Abstract
Flea-borne (murine) typhus is a global rickettsiosis caused by Rickettsia typhi. Although flea-borne typhus is no longer nationally notifiable, cases are reported for surveillance purposes in a few U.S. states. The infection is typically self-limiting, but may be severe or life-threatening in some patients. We performed a retrospective review of confirmed or probable cases of fatal flea-borne typhus reported to the Texas Department of State Health Services during 1985–2015. When available, medical charts were also examined. Eleven cases of fatal flea-borne typhus were identified. The median patient age was 62 years (range, 36–84 years) and 8 (73%) were male. Patients presented most commonly with fever (100%), nausea and vomiting (55%), and rash (55%). Respiratory (55%) and neurologic (45%) manifestations were also identified frequently. Laboratory abnormalities included thrombocytopenia (82%) and elevated hepatic transaminases (63%). Flea or animal contact before illness onset was frequently reported (55%). The median time from hospitalization to administration of a tetracycline-class drug was 4 days (range, 0–5 days). The median time from symptom onset to death was 14 days (range, 1–34 days). Flea-borne typhus can be a life-threatening disease if not treated in a timely manner with appropriate tetracycline-class antibiotics. Flea-borne typhus should be considered in febrile patients with animal or flea exposure and respiratory or neurologic symptoms of unknown etiology.
Introduction
Flea-borne typhus, also known as endemic or murine typhus, is a rickettsial zoonosis caused by Rickettsia typhi that occurs predominantly in warm, coastal areas of the world, including certain parts of the United States.1 During the early 1940s, approximately 2,000–5,000 cases were reported annually, but sanitation and vector control campaigns significantly reduced the number of flea-borne typhus cases in the United States. In 1995, flea-borne typhus was removed from the list of nationally notifiable diseases,2 making the contemporary prevalence of flea-borne typhus in the United States difficult to ascertain3,4; nonetheless, flea-borne typhus remains a reportable disease in 14 U.S. states (CA, HI, IL, IN, MI, MN, MS, NE, NH, OH, OR, PA, TX, and WA). The majority of U.S. cases occur in Texas, which reported 3,048 confirmed or probable cases of flea-borne typhus during 1985–2015 (Texas Department of State Health Services, unpublished data). Flea-borne typhus is often described as a relatively mild and self-limiting rickettsiosis; nonetheless, a spectrum of severe manifestations is also recognized,5–10 and the disease may be fatal in as many as 5% of patients for whom appropriate antibiotic therapy is delayed or not provided.11 The objective of this study was to describe the clinical and epidemiologic features associated with fatal flea-borne typhus cases in Texas.
Methods
Flea-borne typhus cases are reported in Texas by using a standardized case report form that includes demographic information, various clinical characteristics, duration of illness, animal and arthropod exposure, travel history, and outdoor activities prior to illness onset. The data were reviewed for confirmed and probable fatal cases identified during 1985–2015. When available, medical charts were examined for supplemental information relating to comorbidities, clinical presentation, antibiotic therapy, laboratory results, and diagnosis at time of death. Diagnostic test results were generated by multiple commercial laboratories or by state or national reference centers. All personally identifiable information was removed from the case investigation forms and medical charts before review at the Centers for Disease Control and Prevention (CDC). This case series protocol was reviewed and determined to qualify as non-research and was exempt from further review according to the CDC's National Center for Emerging and Zoonotic Infectious Diseases institutional procedures.
A confirmed case was defined as a patient with a clinically compatible illness and 1) a 4-fold or greater rise in antibody titer to R. typhi antigens by using an indirect immunofluorescence antibody (IFA) assay in acute and convalescent phase serum specimens; or 2) a single reciprocal IFA titer ≥ 1,024; or 3) a positive polymerase chain reaction (PCR) assay to R. typhi; or 4) demonstration of typhus group Rickettsia sp. antigens by an immunohistochemical stain in tissue; or 5) isolation of R. typhi from clinical specimen. A probable case was defined as a patient with a clinically compatible illness and a reciprocal IgG or IgM IFA titer of ≥ 64.
