements, and our preliminary data revealed that FA-regulated expression of Nur77 gene expression is conferred by a specific sequence in the Nur77 promoter (Helleboid-Chapman, A., and P. Lefebvre, unpublished data).
The comparative analysis of the transcriptional program regulated by members of the NR4A subfamily in Min6 cells yielded unexpected results in terms of (lack of) functional redundancy between Nur77, Nurr1, and Nor1. Although it is commonly accepted that these receptors must share common functions due to their high degree of homology and strongly overlapping DNA binding specificity, our data rather suggest a functional divergence in pancreatic β-cells. Furthermore, Nur77-regulated clusters of genes functionally significantly diverge from those already described in other metabolically active tissues such as liver, skeletal muscle, and brown adipose tissues. In most instances, NR4A-regulated genes are directly involved in metabolic regulation such as uncoupling protein-1, PPAR gamma coactivator-1α, AMP-regulated kinase (reviewed in Ref. 24). However, most of these studies relied on a gene candidate approach, and it remains to be established whether some common functional features may be found between these tissues and pancreas. NR4A are associated to inflammatory response control in various cell types such as glia (35) and macrophages (36, 37), and palmitate induces an inflammatory response in HPI (38). Although we did not observe any significant regulation of inflammation genes in NR4A-overexpressing Min6 cells, it is worth noting that Nurr1 overexpression in HPI down-regulated a cluster of gene involved in inflammation control (Supplemental Fig. 5). Although the biological outcome of these transcriptional regulations is difficult to predict, this might suggest that pancreatic human Nurr1 is playing a species-specific role in FA-induced inflammation control.
Nur77 stimulates hepatic glucose production (19) and decreases pancreatic β-cell insulin secretion. Combined with the fact that Nur77-null mice challenged with a high-fat diet display an improved hepatic insulin sensitivity and decreased hepatic glucose production, this would argue for designing Nur77 antagonists for T2DM treatment. However, Nur77-null mice exhibit impaired insulin signaling in skeletal muscle and hepatic steatosis, thus calling for caution when extrapolating gene inactivation or overexpression data to a pharmacological intervention strategy. Moreover, Nur77 gene knockout led to a compensatory overexpression of Nor1, and short hairpin RNA-mediated knockdown of a given NR4A receptor in Min6 cells affected the expression of the 2 others (MP and P. Lefebvre, data not shown), indicating that the expression and/or activity of NR4A are tightly linked. A general decreased expression of NR4A has been observed in skeletal muscles of rodent models of T2DM and also in T2DM patients (39, 40). Whether this down-regulation results from a long-term adaptative, protective response that can either be deleterious (skeletal muscle) or beneficial (β-cells) for glucose homeostasis remains to be established by studying tissue-specific gene knockouts.
Materials and Methods
Reagents
Sodium palmitate (P9767) and oleate (O7501) were from Sigma (St. Louis, MO). Stock solutions (100 mm) of sodium palmitate or oleate were prepared in ultrapure water and then added to 10% FA-free BSA to a 5 mm final concentration and filtered.
Antibodies
Anti-HA-Tag (6E2), anti-FoxO1(C29H4), and anti-PDX1 (2437) antibodies were from Cell Signaling Technology (Beverly, MA). Anti-MafA (A300-611A) was from Bethyl Laboratories (Montgomery, TX). Anti-Nurr1(M196, sc-5568), anti-Nur77 (M210, sc5569), and anti-NeuroD (sc-20805) antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-Nor1 antibody (PP-H7833-00) was from R&D Systems (Minneapolis, MN). Antiinsulin antibody (catalog no. 5330–3339) was from AbD Serotec (Oxford, UK). Secondary horseradish peroxidase-conjugated antibodies were from Sigma.
Human and mouse pancreatic islets isolation
Human pancreas were harvested from adult brain-dead donors in accordance with French Regulations and with the local Institutional Ethical Committee. Briefly, pancreatic islets were isolated after ductal distension of the pancreas and digestion of the tissue with GMP Liberase (Roche Diagnostics GmbH, Mannheim, Germany) according to the classic automated method with modifications as described elsewhere (13). Quality controls applied to islets were numbering, viability (dithizone/trypan blue), glucose-stimulated insulin secretion and stimulation index, insulin content, DNA content (Picogreen), apoptosis (Cell Death DetectionR kit, Roche), and ATP content (ATP lite kit; PerkinElmer, Wellesley, MA). A similar procedure was applied to mouse islets (13).
