Abstract
Cadherin-16 was originally identified as a tissue-specific cadherin present exclusively in kidney. Only recently, Cadherin-16 has been detected also on the plasma membrane of mouse thyrocytes. This last finding prompted us to note that the expression profile of Cadherin-16 resembles that of the transcription factor Pax8, a member of the Pax (paired-box) gene family, predominantly expressed in the developing and adult kidney and thyroid. Pax8 has been extensively characterized in the thyroid and shown to be a master gene for thyroid development and differentiation. In this study, we determined the role of the transcription factor Pax8 in the regulation of Cadherin-16 expression. We demonstrate that the Cadherin-16 minimal promoter is transcriptionally active in thyroid cells as well as in kidney cells, that Pax8 is able to activate transcription from a Cadherin-16 promoter reporter construct, and more importantly, that indeed Pax8 is able to bind in vivo the Cadherin-16 promoter region. In addition, by means of Pax8 RNA interference in thyroid cells and by analyzing Pax8 null mice, we demonstrate that Pax8 regulates also in vivo the expression of Cadherin-16. Finally, we reveal that the expression of Cadherin-16 is TSH dependent in FRTL-5 thyroid cells and significantly reduced in mouse thyroid carcinomas. Therefore, we conclude that Cadherin-16 is a novel downstream target of the transcription factor Pax8, likely since the early steps of thyroid development, and that its expression is associated with the fully differentiated state of the thyroid cell.
Cadherins are a family of adhesion molecules that play an important role during embryonic development, maintenance of adult tissue architecture, and growth control during tumorigenesis (1). Typically, cadherins mediate calcium-dependent and homophilic adhesion, thereby promoting association of cells expressing the same cadherin family members. In addition, cadherins assemble into large macromolecular complexes, such as adherent junctions and desmosomes (2). Adherens junctions are cell-cell adhesion complexes that make important contributions to embryogenesis and tissue homeostasis (3).
Cadherin-16, originally named kidney-specific-cadherin, represents a structurally distinct member of the cadherin family (4). It belongs to a new subfamily of cadherins termed the seven domain-cadherins, which are mainly characterized by two structural features: seven extracellular cadherin repeat domains and a highly truncated cytoplasmic tail. Recently, αB-crystallin was identified as a partner of Cadherin-16 by affinity purification of proteins that bind to the cytoplasmic tail of Cadherin-16 in human kidney cells. In addition, both αB-crystallin and Cadherin-16 are associated with the actin cytoskeleton, suggesting a role of this cadherin in maintaining tissue integrity (5). Interestingly, it has also been shown that during the kidney ontogenesis, the expression of the Snail and Cadherin-16 genes is complementary (6).
Being initially identified as the only tissue-specific cadherin present exclusively in kidney, Cadherin-16 was recently detected on the plasma membrane of human and mouse thyrocytes (7). The thyroid gland consists of individual structural and functional units, the thyroid follicles, in which an epithelial cell monolayer surrounds a colloid-filled lumen. The integrity of the follicle structure is essential in preventing leakage of colloid from the lumen. In addition, the polarized organization of the thyroid follicular cells is essential for the proper function of the gland. The cells are coupled together by adhesive and occluding (tight) junctions, thereby preventing passive movement of solutes, fluid, and macromolecules between lumina and the blood compartment (8). Lately, it has been demonstrated that cadherins are important in the maintenance of the thyroid gland structure (9, 10). Lack of E-cadherin in late embryogenesis causes a change in lumen size and shape and affects the level of expression of β-catenin and the distribution of α-catenin but does not impair follicle formation, suggesting that also other cadherins are important in follicular cell adhesion (7).
Interestingly, the expression profile of Cadherin-16 resembles that of the transcription factor Pax8, a member of the paired box (Pax) family of genes encoding for DNA binding proteins that are involved in the regulation of the development of a variety of tissues in different species. In the adult organism, Pax8 is expressed exclusively in thyroid and kidney (11, 12). Furthermore, it has been demonstrated that Pax8 is required for both the morphogenesis of the thyroid gland and the maintenance of the thyroid-differentiated phenotype (13). In Pax8 knockout mice, the thyroid gland is barely visible and lacks the follicular cells, the most abundant cell population of the thyroid gland (14) and consequently the expression of the thyroid-specific markers, such as thyroglobulin (Tg) and thyroperoxidase (TPO), cannot be detected. These in vivo studies led to the conclusion that Pax8 is critical for proper differentiation of the thyrocytes. In parallel, mutations in the Pax8 gene have been associated with congenital hypothyroidism in humans. Patients carrying the mutations are affected by thyroid dysgenesis, indicating an important role for this gene in thyroid organogenesis (15).
The characterization of the promoter of the mouse Cadherin-16 gene revealed that it is a TATA-less promoter, containing multiple GC-boxes and a CAAT box. It also contains consensus recognition sites for several tissue-specific transcription factors, including basic-helix-loop-helix proteins, GATA factors, hepatocyte nuclear factor-1, hepatocyte nuclear factor-3, and CCAAT-enhancer binding protein (16–18). The proximal 250 bp of the promoter have been defined as the minimal promoter required for the expression in transfected renal epithelial cells and EMSA showed that this promoter region contains binding sites for nuclear proteins that are specifically expressed in renal epithelial cells (16). All together, these data were also confirmed in vivo by expressing in transgenic mice the green fluorescent protein reporter gene under the control of the Cadherin-16 minimal promoter (19, 20).
In the present study, we demonstrate that the minimal promoter of Cadherin-16 is active also in thyroid cells, and we show that the transcription factor Pax8 regulates its activity in transient transfection assays in cultured cell lines. In addition, by means of Pax8 RNA interference in rat thyroid cells and by analyzing Pax8 null mice, we demonstrated that Pax8 regulates also in vivo the expression of Cadherin-16.
Results
Cadherin-16 minimal promoter is active in thyroid cells
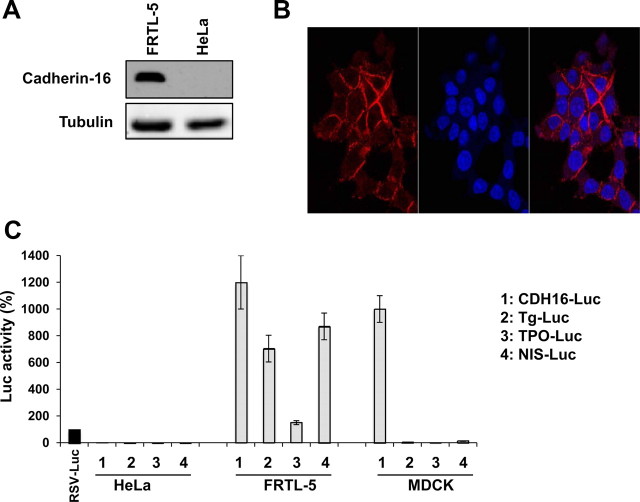
Cadherin-16 was first described as a kidney-specific adhesion molecule and thereafter found expressed also in the thyroid gland (7). Recently, a more detailed analysis showed that mouse thyrocytes express Cadherin-16 on their plasma membrane (Cali, G., S. Mogavero, P. Pallante, R. Nitsch, A. Ferraro, A. Fusco, and L. Nitsch, submitted for publication). Initially, we confirmed the expression of Cadherin-16 in our experimental model system, i.e. the rat-differentiated thyroid cell line FRTL-5, by Western blotting and immunofluorescence. As shown in Fig. 1A, the monoclonal antibody that specifically recognizes Cadherin-16 reveals the presence of a protein of the predicted relative molecular mass in the FRTL-5 protein extract, whereas it does not reveal any band in the HeLa protein extract. In parallel, an immunofluorescence staining with a specific anti-Cadherin-16 monoclonal antibody on FRTL-5 cells followed by confocal microscopy analysis shows the membrane distribution of the protein (Fig. 1B).
Fig. 1.