Results
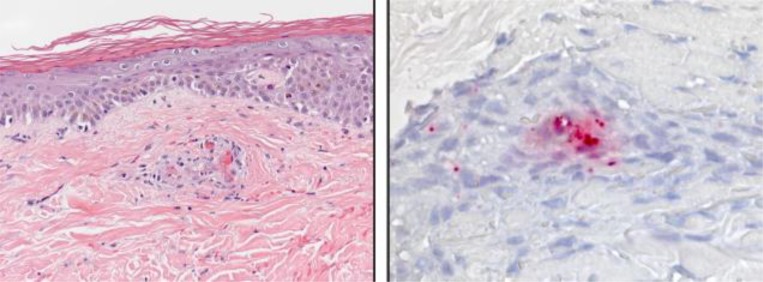
Eleven confirmed or probable fatal cases of flea-borne typhus were reported in Texas during 1985–2015 (Figure 1 ). The median age of the patients was 62 years (range, 36–84 years) and 9 (82%) were > 50 years of age. Eight (73%) patients were male and seven (64%) were of Hispanic ethnicity (Table 1). Six patients met the criteria for a confirmed case; four (67%) patients were diagnosed with single reciprocal IFA titers ≥ 1,024 (range, 1,024–4,096); one patient demonstrated a 4-fold rise in antibody titer; one patient had a skin biopsy specimen that stained positive for antigens of a typhus group Rickettsia sp. (Figure 2 ) and a reciprocal IFA titer of ≥ 4,096 (Table 1). Five patients met the criteria for a probable case and were classified using a reciprocal serologic titer < 1,024 (range, 64–512). Of these, one had reciprocal IgG and IgM titers of 256 and 2,048, respectively, 5 days post-illness onset, and one had reciprocal IgG and IgM titers of 64 and 512, respectively, 9 days after illness onset (Table 1). No patients were diagnosed by PCR or culture isolation. Five (38%) patients were additionally tested by an IFA assay using antigens of Rickettsia rickettsii (the agent of Rocky Mountain spotted fever [RMSF]); 2(40%), 1(20%), and 2(40%) had reciprocal IgG antibody titers to this antigen at < 64, 128, and 512, respectively. Both patients with R. rickettsii titer of 512 had R. typhi titers ≥ 4,096. For the patients with reciprocal titers to both rickettsial antigens, the titer to R. typhi was consistently 4-fold or greater than the titer to R. rickettsii. Two additional patients were identified in 1999 and 2001. These patients each had reciprocal IgG antibody titers to R. typhi of 512; however, no additional case information was available on their clinical course of illness and they were excluded from further analysis.
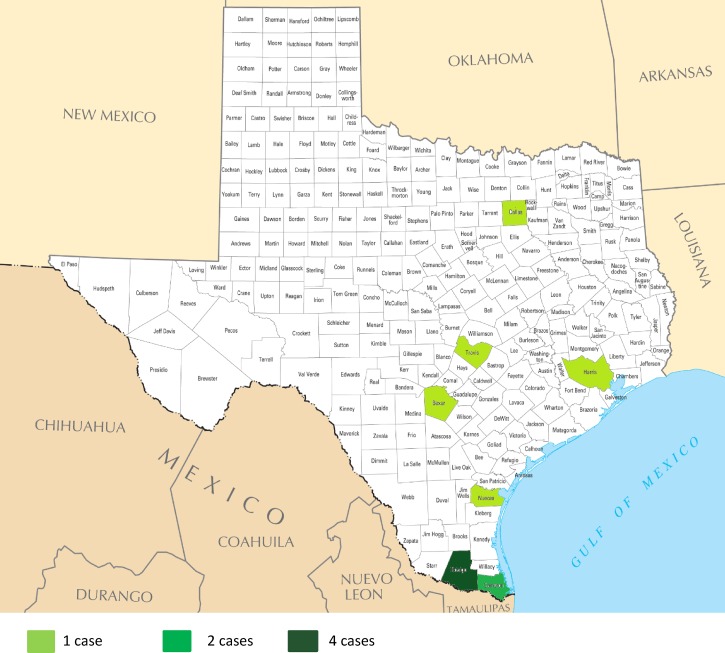
Figure 1.
Texas counties with fatal flea-borne typhus by case count, 1985–2015.
Table 1.
