Abstract
A prerequisite for successful embryo implantation is adequate preparation of receptive endometrium and the establishment and maintenance of a viable embryo. The success of implantation further relies upon a two-way dialogue between the embryo and uterus. However, molecular bases of these preimplantation and implantation processes in humans are not well known. We performed genome expression analyses of human embryos (n = 128) and human endometria (n = 8). We integrated these data with protein-protein interactions in order to identify molecular networks within the endometrium and the embryo, and potential embryo-endometrium interactions at the time of implantation. For that, we applied a novel network profiling algorithm HyperModules, which combines topological module identification and functional enrichment analysis. We found a major wave of transcriptional down-regulation in preimplantation embryos. In receptive-stage endometrium, several genes and signaling pathways were identified, including JAK-STAT signaling and inflammatory pathways. The main curated embryo-endometrium interaction network highlighted the importance of cell adhesion molecules in the implantation process. We also identified cytokine-cytokine receptor interactions involved in implantation, where osteopontin (SPP1), leukemia inhibitory factor (LIF) and leptin (LEP) pathways were intertwining. Further, we identified a number of novel players in human embryo-endometrium interactions, such as apolipoprotein D (APOD), endothelin 1 (END1), fibroblast growth factor 7 (FGF7), gastrin (GAST), kringle containing trnasmembrane protein 1 (KREMEN1), neuropilin 1 (NRP1), serpin peptidase inhibitor clade A member 3 (SERPINA3), versican (VCAN), and others. Our findings provide a fundamental resource for better understanding of the genetic network that leads to successful embryo implantation. We demonstrate the first systems biology approach into the complex molecular network of the implantation process in humans.
Successful embryo implantation is an absolute requirement for the reproduction of mammalian species. In humans, implantation involves complex interactions between the embryo and the maternal endometrium, all of which must be performed within an optimal time frame.
Critical to successful implantation is the embryo's development into an implantation-competent blastocyst and the synchronized transformation of the uterus into a receptive stage (1). The endometrium is receptive to blastocyst implantation only during a spatially and temporally restricted period in the secretory phase of the menstrual cycle, known as the putative “window of implantation” (2, 3). During this window, ovarian estrogen and progesterone induce the endometrial cells to proliferate, differentiate, and secrete molecules that influence trophoblast development. Meanwhile, the presence of an embryo in the uterus triggers specific molecular and cellular responses within the endometrium (4).
The success of embryonic implantation further relies upon a two-way dialogue between the blastocyst and the endometrium, which involves cell-cell and cell-extracellular matrix (ECM) interactions, mediated by integrins, matrix-degrading enzymes and their inhibitors, a variety of growth factors and cytokines, and their receptors and modulator proteins (5). Disturbances in this bidirectional cross talk are believed to represent a major reason why over 60% of all pregnancies are terminated at the end of the periimplantation period (3, 6, 7). Indeed, in assisted reproductive techniques, where often high-quality embryos are transferred, implantation remains the rate-limiting step for the success of treatment (8, 9). Therefore, a better understanding of the implantation process, and the importance of the factors involved, is warranted.
Many studies have been performed to improve understanding of the molecular mechanisms involved in embryo-maternal cross talk. However, most of the information regarding what is believed to occur during human implantation is derived from animal models, largely from studies on mice (10–12), because it is ethically and practically extremely difficult to study human implantation processes in vivo (13). Animal models do provide important clues to the processes regulating human implantation, but because the process varies across species (4), the results cannot always be extrapolated to humans.
With the development of microarray technology, numerous whole-genome expression analyses of the human endometrium have revealed hundreds of simultaneously up- and down-regulated genes that play a role in endometrial receptivity (14–24). Information concerning the molecular basis of human preimplantation development is limited, and only a few studies have been reported (25–30). Although numerous signaling factors and pathways have been found to have a role in the endometrium and/or in the embryo at the time of implantation, the molecular basis of the reciprocal embryo-maternal interactions still remains largely unknown.
In this study, we performed a comprehensive computational analysis of the molecular interaction network underlying the embryo-endometrium interface during implantation. Using a systems biology approach, we integrated embryonic and endometrial transcriptomic profiles with protein-protein interactions. Our analysis revealed a collection of proteins, functional protein modules, and pathways that are activated within the preimplanting blastocyst and in the receptive endometrium. Furthermore, we characterized a high-confidence network of cross-tissue protein interactions that outline the molecular nature of the embryo-endometrium implantation interface.
Results
Transcriptional profiling of the embryo and endometrium
Embryo implantation occurs between blastocyst-stage embryo (d 5 after conception) and the endometrium in its receptive stage (midsecretory phase endometrium). To identify the transcriptional profile within the blastocyst and the receptive endometrium, we used microarrays to profile d-3 vs. d-5 embryos, as well as proliferative vs. midsecretory endometrial tissues, and mapped the transcriptional up- and down-regulation events that coincide with implantation (Fig. 1A and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org).
Fig. 1.
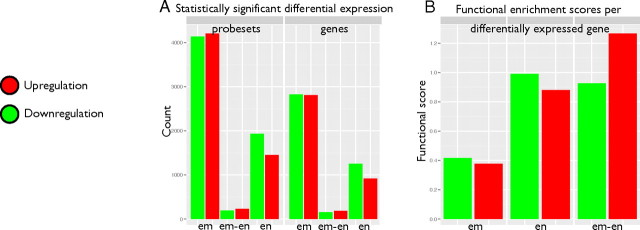
Transcriptional profiling of the embryo-endometrium interface. A, Number of Affymetrix probe sets (left) and corresponding HUGO Gene Nomenclature Committee gene symbols (right) detected in microarray analysis. em, Embryo; en, endometrium; em-en, coregulated in embryo and endometrium; red, up-regulation; green, down-regulation. B, Functional scores of known genes in embryonic and endometrial gene lists from GO and pathway enrichment analysis.
We then applied the incremental enrichment analysis algorithm from the g:Profiler tool (31) to study the functions of the genes involved. This algorithm extends gene set enrichment analysis by assessing the functional significance of increasingly larger sets of genes with the most dramatic transcriptional changes. In contrast to fixed gene set analysis, incremental enrichment analysis detects both specific functions and pathways that exhibit strong transcriptional signals, as well as broader categories that are widely represented within differentially expressed genes. In addition, we combined the above analysis with KEGGanim software, which highlights genes in pathway maps according to their differential expression (32). The results of this analysis are available online at http://biit.cs.ut.ee/KEGGanim (user, embryo; password, endometrium).
Embryonic cells are characterized by a wide transcriptional response, reflected in the activation of 4208 probe sets and inhibition of 4137 probe sets [15% of all probe sets; false discovery rate (FDR), P < 0.05], corresponding to 2812 up-regulated embryonic genes (EM+) and 2824 down-regulated embryonic genes (EM−) (Supplemental Table 1). The EM− subset comprises a large fraction of transcription factors, because a quarter of the regulators of the human transcription factor compendium (33) is inhibited (420 transcription factors, P = 10−36) (Supplemental Table 2). In our previously published study, where we applied different array data analysis tools, these preimplantation embryos also demonstrated a significant number of down-regulated genes involved in transcription regulation (27). Transcriptional and RNA metabolic machinery is known to be active during the embryonic genome activation that takes place before blastocyst formation (34). Relevant Gene Ontology (GO) categories in EM− genes include sexual reproduction (P = 10−11), brain development (P =10−7), and pattern specification processes (P = 10−6), among others. These illustrate the major reprogramming events that define the transition from pluripotent cell mass to differentiated tissues. Strikingly, the functional category histone H3-K4 demethylation (P = 10−4) is detected at the very top of the EM− list, represented by the single gene KDM1B with the strongest down-regulation signal in embryonic tissue. KDM1B is a histone H3-K4 demethylase required to establish maternal genomic imprints during oogenesis in mice (35, 36). Embryos derived from KDM1B-deficient oocytes showed aberrant expression of imprinted genes and so died halfway through gestation (35). EM+ genes are enriched in metabolic processes (P = 10−15), e.g. metabolism of small molecules (P = 10−22), lipids (P = 10−14), alcohol (P = 10−14), and amines (P = 10−7). High expression of lipid metabolic genes in preimplantation embryos confirms our previous observations (27) and also coincides with a very recent study of mural trophectoderm transcriptome of human blastocysts and embryonic stem cell-derived trophoblasts (37). The elevated expression of lipid metabolism in blastocysts may be associated with increased cell proliferation, where newly forming cells require more membrane. Genes related to development (P = 10−11) and localization (P = 10−12) were also enriched in our EM+ list, indicating that certain developmental pathways are regulated in opposite directions. An interesting gene, that for E-cadherin (CDH1), was found in the EM+ list. E-cadherin is a cell adhesion protein with a dual role during embryonic development. It maintains blastocyst structure by participating in cell-cell adhesion and is involved in cell-cell interaction and communication during embryo implantation (38, 39). Further genes of interest in the EM+ list include TGFB1 and IL6ST, which were also detected by Aghajanova et al. (37) in human trophectoderm and embryonic stem cell-derived trophoblasts, both of which are known to be associated with intrauterine lethality in knockout mice (40, 41). Other interesting genes in the EM+ list that are known to be involved in preimplantation development are those for cathepsins (CTSB, CTSH, CTSD, CTSZ, CTSL1, CTSE, and CTSA), prostaglandins (PTGES2, PTGES, PTGR1, and PTGER3), and pregnancy-associated glycoproteins (PSG1, PSG2, PSG4, PSG7, and PSG10) (37, 42).