Cadherin-16 is expressed in rat FRTL-5 thyroid cells. A, Cells were lysed, and proteins were analyzed by Western blotting using a monoclonal anti-Cadherin-16 antibody. A protein of the predicted relative molecular mass (130 kDa) is detected in the protein extract of FRTL-5 cells but not in the protein extract of HeLa cells. The hybridization with α-tubulin assessed the protein uniform loading and integrity. B, To determine the localization of Cadherin-16, FRTL-5 cells were stained by immunofluorescence with the anti-Cadherin-16 antibody and identified using Alexa Fluor 594-conjugated secondary antibody. Cell nuclei were identified by Hoechst staining. Fluorescence was visualized with a Zeiss LSM 510 META confocal microscope. Magnification, ×63. C, Cells were transfected with RSV-Luc, CDH16-Luc, Tg-Luc, TPO-Luc, and NIS-Luc reporter constructs, and after 48 h of transfection luciferase activity was measured. To monitor the transfection efficiency, cells were cotransfected with pRL-TK, and firefly luciferase was normalized to renilla luciferase activity. Normalized firefly luciferase activity is shown relative to RSV-Luc activity, whose value was set at 100%. Data are means ± sd from three independent experiments. Statistical analysis uses t test (P < 0.01).
It was previously reported that the minimal DNA sequence sufficient for promoter activity in transfected renal epithelial cells consists of the 250 bp of the Cadherin-16 5′-flanking region (16). To evaluate whether such minimal promoter could also be active in thyroid cells, we generated a luciferase reporter plasmid containing 282 bp of the Cadherin-16 5′-flanking region (Cadherin-16-Luc) and performed functional assays in FRTL-5, HeLa, and MDCK cells. The latter is a renal epithelial cell line, which endogenously expresses Cadherin-16 and in which the Cadherin-16 minimal promoter is active, as previously reporter (16). Figure 1C shows that the minimal promoter of Cadherin-16 is active in thyroid cells as well as in kidney cells. In contrast, there is no significant stimulation of the luciferase activity upon transfection in HeLa cells. As expected, the reporter vectors of the thyroid-specific genes (Tg, TPO, and sodium-iodide symporter) are active only in FRTL-5 thyroid cells.
The Cadherin-16 promoter region is responsive to Pax8 and associates with Pax8 in vivo
We noticed that the expression profile of Cadherin-16 resembles that of the transcription factor Pax8. To investigate whether Pax8 could activate transcription from the Cadherin-16 minimal promoter, we tested the effect of transient Pax8 expression on the Cadherin-16 minimal promoter activity by luciferase assays. Specifically, we performed transactivation assays in HeLa cells transfecting the reporter construct CDH16-Luc together with increasing concentration of a Pax8 expression vector (CMV-Pax8). As shown in Fig. 2A, Pax8 significantly activates transcription from the Cadherin-16 promoter in a dose-dependent manner in HeLa cells.
Fig. 2.
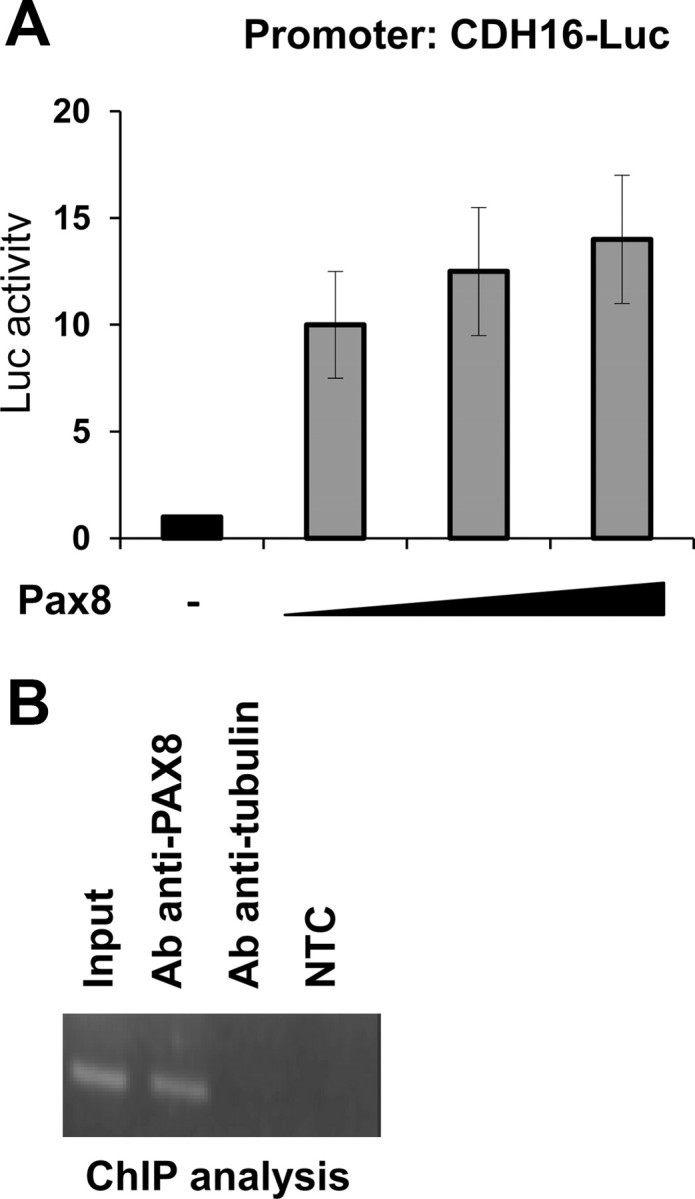
Pax8 activates transcription from the Cadherin-16 promoter. A, HeLa cells were transiently transfected with CDH16-Luc and with increasing concentration (100, 200, and 500 ng) of the expression vector encoding Pax8 (CMV-Pax8). Forty-eight hours after transfection, the transcriptional activity was determined as the firefly over renilla luciferase activity. Data are expressed as fold induction over the transcription obtained with CDH16-Luc, whose value was set at 1.0. Data are means from thee independent experiments, each performed in duplicate. Statistical analysis uses t test (P < 0.01). B, Chromatin extracted from cross-linked FRTL-5 cells was immunoprecipitated in parallel using an unrelated antibody (antitubulin) or an antibody against Pax8. The immunoprecipitates were analyzed by PCR with oligonucleotides corresponding to the rat Cadherin-16 minimal promoter. Parallel PCR were performed with total input DNA obtained from unprecipitated aliquot of similarly treated chromatin sample and from no template control (NTC).
Furthermore, to demonstrate the ability of Pax8 to physically interact with the Cadherin-16 minimal promoter in vivo, we performed chromatin immunoprecipitation (ChIP) assays on rat genomic DNA from FRTL-5 cells. The cross-linked chromatin was immunoprecipitated using an antibody against Pax8. As control, to rule out nonspecific background of the ChIP assay, we performed one reaction using an unrelated antibody. The enrichment of the endogenous Cadherin-16 region was monitored by PCR amplification using specific primers. Indeed, we demonstrate that, in agreement with the transactivation assays data, Pax8 antibody is able to immunoprecipitate the chromatin containing the Cadherin-16 promoter (Fig. 2B).
Identification of a Pax8 binding site on the Cadherin-16 promoter
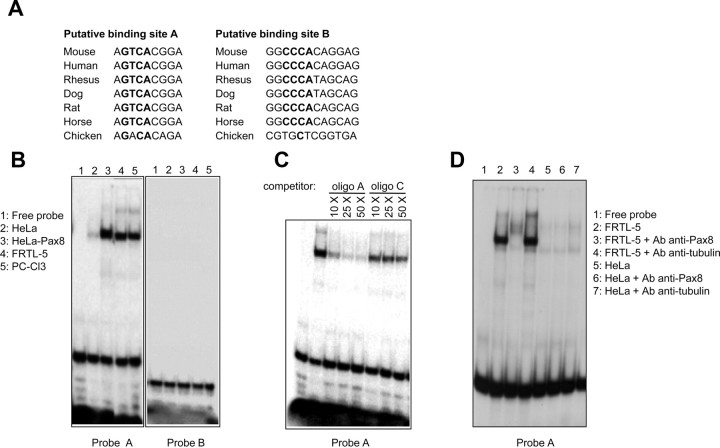
The results so far obtained prompted us to investigate more in details Pax8 binding to the Cadherin-16 promoter and specifically to localize Pax8 binding site(s) within this region. Therefore, we used the MatInspector analysis software (Genomatix, Munich, Germany) to search for transcription factors binding sites, and we found two predicted sites for Pax8 that we named A and B, respectively. Specifically, site A was identified on the positive strand, whereas site B was identified on the negative strand of the DNA sequence, respectively, positioned at −101 and −16 bp from the transcriptional start site (+1) mapped on the mouse promoter (16). The alignment of the core sequence of the two putative Pax8 binding sites shows a high grade of conservation among Cadherin-16 mammalian promoters, highlighting the potential relevance of these sites for the promoter activity (Fig. 3A).
