Abstract
The xenosensing constitutive androstane receptor (CAR) is widely considered to have arisen in early mammals via duplication of the pregnane X receptor (PXR). We report that CAR emerged together with PXR and the vitamin D receptor from an ancestral NR1I gene already in early vertebrates, as a result of whole-genome duplications. CAR genes were subsequently lost from the fish lineage, but they are conserved in all taxa of land vertebrates. This contrasts with PXR, which is found in most fish species, whereas it is lost from Sauropsida (reptiles and birds) and plays a role unrelated to xenosensing in Xenopus. This role is fulfilled in Xenopus by CAR, which exhibits low basal activity and pronounced responsiveness to activators such as drugs and steroids, altogether resembling mammalian PXR. The constitutive activity typical for mammalian CAR emerged first in Sauropsida, and it is thus common to all fully terrestrial land vertebrates (Amniota). The constitutive activity can be achieved by humanizing just two amino acids of the Xenopus CAR. Taken together, our results provide a comprehensive reconstruction of the evolutionary history of the NR1I subfamily of nuclear receptors. They identify CAR as the more conserved and remarkably plastic NR1I xenosensor in land vertebrates. Nonmammalian CAR should help to dissect the specific functions of PXR and CAR in the metabolism of xeno- and endobiotics in humans. Xenopus CAR is a first reported amphibian xenosensor, which opens the way to toxicogenomic and bioaugmentation studies in this critically endangered taxon of land vertebrates.
Efficient disposition of natural and anthropogenic xenobiotics (exogenous chemicals) is essential to survival and reproduction of animals. In humans it additionally affects the therapeutic efficacy of drug treatments and may prevent or cause toxicities. Transcriptional activation of xenobiotic metabolizing enzymes and transporters, predominantly in the liver and small intestine, constitutes the most important adaptive response to xenobiotics exposure. The involved transcription factors are activated by xenobiotics and are therefore collectively referred to as “xenosensors.” The most important human xenosensors are the pregnane X receptor (hsPXR, NR1I2) and constitutive androstane receptor (hsCAR, NR1I3). Together with the vitamin D receptor (VDR), which plays no role in xenosensing in humans (1), CAR and PXR form the NR1I subfamily of nuclear receptors. In addition to activators, CAR and PXR partly share DNA-binding elements and thereby gene targets, including phase I cytochrome P450 such as CYP3A4, CYP2B6, and CYP2C, phase II transferases such as UDP glucuronosyltransferase and glutathione-S-transferase (GST), and drug transporters (2, 3).
In addition to xenobiotic disposition, PXR and especially CAR are increasingly implicated in the metabolism of carbohydrates, lipids, steroids, and bile acids, some of which constitute activators of these receptors (4). In consequence, hsPXR and hsCAR constitute potential pharmacological targets for a wide range of diseases such as dyslipidemia, diabetes, or cholestasis, in addition to transient modulation of drug pharmacokinetics to improve treatment outcomes and reduce drug toxicities. However, the development of pharmacological modulators of hsPXR and hsCAR will require a much better understanding of their specific activation modes and physiological roles. Especially complex is the function of CAR, usually referred to as a constitutively active protein. There is compelling evidence that this activity may reflect artificial translocation of CAR to the nucleus in cell lines, whereas in vivo CAR is confined to the cytosol (2). Indeed, in contrast to PXR ligands, most CAR activators probably do not bind CAR but trigger its translocation to the nucleus indirectly, via a still unknown upstream mechanism (2).
In addition to biochemical analyses (5–7) and studies of humanized and knockout mice (6, 8), a better characterization and differentiation of PXR and CAR functions can be expected from a more exhaustive reconstruction of NR1I evolution. A recent phylogenetic analysis has linked the origin of all three NR1I genes to two rounds of whole-genome duplications (WGD) in early vertebrates (9). This is fully consistent with the identification of VDR genes in the jawless fish sea lamprey (see Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org for binomial names) (1), in bony fishes, amphibians, birds, and mammals (10). In contrast, PXR genes have thus far been reported only in bony fishes, amphibians, and mammals. Even more strikingly, unequivocal CAR genes have been reported exclusively in mammals, and CAR is commonly described as having arisen from PXR in this class of vertebrates (11, 12). Unclear is the orthology status of the chicken X receptor (CXR), protein sequence of which is similarly distant from mammalian CAR and PXR proteins (12).
The relationships between PXR and CAR are additionally complicated by the diversification of their function. Thus, the zebrafish PXR is activated by a subset of human PXR agonists (10, 13), but it does not affect the transcription of CYP3A genes, suggesting substantial differences in the architecture and regulation of xenobiotic response between mammals and other vertebrates. Amphibian PXR (14, 15), termed “BXR” (benzoate X receptor) due to the pronounced benzoate responsiveness, seem to play roles in reproduction and development, but not in xenobiotic disposition, as judged from the activator spectra and tissue expression (16–19). Inexplicably, these relationships leave amphibians and reptiles, i.e. land vertebrates (tetrapods) with highest species extinction rates (20), devoid of a broad-spectrum, NR1I-class xenobiotic sensor. In the following text we provide evidence for the origin of all three NR1I genes via WGD in early vertebrates, which was followed by repeated losses of CAR and PXR. As a result of these losses, CAR is more conserved in land vertebrates than PXR and, thanks to its remarkable functional plasticity, serves as a PXR-like xenosensor in amphibians.
Results
Differential conservation of CAR and PXR genes in land vertebrates and bony fishes
All mammalian CAR genes are flanked by TOMM40L and PCP4L1 genes (Fig. 1 and Supplemental Table 2). We inspected homologous microsyntenic loci from amphibians, reptiles, and birds represented by Western clawed frog (Xenopus tropicalis), green anole lizard (Anolis carolinensis), and the chicken, respectively. CAR orthologs were found adjacent to TMM40L in all three species. The chicken gene is identical to the previously described CXR (21), which is thus a CAR ortholog. The exon-intron structure is conserved between mammalian and nonmammalian genes (data not shown). Neither such microsynthenic loci nor CAR genes at other loci were detected in the four available genomes of bony fishes. Likewise, we found no evidence for CAR genes in the unfinished genomes of the elephant shark (22) and of sea lamprey.
Fig. 1.
Schematic representation of PXR and CAR synthenic regions in 13 vertebrate species. PXR and CAR genes are represented by black arrows and flanking genes are depicted by gray arrows. Chicken CAR and frog PXR are identical to the previously described CXR (21) and BXR (17), respectively.
The PXR-containing genomic region POPDC2-COX-17-C3ORF15-PXR-GSK3B-GPR156 is remarkably conserved in all investigated bony fishes and land vertebrates (Fig. 1 and Supplemental Table 2). PXR genes were found in this region in all bony fishes but the stickleback, and in X. tropicalis. In the chicken, lizard, and stickleback, C3ORF15 genes were found immediately adjacent to GSK3B (Fig. 1). This is consistent with two independent losses of PXR, one in the stem lineage of reptiles and birds, and another one in bony fishes. In addition, a C3ORF15-PXR-GSK3B region containing gaps was found in the unfinished elephant shark sequence (data not shown). Consistent with a previous report (1), we found no PXR in the sea lamprey.