Receptive endometrium is characterized by the activation of 1452 probe sets and the inhibition of 1935 probe sets (FDR, P < 0.05), corresponding to 920 up-regulated endometrial genes (EN+) and 1257 down-regulated endometrial genes (Supplemental Table 1). The down-regulated endometrial gene list is characterized by pregnancy-specific functions, such as gland development (P = 10−5), the progesterone-mediated oocyte maturation pathway (P = 10−6), and maternal process involved in pregnancy (P = 10−6). The strong GO and pathway enrichments in connection with EN+ genes reflect the complex interplay between the invading embryo and the mother's immune system. The aspects involved include response to external stimulus (P = 10−14), positive regulation of the immune system (P = 10−7), ECM-receptor interaction (P = 10−6), acute inflammatory response (P = 10−8), innate immune response (P = 10−7), and macrophage activation during immune response (P = 10−5). The second-strongest induction signal comes from the transcript of the LBP gene, which is involved in leukocyte chemotaxis during an inflammatory response. In fact, a favorable effect of injury-derived inflammation on implantation has been shown (43), and the up-regulation of genes involved in immune responses in receptive endometrium has been highlighted in previous studies (24, 44). Induced genes in functional categories, such as cell adhesion (P = 10−6), ECM-receptor interaction (P = 10−6), integrin cell surface interactions (P = 10−5), and regulation of cell proliferation (P = 10−6), indicate preparation for embryo implantation. Members of KEGG pathways for p53 signaling (P = 10−3) and oocyte meiosis (P = 10−7) were also observed more frequently than expected.
On a single gene level, we detected many of the genes recently implicated in independent microarray analyses of human uterine receptivity for implantation (45, 46), including up-regulated genes such as APOD, CLDN4, C1R, CYP2C9, DKK1, DPP4, EDNRB, GADD45A, GPX3, HABP2, ID4, IL15, LIF, LMOD1, MAOA, MAP3K5, MTNR1A, PAEP, SERPING1, and SPP1 and down-regulated genes such as CCNB1, MSX1, MSX2, and OLFM1. Leukemia inhibitory factor (LIF) involvement in human endometrial receptivity has been studied by several groups [reviewed by Aghajanova et al. (47)]. In fact, we have demonstrated that disturbances in the endometrial LIF signaling pathway could lead to fertility problems in otherwise healthy women (48).
A small number of genes appear to be coregulated in both tissues at the time of implantation, reflecting initiation of the embryo-endometrium interface and cell cycle regulation. The 184 coherently induced genes are concerned with enrichment of anchoring junctions (P = 10−8) and cytoskeletal protein binding (P = 10−6), whereas the 156 coherently inhibited genes are associated with the M phase of the cell cycle (P = 10−5).
We also studied the total functional information attributed to the genes in our embryonic and endometrial gene lists (Fig. 1B). We devised a simple score that reflects all overrepresented GO categories and pathways indicated in up- and down-regulated tissue-specific lists. The score corresponds to the sum of all log-scale P values from enrichment tests, divided by the number of genes in the list. It reflects the average amount of significance in all functional enrichment tests, as a log P value attributed to any single gene in the tissue-specific list. Endometrial genes appeared to have more than 2-fold stronger scores than embryonic genes. As an intuitive explanation, more functions have been assigned to endometrial genes in previous experiments and therefore our gene lists are better explained through functional enrichment. At the same time, human embryonic genes remain less well characterized, and functional enrichment is weaker because of abundant transcripts with unknown or vaguely defined functions. This is to be expected because of the apocryphal nature of human embryonic cells.
Embryonic and endometrial protein-protein interaction networks
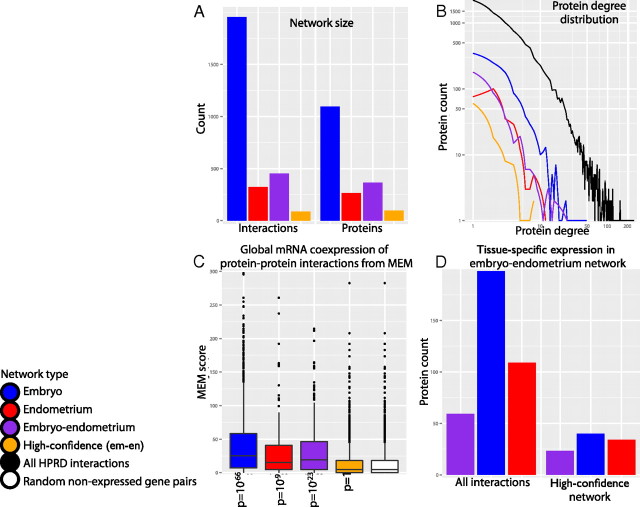
We studied protein-protein interaction networks that are activated within the receptive endometrium and within the d-5 blastocyst. The analysis is based on the assumption that significantly induced genes may establish permanent and transient protein-protein interactions to create protein complexes and initiate signal transduction. To construct embryonic and endometrial interaction networks, EM+ and EN+ genes were mapped to the Human Protein Reference Database (HPRD) (49). The mapping resulted in an embryonic network of 1096 genes and 1956 interactions and an endometrial network of 264 genes and 324 interactions (Fig. 2A, Supplemental Figs. 2 and 3, and Supplemental Tables 3 and 4). The topological structures of our tissue-specific networks closely resemble the raw HPRD network, because these networks appear to follow a similar, approximately log-linear degree distribution (Fig. 2B). The distribution of node (gene) degrees, i.e. the number of their interaction partners, determine global network properties that appear to be shared in many types of biological systems. Log-linear degree distribution implies that the vast majority of genes interact with only one or a couple of other genes. At the same time, a handful of genes interact with hundreds or thousands of others, creating a complex network of global connectivity. Importantly, biological networks appear to be modular, meaning that densely interacting gene groups may share similar functional properties, such as membership of physical protein complexes or signaling cascades.
Fig. 2.
Embryonic and endometrial interaction networks constructed from induced genes with protein-protein interactions. A, Protein counts (right) and interaction counts (left) in constructed networks. blue, Embryonic network (em); red, endometrial network (en); purple, embryo-endometrium network (em-en); orange, high-confidence em-en network. B, Distribution of protein interactions in constructed networks (protein degree, log-scale). Black line represents the global degree distribution of all protein-protein interactions in the HPRD. C, Global coexpression of interacting pairs in constructed networks. The y-axis represents significance score (log10 of P value) of coexpression across hundreds of experiments, as assessed by the MEM tool. Rightmost boxplot (white) shows coexpression for randomly selected pairs of nondifferentially expressed genes. Values of P show that constructed networks tend to have higher coexpression scores than random pairs (one-sided Kolmogorov-Smirnov test). D, Tissue-specific expression of proteins in full embryo-endometrium network (left) and high-confidence embryo-endometrium network (right). Purple bars represent proteins with induced expression in both tissues.
To provide functional interpretation to the intratissue interaction networks, we applied a novel topological clustering algorithm called HyperModules and identified 325 modules in the embryonic network and 144 modules in the endometrial network (Supplemental Figs. 4 and 5). The HyperModules algorithm developed here and implemented in the Graphweb software (50) is based on the assumption that interacting proteins with many shared interactors are biologically more relevant (51, 52). Overlapping modules are of particular biological interest, because proteins can take part in multiple unrelated functions and pathways via distinct sets of interactions. Consequently, HyperModules starts from an initial exhaustive set of modules, where each module consists of one protein and its direct interaction partners. These modules are then merged iteratively in a greedy manner, so that at each interaction, the pair of modules with the highest statistical significance of membership overlap will be merged. Merging is stopped when none of the overlaps are sufficiently significant.