Microarray analysis
The Min6 cell line was provided by J.-I. Miyakazi (Osaka, Japan). Min6 cells were transduced at MOI 30 for 3 h with either Ad-GFP, Ad-Nur77, Ad-Nurr1, or Ad-Nor1 in triplicate, independent experiments. Total RNA was extracted 24 h after transduction and processed to be hybridized to MOE430 2.0 Affymetrix arrays. Data were collected and processed as described elsewhere (41). Each list of differentially expressed genes relative to control was analyzed using DAVID (42) to search for enriched GO terms (high stringency). Venn's diagrams were generated using the Venn's diagram generator at http://www.pangloss.com/seidel/Protocols/venn.cgi.
Plasmids
pCMX-mNur77 was a gift from B.M.Forman (City of Hope National Medical Center, Duarte, CA). pGL3-tk Luc was from Promega Corp. (Madison, WI) (pGL3 promoter), the pGL3-MafA Luc (R1-6, containing the −9389/+56 promoter region of the mouse MafA gene) was a gift from R. Stein (Vanderbilt University Medical Center, Nashville, TN) (43). The rat insulin I promoter (−410 to +1 bp) reporter gene pRIP410 Luc was provided by M. German (University of California, San Francisco, CA) (44) and the FoxO1 expression vector by A. Biola-Vidamment (University of Paris-Sud 11, Paris, France).
Adenovirus production and preparation
PpCMVhNur77 (45) and pAdCMVmNurr1 (46) were gifts from C.J.M. de Vries (Amsterdam, The Netherlands) and S. Tetradis (University of California, Los Angeles, CA), respectively. pAdCMV-rNor1 was generated by cloning the full length Nor1 cDNA into pShuttle (CLONTECH Laboratories, Inc., Mountain View, CA). The expression cassette was transferred into the Adeno-X (CLONTECH) adenoviral genome. Adenoviruses were amplified in human embryonic kidney 293 cells and purified by CsCl centrifugation.
Immunofluorescence, immunohistochemistry, and zinc labeling
Min6 cells and human and mouse pancreata were processed for immunofluorescence and immunohistochemistry as described elsewhere (13, 47).
Min6 cells were labeled with FluoZin-3 as follows: FluoZin-3 was added at 2 μm to the cell medium for 50 min at 37 C. The culture medium was replaced after which cells were further incubated for 2 h. Live cells were examined using a Leica DMI6000 microscope (Leica, Inc., Deerfield, IL) or analyzed by flow cytometry after trypsinization (106 cells/ml).
RT-QPCR analysis
Total RNA was extracted using RNeasy (QIAGEN, Chatsworth, CA) according to the manufacturer's instructions. DNase I-treated total RNA was reverse transcribed using the High-Capacity cDNA archive kit (Applied Biosystems, Foster City, CA). cDNA were analyzed using TaqMan Gene Expression Assays as recommended by the manufacturer. Relative gene expression was calculated by the ΔΔCt method. Final results were expressed as the fold difference in gene expression normalized to 18S rRNA and relative to control conditions. In some instances, QPCR were carried out using SyberGreen-based chemistry (see Supplemental Table 1 in the online appendix for primer sequences).
Coimmunoprecipitation and chromatin immunoprecipitation assays
Min6 TetON-Nur77 cells at 70% confluence were incubated or not with doxycycline (0.5 μg/ml) for 24 h. Chromatin immunoprecipitation assays were then performed as described by Sérandour et al. (48). Antibodies used in chromatin immunoprecipitation and coimmunoprecipitations were raised against Nur77 (sc-5569, Santa-Cruz; and 554088, BD-Pharmingen, Franklin Lakes, NJ), FoxO1 (sc-11350, Santa-Cruz; and 2880, Cell Signaling), RNA PolII (sc-899, Santa-Cruz) and H3K9ac (17–658, Millipore). QPCR primers are described in Supplemental Table 1.
Glucose-stimulated insulin secretion assay
Min6 cells (3 × 105) were grown in 24-well clusters for 3–4 d. GSIS was initiated by starving cells in KRB-BSA buffer [1.2 mm KH2PO4 (pH 7.4), 18.5 mm NaCl, 4.8 mm KCl, 2.7 mm CaCl2, 1.1 mm MgSO4, 7 H2O, 25 mm NaHCO3, 0.5% BSA) for 90 min. KRB was then replaced by low-glucose KRB-BSA (2.8 mm Glc) or high-glucose KRB (25 mm Glc). At the indicated time, plates were cooled at 4 C, and the medium was used to assay secreted insulin. The cell layer was lyzed in cold 0.18 m HCl and 75% ethanol, scraped, and centrifuged. The supernatant was used to determine the intracellular total protein and insulin content. Insulin was assayed using the High Range Rat Insulin ELISA kit from Mercodia (Uppsala, Sweden).