These microsynteny-based results suggested CAR emergence in land vertebrates, which was inconsistent with a recent phylogenetic study linking the origin of all NR1I genes to WGD in early vertebrate evolution (9). We therefore subjected all three human NR1I loci to a macrosynteny analysis, which detects and times duplications based on large-scale conservation of paralogs with known duplication time points (23). Six pairs of genes duplicated in the most recent common ancestor of land vertebrates and fishes (Euteleostomi) were found to be shared between a 71-gene human PXR region and an 87-gene CAR region (Fig. 2 and Supplemental Table 3). Similarly, two pairs of Eutelostomi genes were shared between PXR and VDR regions of 36 and 19 genes, respectively. Such pairs of linked duplicated genes arose via a single duplication, and the intervening genes may have undergone subsequent insertions, deletions, and rearrangements (23). These results demonstrate the existence of all three NR1I loci in the most recent common ancestor of land vertebrates and fishes. We also found evidence for the subsequent loss of CAR in bony fishes represented by medaka (Fig. 2) and stickleback (Supplemental Table 3). Thus, both species contain macrosynteny CAR regions containing apparent orthologs of the MPZ gene found in CAR loci in land vertebrates (Fig. 1), but no CAR genes (Fig. 2 and Supplemental Table 3). In contrast, the VDR macrosynteny regions on medaka chromosomes 7 and 5 contain VDR genes, whereas one of the two PXR macrosynteny regions (on chromosome 21) contains a PXR gene (Fig. 2 and Supplemental Table 3). These results are consistent with a further WGD in the stem lineage of bony fishes (24), followed by the retention of both VDR, and loss of one PXR and of both CAR genes.
Fig. 2.
Macrosynteny among human PXR, CAR, and VDR gene regions and between human and medaka orthologous regions. Human chromosomes (vertical bars) and genes (dots) are in black. Medaka chromosomes and genes are in gray. PXR, CAR, and VDR genes are depicted as red dots in either species. For clarity, only genes with paralogs or orthologs in one of the other presented loci are shown. The approximate number of all genes in the depicted regions can be estimated using the y-axis. The red boxes frame the regions corresponding to the PXR and CAR microsyntenic loci shown in Fig. 1. Black lines connect human paralogs duplicated before the split of bony fishes and land vertebrates. Gray lines connect human and medaka orthologs. Dashed portions of medaka vertical bars indicate large intervening, nonorthologous regions. Chr, Chromosome.
Cloning and expression of nonmammalian CAR
Protein-coding cDNA regions of the putative nonmammalian CAR genes were amplified and subcloned from liver cDNA samples using PCR primers derived from genomic sequences. Twenty positive subclones were sequenced for each species, confirming single CAR genes in A. carolinensis and X. tropicalis, in the following referred to as “acCAR” and “xtCAR.” The 3′-portion of the xtCAR cDNA, including the stop codon predicted by the genomic sequence, is supported by expressed sequence tags (ESTs) DT443910.1 and EL657630.1. Consistent with its tetraploid genome (25), two CAR genes, xlCARa and xlCARb, were amplified from X. laevis. xlCARa was represented by 18 of 20 clones. The first 177 amino acids of the putative xlCARa protein are supported by the EST EB462285.1 and the last 115 amino acids, followed by a stop codon, are supported by EB480326.1. The first 198 amino acids of xlCARb are supported by EST BP707212. In all new CAR genes the putative first methionines are preceded by Kozak sequences (data not shown). Neither EST- nor genomic sequence-derived short upstream open reading frames contain alternative translation initiation sites (data not shown). We found no evidence of further upstream protein-coding exons in the available EST sequences, in agreement with the situation in mammals.
One xlCARa clone contained an insertion of 25 bp whereas two acCAR clones contained a 4-bp deletion in exon 7. No variants were found among the sequenced xtCAR clones, altogether suggesting a low degree of alternative splicing of nonmammalian CAR. The remaining clones encoded putative proteins of 403–425 amino acids, depending on the species (Supplemental Figs. 1 and 2). The larger sizes of nonmammalian CAR are largely caused by their longer N termini and by the presence of interhelical domains between helices 1 and 3. The latter domains are similar in location, although not in sequence, to those found in all reported PXR and VDR proteins. Similarly to these receptors (26), the interhelical domains of nonmammalian CAR display high degrees of intrinsic disorder (data not shown). The C termini of nonmammalian CAR proteins are similar in length to those described in mammals. The new CAR coding sequences were deposited under GenBank accession nos. HM117644–HM117647. When transcribed and translated in vitro, all cDNA yielded similar amounts of proteins of the expected sizes (Supplemental Fig. 3).
Both xlCAR genes are expressed in the digestive tract, liver, kidney, and the skin (Fig. 3). In addition, xlCARa is detectable in the ovary, brain, and heart, suggesting a more ubiquitous expression. acCAR is detected in the intestine, liver, lung, and testes. Green anole and Xenopus CAR genes are thus expressed in tissues most directly exposed to environmental and nutritional xenobiotics. The additional expression of acCAR and xlCARa in reproductive organs and of xlCARa in the brain and heart is suggestive of functions unrelated to xenobiotics disposition.
Fig. 3.
Organ expression of X. laevis and A. carolinensis CAR. Expression of CAR gene transcripts in selected organs of X. laevis and A. carolinensis was investigated by RT-PCR. 18S RNA was used as a reverse transcription and PCR amplification control.
Phylogenetic analyses
Analyzing vertebrate NR1I genes based on an alignment of 312 amino acids using maximum likelihood and Bayesian inference methods produced topologies similar to previously reported ones (1, 10). Consistent with the results of the synteny analyses, CAR and PXR genes form two separate monophyletic groups (Fig. 4 and Supplemental Fig. 4). The support for the former group is stronger than for the latter one, as indicated, respectively, by bootstrap probabilities (BP) of 99 and 75 (Fig. 4) and by posterior probabilities (PP) of 1.00 and 0.92 (Supplemental Fig. 4). The unequivocal placement of Xenopus, lizard, and bird CAR (including chicken CXR) together with mammalian CAR genes confirms their identities as CAR orthologs. In addition, all vertebrate VDR/PXR/CAR genes form a strongly supported group (BP: 85; PP: 0.99; Fig. 4 and Supplemental Fig. 4) without sea squirt NR1K and human LXR (liver X receptor) and FXR (farnesoid X receptor). This result, taken together with the strongly supported grouping of all PXR and CAR genes without VDR genes (BP: 99; PP: 1; Fig. 4 and Supplemental Fig. 4), suggest that the vertebrate NR1 gene family expanded via a VDR-PXR/CAR duplication followed by a PXR-CAR duplication.
Fig. 4.
Maximum-likelihood tree of vertebrate NR1I genes. The sea squirt NR1K and human LXRα and FXR genes were used as outgroup. The losses (X) of PXR in birds and reptiles represented by the chicken, and of CAR in fishes, are shown in gray. The bootstrap values for each group of genes from the same clade of animals are shown above the corresponding branches. See Supplemental Fig. 4 for detailed bootstrap values for each node and for a Bayesian tree.
Low basal activity of amphibian CAR and its determinants
Basal CAR activities were first assessed in the human colon adenocarcinoma LS174T cells (27, 28) toward enhancer/promoter reporter gene constructs derived from the well-characterized hsCAR- and hsPXR-responsive human genes CYP2B6 (29), CYP3A4 (27), and MDR1 (28). In agreement with earlier observations, hsCAR exhibited high constitutive activity toward all promoter constructs tested, as did anole and chicken CAR (Fig. 5A and Supplemental Fig. 5A). In contrast, Xenopus CAR displayed low constitutive activities irrespective of the promoter (Fig. 5A and Supplemental Fig. 5A). xlCARa was less active than hsCAR also in the X. laevis-derived cell line XLK-WG (30) (Supplemental Fig. 5B). Low basal activity of Xenopus CAR was unrelated to the intracellular distribution because, similarly to hsCAR, green fluorescent protein (GFP)-tagged xlCARa accumulated in the nuclei of LS174T cells (Supplemental Fig. 5C). GFP tagging had no effect on the transcriptional activity of these receptors (data not shown).
Fig. 5.
