Abstract
Thyroid hormone (T3) can trigger a massive differentiation of cultured oligodendrocytes precursor cells (OPC) by binding the nuclear T3 receptor α1 (TRα1). Whether this reflects a physiological function of TRα1 remains uncertain. Using a recently generated mouse model, in which CRE/loxP recombination is used to block its function, we show that TRα1 acts at two levels for the in vivo differentiation of OPC in mouse cerebellum. At the early postnatal stage, it promotes the secretion of several neurotrophic factors by acting in Purkinje neurons and astrocytes, defining an environment suitable for OPC differentiation. At later stages, TRα1 acts in a cell-autonomous manner to ensure the complete arrest of OPC proliferation. These data explain contradictory observations made on various models and outline the importance of T3 signaling both for synchronizing postnatal neurodevelopment and restraining OPC proliferation in adult brain.
Oligodendrocytes (OL) synthesize myelin in the vertebrate central nervous system. They develop from oligodendrocyte precursor cells (OPC), which undergo terminal differentiation at a postnatal stage of neurodevelopment in mice (1). The switch between the self-renewal and proliferation of OPC toward differentiation is sensitive to a number of diffusible factors, produced by other neural cell types in their immediate environment. The best studied are platelet-derived growth factors (PDGF) (2), neurotrophin-3 (NT3) (3), brain-derived neurotrophic factor (BDNF) (4), nerve growth factor (NGF) (5), and Sonic hedgehog (Shh) (6). Because OPC and OL also secrete growth factors (7), they participate to a complex network of cellular interactions that coordinates neurodevelopment. OPC also interact with neighboring cells by direct contact, receiving synaptic input from neurons (8). Newly formed OL can respond to these cell contacts and neurotrophins during a short time frame (9). Timely OPC differentiation is important, because myelination, which usually begins just after axonal growth and can last for more than 1 wk, is necessary to maintain axon integrity (10). OPC are maintained in adult brain, and their function and lineage potential are matters of intense investigations (11).
Culture experiments have shown that OPC can be maintained in chemically defined medium, in which purified PDGF and/or NT3 is sufficient to promote their self-renewal (3, 12). Retrieval of these growth factors combined with addition of the active form of thyroid hormone (T3) triggers a rapid and massive differentiation into postmitotic OL. Unlike peptidic neurotrophic factors, T3 acts directly on transcription by binding to TRα1, TRβ1, or TRβ2 nuclear receptors encoded by the THRA and THRB genes (13). THRA is expressed in both OPC and OL, whereas THRB expression starts only at a late stage of OPC differentiation (14). Moreover, circulating T3 peaks at postnatal d 6 (P6) in mice (15). These observations suggest an elegant and simple mechanism for the temporal control of OL differentiation in the rodent brain white matter: OPC proliferation is first stimulated by NT3 and PDGF. Subsequently, as NT3 production decreases and OPC become less sensitive to PDGF (16), a peak of T3 would be sufficient to ensure the timely differentiation of OPC into OL.
The importance of T3 signaling for OPC in vivo differentiation, however, remains unclear. T3 deficiency delays myelination and the onset of OL differentiation, in both mouse and rats (17–19) whereas excess of T3 has the opposite effect (20). OPC differentiation is slightly delayed in THRA, but not THRB, knockout (21) whereas a population of slow cycling OPC persists in the adult optic nerve in mice devoid of all receptors (22). The in vivo consequences of altering T3 signaling are thus much less dramatic than in cultured cells, raising the possibility that other factors can compensate for T3 deficiency. Moreover, THRA knockout can affect OPC differentiation indirectly, as it is expressed in all the major cerebellar cell types including Purkinje neurons, granular neurons, OL, and astrocytes (23–25). In fact, T3 deficiency can affect the ability of these cell types to secrete factors acting on OPC proliferation including NT3, BDNF, and nerve growth factor (26–29).
In the present study, we asked whether the function of T3 signaling in cerebellum OPC differentiation is cell autonomous or indirect, by using the so-called TRαAMI THRA allele (30). Cerebellum was chosen because it is a region in which consequences of impaired T3 signaling are most striking (31), affecting γ-aminobutyric acid (GABA)ergic Purkinje neurons, GABAergic interneurons (32), granular glutamatergic neurons (33), Bergmann glia (34), astrocytes, and oligodendrocytes (35). Cre-mediated recombination of the TRαAMI allele activates the expression of TRα1L400R, a dominant-negative mutant receptor. Unlike what happens in THRA knock-out mice, in which corepressors recruitment by unliganded TRα1 is lost (36, 37), expression of T3 target genes is strongly repressed in TRα1L400R-expressing cells. As a consequence, when TRα1L400R expression is made ubiquitous by early Cre expression, heterozygous mice display many features of congenital hypothyroidism and do not usually survive beyond 3 wk. However, T3 level is not changed and TRβ1/2 functions seem to be preserved (30).
To analyze the consequences of restricted expression of the TRαAMI allele on OPC differentiation, we used a panel of promoter-specific Cre (or the tamoxifen-inducible Cre-ERT2) transgenic mice that allowed expression of TRα1L400R in different cell types and time frames. Our data demonstrate that T3 exerts two completely distinct functions at different stages, explaining apparent contradictions between the conclusions of several previous studies.
Results
Recombinase expression pattern in different Cre and Cre-ERT2 mice
We generated mice expressing the TRα1L400R dominant-negative mutation in a restricted manner by crossing TRαAMI/AMIR26YFP mice with Cre or Cre-ERT2 transgenics. The Cre-dependent R26YFP transgene was introduced to control by immunostaining the Cre recombination pattern and efficiency in all groups of transgenics. We performed yellow fluorescent protein (YFP) immunostaining in the cerebellum at different postnatal stages and used the result as an indirect evidence for TRα1L400R expression, because the mutant receptor cannot be distinguished from its wild-type counterpart still present in heterozygous mice. Previous work, in which broad Cre expression allowed the use of PCR analysis, showed that the TRαAMI and R26YFP display the same pattern and same efficiency of recombination, and that this approach is reliable (Refs. 30 and 38 and Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). We first wanted to generate transgenics able to drive recombination specifically in OPC using the two strains that have been reported to do so (39, 40). Oligodendrocyte transcription factor 2 (Olig2), a basic helix-loop-helix transcription factor, was used as a specific marker for immunostaining of OPC (41, 42) and has been combined with YFP immunostaining (Fig. 1). To our surprise neither Cnp-Cre nor PDGFRα-CreERT2 restricted YFP expression to OPC in the cerebellum. First, YFP was observed in approximately 100% of Purkinje cells in Cnp-CrexR26YFP and Cnp-CrexR26YFPxTRαAMI mice after P8 but not at P3 (Fig. 1 and Table 1). This is in agreement with published data (43) and the Allen Brain Atlas information. Second, when PDGFRα-CreERT2xR26YFP mice were treated with tamoxifen either at P2, P5, or P30, the YFP expression pattern, expressed several days later, was much broader than expected, being found not only in OPC and OL but also in astrocytes and most if not all neuronal cell types, including Purkinje and granular cells. The weak PDGFRα expression found outside OPC (44) is thus sufficient to promote recombination at this stage. These unexpected results prompted us to use several other transgenics, to reach a conclusion on a possible cell-autonomous effect of TRα1L400R expression in OPC. In particular L7-Cre allowed recombination only in Purkinje neurons, at P8 but not at P3, and provides a satisfying control for Cnp-Cre. We also used transgenes recombining in all GABAergic neurons, including Purkinje and GABAergic interneurons (Ptf1a-Cre) or astrocytes lineage (Glast-Cre-ERT2) to address the possible influence of a cerebellar environment expressing TRα1L400R on wild-type OPC differentiation. Comparisons within this collection of Cre and Cre-ERT2 transgenic mice should allow identification of the main glial and neuronal cell types in which TRα1L400R has cell-autonomous consequences, with the noticeable exception of GABAergic interneurons, the influence of which before P8 cannot be separated from that of Purkinje cells. Expression patterns, recombination efficiencies, and simplified nomenclature are summarized in Table 1 and in Fig 1, panel I.