Transient transfection
Min6 cells (3 × 105) were seeded in 12-well plates and transfected 48 h later with Lipofectamine (Invitrogen, Carlsbad, CA) using 1.5 μg of reporter vector and varying quantities of Nur77 and/or FoxO1 expression vector and/or empty plasmid vector (pcDNA3). The medium was replaced 5 h later by fresh DMEM with 10% fetal calf serum. Luciferase activity was measured 24 h later (Luciferase assay system; Promega Corp., Madison, WI) with a Victor Light (PerkinElmer) plate reader and normalized to cellular DNA. Transfection experiments were done at least three times in triplicate.
Min6 Tet-On cell line and ΔAF1-Nur77 Min6
The pRetroX HA-Nur77 was generated by inserting the HA-tagged mNur77 cDNA into pRetroX-Tight-Pur (CLONTECH Laboratories). This vector and the pRetroX-TetON Advanced-rtTA were packaged into retroviral particles in Phoenix cells and used for coinfection. Pools of clones displaying distinct levels of expression of Nur77 were characterized. Sequences are available upon request. The Min6-Retro control cell line was generated using a retroviral empty pSM2 vector. The ΔAF1-Nur77 Min6 cell line was generated by retroviral infection using pBabe-DN Nur77 (49).
Statistical analysis
Statistical analysis was performed using Prism 5.0 (GraphPad Software, La Jolla, CA). Values are expressed as the mean ± sem. Statistical significance was evaluated using either an unpaired, two-tailed t test or by one-way ANOVA followed by Tukey's or Dunnett's post hoc tests. P < 0.05 (*), 0.005 (**), or 0.001 (***) was considered as significant, very significant or highly significant, respectively.
Acknowledgments
We thank Drs C. M. deVries (University of Amsterdam, Amsterdam, The Netherlands), J. J. Rochford (University of Cambridge, Cambridge, UK), A. Winoto (University of California, Berkeley, CA), R. Stein (Vanderbilt University Medical Center, Nashville, TN), M. German (University of California, San Francisco, CA), A. Biola-Vidamment (University of Paris-Sud 11, Paris, France), B. Forman (City of Hope National Medical Center, Duarte, CA) and J.-I. Miyakazi (Osaka University Medical School, Osaka, Japan) for expression vectors and cell lines; S. Deledicque and J. Brozek (Genfit SA, Loos, France) for Affymetrix array processing and analysis, E. Machez (IMPRT, Lille, France) for fluorescence-activated cell sorting analysis, M. Tardivel (IMPRT and BICeL facility) for access to confocal microscope and technical advice, and P. Sacchetti (Mount Holyoke College, Mt. Holyoke, MA) for initial help and constant interest in this work.
This work was supported by grants from Institut National de la Santé et de la Recherche Médicale, University of Lille 2, Région Nord-Pas de Calais/FEDER, and Fondation Coeur et Artères (ChOBeCell program).
Present address for R.C.: Horm Unit, ICP-DeDuve Institute, 75 avenue Hippocrate, 7529 B1200 Bruxelles, Belgium.
Disclosure Summary: No potential conflict of interest related to this work is reported.
Footnotes
- FA
- Fatty acid
- FFA
- free fatty acid
- FKHRE
- FoxO1-dependent enhancer
- GFP
- green fluorescent protein
- GO
- gene ontology
- GSIS
- glucose-stimulated insulin secretion
- GST
- glutathione-S-transferase
- HA
- hemagglutinin
- HPI
- purified human islets
- MPI
- purified mouse islets
- NBRE
- Nur77-binding response element
- NR
- nuclear receptor
- PPAR
- peroxisome proliferator-activated receptor
- QPCR
- quantitative PCR
- RT-QPCR
- reverse transcription-quantitative PCR
- T2DM
- type 2 diabetes
- TSS
- transcriptional start site.