The determinants of interspecies differences in CAR activities. A, Basal CAR activities of the indicated nonmammalian CAR toward the CYP2B6 promoter in comparison with hsCAR. B, RXRa-dependent binding of wild-type CAR proteins to the proximal ER6 of CYP3A4. The amounts of CAR proteins were equal in all lanes. The probe fractions bound by CAR-RXRa heterodimers in the individual lanes are shown relative to the fraction bound by hsCAR designated as 1. C, Constitutive and exogenous ligand-dependent activities of CAR toward hsRXRa (left panel) and toward hsPGC1a in the presence or absence of RXRa (right panel) tested in two-hybrid assays (Arte, artemisinin; Andro, androstanol; Preg, pregnenolone). The activity of pGL3–G5 promoter in cells transfected with empty pVP16-AD vector (AD) and treated with DMSO only was designated as 1. D, Basal (left panel) and drug-induced (right panel) activities of wild-type and mutant xlCARa toward the DR4-TK promoter in comparison with hsCAR. All numerical data are presented as means ± sem. Statistically significant differences (P < 0.05) are indicated by asterisks.
As expected, all CAR bound in EMSA to PXR/CAR response elements derived from the aforementioned promoters in an retinoid X receptor-α (RXRa)-dependent manner. The binding of Xenopus CAR was much weaker in comparison to hsCAR and acCAR (Fig. 5B and Supplemental Fig. 6A). To clarify whether this was responsible for the lower basal activity of the amphibian receptors, we replaced the DNA-binding domain (DBD) of xlCARa and -b with the DBD of hsCAR. This strengthened binding of the chimeric proteins to DR4 binding element of MDR1, even exceeding the level observed with hsCAR, but the low basal activity of the chimeric proteins toward this response element remained unchanged (Supplemental Fig. 6B). This suggested that the low basal activity of Xenopus CAR was determined by their ligand-binding domains (LBD) rather than DBD. To address this hypothesis, LBD of the individual CAR fused to the activation domain of VP16 were expressed in COS cells together with fusion proteins of GAL4-DBD with the LBD of hsRXRa, or with the receptor interaction domain of the coactivator of nuclear receptors hsPGC1a. Xenopus CAR-LBD indeed showed weaker constitutive two-hybrid interactions than hsCAR- and acCAR-LBD toward each one of these proteins (Fig. 5C). Interactions between Xenopus CAR and hsPGC1a were observed only in the presence of RXRa. Exposure to the established NR1I ligands artemisinin, androstanol, and pregnenolone increased 3- to 7-fold the interactions of Xenopus CAR LBD with RXRa and PGC1a as compared with dimethylsulfoxide (DMSO). acCAR responded more weakly (<2-fold increase) and hsCAR responded not at all to these activators (Fig. 5C). The weak basal activity and strong response of amphibian CAR to these chemicals was not an artifact due to the use of the human (i.e. heterologous) RXR-LBD, as similar results were obtained with xtRXRa (Supplemental Fig. 6C). The ligand- and RXRa-dependent recruitment of coactivators by Xenopus CAR was not limited to PGC1a, because it was also seen with hsSRC1 and xtSRC1 (data not shown).
The combination of low basal activity and pronounced activator responsiveness displayed by Xenopus CAR strongly resembled the recently reported Phe243Ala mutant of hsCAR (31). Humanizing the corresponding xlCARa amino acid 316 (Met316Phe mutant) increased 5-fold the basal activity of xlCARa (Fig. 5D, left panel). A similar, 3-fold increase was elicited by the humanizing Met272Val mutant (Fig. 5D, left panel), expected to enhance the interactions between the helices 5 and 12. A double Met272Val-Met316Phe mutant surpassed the basal activity of hsCAR (Fig. 5D, left panel). For all three mutants the increased basal activity was accompanied by diminished responsiveness of xlCARa to chemically diverse activators, with that of the double mutant being indistinguishable from hsCAR (Fig. 5D, right panel).
CAR activator spectra
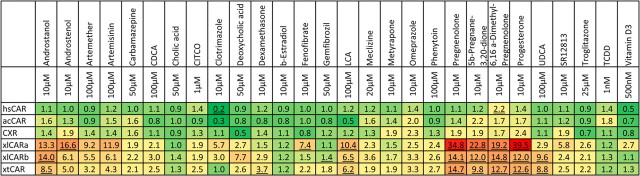
CAR activation by drugs, steroids, and bile acids was first determined in LS174T cells transfected with the individual full-length CAR and the MDR1-derived DR4 cloned as a tandem repeat upstream of a thymidine kinase promoter (28). Due to the substantial differences in CAR basal activities, some of the results are presented in Supplemental Table 4 as normalized firefly luciferase activity, in addition to fold changes shown in Fig. 6. Xenopus CAR responded to more compounds than the other CAR, and they showed much higher fold changes in activity. The highest, 40-fold activation was observed with xlCARa in response to progesterone (Fig. 6). EC50 values were determined for six diverse strong activators of xlCARa, and they were (in μm concentration): androstenol, 8.5; artemisinin, more than 60; fenofibrate, more than 10; lithocholic acid (LCA), 75; pregnenolone, 1.4; and progesterone, 0.1.
Fig. 6.
Heat map of CAR-dependent induction of the DR4-TK promoter by 28 potential activators. The numbers indicate mean fold inductions over vehicle (DMSO)-treated LS174T cells transfected with the indicated CAR. Statistically significant inductions (P < 0.05) are underlined. Suppression and activation are marked with increasing intensities of green and red, respectively.
In agreement with two-hybrid data shown in Fig. 5C, higher fold-change activation values of Xenopus CAR were accompanied by lower basal activities, whereas maximal levels of induced firefly luciferase were similar for all receptors tested (Supplemental Table 4). For hsCAR and xlCARa, these results were verified and confirmed in XLK-WG cells (Supplemental Table 5), and in the two-hybrid assay with hsRXRa in COS7 cells. xlCARa fold activation values from the latter assay were plotted against corresponding values from Fig. 6. This revealed excellent concordance for most inducing chemicals, including the steroids androstanol, androstenol, three pregnanes, and the pregnane derivative progesterone, artemether, artemisinin, SR12813, and fenofibrate (Fig. 7). The only discrepancies were that in the two-hybrid assay LCA was inactive toward xlCAR, whereas clotrimazol was particularly active.
Fig. 7.
xlCARa activation in LS174T cells compared with two-hybrid assay in COS1 cells. xlCAR activity data toward the DR4-TK promoter (from Fig. 6) are plotted against data on interaction between xlCAR-LBD and RXRa in response to the same 28 activators.
Hepatic targets of Xenopus CAR
To identify physiological targets of the Xenopus CAR genes, we investigated global gene expression changes in livers of X. laevis treated on two consecutive days with artemisinin or pregnenolone, identified above by two different methods as structurally different activators of Xenopus CAR (Figs. 6 and 7). Treatment-induced gene expression changes were determined by comparing treated with untreated animals. To minimize the contribution of genes altered in their expression via CAR-independent mechanisms, we considered only genes similarly affected by either activator and changed at least 2-fold by one activator and at least 5-fold by the other one. This resulted in eight up-regulated and 11 down-regulated genes (Table 1). The former group contained three genes belonging to phase I–III gene families AKR1C, GSTP, and SLC16a. Down-regulated genes included glucose 6 phosphatase, amylase, chymotrypsin, trypsin, and elastase. The induction of the GSTP and SLC16a gene family members was verified and confirmed using SYBR Green-based quantitative PCR (Supplemental Table 6).
Table 1.