Fig. 1.
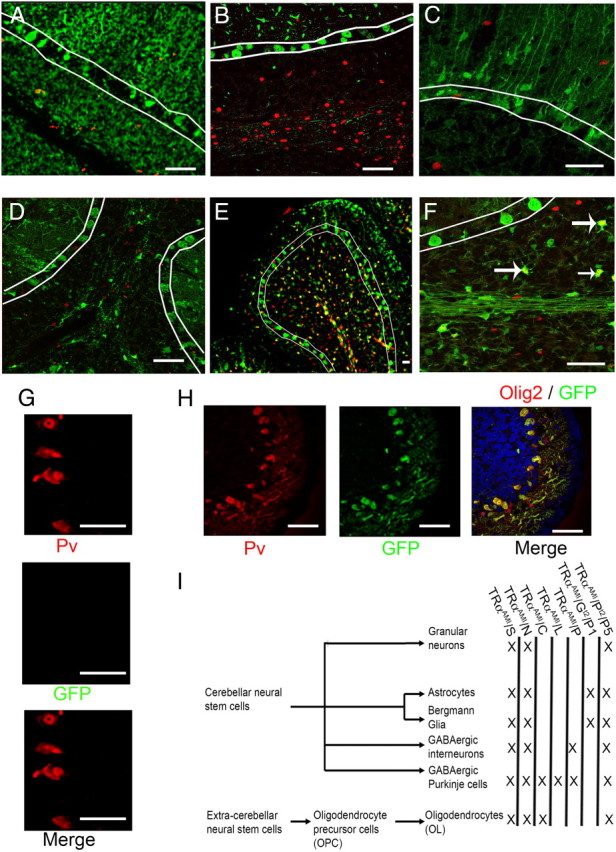
Expression pattern of different Cre-mice in the cerebellum, using ROSA26-lox-STOP-lox-EYFP (R26YFP) as reporter. A–F, Olig2 immunostaining in red; YFP immunostaining in green,. Colocalization between YFP and Olig2 (yellow) indicates Cre-mediated recombination in OPC. White lines delineate the Purkinje cell layer. A, PDGFRα-Cre ERT2 (tamoxifen injection at P1. Immunostaining at P15) indicates cre-mediated recombination in all cerebellar cell types including oligodendrocyte lineage. B, L7-Cre (P15). Recombination is restricted to Purkinje cells. C, Glast-Cre ERT2 (tamoxifen injection at P1. Immunostaining at P15). Recombination is restricted to the astrocytes and not found in Olig2+ OPC. D, Ptfa1-Cre (P15): Recombination in GABAergic neurons, but not in oligodendrocytes lineage at P15. E and F, Cnp-Cre (P15). Recombination limited to the oligodendrocytes lineage and Purkinje cells. Stars indicate YFP+Olig2+ OPC. G, Cnp-Cre (P8, green; YFP, red; parvalbumin, blue: DAPI) indicates an expression of Cre recombinase from in Purkinje cells at this stage. H, Cnp-Cre (P3 green: YFP, red: parvalbumin). Absence of recombination at P3 in Purkinje cells at this stage. Scale bar, 100 μm. I, Cell lineages in the cerebellum and recombination patterns observed in various Cre and Cre-ERT2 transgenic mice. X indicates Cre expression. Counting and nomenclature are summarized in Table 1. GFP, Green fluorescent protein.
Table 1.
Genotypes, mice, and nomenclature
| Promoter | Nomenclature | Granular neurons | GABAergic neurons |
Glia |
Ref. | ||
|---|---|---|---|---|---|---|---|
| Purkinje cells | GABAergic inter-neurons | OL | Astrocytes | ||||
| Sycp1 (synaptonemal complex protein 1) | TRαAMI/S | 100 | 100 | 100 | 100 | 100 | 69 |
| Nes (Nestin) | TRαAMI/N | 100 | 100 | 100 | 100 | 100 | 70 |
| Cnp (2′,3′-cyclic nucleotide 3′- phosphodiesterase) | TRαAMI/C | 0 | 74.1 ± 11.4 | 0% | 91.6 ± 7.2 | 0 | 39 |
| Pcp2 (L7, Purkinje cell protein 2) | TRαAMI/liter | 0 | 98.3 ± 4.1 | 0 | 0 | 0 | 71 |
| Ptf1a (pancreas transcription factor 1a) | TRαAMI/P | 0 | 72.5 ± 17.3 | 87.3 ± 7.1 | 0 | 0 | 72 |
| Tamoxifen inducible CreERt2 | |||||||
| Slc1a3 (Glast, solute carrier 1a3) | TRαAMI/S/Gt2/Pna | 0 | 0 | 0 | 0 | 92.8 ± 4.9 | 73 |
| Pdgfra (platelet-derived growth factor receptor, α polypeptide) | TRαAMI/S/Pt2/Pna | >99b | >99b | >99b | 98.7 ± 2.3 | >99b | 40 |
% of YFP+ observed at P15 in TRαAMI/ROSAYFP/CRE cerebellum.
Pn, Postnatal day of tamoxifen treatment (P1 for initial characterization); OL, oligodendrocyte lineage, characterized using Olig2 staining. n = 3 for each phenotype.
No DAPI+/YFP− cells detected.
TRα1L400R expression increases OPC postnatal proliferation in an indirect manner
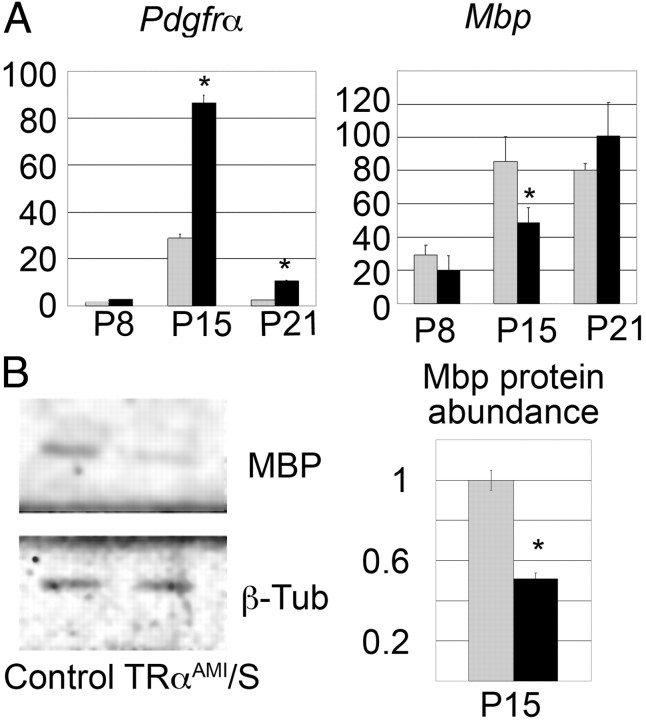
First, study of TRαAMI/S mice was performed to address the influence of ubiquitous expression of TRα1L400R on OPC differentiation and proliferation. Because the intact TRαAMI allele is a nul allele, expressing none of the known TRα isoforms, we used heterozygous TRαAMI/+ mice as controls rather than wild-type animals. Quantitative RT-PCR analysis of whole cerebellum RNA indicated increased expression of PDGFRα, a marker of OPC encoding the platelet-derived growth factor receptor α, and decreased expression of Mbp, a specific marker of mature oligodendrocyte encoding myelin basic protein (Fig. 2A). Quantitative Western blot analysis showed an equivalent reduction in the MBP protein level at the P15 (Fig. 2B). Because these methods provide only indirect indications that OPC differentiation is affected, we used immunostaining to count Olig2+ cells in cerebellum lobule VIII of TRαAMI/S. This revealed a slight excess of OPC at P15 (Fig. 3A and Table 1) and confirmed that increased proliferation and/or delayed OPC differentiation change the cellular composition of TRαAMI/S cerebellum. Pan-neural expression of TRα1L400R in TRαAMI/N and TRαAMI/PT2/P5 (tamoxifen injection at P5) mice also resulted in an increased Olig2+ cell numbers (Fig. 3A and Table 2). Delay in the onset of OL appearance was confirmed by using the CC-1 monoclonal antibody, which marks mature OL (45) and reveals a reduced density at P15 (Supplemental Fig. 1). Altogether, these data indicate that ubiquitous, pan-neural and pan-cerebellar (from P5) expression of TRαAMI allele delays OPC differentiation. This phenotype is mainly visible at P15.