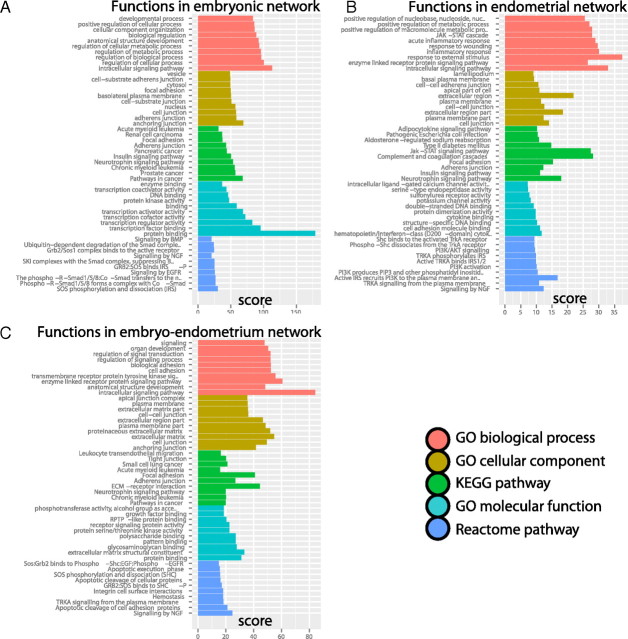
To assess the functional importance of detected gene modules, we applied enrichment analysis in GraphWeb and identified 10 of the most significant biological processes, cell components, molecular functions, and pathways for embryonic and endometrial networks (Fig. 3, A and B). A number of relevant functions and pathways was detected within the embryo, including transcription regulation, developmental processes, regulation of cellular metabolic processes, and pathways in cancer, and within the endometrium, various immune responses, the JAK-STAT signaling pathway, cell-cell adherens junctions, focal adhesion, and complement and coagulation cascades. The latter functional enrichment confirms our previous observations of the involvement of coagulation factors in endometrial receptivity (53, 54).
Fig. 3.
Functional enrichment analysis of embryonic and endometrial interaction networks: embryonic (A), endometrial (B), and embryo-endometrium (C). Bar color denotes different types of evidence from GO and pathway databases. The x-axis denotes functional enrichment score, computed as log10 sum of related P values from all topological modules, as identified by the HyperModules algorithm. The 10 most significant functional categories are shown for each source of evidence.
To gain additional confidence in our networks, we investigated global mRNA coexpression patterns of interacting proteins (Fig. 2C). Permanent physical protein-protein interactions are known to be associated with strong coexpression at the mRNA level across many cell types and conditions (55). To validate this observation, we used our recently developed Multi Experiment Matrix (MEM) software (56) to analyze our interaction networks. Briefly, MEM uses novel rank aggregation methods to find genes that exhibit similar expression patterns across a collection of several thousand microarray datasets. We applied MEM to measure relative coexpression of interacting gene pairs in embryonic, endometrial, and cross-tissue networks (see below) and compared these with randomly selected pairs of nonspecifically expressed genes. Here, we show that protein interactions indicated in our networks have considerably higher coexpression scores than those of random gene pairs (t tests: embryonic, P = 10−66; endometrial, P = 10−9; embryo-endometrium, P = 10−23). This analysis creates added confidence in our interaction networks, because protein-protein interactions with strong transcript-level coexpression are more likely to represent biologically relevant in vivo interactions.
Embryo-endometrium interaction network
Next, we set out to describe the intertissue interface that is initiated during implantation. We constructed an embryo-endometrium interaction network that encompasses genes induced in both endometrial and embryonic tissues (Fig. 2, A and B, Supplemental Fig. 6, and Supplemental Table 5). We extracted known protein-protein interactions from the HPRD that spanned the two tissues, such that each interaction comprised one gene induced in the embryo and the other induced in the endometrium. The majority of nodes in this network (54%) originate from the embryonic list of genes, whereas there is also a considerable fraction of endometrial genes (30%) and genes simultaneously induced in both tissues (16%) (Fig. 2D). The interactions in the embryo-endometrium interaction network were further filtered using GO cell component annotations. We focused on proteins known to be localized near the outer cell boundaries, such as membranes and the ECM, and excluded proteins localized within the cell cytoplasm, organelles, and nucleus (Supplemental Table 6). Proteins with no cellular component annotations were also included in the analysis.
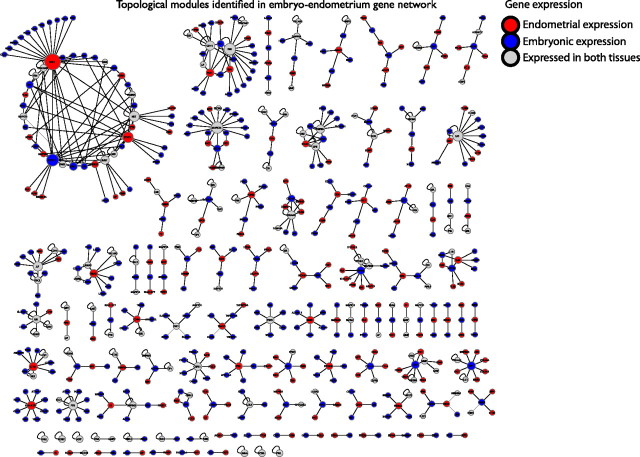
The embryo-endometrium interaction network was then analyzed by means of HyperModules to provide functional interpretation to the interaction networks, and 105 modules were identified (Fig. 4 and Supplemental Table 7). Numerous relevant functions and pathways were detected in functional enrichment analysis; for instance, cell adhesion, focal adhesion, cell-cell junctions, tight junctions, integrin cell surface interactions, ECM structural constituents, and others (Fig. 3C).
Fig. 4.
One hundred and five topological protein modules identified from the embryo-endometrium interaction network. Node color represents tissue-specific differential gene expression. blue, Expressed in embryo; red, expressed in endometrium; gray, expressed in both tissues. Node size represents number of interaction partners in the module.
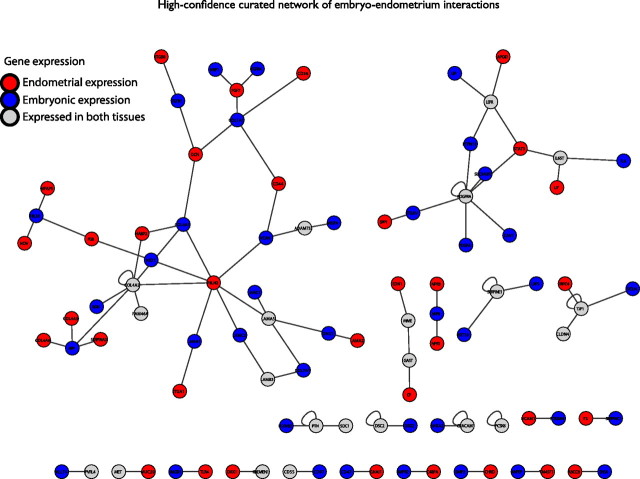
We then created a high-confidence variant of the embryo-endometrium interface by careful literature curation (Fig. 5 and Supplemental Table 8). The high-confidence network comprises 96 genes, 88 interactions, and 22 connected network components. The largest curated network is built up of 35 interacting molecules between the two tissues belonging to the protein families of collagens (COL1A1, COL4A1, COL4A2, COL4A5, COL4A6, and COL7A1), integrins (ITGA1 and ITGB8), laminins (LAMA1, LAMA2, LAMA5, LAMB3, LAMC1, and LAMC2), and fibulins (FBLN1 and FBLN2), together with other molecules involved in cell adhesion [CD36, CD44, HABP2, transforming growth factor beta 1 (TGFB1), VCAN, and vascular endothelial growth factor A). Activation of TGFB signaling during porcine implantation has been shown previously (57), and CD44 involvement in blastocyst adhesion has also been proposed earlier (58–60). Interestingly, HABP2 is one of the few genes identified in a number of studies in receptive endometrium (24, 45, 53). Vascular endothelial growth factor A synthesis by blastocysts has been demonstrated (61, 62), and its expression level in follicular fluid has been correlated with pregnancy outcome in in vitro fertilization (IVF) treatment (63). Integrins are expressed by both blastocyst-derived trophoblast cells and endometrial epithelial cells and are intimately involved in mediating embryo adhesion (59, 60, 64). The role of integrins in implantation has been widely reviewed (19, 65, 66). Endometrial collagen and laminin expression is believed to regulate embryo implantation (19, 60, 67). A role of fibulin in endometrial preparation toward implantation has been also suggested (68), but its involvement in the implantation process has not been demonstrated before. In addition, within the large network, we identified several new players in human embryo-endometrium interactions, which have been suggested to have roles in implantation in animal models, such as FGF7 (69), fibroblast growth factor receptor 4 (70), VCAN (71), NRP1 (72), biglycan (73), and SERPINA3 (74).