Gene expression changes in livers of female X. laevis treated with artemisinin or pregenolone
| Gene symbol | Gene name | UniGene ID | Artemisinin | Pregnenolone |
|---|---|---|---|---|
| SLC16A6 | Solute carrier family 16, member 6 | Xl.16655 | 3.0 | 19.9 |
| GSTP1 | Glutathione S-transferase P1 | Xl.54920 | 20.4 | 11.7 |
| LOC443699 | Transcobalamin II | Xl.25998 | 4.0 | 7.2 |
| PIM3 | Pim3 oncogene | Xl.23800 | 4.9 | 6.4 |
| SLC1A5 | Solute carrier family 1, member 5 | Xl.75429 | 6.2 | 5.6 |
| HIGD1ab | HIG1 hypoxia inducible | Xl.25757 | 5.3 | 3.5 |
| HSD15B5/AKR1C3 | Hydroxysteroid (17β) dehydrogenase 5 | Xl.14135 | 31.2 | 3.2 |
| PTGS2/COX2 | Cyclooxygenase 2 | Xl.78035 | 14.7 | 3.0 |
| CPA1 | Carboxypeptidase A1 | Xl.73839 | 0.2 | 0.2 |
| RAB11-FIP3 | RAB11 family-interacting protein 3 | Xl.3719 | 0.5 | 0.2 |
| G6PC | Glucose-6-phosphatase | Xl.5979 | 0.2 | 0.1 |
| TRY | Trypsin | Xl.18278 | 0.1 | 0.1 |
| NAT | N-acetyltransferase | Xl.14656 | 0.5 | 0.1 |
| AMY2A | α-Amylase 2A | Xl.9243 | 0.1 | 0.1 |
| CELA3B | Chymotrypsin-like elastase 3B | Xl.2956 | 0.1 | 0.1 |
| CTRC | Chymotrypsin C (caldecrin) | Xl.23779 | 0.0 | 0.1 |
| CELA1 | Chymotrypsin-like elastase 1 | Xl.3275 | 0.0 | 0.0 |
| CELA3A | Chymotrypsin-like elastase family 3A | Xl.4030 | 0.0 | 0.0 |
| LOC495461 | MHC class II DR α | Xl.4415 | 0.5 | 0.0 |
Data are presented as fold changes compared with solvent-treated controls. Only genes similarly affected by either activator and changed at least 2-fold by one activator and at least 5-fold by the other one are included. ID, Identification; MHC, major histocompatability complex.
Discussion
The origin of NR1I genes via WGD in early vertebrates
Consistent with a previous analysis (9), our phylogenetic data suggest that the PXR-CAR duplication took place after the split of sea squirt and vertebrates but before that of fishes and land vertebrates. This time point is further corroborated by the presented macrosynteny data. Macrosynteny analyses detect duplications based on large-scale conservation of paralogs with known duplication time points (23). For all three NR1I genes we have identified far-flanking genes with paralogs that originated before the split of fishes and land vertebrates. Crucially, pairs of such paralogs flank more than one NR1I gene. This is consistent with the origin of all three NR1I paralogs from an ancestral NR1I gene (32) before the separation of fishes and land vertebrates. The large sizes of the syntenic regions strongly suggest WGD to be the mechanism underlying NR1I gene duplications. This interpretation is in agreement with a recent genome-wide scan for WGD-derived genes, which lists NR1I genes as WGD-derived, but provides no supporting data (33). These results place CAR origin to the stem lineage of vertebrates, i.e. some 300 million years earlier than their hitherto assumed origin in the stem lineage of mammals (11, 12).
Typically for WGD-derived genes (33), NR1I genes underwent no further duplications, with the exception of the retention of two VDR copies resulting from a WGD in bony fishes (24). In contrast, CAR underwent at least one and PXR underwent at least three independent losses in vertebrate evolution. Because whole-genome sequences are currently available only for bony fishes, it is unclear whether the CAR gene loss detected on medaka chromosome 17 occurred in this taxon or earlier, i.e. during the differentiation of ray-finned fishes (Actinopterygii). The same applies to the PXR loss from the locus on medaka chromosome 20. In contrast, the loss of the second PXR gene copy in the stickleback appears to be species specific (Fig. 1). The third loss of PXR took place in a common ancestor of reptiles and birds (Sauropsida). Further losses may have occurred in the lineages leading to lamprey, which lacks both PXR and CAR, and to elephant shark, which lacks CAR. Alternatively, these apparent losses may reflect the incompleteness of the currently available genomic sequences from these species (22, 34).
Leaving aside the nonxenosensing Xenopus BXR/PXR, CAR is thus the predominating xenosensor in land vertebrates and PXR in fishes. An exception are mammals, which utilize both receptors as xenosensors (3). Because mammalian CAR additionally generate functionally distinct splice variants such as hsCAR3 (35, 36), mammals probably possess the most complex and functionally versatile set of NR1I receptors of all vertebrates. It is tempting to speculate that this complexity enhanced the mammalian ability to adapt to different environments. Lastly, we note that among land vertebrates, only mammals express a bile acid-sensing PXR (13). The composition of bile acids as a major factor driving PXR evolution (37) may therefore need to be reassessed.
The xenosensing function of amphibian CAR
In nonmammalian land vertebrates, most evidently in amphibians, the xenosensing function may be fulfilled by CAR. We investigated Xenopus CAR in more detail because of their unusual, PXR-like properties but also because of the current amphibian extinction rate, which is 200 times higher than the mean extinction rate for all species over the last 350 million years (38). The environmental pollution likely contributes to amphibian extinction (39), but the underlying receptors and downstream pathways are unknown. The identification of CAR as a first Xenopus xenosensor provides a starting point to investigate this issue on a molecular level. Conversely, targeted stimulation of xenobiotic disposition could supplement bioaugmentation strategies such as those currently tested in some endangered amphibian species (40).
Xenopus CAR respond to typical activators of mammalian CAR, such as artemisinin, but also to PXR activators such as pregnanes (3). Taken together with xlCAR expression in the ovary and testis, the pronounced responsiveness to sex steroids and their precursors suggests a regulatory role in the latter ones' homoestasis. Consistent with their nuclear localization upon transfection, Xenopus CAR are irresponsive to the indirect (cytoplasmatic) activator phenytoin (41). Clotrimazol has been variously reported as an hsCAR agonist (42) as well as an inverse agonist (43), and similarly contradictory responses to this chemical are seen in our CAR panel.
At a first glance, the wide activator spectrum of Xenopus CAR seems to be related to the presence of a PXR-like interhelical domain between H1 and H3. This domain has been suggested to underlie PXR promiscuity by expanding the ligand-binding pocket (3). However, similar interhelical domains are contained in the much less activator-responsive acCAR and chicken CAR (CXR), as well as in the even more ligand-restricted VDR. Conversely, CAR3 shows a wide activator spectrum (44) and lacks such a domain. This suggests that the importance of the H1–H3 interhelical domain in the determination of PXR and CAR activator spectra may require reassessment and further studies.
In contrast to most mammalian CAR (44), the activation of Xenopus CAR seems to be translocation independent, because these receptors accumulate in the nucleus already in the absence of activators. This is also supported by two-hybrid experiments. Both VP16 and GAL4 fusion proteins contain nuclear localization sequences and yet high activity is observed only upon exposure to exogenous compounds. This establishes Xenopus CAR as a much-needed model to investigate CAR activation unrelated to translocation from the cytoplasm to the nucleus.
To determine xlCAR gene targets in Xenopus livers, we applied two structurally different activators and considered only genes similarly and strongly affected by either chemical. Despite these precautions, the possibility of the involvement of a different receptor cannot be fully excluded. The genes induced by CAR in Xenopus livers comprise several phase I–III proteins. Some of them possess mammalian orthologs regulated by NR1I. This applies to the phase II enzyme GSTP1 (45) and the phase I enzyme human hydroxysteroid (17-β) dehydrogenase 5/AKR1C, which in mammals detoxifies xenobiotic-derived aldehydes and ketones (2, 46). In contrast, we found no induction of the classical mammalian phase I NR1I targets CYP3A and CYP2B. This is consistent with the absence of the apparently essential proximal ER6 PXR/CAR response elements from nonmammalian CYP3A promoters (47). Taken together with the observation that zebrafish CYP3A inducers PCN and rifampin do not activate zebrafish PXR (48), the induction of nonmammalian CYP3A may be mediated by NR1I-independent mechanisms.
