Abstract
Emerging data suggest that off-target cannabinoid effects may be mediated via novel seven-transmembrane spanning/G protein-coupled receptors. Due to its cannabinoid sensitivity, the G protein-coupled receptor 55 (GPR55) was recently proposed as a candidate; however, GPR55 is phylogenetically distinct from the traditional cannabinoid receptors, and the conflicting pharmacology, signaling, and functional data have prevented its classification as a novel cannabinoid receptor. Indeed, the most consistent and potent agonist to date is the noncannabinoid lysophospholipid, lysophosphatidylinositol. Here we present new human GPR55 mRNA expression data, providing supportive evidence of GPR55 expression in a vast array of tissues and cell types. Moreover, we summarize major recent developments in GPR55 research and aim to update the reader in the rapidly expanding fields of GPR55 pharmacology, physiology, and pathology.
Classically, cannabinoid ligands interact with two seven-transmembrane spanning (7TM)/G protein-coupled receptors (GPCR); cannabinoid receptor type 1 and type 2 (CB1 and CB2); however, pharmacological data and studies exploiting knockout animals suggest that additional cannabinoid-sensitive targets exist (1, 2). Given the prominent role of the endocannabinoid system in normal and pathological conditions, there has been considerable interest in the identification of these novel targets, both within academia and the pharmaceutical industry. Indeed, it was AstraZeneca and GlaxoSmithKline (3) that first thrust G protein-coupled receptor-55 (GPR 55) into the limelight as a novel candidate. However, academic laboratories soon contributed, with the revelation that GPR55 is activated by a novel, endogenous lipid known as lysophosphatidylinositol (LPI) (4). These studies effectively assimilated the fields of cannabinoid, GPR55, and LPI research; however, they quickly conflicted, resulting in a contradictory cannabinoid pharmacology, which has been reviewed extensively elsewhere (5–10).
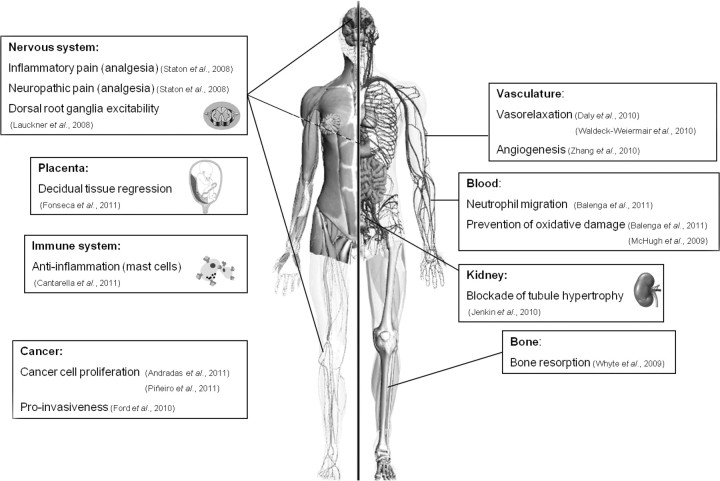
Since the seminal report by Oka et al. (4), LPI has been confirmed with ever-increasing consistency as a potent and direct agonist of GPR55. LPI was first identified in the early 1960s (11, 12), but it was not until 20 yr later that a potential signaling role was suggested, when it was shown to stimulate the release of insulin from pancreatic cells (13). In the 1980s and early 1990s, a number of papers detailing the physiology and production of LPI in various cellular systems, including cancer cell lines, were published (13–19). Interestingly, subsequent studies reinforced the idea that LPI can be released from cells, and one group proposed that blood LPI levels might be a novel biomarker for certain cancers and gynecological diseases, with LPI levels 3-fold higher in patients than controls (20–22). LPI was shown to be neuroprotective in a model of global cerebral ischemia and glutamate excitotoxicity in neuronal cultures (23). When injected 30 min before or after the onset of ischemia, LPI induced a prolonged brain tolerance to ischemic damage and pretreatment with LPI 1–3 d before ischemic challenge resulted in a 98% survival rate of CA1 pyramidal cells (23). The authors suggested the two-pore domain K+ channels TREK and TRAAK (background K+ channels) may explain the effect because they were previously shown to be sensitive to certain lysophospholipids, including LPI (24), although no direct evidence was presented to confirm this. Finally in 2007, the study by Oka et al. (4) revealed the first elusive receptor target for LPI to be GPR55. LPI-dependent GPR55 signaling is now thought to be important in a vast array of physiological and pathological processes including cancer progression, bone regulation, endothelial function, inflammation, and pain (Fig. 1). Therefore, it seems GPR55 should be described as the first recognized LPI receptor, although it undoubtedly retains some cannabinoid ligand sensitivity.
Fig. 1.
Pathophysiological relevance of GPR55 expression. Recent evidence suggests that GPR55 is involved in the control of a variety of physiological functions. In the nervous system, GPR55 regulates dorsal root ganglia excitability (49) and controls inflammatory and neuropathic pain (63). In blood, GPR55 regulates neutrophil migration (48) and may prevent oxidative damage (48, 84). GPR55 is also involved in bone metabolism, specifically inducing bone resorption (87). Other studies have suggested additional roles for GPR55 in modulating vascular function [by inducing vasorelaxation (53, 76) and controlling angiogenesis (37), renal tubule hypertrophy (109), decidual tissue regression during pregnancy (105), and mast cell-mediated antiinflammatory actions (104)]. However, additional experimental evidence is required to support these hypotheses. Besides controlling these (and most probably many other) physiological functions, GPR55 seems to play an important role in cancer progression by modulating cancer cell proliferation (52, 102) and migration (43).
Functional and pharmacological GPR55 data are emerging at an ever-increasing pace, and after some major recent developments, this review aims to summate these findings, bring the reader up to date with the rapidly expanding field of GPR55 physiology, and introduce potential therapeutic strategies exploiting current knowledge of GPR55 pathophysiology.
GPR55 structure
Human GPR55 (hGPR55; NM_005683.3) is a 319-amino acid protein and a member of the rhodopsin-like 7TM/GPCR family. hGPR55 was first isolated and cloned in 1999 and mapped to human chromosome 2q37 (25). Different sequences have been reported for hGPR55, most likely due to polymorphisms or technical errors during cloning or sequencing. So far, orthologs of GPR55 have been identified in the genomes of rat, mouse, dog, cow, chimpanzee, zebrafish, pufferfish, and humans (3, 26, 27). The closest homologs to GPR55, based on its amino acid sequence, are the purinergic receptor P2Y5 (29%, NM_005767.4), the purinoceptor-like orphan receptors GPR23 (30%, NM_005296.2), and GPR35 (27% NM_005301.2) and the chemokine receptor CCR4 (23%, NM_005508.4) (25). Interestingly GPR55 shows low sequence identity with the two traditional cannabinoid receptors CB1 (13.5%) and CB2 (14.4%).
Sequence alignment analysis with rhodopsin shows conserved patterns in transmembrane (TM)-1, 2, 4, and 5, but instead of the highly conserved DRY motif (28) in TM3, GPR55 exhibits a DRF motif. GPR55 contains a conserved proline in TM5, which is absent in CB1 and CB2. Furthermore, the highly conserved NPXXY motif found in TM7 of rhodopsin, CB1 and CB2 sequences, is altered in the GPR55 structure (10, 29). GPR55 comprises several protein kinase A and protein kinase C phosphorylation sites as well as conserved patterns for glycosylation, and conserved cysteines are located in extracellular loops 1 and 2 (25).
Interestingly, although several cannabinoid ligands can activate GPR55, it lacks the classical cannabinoid binding pocket (30). Using sequence to structure alignments with the well-known β2-adrenergic receptor (PDB: 2RH1) structure (31), a homology model of GPR55 in both its active and inactive state was constructed (29). These models were used to examine the ligand binding pocket of GPR55 and identify important amino acid interactions during ligand binding. The active conformation model of GPR55 was built in a hydrated lipid bilayer, which resulted in a deep, vertical, and highly hydrated binding pocket. This ligand binding region consists of many hydrophilic residues, which is in contrast to the highly hydrophobic CB1 and CB2 receptor binding pockets.
Microsecond time-scale molecular dynamic simulations and isothiocyanate covalent labeling studies suggest an entry pathway for lipid ligands between TM6 and TM7 for CB1 and CB2 (32–34). The third extracellular loop of GPR55 connects TM6 and TM7 and is significantly longer than the third extracellular loops of the CB1 and CB2 receptors. Furthermore, it consists of many charged amino acids, indicating that the loop will be mainly solvated in water thus enabling ligands with large head groups, such as LPI to enter between TM6 and TM7 (35).
Taken together, the data suggest that GPR55 is quite distinct from the traditional cannabinoid receptors and is well primed for receiving the LPI family of lipids as ligands due to its deep elongated binding pocket and three-dimensional protein conformation.
GPR55 pharmacology
The multifarious pharmacology of GPR55 has generated fierce debate over its classification as a novel cannabinoid receptor (36). One argument in support is that GPR55 exhibits sensitivity to distinct cannabinoid ligands in a multitude of cell lines and assays [reviewed extensively elsewhere (5–10)]. However, there is significant inconsistency between studies using cannabinoid ligands, which are variously reported as agonists or antagonists or to have no effect. In contrast, LPI has been confirmed as an agonist in all studies tested. Furthermore, emerging data suggest that other endogenous lipid ligands may possibly interact with GPR55. For example, N-arachidonoyl serine (ARA-S) induces a variety of effects in the Human Dermal Microvascular Endothelial Cell line (HMVEC), including modulation of proliferation and migration, which are inhibited upon silencing of GPR55 (37). However, ARA-S exhibited no functional activity in another GPR55 assay (121); thus, its status as a true endogenous GPR55 ligand is debatable.