Fig. 5.
High-confidence embryo-endometrium interaction network from protein-protein interaction data and literature curation. Node color represents tissue-specific differential gene expression. blue, Expressed in embryo; red, expressed in endometrium; gray, expressed in both tissues.
The second largest interaction network, of 14 genes, represents proteins involved in cytokine-cytokine receptor interaction, where osteopontin, apolipoprotein D, leptin, and LIF pathways intertwine. Osteopontin binds directly to specific integrins and thus promotes trophectoderm cell migration and attachment to luminal epithelium. This complex has been proposed to be important in promoting embryo attachment (75, 76). The expression of APOD in human receptive endometrium has been highlighted (17). LIF and LEP signaling pathways in implantation/embryo-maternal communication have been extensively studied, and they are well established (summarized in Refs. 47, 77, 78).
The third largest interaction network unites four molecules that are involved in tight junctions, including tight junction protein 1, occludin (OCLN), and claudin 4. The presence of OCLN and claudin 4 in tight junctions at the time of implantation has been shown (65, 79). Data from experiments on mice suggest that during the early steps of implantation, trophoblast-induced expression of tight junctions results in a temporary barrier to protect the embryo from maternal injurious stimuli, such as Ig (80).
The next network in size demonstrates a novel interaction network in the human implantation process, comprising the hormone gastrin, the metalloprotein ceruloplasmin, membrane metallo-endopeptidase, and endothelin 1 (EDN1). These molecules have not been associated with implantation before, but the level of expression of EDN1 in follicular fluid was correlated with successful pregnancies in IVF treatment in a recent study (63).
Several additional novel interactors in the embryo-endometrium interface were detected in our study, including the molecules Dickkopf 1, kringle containing transmembrane protein 1 (KREMEN1), and carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1). Recent studies have demonstrated the involvement of DKK1 gene expression in embryo attachment and implantation in an in vitro coculture model (81), and aberrant endometrial expression of DKK1 has been associated with IVF failure (82). Furthermore, the importance of Dickkopf 1-KREMEN1 interaction in implantation has been demonstrated in mice (83). CEACAM1 is an adhesion molecule whose expression at the apical pole of endometrial epithelial cells and by extravillous trophoblast at the implantation site has been shown (84). Given its specific expression pattern, CEACAM1 has been proposed as a useful marker in mediating embryo-endometrial interactions (84), a notion which we support.
Discussion
We describe the first comprehensive computational study into the complex molecular networks of the implantation process in humans. The cellular events that define various stages of implantation have been described earlier (4), but the molecules and molecular genetic pathways that are crucial to this process (and how they interact) are not well known. Here, we performed an integrative systems biology analysis to uncover the complex molecular networks of human embryo-endometrium interface at the time of implantation, by combining tissue-specific transcriptome profiles with molecular interaction networks. We used an original network profiling strategy HyperModules to reduce the complexity of implantation networks and uncover pathways and functional protein modules that contribute to successful implantation. Furthermore, we outlined a high-confidence embryo-endometrium interaction network that represents the intertissue molecular interface at the time of implantation. Our findings serve as a resource for studying human implantation at the molecular level through hypothesis generation and functional validation.
Both adequate preparation of receptive endometrium and the establishment and maintenance of a viable embryo before reaching the endometrium are essential for successful implantation. Preimplantation development of embryos includes critical events, such as the transition from maternal to embryonic genome activation, compaction, cavitation, and blastocyst formation (85). Maternal to embryonic gene activation shows, in parallel with degradation of maternal transcripts, two principal transient waves of de novo transcription, as seen in mice, where the first wave peaks between the two- and four-cell stages and the second wave peaks at the eight-cell stage and precedes morula-to-blastocyst formation (86). The existence of these programmed waves of induced and inhibited gene expression patterns explains well our major finding that differentially expressed genes in blastocyst-stage embryos are involved in transcription regulation and especially transcriptional down-regulation.
Simultaneously with embryo development into the blastocyst stage, ovarian steroid hormones and downstream factors for growth and differentiation transform the endometrium into its receptive stage (46). In the current study, we confirm the involvement of many genes that have previously been identified in connection with uterine receptivity, such as LIF, HABP2, IL15, PAEP, SPP1, and others. In addition, we identify several relevant gene networks that are known to be involved in the adequate preparation of receptive endometrium, such as those connected with the JAK-STAT signaling pathway, complement and coagulation cascades, focal adhesion, adherens junctions, and inflammatory responses.
One of the most elegant and fascinating interactions in human physiology takes place between an embryo and the endometrium to initiate and maintain the process of implantation (87). We are the first to model the complex interaction pattern between the implanting embryo and the endometrium in humans. The main interaction network in our study highlights the importance of cell adhesion molecules, including integrins, collagens, and laminins in the implantation process. Indeed, in the initial stage of implantation, the blastocyst interacts with the endometrium using adhesion molecules, followed by stable adhesion (38). The polarized interaction between blastocyst and endometrium is established and becomes stronger, a process mediated by adhesion molecules, immune cells, and cytokines (88). Also in focus among the first interacting molecules, we found cytokine-cytokine receptor interactions to be important, where osteopontin and LIF and LEP pathways intertwine. We also propose several new players in human embryo-endometrium interaction, including apolipoprotein D, biglycan, EDN1, FBLN2, FGF7, gastrin, KREMEN1, NRP1, SERPINA3, VCAN, and others.
In the attempt to reveal the initial steps of the implantation, a recent study presented the global gene expression comparison in exogenous gonadotrophin-stimulated endometrium and blastocyst trophectoderm cells during the implantation period in women undergoing infertility treatment in IVF (60). A number of interacting molecules identified are also present in our study, such as LAMA1 in embryo and ITGB8, LAMA2, DCN, CEACAM1, CD44, and collagens 1 and 4. Nevertheless, their study approach was different, because they contrasted gene expression in two distinct tissues, trophectoderm cells and endometrial cells. Furthermore, they analyzed endometrium in IVF conditions that has been shown to alter endometrial receptivity (89–91). Further, a very recent study analyzed the transcriptome of mural trophectoderm cells from human blastocysts and compared the pattern with human embryonic stem cell-derived trophoblasts, offering a new view into the players in the very early stages of human implantation process (37). Interestingly, several of these proteins are present in our embryos that intertwine in our high-confidence embryo-endometrium interaction networks, such as IL-6, IL-6ST, LEPR, OCLN, SERPINE1, TGFB1, and VCAN.
One of the most important and studied genes related to implantation is that for LIF, because its crucial role in mice was demonstrated (92). LIF pathway involvement in human embryo implantation was clearly seen in our study. However, although LIF expression is an indicator of receptive endometrium, its role in the assessment of implantation potential in humans is controversial (47), and use of recombinant human LIF has failed to improve the outcome of IVF treatment in women with recurrent implantation failure (93). Although the role of LIF in the human implantation process has been proved to be important, it seems not to be crucial but rather a part of a highly coordinated orchestra.
In the current study, we present a novel systems biology approach for investigating the complex implantation process, although we have to acknowledge the limitation of microarray technology, with its focus on a static snapshot analysis of a dynamic process, and its unilateral analysis of either the embryo or the endometrium. Further, for ethical reasons, it is possible to use only in vitro cultured human embryos, which might not reflect fully the in vivo processes. Altogether, we cannot exclude the possibility that the expression profiles that we identified as potentially involved in these early dialogues between the embryo and the endometrium could, in some extent, differ from the conditions in natural conception.
It is an important challenge to elucidate the processes within the embryo and endometrium adjacent to implantation and to understand the complex cross talk between the implanting embryo and the endometrium in humans. Fifteen percent of couples worldwide are childless because of infertility (94). Although many underlying causes of human infertility have been overcome by a variety of assisted reproductive techniques, implantation remains the rate-limiting step for the success of IVF treatments (9). There is, therefore, a continuing need to unravel the complexities of uterine receptivity and preimplantation embryonic development, and subsequent implantation, to address two contrasting global issues: to improve infertility and to design new and improved contraceptives.
In conclusion, our findings and database provide a fundamental resource for better understanding of the complex genetic network that leads to successful embryo implantation. We have detected new molecular aspects in blastocyst preimplantation development and confirm several molecules and pathways important for endometrial receptivity. With our computational analysis, we highlight the first interacting molecules and their networks in initiating the implantation process between the blastocyst and the receptive endometrium. Furthermore, the methodology presented herein could serve to inspire new analysis approaches to unravel complex networks in human physiology.