Fig. 2.
Delayed postnatal differentiation of OPC after ubiquitous TRα1L400R expression. A, Quantification of mRNA relative abundance in whole cerebellum at different ages (P8, P15, and P21). Comparison between ubiquitous mutants (TRαAMI/S) mice (n = 6, in black) and control littermates (n = 6, in gray). PDGFRα is specific of OPC, and Mbp is a marker of mature OL. P15 wild-type whole cerebellum RNA was used as a reference. B, Myelin basic protein quantification at P15 by Western blot analysis (n = 5), normalized by β-tubulin (β-Tub) indicates a delay in myelination in mutant expressing the TRα1L400R ubiquitously (TRαAMI/S). Data are expressed ± sd. *, Significant difference between mutants and wild types, P < 0.05. MBP, Myelin basic protein.
Fig. 3.
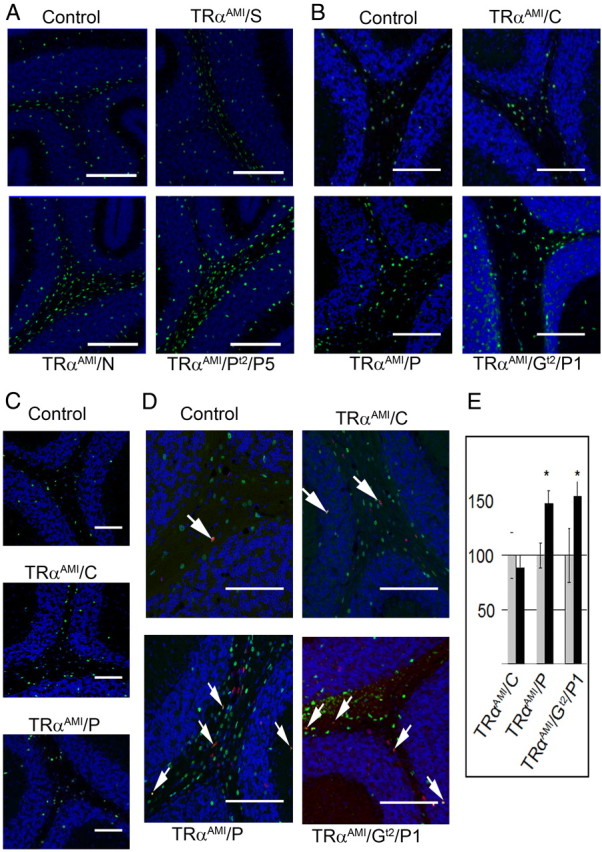
An indirect effect of T3 for OPC differentiation at P15. A, Olig2 staining at P15 (in green. DAPI Counterstain blue) indicates an increased density of OPC in TRαAMI/S (ubiquitous expression), TRαAMI/N (brain specific), and TRαAMI/PT2/P5 (pan-cerebellar expression from P5) compared with littermate control. See Table 2 for counts. B, Olig2 staining in the white matter of control (without Cre), oligodendrocyte specific (TRαAMI/C), GABAergic lineage specific (Purkinje cell and GABAergic interneurons, TRαAMI/P), and astrocyte specific from P1 (TRαAMI/GT2/P1) indicate a not cell-autonomous effect of thyroid hormone in oligodendrocyte differentiation at P15. C, Same staining at P21 indicates a transient phenotype, mainly observable at P15. D, 24 h BrdU labeling of proliferating cells. Double BrdU/Olig2+ (red/green, colocalization in yellow) E, Counting of double BrdU/Olig2+ cells indicates an increased density in proliferating OPC at P15 when TRα1L400R is expressed in GABAergic neurons (TRαAMI/P) mutants or in astrocytes (TRαAMI/GT2/P1) but not in OPC (TRαAMI/C). Data are expressed ± sd. *, Significant difference between mutants and wild types, P < 0.05. Scale bar, 50 μm.
Table 2.
Counts of olig2+ OPC on cerebellum lobule VIII slices
| Genotype | Relative Olig2+ density at P15 mean ± sd (±sd of control littermates) |
Relative Olig2+ density at P21 mean ± sd (±sd of control littermates) |
||||
|---|---|---|---|---|---|---|
| White matter | Total | n | White matter | Total | N | |
| TRαAMI/S | 0.94 ± 0.30 (±0.01) | 1.39 ± 0.21 (±0.06)a | 3 | 0.85 ± 0.1 (±0.01)a | 0.93 ± 0.14 (±0.15) | 2 |
| TRαAMI/N | 1.24 ± 0.11 (±0.13)a | 1.23 ± 0.14 (±0.18)a | 4 | 0.91 ± 0.11 (±0.06) | 1.14 ± 0.27 (±0.22) | 4 |
| TRαAMI/C | 1.12 ± 0.11 (±0.16) | 1.19 ± 0.19 (±0.16) | 5 | 0.97 ± 0.25 (±0.24) | 1.09 ± 0.33 (±0.20) | 6 |
| TRαAMI/liter | 0.98 ± 0.14 (±0.12) | 0.93 ± 0.13 (±0.29) | 4 | 1.06 ± 0.19 (±0.19) | 1.07 ± 0.21 (±0.36) | 3 |
| TRαAMI/P | 1.61 ± 0.22 (±0.18)a | 1.57 ± 0.15 (±0.11)a | 6 | 1.09 ± 0.10 (±0.09) | 1.11 ± 0.06 (±0.06) | 5 |
| TRαAMI/GT2/P1 | 1.53 ± 0.36 (±0.12) | 2.39 ± 0.46 (±0.05)a | 3 | 1.21 ± 0.17 (±0.01) | 1.19 ± 0.17 (±0.01) | 4 |
| TRαAMI/PT2/P5 | 1.49 ± 0.28 (±0.22)a | 1.51 ± 0.30 (±0.13)a | 5 | Not determined | Not determined | |
Relative density was measured on cerebellum slices as described in Materials and Methods.
Significant changes, compared with control littermates, are in bold (P < 0.05).
We next asked whether the changes observed at P15 in mice expressing TRα1L400R ubiquitously were due to the cell-autonomous inhibition of T3 signaling. We therefore counted Olig2+ cells of mice cerebellum expressing TRα1L400R in a restricted manner. Surprisingly Olig2+ cell density remained unchanged when TRα1L400R expression was in OPC (TRαAMI/C; Fig. 3B and Table 2), suggesting that the observed phenotype is not a cell-autonomous consequence of TRα1L400R expression. Conversely, Olig2+ cell density was increased at P15), at the expense of OL (Supplemental Fig. 1), when expression was either in Purkinje neurons and GABAergic interneurons (TRαAMI/P) or in astrocytes and Bergmann glia (TRαAMI/GT2/P1, Fig. 3B and Table 2). Although we cannot formally rule out a possible intervention of GABAergic interneurons in the determination of the TRαAMI/P phenotype, Purkinje cells are more likely to be involved (see Discussion). If this is true, expression of TRα1L400R in Purkinje neurons impacts OPC differentiation when it occurs early in development (TRαAMI/P) but not after P8 (TRαAMI/L, TRαAMI/C). Therefore, we conclude that TRα1L400R expression impairs OPC differentiation at this stage in an indirect manner, by acting on both astrocytes and Purkinje cells, and perhaps GABAergic interneurons, before P8. This phenotype is mainly visible at P15, a recovery being apparent at P21 (Fig. 3C and Table 2).