Fig. 3.
Cadherin-16 minimal promoter contains a binding site for Pax8. A, Graphic output of the sequence analysis showing the conservation in different species of the core consensus sequences (in bold) of the Pax8 binding sites predicted by the MatInspector software, designed A (on the positive strand) and B (on the negative strand), respectively. The sequence alignment was obtained using whole genome comparative analysis from VISTA (reference genome, mouse July 2007). B, 32P-labeled oligo probes A and B were challenged in EMSA with total protein extracts prepared from FRTL-5 (lane 4) and PC Cl3 (lane 5) cells. We used total protein extracts of HeLa (lane 2) and Pax8-transfected HeLa cells (lane 3), as negative and positive control of the EMSA. C, The specificity of the complexes observed with the FRTL-5 protein extract and probe A was tested by competition analysis, using an increasing amount (from 10- to 50-fold molar excesses) of unlabeled oligonucleotide A or unlabeled oligonucleotide C, which contains the binding site for Pax8 on the Tg promoter. D, Protein extract of FRTL-5 and HeLa cells were incubated, alone or together with the antibody (Ab) against Pax8 or tubulin (as control), in a supershift EMSA with labeled probe A. A FRTL-5 specific supershift with the antibody against PAX8 is clearly visible.
To further characterize and verify the prediction obtained by the MatInspector analysis, we designed two oligo probes, containing the putative binding sites A and B, and we performed gel mobility shift assays. Initially, we incubated the A and B 32P-labeled oligo probes with total protein extracts prepared from PC Cl3 and FRTL-5 thyroid cells. We used total protein extracts of wild-type and Pax8-transfected HeLa cells, as negative and positive control, respectively. Interestingly, the retarded band observed when PC Cl3 and FRTL-5 protein extracts were incubated with oligo A (Fig. 3B, probe A, lanes 4 and 5) is identical to the retarded band of the positive control (Fig. 3B, probe A, lane 3). In contrast, the predicted binding site B could not be confirmed as a Pax8 site (Fig. 3B, probe B).
We further demonstrated by competition assays that the retarded band observed when the FRTL-5 extract is incubated with oligo probe A is the result of a specific interaction. We tested the binding specificity of the oligo probe A by competition with unlabeled oligo A and with a control oligo C, which contains a well-known Pax8 binding site (21). The Pax8 protein complex was specifically inhibited by competition with 25- and 50-fold molar excesses of unlabeled “self” and unlabeled C oligo (Fig. 3C). The same results were obtained in reciprocal EMSA in which the oligo C was radiolabeled and the retarded band was competed with an excess of unlabeled oligo A (data not shown).
Further consolidation of the data of Pax8 binding on the oligo probe A was obtained performing supershift assays with an anti-Pax8-specific antibody. In this case, as shown in Fig. 3D, we observed that a supershifted band is produced after the incubation of probe A with FRTL-5 protein extract and the anti-Pax8 antibody. The specificity of this supershifted band is confirmed by the reduction of the expected retarded band corresponding to the Pax8-oligo complex and by the absence of a supershift when the HeLa protein extract or the antitubulin antibody is used.
Mutation of Pax8 binding site strongly reduces Cadherin-16 promoter activity
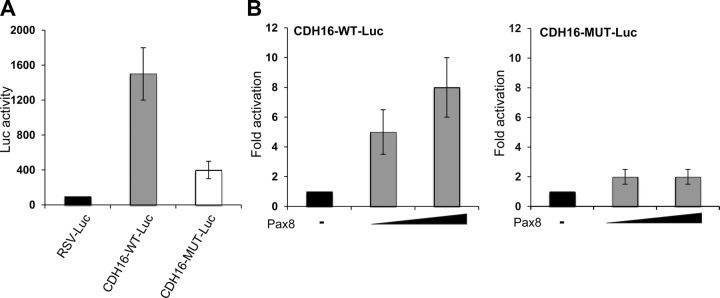
To determine whether mutations in the consensus core of Pax8 binding site could affect the activity of the Cadherin-16 minimal promoter, we generated a luciferase reporter plasmid containing a mutant promoter sequence (CDH16-MUT-Luc). First, we determined that such mutation was able to interfere with Pax8 binding to DNA in EMSA (data not shown). Afterwards, luciferase reporter plasmids containing the wild-type and mutant Cadherin-16 promoter were transiently transfected into FRTL-5 cells, and luciferase activity was measured 48 h later. As shown in Fig. 4A, transfections of the wild-type promoter produced high levels of luciferase activity (Fig. 4A, gray bar), whereas the mutations introduced in Pax8 binding site resulted in a substantial loss of activity (75–80% of wild type) (Fig. 4A, white bar), indicating that the binding site that we have identified plays an important functional role in Cadherin-16 promoter activity.
Fig. 4.
Pax8 binding site is necessary for the activity of the Cadherin-16 promoter. A, Site-directed mutagenesis was performed to generate the construct CDH16-MUT-Luc, containing the GTCA to TGAC mutation in the core of Pax8 binding site. FRTL-5 cells were transfected with RSV-Luc (black bar), CDH16-Luc (gray bars), and CDH16-MUT-Luc (white bars), and 48 h after transfection, the transcriptional activity was determined as firefly over renilla luciferase activity. Normalized firefly luciferase activity is shown relative to RSV-Luc activity, whose value was set at 100%. Data are means ± sd from three independent experiments. Statistical analysis uses t test (P < 0.01). B, HeLa cells were transiently transfected with CDH16-Luc or with CDH16-MUT-Luc and increasing concentration (100 and 500 ng) of the expression vector encoding Pax8 (CMV-Pax8). Forty-eight hours after transfection, the transcriptional activity was determined as the firefly over renilla luciferase activity. Data are expressed as fold induction over the transcription obtained with CDH16-Luc or CDH16-MUT-Luc, whose value was set at 1.0. Data are means from three independent experiments, each performed in duplicate. Statistical analysis uses t test (P < 0.01).
These data were confirmed by transactivation assays in HeLa cells, in which the expression vector encoding Pax8 and the luciferase reporter plasmids containing the wild-type Cadherin-16 or mutated Cadherin-16 promoter were cotransfected. Figure 4B shows that Pax8 transcriptional activation from the mutated promoter is strongly reduced with respect to that observed from the wild-type promoter. All together, these results demonstrate that the identified Pax8 binding site is required for Pax8-mediated transcriptional activation of the Cadherin-16 minimal promoter.
Lack of Pax8 in vivo significantly affects Cadherin-16 expression in thyroid cells
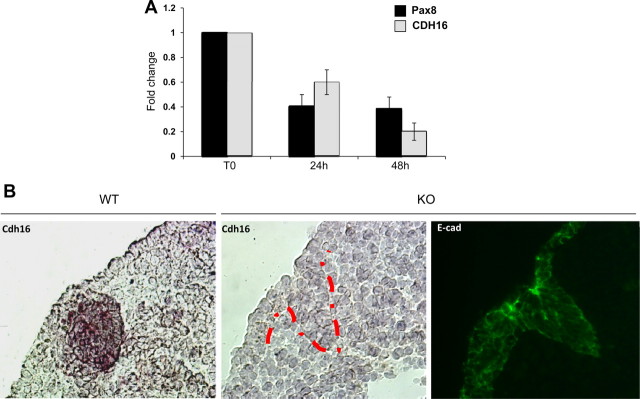
To demonstrate the endogenous relationship between Pax8 and Cadherin-16 expression, we knocked down Pax8 expression in thyroid cells and determined the intracellular mRNA levels of Cadherin-16. To this purpose, we transfected FRTL-5 cells with small interfering RNA (siRNA) that specifically targets the Pax8 coding region. As control, we used a transfection with a scrambled siRNA. Quantitative RT-PCR (qRT-PCR) was performed 24 and 48 h after the transfection to confirm the reduction of Pax8 expression and to analyze the expression of Cadherin-16. As shown in Fig. 5, we demonstrated that the reduction of Pax8 expression clearly corresponds a significant reduction of Cadherin-16 expression. No change in the expression of Pax8 and Cadherin-16 was observed with the scrambled siRNA transfection (data not shown).