References
- 1. Cabrera O , Berman DM , Kenyon NS , Ricordi C , Berggren PO , Caicedo A. 2006. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prentki M , Nolan CJ. 2006. Islet β cell failure in type 2 diabetes. J Clin Invest 116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aramata S , Han SI , Kataoka K. 2007. Roles and regulation of transcription factor MafA in islet β-cells. Endocr J 54:659–666 [DOI] [PubMed] [Google Scholar]
- 4. Remedi MS , Nichols CG. 2009. Hyperinsulinism and diabetes: genetic dissection of β cell metabolism-excitation coupling in mice. Cell Metab 10:442–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muoio DM , Newgard CB. 2006. Obesity-related derangements in metabolic regulation. Annu Rev Biochem 75:367–401 [DOI] [PubMed] [Google Scholar]
- 6. Itoh Y , Kawamata Y , Harada M , Kobayashi M , Fujii R , Fukusumi S , Ogi K , Hosoya M , Tanaka Y , Uejima H , Tanaka H , Maruyama M , Satoh R , Okubo S , Kizawa H , Komatsu H , Matsumura F , Noguchi Y , Shinohara T , Hinuma S , Fujisawa Y , Fujino M. 2003. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 422:173–176 [DOI] [PubMed] [Google Scholar]
- 7. Colditz GA , Willett WC , Rotnitzky A , Manson JE. 1995. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 122:481–486 [DOI] [PubMed] [Google Scholar]
- 8. Donath MY , Størling J , Maedler K , Mandrup-Poulsen T. 2003. Inflammatory mediators and islet β-cell failure: a link between type 1 and type 2 diabetes. J Mol Med 81:455–470 [DOI] [PubMed] [Google Scholar]
- 9. Eizirik DL , Sandler S , Welsh N , Cetkovic-Cvrlje M , Nieman A , Geller DA , Pipeleers DG , Bendtzen K , Hellerström C. 1994. Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest 93:1968–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tulachan SS , Doi R , Kawaguchi Y , Tsuji S , Nakajima S , Masui T , Koizumi M , Toyoda E , Mori T , Ito D , Kami K , Fujimoto K , Imamura M. 2003. All-trans retinoic acid induces differentiation of ducts and endocrine cells by mesenchymal/epithelial interactions in embryonic pancreas. Diabetes 52:76–84 [DOI] [PubMed] [Google Scholar]
- 11. Micallef SJ , Janes ME , Knezevic K , Davis RP , Elefanty AG , Stanley EG. 2005. Retinoic acid induces Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes 54:301–305 [DOI] [PubMed] [Google Scholar]
- 12. Sugden MC , Holness MJ. 2008. Role of nuclear receptors in the modulation of insulin secretion in lipid-induced insulin resistance. Biochem Soc Trans 36:891–900 [DOI] [PubMed] [Google Scholar]
- 13. Popescu IR , Helleboid-Chapman A , Lucas A , Vandewalle B , Dumont J , Bouchaert E , Derudas B , Kerr-Conte J , Caron S , Pattou F , Staels B. 2010. The nuclear receptor FXR is expressed in pancreatic β-cells and protects human islets from lipotoxicity. FEBS Lett 584:2845–2851 [DOI] [PubMed] [Google Scholar]
- 14. Pei L , Castrillo A , Chen M , Hoffmann A , Tontonoz P. 2005. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem 280:29256–29262 [DOI] [PubMed] [Google Scholar]
- 15. Arkenbout EK , de Waard V , van Bragt M , van Achterberg TA , Grimbergen JM , Pichon B , Pannekoek H , de Vries CJ. 2002. Protective function of transcription factor TR3 orphan receptor in atherogenesis: decreased lesion formation in carotid artery ligation model in TR3 transgenic mice. Circulation 106:1530–1535 [DOI] [PubMed] [Google Scholar]
- 16. Busch AK , Cordery D , Denyer GS , Biden TJ. 2002. Expression profiling of palmitate- and oleate-regulated genes provides novel insights into the effects of chronic lipid exposure on pancreatic β-cell function. Diabetes 51:977–987 [DOI] [PubMed] [Google Scholar]
- 17. Laybutt DR , Preston AM , Akerfeldt MC , Kench JG , Busch AK , Biankin AV , Biden TJ. 2007. Endoplasmic reticulum stress contributes to β cell apoptosis in type 2 diabetes. Diabetologia 50:752–763 [DOI] [PubMed] [Google Scholar]