To better characterize the indirect consequences of expressing TRα1L400R in astrocytes or Purkinje neurons, mitotic activity of OPC was assayed by treating mice with bromodeoxyuridine (BrdU) 24 h before histochemical analysis (Fig. 3, D and E). The number of Olig2+/BrdU+ cells was increased when TRα1L400R expression was in Purkinje neurons (TRαAMI/P) or astrocytes (TRαAMI/GT2/P1), indicating that the increase of Olig2+ cells number correlated with an increase in OPC mitotic activity (Fig. 3E). In an attempt to identify signaling pathways involved in these indirect effects, we performed gene expression profiling by quantitative RT-PCR at P8. Pan-neural expression of TRα1L400R in TRαAMI/N mice correlated with low Ntf3, Bdnf, Pdgf, P75ntr mRNA levels, and down-regulation of three genes encoding component of the hedgehog pathways: Shh, Ptch1, and Gli2 (Table 3). Decrease in NT3, BDNF, and Shh signaling suggests a global deficiency in the cerebellum developmental process. Among other cell types, it can thus affect granular neurons, which express Ntf3 and Bdnf, and Purkinje cells, which express Shh. OPC differentiation can then be indirectly influenced because it is sensitive to the corresponding signaling pathways. However, TRα1L400R expression in early Purkinje neurons (TRαAMI/P mice) affects only Ntf3 mRNA level, indicating that Bdnf, Shh, and Ntf3 deregulation, as observed in TRαAMI/N mice, is not a consequence of TRα1L400R expression in Purkinje cells. Because Ntf3 expression gradually decreases during normal cerebellar development, we addressed the possibility that changes in Ntf3 mRNA level at P15 were only a consequence of delayed granular neurons maturation, by measuring Gabra6 mRNA level. This gene encodes the α6-subunit of the GABA receptor, and its expression, restricted to inner granular layer neurons, increases gradually during postnatal maturation. Gabra6 mRNA level was changed by broad TRα1L400R expression (TRαAMI/N) but not when it was in Purkinje neurons (TRαAMI/P). Therefore, delayed granular neurons maturation cannot be the only explanation for the observed changes in Ntf3 expression (Table 3). The mechanism underlying the indirect effect of TRα1L400R on early OPC therefore involves several cell types, including astrocytes, Purkinje neurons, and granular neurons and permanent interactions mediated by cell contacts and exchange of diffusible factors.
Table 3.
Quantitative RT-PCR analysis of gene expression in whole cerebellum at P8 and P60
| A. Total cerebellar mRNA at P8 | |||
|---|---|---|---|
| Gene | Control group mean (±sd) (n = 6) | TRαAMI/N mean (±sd) (n = 6) | TRαAMI/P mean (±sd) (n = 6) |
| Ntf-3 | 100 ± 6.2 | 67 ± 6.6a | 144.0 ± 14.9a |
| Ngf | 100 ± 28.2 | 99.3 ± 12.3 | 96.6 ± 11.1 |
| Bdnf | 100 ± 49.6 | 20 ± 2.4a | 152.0 ± 54 |
| Pdgf | 100 ± 5.4 | 79 ± 8.4a | 101.9 ± 22.1 |
| p75 | 100 ± 6.5 | 37 ± 4.4a | 89.3 ± 5.1 |
| Shh | 100 ± 13.2 | 46 ± 4.7a | 105.4 ± 35 |
| Gli2 | 100 ± 8.9 | 26 ± 3.4a | 126.8 ± 39.7 |
| Ptch1 | 100 ± 18.1 | 33 ± 5.0a | 114.8 ± 16.5 |
| Gabra6 | 100 ± 16.5 | 42 ± 18.3 | 109 ± 8.7 |
| B. Total cerebellar mRNA at P60 | |||
|---|---|---|---|
| Gene | Control group cre-mean (±sd) (n = 5) | TRαAMI/Pt2/P30 mean (±sd) (n = 5) | TRαAMI /Pt2 no tamoxifen mean (±sd) (n = 5) |
| Olig2 | 100 ± 25.3 | 198.0 ± 34.2a | 93.0 ± 16.2 |
| Pdgf-aa | 100 ± 14.2 | 102.3 ± 34.5 | 107.8 ± 20.1 |
| Plp | 100 ± 16.5 | 92.5 ± 43.9 | 128.0 ± 17.1 |
| Mbp | 100 ± 16.3 | 76.1 ± 15.3 | 97.8 ± 19.0 |
| Golli Mbp | 100 ± 24.0 | 78.3 ± 14.7 | 108.2 ± 25.3 |
| TRa1 | 100 ± 20.8 | 135.0 ± 25.6 | 106.3 ± 12.5 |
Data normalized using Hprt expression as a reference.
Significant difference (bold characters), P < 0.05.
A late cell-autonomous effect of T3 on OPC
In all mice, OPC differentiation appeared to be only delayed, as, by P21, Olig2+ OPC cell numbers fell within control values in all genotypes (Fig. 2C and Table 2). Broad expression of TRα1L400R, which occurs in TRαAMI/S, TRαAMI/N, or TRαAMI/PT2/P5 mice is usually lethal before P25, precluding any analysis at a later stage. However, the long-term consequences of TRα1L400R expression on cerebellum OPC fate can be addressed in the mice expressing TRα1L400R in a restricted manner, or only after the critical weaning period, by activating recombination with tamoxifen at a late stage (TRαAMI/PT2/P30) (Fig. 4). Including the R26YFP reporter transgene allowed us to verify that Cre recombination patterns at P60 were identical to those observed at early stages for TRαAMI/C, TRαAMI/P (data not shown) or TRαAMI/PT2/P30 (Fig. 4I) and to trace the fate of cells undergoing recombination. In adults, few Olig2+ OPC were present in wild-type cerebellum white matter (Fig. 4, A and E). By contrast to what was observed at P15, the density of Olig2+ OPC was not increased in TRαAMI/P mice (Fig. 4, D and H), confirming that expression of TRα1L400R in Purkinje cells has only a transient influence on OPC differentiation. Conversely, when OPC express TRα1L400R (TRαAMI/C), density dramatically increased at P60, indicating that this late effect of TRα1L400R on OPC proliferation is cell autonomous (Fig. 4, B and F). Interestingly, the accumulation of OPC-derived cells, as judged by YFP staining, was not observed in the white matter of other brain areas, including corpus callosum, in which suggestive observations have been made in hypothyroid rats (46).
Fig. 4.