The constitutive CAR activity in Amniota
The low basal activity and strong response to chemicals of Xenopus CAR contrast with the constitutive activity and weak activator response of Amniota (reptiles, birds, and mammals) CAR, except the human splice variant CAR3 (44). Xenopus CAR may have secondarily acquired PXR-like characteristics to compensate the loss of xenosensing by BXR/PXR. A more parsimonious alternative is that the high constitutive activity seen in Amniota CAR arose after the separation from the amphibian lineage. The activity switch may have involved a limited number of mutations, because it can be achieved by humanizing just two amino acids of xlCARa. In a homology model based on the xlCARa LBD, to be published elsewhere, both mutated amino acids are part of the ligand-binding pocket. Met272 is located at the LBD-helix 12 interface on helix 5 whereas Met316 is part of helix 7 (Fig. 8). Mutation Met272Val is expected to increase van der Waals interactions with amino acids of helix 12, because the branched side chain of valine provides more interaction points compared with the elongated methionine, predicted to point into the ligand-binding pocket. This may place helix 12 in an active conformation typical for mammalian CAR (3). The Met316Phe mutant probably stabilizes the orientation of helix 7, which is expected to limit the flexibility of the neighboring helix 10/11. This, in turn, is expected to enhance interactions with helix 12, further contributing to its active conformation. This explanation is consistent with molecular dynamics simulations of an hsCAR LBD homology model performed to explain the diminished basal activity found in several single-amino acid mutants (49). Therein, a substitution of phenylalanine on the corresponding hsCAR amino acid position 243 with alanine diminished van der Waals interactions between Tyr326 on helix 10/11 and Leu343 on helix 12, which are considered crucial for basal activity. These observations have been subsequently verified in vitro using a Phe243Ala mutant of hsCAR (31).
Fig. 8.
X-ray crystal structure of human CAR (PDB code 1xv9). The protein backbone is shown as ribbon. The bound agonist pregnanedione is depicted in spacefill representation. Amino acids humanized in xlCARa are shown as capped sticks. The corresponding amino acids in wild-type xlCARa are given in brackets. Parts of the structure have been removed for the sake of clarity.
Because constitutive CAR activity is found exclusively in Amniota, it is tempting to speculate that it may have been important for the acquisition of a fully terrestrial life style. Although walking and running require approximately 10 times more energy than swimming, Amniota are capable of much stronger locomotion bursts than fishes and amphibians. This is enabled by a higher glycolytic capacity (50). Remarkably, CAR regulates the metabolism of glucose and ketones, in agreement with the mounting evidence for its involvement in metabolic processes beyond xenobiotic response (4). The relevant CAR gene targets common to amphibians (Table 1) and mammals include the key regulator of blood glucose glucose 6-phosphatase (4), AKR1C, which detoxifies endobiotic ketones (2), and SLC16A, which transport lactate, pyruvate, ketones, and ketoacids (51). Constitutive CAR activity may have increased the capacity of glycolysis and ketone utilization in land vertebrates. This could be advantageous via increased burst speed and better adaptation to prolonged starvation periods. This and alternative possibilities can be now investigated using the CAR described in our study.
Materials and Methods
Genome and phylogenetic analyses
The fine-scale genomic synteny among NR1I gene loci was determined in whole-genome alignments generated using BLASTZ (52) and accessed through the Chain and Net tracks in UCSC genome browser (53). The gene contents and linkages within CAR and PXR loci were confirmed by aligning protein sequences of CAR and PXR as well as of the flanking genes to the investigated genomes using Blat (54). Considering that fine-scale synteny strongly erodes in evolution, this analysis was complemented by macrosynteny comparisons (23) among human PXR, CAR, and VDR gene regions. To this end, human paralogous gene pairs as well as human-fish orthologous gene pairs were downloaded from ENSEMBL release 63 (http://useast.ensembl.org/) together with the corresponding gene duplication age information. Human genes on chromosome 1 (CAR), 3 (PXR), and 12 (VDR) were sorted by their relative positions on the chromosomes. CAR-, PXR-, and VDR-flanking regions were manually examined for matched multigene segments duplicated on any other chromosome. Two genomic intervals (harboring ≤100 genes) were considered as products of WGD before the divergence of Actinopterygii (ancestors of fishes) and Sarcopterygii (ancestor of human), if sharing two or more pairs of genes duplicated before Actinopterygii-Sarcopterygii split and after vertebrate emergence (23). The orthologous regions between human and medaka were identified in a similar way based on human-fish orthologous gene pairs.
The 27 nuclear receptor protein sequences (Supplemental Table 1) were aligned using clustalW function implemented in BioEdit (55), and the resulting alignment was manually inspected to remove ambiguously aligned regions. The amino acid substitution model that best describes the evolution of the alignment was selected using ProtTest version 3.0 (56). The JTT+I+Γ+F model was selected as the best model. In this model, the substitution rates between pairs of amino acids are defined by the JTT substitution matrix (57). It further assumes a proportion of invariable sites in the proteins (+I) and that the rates of changes among the remaining sites follow γ-distributions (+I′). Further, the equilibrium amino acid frequencies in the model are estimated from the data (+F). Under this model, we constructed maximum-likelihood tree using PhyML version 3 (58) assuming four categories of γ-distributed discrete site classes. The support values for individual nodes were estimated with 1000 bootstrap replicates. We also implemented the model for bayesian inference using MrBayes version 3.1.2 (59) with 4 million generations in two parallel runs. Trees were sampled every 100 generations, and the consensus tree was generated after removal of the first 25% sampled trees.
Cloning, mutagenesis, and in vitro and in vivo expression of CAR genes
Liver samples were obtained from one female X. laevis and one female Anolis carolinensis. Total RNA was isolated using peqGOLD TriFast (Peqlab, Wilmington, DE). X. tropicalis liver RNA was obtained from Professor Thomas Hankeln (University Mainz). Reverse transcription was performed using the High-Capacity cDNA Reverse Transcription system (Applied Biosystems, Foster City, CA). Primers used for PCR amplification of the protein-coding regions of the individual CAR genes from liver cDNA are listed in Supplemental Table 7. PCR amplicons were subcloned into the pcDNA3 plasmid (Invitrogen, Carlsbad, CA) and sequenced. Mutants were produced with the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions and verified by sequencing. Plasmids expressing GFP-CAR fusion proteins were generated by subcloning full-length CAR into XhoI and EcoRI sites of pEGFP-C1 (CLONTECH Laboratories, Inc., Mount View, CA). Plasmids used in the two-hybrid interaction assay (Supplemental Table 7) were cloned analogously to those described elsewhere (35). The expression of all proteins was verified using the TNT T7 Quick Coupled transcription/translation system (Promega Corp., Madison, WI) either in the presence of [35S]methionine (MP Biomedicals, Irvine, CA), or of biotinylated lysine (Transcend Non-Radioactive Translation Detection Systems, Promega). [35S]-labeled proteins were resolved on a 10% SDS-polyacrylamide gel and dried, and the expression was quantified using a BAS1800II phosphor-storage scanner (Fuji, Stanford, CT) and AIDA software (Raytest, Straubenhardt, Germany). Biotinylated proteins were detected in a standard Western blot using streptavidin-horseradish peroxidase (Transcend Nonradioactive Translation Detection Systems, Promega). Primers used for semiquantitative measurement of tissue expression of the individual CAR genes by PCR (35 cycles) are listed in Supplemental Table 7. The gene specificity of primers for the two X. laevis genes was confirmed using subcloned cDNA of these genes. The expression of 18s RNA was used to detect any differences in the reverse transcription or PCR amplification efficiency among the samples.