GPR55 pharmacological studies have proven problematic to date due to lack of ligand specificity, with effects on other targets such as cannabinoid receptors, transient receptor potential vanilloid channels, and peroxisome proliferator-activated receptors. However, recent studies have provided new exciting tools for future GPR55 research. A study by Brown et al. (38) reported a number of synthetic compounds that appear highly selective for GPR55. These structurally related benzoylpiperazine compounds are inactive at CB1 and CB2 and were initially produced as inhibitors of the glycine transporter subtype 1; however, the most GPR55-specific ligand (GSK575594A) is greater than 60-fold selective for GPR55 than glycine transporter subtype 1. Intriguingly, the authors report that LPI is equipotent at human and rodent GPR55, although the new benzoylpiperazine compounds specifically activated human receptors, suggesting that these ligands may have distinct binding domains, which are divergent between species.
Kotsikorou et al. (29) have also recently described novel GPR55-specific compounds and have used these to map the putative binding site within GPR55. Importantly, some compounds in this study share a similar structure to those reported by Brown et al. (38), providing welcome consistency. LPI adopts an inverted L shape and interacts with the receptor by inserting its fatty acid tail deep within TM helices 2, 3, 5, 6, and 7 [in line with previous reports, suggesting LPI exhibits an extended acyl chain conformation (35)] and crucially binds K2.60 at the extracellular side of TM2 via its electronegative head group (29). This residue on TM2 is critical for GPR55 activation as all ligands modeled in the study appear to interact here, and furthermore, unpublished mutagenesis data by the group reinforce this significance (29). Although the compounds described in both studies are not identical, Kotsikorou et al. (29) have shown that they can adopt similar three-dimensional conformations and interact with the residues critical for ligand-induced activation. Furthermore, their data provide strong evidence that the cannabinoid antagonist AM251, is a bone fide GPR55 agonist as previously reported (26, 39–42), which can adopt a similar conformation and interact with the critical residues required for activation.
These new tools and modeling data will ultimately aid future targeted drug design and produce more specific compounds, allowing greater exploration of the pharmacology and physiology of GPR55.
Trafficking and intracellular sorting of GPR55
To date, most GPR55 trafficking studies have been performed in recombinant cell systems, such as human embryonic kidney (HEK)-293, U2OS, and MCF-7 cells, transiently or stably expressing hGPR55. Before agonist treatment the receptor is predominantly found at the cell surface but markedly internalizes following agonist challenge (39–41, 43). The intracellular sorting of internalized 7TM/GPCR is a highly regulated phenomena, typically resulting in recycling of the receptor back to the cell surface or enzymatic degradation and removal. Kargl et al. (44) have recently shown that GPR55 is significantly down-regulated via targeted degradation after prolonged agonist stimulation in a process involving the GPCR-associated sorting protein-1 (GASP-1). Furthermore, they demonstrated that in the absence of GASP-1, GPR55 recycles back to the cell surface, largely avoiding intracellular degradation (44). These data suggest GASP-1 dynamically regulates cell surface GPR55, thus playing an important role in GPR55 availability and subsequent cellular physiology (44).
GPR55 signaling
In accordance with the diverse and complex pharmacology of GPR55, the current literature regarding the downstream signaling of the receptor is equally disparate. A vast array of cell lines and assays has been used, potentially explaining some of the controversies. The complex nature of current signaling data may be due to several factors, including endogenous, transient, or stable receptor expression; variation in cellular background; ligand biased signaling or the interaction of endogenous proteins modulating GPR55 function.
In the early study by Ryberg et al. (26), GTPγS binding and FLIPR Ca2+ assays were exploited to unravel the downstream signaling of GPR55. Preincubation of membranes with blocking peptide (the last 12 amino acids) or antibody against the C terminus of Gα13 inhibited GTPγS binding in a dose-dependent manner, but those against Gαi1/2, Gαi3, and Gαs had no effect. The involvement of Gαq was ruled out because none of the ligands induced Ca2+ release in the FLIPR assay. Henstridge et al. (39) completed a comprehensive study in HEK-hGPR55 cells, which reinforced the role of Gα13 in GPR55-mediated Ca2+ release from endoplasmic reticulum stores. The single-cell Ca2+ imaging approach revealed an oscillatory Ca2+ response, a novel finding for 7TM/GPCR, which has previously only been shown for the tyrosine kinase insulin receptor (45, 46). The upstream signaling cascade involved Gα13-RhoA-ROCK, which in turn induced phospholipase C (PLC)-mediated inositol 1,4,5-triphosphate formation and subsequent release of Ca2+ from internal stores. Furthermore, a dynamic mass redistribution assay was used to confirm the pharmacology and G proteins implicated in GPR55 signaling pathways (41, 47). A distinct signature of mass redistribution was shown for GPR55 in comparison with Gαi-, Gαs-, or Gαq-coupled 7TM/GPCR. The GPR55-mediated redistribution was blocked in the presence of dominant negative Gα13, whereas the Gαi inhibitor pertussis toxin (PTX), Gαs inhibitor cholera toxin, and the Gαq inhibitor YM254890 had no effect. The coupling of GPR55 to Gα13-RhoA-ROCK and the consequent remodeling of the cytoskeleton was recently described in HEK293 cells and human neutrophils (48). Activation of GPR55 led to the formation of filamentous actin in HEK293 cells, which was dependent on the presence of functional Gα13, RhoA, and ROCK. Yet further reinforcement of Gα13 coupling was recently published by Brown et al. (38). Using a yeast cell line coexpressing GPR55 and various chimeric G proteins, they show specific coupling of GPR55 to Gα13 and not to Gαi, Gαs, or Gαq (38).
However, not all studies have reported specific coupling to Gα13. Lauckner et al. (49) also used single-cell Ca2+ imaging to assess GPR55 signaling cascades in HEK293 cells and mouse dorsal root ganglia. Ca2+ responses in these cells were mediated by both Gαq and Gα12 and a phosphatidylinositol-specific PLC. The role of Gαi and its Gβ/γ subunits was excluded due to the PTX-insensitive nature of the Ca2+ rise. Furthermore, the presence of an intact cytoskeleton and functional RhoA GTPase were suggested as critical elements in the response.
The endogenous GPR55 ligand LPI was discovered by screening a panel of lysolipids and cannabinoids and measuring GPR55-induced ERK1/2 activation (4). ERK phosphorylation has since been confirmed in a number of studies (41, 50–52). Furthermore, Oka et al. (50) have also shown that p38 MAPK are activated downstream of GPR55. Henstridge et al. (41) analyzed a variety of signaling readouts for GPR55 and in addition to single-cell Ca2+ imaging and ERK phosphorylation, nuclear factor of activated T cells, and nuclear factor κB reporter gene assays appear to be robust readouts of GPR55 activation. The GPR55-induced activation of nuclear factor of activated T cells has also been reported in an endothelial cell line (53).
In summary, GPR55 appears primarily a Gα13-coupled 7TM/GPCR that activates RhoA and ROCK and induces prolonged and oscillatory Ca2+ release from intracellular stores, culminating in the induction of a variety of transcription factors with the potential to significantly alter cellular physiology.
(Patho)physiology of GPR55
GPR55 in the nervous system
Given that CB1 is the most abundant GPCR in the brain and plays a fundamental role in regulating neurotransmission, it is of no great surprise that alterations in the endocannabinoid system have been implicated in a number of severe neurological diseases (54). However, since the discovery of the endocannabinoid system, pharmacological and functional data suggest that additional non-CB1/non-CB2 cannabinoid targets exist in the central nervous system (CNS) (1–3). Furthermore, an increasing number of studies describe the importance of glial cannabinoid receptors in regulating neuroimmunological processes and influencing neuronal communication (55, 56).
Anandamide and the synthetic cannabinoid agonist R-(+)-WIN55212 stimulate GTPγS binding in brain homogenates from both wild-type and CB1 knockout mice (57) and endocannabinoid-induced-long term depression in the CA1 region of the hippocampus is present in both CB1 knockout mice and wild-type littermates (58). Furthermore, other groups have reported that R-(+)-WIN55212 could equally affect neurotransmission in brain slices from wild-type and CB1 knockout rodents, and effects were blocked by Rimonabant but not by AM251 (59–61). Also, further studies have shown that primary microglia and astrocytes express unidentified cannabinoid targets that regulate cellular responses to excitotoxicity, cell migration, and cytokine release (56). Together these data suggest the presence of non-CB1/non-CB2 receptors within the CNS sensitive to cannabinoids.