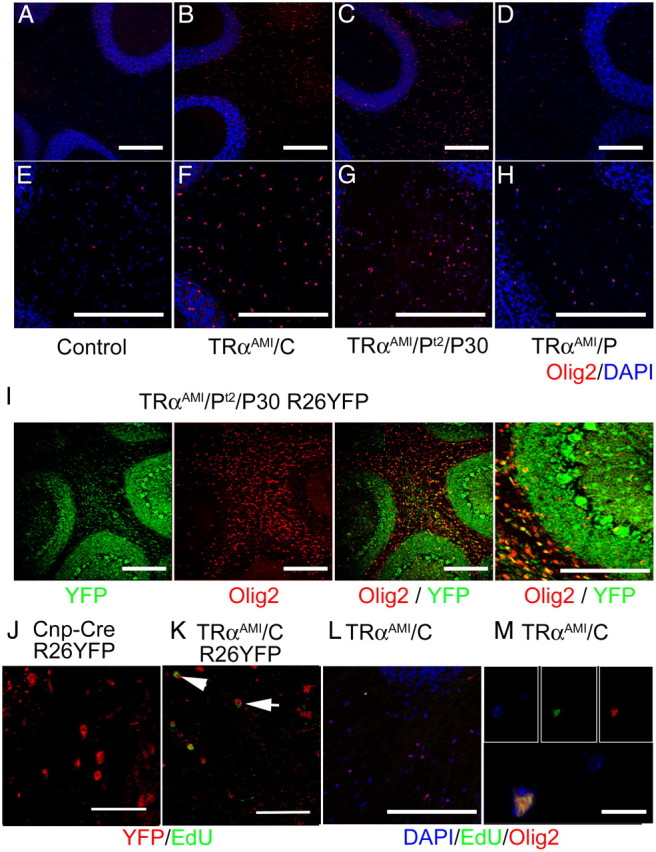
TRα1L400R stimulates adult OPC proliferation in a cell-autonomous manner. A–H, Immunostaining at P60 of Olig2+ OPC (red; DAPI counterstain in blue) compared with control (A and E). Density is increased when TRα1L400R is expressed in the oligodendrocyte lineage (B and F, TRαAMI/C, 12-fold increase compared with littermate controls) or in all cell types starting from P30 (C and G, TRαAMI/PT2/P30, 14-fold increase compared with littermate controls), but not if expression is restricted to GABAergic neurons (D and H, TRαAMI/P). I, YFP/Olig2 immunostaining confirms that Cre recombination occurs in the oligodendrocyte lineage of TRαAMI/PT2/P30/R26YFP mice (green, YFP; red, Olig2; yellow, merge). J and K, Expression of TRα1L400R in oligodendrocyte lineage increases the number of YFP+/EdU+ cells at P60, 7 d after EdU injection (YFP, red; and EdU, green). J, Control CrexR26YFP K, TRαAMI/CxR26YFP. L and M, Colocalization of Olig2 and EdU in TRαAMI/C white matter (blue, DAPI; gree:, EdU; red, Olig2). n = 4 for each genotype. Scale bar, 200 μm (panels A–L), 10 μm (panel M).
We then asked whether the late accumulation of OPC in cerebellum white matter requires the permanent expression of TRα1L400R, by inducing a broad expression only after cerebellum maturation (TRαAMI/PT2/P30). The important increase of Olig2+ cell density found in these mice 30 d later showed that proliferation is stimulated by TRα1L400R, even when expression starts after the completion of the myelination process (Fig. 4, C and G). R26YFP reporter transgene indicated that, in the white matter, most of the cells that underwent recombination were OPC, which did not differentiate within 30 d (Fig. 4I). TRαAMI/PT2/P30 cerebellum also showed an increase in Olig2 mRNA level at P60, correlating with the change in cellular content, but mRNA levels were not changed for Mbp, GolliMBP, and Plp, which mark differentiated OL (Table 3). These observations further support the idea that adult OPC expressing TRα1L400R are unable to differentiate.
5-Ethynyl-2′ deoxyuridine (EdU) injection at P53, i.e. 7 d before analysis, revealed the presence of proliferating cells among the OPC that expressed TRα1L400R (TRαAMI/C/R26YFP) indicating that a permanent but slow proliferation of Olig2+ OPC was responsible for the observed accumulation of Olig2+ cells in the white matter (Fig. 4, J, K, L, and M). As recombination takes place at an early stage for these TRαAMI/C/R26YFP mice, OL also express YFP. Taken together, these data lead us to conclude that T3 exerts a permanent control of OPC proliferation in adults, in nonpathological situations. This control is mediated by TRα1 and is cell autonomous.
Discussion
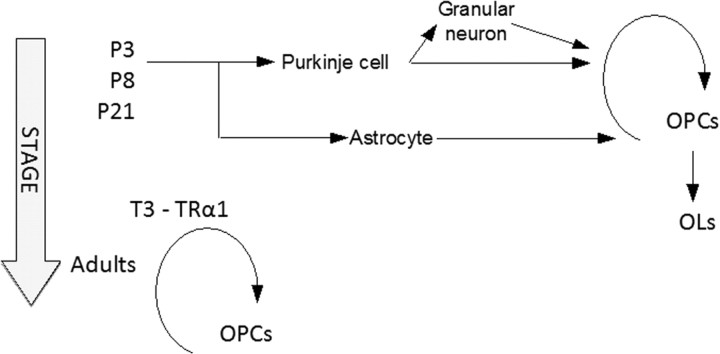
We previously reported that, like T3 deficiency, ubiquitous expression of TRα1L400R mutation retards myelin formation (47). Because the phenotype was observed in heterozygous mice, and T3 level was unchanged in this transgenic model, other TR isoforms (TRβ1, TRβ2, TRα2, TRΔα1, etc.) were not involved. However, the ubiquitous expression of TRα1L400R being usually lethal, we could not decide whether systemic disorders could be compromising neurodevelopment in an indirect and not specific manner. By using somatic recombination we show here that a combination of direct and indirect effects explains the influence of TRα1 on the oligodendrocyte lineage. When T3 is present at physiological levels, TRα1 promotes OPC cell cycle exit and commitment to differentiation both in juveniles and adults. However, TRα1 exerts its function at different stages in two very different manners. Soon after birth, liganded TRα1 acts primarily on Purkinje cells and astrocytes, triggering a combination of neurotrophic factors secretion and an intricate network of cellular interactions involving granular neurons, Purkinje neurons, and astrocytes. These cellular interactions indirectly ensure the timely differentiation of OPC. Because we are unable to restrict TRα1L400R to Purkinje cells before P8, we cannot rule out an involvement of immature GABAergic interneurons at an early stage. Based on the parsimony principle, we believe that such a possibility should not be taken into account at this point because, unlike Purkinje cells, GABAergic interneurons are not known to secrete neurotrophins. Second, they differentiate mainly at a later stage. This prompts us to believe that the TRα1 effect on early OPC differentiation is only relayed by Purkinje cells and Bergmann glia paracrine activity. Interestingly, Purkinje cells effect is only transient and ceases between P5 and P8. This conclusion is based on the observation that OPC density at P15 is increased in TRαAMI/PT2/P5 mice, which express TRα1L400R from P5, but not in TRαAMI/L and TRαAMI/C, which express the mutation from P8 in Purkinje cells. In vitro experiments also indicate that activation of TRα1 by T3 is required for their early morphological maturation (48, 49). Recent evidence suggests that TRα1 expression is high in Purkinje cells and then drops (23), whereas TRβ1 expression increases and might become necessary for the maintenance of the function of these cells (50). Impairment of Purkinje cells maturation by early TRα1L400R expression consequently impacts their environment, increasing Ntf3 expression in granular neurons and delaying OPC differentiation. When TRα1L400R expression is ubiquitous (TRαAMI/N), a delay in granular neurons differentiation exerts an additional indirect influence on Ntf3 mRNA level, and probably on NT3 secretion. Although the heterogeneous astrocyte lineage is poorly characterized, it is also sensitive to the direct action of liganded TRα1 (34, 51) and can synthesize a number of factors required for proper cerebellum development and myelin formation (9). The response of astrocytes to T3 appears to be also required for timely OPC differentiation. T3 at this early stage appears therefore to be able to synchronize a complex network of cellular interactions involving several glial and neuronal cell types (52, 53) but does not act directly on OPC differentiation.
Our second finding is that T3/TRα1 signaling is also required to prevent the expansion of a population of slowly proliferative OPC in adults. This second function becomes obvious only at a late stage of development, when most OPC differentiation is normally terminated, and requires the cell-autonomous function of TRα1. This persistence of cycling OPC in adult mice has already been reported in the optic nerve for THRA/THRB double knockout, which are devoid of all T3 receptors but not for THRA single knockout (22). Similarity between the phenotypes of TRαAMI/C and THRA/THRB knockout, but not single THRA knockout, can be explained in several ways. First, most manifestations of T3 deficiency during neurodevelopment are due to permanent negative regulation exerted by unliganded TRα1, rather than loss of transactivation. Knock-in mutations are thus more detrimental than knock-out in which negative regulation is lost (54). Second THRA individual knockout might artificially broaden the repertoire of TRβ1 target genes, as demonstrated for other nuclear receptors (55). Finally TRα1L400R could act as a TRβ antagonist, although this is not usually the case, in cell types in which both receptors are present (30). We thus propose a hypothetical model in which liganded TRα1 exerts two distinct functions: an early indirect effect involving several neurotrophins and a cell-autonomous regulation in adult OPC (Fig. 5). At this point the early influence is very unclear, but certainly involves several glial and neuronal cell populations, and neurotrophin factors, including NT3, the individual contribution of which was not precisely defined.