Fig. 5.
Silencing of Pax8 affects Cadherin-16 expression both in vitro and in vivo. A, FRTL-5 cells were transfected with a PAX8 siRNA that specifically targets the rat Pax8 coding region. Pax8 and Cadherin-16 (CDH16) expression levels were measured on total RNA by qRT-PCR 24 and 48 h after siRNA transfection. The values are means ± sd of three independent experiments in duplicate, normalized by the expression of β-actin, and expressed as fold change with respect to the untransfected FRTL-5 cells, whose value was set at 1.0. Statistical analysis uses t test (P < 0.01). B, In situ hybridization using DIG-labeled Cadherin-16 riboprobe was performed on sagittal sections of E10.5 wild-type (WT) and Pax8−/− (KO) embryos. A strong Cadherin-16 expression is observed in the wild-type developing thyroid, whereas thyroid precursor cells lack the expression of Cadherin-16 in the Pax8−/− thyroid bud. In parallel, an immunofluorescence analysis was performed on sagittal sections of E10.5 Pax8−/− embryos, with the anti-E-cadherin antibody, to confirm the presence of epithelial cells in the forming thyroid primordium.
To confirm also in vivo the involvement of Pax8 in Cadherin-16 expression, an in situ hybridization analysis was carried out on thyroid sections prepared from wild-type and knockout Pax8 mice. As demonstrated in previous studies, in the thyroid gland of Pax8−/− mouse embryos, the follicular cell population is seriously affected. In particular, soon after their evagination [embryonic day (E)11.5–E12.0], the follicular precursor cells disappear and are not detected in E12.0. Consequently, the thyroid is smaller compared with wild-type embryos, and no follicles can be detected (14). Taking into account these data, our in situ analysis was performed at E10.5. As shown in Fig. 5B, at E10.5, the Cadherin-16 probe stains the cells in the developing thyroid bud in the wild-type tissue. In contrast, in Pax8 null embryos, Cadherin-16 transcript cannot be detected. To confirm the presence of epithelial cells forming the thyroid primordium, an immunofluorescence analysis was performed on the Pax8−/− thyroid sections using an anti-E-cadherin antibody. As shown in Fig. 5B, the expression of E-cadherin is maintained in the epithelial cells of the thyroid bud.
These data clearly indicate that the absence of Pax8 affects Cadherin-16 expression in thyroid cells and in the developing thyroid.
TSH regulates Cadherin-16 expression in thyroid cells
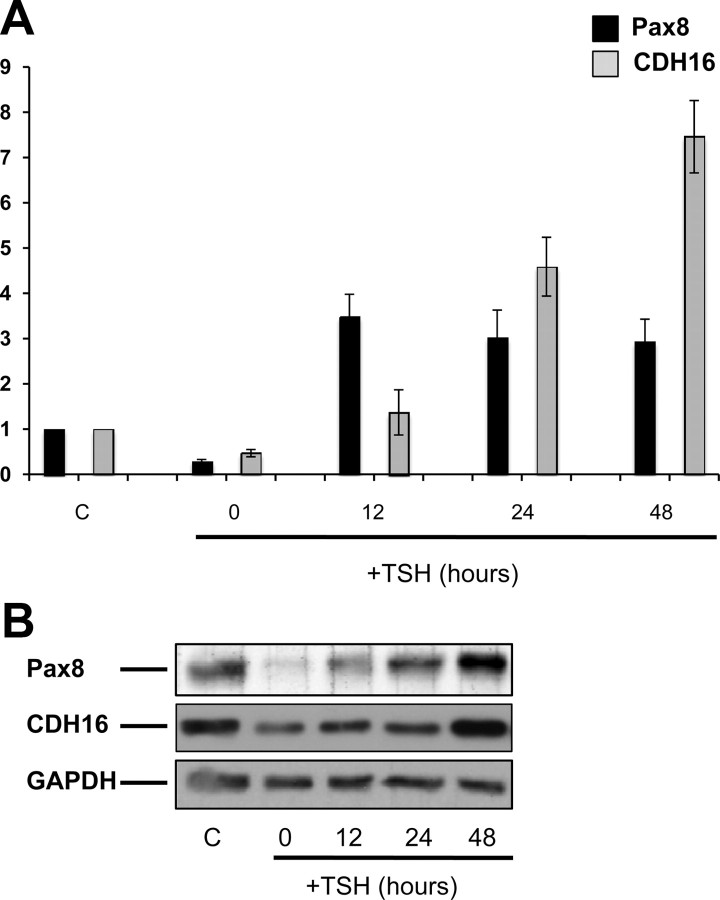
In the past, we observed that in rat thyroid cells cultured in the absence of TSH, Pax8 mRNA and protein rapidly disappear, whereas upon TSH stimulation, both mRNA and protein are newly synthesized (22). To investigate the role of TSH in the expression of Cadherin-16, differentiated rat thyroid FRTL-5 cells were cultured for 7 d in serum-free medium containing 0.2% BSA and then treated with TSH for different times (12, 24, and 48 h). The expression of both Pax8 and Cadherin-16 was analyzed at mRNA and protein level by qRT-PCR and Western blotting, respectively. As expected, Pax8 expression was strongly reduced upon starvation of the cells and reinduced upon TSH stimulation (Fig. 6, A and B). Similarly, also the expression of Cadherin-16 turned out to be modulated by TSH, and the kinetic of induction upon TSH stimulation well correlates with that of Pax8, suggesting that TSH regulation of Cadherin-16 expression could be mediated by Pax8. Indeed, Pax8 mRNA is highly expressed already 12 h after TSH stimulation, whereas Cadherin-16 expression starts to be detected at the same time point but peaks after 48 h of treatment, when Pax8 protein levels are back to wild-type conditions (Fig. 6, A and B).
Fig. 6.
Cadherin-16 expression is modulated by TSH in FRTL-5 thyroid cells. A, FRTL-5 cells were cultured in regular medium (C) or maintained in starvation medium for 7 d (T0) and then treated with 1 mU/ml of TSH for different times (12, 24, and 48 h) and analyzed by qRT-PCR analysis to measure the expression of Pax8 (black bars) and Cadherin-16 (CDH16) (gray bars) mRNA. The values are means ± sd of three independent experiments in duplicate, normalized by the expression of β-actin, and expressed as fold change with respect to the control, whose value was set at 1.0. Statistical analysis uses t test (P < 0.01). B, FRTL-5 cells were cultured in regular medium (lane C) or maintained in starvation medium for 7 d (lane 0) and then treated with 1 mU/ml TSH for different times (12, 24, and 48 h) and analyzed by Western blotting to detect Pax8 and Cadherin-16 proteins. The hybridization with α-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) assessed the protein uniform loading and integrity.
Cadherin-16 expression is down-regulated in dedifferentiated thyroid cells and in mouse thyroid cancer
To investigate whether the expression of Cadherin-16 is modified when the thyroid cell is dedifferentiated, we used an inducible system consisting of FRTL-5 cells expressing a conditional RAS oncoprotein, obtained by fusing H-RASV12 downstream of a 4-hydroxytamoxifen (4OHT)-sensitive mutant of the estrogen receptor ligand binding domain (ERTM-RAS). Previously, it has been reported that in this RAS-transformed cell line, there is a clear loss of thyroid differentiation (23).