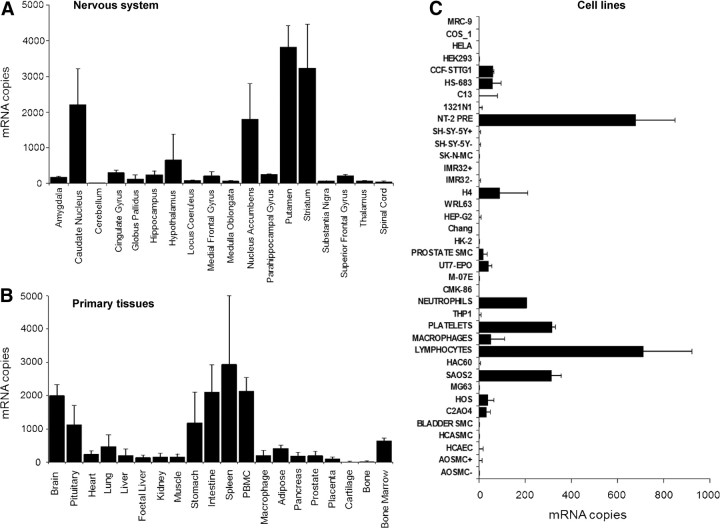
Despite the extensive mRNA data showing CNS expression of GPR55 (25, 26, 51, 62, 63), protein localization has yet to be confirmed in the brain. Intriguingly, Ryberg et al. (26) suggest that in the striatum, hypothalamus and brain stem, the expression of GPR55 mRNA is comparable with that of CB1, which may indicate an important role for GPR55 in these regions. Furthermore, we observed high hGPR55 mRNA expression in the striatum and nucleus accumbens and detectable levels in the hippocampus (Fig. 2A). Pietr et al. (51) used real-time PCR to confirm the expression of GPR55 in primary microglial cells, signifying a potential role for GPR55 in neuroimmunological regulation. Immunohistochemistry has revealed GPR55 protein expression in mouse large diameter dorsal root ganglia neurons (49) and activation of GPR55 induced significant inhibition of the potassium M-current (49), which can potentially enhance the excitability of sensory neurons.
Fig. 2.
GPR55 mRNA expression in the CNS, peripheral tissues, and cell lines. TaqMan quantitative RT-PCR analysis of GPR55 in human tissues and cells was conducted. The level of mRNA expression in poly A+ RNA from 18 regions of the brain and nervous system (A) and 20 primary peripheral tissues (B) were determined as described previously (118) using a hGPR55-specific primer set: forward primer, 5′-TCTTCCCGCTGGAGGTGTTT-3′, reverse primer, 5′-CAGGATGTGGATGCTCCTGG-3′, TaqMan probe, 5′-CTTCCTCCTTCCCATGGGCATCATGG-3′. Data show the mean (±sd) copies of mRNA detected in samples from three or four individuals per tissue (two males/two females for all tissues except two males/one female for globus pallidus and four males for prostate). No trends suggestive of sex-specific expression were observed. Levels of β-actin in these samples vary within a normal range and have been described previously (118). The same hGPR55-specific primers were used to measure mRNA levels in 37 distinct human cell lines and primary cells (C). Data show mean (±sd) copies per nanogram mRNA detected in duplicate from a single batch of cells. The mRNA levels of three housekeeping genes (β-actin, glyceraldehyde-3-phosphate dehydrogenase, and cyclophilin) in these samples vary within a normal range, confirming the integrity of each RNA sample, and has been presented previously (119). +, Differentiated (IMR32 and SH-SY-5Y) or serum-starved (AOSMC) cells; −, undifferentiated or unstimulated cells. The origin of cells has been described elsewhere (120).
The endocannabinoid system is believed to be critical for successful brain development and shaping neuronal connectivity (64, 65). A recent study assessed the role of cannabinoid receptors in the development of corticothalamic and thalamocortical axonal projections (66). GPR55 knockout mice showed no perturbation in the targeting or fasiculation of these axonal fibres, although the CB1 receptor was shown to be vital for correct axonal path finding.
Despite finding little or no GPR55 mRNA expression in human cerebellum (Fig. 2A), a recent study has reported GPR55 mRNA in cultured rat cerebellar granule cells (67). Lipopolysaccharide treatment induced significant up-regulation of inflammatory mediators such as IL-1b, IL-6, and TNF-α, and this effect was dose dependently blocked by pretreatment with CP55940. The authors suggest that the effect of CP55940 was not via CB1 or CB2; however, also reason that based on their pharmacological evidence, GPR55 is also unlikely to be the CP55940-sensitive target in the cerebellum.
Given that LPI is a potent endogenous agonist of GPR55 and both are found in the brain, it is of interest to note that recent studies have shown LPI application to rat neuroendocrine PC12 cells results in increased exocytosis and subsequent catecholamine release (68). Furthermore, LPI application resulted in a significant intracellular Ca2+ rise and LPI-induced effects were abolished after thapsigargin treatment. The effects were not observed with closely related lipid molecules (lysophosphatidylcholine and lysophosphatidylserine); however, GPR55 expression has yet to be assessed in this cell line.
Knowledge of GPR55 protein expression and functional significance in the nervous system are currently limited, however it appears that, GPR55 can regulate dorsal root ganglia excitability and may modulate certain neuroinflammatory conditions. With greater access to GPR55 antibodies and the new selective ligands, plus extensive evaluation of the knockout mice, the true significance of GPR55 in the nervous system will soon be ascertained.
GPR55: a novel target for pain?
Initial GPR55 studies within the pharmaceutical industry provoked great excitement as inflammatory and neuropathic pain were identified as potential therapeutic targets (63). Indeed, GPR55 knockout animals exhibit a striking pain phenotype, being markedly resistant to mechanical hyperalgesia associated with Freund's complete adjuvant (FCA)-induced inflammation or partial nerve ligation, a model of neuropathic hypersensitivity (63). In GPR55 knockout mice, mechanical hyperalgesia was completely absent for up to 2 wk after FCA injection and up to 28 d after ligation. Interestingly, GPR55 does not appear to modulate normal nociceptive pathways because they were largely unaffected in the knockout animals. Although GPR55 is present in dorsal root ganglia, which are an integral component of nociceptive neurocircuitry, it is specifically located in large diameter neurons (49), which typically detect innocuous stimuli. The mechanism of action underlying the effect of GPR55 on inflammatory pain is not clear at present but may reflect an altered immune response. Indeed cytokine profiling revealed increased levels of IL-4, IL-10, interferon-γ, and granulocyte macrophage colony-stimulating factor in paws from FCA-injected GPR55 knockout mice (63). Furthermore, we discovered significant GPR55 mRNA expression in spleen, bone marrow, lymphocytes (peripheral blood mononuclear cells), platelets, and neutrophils consist with a role in inflammatory processes (Fig. 2, B and C). A recent study using noxious rotation of an inflamed rat knee joint, has shown that local administration of O-1602 significantly reduced the mechanosensitivity of the joint afferent fibers (69). More specifically, the effect was observed only in the unmyelinated C fibers was quick in onset and was unaffected by coadministration of CB1 and CB2 antagonists (AM281 and AM630, respectively). However, the effect of O-1602 was completely blocked following coadministration of O-1918 (69). Whether these effects involve GPR55 or GPR18 for example, remains to be established, and it would be interesting to repeat these studies with the new GPR55 compounds described by Brown et al. (38) and Kotsikorou et al. (29).
GPR55 in the vasculature
Studies using rat mesenteric artery preparations suggest that certain cannabinoids target a distinct non-CB1/non-CB2 vascular receptor. For example, Δ9-THC-induced vasorelaxation could not be blocked by the CB1 antagonist Rimonabant, even at high concentrations (70). Furthermore, abnormal-cannabidiol (abn-CBD) was shown to induce mesenteric vascular relaxation in CB1 and CB2 knockout mice (71), and intriguingly, these effects were antagonized by a cannabidiol analog lacking CB1/CB2 activity (O-1918) (72). The current data suggest the presence of a non-CB1/non-CB2 Gαi-coupled receptor that is sensitive to Δ9-THC, anandamide, and abn-CBD (71, 73, 74). However, Johns et al. (75) have shown that abn-CBD induces a similar decrease in mean arterial pressure in both wild-type and GPR55 knockout mice, which could be antagonized by O-1918, suggesting that abn-CBD affects a distinct target from GPR55 in the endothelium. In support of this theory is the well-described data describing GPR55 coupling to Gα13, which contradicts the PTX-sensitive effects of abn-CBD and anandamide in endothelium (71).
Daly et al. (76) have recently shown using a fluorescent ligand binding approach that GPR55 may be expressed in mouse mesenteric arteries (MMA). T1117 is a derivative of AM251 with a fluorescent tetramethylrhodamine group added. The addition of this fluorescent group renders the compound unable to bind CB1 yet retain its agonist properties at GPR55 (76). T1117 bound to all three vascular layers of the MMA, and preincubation of excess unlabeled AM251 inhibited T1117 binding. Furthermore, prior treatment of the MMA preparation with O-1602 resulted in a significant loss of T1117 labeling, suggesting that O-1602 internalizes GPR55, thus removing the T1117 binding target.
Interestingly, endothelial cells may regulate GPR55-mediated physiology via an autocrine release of LPI. Early studies show that endothelial cells can release LPI into the extracellular milieu after intracellular PLA2-mediated lipid breakdown (77). Anandamide is also produced by vascular endothelial cells (78) and activates GPR55 in some recombinant systems, suggesting that in principle, anandamide can bind both CB1 and GPR55 in endothelial cells. Waldeck-Weiermair et al. (53) have shown in human umbilical vein endothelial cells (HUVEC), that anandamide induces an increase in intracellular Ca2+, which can be blocked by the CB1 antagonist Rimonabant and CB1-insensitive O-1918. Furthermore, O-1602 evoked GPR55-mediated Ca2+ elevation, thus suggesting that both CB1 and GPR55 may contribute to anandamide-induced Ca2+ release in endothelium.