Fig. 5.
Hypothetical model proposed for the influence of T3 signaling on OPC differentiation. During the first postnatal week, T3 exerts its cell-autonomous function only in Purkinje cells and astrocytes, modifying neurotrophic factor secretion and cell contacts, and indirectly favoring the commitment of proliferating OPC toward terminal differentiation. Although ubiquitous expression of TRα1L400R affects BDNF, Shh, and PDGF secretion, only secretion of NT3 by granular neurons is presented. By contrast, adult OPC are sensitive to the cell-autonomous signaling by T3/TRα1, which is required to prevent uncontrolled proliferation (second mode of action).
It is likely that the early and late OPC phenotypes involve distinct cell populations. The origin of murine cerebellar OPC is unknown (56) but recent quail/chick chimera experiments indicate that in birds they migrate from the midbrain alar plate (57). Whether adult OPC derive from the maturation of perinatal OPC (58) or from the expansion of a distinct small population of cells already present at birth (59) remains unclear. The early OPC population cycles rapidly and ensures most of initial myelination, after few symmetric divisions (60). We found that it is mostly sensitive to the indirect effect of T3. If early OPC and late OPC are truly distinct cell types, they would not necessarily respond in the same manner to T3 stimulation.
Interestingly, an early in vitro study concluded that purified newborn OPC are poorly sensitive to T3-mediated differentiation (61). This prompts us to believe that most of these previous studies, performed in vitro, which very convincingly demonstrated that T3 is a key factor triggering proliferation arrest and terminal differentiation, are relevant to adult OPC. This second population of OPC has a very different behavior. It is composed of slow cycling cells, which mostly perform asymmetric divisions (58, 60) and have a poor capacity to make myelin (59). This slow cycling can be maintained for months in culture medium without T3 (12, 62). Adult OPC recently received considerable attention not only because ischemia and demyelination reactivate their proliferation (63) but also because they make synapses with the climbing fibers that excite Purkinje cells (64). They are also believed to constitute a distinct population of dispersed neural stem cells (11, 40, 42, 65), although their ability to generate neurons has been recently challenged (66). The fact that liganded TRα1 exerts a direct and permanent control on adult OPC proliferation suggests a number of interesting possibilities for later investigations.
Materials and Methods
Animals
All animal experimentations were conducted under animal care procedures in accordance with the guidelines set by the European Community Council Directives (86/609/EEC). All mice were of C57/Bl6 genetic background, with a 129Sv contribution. ROSA26-lox-STOP-lox-EYFP (R26YFP) (67) was introduced in TRαAMI/Cre mice to define recombination patterns (see Table 1c). YFP immunostaining was systematically used after tamoxifen treatment to rule out any variation between treated animals. Mice were genotyped by PCR for Cre, R26YFP, and TRαAMI alleles using specific primers. Tamoxifen (1 mg/40 g of body weight in 100 μl of sunflower oil), 100 mg BrdU/kg of mice in 100 μl 0.9% NaCl injectable solution, or 25 mg EdU/kg of mice were injected ip. For immunohistochemistry, animals were first anesthetized by ip injection of a lethal dose of ketamine/xylazine and perfused with paraformaldehyde (4% in PBS). Cerebellums were postfixed for 2 h and washed, and sections (50 μm thick) were performed with a vibratome (Campden Instruments, Lafayette, IN).
Immunohistochemistry, cell labeling, and Western blotting
Sections were washed in PBS (pH 7.4) and then blocked in 10% normal donkey serum, 2% gelatin from cold water Fish Skin (Sigma, St. Louis, MO), and 0.02% Triton X-100 in PBS. Sections were incubated overnight at 4 C with primary antibodies (Supplemental Table 2) diluted in 1% dimethylsulfoxide, 1% donkey serum, 1% goat fetal serum in PBS, washed at least three times in PBS, and incubated for 2 h at room temperature with 4′,6-diamidino-2-phenylindole (DAPI) (1:1000; Invitrogen, Carlsbad, CA) and secondary antibodies coupled to a fluorescent dye diluted in 1% donkey serum, 1% dimethylsulfoxide in PBS. For BrdU labeling, HCl treatment (2 m, 30 min, 37 C) was performed before the blocking step. EdU staining was done as previously described (68). Microscopic observations were done using Axioplan2 (Carl Zeiss, Thornwood, NY) or SP5 confocal microscope (Leica Microsystems, Deerfield, IL). Cell counting was performed using Metamorph and ImageJ softwares. Two series of two optical sections (1 μm along the z-axis) were acquired in each slice. Section series were projected using the ImageJ z-projection function set for maximum intensity. Counting was performed manually, and the region of interest was determined using DAPI staining.
For Western blotting, protein extracts (10 μg) were resolved on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes before incubation with antibody and then IRDye secondary antibody. Immunoreactive bands were detected and quantified using Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE). All antibodies are listed in Supplemental Table 1.
RNA analysis
RNA was extracted from whole cerebellum using a mini-extraction kit (QIAGEN, Chatsworth, CA). cDNA were prepared from 1 μg of RNA using M-MLV reverse transcriptase (Promega Corp., Madison, WI) and random 6-mer primers in 20 μl. cDNA (1 ng) was used for quantitative PCR, using sybrgreen (QIAGEN). Quantification was performed in triplicate using HPRT as internal standard and the 2–ΔΔCt method for analysis.
Statistical analysis
All statistical analyses were done using R software. ANOVA analysis was used to determine significant difference between Olig2+ cell number or mRNA abundance in different genotypes. Data are presented ± sd. The number of mice in each experiment was at least four per genotype, originating from two different litters, providing both mutant and control mice. All statistical analyses were performed between animals from the same litter.
Acknowledgments
We thank W.D. Richardson, F. Tronche, K.A. Nave, and F.W. Pfrieger for the kind gift of transgenic mice, and K. Gauthier, I. Dusart, P. Godement, and J. Burden for the critical reading of the manuscript. We especially thank N. Aguilera for mouse breeding and the IFR128 facilities, PBES and PLATIM, for animal care and microscopy.
This work was supported by the CRESCENDO European integrated project (LSHM-CT-2005-018652), CASCADE network of excellence (FOOD-CT-2004-506319), and Agence Nationale pour la Recherche (Neuro 2007/Projet Switch). F. Picou was supported by a Fondation pour la Recherche Médicale fellowship; F. Chatonnet was supported by Association pour la Recherche contre le Cancer, and T. Fauquier was supported by CRESCENDO.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BDNF
- Brain-derived neurotrophic factor
- BrdU
- bromodeoxyuridine
- DAPI
- 4′,6-diamidino-2-phenylindole
- EdU
- 5-Ethynyl-2′ deoxyuridine
- GABA
- γ-aminobutyric acid
- NT3
- neurotrophin-3
- OL
- oligodendrocytes
- Olig2
- oligodendrocyte transcription factor 2
- OPC
- oligodendrocyte precursor cells
- P6
- postnatal d 6
- PDGF
- platelet-derived growth factors
- Shh
- sonic hedgehog
- TRα1
- T3 receptor α1
- YFP
- yellow fluorescent protein.