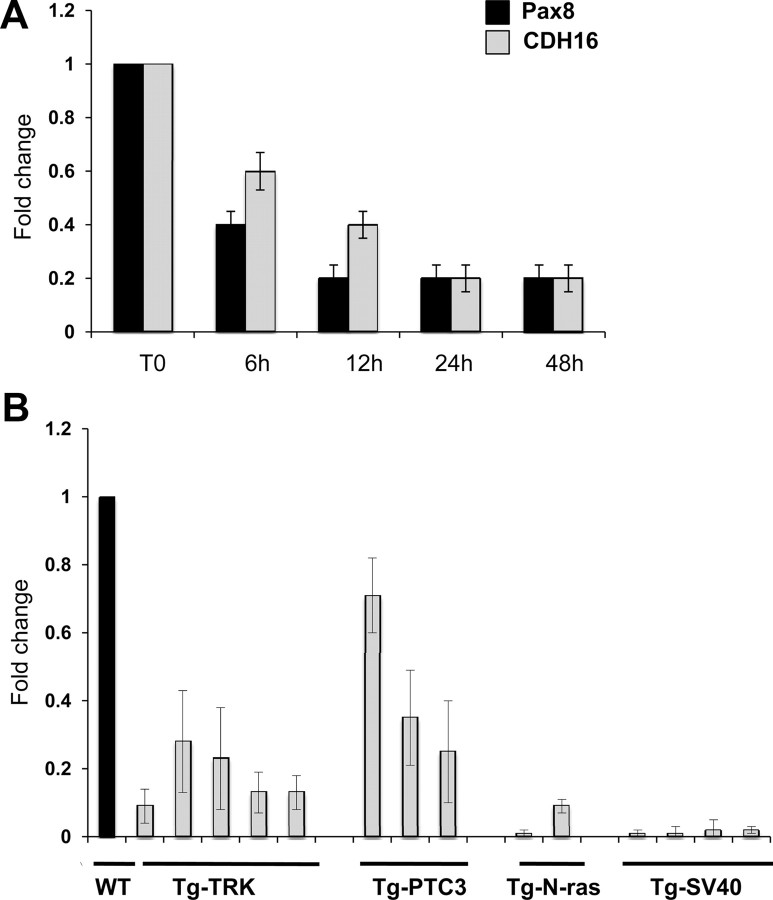
For our experiments, we used one representative high-expressing RAS clone and measured the expression of Pax8 and Cadherin-16 by qRT-PCR 6, 12, 24, and 48 h after RAS induction by tamoxifen. As control, we used FRTL-5 wild-type cells treated at same extent. Figure 7A shows the results of the qRT-PCR analysis with expression values normalized for β-actin expression and reported as fold change with respect to the value in the absence of 4OHT (T0, time 0). Upon a 6-h tamoxifen treatment, the expression of Pax8 is reduced and well correlates with the down-regulation of Cadherin-16 expression. At the same time, wild-type FRTL-5 cells do not show any difference after tamoxifen treatment.
Fig. 7.
Cadherin-16 expression is down-regulated in dedifferentiated thyroid cells. A, qRT-PCR analysis was performed on total RNA prepared from FRTL-5 and FRTL-5/ERTM-RAS cells. The expression of Pax8 (black bars) and Cadherin-16 (CDH16) (gray bars) mRNA was measured after a time course of 4OHT treatment (6, 12, 24, and 48 h). The values are means ± sd of three independent experiments in duplicate, normalized by the expression of β-actin, and expressed as fold change with respect to the T0, whose value was set at 1.0. Statistical analysis uses t test (P < 0.01). B, qRT-PCR analysis was performed on total RNA prepared from thyroid carcinomas developed in Tg-TRK, Tg-PTC3, and Tg-N-ras transgenic mice expressing the TRK, RET/PTC3, and N-ras oncogenes under the transcriptional control of the Tg promoter. Relative quantities of Cadherin-16 mRNA were normalized to the cyclophilin-A as reference gene. Each amplification was performed in duplicate, and the data are expressed as fold change with respect to the normal mouse thyroid tissues used as a control (WT), whose value was set at 1.0. Statistical analysis uses t test (P < 0.01).
To further assess whether the expression of Cadherin-16 is altered in thyroid cancer, we evaluated its expression in thyroid neoplasia developed in transgenic mouse lines expressing different oncogenes under the transcriptional control of the Tg promoter. Specifically, we analyzed transgenic mice carrying TRK and RET/PTC3 oncogenes, which develop papillary thyroid carcinomas (PTC) (24, 25) and N-ras mice that develop thyroid follicular tumors that undergo dedifferentiation, predominantly follicular thyroid carcinomas (26). Anaplastic thyroid carcinomas were obtained from mice carrying the simian virus 40 large T antigen (27). Figure 7B shows the results of the qRT-PCR analysis with expression values normalized for cyclophilin-A expression and reported as fold change with respect to the normal mouse thyroid used as a control. Cadherin-16 expression was significantly reduced in all the PTC and follicular thyroid carcinoma samples derived from TRK, RET/PTC3, and N-ras mice, and it was almost undetectable in the anaplastic thyroid carcinoma samples derived from the simian virus 40 large T mice.
Discussion
A correct process of thyroid organogenesis, morphogenesis, and follicular cell differentiation is required for the maintenance of ordered architecture and function of the differentiated thyroid gland. Many data suggest that the development of the embryonic thyroid gland and its migration require an interplay between four transcription factors, Pax8 (TTF-1/Nkx2.1), Foxe1, Hhex, and Nkx2.5 (28, 29). In particular, the transcription factor Pax8 is a master gene for thyroid differentiation, being necessary for the transcriptional activation of all the known differentiation markers (30). Moreover, Pax8 knockout mice show a severe thyroid phenotype (14), and patients carrying mutations in the Pax8 gene suffer from congenital hypothyroidism (31).
Together with the transcription factor Pax2, Pax8 is also a central regulator of both nephron differentiation and branching morphogenesis in the developing kidney. Both transcription factors are necessary and sufficient for the formation of the pronephros and all subsequent kidney structures, regulating key target genes involved in pro/mesonephros formation (32, 33).
Intriguingly, Cadherin-16 was initially described as a kidney-specific cadherin, but recently, it has been detected also in the thyroid tissue (7), although its physiological role in both tissues has not been thoroughly studied. In this paper, we show that Pax8 regulates the expression of Cadherin-16 at the transcriptional level. By EMSA and transfection assays, we show that Pax8 binds to the Cadherin-16 promoter and activates transcription from it. The Pax8 DNA binding site identified in this study is functionally relevant as demonstrated by the reduced activity of a mutated Cadherin-16 promoter unable to bind Pax8. In addition, Cadherin-16 mRNA is not expressed in the developing thyroid bud of Pax8−/− mice, indicating that Pax8 is required for Cadherin-16 expression also in vivo during thyroid development.
TSH is the main regulator of thyroid differentiation. Its role in this process has been studied in different model systems (34), and the expression of several thyroid-specific genes, such as Tg, TPO, TSH receptor (TSHR), and sodium-iodide symporter (NIS) has been shown to be modulated by TSH/cAMP. In the past, we have demonstrated that in PC Cl3 cells, TSH regulates the expression of Tg and Pax8 at the transcriptional level by cAMP-mediated mechanism (22). Here, we show that in FRTL-5 rat thyroid cells also the expression of Cadherin-16 is under the control of TSH and that this control is likely mediated by Pax8. In fact, TSH deprivation causes a dramatic reduction of Pax8 and Cadherin-16 expression, whereas TSH stimulation efficiently restores it with Pax8 reexpression preceding that of Cadherin-16 as expected for a target gene. Interestingly, in the past, it has been demonstrated that in primary cultured pig thyrocytes, TSH stabilizes E-cadherin at the cell surface and prevents its accelerated turnover to support the maintenance of thyroid follicular integrity (35). Therefore, we would like to propose that TSH promotes and ensures the maintenance of the architecture and integrity of the follicles also regulating the expression of Cadherin-16. Obviously, at this stage, this is only a hypothesis that needs to be further investigated and experimentally verified.