Epidemiological and laboratory data of patients with confirmed or probable fatal flea-borne typhus in Texas, 1985–2015
| Patient (age/sex) | Ethnicity | Animal exposure | Flea exposure | Onset (month/year) | Days from onset to death | Case status | Reciprocal IgG IFA titer(s) to Rickettsia typhi |
|---|---|---|---|---|---|---|---|
| 81/F | Hispanic | Yes*† | Unknown | June 1985 | 11 | Confirmed | 64, 1,024 |
| 67/M | Non-Hispanic | No | No | September 1986 | 17 | Probable | 256 |
| 36/M | Hispanic | Unknown | Unknown | June 1991 | 8 | Confirmed | ≥1,024 |
| 72/M | Hispanic | Unknown | Unknown | June 1995 | 34 | Confirmed | ≥1,024 |
| 73/F | Hispanic | Yes† | Yes | December 1998 | 6 | Probable | 512§ |
| 53/M | Hispanic | Unknown | Unknown | January 2007 | 6 | Confirmed | ≥1,024 |
| 36/M | Non-Hispanic | Yes*‡ | Yes | April 2012 | 13 | Confirmed | ≥4,096 |
| 50/M | Non-Hispanic | Yes† | Unknown | May 2013 | 20 | Confirmed | ≥4,096¶ |
| 55/M | Hispanic | Yes†‡ | Yes | May 2013 | 14 | Probable | 256 |
| 84/F | Hispanic | Unknown | Unknown | November 2014 | 12 | Probable | 64∥ |
| 62/M | Unknown | Yes‡ | Unknown | January 2015 | 9 | Probable | 256** |
IFA = indirect immunofluorescence antibody.
Reported exposure to: * opossum, † cat, ‡ dog.
Patient also had 1:40 titer to R. typhi by slide agglutination assay.
Patient also had a skin biopsy specimen positive for a typhus group Rickettsia sp.by immunohistochemical stain.
Patient also had a reciprocal IgM IFA titer of 512 on same date.
Patient also had a reciprocal IgM IFA titer of 2,048 on same date.
Figure 2.
Histopathological and immunohistochemical appearance of a skin biopsy specimen from a patient with a fatal typhus group rickettsiosis.
Eight of 11 (73%) case report forms contained information on flea or animal contact in the 2 weeks before illness onset. Three (27%) patients reported fleas in the home, three (27%) patients had at least one dog, and four (36%) patients either owned a cat or cared for stray cats. Two (18%) patients reported an opossum living within or next to their home (Table 1). One (9%) patient reported hunting in south Texas before illness onset, but no specific animal species were named. Cases occurred year-round with no apparent seasonality (Table 1).
All patients were hospitalized. At hospital presentation, fever was reported for 11 (100%) patients. The median temperature was 101.9°F (range, 99–104.6°F). Thrombocytopenia (82%) and elevated hepatic transaminase levels (64%) were the most frequently reported laboratory abnormalities. Six (55%) patients reported one or more respiratory-related symptom (i.e., cough), or diagnosis (i.e., pneumonia, pulmonary edema, or acute respiratory distress syndrome). Rash was reported in 55% of patients, and was described as macular and maculopapular (50%) or petechial (50%), and was distributed across the palms and soles (33%), arms and legs (33%), and trunk (67%) (Table 2). Rash was frequently reported as a late finding, occurring > 7 days after initial illness onset.
Table 2.
Clinical characteristics of 11 patients with fatal flea-borne typhus in Texas, 1985–2015
| Patient (age/sex) | Fever | Thrombocytopenia | Elevated hepatic transaminases | Anorexia | Nausea/vomiting | Rash | Pneumonia | Headache | Coma | Cough | Encephalopathy | Pulmonary edema | Acute kidney injury | Meningitis | Vertigo | Acute respiratory distress syndrome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 81/F*† | √ | √ | √ | √ | ||||||||||||
| 67/M | √ | √ | √ | √ | √ | |||||||||||
| 36/M‡ | √ | √ | √ | |||||||||||||
| 72/M* | √ | √ | √ | √ | √ | √ | ||||||||||
| 73/F*‡ | √ | √ | √ | √ | √ | |||||||||||
| 53/M‡ | √ | √ | √ | √ | √ | √ | √ | |||||||||
| 36/M‡ | √ | √ | √ | √ | √ | √ | √ | |||||||||
| 50/M*‡ | √ | √ | √ | √ | ||||||||||||
| 55/M*‡ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||
| 84/F*†‡ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||
| 62/M*‡ | √ | √ | √ | √ | √ | √ | √ | |||||||||
| Total (%) | 11 (100) | 9 (82) | 7 (63) | 6 (55) | 6 (55) | 6 (55) | 3 (27) | 3 (27) | 3 (27) | 2 (18) | 2 (18) | 2 (18) | 2 (18) | 1 (9) | 1 (9) | 1 (9) |
Patients that received tetracycline-class antibiotic during hospitalization.
Patients that received sulfa drug before hospitalization.
Medical charts available for review.