References
- 1. Li H , He Y , Richardson WD , Casaccia P. 2009. Two-tier transcriptional control of oligodendrocyte differentiation. Curr Opin Neurobiol 19:479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calver AR , Hall AC , Yu WP , Walsh FS , Heath JK , Betsholtz C , Richardson WD. 1998. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron 20:869–882 [DOI] [PubMed] [Google Scholar]
- 3. Barres BA , Raff MC , Gaese F , Bartke I , Dechant G , Barde YA. 1994. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature 367:371–375 [DOI] [PubMed] [Google Scholar]
- 4. Van't Veer A , Du Y , Fischer TZ , Boetig DR , Wood MR , Dreyfus CF. 2009. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J Neurosci Res 87:69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan JR , Watkins TA , Cosgaya JM , Zhang C , Chen L , Reichardt LF , Shooter EM , Barres BA. 2004. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron 43:183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Merchán P , Bribián A , Sánchez-Camacho C , Lezameta M , Bovolenta P , de Castro F. 2007. Sonic hedgehog promotes the migration and proliferation of optic nerve oligodendrocyte precursors. Mol Cell Neurosci 36:355–368 [DOI] [PubMed] [Google Scholar]
- 7. Du Y , Dreyfus CF. 2002. Oligodendrocytes as providers of growth factors. J Neurosci Res 68:647–654 [DOI] [PubMed] [Google Scholar]
- 8. Kukley M , Nishiyama A , Dietrich D. 2010. The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J Neurosci 30:8320–8331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watkins TA , Emery B , Mulinyawe S , Barres BA. 2008. Distinct stages of myelination regulated by γ-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron 60:555–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nave KA , Trapp BD. 2008. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci 31:535–561 [DOI] [PubMed] [Google Scholar]
- 11. Nishiyama A , Komitova M , Suzuki R , Zhu X. 2009. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10:9–22 [DOI] [PubMed] [Google Scholar]
- 12. Tang DG , Tokumoto YM , Raff MC. 2000. Long-term culture of purified postnatal oligodendrocyte precursor cells. Evidence for an intrinsic maturation program that plays out over months. J Cell Biol 148:971–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yen PM. 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- 14. Billon N , Tokumoto Y , Forrest D , Raff M. 2001. Role of thyroid hormone receptors in timing oligodendrocyte differentiation. Dev Biol 235:110–120 [DOI] [PubMed] [Google Scholar]
- 15. Hadj-Sahraoui N , Seugnet I , Ghorbel MT , Demeneix B. 2000. Hypothyroidism prolongs mitotic activity in the post-natal mouse brain. Neurosci Lett 280:79–82 [DOI] [PubMed] [Google Scholar]
- 16. Raff MC , Lillien LE , Richardson WD , Burne JF , Noble MD. 1988. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature 333:562–565 [DOI] [PubMed] [Google Scholar]
- 17. Ibarrola N , Rodríguez-Peña A. 1997. Hypothyroidism coordinately and transiently affects myelin protein gene expression in most rat brain regions during postnatal development. Brain Res 752:285–293 [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez-Peña A , Ibarrola N , Iniguez MA , Muñoz A , Bernal J. 1993. Neonatal hypothyroidism affects the timely expression of myelin-associated glycoprotein in the rat brain. J Clin Invest 91:812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahlgren SC , Wallace H , Bishop J , Neophytou C , Raff MC. 1997. Effects of thyroid hormone on embryonic oligodendrocyte precursor cell development in vivo and in vitro. Mol Cell Neurosci 9:420–432 [DOI] [PubMed] [Google Scholar]
- 20. Marta CB , Adamo AM , Soto EF , Pasquini JM. 1998. Sustained neonatal hyperthyroidism in the rat affects myelination in the central nervous system. J Neurosci Res 53:251–259 [DOI] [PubMed] [Google Scholar]
- 21. Billon N , Jolicoeur C , Tokumoto Y , Vennström B , Raff M. 2002. Normal timing of oligodendrocyte development depends on thyroid hormone receptor α 1 (TRα1). EMBO J 21:6452–6460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baas D , Legrand C , Samarut J , Flamant F. 2002. Persistence of oligodendrocyte precursor cells and altered myelination in optic nerve associated to retina degeneration in mice devoid of all thyroid hormone receptors. Proc Natl Acad Sci USA 99:2907–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wallis K , Dudazy S , van Hogerlinden M , Nordström K , Mittag J , Vennström B. 2010. The thyroid hormone receptor α 1 protein is expressed in embryonic postmitotic neurons and persists in most adult neurons. Mol Endocrinol 24:1904–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradley DJ , Towle HC , Young WS. 1992. Spatial and temporal expression of α- and β-thyroid hormone receptor mRNAs, including the β 2-subtype, in the developing mammalian nervous system. J Neurosci 12:2288–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manzano J , Bernal J , Morte B. 2007. Influence of thyroid hormones on maturation of rat cerebellar astrocytes. Int J Dev Neurosci 25:171–179 [DOI] [PubMed] [Google Scholar]
- 26. Lindholm D , Castrén E , Tsoulfas P , Kolbeck R , Berzaghi Mda P , Leingärtner A , Heisenberg CP , Tessarollo L , Parada LF , Thoenen H , Tesarollo L. 1993. Neurotrophin-3 induced by tri-iodothyronine in cerebellar granule cells promotes Purkinje cell differentiation. J Cell Biol 122:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neveu I , Arenas E. 1996. Neurotrophins promote the survival and development of neurons in the cerebellum of hypothyroid rats in vivo. J Cell Biol 133:631–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poguet AL , Legrand C , Feng X , Yen PM , Meltzer P , Samarut J , Flamant F. 2003. Microarray analysis of knockout mice identifies cyclin D2 as a possible mediator for the action of thyroid hormone during the postnatal development of the cerebellum. Dev Biol 254:188–199 [DOI] [PubMed] [Google Scholar]
- 29. Sinha RA , Pathak A , Kumar A , Tiwari M , Shrivastava A , Godbole MM. 2009. Enhanced neuronal loss under perinatal hypothyroidism involves impaired neurotrophic signaling and increased proteolysis of p75(NTR). Mol Cell Neurosci 40:354–364 [DOI] [PubMed] [Google Scholar]
- 30. Quignodon L , Vincent S , Winter H , Samarut J , Flamant F. 2007. A point mutation in the activation function 2 domain of thyroid hormone receptor α1 expressed after CRE-mediated recombination partially recapitulates hypothyroidism. Mol Endocrinol 21:2350–2360 [DOI] [PubMed] [Google Scholar]
- 31. Koibuchi N. 2008. Hormonal regulation of cerebellar development and plasticity. Cerebellum 7:1–3 [DOI] [PubMed] [Google Scholar]
- 32. Manzano J , Cuadrado M , Morte B , Bernal J. 2007. Influence of thyroid hormone and thyroid hormone receptors in the generation of cerebellar γ-aminobutyric acid-ergic interneurons from precursor cells. Endocrinology 148:5746–5751 [DOI] [PubMed] [Google Scholar]
- 33. Nicholson JL , Altman J. 1972. The effects of early hypo- and hyperthyroidism on the development of the rat cerebellar cortex. II. Synaptogenesis in the molecular layer. Brain Res 44:25–36 [DOI] [PubMed] [Google Scholar]
- 34. Morte B , Manzano J , Scanlan TS , Vennström B , Bernal J. 2004. Aberrant maturation of astrocytes in thyroid hormone receptor α 1 knockout mice reveals an interplay between thyroid hormone receptor isoforms. Endocrinology 145:1386–1391 [DOI] [PubMed] [Google Scholar]
- 35. Rodríguez-Peña A. 1999. Oligodendrocyte development and thyroid hormone. J Neurobiol 40:497–512 [DOI] [PubMed] [Google Scholar]