Cell-cell adhesion determines cell polarity and participates to cell differentiation and in the establishment and maintenance of tissue homeostasis. During oncogenesis, this organized adhesion is disturbed, resulting in changes in signaling, loss of contact inhibition and altered cell migration and stromal interactions. Recently, it has been demonstrated that the cytoplasmic tail of Cadherin-16 binds the N-terminal domain of αB-crystallin. It is well known to interact with such cytoskeletal proteins as actin, vimentin, and desmin in lens epithelium and was found to play a role in the regulation of actin dynamics during cell migration and adhesion processes. In addition, Cadherin-16 has been proposed as a potential new marker for discriminating PTC from benign and normal thyroid samples (36). In agreement with these data, we show that in mouse thyroid carcinomas resembling papillary, follicular, and anaplastic carcinomas, the expression of Cadherin-16 is severely reduced. Therefore, the down-regulation of Cadherin-16 expression that we observe upon Ras activation in FRTL-5 cells treated is consistent with the hypothesis that the presence of Cadherin-16 is associated with the fully differentiated state of the thyroid cell and that its expression is reduced or lost when the cell becomes dedifferentiated. Moreover, it has been demonstrated that the expression of Snail and Cadherin-16 is complementary during kidney ontogenesis, because Snail genes act as repressors of Cadherin-16 expression (6). It is well known that the Snail gene family of transcription factors triggers epithelium to mesenchymal transition, converting epithelial cells into mesenchymal cells with migratory properties, influencing the acquisition of invasive properties in epithelial tumors. Intriguingly, SNAI1 and SNAI2 are highly expressed in human thyroid carcinoma samples and in their metastases and cause the reduction in the CDH1 expression (37). Therefore, we propose that in thyroid cells, as in kidney cells, the expression of Cadherin-16 correlates with the integrity of the epithelial phenotype and is reduced when this integrity is perturbed. Further investigation is now required to define the function of Cadherin-16 in the maintenance of the thyroid structure during development and in the adult gland as well as its role during thyroid carcinogenesis.
Materials and Methods
Constructs generation
The fragment containing the minimal promoter of the mouse Cadherin-16 gene was amplified by PCR using the following primers: forward, 5′-CGGGGTACCCCGAGTGGGAGCCAAGTCTGAACACAC-3′ and reverse, 5′-CCGCTCGAGCGGAGGTAGGCGGTCTGGCCGTGC-3′. The fragment was cloned into KpnI/XhoI sites of the pGL3basic vector (Promega, Madison, WI) upstream the Luciferase reporter gene (pGL3-LUC) to generate the Cadherin-16-Luc construct (CDH16-Luc).
The Tg-Luc construct contains the minimal Tg promoter (38) subcloned into XhoI/HindIII sites of the pGL3basic vector (Promega) upstream the Luciferase reporter gene. The TPO-Luc, NIS-Luc, and CMV-Pax8 constructs were previously described (21, 39, 40).
The mutant Cadherin-16-MUT-Luc was generated using the QuikChange XL Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) using as template the Cadherin-16-Luc to introduce the GTCA to TGAC mutation in the core of Pax8 binding site. The presence of the mutation was confirmed by sequence analysis.
Protein extracts and immunoblotting
Cells were washed twice with ice-cold PBS and lysed in a buffer containing 50 mm HEPES (pH 7.5), 150 mm NaCl, 5 mm EGTA (pH 7.8), 10% glycerol, 1% triton, 1 mm dithiothreitol, and 1 mm phenylmetilsulfonil fluoride. The protein concentration was determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA).
For Western blot analysis, proteins were separated on SDS-PAGE, and gels were blotted onto Immobilon P (Millipore, Bedford, MA). The primary antibodies used were: mouse monoclonal anti-Cadherin-16 (USB Biological, Massachusetts, MA), rabbit policlonal anti-Pax8 (kindly provided by R. Di Lauro), mouse monoclonal antitubulin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and antimouse glyceraldehyde-3-phosphate dehydrogenase (Santa Cruz Biotechnology, Inc.). The filters were developed using an enhanced chemiluminescence detection method (Pierce, Rockford, IL) according to the manufacturer's directions.
Cell culture and transfection assay
Rat thyroid follicular FRTL-5 and PC Cl3 cells were maintained in Coon's modified F-12 medium (EuroClone, Milan, Italy) supplemented with 5% newborn bovine serum (HyClone, Logan, UT) and a six-hormone mixture as previously described (41). The starvation medium consisted of Coon's modified Ham's F12 medium supplemented only with 0·2% BSA. TSH treatment was performed by addition of 1 mU/ml TSH (Sigma-Aldrich, St. Louis, MO) to the culture medium at 12, 24, and 48 h after starvation. 4OHT treatment was performed by addition of 100 nm 4OHT (Sigma-Aldrich) to the culture medium. The FRTL-5-ERTM-RAS cells were kindly provided by R. Di Lauro.
HeLa cells and MDCK were grown in DMEM (EuroClone) supplemented with 10% vol/vol fetal calf serum (HyClone). Transfections were carried out with the FuGENE 6 reagent (Roche Diagnostics, Munich, Germany) according to the manufacturer's directions. The DNA/FuGENE ratio was 1:3 in all the experiments. The plasmid pRL-TK (Promega) was used as internal control in the transfection assays. Cells extracts were prepared 48 h after transfection to determine the levels of the firefly and renilla luciferase with the Luciferase and Renilla Reporter Assay System (Promega). Transfection experiments were done in duplicate and repeated at least three times. Statistical analysis has been performed by means of an unpaired two-tailed Student's t test to obtain the P value associated with the observed fold of activation differences.
ChIP assays
The procedure was previously described (42). The primary antibodies used were: rabbit policlonal anti-Pax8 (kindly provided by R. Di Lauro) and mouse monoclonal antitubulin (Santa Cruz Biotechnology, Inc.). PCR was performed using the following specific primers: forward, 5′-AGAAGTGGGGCCAAGTCTGAAGCC-3′ and reverse, 5′-GGGGCGAGGCAAGGTGGACACTT-3′.
Electrophoretic mobility shift assay
Double-strand oligonucleotides were labeled with γ-32P ATP and T4 polynucleotide kinase and used as probes. The binding reactions were carried out in a buffer containing 20 mm Tris-HCl (pH 7.6),75 mm KCl, 1 mm dithiothreitol, 10% glycerol, 1 mg/ml BSA, and 3 mg/ml polydeoxyinosinic deoxycytidylic acid. After 30 min of incubation at room temperature, free DNA and DNA-protein complexes were resolved on a 6% nondenaturating polyacrylamide gel and visualized with a PhosphorImager (Molecular Dynamics, Piscataway, NJ) or by autoradiography.
The oligonucleotide used in the competition assay and the antibody used in the supershift experiments were incubated with the protein extract for 20 min before adding the probe.
Oligonucleotides derived from the MatInspector analysis were: probe A, 5′-GCTGGCAGTCACGGATGCTGAGCAGATCTG-3′ and probe B, 5′-ACCGGGGCCCACAGGAGCAGGCCTGGCCCC-3′.
RNA interference
FRTL-5 cells were plated (8 × 104 well) in a 24-well plate and were transfected in triplicate with 100 nm Pax8 siGENOME siRNA or siGENOME Non-Targeting #3 (Dharmacon, Lafayette, CO) as scramble using DharmaFECT 1 transfection reagent, following the manufacturer's protocol. Sequences corresponding to the siRNA of Pax8 were: sense, 5′-CCAUAUUAUUACAGCUCUA-3′ and antisense, 5′-UAGAGCUGUAAUAAUAUGG-3′. Twenty-four and 48 h after transfection, the cells were harvested and the total RNA was extracted.
RNA extraction, cDNA synthesis, real-time PCR, and RNA probe synthesis
Total RNA was prepared using TRIzol Reagent (Invitrogen, San Diego, CA) according to the manufacturer's directions. Total RNA (1 μg) was retrotranscribed using the iScript cDNA Synthesis kit (Bio-Rad Laboratories). Real-time PCR analysis was performed using an iCycler-iQ real-time detection system and SYBR green chemistry (Bio-Rad Laboratories). To design the qRT-PCR assays, we used the Primer Express software, and the primers sequences are reported in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org. Reactions were carried out in duplicate in three independent experiments. For each gene, values are means ± sd of three independent experiments, normalized by the expression of housekeeping gene, and expressed as a percentage of the value measured in parental FRTL-5 cells. To calculate the relative expression levels, we used the 2−DDCT method (43).
For RNA probe synthesis, PCR was performed using the following primers: forward, 5′-CGATTTAGGTGACACTATAGAAGCCGACACCTCTGTACACC-3′ and reverse, 5′-GAATTTAATACGACTCACTATAGGGAGAGTGCATCTTGGGTACTGGGTC-3′. In vitro transcription reaction for the synthesis of antisense RNA labeled probes was carried out according to the manufacturer's specifications (Roche Diagnostics).