The median time from symptom onset to hospitalization was 8 days (range, 1–21 days), and the median time from symptom onset to death was 14 days (range, 1–34 days). Four (36%) patients sought medical care during their first week of illness; two cases were diagnosed with urinary tract infections unrelated to R. typhi infection and prescribed non-tetracycline class antibiotics, one patient received an unknown antibiotic for an unspecified illness, and the fourth patient had no information available. Two (18%) patients received a sulfa-containing antibiotic during their illness. Seven (64%) patients received a tetracycline-class antibiotic (doxycycline or tetracycline) during hospitalization. The median number of days from hospital admission to initiation of a tetracycline-class antibiotic was 4 (range, 0–5 days). The median time from symptom onset to initiation of a tetracycline-class drug was 9 days (range, 4–17 days).
Additional data abstracted from medical charts were available for eight patients. Of these, neurologic complications were present at hospital admission in five (63%) patients and included meningitis, encephalitis, vertigo, dizziness, seizure, and coma. Two of the five patients were obtunded on presentation and never regained consciousness. At hospital presentation, two (25%) patients had acute kidney injury. Comorbidities were for five (63%) of these patients including three (37%) with a history of alcohol abuse, one (13%) with chronic renal disease, congestive heart failure, and Type 2 diabetes mellitus, and one (13%) with a preexisting seizure disorder.
Discussion
To our knowledge, this is the largest series of patients with fatal flea-borne typhus. The case fatality rate (CFR) in Texas during this period (0.4%) was not significantly different from the CFR described for 200 cases of flea-borne typhus from Texas that occurred during 1980–198412 (1%, P = 0.30). During the pre-antibiotic era, CFRs of flea-borne typhus varied regionally, but the overall CFR for 18,337 U.S. cases during 1940–1944 was 4.6%.11–14 Among the cases reviewed herein, a high proportion were male (73%) and over the age of 50 years (82%). More than 70% of the patients in this series who were treated with a tetracycline-class antibiotic did not receive this drug until the second week of illness, emphasizing the need for prompt administration of appropriate antimicrobial therapy to reduce fatal outcome.
Respiratory signs or symptoms were noted for more than half of the patients in this series. The frequency of respiratory manifestations have been recognized for many years; indeed, an early summary of flea-borne typhus in Texas noted, “few diseases present so much subjective respiratory symptoms with so little objective findings.”15 Previous literature reported 14–59% of flea-borne typhus patients had respiratory symptoms.12,16,17 Neurologic manifestations were also seen in more than half of the patients for whom additional data were available, and previous literature has described neurologic manifestations in 15–45% of patients.17,18 Our findings are consistent with previous literature and suggest that respiratory and neurologic complications from R. typhi infection may occur frequently in severe cases.6,16,18–21 The frequency of respiratory and neurologic manifestations identified in this series may have been confounded by the advanced stage of disease that patients presented at initial hospital presentation. Previous literature reports risk of complications increases with age. Therefore, older patients (> 50 years) may be at greater risk for poor outcomes.20,22,23
Of the seven patients with elevated hepatic transaminases, three (43%) had a history of alcohol abuse. Clinicians may have attributed elevated hepatic transaminases to complications from alcohol abuse; however, hepatic inflammation has also been documented with severe flea-borne typhus.24,25 Two patients in this case series were treated with sulfa-containing antibiotic after illness onset and before hospital admission. Multiple historical and contemporary case reports describe adverse outcomes in flea-borne typhus patients treated with sulfa-containing antibiotics.11,18,26 Similar observations have been identified in patients with other rickettsial diseases, particularly RMSF and Ehrlichia chaffeensis ehrlichiosis.27 Case–control studies are needed to better assess the potential contraindication of sulfa drugs in the treatment of patients with rickettsiosis, including flea-borne typhus. Glucose-6-phosphate dehydrogenase deficiency was not identified or assessed as a comorbidity in any of the patients in this series; nonetheless, this condition has been previously associated with life-threatening disease in patients with flea-borne typhus28 as well as other rickettsioses including RMSF29 and Mediterranean spotted fever (caused by Rickettsia conorii)30 and scrub typhus (caused by Orientia tsutsugamushi).28
Five cases of fatal flea-borne typhus have been reported previously in detail.20,26,31 These patients, from the United States and Spain, presented with similar demographic and clinical characteristics to the patients in our case series. Their median age was 56 years (range, 46–81 years), three (60%) were male, one (20%) reported contact with rodents, four (80%) reported fever, one (20%) had rash, two (40%) had thrombocytopenia, one (20%) developed respiratory manifestations, and one (20%) developed neurologic manifestations. The median time from illness onset to death was 13 days (range, 11–15 days). Importantly, two (40%) received a sulfa-containing antibiotic.