Materials and Methods
Embryonic and endometrial samples
In total, 128 in vitro cultured embryos were used in the current study, 68 d-3 eight-cell embryos and 60 d-5 blastocysts. These large numbers of unique preimplantation human embryos, collected at IVF units at Örebro University Hospital and Uppsala University Hospital, were donated for research and were not used in infertility treatment. The donated embryos had been frozen for future infertility treatment, and when there was a wish for no further infertility treatment, they were donated for research. The Ethics Committees of Karolinska Institutet and Örebro University approved the study, and informed consent was obtained from the donating couples. Detailed information about the infertility treatment protocols and further embryo culture has been published previously (27). Evaluation of blastocysts was performed at the IVF unit using the system described by Gardner (95). The blastocoel was graded from one to six as follows: 1) early blastocyst with a blastocoel of less than 50% of embryo volume, 2) early blastocyst with a blastocoel of 50–80%, 3) fully developed blastocoel of at least 80% of embryo volume, 4) expanded blastocyst, 5) hatching blastocyst, and 6) fully hatched blastocyst. The inner cell mass was graded as follows: 1) many cells and tightly packed, 2) average number of cells, and 3) few cells and loosely packed. The trophectoderm was graded: 1) for many cells, equal in size, and 2) for uneven cells and 3) for few cells. Expanded blastocysts with a good inner cell mass and trophectoderm, i.e. at least 4AB were considered to be of high quality, and blastocysts scoring at least 3AA were considered to be of good quality. We did not use developmentally poor embryos; those included in our study were all of high or good quality.
Endometrial samples from healthy volunteers were collected at the Department of Obstetrics and Gynaecology (Uppsala University). The Ethics Committee of Uppsala University approved the study, and informed consent was obtained from every woman. In total, eight endometrial samples were collected, four samples from the proliferative phase of the natural menstrual cycle (cycle d 7, nonreceptive endometrium) and four samples from the midsecretory phase (LH+7, receptive endometrium). The endometrial biopsy samples were obtained from the anterior wall of the uterine cavity, without dilatation of the cervix, using a Pipelle catheter (Genetics, Namont-Achel, Belgium). The detection of LH in morning urine (Unipath Ltd., Bedford, UK) was used to determine the day of the LH surge (day LH+0). Histological evaluation of the samples showed normal maturation in relation to the cycle day, according to the criteria described by Noyes et al. (96). The mean age of these women was 34.0 ± 10.0 (sd), they were healthy, with no gynecological complications, nonsmoking, and with proven fertility (para 2.4 ± 1.5), except for two women who were young and had not yet had children. Their mean body mass index was 25.7 ± 7.2 kg/m2, mean cycle length was 27.6 ± 1.1 d, and the mean duration of menses was 5.3 ± 1.6 d.
Total RNA isolation and oligonucleotide microarray
Total RNA was isolated from embryos and endometrial biopsy samples using RNeasy Mini kits (QIAGEN, Venlo, The Netherlands). The quality of the RNA was assessed by using an A2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). For embryo samples of both stages, RNA samples were pooled into one biological sample, and two independent biological samples were used as replicates (d 3, 32 and 36 embryos; d 5, 27 and 33 embryos). Plots of correlations between the biological duplicates have been illustrated previously [Zhang et al. (27)]. In total, four samples (d 3, n = 2; d 5, n = 2) were used for microarray analysis. Endometrial samples were not pooled, and in total eight microarray assays were performed (proliferative phase endometrium, n = 4; midsecretory phase endometrium, n = 4).
For all samples, 50 ng of total RNA was reverse transcribed, amplified, labeled, and hybridized according to the Affymetrix two-cycle GeneChip Eukaryotic small sample target labeling assay, version II (Affymetrix, Santa Clara, CA). For hybridization, an Affymetrix HG-U133 Plus 2.0 array was used.
Microarray analysis
Microarray data analysis was performed by using R Bioconductor software. First, array normalization was carried out using the robust multiarray average method of the Affy package. Differential expression for each probe set was then quantified with linear models with the Limma package (97). Two contrasts were applied, covering changes between proliferative and receptive endometrium and changes between d-3 and d-5 embryos. Statistical significance of differential expression was assessed using empirical Bayes-moderated t statistics of the Limma package. Resulting P values were globally corrected for multiple testing (FDR), and a cut-off (P < 0.05) was applied to distinguish probe sets with significant differential expression. Affymetrix probe set ID were converted to Human Genome Nomenclature symbols with g:Profiler software (31). Enrichment analysis and incremental enrichment analysis for GO categories and KEGG pathways was also carried out with the g:Profiler software.
Our primary and processed microarray data are available in the public database ArrayExpress repository (accession no. E-MEXP-3111), and previous microarray analysis of embryo tissue [Zhang et al. (27)] is also available (accession no. E-MEXP-2359). Microarray data have been validated in our previous study using real-time PCR analysis (98).
Construction of embryonic and endometrial interaction networks
The manually curated collection of human protein-protein interactions was retrieved from the HPRD (49). Endometrial (embryonic) interaction networks consisted of HPRD interactions, in which mRNA levels of both interactors were significantly up-regulated in endometrial (embryonal) microarray analysis. To construct the embryo-endometrium interaction network, we considered interaction pairs where one of the interacting proteins was significantly up-regulated in the embryo and the other in the endometrium, or either of the proteins in both tissues. Proteins in the embryo-endometrium network were further filtered on the basis of HPRD GO cellular component annotations, and proteins with known roles in unrelated compartments were removed (Supplemental Table 6).
Each network was partitioned into partially overlapping topological protein modules. Detected interaction modules were profiled with functional enrichments of GO terms and pathways with g:Profiler software (31). The final score for each recovered category was computed as the log10 sum of P values from all topological modules within the respective category. Network visualization was carried out using Cytoscape software (99).
Assessment of mRNA coexpression in network interactions
mRNA-level coexpression of interacting protein pairs was assessed by using the MEM web tool (56). In brief, the MEM web tool involves use of correlation-based measures and novel rank aggregation strategies to rank coexpressed genes to a given gene, assess the statistical significance of detected coexpression, and select microarray datasets that contribute most to observed coexpression. In this analysis, MEM was applied to study coexpression of physically interacting proteins. For a given pair of interactors, all related microarray probe sets were retrieved, paired appropriately, and assessed for coexpression, using the MEM web tool. The probe set with the best P value was selected as a representative of the current pair of interactors. To obtain MEM scores, P values from the above were corrected by using the Holm multiple testing procedure, log-transformed, and subjected to significance cut-off (P = 0.05). Random pairs of interactors were combined from nondifferentially expressed subsets of embryonic and endometrial genes and subjected to the same selection, correction, and cut-off criteria. MEM scores for interaction networks and random gene pairs were compared by using one-sided Kolmogorov-Smirnov tests.
The HyperModules algorithm
The constructed interaction networks were dissected into partially overlapping modules using a novel probabilistic algorithm called HyperModules. HyperModules involves commonly targeted interacting partners of genes. We use a “greedy” approach to build modules of genes whose interaction partners significantly overlap and merge modules iteratively until convergence. At each interaction, we merge the two modules with the greatest overlap as defined by the cumulative hypergeometric test. Convergence occurs when the significance of merging events falls below a predefined cut-off value (P = 0.05).
More specifically, the algorithm involves the following steps. 1) Set the initial collection of gene modules. Every initial module consists of a gene and its direct interaction partners. For each gene in the network, there exists an initial module with itself and all its interactors. 2) Study all pairs of modules and focus on those that include overlapping sets of genes. Calculate the statistical significance of enrichment of overlapping genes, using the hypergeometric test. Perform multiple testing correction (FDR) for all tests. Merge the pair of modules where the statistical significance as regards enrichment of common member genes is the greatest. 3) Repeat step 3 until no more modules can be merged with a statistically significant P value (P = 0.05).
We have made the algorithm available in our GraphWeb tool (50).
Acknowledgments
We thank all of the participants and study personnel who took part in this work. We thank Erja Kerkelä from the University of Tampere and Fredwell Hambiliki from Karolinska Institutet for their work with embryo material, Marco Zucchelli from Karolinska Institutet for help in embryo array data, Viljar Jaks from the University of Tartu for the help in data analysis, and Priit Adler, Raivo Kolde, and Laur Tooming from the University of Tartu for discussion and software-related contributions.