- 18. Zhang XK. 2007. Targeting Nur77 translocation. Expert Opin Ther Targets 11:69–79 [DOI] [PubMed] [Google Scholar]
- 19. Pei L , Waki H , Vaitheesvaran B , Wilpitz DC , Kurland IJ , Tontonoz P. 2006. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med 12:1048–1055 [DOI] [PubMed] [Google Scholar]
- 20. Evans-Molina C , Garmey JC , Ketchum R , Brayman KL , Deng S , Mirmira RG. 2007. Glucose regulation of insulin gene transcription and pre-mRNA processing in human islets. Diabetes 56:827–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Docherty HM , Hay CW , Ferguson LA , Barrow J , Durward E , Docherty K. 2005. Relative contribution of PDX-1, MafA and E47/β2 to the regulation of the human insulin promoter. Biochem J 389:813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirota K , Daitoku H , Matsuzaki H , Araya N , Yamagata K , Asada S , Sugaya T , Fukamizu A. 2003. Hepatocyte nuclear factor-4 is a novel downstream target of insulin via FKHR as a signal-regulated transcriptional inhibitor. J Biol Chem 278:13056–13060 [DOI] [PubMed] [Google Scholar]
- 23. Dowell P , Otto TC , Adi S , Lane MD. 2003. Convergence of peroxisome proliferator-activated receptor γ and Foxo1 signaling pathways. J Biol Chem 278:45485–45491 [DOI] [PubMed] [Google Scholar]
- 24. Zhao Y , Bruemmer D. 2010. NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler Thromb Vasc Biol 30:1535–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Susini S , Roche E , Prentki M , Schlegel W. 1998. Glucose and glucoincretin peptides synergize to induce c-fos, c-jun, junB, zif-268, and nur-77 gene expression in pancreatic β(INS-1) cells. FASEB J 12:1173–1182 [PubMed] [Google Scholar]
- 26. Roche E , Buteau J , Aniento I , Reig JA , Soria B , Prentki M. 1999. Palmitate and oleate induce the immediate-early response genes c-fos and nur-77 in the pancreatic β-cell line INS-1. Diabetes 48:2007–2014 [DOI] [PubMed] [Google Scholar]
- 27. Giacca A , Xiao C , Oprescu AI , Carpentier AC , Lewis GF. 2011. Lipid-induced pancreatic β-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab 300:E255–E262 [DOI] [PubMed] [Google Scholar]
- 28. Poitout V , Amyot J , Semache M , Zarrouki B , Hagman D , Fontés G. 2010. Glucolipotoxicity of the pancreatic β cell. Biochim Biophys Acta 1801:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brunham LR , Kruit JK , Hayden MR , Verchere CB. 2010. Cholesterol in β-cell dysfunction: the emerging connection between HDL cholesterol and type 2 diabetes. Curr Diab Rep 10:55–60 [DOI] [PubMed] [Google Scholar]
- 30. Wang H , Brun T , Kataoka K , Sharma AJ , Wollheim CB. 2007. MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia 50:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kitamura YI , Kitamura T , Kruse JP , Raum JC , Stein R , Gu W , Accili D. 2005. FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab 2:153–163 [DOI] [PubMed] [Google Scholar]
- 32. Haeusler RA , Kaestner KH , Accili D. 2010. FoxOs function synergistically to promote glucose production. J Biol Chem 285:35245–35248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi JW , Park SC , Kang GH , Liu JO , Youn HD. 2004. Nur77 activated by hypoxia-inducible factor-1α overproduces proopiomelanocortin in von Hippel-Lindau-mutated renal cell carcinoma. Cancer Res 64:35–39 [DOI] [PubMed] [Google Scholar]
- 34. Lam BY , Zhang W , Ng DC , Maruthappu M , Roderick HL , Chawla S. 2010. CREB-dependent Nur77 induction following depolarization in PC12 cells and neurons is modulated by MEF2 transcription factors. J Neurochem 112:1065–1073 [DOI] [PubMed] [Google Scholar]
- 35. Saijo K , Winner B , Carson CT , Collier JG , Boyer L , Rosenfeld MG , Gage FH , Glass CK. 2009. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pei L , Castrillo A , Tontonoz P. 2006. Regulation of macrophage inflammatory gene expression by the orphan nuclear receptor Nur77. Mol Endocrinol 20:786–794 [DOI] [PubMed] [Google Scholar]