In previous outbreaks, as many as 70% of patients of flea-borne typhus have required hospitalization and as many as 30% of these patients require admission to an intensive care unit32; nonetheless, even in areas such as Texas where the disease is endemic, a correct diagnosis of flea-borne typhus is often delayed for 10 or more days.33 The signs and symptoms of even advanced disease may be nonspecific and can hamper a timely diagnosis. Delay in treatment with a tetracycline-class antibiotic is associated with poorer outcomes including death. Doxycycline is the drug of choice for treating rickettsiosis in patients of all ages.34 Flea-borne typhus should be considered in patients with a history of animal or flea exposure and a nonspecific febrile illness of > 3 days.22,31,34 Absence of rash should not exclude a presumptive diagnosis of flea-borne typhus, particularly as it often occurs late in the development of disease, as identified in this series. A history of recent exposure to fleas or animals can provide valuable clues into potential etiology of unknown illness; however, absence of known animal or flea exposures does not rule out flea-borne typhus in an endemic area, as patients may be unaware of these exposures.
This case series had several limitations inherent to studies that use data acquired predominantly or exclusively through passive surveillance, including incompleteness of information reported to the state health department, and inability to review medical charts for some of the patients. In contrast to many other rickettsioses endemic in the United States, typhus group rickettsioses are not nationally notifiable. In that context, there are no nationally recognized laboratory criteria to define confirmed or probable cases of typhus group rickettsioses. Because each of the fatal cases were from Texas, we applied the case definitions developed by the Texas Department of State Health Services. Nonetheless, a single antibody titer is not a universally accepted laboratory criterion for a confirmed case. Furthermore, because this was a retrospective study, the serological data were generated by multiple laboratories during a 30-year period and therefore lack consistency with respect to reagents, methods, and interpretation by laboratory personnel. Finally, this study lacked a control group to comparatively assess risk factors. Future case–control studies could help identify risk factors for severe and fatal outcomes in patients with flea-borne typhus.
Infection with Rickettsia felis, a typhus-like Rickettsia, has been documented in at least one patient in Texas.35 Because R. typhi and R. felis occur sympatrically in fleas in Texas, it is possible that R. felis may be responsible for some cases of flea-borne typhus36–38; however, vertebrates infected with R. felis characteristically produce antibodies that cross-react with antigens of spotted fever group rickettsiae rather than typhus group rickettsiae.39,40 Antibodies reactive with spotted fever group rickettsiae were detected in five of the patients in this series for whom these tests were performed, but each of these patients had corresponding typhus group titers that were at least 4-fold higher. Additionally, R. typhi typically causes more severe disease in humans than R. felis,6,19,22 and to our knowledge there are no reported fatal cases of human infection with R. felis. A previous study of Texas patients for whom a flea-borne rickettsiosis was suspected found evidence of infection with R. typhi in 22% versus infection with R. felis in only 2%, by using species-specific recombinant antigens, to suggest that infections with R. typhi are more prevalent in humans in Texas than are infections with R. felis.41 In this context, we believe each of the fatal cases described herein represented infection with R. typhi rather than R. felis. A confirmed human infection with Rickettsia prowazekii, another typhus group Rickettsia species, was described in 2001 that may have been acquired in south Texas42 and serological evidence of infection with R. prowazekii was confirmed by cross-adsorption assays in two of 19 homeless persons in Houston, TX, with antibodies reactive to typhus group rickettsiae.43 Rickettsia prowazekii has been detected in an Amblyomma tick collected in northern Mexico,44 and this species is also found in south Texas.45 In this context, it is also possible that some of the cases in this case series were caused by R. prowazekii rather than R. typhi.
Clinicians may want to consider older age, delay in treatment, and a history of alcohol abuse as potential risk factors for severe flea-borne typhus; however, case–control studies need to be conducted to identify risk factors for severe disease. Severity of disease may be mitigated by early diagnosis and initiation of appropriate tetracycline-class antibiotic therapy. Clinicians should be aware that flea-borne typhus is a potentially serious illness and should be considered in febrile patients with animal or flea exposure and respiratory or neurologic signs of unknown etiology.