Integrins play a major role in the anandamide response, as revealed with the use of Mn2+, fibronectin, and the ROCK inhibitor Y27632, which all modulate integrin clustering (53). If CB1 and GPR55 are both found within the same cell type and can be activated by the same ligands, this suggests there may be interactions between their downstream signaling cascades, as observed in neutrophils (48), and this appears to be the case in the endothelium. When integrins are unclustered, anandamide activates the CB1-Gαi-Syk pathway, which inhibits the GPR55-phosphatidylinositol 3-kinase -Bmx-PLC-Ca2+ cascade at the level of Syk (53). However, when integrins cluster, this uncouples CB1 from β1 integrin and unleashes the uninhibited GPR55-phosphatidylinositol 3-kinase-Bmx-PLC-Ca2+ cascade after anandamide stimulation.
Intriguingly, a recent study has shown that LPI-induced activation of nonselective cation channels and endothelial cell depolarization are independent of GPR55 (79). The authors followed up this observation with new reports describing further direct effects of LPI on endothelial cation channels (80, 81); however, these non-GPR55-mediated effects currently appear to be endothelium specific.
GPR55 in blood
Cannabinoids induce diverse responses in blood cells, such as migration, proliferation, cytokine production, apoptosis, reactive oxygen species production, and chemotaxis. Indeed, certain phytocannabinoids and synthetic CB2 ligands exert potentially therapeutic immunosuppressant actions (82, 83). The role of cannabinoids in regulating neutrophil chemotaxis and migration is contentious, and although Balenga et al. (48) recently demonstrated 2-arachidonoyl glycerol (2-AG)-induced chemotaxis via CB2, other studies have not reported this effect (84). Furthermore, increasing evidence in the immune system suggests the presence of non-CB1/non-CB2 cannabinoid and associated lipid targets (82, 84, 85). Two lipid-sensitive orphan 7TM/ GPCR have been implicated as major candidates, GPR18 and GPR55.
Our mRNA expression data confirm GPR55 in a number of diverse human immune cells, including neutrophils (Fig. 2C). However, it was recently shown in a model of experimental colitis that O-1602 could inhibit neutrophil recruitment, independently of GPR55, implicating the potential role of the O-1602-sensitive receptor GPR18 in the effects (86). Despite this, Balenga et al. (48) have shown for the first time that LPI and AM251 act as GPR55 agonists in neutrophils, promoting RhoA-dependent chemotaxis. Furthermore, Balenga et al. identified novel downstream interactions between GPR55 (activated with LPI or AM251) and CB2 (activated with 2-AG) receptor signaling in neutrophils. When both receptors were stimulated in tandem, pathways involving RhoA and Cdc42 were significantly enhanced with clear synergistic potentiation observed between AM251 and 2-AG induction of RhoA-mediated chemotaxis (48). Thus, the data suggest that CB2 and GPR55 initially cooperate by mutually enhancing RhoA-induced migration to hunt for inflammatory loci.
Interestingly, a negative interaction between GPR55 and CB2 was observed during neutrophil respiratory burst at the level of reactive oxygen species generation. Also, LPI significantly reduced the 2-AG-induced activation of the GTPase Rac2. Intriguingly, previous data have described a novel Rimonabant-sensitive non-CB1/non-CB2 target in neutrophils, exhibiting negative cooperativity with CB2 receptors (84). Thus, after initial functional cooperation in inducing chemotaxis, GPR55 and CB2 disengage, and in a refined process of functional repression, GPR55 restricts excessive CB2-mediated oxidative damage. Microglia express both GPR55 and CB2; thus, in future studies it would be interesting to assess GPR55-CB2 receptor cross talk and any functional relevance of such interaction.
GPR55 in bone
Recently GPR55 has been found in cells that regulate bone metabolism (87) and thus may represent a novel target for osteoporosis and bone loss associated with arthritis. GPR55 is present in cells that both generate (osteoblasts) and resorb (osteoclasts) bone. In osteoclasts, GPR55 activation results in osteoclastogenesis, cell polarization and bone resorption; however, the function of GPR55 in osteoblasts is unclear (87). Male GPR55 knockout mice exhibit a clear osteopetrotic phenotype with high bone mass, although this is not observed in females; the reasons for this striking difference are intriguing but remain to be established (87). In male GPR55 knockouts, osteoclast numbers are increased significantly; however, osteoclast function is impaired overall, which is consistent with other osteopetrotic phenotypes and may be a homeostatic response (88). Furthermore, the authors also show that in GPR55 knockout mice, cartilaginous remnant (chondrocytes) levels are doubled within the trabecular bone. In addition, a recent conference abstract has confirmed the presence of GPR55 in human chondrocytes (89). Using RT-PCR, the authors suggest that GPR55 is present in normal human chondrocytes and those extracted from osteoarthritic patients. However, to date the role of GPR55 in these cells or whether the receptor is important in osteoarthritis development remains to be determined. Despite finding very low levels of GPR55 mRNA in human bone and cartilage samples, cell lines derived from these tissues expressed significant levels of the receptor in our hands (Fig. 2, B and C).
GPR55 signaling in osteoclasts is in line with most other cell types, in that there is prominent activation of RhoA and ERK1/2, together with effects on the actin cytoskeleton (87). Much of the work on bone metabolism was carried out using the ligands O-1602 and LPI, which exhibit bell-shaped concentration-response relationships in this system. However, the effects of O-1602 and LPI on RhoA signaling are absent in GPR55 knockout mice and O-1602 responses are inhibited by the putative GPR55 antagonist cannabidiol (87).
GPR55 in cancer
Several lines of evidence point to a potential role for GPR55 in the control of cancer cell proliferation. First, certain cannabinoids can modulate GPR55 activity (5, 7, 9, 10, 36, 90), and it is well established that cannabinoids can control cancer cell proliferation (91–93). This property has been demonstrated in several models of cancer, ranging from the simplest (cell cultures) to the most complex [genetically engineered mice (94–96)], and in tumors from very different origins [brain, breast, pancreas, hematopoietic system, etc. (91–93)]. Second, the levels of the endogenous GPR55 ligand LPI (4) are augmented in plasma and ascites from patients with ovarian cancer compared with healthy women or with women with noncancerous pathologies (22, 97). Interestingly, the highest elevated LPI species found in cancer patients is arachidonoyl-LPI (22), and this particular LPI is the most biologically active at GPR55 (98). Moreover, Falasca and Corda demonstrated some 15 yr ago that epithelial cells (18) and fibroblasts (99) are able to generate LPI when transformed with ras oncogene and that this LPI induces cell proliferation. Finally, GPR55 predominantly couples to Gα12/13 proteins (26, 38, 39, 48, 49), which are known to signal oncogenesis (100, 101). For example, overexpression of these Gα proteins induces fibroblast transformation, and their activation (by binding of ligands to their corresponding GPCRs) enhances invasive potential and angiogenic responses (100, 101).
The expression of GPR55 has been confirmed in a diverse range of human cancer cell lines including breast (43, 102), ovary, prostate (52), brain, skin, cervix, liver, blood, pancreas (102), and bile ducts (103). Interestingly, in human tumors the expression of GPR55 correlates with their aggressiveness. Higher GPR55 mRNA levels were found in high histological grade breast, pancreas, and brain tumors compared with low-grade tumors and healthy tissue (102). Moreover, elevated GPR55 mRNA was associated with decreased overall glioma patient survival (102). These data suggest that GPR55 expression and/or activation confers an oncogenic capability on cancer cells. In fact, this hypothesis has been proven correct and results obtained so far indicate that this capability is invested in an increased proliferative potential. In addition, genetic blockade of GPR55 [using selective small interfering RNA (siRNA)] in ovarian, prostate (52), breast and brain (102) cancer cells decreased their proliferation in culture, whereas GPR55 overexpression in breast and brain cancer cells had the opposite effect (102). Importantly, in vivo silencing of GPR55 reduced tumor growth in a xenograft model of glioblastoma, an effect that was accompanied by a decrease in the number of proliferating cells within the tumors (102).
Together these results indicate that GPR55 expression enhances the proliferative capability of cancer cells. However, a recent paper shows that activation of this receptor in cholangiocarcinoma cells exerts an antiproliferative action both in vitro and in vivo (103). In this case, anandamide and O-1602 activated GPR55, induced coupling to Gα12 proteins, activation of c-Jun N-terminal kinase, cell death by apoptosis, and the consequent decrease in the number of viable cancer cells (103). This process was accompanied by the recruitment of Fas death receptor into lipid rafts (103). Additional research is required to determine whether this discrepancy with previous data is the reflection of the complex pharmacology of GPR55; an indirect, cell type-, ligand-, or dose-specific effect; or the combination of these and other factors.
It is important to note that experiments describing a proliferation-inducing effect were performed in the absence of exogenously applied GPR55 agonists, which raises the question of whether GPR55 is activated by endogenously produced ligands or is constitutively active. Although the latter possibility cannot be ruled out, experimental evidence supports the existence of an endogenous GPR55 agonist tone. Pharmacological or genetic inhibition of the cytosolic phospholipase A2, an enzyme that was previously described to generate mitogenic LPI in ras-transformed cells (18, 99), reduced proliferation in prostate cancer cells (52) and GPR55-overexpressing HEK293 cells (102). Both in vitro and in vivo approaches point to the ERK pathway as a major GPR55 downstream signaling pathway involved in its proliferation-promoting action. For example, Piñeiro et al. (52) demonstrated that LPI induces ERK activation in prostate cancer cells in culture and that this effect can be prevented by genetic (siRNA) or pharmacological (cannabidiol) blockade of GPR55. Andradas et al. (102) also demonstrated the modulation of this GPR55 signaling cascade by silencing and overexpressing the receptor in breast and brain cancer cells, both in cell cultures and in vivo.