This work was supported by grants from Karolinska Institutet, the Swedish Research Council, R&D funding from Karolinska Institutet and Stockholm County Council (ALF), the Enterprise Estonia Grant EU30200, the Estonian Doctoral School of Information and Communication Technology, the Estonian Centre of Excellence in Computer Science, the Estonian Ministry of Education and Science Core Grants SF0180044s09 and MEM project ETF7477, the Swedish Research Council Grant 2005-7293, Uppsala University, and the Family planning foundation, Uppsala.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- APOD
- apolipoprotein D
- CEACAM1
- carcinoembryonic antigen-related cell adhesion molecule 1
- COL
- collagen
- ECM
- Extracellular matrix
- EDN1
- endothelin 1
- EM−
- down-regulated embryonic gene
- EM+
- up-regulated embryonic gene
- EN+
- up-regulated endometrial gene
- FBLN
- fibulin
- FDR
- false discovery rate
- FGF7
- fibroblast growth factor 7
- GAST
- gastrin GO, Gene Ontology
- HPRD
- Human Protein Reference Database
- IVF
- in vitro fertilization
- ITG
- integrin
- JAK-STAT
- Janus kinase-signal transducer and activator of transcription
- KEGG
- Kyoto Encyclopedia of Genes and Genomes
- KREMEN1
- kringle containing transmembrane protein 1
- LAM
- laminin
- LEP
- leptin
- LIF
- leukemia inhibitory factor
- MEM
- Multi Experiment Matrix
- NRP1
- neuropilin 1
- OCLN
- occludin
- SERPINA3
- serpin peptidase inhibitor, clade A, member 3
- SPP1
- osteopontin
- VCAN
- versican.
References
- 1. Giudice LC. 1999. Genes associated with embryonic attachment and implantation and the role of progesterone. J Reprod Med 44:165–171 [PubMed] [Google Scholar]
- 2. Harper MJ. 1992. The implantation window. Baillieres Clin Obstet Gynaecol 6:351–371 [DOI] [PubMed] [Google Scholar]
- 3. Wilcox AJ , Baird DD , Weinberg CR. 1999. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 340:1796–1799 [DOI] [PubMed] [Google Scholar]
- 4. Carson DD , Bagchi I , Dey SK , Enders AC , Fazleabas AT , Lessey BA , Yoshinaga K. 2000. Embryo implantation. Dev Biol 223:217–237 [DOI] [PubMed] [Google Scholar]
- 5. Giudice LC. 1999. Potential biochemical markers of uterine receptivity. Hum Reprod 14(Suppl 2):3–16 [DOI] [PubMed] [Google Scholar]
- 6. Herrler A , von Rango U , Beier HM. 2003. Embryo-maternal signalling: how the embryo starts talking to its mother to accomplish implantation. Reprod Biomed Online 6:244–256 [DOI] [PubMed] [Google Scholar]
- 7. Macklon NS , Geraedts JP , Fauser BC. 2002. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update 8:333–343 [DOI] [PubMed] [Google Scholar]
- 8. Edwards RG. 1995. Clinical approaches to increasing uterine receptivity during human implantation. Hum Reprod 10(Suppl 2):60–66 [DOI] [PubMed] [Google Scholar]
- 9. Macklon NS , Stouffer RL , Giudice LC , Fauser BC. 2006. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev 27:170–207 [DOI] [PubMed] [Google Scholar]
- 10. Dimitriadis E , White CA , Jones RL , Salamonsen LA. 2005. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update 11:613–630 [DOI] [PubMed] [Google Scholar]
- 11. Tranguch S , Daikoku T , Guo Y , Wang H , Dey SK. 2005. Molecular complexity in establishing uterine receptivity and implantation. Cell Mol Life Sci 62:1964–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang H , Dey SK. 2005. Lipid signaling in embryo implantation. Prostaglandins Other Lipid Mediat 77:84–102 [DOI] [PubMed] [Google Scholar]
- 13. Lee KY , DeMayo FJ. 2004. Animal models of implantation. Reproduction 128:679–695 [DOI] [PubMed] [Google Scholar]
- 14. Riesewijk A , Martín J , van Os R , Horcajadas JA , Polman J , Pellicer A , Mosselman S , Simón C. 2003. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod 9:253–264 [DOI] [PubMed] [Google Scholar]
- 15. Borthwick JM , Charnock-Jones DS , Tom BD , Hull ML , Teirney R , Phillips SC , Smith SK. 2003. Determination of the transcript profile of human endometrium. Mol Hum Reprod 9:19–33 [DOI] [PubMed] [Google Scholar]
- 16. Carson DD , Lagow E , Thathiah A , Al-Shami R , Farach-Carson MC , Vernon M , Yuan L , Fritz MA , Lessey B. 2002. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod 8:871–879 [DOI] [PubMed] [Google Scholar]
- 17. Kao LC , Tulac S , Lobo S , Imani B , Yang JP , Germeyer A , Osteen K , Taylor RN , Lessey BA , Giudice LC. 2002. Global gene profiling in human endometrium during the window of implantation. Endocrinology 143:2119–2138 [DOI] [PubMed] [Google Scholar]
- 18. Mirkin S , Arslan M , Churikov D , Corica A , Diaz JI , Williams S , Bocca S , Oehninger S. 2005. In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod 20:2104–2117 [DOI] [PubMed] [Google Scholar]
- 19. Haouzi D , Mahmoud K , Fourar M , Bendhaou K , Dechaud H , De Vos J , Rème T , Dewailly D , Hamamah S. 2009. Identification of new biomarkers of human endometrial receptivity in the natural cycle. Hum Reprod 24:198–205 [DOI] [PubMed] [Google Scholar]
- 20. Horcajadas JA , Riesewijk A , Martín J , Cervero A , Mosselman S , Pellicer A , Simón C. 2004. Global gene expression profiling of human endometrial receptivity. J Reprod Immunol 63:41–49 [DOI] [PubMed] [Google Scholar]
- 21. Feroze-Zaidi F , Fusi L , Takano M , Higham J , Salker MS , Goto T , Edassery S , Klingel K , Boini KM , Palmada M , Kamps R , Groothuis PG , Lam EW , Smith SK , Lang F , Sharkey AM , Brosens JJ. 2007. Role and regulation of the serum- and glucocorticoid-regulated kinase 1 in fertile and infertile human endometrium. Endocrinology 148:5020–5029 [DOI] [PubMed] [Google Scholar]
- 22. Talbi S , Hamilton AE , Vo KC , Tulac S , Overgaard MT , Dosiou C , Le Shay N , Nezhat CN , Kempson R , Lessey BA , Nayak NR , Giudice LC. 2006. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 147:1097–1121 [DOI] [PubMed] [Google Scholar]
- 23. Simon C , Oberyé J , Bellver J , Vidal C , Bosch E , Horcajadas JA , Murphy C , Adams S , Riesewijk A , Mannaerts B , Pellicer A. 2005. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum Reprod 20:3318–3327 [DOI] [PubMed] [Google Scholar]
- 24. Altmäe S , Martínez-Conejero JA , Salumets A , Simón C , Horcajadas JA , Stavreus-Evers A. 2010. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod 16:178–187 [DOI] [PubMed] [Google Scholar]
- 25. Dobson AT , Raja R , Abeyta MJ , Taylor T , Shen S , Haqq C , Pera RA. 2004. The unique transcriptome through day 3 of human preimplantation development. Hum Mol Genet 13:1461–1470 [DOI] [PubMed] [Google Scholar]
- 26. Jaroudi S , Kakourou G , Cawood S , Doshi A , Ranieri DM , Serhal P , Harper JC , SenGupta SB. 2009. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays. Hum Reprod 24:2649–2655 [DOI] [PubMed] [Google Scholar]
- 27. Zhang P , Zucchelli M , Bruce S , Hambiliki F , Stavreus-Evers A , Levkov L , Skottman H , Kerkelä E , Kere J , Hovatta O. 2009. Transcriptome profiling of human pre-implantation development. PLoS One 4:e7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamatani T , Ko MSh , Yamada M , Kuji N , Mizusawa Y , Shoji M , Hada T , Asada H , Maruyama T , Yoshimura Y. 2006. Global gene expression profiling of preimplantation embryos. Hum Cell 19:98–117 [DOI] [PubMed] [Google Scholar]
- 29. Kocabas AM , Crosby J , Ross PJ , Otu HH , Beyhan Z , Can H , Tam WL , Rosa GJ , Halgren RG , Lim B , Fernandez E , Cibelli JB. 2006. The transcriptome of human oocytes. Proc Natl Acad Sci USA 103:14027–14032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wells D , Bermudez MG , Steuerwald N , Thornhill AR , Walker DL , Malter H , Delhanty JD , Cohen J. 2005. Expression of genes regulating chromosome segregation, the cell cycle and apoptosis during human preimplantation development. Hum Reprod 20:1339–1348 [DOI] [PubMed] [Google Scholar]