- 37. Bonta PI , van Tiel CM , Vos M , Pols TW , van Thienen JV , Ferreira V , Arkenbout EK , Seppen J , Spek CA , van der Poll T , Pannekoek H , de Vries CJ. 2006. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol 26:2288–2294 [DOI] [PubMed] [Google Scholar]
- 38. Igoillo-Esteve M , Marselli L , Cunha DA , Ladrière L , Ortis F , Grieco FA , Dotta F , Weir GC , Marchetti P , Eizirik DL , Cnop M. 2010. Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by β cells in type 2 diabetes. Diabetologia 53:1395–1405 [DOI] [PubMed] [Google Scholar]
- 39. Fu Y , Luo L , Luo N , Zhu X , Garvey WT. 2007. NR4A orphan nuclear receptors modulate insulin action and the glucose transport system: potential role in insulin resistance. J Biol Chem 282:31525–31533 [DOI] [PubMed] [Google Scholar]
- 40. Kanzleiter T , Preston E , Wilks D , Ho B , Benrick A , Reznick J , Heilbronn LK , Turner N , Cooney GJ. 2010. Overexpression of the orphan receptor Nur77 alters glucose metabolism in rat muscle cells and rat muscle in vivo. Diabetologia 53:1174–1183 [DOI] [PubMed] [Google Scholar]
- 41. Lefebvre B , Benomar Y , Guédin A , Langlois A , Hennuyer N , Dumont J , Bouchaert E , Dacquet C , Pénicaud L , Casteilla L , Pattou F , Ktorza A , Staels B , Lefebvre P. 2010. Proteasomal degradation of retinoid X receptor α reprograms transcriptional activity of PPARγ in obese mice and humans. J Clin Invest 120:1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang da W , Sherman BT , Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57 [DOI] [PubMed] [Google Scholar]
- 43. Raum JC , Gerrish K , Artner I , Henderson E , Guo M , Sussel L , Schisler JC , Newgard CB , Stein R. 2006. FoxA2, Nkx2.2, and PDX-1 regulate islet β-cell-specific mafA expression through conserved sequences located between base pairs −8118 and −7750 upstream from the transcription start site. Mol Cell Biol 26:5735–5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. German MS , Wang J , Chadwick RB , Rutter WJ. 1992. Synergistic activation of the insulin gene by a LIM-homeo domain protein and a basic helix-loop-helix protein: building a functional insulin minienhancer complex. Genes Dev 6:2165–2176 [DOI] [PubMed] [Google Scholar]
- 45. Pols TW , Ottenhoff R , Vos M , Levels JH , Quax PH , Meijers JC , Pannekoek H , Groen AK , de Vries CJ. 2008. Nur77 modulates hepatic lipid metabolism through suppression of SREBP1c activity. Biochem Biophys Res Commun 366:910–916 [DOI] [PubMed] [Google Scholar]
- 46. Pirih FQ , Tang A , Ozkurt IC , Nervina JM , Tetradis S. 2004. Nuclear orphan receptor Nurr1 directly transactivates the osteocalcin gene in osteoblasts. J Biol Chem 279:53167–53174 [DOI] [PubMed] [Google Scholar]
- 47. Lalloyer F , Vandewalle B , Percevault F , Torpier G , Kerr-Conte J , Oosterveer M , Paumelle R , Fruchart JC , Kuipers F , Pattou F , Fiévet C , Staels B. 2006. Peroxisome proliferator-activated receptor α improves pancreatic adaptation to insulin resistance in obese mice and reduces lipotoxicity in human islets. Diabetes 55:1605–1613 [DOI] [PubMed] [Google Scholar]
- 48. Sérandour AA , Avner S , Percevault F , Demay F , Bizot M , Lucchetti-Miganeh C , Barloy-Hubler F , Brown M , Lupien M , Métivier R , Salbert G , Eeckhoute J. 2011. Epigenetic switch involved in activation of pioneer factor FOXA1-dependent enhancers. Genome Res 21:555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Au WS , Payne VA , O'Rahilly S , Rochford JJ. 2008. The NR4A family of orphan nuclear receptors are not required for adipogenesis. Int J Obes (Lond) 32:388–392 [DOI] [PubMed] [Google Scholar]
- 50. Wang X , Li H , De Leo D , Guo W , Koshkin V , Fantus IG , Giacca A , Chan CB , Der S , Wheeler MB. 2004. Gene and protein kinase expression profiling of reactive oxygen species-associated lipotoxicity in the pancreatic β-cell line MIN6. Diabetes 53:129–140 [DOI] [PubMed] [Google Scholar]