Mice and breeding
Pax8 knockout mice have been previously described (14). Heterozygous male and female mice in C57BL/6 background were mated overnight, and the appearance of the vaginal plug was taken as d 0.5 of embryogenesis (E0.5). Pregnant mice were killed by cervical disclocation on E10.5.
Genotypes of Pax8 mutant embryos were determined by PCR using genomic DNA isolated from yolk sacs as described (14).
RNA samples of mouse thyroid carcinomas were kindly provided by Alfredo Fusco.
Immunofluorescence and in situ hybridization analysis
FRTL-5 cells cultured on glass coverslips for 48–72 h were fixed by 4% paraformaldehyde (PFA) in PBS for 20 min at room temperature and then permeabilized with 0.2% Triton X-100 solution for 10 min. After blocking with 0.5% BSA in PBS for 1 h, cells were incubated with mouse monoclonal anti-Cadherin-16 (USB Biological) for 1 h at room temperature. After that, cells were washed and incubated with Alexa Fluor 594-conjugated secondary antibody (Invitrogen) for 30 min. Cell nuclei were identified by Hoechst staining. Images were collected with a Zeiss LSM 510 confocal laser scanning microscope (Zeiss, Oberkochen, Germany), equipped with a 543-nm HeNe laser, and a Plan-Apochromat 63/1.4 oil-immersion objective.
For mouse handling, immediately after dissection in ice-cool PBS, embryos were immersion fixed in 4% PFA in PBS. Then, embryos were immersed (after washing 2 × 15 min in PBS) in 30% sucrose/PBS at 4 C with gentle rocking until they sink. Embedding was done in optimum cutting temperature compound (Sakura, Torrance, CA), and care was taken to obtain proper orientation of the embryos in the molds. Freezing of embryos was done over an ethyl alcohol/dry ice slurry; 10-μm-thick sections were cut on a cryostat microtome (Microm HM 500M) and collected on polylysine glass slides (Menzel-Gläser, Braunschweig, Germany).
For immunofluorescence analysis, air-dried sections were permeabilized by incubation with 0.1% Triton X-100 for 20 min, incubated in blocking buffer (PBS with 2% normal donkey serum; Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature, and then incubated overnight at 4 C with primary antibody diluted in blocking buffer (anti-CDH1, 205604; Calbiochem, San Diego, CA). Immunolabeled sections were incubated with secondary antibodies diluted in blocking buffer for 1 h and thereafter with Streptavidin-fluorescein isothiocyanate for 30 min at room temperature. All incubation steps were followed by washing in 0.1% Triton X-100 for 3× 5 min. Microscopy and imaging were performed in a Zeiss AttoArc II epifluorescence microscope or a Bio Radiance 2000 Laser Scanning Microscope.
For in situ hybridization analysis, the sections were fixed in 4% PFA, washed in PBS-DEPC (diethyl-pyrocarbonate) and treated with 1 mg/ml of Proteinase K (Roche Diagnostics) in PBS-DEPC for 10 min at room temperature. The sections were then subjected to acetylation step using 0.25% acetic anhydride (Sigma-Aldrich) in 1 m triethanolamine (Sigma-Aldrich)-hydrochloride (J.T. Baker, Center Valley, PA) for 10 min. Thereafter, slides were hybridized overnight at 68 C in the hybridization mix [50% formamide (EuroClone), 5× SSC (pH 4.5) (AppliChem, Darmstadt, Germany), 50 mg/ml yeast tRNA (Roche Diagnostics), 1% sodium dodecyl sulfate, and 50 mg/ml heparin (Sigma-Aldrich)], using a probe concentration of 0.5–1 mg/ml. Sections were incubated with antidigoxigenin alkaline phosphatase-conjugated Fab fragments (Roche Diagnostics) at 1:4000 dilution. Staining was developed for 24/48 h, according to probe signal, with BM Purple AP Substrate (Roche Diagnostics). Finally, slides were fixed in 4% PFA and 0.2% gluteraldehyde (Sigma-Aldrich) and mounted in glycerol (Dako, Glostrup, Denmark). The in situ reaction was controlled under an AXIOPLAN 2 microscope equipped with Axiocam digital camera (Zeiss), and the images were processed using Axion Vision software and edited using ImageJ software.
Acknowledgments
We deeply thank L. Nitsch for sharing with us unpublished results and for helpful discussion.
This work was supported by a grant from the Associazione Italiana per la Ricerca sul Cancro (M.Z.) and by a grant from the Italian Ministry of Economy and Finance to the National Research Council for the Project FaReBio di Qualità.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ChIP
- Chromatin immunoprecipitation
- E
- embryonic day
- NIS
- sodium-iodide symporter
- Pax
- paired box
- PFA
- paraformaldehyde
- PTC
- papillary thyroid carcinoma
- qRT-PCR
- quantitative RT-PCR
- siRNA
- small interfering RNA
- Tg
- thyroglobulin
- TPO
- thyroperoxidase
- TSHR
- TSH receptor.
References
- 1. Hulpiau P , van Roy F. 2009. Molecular evolution of the cadherin superfamily. Int J Biochem Cell Biol 41:349–369 [DOI] [PubMed] [Google Scholar]
- 2. Pokutta S , Weis WI. 2007. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol 23:237–261 [DOI] [PubMed] [Google Scholar]
- 3. Harris TJ , Tepass U. 2010. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol 11:502–514 [DOI] [PubMed] [Google Scholar]
- 4. Wendeler MW , Praus M , Jung R , Hecking M , Metzig C , Gessner R. 2004. Ksp-cadherin is a functional cell-cell adhesion molecule related to LI-cadherin. Exp Cell Res 294:345–355 [DOI] [PubMed] [Google Scholar]
- 5. Thedieck C , Kalbacher H , Kratzer U , Lammers R , Stevanovic S , Klein G. 2008. αB-crystallin is a cytoplasmic interaction partner of the kidney-specific cadherin-16. J Mol Biol 378:145–153 [DOI] [PubMed] [Google Scholar]
- 6. Boutet A , De Frutos CA , Maxwell PH , Mayol MJ , Romero J , Nieto MA. 2006. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J 25:5603–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calì G , Zannini M , Rubini P , Tacchetti C , D'Andrea B , Affuso A , Wintermantel T , Boussadia O , Terracciano D , Silberschmidt D , Amendola E , De Felice M , Schütz G , Kemler R , Di Lauro R , Nitsch L. 2007. Conditional inactivation of the E-cadherin gene in thyroid follicular cells affects gland development but does not impair junction formation. Endocrinology 148:2737–2746 [DOI] [PubMed] [Google Scholar]
- 8. Missero C , Cobellis G , De Felice M , Di Lauro R. 1998. Molecular events involved in differentiation of thyroid follicular cells. Mol Cell Endocrinol 140:37–43 [DOI] [PubMed] [Google Scholar]
- 9. Brabant G , Hoang-Vu C , Behrends J , Cetin Y , Pötter E , Dumont JE , Maenhaut C. 1995. Regulation of the cell-cell adhesion protein, E-cadherin, in dog and human thyrocytes in vitro. Endocrinology 136:3113–3119 [DOI] [PubMed] [Google Scholar]
- 10. Nilsson M , Fagman H , Ericson LE. 1996. Ca2+-dependent and Ca2+-independent regulation of the thyroid epithelial junction complex by protein kinases. Exp Cell Res 225:1–11 [DOI] [PubMed] [Google Scholar]
- 11. Plachov D , Chowdhury K , Walther C , Simon D , Guenet JL , Gruss P. 1990. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development 110:643–651 [DOI] [PubMed] [Google Scholar]
- 12. Poleev A , Fickenscher H , Mundlos S , Winterpacht A , Zabel B , Fidler A , Gruss P , Plachov D. 1992. PAX8, a human paired box gene: isolation and expression in developing thyroid, kidney and Wilms' tumors. Development 116:611–623 [DOI] [PubMed] [Google Scholar]