CAR activity and localization measurements in transfected cell lines
The human colon adenocarcinoma cells LS174T (60) were grown, transfected, and processed as described previously (61), as were the African green monkey (Chlorocebus aethiops)-derived COS1 cells. The XLK-WG cell line derived from the kidney of a X. laevis (30) and obtained from American Type Culture Collection (Manassas, VA) was maintained at 32 C with 5% CO2 and RPMI-1640 medium with 2 mm l-glutamine (Sigma-Aldrich, St. Louis, MO) adjusted to contain 1.5 g/liter sodium bicarbonate, 4.5 g/liter glucose, 10 mm HEPES, and 1.0 mm sodium pyruvate and 20% fetal bovine serum. Cells were plated into 24-, 48-, or 96-well plates 24 h before transfection, which was performed using GeneJuice transfection reagent (Novagen, Madison, WI). Constructs expressing the reporter gene firefly luciferase under the control of CAR- and PXR-responsive regulatory elements of CYP2B6 (pB-1.6k/PB/XREM), CYP3A4 (pGL3-CYP3A4(-7830Δ7208–364)), and MDR1 (p-7975(Δ7012–1804)MDR, pDR4(I)2TK), were as described elsewhere (27–29). For two-hybrid assays, COS1 cells plated onto 24-well plates were transfected (per well) with 110 ng of the reporter gene plasmid pGL3-G5, 10 ng of an expression plasmid encoding GAL4-DBD/RXRα-LBD, or GAL4-DBD/coactivator-receptor interaction domain fusion protein, 80 ng of expression plasmids encoding the respective VP16-AD/CAR-LBD fusions, and 20 ng of the pCMVb per well. Induction by drugs and other compounds was started 6 h after transfection and assessed in cells lysed after another 40 h. Basal nuclear receptor activities were assessed at the same time point as ratios of firefly luciferase activities in nuclear receptor-transfected to empty vector-transfected cells. Differences in transfection efficiencies were normalized by dividing firefly luciferase activities by those of the cotransfected constitutive Renilla luciferase expression construct pRL-EF1a (62) or constitutive ß-galactosidase expression plasmid pCMVβ (CLONTECH) in two-hybrid experiments. The subcellular localization of CAR-GFP was assessed on living cells using standard laser-scanning microscopy.
To identify statistically significant differences, one-way ANOVA with Student-Newman-Keuls posttest was performed with the mean values of at least three independent experiments done in triplicates using GraphPad InStat version 3.05 for Windows 95 (GraphPad Software, San Diego, CA).
Chemicals tested for induction were: androstanol (5a-androstan-3b-ol), androstenol (5a-androst-16en-3a-ol), arteether, artemether, artemisinin, carbamazepine, chenodeoxycholic acid, cholic acid, 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime, clotrimazol, desoxycholic acid, dexamethason, β-estradiol, fenofibrate, gemfibrozil, LCA, medizine, metapyrone, omeprazol, phenytoin, pregnenolone, 5b-pregnane-3,20-dione, 6,16a-dimethyl-pregnenolone, progesterone, ursodeoxycholic acid, troglitazone, 2,3,7,8-terachloridbenzodioxin, vitamin D3 (1α25-dihydroxyvitamin D3). Chemicals were dissolved in DMSO. 6-(4-Chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime was purchased from BIOMOL Research Laboratories, Inc., and artemisinin, arteether, and artemether were kindly provided by Dafra Pharma (Turnhout, Belgium). All the other chemicals were purchased from Sigma-Aldrich. The EC50 values were determined by nonlinear regression analysis (GraphPad Prism Software) using a three-parameter dose-response curve from 10 concentrations measured as triplicates in four independent experiments.
EMSA
CAR, PXR, and RXRa proteins were expressed in vitro using the respective expression plasmids as described above; however, in the absence of any labeled amino acids. Equal volumes of TNT reactions were added to gel shift reactions. Considering the similar expression efficiencies of the individual CAR-expressing plasmids (Supplemental Fig. 3), roughly equal amounts of CAR proteins were present in all reactions. Nuclear receptor response elements were generated by annealing 1 nmol each of two complementary oligonucleotides in 25 mm Tris-Cl (pH 7.5), 25 mm NaCl, 5 mm MgCl2 in a total volume of 200 μl. Radioactive labeling was performed by incubating 10 pmol of the annealed double-stranded oligonucleotide with 2 U of Klenow fragment and 25 μCi [α-32P]dCTP in 50 mm Tris-Cl (pH 7.5), 50 mm NaCl, 10 mm MgCl2, 0.2 mm each of dATP, dGTP, dTTP in a total volume of 50 μl at 37 C for 60 min. The labeled double-stranded oligonucleotides were purified through ProbeQuant sephadex G-50 micro columns (GE Healthcare, Piscataway, NJ). Binding reactions and gel electrophoresis were performed as described elsewhere (28). Retarded complexes and free probes were quantified with the BAS1800 II phosphor-storage scanner (Fuji) and AIDA software (Raytest). The probe fractions bound by CAR-RXRa heterodimers in the individual lanes were calculated and expressed relative to the fraction bound by hsCAR designated as 1.
RNA microarray analysis
Nine adult X. laevis females with an average weight of 40 g were obtained from Professor Jürgen Markl (University Mainz). Arteminisin (200 mg/kg) or pregnenolone (50 mg/kg), each dissolved in DMSO, were injected ip into three frogs on two consecutive days. The control group received in parallel two DMSO injections. All frogs were killed 24 h after the second injection by decapitation, and livers were immediately frozen in liquid nitrogen. These procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, and they were approved by the responsible local ethics committee.
Total RNA was isolated from livers using peqGOLD TriFast (PeqLab). RNA specimens within the same experimental group were pooled and used for double-strand complementary DNA synthesis using reverse transcription (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems). Biotin-labeled cRNA was in vitro synthesized, purified, and fragmented. cRNA (15 μg) was used for hybridization to GeneChip X. laevis 2.0 arrays (Affymetrix, Santa Clara, CA). The raw data obtained from scanned chips were normalized by the robust multiarray analysis and deposited in NCBI's Gene Expression Omnibus (63) under the GEO Series accession no. GSE28510. For more stringency, only genes with expression levels changed at least 2-fold by one activator and at least 5-fold by the other one were defined as differentially expressed. For the selected genes GSTP1 and SLC16A6, microarray results were verified in RNA samples from the individual animals using a ΔΔCt variant of quantitative real-time PCR with SYBR Green (Sigma-Aldrich) and oligonucleotides given in Supplemental Table 7. 18S RNA was used for normalization. The reverse transcription for these analyses was conducted using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems).
Acknowledgments
We thank Dr. Hongbing Wang (University Maryland, School of Pharmacy, Baltimore, MD 21201) for permission to use the CYP2B6 reporter gene construct pB-1.6k/PB/XREM, Professor Jürgen Markl (Department of Zoology, Johannes Gutenberg University Mainz, 55101 Mainz, Germany) for providing the X. laevis, and Professor Thomas Hankeln (Department of Molecular Genetics, Johannes Gutenberg University Mainz, 55101 Mainz, Germany) for the X. tropicalis RNA. We thank Professor Urs A. Meyer (Biocenter, University Basel, 5056 Basel, Switzerland) and Dr. Holger Herlyn (Department of Anthropology, Johannes Gutenberg University Mainz, 55101 Mainz, Germany) for comments on the manuscript. This publication contains data to be included in the doctoral thesis of Christian Nußhag.