Interestingly, GPR55 expression/activation may confer additional capabilities to cancer cells apart from an increased proliferation potential. For instance, it has been reported that MCF-7 breast cancer cells increase their migration toward chemoattractants when GPR55 is ectopically overexpressed and that these cells migrate in response to LPI via a mechanism selectively blocked by GPR55 silencing (43). Furthermore, activation of GPR55 induces endothelial cell proliferation, migration, tube formation, and an increase in vascular endothelial growth factor in culture (37). Although further research is needed, these results suggest that GPR55 may enhance the metastatic potential of cancer cells and induce/sustain angiogenesis in tumors.
Recent GPR55 expression data in other tissues and cell lines
Mast cells
Human mastocytic cells treated with phorbol 12-myristate 13-acetate induce significant release of nerve growth factor, which is thought to further enhance inflammatory responses via angiogenesis (104). Inflammation-induced nerve growth factor release from a human mast cell line (HMC-1) was specifically attenuated by preincubation with the cannabinoid-like molecule palmitoylethanolamide (PEA). PEA has been shown in one study to be a potent GPR55 agonist (26), and the receptor is expressed in HMC-1 cells, thus potentially regulating the antiinflammatory effect of PEA. CB1 and CB2 receptors were not found in HMC-1 cells, and targeted GPR55 siRNA could specifically knock down the antiinflammatory effect.
These data further enforce the idea that GPR55 may play a role following an inflammatory insult.
Placenta
Recently GPR55 expression in uterine tissues from d 10 until the end of pregnancy was assessed using real-time quantitative PCR, Western blot, and immunohistochemistry (105). PCR revealed peak GPR55 transcript expression at d 12, although protein levels peaked slightly later, at d 14. Delayed protein expression in uterine tissue is not unprecedented and has been previously described for the important endocannabinoid regulating enzyme fatty acid amide hydrolase (106).
GPR55 was significantly expressed in decidual tissue, which is crucial for pregnancy success and undergoes a regressive process during pregnancy development (105). Intriguingly, the authors suggested that GPR55 may play a role in these critical regression processes by inducing apoptotic death of decidual cells because the GPR55 agonist AM251 promoted cell death.
Kidney
Diabetes is becoming a major health issue in our modern indulgent society, and diabetic patients often exhibit elevated endocannabinoid levels (107). The onset of diabetic nephropathy is often preceded by proximal tubule hypertrophy, and research has revealed a role for CB1 in inducing hypertrophic events in the renal tubules (108). A recent study has used RT-PCR and Western blotting to reveal GPR55 expression in human proximal tubule cells [HK2 (109)]. Treating HK2 cells with anandamide resulted in significant cell hypertrophy. To assess the role of CB1, the authors pretreated the cells with AM251. AM251 alone reduced hypertrophy below baseline and blocked anandamide-induced hypertrophy. However, this effect could be attributed to GPR55 interaction because AM251 is a GPR55 agonist at the concentrations applied (0.5–5 μm) (41). To assess the role of GPR55, the authors attempted to block the effects of anandamide with O-1918. This is a cannabidiol analog that may antagonize GPR55 (53) or act via GPR18 (85). Therefore, GPR55 effects in this cell line cannot be ruled out, although further pharmacological evaluation is required.
Summary and concluding remarks
Interaction with GPR55 may explain some of the off-target effects of certain cannabinoid ligands; however, other novel cannabinoid targets in various tissues remain to be characterized. For example, the R-(+)-WIN55212-sensitive brain receptor (57, 59) awaits isolation, and the vascular system retains the so called abn-CBD-sensitive endothelial receptor awaiting precise characterization. Furthermore, intriguing data, especially from endothelial cells, suggest LPI and the putative GPR55 ligand ARA-S have non-GPR55 targets, distinct from CB1 and CB2 (37, 79). Moreover, another putative GPR55 ligand O-1602 has non-GPR55 effects in experimental colitis and neutrophil migration, potentially acting via GPR18 (86). Therefore, although our current knowledge of GPR55 pharmacology and physiology has significantly improved our understanding of lipid signaling, a number of questions still remain unanswered.
Lysophospholipid receptors and the traditional cannabinoid receptors are widely expressed in a variety of tissues and cells throughout the body and have considerable influence over vast physiological processes (36, 110). There are currently more than 4000 publications detailing the similar lipid-sensitive lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) receptor families, which are implicated in numerous pathophysiological states ranging from cancer, multiple sclerosis, cardiovascular disease, and asthma (111, 112). Intriguingly, LPA and S1P can be released from the host cell in a similar manner to LPI and can act on specific receptors in an autocrine or paracrine fashion and play a significant role in disease prognosis (113). Given that LPI can be released in a similar manner and exerts similar autocrine actions (52), it raises the exciting yet speculative hypothesis that LPI/GPR55 signaling may play an equally important role in cellular physiology. Furthermore, the role of these lipid-sensitive receptors in human pathology is currently under consideration for novel therapeutic exploitation (111, 114). For example, the S1P ligand fingolimod has recently been approved in a number of countries as a treatment for relapsing forms of multiple sclerosis (115).
Therefore, GPR55 should also be considered as a novel therapeutic target in the numerous pathologies in which it is involved. For example, GPR55 plays an important role in tumor progression and could be exploited as a new biomarker and/or therapeutic target in oncology. GPR55 antagonists may prove beneficial in slowing tumor proliferation, angiogenesis, and cancer pain (116). Furthermore, the ability of GPR55 to regulate bone metabolism could be exploited therapeutically. However, because the osteopetrotic phenotype is absent in female GPR55 knockout mice, this may be of limited utility. Interestingly, in male mice, an 8-wk in vivo treatment paradigm with cannabidiol significantly reduced serum type 1 collagen C-terminal telopeptide fragments, a biochemical marker of bone resorption (87). Arthritis may prove to be a more tractable target because GPR55 has the potential to act at multiple levels in this pathological state, regulating the underlying immune response as well as bone resorption and pain. Indeed, in vivo studies suggest that cannabidiol is an effective oral antiarthritic therapeutic in murine collagen-induced arthritis (117). The role of GPR55 antagonism in this effect of cannabidiol remains to be established. Additionally, there is need for more academic research into the role of GPR55 in inflammatory pain, and it will be of interest to discover whether the sites of pain exhibit elevated levels of LPI. Whether GPR55 itself continues to be explored as a therapeutic pain target by the pharmaceutical industry may be limited by the inherent drugability of the receptor rather than the underlying physiology, although phytocannabinoids such as cannabidiol may offer potential here. Other aspects of the LPI-GPR55-pain pathway may be worth exploring, for example, regulating LPI metabolism or reducing the availability of endogenous ligands in plasma using molecular sequestration approaches.
In summary, GPR55 has emerged as an intriguing addition to the lipid-sensitive receptor family and is involved in numerous cellular processes and pathologies, which may represent areas for novel therapeutic intervention in the future.
Acknowledgments
We thank Paul Murdock (GlaxoSmithKline) for providing the hGPR55 mRNA expression data.
This work was supported by grants from the Austrian Science Fund (Grant P18723, to M.W.); the Jubiläumsfonds of the Austrian National Bank and the Lanyar Stiftung Graz (all to M.W.); and the Molecular Medicine PhD program from the Medical University of Graz, Graz, Austria, and a BaCa Visiting Scientists program (all to N.A.B.B.). C.M.H. is a recipient of a European Molecular Biology Organization Long-Term Fellowship. J.K. was supported by the PhD program from the Medical University of Graz and a research fellowship from the Austrian Government. A.I. and C.M.H. were also supported by the Medical Research Council. This work was also supported by the Spanish Ministry of Science and Innovation (to C.S.), the Spanish Association Against Cancer (to C.S.), the Mutua Madrileña (to C.S.) and GW and the Otsuka Pharmaceuticals (to C.S.). This work was also supported by an ‘Acciones Integradas’ grant between Austria (M.W.) and Spain (C.S.).
Current address for M.W.: Hagedorn Research Institute, Novo Nordisk A/S, Gentofte, Denmark.
Current address for N.A.B.B.: Molecular and Signal Transduction Section, Laboratory of Allergic Diseases, National Institute of Allergy and Infectious Diseases/National Institutes of Health, 10 Center Drive, Room 11N242, Bethesda, Maryland.
Disclosure Summary: A.J.B. is an employee of GlaxoSmithKline and holds a patent on GPR55 (WO/2001/086305). All other authors have nothing to disclose.
Footnotes
- abn-CBD
- Abnormal-cannabidiol
- 2-AG
- 2-arachidonoyl glycerol
- ARA-S
- N-arachidonoyl serine
- CNS
- central nervous system
- FCA
- Freund's complete adjuvant
- GASP-1
- GPCR-associated sorting protein-1
- GPCR
- G protein-coupled receptor
- GPR55
- G protein-coupled receptor-55
- HEK
- human embryonic kidney
- hGPR55
- human GPR55
- LPA
- lysophosphatidic acid
- LPI
- lysophosphatidylinositol
- MMA
- mouse mesenteric artery
- PEA
- palmitoylethanolamide
- PLC
- phospholipase C
- PTX
- pertussis toxin
- siRNA
- small interfering RNA
- S1P
- sphingosine-1-phosphate
- TM
- transmembrane
- 7TM
- seven-TM spanning.