- 36. Morte B , Manzano J , Scanlan T , Vennström B , Bernal J. 2002. Deletion of the thyroid hormone receptor α 1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc Natl Acad Sci USA 99:3985–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Flamant F , Poguet AL , Plateroti M , Chassande O , Gauthier K , Streichenberger N , Mansouri A , Samarut J. 2002. Congenital hypothyroid Pax8(−/−) mutant mice can be rescued by inactivating the TRα gene. Mol Endocrinol 16:24–32 [DOI] [PubMed] [Google Scholar]
- 38. Vujovic M , Nordström K , Gauthier K , Flamant F , Visser TJ , Vennström B , Mittag J. 2009. Interference of a mutant thyroid hormone receptor α1 with hepatic glucose metabolism. Endocrinology 150:2940–2947 [DOI] [PubMed] [Google Scholar]
- 39. Lappe-Siefke C , Goebbels S , Gravel M , Nicksch E , Lee J , Braun PE , Griffiths IR , Nave KA. 2003. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet 33:366–374 [DOI] [PubMed] [Google Scholar]
- 40. Rivers LE , Young KM , Rizzi M , Jamen F , Psachoulia K , Wade A , Kessaris N , Richardson WD. 2008. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci 11:1392–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ligon KL , Kesari S , Kitada M , Sun T , Arnett HA , Alberta JA , Anderson DJ , Stiles CD , Rowitch DH. 2006. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci USA 103:7853–7858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dimou L , Simon C , Kirchhoff F , Takebayashi H , Götz M. 2008. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci 28:10434–10442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cho SJ , Jung JS , Jin I , Moon IS. 2003. 2′,3′-cyclic nucleotide 3′-phosphodiesterase is expressed in dissociated rat cerebellar cells and included in the postsynaptic density fraction. Mol Cells 16:128–135 [PubMed] [Google Scholar]
- 44. Nait Oumesmar B , Vignais L , Baron-Van Evercooren A. 1997. Developmental expression of platelet-derived growth factor α-receptor in neurons and glial cells of the mouse CNS. J Neurosci 17:125–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu GY , Liu S , Hughes MG , McAdoo DJ. 2008. Glutamate-induced losses of oligodendrocytes and neurons and activation of caspase-3 in the rat spinal cord. Neuroscience 153:1034–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goodman JH , Gilbert ME. 2007. Modest thyroid hormone insufficiency during development induces a cellular malformation in the corpus callosum: a model of cortical dysplasia. Endocrinology 148:2593–2597 [DOI] [PubMed] [Google Scholar]
- 47. Fauquier T , Romero E , Picou F , Chatonnet F , Nguyen XN , Quignodon L , Flamant F. 2011. Severe impairment of cerebellum development in mice expressing a dominant-negative mutation inactivating thyroid hormone receptor α1 isoform. Dev Biol 356:350–358 [DOI] [PubMed] [Google Scholar]
- 48. Boukhtouche F , Janmaat S , Vodjdani G , Gautheron V , Mallet J , Dusart I , Mariani J. 2006. Retinoid-related orphan receptor α controls the early steps of Purkinje cell dendritic differentiation. J Neurosci 26:1531–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heuer H , Mason CA. 2003. Thyroid hormone induces cerebellar Purkinje cell dendritic development via the thyroid hormone receptor α1. J Neurosci 23:10604–10612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hashimoto K , Curty FH , Borges PP , Lee CE , Abel ED , Elmquist JK , Cohen RN , Wondisford FE. 2001. An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc Natl Acad Sci USA 98:3998–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trentin AG. 2006. Thyroid hormone and astrocyte morphogenesis. J Endocrinol 189:189–197 [DOI] [PubMed] [Google Scholar]
- 52. Mathis C , Collin L , Borrelli E. 2003. Oligodendrocyte ablation impairs cerebellum development. Development 130:4709–4718 [DOI] [PubMed] [Google Scholar]
- 53. Delaney CL , Brenner M , Messing A. 1996. Conditional ablation of cerebellar astrocytes in postnatal transgenic mice. J Neurosci 16:6908–6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mittag J , Wallis K , Vennström B. 2010. Physiological consequences of the TRα1 aporeceptor state. Heart Fail Rev 15:111–115 [DOI] [PubMed] [Google Scholar]
- 55. Taneja R , Roy B , Plassat JL , Zusi CF , Ostrowski J , Reczek PR , Chambon P. 1996. Cell-type and promoter-context dependent retinoic acid receptor (RAR) redundancies for RAR β 2 and Hoxa-1 activation in F9 and P19 cells can be artefactually generated by gene knockouts. Proc Natl Acad Sci USA 93:6197–6202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grimaldi P , Parras C , Guillemot F , Rossi F , Wassef M. 2009. Origins and control of the differentiation of inhibitory interneurons and glia in the cerebellum. Dev Biol 328:422–433 [DOI] [PubMed] [Google Scholar]
- 57. Mecklenburg N , Garcia-Lopez R , Puelles E , Sotelo C , Martinez S. 2011. Cerebellar oligodendroglial cells have a mesencephalic origin. Glia [DOI] [PubMed] [Google Scholar]
- 58. Wren D , Wolswijk G , Noble M. 1992. In vitro analysis of the origin and maintenance of O-2Aadult progenitor cells. J Cell Biol 116:167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bouslama-Oueghlani L , Wehrlé R , Sotelo C , Dusart I. 2005. Heterogeneity of NG2-expressing cells in the newborn mouse cerebellum. Dev Biol 285:409–421 [DOI] [PubMed] [Google Scholar]
- 60. Zhu X , Hill RA , Dietrich D , Komitova M , Suzuki R , Nishiyama A. 2011. Age-dependent fate and lineage restriction of single NG2 cells. Development 138:745–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barres BA , Lazar MA , Raff MC. 1994. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120:1097–1108 [DOI] [PubMed] [Google Scholar]
- 62. Tang DG , Tokumoto YM , Apperly JA , Lloyd AC , Raff MC. 2001. Lack of replicative senescence in cultured rat oligodendrocyte precursor cells. Science 291:868–871 [DOI] [PubMed] [Google Scholar]
- 63. Levine JM , Reynolds R , Fawcett JW. 2001. The oligodendrocyte precursor cell in health and disease. Trends Neurosci 24:39–47 [DOI] [PubMed] [Google Scholar]
- 64. Lin SC , Huck JH , Roberts JD , Macklin WB , Somogyi P , Bergles DE. 2005. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron 46:773–785 [DOI] [PubMed] [Google Scholar]
- 65. Guo F , Ma J , McCauley E , Bannerman P , Pleasure D. 2009. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci 29:7256–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kang SH , Fukaya M , Yang JK , Rothstein JD , Bergles DE. 2010. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68:668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Srinivas S , Watanabe T , Lin CS , William CM , Tanabe Y , Jessell TM , Costantini F. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Salic A , Mitchison TJ. 2008. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA 105:2415–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chung SS , Cuzin F , Rassoulzadegan M , Wolgemuth DJ. 2004. Primary spermatocyte-specific Cre recombinase activity in transgenic mice. Transgenic Res 13:289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tronche F , Kellendonk C , Kretz O , Gass P , Anlag K , Orban PC , Bock R , Klein R , Schütz G. 1999. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23:99–103 [DOI] [PubMed] [Google Scholar]
- 71. Barski JJ , Dethleffsen K , Meyer M. 2000. Cre recombinase expression in cerebellar Purkinje cells. Genesis 28:93–98 [PubMed] [Google Scholar]
- 72. Pascual M , Abasolo I , Mingorance-Le Meur A , Martínez A , Del Rio JA , Wright CV , Real FX , Soriano E. 2007. Cerebellar GABAergic progenitors adopt an external granule cell-like phenotype in the absence of Ptf1a transcription factor expression. Proc Natl Acad Sci USA 104:5193–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Slezak M , Göritz C , Niemiec A , Frisén J , Chambon P , Metzger D , Pfrieger FW. 2007. Transgenic mice for conditional gene manipulation in astroglial cells. Glia 55:1565–1576 [DOI] [PubMed] [Google Scholar]