This work was supported by DFG grant WO505/2-2 (to L.W.) and by a DFG research training fellowship GRK1034 (to M.M.) (http://www.dfg.de).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BXR
- Benzoate X receptor
- CAR
- constitutive androstane receptor
- CXR
- chicken X receptor
- DBD
- DNA-binding domain
- DMSO
- dimethylsulfoxide
- EST
- expressed sequence tag
- FXR
- farnesoid X receptor
- GFP
- green fluorescent protein
- GST
- glutathione-S-transferase
- LBD
- ligand-binding domain
- LCA
- lithocholic acid
- LXR
- liver X receptor
- PXR
- pregnane X receptor
- RXRa
- retinoid X receptor-α
- VDR
- vitamin D receptor
- WGD
- whole-genome duplications.
References
- 1. Reschly EJ , Bainy AC , Mattos JJ , Hagey LR , Bahary N , Mada SR , Ou J , Venkataramanan R , Krasowski MD. 2007. Functional evolution of the vitamin D and pregnane X receptors. BMC Evol Biol 7:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tolson AH , Wang H. 2010. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv Drug Deliv Rev 62:1238–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. di Masi A , De Marinis E , Ascenzi P , Marino M. 2009. Nuclear receptors CAR and PXR: Molecular, functional, and biomedical aspects. Mol Aspects Med 30:297–343 [DOI] [PubMed] [Google Scholar]
- 4. Moreau A , Vilarem MJ , Maurel P , Pascussi JM. 2008. Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol Pharm 5:35–41 [DOI] [PubMed] [Google Scholar]
- 5. Milnes MR , Garcia A , Grossman E , Grün F , Shiotsugu J , Tabb MM , Kawashima Y , Katsu Y , Watanabe H , Iguchi T , Blumberg B. 2008. Activation of steroid and xenobiotic receptor (SXR, NR1I2) and its orthologs in laboratory, toxicologic, and genome model species. Environ Health Perspect 116:880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xia X , Xie Z. 2001. DAMBE: software package for data analysis in molecular biology and evolution. J Hered 92:371–373 [DOI] [PubMed] [Google Scholar]
- 7. Watkins RE , Wisely GB , Moore LB , Collins JL , Lambert MH , Williams SP , Willson TM , Kliewer SA , Redinbo MR. 2001. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science 292:2329–2333 [DOI] [PubMed] [Google Scholar]
- 8. Scheer N , Ross J , Rode A , Zevnik B , Niehaves S , Faust N , Wolf CR. 2008. A novel panel of mouse models to evaluate the role of human pregnane X receptor and constitutive androstane receptor in drug response. J Clin Invest 118:3228–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuraku S , Meyer A , Kuratani S. 2009. Timing of genome duplications relative to the origin of the vertebrates: did cyclostomes diverge before or after? Mol Biol Evol 26:47–59 [DOI] [PubMed] [Google Scholar]
- 10. Ekins S , Reschly EJ , Hagey LR , Krasowski MD. 2008. Evolution of pharmacologic specificity in the pregnane X receptor. BMC Evol Biol 8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krasowski MD , Ni A , Hagey LR , Ekins S. 2011. Evolution of promiscuous nuclear hormone receptors: LXR, FXR, VDR, PXR, and CAR. Mol Cell Endocrinol 334:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Handschin C , Blättler S , Roth A , Looser R , Oscarson M , Kaufmann MR , Podvinec M , Gnerre C , Meyer UA. 2004. The evolution of drug-activated nuclear receptors: one ancestral gene diverged into two xenosensor genes in mammals. Nucl Recept 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moore LB , Maglich JM , McKee DD , Wisely B , Willson TM , Kliewer SA , Lambert MH , Moore JT. 2002. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol Endocrinol 16:977–986 [DOI] [PubMed] [Google Scholar]
- 14. Blumberg B , Mangelsdorf DJ , Dyck JA , Bittner DA , Evans RM , De Robertis EM. 1992. Multiple retinoid-responsive receptors in a single cell: families of retinoid “X” receptors and retinoic acid receptors in the Xenopus egg. Proc Natl Acad Sci USA 89:2321–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishikawa J , Saito K , Sasaki M , Tomigahara Y , Nishihara T. 2000. Molecular cloning and functional characterization of a novel nuclear receptor similar to an embryonic benzoate receptor BXR. Biochem Biophys Res Commun 277:209–215 [DOI] [PubMed] [Google Scholar]
- 16. Heath LA , Jones EA , Old RW. 2000. Expression pattern of BXR suggests a role for benzoate ligand-mediated signalling in hatching gland function. Int J Dev Biol 44:141–144 [PubMed] [Google Scholar]
- 17. Blumberg B , Kang H , Bolado J , Chen H , Craig AG , Moreno TA , Umesono K , Perlmann T , De Robertis EM , Evans RM. 1998. BXR, an embryonic orphan nuclear receptor activated by a novel class of endogenous benzoate metabolites. Genes Dev 12:1269–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grün F , Venkatesan RN , Tabb MM , Zhou C , Cao J , Hemmati D , Blumberg B. 2002. Benzoate X receptors α and β are pharmacologically distinct and do not function as xenobiotic receptors. J Biol Chem 277:43691–43697 [DOI] [PubMed] [Google Scholar]
- 19. Smith DP , Mason CS , Jones EA , Old RW. 1994. A novel nuclear receptor superfamily member in Xenopus that associates with RXR, and shares extensive sequence similarity to the mammalian vitamin D3 receptor. Nucleic Acids Res 22:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. May RM. 2010. Ecological science and tomorrow's world. Philos Trans R Soc Lond B Biol Sci 365:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Handschin C , Podvinec M , Meyer UA. 2000. CXR, a chicken xenobiotic-sensing orphan nuclear receptor, is related to both mammalian pregnane X receptor (PXR) and constitutive androstane receptor (CAR). Proc Natl Acad Sci USA 97:10769–10774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Venkatesh B , Kirkness EF , Loh YH , Halpern AL , Lee AP , Johnson J , Dandona N , Viswanathan LD , Tay A , Venter JC , Strausberg RL , Brenner S. 2007. Survey sequencing and comparative analysis of the elephant shark (Callorhinchus milii) genome. PLoS Biol 5:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dehal P , Boore JL. 2005. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol 3:e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaillon O , Aury JM , Brunet F , Petit JL , Stange-Thomann N , Mauceli E , Bouneau L , Fischer C , Ozouf-Costaz C , Bernot A , Nicaud S , Jaffe D , Fisher S , Lutfalla G , Dossat C , Segurens B , Dasilva C , Salanoubat M , Levy M , Boudet N , Castellano S , Anthouard V , Jubin C , Castelli V , Katinka M , et al. 2004. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature 431:946–957 [DOI] [PubMed] [Google Scholar]
- 25. Hughes MK , Hughes AL. 1993. Evolution of duplicate genes in a tetraploid animal, Xenopus laevis. Mol Biol Evol 10:1360–1369 [DOI] [PubMed] [Google Scholar]
- 26. Krasowski MD , Reschly EJ , Ekins S. 2008. Intrinsic disorder in nuclear hormone receptors. J Proteome Res 7:4359–4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hustert E , Zibat A , Presecan-Siedel E , Eiselt R , Mueller R , Fuss C , Brehm I , Brinkmann U , Eichelbaum M , Wojnowski L , Burk O. 2001. Natural protein variants of pregnane x receptor with altered transactivation activity toward cyp3a4. Drug Metab Dispos 29:1454–1459 [PubMed] [Google Scholar]
- 28. Geick A , Eichelbaum M , Burk O. 2001. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem 276:14581–14587 [DOI] [PubMed] [Google Scholar]
- 29. Wang H , Faucette S , Sueyoshi T , Moore R , Ferguson S , Negishi M , LeCluyse EL. 2003. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem 278:14146–14152 [DOI] [PubMed] [Google Scholar]