ACKNOWLEDGMENTS
We would like to acknowledge the contribution of the local, regional, and state public health personnel for their assistance in reporting and investigating cases of flea-borne typhus.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Emily G. Pieracci, Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, and Epidemic Intelligence Service, Center for Surveillance, Epidemiology and Laboratory Services, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: epieracci@cdc.gov. Nicole Evert, Bonny Mayes, and Inger Vilcins, Texas Department of State Health Services, Austin, TX, E-mails: nicole.evert@dshs.state.tx.us, bonny.mayes@dshs.state.tx.us, and inger.vilcins@dshs.state.tx.us. Naomi A. Drexler, Casey Barton Behravesh, and Christopher D. Paddock, Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: ndrexler@cdc.gov, cbartonBehravesh@cdc.gov, and cpaddock@cdc.gov. Philip Huang and Jill Campbell, Austin/Travis County Health and Human Services Department, Austin, TX, E-mails: philip.huang@austintexas.gov and jill.campbell@dshs.state.tx.us.
References
- 1.Azad AF. Epidemiology of murine typhus. Annu Rev Entomol. 1990;35:553–569. doi: 10.1146/annurev.en.35.010190.003005. [DOI] [PubMed] [Google Scholar]
- 2.CDC Summary of notifiable diseases—United States, 2002. MMWR Morb Mortal Wkly Rep. 2004;51:74. [PubMed] [Google Scholar]
- 3.Evert N, Mayes B. Flea-Borne Typhus in Texas (2003–2013); 65th James Steele Conference on Diseases in Nature Transmissible to Man, 2015; Galveston, TX: 2015. p. 57. [Google Scholar]
- 4.Boostrom A, Beier MS, Macaluso JA, Macaluso KR, Sprenger D, Hayes J, Radulovic S, Azad AF. Geographic association of Rickettsia felis-infected opossums with human murine typhus, Texas. Emerg Infect Dis. 2002;8:549–554. doi: 10.3201/eid0806.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteford SF, Taylor JP, Dumler JS. Clinical, laboratory, and epidemiologic features of murine typhus in 97 Texas children. Arch Pediatr Adolesc Med. 2001;155:396–400. doi: 10.1001/archpedi.155.3.396. [DOI] [PubMed] [Google Scholar]
- 6.Carr SB, Bergamo DF, Emmanuel PJ, Ferreira JA. Murine typhus as a cause of cognitive impairment: case report and a review of the literature. Pediatr Neurol. 2014;50:265–268. doi: 10.1016/j.pediatrneurol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Fergie JE, Purcell K, Wanat D. Murine typhus in south Texas children. Pediatr Infect Dis J. 2000;19:535–538. doi: 10.1097/00006454-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto N, Nakamura-Uchiyama F, Kobayashi K, Takasaki T, Ogasawara Y, Ando S, Iwabuchi S, Ohnishi K. Severe murine typhus with shock and acute respiratory failure in a Japanese traveler after returning from Thailand. J Travel Med. 2013;20:50–53. doi: 10.1111/j.1708-8305.2012.00678.x. [DOI] [PubMed] [Google Scholar]
- 9.Dittrich S, Rattanavong S, Lee SJ, Panyanivong P, Craig SB, Tulsiani SM, Blacksell SD, Dance DA, Dubot-Peres A, Sengduangphachanh A, Phoumin P, Paris DH, Newton PN. Orientia, Rickettsia, and leptospira pathogens as causes of CNS infections in Laos: a prospective study. Lancet Glob Health. 2015;3:e104–e112. doi: 10.1016/S2214-109X(14)70289-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adjemian J, Parks S, McElroy K, Campbell J, Eremeeva ME, Nicholson WL, McQuiston J, Taylor J. Murine typhus in Austin, Texas, USA, 2008. Emerg Infect Dis. 2010;16:412–417. doi: 10.3201/eid1603.091028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson JC, Overall JC, Shapiro JL. Rickettsial diseases of childhood; a clinical pathologic study of tick typhus, Rocky Mountain spotted fever and murine typhus, endemic typhus. J Pediatr. 1947;30:495–528. doi: 10.1016/s0022-3476(47)80046-0. [DOI] [PubMed] [Google Scholar]