- 31. Reimand J , Arak J , Vilo J. 2011. g:Profiler—a web server for functional interpretation of gene lists. Nucleic Acids Res 39:W307–W315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adler P , Reimand J , Jänes J , Kolde R , Peterson H , Vilo J. 2008. KEGGanim: pathway animations for high-throughput data. Bioinformatics 24:588–590 [DOI] [PubMed] [Google Scholar]
- 33. Vaquerizas JM , Kummerfeld SK , Teichmann SA , Luscombe NM. 2009. A census of human transcription factors: function, expression and evolution. Nat Rev Genet 10:252–263 [DOI] [PubMed] [Google Scholar]
- 34. Zeng F , Baldwin DA , Schultz RM. 2004. Transcript profiling during preimplantation mouse development. Dev Biol 272:483–496 [DOI] [PubMed] [Google Scholar]
- 35. Ciccone DN , Su H , Hevi S , Gay F , Lei H , Bajko J , Xu G , Li E , Chen T. 2009. KDM1B is a histone H3K4 demethylase required to establish maternal genomic imprints. Nature 461:415–418 [DOI] [PubMed] [Google Scholar]
- 36. Karytinos A , Forneris F , Profumo A , Ciossani G , Battaglioli E , Binda C , Mattevi A. 2009. A novel mammalian flavin-dependent histone demethylase. J Biol Chem 284:17775–17782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aghajanova L , Shen S , Rojas AM , Fisher SJ , Irwin JC , Giudice LC. 24 August 2011. Comparative transcriptome analysis of human trophectoderm and embryonic stem cell-derived trophoblasts reveal key participants in early implantation. Biol Reprod 10.1095/biolreprod.111.092775 [DOI] [PubMed] [Google Scholar]
- 38. Aplin JD. 1997. Adhesion molecules in implantation. Rev Reprod 2:84–93 [DOI] [PubMed] [Google Scholar]
- 39. Chen HW , Chen JJ , Yu SL , Li HN , Yang PC , Su CM , Au HK , Chang CW , Chien LW , Chen CS , Tzeng CR. 2005. Transcriptome analysis in blastocyst hatching by cDNA microarray. Hum Reprod 20:2492–2501 [DOI] [PubMed] [Google Scholar]
- 40. Kulkarni AB , Karlsson S. 1993. Transforming growth factor-β1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol 143:3–9 [PMC free article] [PubMed] [Google Scholar]
- 41. Yoshida K , Taga T , Saito M , Suematsu S , Kumanogoh A , Tanaka T , Fujiwara H , Hirata M , Yamagami T , Nakahata T , Hirabayashi T , Yoneda Y , Tanaka K , Wang WZ , Mori C , Shiota K , Yoshida N , Kishimoto T. 1996. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA 93:407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Camolotto S , Racca A , Rena V , Nores R , Patrito LC , Genti-Raimondi S , Panzetta-Dutari GM. 2010. Expression and transcriptional regulation of individual pregnancy-specific glycoprotein genes in differentiating trophoblast cells. Placenta 31:312–319 [DOI] [PubMed] [Google Scholar]
- 43. Dekel N , Gnainsky Y , Granot I , Mor G. 2010. Inflammation and implantation. Am J Reprod Immunol 63:17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giudice LC. 2006. Application of functional genomics to primate endometrium: insights into biological processes. Reprod Biol Endocrinol 4(Suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Horcajadas JA , Pellicer A , Simón C. 2007. Wide genomic analysis of human endometrial receptivity: new times, new opportunities. Hum Reprod Update 13:77–86 [DOI] [PubMed] [Google Scholar]
- 46. Wang H , Dey SK. 2006. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 7:185–199 [DOI] [PubMed] [Google Scholar]
- 47. Aghajanova L. 2010. Update on the role of leukemia inhibitory factor in assisted reproduction. Curr Opin Obstet Gynecol 22:213–219 [DOI] [PubMed] [Google Scholar]
- 48. Aghajanova L , Altmäe S , Bjuresten K , Hovatta O , Landgren BM , Stavreus-Evers A. 2009. Disturbances in the LIF pathway in the endometrium among women with unexplained infertility. Fertil Steril 91:2602–2610 [DOI] [PubMed] [Google Scholar]
- 49. Keshava Prasad TS , Goel R , Kandasamy K , Keerthikumar S , Kumar S , Mathivanan S , Telikicherla D , Raju R , Shafreen B , Venugopal A , Balakrishnan L , Marimuthu A , Banerjee S , Somanathan DS , Sebastian A , Rani S , Ray S , Harrys Kishore CJ , Kanth S , Ahmed M , Kashyap MK , Mohmood R , Ramachandra YL , Krishna V , Rahiman BA , Mohan S , Ranganathan P , Ramabadran S , Chaerkady R , Pandey A. 2009. Human protein reference database—2009 update. Nucleic Acids Res 37:D767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reimand J , Tooming L , Peterson H , Adler P , Vilo J. 2008. GraphWeb: mining heterogeneous biological networks for gene modules with functional significance. Nucleic Acids Res 36:W452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goldberg DS , Roth FP. 2003. Assessing experimentally derived interactions in a small world. Proc Natl Acad Sci USA 100:4372–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bader JS , Chaudhuri A , Rothberg JM , Chant J. 2004. Gaining confidence in high-throughput protein interaction networks. Nat Biotechnol 22:78–85 [DOI] [PubMed] [Google Scholar]
- 53. Altmäe S , Kallak TK , Fridén B , Stavreus-Evers A. 2011. Variation in hyaluronan-binding protein 2 (HABP2) promoter region is associated with unexplained female infertility. Reprod Sci 18:485–492 [DOI] [PubMed] [Google Scholar]
- 54. Altmäe S , Salumets A , Bjuresten K , Kallak TK , Wånggren K , Landgren BM , Hovatta O , Stavreus-Evers A. 2011. Tissue factor (TF) and tissue factor pathway inhibitors TFPI and TFPI2 in human secretory endometrium—possible link to female infertility. Reprod Sci 18:666–678 [DOI] [PubMed] [Google Scholar]
- 55. Jansen R , Greenbaum D , Gerstein M. 2002. Relating whole-genome expression data with protein-protein interactions. Genome Res 12:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Adler P , Kolde R , Kull M , Tkachenko A , Peterson H , Reimand J , Vilo J. 2009. Mining for coexpression across hundreds of datasets using novel rank aggregation and visualization methods. Genome Biol 10:R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Massuto DA , Kneese EC , Johnson GA , Burghardt RC , Hooper RN , Ing NH , Jaeger LA. 2010. Transforming growth factor β (TGFB) signaling is activated during porcine implantation: proposed role for latency-associated peptide interactions with integrins at the conceptus-maternal interface. Reproduction 139:465–478 [DOI] [PubMed] [Google Scholar]
- 58. Kimber SJ , Spanswick C. 2000. Blastocyst implantation: the adhesion cascade. Semin Cell Dev Biol 11:77–92 [DOI] [PubMed] [Google Scholar]
- 59. Lessey BA , Castelbaum AJ. 2002. Integrins and implantation in the human. Rev Endocr Metab Disord 3:107–117 [DOI] [PubMed] [Google Scholar]
- 60. Haouzi D , Dechaud H , Assou S , Monzo C , de Vos J , Hamamah S. 2011. Transcriptome analysis reveals dialogues between human trophectoderm and endometrial cells during the implantation period. Hum Reprod 26:1440–1449 [DOI] [PubMed] [Google Scholar]
- 61. Staun-Ram E , Shalev E. 2005. Human trophoblast function during the implantation process. Reprod Biol Endocrinol 3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Artini PG , Valentino V , Monteleone P , Simi G , Parisen-Toldin MR , Cristello F , Cela V , Genazzani AR. 2008. Vascular endothelial growth factor level changes during human embryo development in culture medium. Gynecol Endocrinol 24:184–187 [DOI] [PubMed] [Google Scholar]
- 63. Zhao M , Chang C , Liu Z , Chen LM , Chen Q. 2010. The level of vascular endothelial cell growth factor, nitric oxide, and endothelin-1 was correlated with ovarian volume or antral follicle counts: a potential predictor of pregnancy outcome in IVF. Growth Factors 28:299–305 [DOI] [PubMed] [Google Scholar]
- 64. Reddy KV , Mangale SS. 2003. Integrin receptors: the dynamic modulators of endometrial function. Tissue Cell 35:260–273 [DOI] [PubMed] [Google Scholar]