- 30. Martin OC , Gunawardane RN , Iwamatsu A , Zheng Y. 1998. Xgrip109: a γ tubulin-associated protein with an essential role in γ tubulin ring complex (γTuRC) assembly and centrosome function. J Cell Biol 141:675–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jyrkkärinne J , Windshügel B , Rönkkö T , Tervo AJ , Küblbeck J , Lahtela-Kakkonen M , Sippl W , Poso A , Honkakoski P. 2008. Insights into ligand-elicited activation of human constitutive androstane receptor based on novel agonists and three-dimensional quantitative structure-activity relationship. J Med Chem 51:7181–7192 [DOI] [PubMed] [Google Scholar]
- 32. Bertrand S , Brunet FG , Escriva H , Parmentier G , Laudet V , Robinson-Rechavi M. 2004. Evolutionary genomics of nuclear receptors: from twenty-five ancestral genes to derived endocrine systems. Mol Biol Evol 21:1923–1937 [DOI] [PubMed] [Google Scholar]
- 33. Makino T , McLysaght A. 2010. Ohnologs in the human genome are dosage balanced and frequently associated with disease. Proc Natl Acad Sci USA 107:9270–9274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith JJ , Antonacci F , Eichler EE , Amemiya CT. 2009. Programmed loss of millions of base pairs from a vertebrate genome. Proc Natl Acad Sci USA 106:11212–11217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arnold KA , Eichelbaum M , Burk O. 2004. Alternative splicing affects the function and tissue-specific expression of the human constitutive androstane receptor. Nucl Recept 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Auerbach SS , Ramsden R , Stoner MA , Verlinde C , Hassett C , Omiecinski CJ. 2003. Alternatively spliced isoforms of the human constitutive androstane receptor. Nucleic Acids Res 31:3194–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krasowski MD , Yasuda K , Hagey LR , Schuetz EG. 2005. Evolution of the pregnane x receptor: adaptation to cross-species differences in biliary bile salts. Mol Endocrinol 19:1720–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roelants K , Gower DJ , Wilkinson M , Loader SP , Biju SD , Guillaume K , Moriau L , Bossuyt F. 2007. Global patterns of diversification in the history of modern amphibians. Proc Natl Acad Sci USA 104:887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hayes TB , Falso P , Gallipeau S , Stice M. 2010. The cause of global amphibian declines: a developmental endocrinologist's perspective. J Exp Biol 213:921–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harris RN , Brucker RM , Walke JB , Becker MH , Schwantes CR , Flaherty DC , Lam BA , Woodhams DC , Briggs CJ , Vredenburg VT , Minbiole KP. 2009. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J 3:818–824 [DOI] [PubMed] [Google Scholar]
- 41. Wang H , Faucette S , Moore R , Sueyoshi T , Negishi M , LeCluyse E. 2004. Human constitutive androstane receptor mediates induction of CYP2B6 gene expression by phenytoin. J Biol Chem 279:29295–29301 [DOI] [PubMed] [Google Scholar]
- 42. Mäkinen J , Frank C , Jyrkkärinne J , Gynther J , Carlberg C , Honkakoski P. 2002. Modulation of mouse and human phenobarbital-responsive enhancer module by nuclear receptors. Mol Pharmacol 62:366–378 [DOI] [PubMed] [Google Scholar]
- 43. Moore LB , Parks DJ , Jones SA , Bledsoe RK , Consler TG , Stimmel JB , Goodwin B , Liddle C , Blanchard SG , Willson TM , Collins JL , Kliewer SA. 2000. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem 275:15122–15127 [DOI] [PubMed] [Google Scholar]
- 44. Chen T , Tompkins LM , Li L , Li H , Kim G , Zheng Y , Wang H. 2010. A single amino acid controls the functional switch of human constitutive androstane receptor (CAR) 1 to the xenobiotic-sensitive splicing variant CAR3. J Pharmacol Exp Ther 332:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gong H , Singh SV , Singh SP , Mu Y , Lee JH , Saini SP , Toma D , Ren S , Kagan VE , Day BW , Zimniak P , Xie W. 2006. Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Mol Endocrinol 20:279–290 [DOI] [PubMed] [Google Scholar]
- 46. Oscarson M , Burk O , Winter S , Schwab M , Wolbold R , Dippon J , Eichelbaum M , Meyer UA. 2007. Effects of rifampicin on global gene expression in human small intestine. Pharmacogenet Genomics 17:907–918 [DOI] [PubMed] [Google Scholar]
- 47. Qiu H , Mathäs M , Nestler S , Bengel C , Nem D , Gödtel-Armbrust U , Lang T , Taudien S , Burk O , Wojnowski L. 2010. The unique complexity of the CYP3A4 upstream region suggests a nongenetic explanation of its expression variability. Pharmacogenet Genomics 20:167–178 [DOI] [PubMed] [Google Scholar]
- 48. Iyer M , Reschly EJ , Krasowski MD. 2006. Functional evolution of the pregnane X receptor. Expert Opin Drug Metab Toxicol 2:381–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Windshügel B , Jyrkkärinne J , Poso A , Honkakoski P , Sippl W. 2005. Molecular dynamics simulations of the human CAR ligand-binding domain: deciphering the molecular basis for constitutive activity. J Mol Model 11:69–79 [DOI] [PubMed] [Google Scholar]
- 50. Bennett AF. 1991. The evolution of activity capacity. J Exp Biol 160:1–23 [DOI] [PubMed] [Google Scholar]
- 51. Halestrap AP , Meredith D. 2004. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch 447:619–628 [DOI] [PubMed] [Google Scholar]
- 52. Schwartz S , Kent WJ , Smit A , Zhang Z , Baertsch R , Hardison RC , Haussler D , Miller W. 2003. Human-mouse alignments with BLASTZ. Genome Res 13:103–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hinrichs AS , Karolchik D , Baertsch R , Barber GP , Bejerano G , Clawson H , Diekhans M , Furey TS , Harte RA , Hsu F , Hillman-Jackson J , Kuhn RM , Pedersen JS , Pohl A , Raney BJ , Rosenbloom KR , Siepel A , Smith KE , Sugnet CW , Sultan-Qurraie A , Thomas DJ , Trumbower H , Weber RJ , Weirauch M , Zweig AS , Haussler D , Kent WJ. 2006. The UCSC Genome Browser Database: update 2006. Nucleic Acids Res 34:D590–D598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kent WJ. 2002. BLAT–the BLAST-like alignment tool. Genome Res 12:656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis. Nucl Acids Symp Ser 41:95–98 [Google Scholar]
- 56. Abascal F , Zardoya R , Posada D. 2005. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21:2104–2105 [DOI] [PubMed] [Google Scholar]
- 57. Jones DT , Taylor WR , Thornton JM. 1992. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8:275–282 [DOI] [PubMed] [Google Scholar]
- 58. Guindon S , Dufayard JF , Lefort V , Anisimova M , Hordijk W , Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321 [DOI] [PubMed] [Google Scholar]
- 59. Huelsenbeck JP , Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755 [DOI] [PubMed] [Google Scholar]
- 60. Tom BH , Rutzky LP , Jakstys MM , Oyasu R , Kaye CI , Kahan BD. 1976. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro 12:180–191 [DOI] [PubMed] [Google Scholar]
- 61. Burk O , Koch I , Raucy J , Hustert E , Eichelbaum M , Bröckmoller J , Zanger UM , Wojnowski L. 2004. The induction of cytochrome P450 3A5 (CYP3A5) in the human liver and intestine is mediated by the xenobiotic sensors pregnane X receptor (PXR) and constitutively activated receptor (CAR). J Biol Chem 279:38379–38385 [DOI] [PubMed] [Google Scholar]
- 62. Bros M , Ross XL , Pautz A , Reske-Kunz AB , Ross R. 2003. The human fascin gene promoter is highly active in mature dendritic cells due to a stage-specific enhancer. J Immunol 171:1825–1834 [DOI] [PubMed] [Google Scholar]
- 63. Edgar R , Domrachev M , Lash AE. 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30:207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]