- 13. Pasca di Magliano M , Di Lauro R , Zannini M. 2000. Pax8 has a key role in thyroid cell differentiation. Proc Natl Acad Sci USA 97:13144–13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mansouri A , Chowdhury K , Gruss P. 1998. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet 19:87–90 [DOI] [PubMed] [Google Scholar]
- 15. Macchia PE , Lapi P , Krude H , Pirro MT , Missero C , Chiovato L , Souabni A , Baserga M , Tassi V , Pinchera A , Fenzi G , Grüters A , Busslinger M , Di Lauro R. 1998. PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet 19:83–86 [DOI] [PubMed] [Google Scholar]
- 16. Whyte DA , Li C , Thomson RB , Nix SL , Zanjani R , Karp SL , Aronson PS , Igarashi P. 1999. Ksp-cadherin gene promoter. I. Characterization and renal epithelial cell-specific activity. Am J Physiol 277:F587–F598 [DOI] [PubMed] [Google Scholar]
- 17. Thedieck C , Kuczyk M , Klingel K , Steiert I , Müller CA , Klein G. 2005. Expression of Ksp-cadherin during kidney development and in renal cell carcinoma. Br J Cancer 92:2010–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bai Y , Pontoglio M , Hiesberger T , Sinclair AM , Igarashi P. 2002. Regulation of kidney-specific Ksp-cadherin gene promoter by hepatocyte nuclear factor-1β. Am J Physiol Renal Physiol 283:F839–F851 [DOI] [PubMed] [Google Scholar]
- 19. Igarashi P , Shashikant CS , Thomson RB , Whyte DA , Liu-Chen S , Ruddle FH , Aronson PS. 1999. Ksp-cadherin gene promoter. II. Kidney-specific activity in transgenic mice. Am J Physiol 277:F599–F610 [DOI] [PubMed] [Google Scholar]
- 20. Shao X , Johnson JE , Richardson JA , Hiesberger T , Igarashi P. 2002. A minimal Ksp-cadherin promoter linked to a green fluorescent protein reporter gene exhibits tissue-specific expression in the developing kidney and genitourinary tract. J Am Soc Nephrol 13:1824–1836 [DOI] [PubMed] [Google Scholar]
- 21. Zannini M , Francis-Lang H , Plachov D , Di Lauro R. 1992. Pax-8, a paired domain-containing protein, binds to a sequence overlapping the recognition site of a homeodomain and activates transcription from two thyroid-specific promoters. Mol Cell Biol 12:4230–4241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mascia A , Nitsch L , Di Lauro R , Zannini M. 2002. Hormonal control of the transcription factor Pax8 and its role in the regulation of thyroglobulin gene expression in thyroid cells. J Endocrinol 172:163–176 [DOI] [PubMed] [Google Scholar]
- 23. De Vita G , Bauer L , da Costa VM , De Felice M , Baratta MG , De Menna M , Di Lauro R. 2005. Dose-dependent inhibition of thyroid differentiation by RAS oncogenes. Mol Endocrinol 19:76–89 [DOI] [PubMed] [Google Scholar]
- 24. Powell DJ , Russell J , Nibu K , Li G , Rhee E , Liao M , Goldstein M , Keane WM , Santoro M , Fusco A , Rothstein JL. 1998. The RET/PTC3 oncogene: metastatic solid-type papillary carcinomas in murine thyroids. Cancer Res 58:5523–5528 [PubMed] [Google Scholar]
- 25. Russell JP , Powell DJ , Cunnane M , Greco A , Portella G , Santoro M , Fusco A , Rothstein JL. 2000. The TRK-T1 fusion protein induces neoplastic transformation of thyroid epithelium. Oncogene 19:5729–5735 [DOI] [PubMed] [Google Scholar]
- 26. Vitagliano D , Portella G , Troncone G , Francione A , Rossi C , Bruno A , Giorgini A , Coluzzi S , Nappi TC , Rothstein JL , Pasquinelli R , Chiappetta G , Terracciano D , Macchia V , Melillo RM , Fusco A , Santoro M. 2006. Thyroid targeting of the N-ras(Gln61Lys) oncogene in transgenic mice results in follicular tumors that progress to poorly differentiated carcinomas. Oncogene 25:5467–5474 [DOI] [PubMed] [Google Scholar]
- 27. Ledent C , Dumont J , Vassart G , Parmentier M. 1991. Thyroid adenocarcinomas secondary to tissue-specific expression of simian virus-40 large T-antigen in transgenic mice. Endocrinology 129:1391–1401 [DOI] [PubMed] [Google Scholar]
- 28. Damante G , Tell G , Di Lauro R. 2001. A unique combination of transcription factors controls differentiation of thyroid cells. Prog Nucleic Acid Res Mol Biol 66:307–356 [DOI] [PubMed] [Google Scholar]
- 29. Parlato R , Rosica A , Rodriguez-Mallon A , Affuso A , Postiglione MP , Arra C , Mansouri A , Kimura S , Di Lauro R , De Felice M. 2004. An integrated regulatory network controlling survival and migration in thyroid organogenesis. Dev Biol 276:464–475 [DOI] [PubMed] [Google Scholar]
- 30. Di Lauro R , Damante G , De Felice M , Arnone MI , Sato K , Lonigro R , Zannini M. 1995. Molecular events in the differentiation of the thyroid gland. J Endocrinol Invest 18:117–119 [DOI] [PubMed] [Google Scholar]
- 31. Montanelli L , Tonacchera M. 2010. Genetics and phenomics of hypothyroidism and thyroid dys- and agenesis due to PAX8 and TTF1 mutations. Mol Cell Endocrinol 322:64–71 [DOI] [PubMed] [Google Scholar]
- 32. Bouchard M , Souabni A , Mandler M , Neubüser A , Busslinger M. 2002. Nephric lineage specification by Pax2 and Pax8. Genes Dev 16:2958–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Narlis M , Grote D , Gaitan Y , Boualia SK , Bouchard M. 2007. Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol 18:1121–1129 [DOI] [PubMed] [Google Scholar]
- 34. Dumont JE , Lamy F , Roger P , Maenhaut C. 1992. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev 72:667–697 [DOI] [PubMed] [Google Scholar]
- 35. Larsson F , Fagman H , Nilsson M. 2004. TSH receptor signaling via cyclic AMP inhibits cell surface degradation and internalization of E-cadherin in pig thyroid epithelium. Cell Mol Life Sci 61:1834–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fontaine JF , Mirebeau-Prunier D , Raharijaona M , Franc B , Triau S , Rodien P , Goëau-Brissonniére O , Karayan-Tapon L , Mello M , Houlgatte R , Malthiery Y , Savagner F. 2009. Increasing the number of thyroid lesions classes in microarray analysis improves the relevance of diagnostic markers. PLoS One 4:e7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hardy RG , Vicente-Dueñas C , González-Herrero I , Anderson C , Flores T , Hughes S , Tselepis C , Ross JA , Sánchez-García I. 2007. Snail family transcription factors are implicated in thyroid carcinogenesis. Am J Pathol 171:1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sinclair AJ , Lonigro R , Civitareale D , Ghibelli L , Di Lauro R. 1990. The tissue-specific expression of the thyroglobulin gene requires interaction between thyroid-specific and ubiquitous factors. Eur J Biochem 193:311–318 [DOI] [PubMed] [Google Scholar]
- 39. Ohno M , Zannini M , Levy O , Carrasco N , di Lauro R. 1999. The paired-domain transcription factor Pax8 binds to the upstream enhancer of the rat sodium/iodide symporter gene and participates in both thyroid-specific and cyclic-AMP-dependent transcription. Mol Cell Biol 19:2051–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Francis-Lang H , Price M , Polycarpou-Schwarz M , Di Lauro R. 1992. Cell-type-specific expression of the rat thyroperoxidase promoter indicates common mechanisms for thyroid-specific gene expression. Mol Cell Biol 12:576–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ambesi-Impiombato FS , Coon HG. 1979. Thyroid cells in culture. Int Rev Cytol 10(Suppl):163–172 [DOI] [PubMed] [Google Scholar]
- 42. Nitsch R , Di Dato V , di Gennaro A , de Cristofaro T , Abbondante S , De Felice M , Zannini M , Di Lauro R. 2010. Comparative genomics reveals a functional thyroid-specific element in the far upstream region of the PAX8 gene. BMC Genomics 11:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Livak KJ , Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]