- 12.Miller ES, Beeson PB. Murine typhus fever. Medicine. 1946;25:1–15. doi: 10.1097/00005792-194602000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Beck D, Van Allen A. Typhus Fever in California, 1916–1948, Inclusive. San Francisco: CA; State of California, Department of Public Health: 1950. [Google Scholar]
- 14.Joseph C. Typhus fever in Charity Hospital. New Orleans Med Surg J. 1942;95:53–60. [Google Scholar]
- 15.Kemp HA. Endemic typhus fever in Texas: an epidemiological and clinical comparison with forms of typhus seen elsewhere. Am J Trop Med Hyg. 1939;S1–19:109–129. [Google Scholar]
- 16.van der Vaart TW, van Thiel PP, Juffermans NP, van Vugt M, Geerlings SE, Grobusch MP, Goorhuis A. Severe murine typhus with pulmonary system involvement. Emerg Infect Dis. 2014;20:1375–1377. doi: 10.3201/eid2008.131421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuart BM, Pullen RL. Endemic (murine) typhus fever: clinical observations of 180 cases. Ann Intern Med. 1945;23:520–536. [Google Scholar]
- 18.Dumler JS, Taylor JP, Walker DH. Clinical and laboratory features of murine typhus in south Texas, 1980 through 1987. JAMA. 1991;266:1365–1370. [PubMed] [Google Scholar]
- 19.Chaliotis G, Kritsotakis EI, Psaroulaki A, Tselentis Y, Gikas A. Murine typhus in central Greece: epidemiological, clinical, laboratory, and therapeutic-response features of 90 cases. Int J Infect Dis. 2012;16:e591–e596. doi: 10.1016/j.ijid.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Walker DH, Parks FM, Betz TG, Taylor JP, Muehlberger JW. Histopathology and immunohistologic demonstration of the distribution of Rickettsia typhi in fatal murine typhus. Am J Clin Pathol. 1989;91:720–724. doi: 10.1093/ajcp/91.6.720. [DOI] [PubMed] [Google Scholar]
- 21.Bernabeu-Wittel M, Villanueva-Marcos JL, de Alarcon-Gonzalez A, Pachon J. Septic shock and multiorganic failure in murine typhus. Eur J Clin Microbiol Infect Dis. 1998;17:131–132. doi: 10.1007/BF01682172. [DOI] [PubMed] [Google Scholar]
- 22.Tsioutis C, Chaliotis G, Kokkini S, Doukakis S, Tselentis Y, Psaroulaki A, Gikas A. Murine typhus in elderly patients: a prospective study of 49 patients. Scand J Infect Dis. 2014;46:779–782. doi: 10.3109/00365548.2014.943283. [DOI] [PubMed] [Google Scholar]
- 23.Irons JV, Cox GW. An epidemiological investigation of typhus fever in Texas, 1943–1945. Tex State J Med. 1946;42:332–336. [PubMed] [Google Scholar]
- 24.Silpapojakul K, Mitarnun W, Ovartlarnporn B, Chamroonkul N, Khow-Ean U. Liver involvement in murine typhus. Q J Med. 1996;89:623–629. doi: 10.1093/qjmed/89.8.623. [DOI] [PubMed] [Google Scholar]
- 25.Botelho-Nevers E, Raoult D. Host, pathogen and treatment-related prognostic factors in rickettsioses. Eur J Clin Microbiol Infect Dis. 2011;30:1139–1150. doi: 10.1007/s10096-011-1208-z. [DOI] [PubMed] [Google Scholar]
- 26.Binford CH, Ecker HD. Endemic (murine) typhus; report of autopsy findings in three cases. Am J Clin Pathol. 1947;17:797–806. doi: 10.1093/ajcp/17.10.797. [DOI] [PubMed] [Google Scholar]
- 27.Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS, Folk SM, Kato CY, Lash RR, Levin ML, Massung RF, Nadelman RB, Nicholson WL, Paddock CD, Pritt BS, Traeger MS. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Morb Mortal Wkly Rep. 2016;65:1–44. doi: 10.15585/mmwr.rr6502a1. [DOI] [PubMed] [Google Scholar]
- 28.Whelton A, Donadio JV, Jr, Elisberg BL. Acute renal failure complicating rickettsial infections in glucose-6-phosphate dehydrogenase-deficient individuals. Ann Intern Med. 1968;69:323–328. doi: 10.7326/0003-4819-69-2-323. [DOI] [PubMed] [Google Scholar]
- 29.Walker DH, Kirkman HN. Rocky Mountain spotted fever and deficiency in glucose-6-phosphate dehydrogenase. J Infect Dis. 1980;142:771. doi: 10.1093/infdis/142.5.771. [DOI] [PubMed] [Google Scholar]
- 30.Raoult D, Lena D, Perrimont H, Gallais H, Walker DH, Casanova P. Haemolysis with Mediterranean spotted fever and glucose-6-phosphate dehydrogenase deficiency. Trans R Soc Trop Med Hyg. 1986;80:961–962. doi: 10.1016/0035-9203(86)90272-5. [DOI] [PubMed] [Google Scholar]