References
- 1. Mackie K , Stella N. 2006. Cannabinoid receptors and endocannabinoids: evidence for new players. AAPS J 8:E298–E306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown AJ. 2007. Novel cannabinoid receptors. Br J Pharmacol 152:567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker D , Pryce G , Davies WL , Hiley CR. 2006. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci 27:1–4 [DOI] [PubMed] [Google Scholar]
- 4. Oka S , Nakajima K , Yamashita A , Kishimoto S , Sugiura T. 2007. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun 362:928–934 [DOI] [PubMed] [Google Scholar]
- 5. Brown AJ , Robin Hiley C. 2009. Is GPR55 an anandamide receptor? Vitam Horm 81:111–137 [DOI] [PubMed] [Google Scholar]
- 6. Godlewski G , Offertáler L , Wagner JA , Kunos G. 2009. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat 89:105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moriconi A , Cerbara I , Maccarrone M , Topai A. 2010. GPR55: current knowledge and future perspectives of a purported “type-3” cannabinoid receptor. Curr Med Chem 17:1411–1429 [DOI] [PubMed] [Google Scholar]
- 8. Nevalainen T , Irving AJ. 2010. GPR55, a lysophosphatidylinositol receptor with cannabinoid sensitivity? Curr Top Med Chem 10:799–813 [DOI] [PubMed] [Google Scholar]
- 9. Ross RA. 2009. The enigmatic pharmacology of GPR55. Trends Pharmacol Sci 30:156–163 [DOI] [PubMed] [Google Scholar]
- 10. Sharir H , Abood ME. 2010. Pharmacological characterization of GPR55, a putative cannabinoid receptor. Pharmacol Ther 126:301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keenan RW , Hokin LE. 1962. The identification of lysophosphatidylinositol and its enzymic conversion to phosphatidylinositol. Biochim Biophys Acta 60:428–430 [DOI] [PubMed] [Google Scholar]
- 12. Keenan RW , Hokin LE. 1964. The enzymatic acylation of lysophosphatidylinositol. J Biol Chem 239:2123–2129 [PubMed] [Google Scholar]
- 13. Metz SA. 1986. Lysophosphatidylinositol, but not lysophosphatidic acid, stimulates insulin release. A possible role for phospholipase A2 but not de novo synthesis of lysophospholipid in pancreatic islet function. Biochem Biophys Res Commun 138:720–727 [DOI] [PubMed] [Google Scholar]
- 14. Metz SA. 1988. Mobilization of cellular Ca2+ by lysophospholipids in rat islets of Langerhans. Biochim Biophys Acta 968:239–252 [DOI] [PubMed] [Google Scholar]
- 15. Zoeller RA , Wightman PD , Anderson MS , Raetz CR. 1987. Accumulation of lysophosphatidylinositol in RAW 264.7 macrophage tumor cells stimulated by lipid A precursors. J Biol Chem 262:17212–17220 [PubMed] [Google Scholar]
- 16. Baran DT , Kelly AM. 1988. Lysophosphatidylinositol: a potential mediator of 1,25-dihydroxyvitamin D-induced increments in hepatocyte cytosolic calcium. Endocrinology 122:930–934 [DOI] [PubMed] [Google Scholar]
- 17. Smith DM , Waite M. 1992. Phosphatidylinositol hydrolysis by phospholipase A2 and C activities in human peripheral blood neutrophils. J Leukoc Biol 52:670–678 [DOI] [PubMed] [Google Scholar]
- 18. Falasca M , Corda D. 1994. Elevated levels and mitogenic activity of lysophosphatidylinositol in k-ras-transformed epithelial cells. Eur J Biochem 221:383–389 [DOI] [PubMed] [Google Scholar]
- 19. Falasca M , Silletta MG , Carvelli A , Di Francesco AL , Fusco A , Ramakrishna V , Corda D. 1995. Signalling pathways involved in the mitogenic action of lysophosphatidylinositol. Oncogene 10:2113–2124 [PubMed] [Google Scholar]
- 20. Xiao Y , Chen Y , Kennedy AW , Belinson J , Xu Y. 2000. Evaluation of plasma lysophospholipids for diagnostic significance using electrospray ionization mass spectrometry (ESI-MS) analyses. Ann NY Acad Sci 905:242–259 [DOI] [PubMed] [Google Scholar]
- 21. Shen Z , Wu M , Elson P , Kennedy AW , Belinson J , Casey G , Xu Y. 2001. Fatty acid composition of lysophosphatidic acid and lysophosphatidylinositol in plasma from patients with ovarian cancer and other gynecological diseases. Gynecol Oncol 83:25–30 [DOI] [PubMed] [Google Scholar]
- 22. Sutphen R , Xu Y , Wilbanks GD , Fiorica J , Grendys EC , LaPolla JP , Arango H , Hoffman MS , Martino M , Wakeley K , Griffin D , Blanco RW , Cantor AB , Xiao YJ , Krischer JP. 2004. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol Biomarkers Prev 13:1185–1191 [PubMed] [Google Scholar]
- 23. Blondeau N , Lauritzen I , Widmann C , Lazdunski M , Heurteaux C. 2002. A potent protective role of lysophospholipids against global cerebral ischemia and glutamate excitotoxicity in neuronal cultures. J Cereb Blood Flow Metab 22:821–834 [DOI] [PubMed] [Google Scholar]
- 24. Maingret F , Patel AJ , Lesage F , Lazdunski M , Honoré E. 2000. Lysophospholipids open the two-pore domain mechano-gated K(+) channels TREK-1 and TRAAK. J Biol Chem 275:10128–10133 [DOI] [PubMed] [Google Scholar]
- 25. Sawzdargo M , Nguyen T , Lee DK , Lynch KR , Cheng R , Heng HH , George SR , O'Dowd BF. 1999. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res Mol Brain Res 64:193–198 [DOI] [PubMed] [Google Scholar]
- 26. Ryberg E , Larsson N , Sjögren S , Hjorth S , Hermansson NO , Leonova J , Elebring T , Nilsson K , Drmota T , Greasley PJ. 2007. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152:1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McPartland JM , Glass M , Matias I , Norris RW , Kilpatrick CW. 2007. A shifted repertoire of endocannabinoid genes in the zebrafish (Danio rerio). Mol Genet Genomics 277:555–570 [DOI] [PubMed] [Google Scholar]
- 28. Rovati GE , Capra V , Neubig RR. 2007. The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Mol Pharmacol 71:959–964 [DOI] [PubMed] [Google Scholar]
- 29. Kotsikorou E , Madrigal KE , Hurst DP , Sharir H , Lynch DL , Heynen-Genel S , Milan LB , Chung TD , Seltzman HH , Bai Y , Caron MG , Barak L , Abood ME , Reggio PH. 2011. Identification of the GPR55 agonist binding site using a novel set of high potency GPR55 selective ligands. Biochemistry 50:5633–5647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petitet F , Donlan M , Michel A. 2006. GPR55 as a new cannabinoid receptor: still a long way to prove it. Chem Biol Drug Des 67:252–253 [DOI] [PubMed] [Google Scholar]
- 31. Cherezov V , Rosenbaum DM , Hanson MA , Rasmussen SG , Thian FS , Kobilka TS , Choi HJ , Kuhn P , Weis WI , Kobilka BK , Stevens RC. 2007. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318:1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hurst DP , Grossfield A , Lynch DL , Feller S , Romo TD , Gawrisch K , Pitman MC , Reggio PH. 2010. A lipid pathway for ligand binding is necessary for a cannabinoid G protein-coupled receptor. J Biol Chem 285:17954–17964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pei Y , Mercier RW , Anday JK , Thakur GA , Zvonok AM , Hurst D , Reggio PH , Janero DR , Makriyannis A. 2008. Ligand-binding architecture of human CB2 cannabinoid receptor: evidence for receptor subtype-specific binding motif and modeling GPCR activation. Chem Biol 15:1207–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Picone RP , Khanolkar AD , Xu W , Ayotte LA , Thakur GA , Hurst DP , Abood ME , Reggio PH , Fournier DJ , Makriyannis A. 2005. (−)-7′-Isothiocyanato-11-hydroxy-1′,1′-dimethylheptylhexahydrocannabinol (AM841), a high-affinity electrophilic ligand, interacts covalently with a cysteine in helix six and activates the CB1 cannabinoid receptor. Mol Pharmacol 68:1623–1635 [DOI] [PubMed] [Google Scholar]
- 35. Kotsikorou E , Lynch DL , Abood ME , Reggio PH. 2011. Lipid bilayer molecular dynamics study of lipid-derived agonists of the putative cannabinoid receptor, GPR55. Chem Phys Lipids 164:131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pertwee RG , Howlett AC , Abood ME , Alexander SP , Di Marzo V , Elphick MR , Greasley PJ , Hansen HS , Kunos G , Mackie K , Mechoulam R , Ross RA. 2010. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 62:588–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang X , Maor Y , Wang JF , Kunos G , Groopman JE. 2010. Endocannabinoid-like N-arachidonoyl serine is a novel pro-angiogenic mediator. Br J Pharmacol 160:1583–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown AJ , Daniels DA , Kassim M , Brown S , Haslam CP , Terrell VR , Brown J , Nichols PL , Staton PC , Wise A , Dowell SJ. 2011. Pharmacology of GPR55 in yeast and identification of GSK494581A as a mixed-activity glycine transporter subtype 1 inhibitor and GPR55 agonist. J Pharmacol Exp Ther 337:236–246 [DOI] [PubMed] [Google Scholar]
- 39. Henstridge CM , Balenga NA , Ford LA , Ross RA , Waldhoer M , Irving AJ. 2009. The GPR55 ligand l-α-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J 23:183–193 [DOI] [PubMed] [Google Scholar]