- 65. Aplin JD. 2006. Embryo implantation: the molecular mechanism remains elusive. Reprod Biomed Online 13:833–839 [DOI] [PubMed] [Google Scholar]
- 66. Aplin JD , Kimber SJ. 2004. Trophoblast-uterine interactions at implantation. Reprod Biol Endocrinol 2:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tanaka T , Wang C , Umesaki N. 2009. Remodeling of the human endometrial epithelium is regulated by laminin and type IV collagen. Int J Mol Med 23:173–180 [PubMed] [Google Scholar]
- 68. Nakamoto T , Okada H , Nakajima T , Ikuta A , Yasuda K , Kanzaki H. 2005. Progesterone induces the fibulin-1 expression in human endometrial stromal cells. Hum Reprod 20:1447–1455 [DOI] [PubMed] [Google Scholar]
- 69. Kim M , Seo H , Choi Y , Hwang W , Lee CK , Ka H. 2009. Aberrant expression of retinol-binding protein, osteopontin and fibroblast growth factor 7 in the porcine uterine endometrium of pregnant recipients carrying embryos produced by somatic cell nuclear transfer. Anim Reprod Sci 112:172–181 [DOI] [PubMed] [Google Scholar]
- 70. Rappolee DA , Patel Y , Jacobson K. 1998. Expression of fibroblast growth factor receptors in peri-implantation mouse embryos. Mol Reprod Dev 51:254–264 [DOI] [PubMed] [Google Scholar]
- 71. San Martin S , Soto-Suazo M , Zorn TM. 2003. Distribution of versican and hyaluronan in the mouse uterus during decidualization. Braz J Med Biol Res 36:1067–1071 [DOI] [PubMed] [Google Scholar]
- 72. Halder JB , Zhao X , Soker S , Paria BC , Klagsbrun M , Das SK , Dey SK. 2000. Differential expression of VEGF isoforms and VEGF(164)-specific receptor neuropilin-1 in the mouse uterus suggests a role for VEGF(164) in vascular permeability and angiogenesis during implantation. Genesis 26:213–224 [PubMed] [Google Scholar]
- 73. Ace CI , Okulicz WC. 2004. Microarray profiling of progesterone-regulated endometrial genes during the rhesus monkey secretory phase. Reprod Biol Endocrinol 2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sherwin JR , Sharkey AM , Cameo P , Mavrogianis PM , Catalano RD , Edassery S , Fazleabas AT. 2007. Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology 148:618–626 [DOI] [PubMed] [Google Scholar]
- 75. Johnson GA , Burghardt RC , Bazer FW , Spencer TE. 2003. Osteopontin: roles in implantation and placentation. Biol Reprod 69:1458–1471 [DOI] [PubMed] [Google Scholar]
- 76. Casals G , Ordi J , Creus M , Fábregues F , Casamitjana R , Quinto L , Campo E , Balasch J. 2008. Osteopontin and αvβ3 integrin expression in the endometrium of infertile and fertile women. Reprod Biomed Online 16:808–816 [DOI] [PubMed] [Google Scholar]
- 77. González RR , Simón C , Caballero-Campo P , Norman R , Chardonnens D , Devoto L , Bischof P. 2000. Leptin and reproduction. Hum Reprod Update 6:290–300 [DOI] [PubMed] [Google Scholar]
- 78. van Mourik MS , Macklon NS , Heijnen CJ. 2009. Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J Leukoc Biol 85:4–19 [DOI] [PubMed] [Google Scholar]
- 79. Nicholson MD , Lindsay LA , Murphy CR. 2010. Ovarian hormones control the changing expression of claudins and occludin in rat uterine epithelial cells during early pregnancy. Acta Histochem 112:42–52 [DOI] [PubMed] [Google Scholar]
- 80. Wang X , Matsumoto H , Zhao X , Das SK , Paria BC. 2004. Embryonic signals direct the formation of tight junctional permeability barrier in the decidualizing stroma during embryo implantation. J Cell Sci 117:53–62 [DOI] [PubMed] [Google Scholar]
- 81. Liu Y , Kodithuwakku SP , Ng PY , Chai J , Ng EH , Yeung WS , Ho PC , Lee KF. 2010. Excessive ovarian stimulation up-regulates the Wnt-signaling molecule DKK1 in human endometrium and may affect implantation: an in vitro co-culture study. Hum Reprod 25:479–490 [DOI] [PubMed] [Google Scholar]
- 82. Koler M , Achache H , Tsafrir A , Smith Y , Revel A , Reich R. 2009. Disrupted gene pattern in patients with repeated in vitro fertilization (IVF) failure. Hum Reprod 24:2541–2548 [DOI] [PubMed] [Google Scholar]
- 83. Li J , Liu WM , Cao YJ , Peng S , Zhang Y , Duan EK. 2008. Roles of Dickkopf-1 and its receptor Kremen1 during embryonic implantation in mice. Fertil Steril 90:1470–1479 [DOI] [PubMed] [Google Scholar]
- 84. Horne AW , White JO , Lalani el-N. 2002. Adhesion molecules and the normal endometrium. BJOG 109:610–617 [DOI] [PubMed] [Google Scholar]
- 85. Bell CE , Calder MD , Watson AJ. 2008. Genomic RNA profiling and the programme controlling preimplantation mammalian development. Mol Hum Reprod 14:691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hamatani T , Carter MG , Sharov AA , Ko MS. 2004. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell 6:117–131 [DOI] [PubMed] [Google Scholar]
- 87. Banerjee P , Fazleabas AT. 2010. Endometrial responses to embryonic signals in the primate. Int J Dev Biol 54:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dominguez F , Yáñez-Mó M , Sanchez-Madrid F , Simón C. 2005. Embryonic implantation and leukocyte transendothelial migration: different processes with similar players? FASEB J 19:1056–1060 [DOI] [PubMed] [Google Scholar]
- 89. Horcajadas JA , Mínguez P , Dopazo J , Esteban FJ , Domínguez F , Giudice LC , Pellicer A , Simón C. 2008. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab 93:4500–4510 [DOI] [PubMed] [Google Scholar]
- 90. Haouzi D , Assou S , Mahmoud K , Tondeur S , Rème T , Hedon B , De Vos J , Hamamah S. 2009. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Hum Reprod 24:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Haouzi D , Assou S , Dechanet C , Anahory T , Dechaud H , De Vos J , Hamamah S. 2010. Controlled ovarian hyperstimulation for in vitro fertilization alters endometrial receptivity in humans: protocol effects. Biol Reprod 82:679–686 [DOI] [PubMed] [Google Scholar]
- 92. Stewart CL , Kaspar P , Brunet LJ , Bhatt H , Gadi I , Köntgen F , Abbondanzo SJ. 1992. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359:76–79 [DOI] [PubMed] [Google Scholar]
- 93. Brinsden PR , Alam V , de Moustier B , Engrand P. 2009. Recombinant human leukemia inhibitory factor does not improve implantation and pregnancy outcomes after assisted reproductive techniques in women with recurrent unexplained implantation failure. Fertil Steril 91:1445–1447 [DOI] [PubMed] [Google Scholar]
- 94. Boivin J , Bunting L , Collins JA , Nygren KG. 2007. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 22:1506–1512 [DOI] [PubMed] [Google Scholar]
- 95. Gardner DK. 2000. Blastocyst culture: toward single embryo transfers. Human Fertility 3:229–237 [DOI] [PubMed] [Google Scholar]
- 96. Noyes RW , Hertig AT , Rock J. 1975. Dating the endometrial biopsy. Am J Obstet Gynecol 122:262–263 [DOI] [PubMed] [Google Scholar]
- 97. Smyth GK. 2004. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3:Article3 [DOI] [PubMed] [Google Scholar]
- 98. Zhang P , Dixon M , Zucchelli M , Hambiliki F , Levkov L , Hovatta O , Kere J. 2008. Expression analysis of the NLRP gene family suggests a role in human preimplantation development. PLoS One 3:e2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cline MS , Smoot M , Cerami E , Kuchinsky A , Landys N , Workman C , Christmas R , Avila-Campilo I , Creech M , Gross B , Hanspers K , Isserlin R , Kelley R , Killcoyne S , Lotia S , Maere S , Morris J , Ono K , Pavlovic V , Pico AR , Vailaya A , Wang PL , Adler A , Conklin BR , Hood L , Kuiper M , Sander C , Schmulevich I , Schwikowski B , Warner GJ , Ideker T , Bader GD. 2007. Integration of biological networks and gene expression data using cytoscape. Nat Protoc 2:2366–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]