- 31.Pether JV, Jones W, Lloyd G, Rutter DA, Barry M. Fatal murine typhus from Spain. Lancet. 1994;344:897–898. doi: 10.1016/s0140-6736(94)92875-4. [DOI] [PubMed] [Google Scholar]
- 32.CDC Outbreak of Rickettsia typhi infection: Austin, Texas, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1267–1270. [PubMed] [Google Scholar]
- 33.Carter CN, Ronald NC, Steele JH, Young E, Taylor JP, Russell LH, Jr, Eugster AK, West JE. Knowledge-based patient screening for rare and emerging infectious/parasitic diseases: a case study of brucellosis and murine typhus. Emerg Infect Dis. 1997;3:73–76. doi: 10.3201/eid0301.970111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todd SR, Dahlgren FS, Traeger MS, Beltran-Aguilar ED, Marianos DW, Hamilton C, McQuiston J, Regan JJ. No visible dental staining in children treated with doxycycline for suspected Rocky Mountain spotted fever. J Pediatr. 2015;166:1246–1251. doi: 10.1016/j.jpeds.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Azad AF, Radulovic S, Higgins JA, Noden BH, Troyer JM. Flea-borne rickettsioses: ecologic considerations. Emerg Infect Dis. 1997;3:319–327. doi: 10.3201/eid0303.970308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eremeeva ME, Warashina WR, Sturgeon MM, Buchholz AE, Olmsted GK, Park SY, Effler PV, Karpathy SE. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis. 2008;14:1613–1615. doi: 10.3201/eid1410.080571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanton LS, Vohra RF, Bouyer DH, Walker DH. Reemergence of murine typhus in Galveston, Texas, USA, 2013. Emerg Infect Dis. 2015;21:484–486. doi: 10.3201/eid2103.140716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basra G, Berman MA, Blanton LS. Murine typhus: an important consideration for the nonspecific febrile illness. Case Rep Med. 2012;2012:134601. doi: 10.1155/2012/134601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang R, Raoult D. Antigenic classification of Rickettsia felis by using monoclonal and polyclonal antibodies. Clin Diagn Lab Immunol. 2003;10:221–228. doi: 10.1128/CDLI.10.2.221-228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maina AN, Fogarty C, Krueger L, Macaluso KR, Odhiambo A, Nguyen K, Farris CM, Luce-Fedrow A, Bennett S, Jiang J, Sun S, Cummings RF, Richards AL. Rickettsial infections among Ctenocephalides felis and host animals during a flea-borne typhus rickettsioses outbreak in Orange County, California. PLoS One. 2016;11:e0160604. doi: 10.1371/journal.pone.0160604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiggers RJ, Martin MC, Bouyer D. Rickettsia felis infection rates in an east Texas population. Tex Med. 2005;101:56–58. [PubMed] [Google Scholar]
- 42.Massung RF, Davis LE, Slater K, McKechnie DB, Puerzer M. Epidemic typhus meningitis in the southwestern United States. Clin Infect Dis. 2001;32:979–982. doi: 10.1086/319351. [DOI] [PubMed] [Google Scholar]
- 43.Reeves WK, Murray KO, Meyer TE, Bull LM, Pascua RF, Holmes KC, Loftis AD. Serological evidence of typhus group Rickettsia in a homeless population in Houston, Texas. J Vector Ecol. 2008;33:205–207. doi: 10.3376/1081-1710(2008)33[205:seotgr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 44.Medina-Sanchez A, Bouyer DH, Alcantara-Rodriguez V, Mafra C, Zavala-Castro J, Whitworth T, Popov VL, Fernandez-Salas I, Walker DH. Detection of a typhus group Rickettsia in Amblyomma ticks in the state of Nuevo Leon, Mexico. Ann N Y Acad Sci. 2005;1063:327–332. doi: 10.1196/annals.1355.052. [DOI] [PubMed] [Google Scholar]
- 45.Hilburn LR, Gunn SJ, Castillo C. Comparison of the isozyme phenotypes of the morphologically similar ticks Amblyomma cajennense and A. imitator (Acari: Ixodidae) from south Texas. J Med Entomol. 1989;26:23–29. doi: 10.1093/jmedent/26.1.23. [DOI] [PubMed] [Google Scholar]