- 40. Kapur A , Zhao P , Sharir H , Bai Y , Caron MG , Barak LS , Abood ME. 2009. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J Biol Chem 284:29817–29827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Henstridge CM , Balenga NA , Schröder R , Kargl JK , Platzer W , Martini L , Arthur S , Penman J , Whistler JL , Kostenis E , Waldhoer M , Irving AJ. 2010. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br J Pharmacol 160:604–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yin H , Chu A , Li W , Wang B , Shelton F , Otero F , Nguyen DG , Caldwell JS , Chen YA. 2009. Lipid G protein-coupled receptor ligand identification using β-arrestin PathHunter assay. J Biol Chem 284:12328–12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ford LA , Roelofs AJ , Anavi-Goffer S , Mowat L , Simpson DG , Irving AJ , Rogers MJ , Rajnicek AM , Ross RA. 2010. A role for l-α-lysophosphatidylinositol and GPR55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br J Pharmacol 160:762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kargl J , Balenga N , Platzer W , Martini L , Whistler J , Waldhoer M. 30 June 2011. The GPCR-associated sorting protein 1 regulates ligand-induced downregulation of GPR55. Br J Pharmacol 10.1111/j.1476-5381.2011.01562.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baltrusch S , Lenzen S. 2007. Regulation of [Ca2+]i oscillations in mouse pancreatic islets by adrenergic agonists. Biochem Biophys Res Commun 363:1038–1043 [DOI] [PubMed] [Google Scholar]
- 46. Soria B , Martin F. 1998. Cytosolic calcium oscillations and insulin release in pancreatic islets of Langerhans. Diabetes Metab 24:37–40 [PubMed] [Google Scholar]
- 47. Schröder R , Janssen N , Schmidt J , Kebig A , Merten N , Hennen S , Müller A , Blättermann S , Mohr-Andrä M , Zahn S , Wenzel J , Smith NJ , Gomeza J , Drewke C , Milligan G , Mohr K , Kostenis E. 2010. Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat Biotechnol 28:943–949 [DOI] [PubMed] [Google Scholar]
- 48. Balenga NA , Aflaki E , Kargl J , Platzer W , Schröder R , Blättermann S , Kostenis E , Brown AJ , Heinemann A , Waldhoer M. 5 April 2011. GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell Res 10.1038/cr.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lauckner JE , Jensen JB , Chen HY , Lu HC , Hille B , Mackie K. 2008. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci USA 105:2699–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oka S , Kimura S , Toshida T , Ota R , Yamashita A , Sugiura T. 2010. Lysophosphatidylinositol induces rapid phosphorylation of p38 mitogen-activated protein kinase and activating transcription factor 2 in HEK293 cells expressing GPR55 and IM-9 lymphoblastoid cells. J Biochem 147:671–678 [DOI] [PubMed] [Google Scholar]
- 51. Pietr M , Kozela E , Levy R , Rimmerman N , Lin YH , Stella N , Vogel Z , Juknat A. 2009. Differential changes in GPR55 during microglial cell activation. FEBS Lett 583:2071–2076 [DOI] [PubMed] [Google Scholar]
- 52. Piñeiro R , Maffucci T , Falasca M. 2011. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 30:142–152 [DOI] [PubMed] [Google Scholar]
- 53. Waldeck-Weiermair M , Zoratti C , Osibow K , Balenga N , Goessnitzer E , Waldhoer M , Malli R , Graier WF. 2008. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J Cell Sci 121:1704–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Katona I , Freund TF. 2008. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 14:923–930 [DOI] [PubMed] [Google Scholar]
- 55. Navarrete M , Araque A. 2010. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68:113–126 [DOI] [PubMed] [Google Scholar]
- 56. Stella N. 2010. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58:1017–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Breivogel CS , Griffin G , Di Marzo V , Martin BR. 2001. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol 60:155–163 [PubMed] [Google Scholar]
- 58. Rouach N , Nicoll RA. 2003. Endocannabinoids contribute to short-term but not long-term mGluR-induced depression in the hippocampus. Eur J Neurosci 18:1017–1020 [DOI] [PubMed] [Google Scholar]
- 59. Hájos N , Ledent C , Freund TF. 2001. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience United States 106:1–4 [DOI] [PubMed] [Google Scholar]
- 60. Hájos N , Freund TF. 2002. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem Phys Lipids 121:73–82 [DOI] [PubMed] [Google Scholar]
- 61. Pistis M , Perra S , Pillolla G , Melis M , Gessa GL , Muntoni AL. 2004. Cannabinoids modulate neuronal firing in the rat basolateral amygdala: evidence for CB1- and non-CB1-mediated actions. Neuropharmacology 46:115–125 [DOI] [PubMed] [Google Scholar]
- 62. Brown AJ , Wise A. 2001. GlaxoSmithKline. Identification of modulators of GPR55 activity 2001. Patent WO/2001/86305
- 63. Staton PC , Hatcher JP , Walker DJ , Morrison AD , Shapland EM , Hughes JP , Chong E , Mander PK , Green PJ , Billinton A , Fulleylove M , Lancaster HC , Smith JC , Bailey LT , Wise A , Brown AJ , Richardson JC , Chessell IP. 2008. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain 139:225–236 [DOI] [PubMed] [Google Scholar]
- 64. Harkany T , Mackie K , Doherty P. 2008. Wiring and firing neuronal networks: endocannabinoids take center stage. Curr Opin Neurobiol 18:338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Berghuis P , Rajnicek AM , Morozov YM , Ross RA , Mulder J , Urbán GM , Monory K , Marsicano G , Matteoli M , Canty A , Irving AJ , Katona I , Yanagawa Y , Rakic P , Lutz B , Mackie K , Harkany T. 2007. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science (New York, NY) 316:1212–1216 [DOI] [PubMed] [Google Scholar]
- 66. Wu CS , Zhu J , Wager-Miller J , Wang S , O'Leary D , Monory K , Lutz B , Mackie K , Lu HC. 2010. Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. Eur J Neurosci 32:693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chiba T , Ueno S , Obara Y , Nakahata N. 2011. A synthetic cannabinoid, CP55940, inhibits lipopolysaccharide-induced cytokine mRNA expression in a cannabinoid receptor-independent mechanism in rat cerebellar granule cells. J Pharm Pharmacol 63:636–647 [DOI] [PubMed] [Google Scholar]
- 68. Ma MT , Yeo JF , Farooqui AA , Zhang J , Chen P , Ong WY. 2010. Differential effects of lysophospholipids on exocytosis in rat PC12 cells. J Neural Transm 117:301–308 [DOI] [PubMed] [Google Scholar]
- 69. Schuelert N , McDougall JJ. 2011. The abnormal cannabidiol analogue O-1602 reduces nociception in a rat model of acute arthritis via the putative cannabinoid receptor GPR55. Neurosci Lett 500:72–76 [DOI] [PubMed] [Google Scholar]
- 70. O'Sullivan SE , Kendall DA , Randall MD. 2005. The effects of Δ9-tetrahydrocannabinol in rat mesenteric vasculature, and its interactions with the endocannabinoid anandamide. Br J Pharmacol 145:514–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Járai Z , Wagner JA , Varga K , Lake KD , Compton DR , Martin BR , Zimmer AM , Bonner TI , Buckley NE , Mezey E , Razdan RK , Zimmer A , Kunos G. 1999. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA 96:14136–14141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Offertáler L , Mo FM , Bátkai S , Liu J , Begg M , Razdan RK , Martin BR , Bukoski RD , Kunos G. 2003. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol 63:699–705 [DOI] [PubMed] [Google Scholar]
- 73. Ho WS , Hiley CR. 2003. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol 138:1320–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wagner JA , Varga K , Járai Z , Kunos G. 1999. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension 33:429–434 [DOI] [PubMed] [Google Scholar]
- 75. Johns DG , Behm DJ , Walker DJ , Ao Z , Shapland EM , Daniels DA , Riddick M , Dowell S , Staton PC , Green P , Shabon U , Bao W , Aiyar N , Yue TL , Brown AJ , Morrison AD , Douglas SA. 2007. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol 152:825–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Daly CJ , Ross RA , Whyte J , Henstridge CM , Irving AJ , McGrath JC. 2010. Fluorescent ligand binding reveals heterogeneous distribution of adrenoceptors and ‘cannabinoid-like’ receptors in small arteries. Br J Pharmacol 159:787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kaya H , Patton GM , Hong SL. 1989. Bradykinin-induced activation of phospholipase A2 is independent of the activation of polyphosphoinositide-hydrolyzing phospholipase C. J Biol Chem 264:4972–4977 [PubMed] [Google Scholar]
- 78. Deutsch DG , Goligorsky MS , Schmid PC , Krebsbach RJ , Schmid HH , Das SK , Dey SK , Arreaza G , Thorup C , Stefano G , Moore LC. 1997. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest 100:1538–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bondarenko A , Waldeck-Weiermair M , Naghdi S , Poteser M , Malli R , Graier WF. 2010. GPR55-dependent and -independent ion signalling in response to lysophosphatidylinositol in endothelial cells. Br J Pharmacol 161:308–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bondarenko AI , Malli R , Graier WF. 2011. The GPR55 agonist lysophosphatidylinositol acts as an intracellular messenger and bidirectionally modulates Ca2+ -activated large-conductance K+ channels in endothelial cells. Pflugers Arch 461:177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bondarenko AI , Malli R , Graier WF. 2011. The GPR55 agonist lysophosphatidylinositol directly activates intermediate-conductance Ca(2+)-activated K (+) channels. Pflugers Arch 462:245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pacher P , Mechoulam R. 2011. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res 50:193–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tanasescu R , Constantinescu CS. 2010. Cannabinoids and the immune system: an overview. Immunobiology 215:588–597 [DOI] [PubMed] [Google Scholar]
- 84. McHugh D , Ross RA. 2009. Endogenous cannabinoids and neutrophil chemotaxis. Vitam Horm 81:337–365 [DOI] [PubMed] [Google Scholar]
- 85. McHugh D , Hu SS , Rimmerman N , Juknat A , Vogel Z , Walker JM , Bradshaw HB. 2010. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci 11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schicho R , Bashashati M , Bawa M , McHugh D , Saur D , Hu HM , Zimmer A , Lutz B , Mackie K , Bradshaw HB , McCafferty DM , Sharkey KA , Storr M. 2011. The atypical cannabinoid O-1602 protects against experimental colitis and inhibits neutrophil recruitment. Inflamm Bowel Dis 17:1651–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Whyte LS , Ryberg E , Sims NA , Ridge SA , Mackie K , Greasley PJ , Ross RA , Rogers MJ. 2009. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc Natl Acad Sci USA 106:16511–16516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sims NA , Gooi JH. 2008. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol 19:444–451 [DOI] [PubMed] [Google Scholar]
- 89. Andersson J , Sophocleous A , Zhou Y , Rischitor G , Ralston S , Salter D. 2011. Expression of cannabinoid receptors by human articular chondrocytes. Bone 48:S141–S141 [Google Scholar]
- 90. Pertwee RG. 2010. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem 17:1360–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Guzmán M. 2003. Cannabinoids: potential anticancer agents. Nat Rev Cancer 3:745–755 [DOI] [PubMed] [Google Scholar]
- 92. Sarfaraz S , Adhami VM , Syed DN , Afaq F , Mukhtar H. 2008. Cannabinoids for cancer treatment: progress and promise. Cancer Res 68:339–342 [DOI] [PubMed] [Google Scholar]
- 93. Velasco G , Carracedo A , Blázquez C , Lorente M , Aguado T , Haro A , Sánchez C , Galve-Roperh I , Guzmán M. 2007. Cannabinoids and gliomas. Mol Neurobiol 36:60–67 [DOI] [PubMed] [Google Scholar]
- 94. Caffarel MM , Andradas C , Mira E , Pérez-Gómez E , Cerutti C , Moreno-Bueno G , Flores JM , García-Real I , Palacios J , Mañes S , Guzmán M , Sánchez C. 2010. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol Cancer 9:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Qamri Z , Preet A , Nasser MW , Bass CE , Leone G , Barsky SH , Ganju RK. 2009. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol Cancer Ther 8:3117–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang D , Wang H , Ning W , Backlund MG , Dey SK , DuBois RN. 2008. Loss of cannabinoid receptor 1 accelerates intestinal tumor growth. Cancer Res 68:6468–6476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Xiao YJ , Schwartz B , Washington M , Kennedy A , Webster K , Belinson J , Xu Y. 2001. Electrospray ionization mass spectrometry analysis of lysophospholipids in human ascitic fluids: comparison of the lysophospholipid contents in malignant vs nonmalignant ascitic fluids. Anal Biochem 290:302–313 [DOI] [PubMed] [Google Scholar]
- 98. Oka S , Toshida T , Maruyama K , Nakajima K , Yamashita A , Sugiura T. 2009. 2-Arachidonoyl-sn-glycero-3-phosphoinositol: a possible natural ligand for GPR55. J Biochem 145:13–20 [DOI] [PubMed] [Google Scholar]
- 99. Falasca M , Iurisci C , Carvelli A , Sacchetti A , Corda D. 1998. Release of the mitogen lysophosphatidylinositol from H-Ras-transformed fibroblasts; a possible mechanism of autocrine control of cell proliferation. Oncogene 16:2357–2365 [DOI] [PubMed] [Google Scholar]
- 100. Kelly P , Moeller BJ , Juneja J , Booden MA , Der CJ , Daaka Y , Dewhirst MW , Fields TA , Casey PJ. 2006. The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proc Natl Acad Sci USA 103:8173–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lappano R , Maggiolini M. 2011. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov 10:47–60 [DOI] [PubMed] [Google Scholar]
- 102. Andradas C , Caffarel MM , Pérez-Gómez E , Salazar M , Lorente M , Velasco G , Guzmán M , Sánchez C. 2011. The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 30:245–252 [DOI] [PubMed] [Google Scholar]
- 103. Huang L , Ramirez JC , Frampton GA , Golden LE , Quinn MA , Pae HY , Horvat D , Liang LJ , DeMorrow S. 2011. Anandamide exerts its antiproliferative actions on cholangiocarcinoma by activation of the GPR55 receptor. Lab Invest 91:1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cantarella G , Scollo M , Lempereur L , Saccani-Jotti G , Basile F , Bernardini R. 2011. Endocannabinoids inhibit release of nerve growth factor by inflammation-activated mast cells. Biochem Pharmacol 82:380–388 [DOI] [PubMed] [Google Scholar]
- 105. Fonseca BM , Teixeira NA , Almada M , Taylor AH , Konje JC , Correia-da-Silva G. 2011. Modulation of the novel cannabinoid receptor—GPR55—during rat fetoplacental development. Placenta 32:462–469 [DOI] [PubMed] [Google Scholar]
- 106. Fonseca BM , Correia-da-Silva G , Taylor AH , Lam PM , Marczylo TH , Konje JC , Bell SC , Teixeira NA. 2010. N-acylethanolamine levels and expression of their metabolizing enzymes during pregnancy. Endocrinology 151:3965–3974 [DOI] [PubMed] [Google Scholar]
- 107. Matias I , Di Marzo V. 2007. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab 18:27–37 [DOI] [PubMed] [Google Scholar]
- 108. Janiak P , Poirier B , Bidouard JP , Cadrouvele C , Pierre F , Gouraud L , Barbosa I , Dedio J , Maffrand JP , Le Fur G , O'Connor S , Herbert JM. 2007. Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int 72:1345–1357 [DOI] [PubMed] [Google Scholar]
- 109. Jenkin KA , McAinch AJ , Grinfeld E , Hryciw DH. 2010. Role for cannabinoid receptors in human proximal tubular hypertrophy. Cell Physiol Biochem 26:879–886 [DOI] [PubMed] [Google Scholar]
- 110. Chun J , Hla T , Lynch KR , Spiegel S , Moolenaar WH. 2010. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev 62:579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Takabe K , Paugh SW , Milstien S , Spiegel S. 2008. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60:181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xu Y , Xiao YJ , Zhu K , Baudhuin LM , Lu J , Hong G , Kim KS , Cristina KL , Song L , S Williams F , Elson P , Markman M , Belinson J. 2003. Unfolding the pathophysiological role of bioactive lysophospholipids. Curr Drug Targets Immune Endocr Metab Disord 3:23–32 [PubMed] [Google Scholar]
- 113. Panupinthu N , Lee HY , Mills GB. 2010. Lysophosphatidic acid production and action: critical new players in breast cancer initiation and progression. Br J Cancer 102:941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lin ME , Herr DR , Chun J. 2010. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat 91:130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Brinkmann V , Billich A , Baumruker T , Heining P , Schmouder R , Francis G , Aradhye S , Burtin P. 2010. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 9:883–897 [DOI] [PubMed] [Google Scholar]
- 116. Ross RA. 2011. l-α-Lysophosphatidylinositol meets GPR55: a deadly relationship. Trends Pharmacol Sci 32:265–269 [DOI] [PubMed] [Google Scholar]
- 117. Malfait AM , Gallily R , Sumariwalla PF , Malik AS , Andreakos E , Mechoulam R , Feldmann M. 2000. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA 97:9561–9566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chapman CG , Meadows HJ , Godden RJ , Campbell DA , Duckworth M , Kelsell RE , Murdock PR , Randall AD , Rennie GI , Gloger IS. 2000. Cloning, localisation and functional expression of a novel human, cerebellum specific, two pore domain potassium channel. Brain Res Mol Brain Res 82:74–83 [DOI] [PubMed] [Google Scholar]
- 119. Moore DJ , Murdock PR , Watson JM , Faull RL , Waldvogel HJ , Szekeres PG , Wilson S , Freeman KB , Emson PC. 2003. GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res Mol Brain Res 118:10–23 [DOI] [PubMed] [Google Scholar]
- 120. Moore DJ , Chambers JK , Wahlin JP , Tan KB , Moore GB , Jenkins O , Emson PC , Murdock PR. 2001. Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription-polymerase chain reaction study. Biochim Biophys Acta 1521:107–119 [DOI] [PubMed] [Google Scholar]
- 121. Cohen-Yeshurun A , Trembovler V , Alexandrovich A , Ryberg E , Greasley PJ , Mechoulam R , Shohami E , Leker RR. 2011. N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis. J Cereb Blood Flow Metab 8:1768–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]