Abstract
Isolated envelope membranes of spinach chloroplasts (Spinacia oleracea L. var. Viroflay) exhibited selective permeability. Metabolites such as 3-phosphoglycerate, bicarbonate, glyoxylate, and acetate were transported rapidly; 6-phosphogluconate, glycolate, glycine, l-malate, and succinate were intermediate; whereas glucose 6-phosphate, fructose 1,6-diphosphate, and sucrose were hardly transported. Transport rates, metabolite accumulations within the membrane vesicles, and the internal water volume of isolated and in situ envelope membranes were compared and found to show similar trends.
Full text
PDF
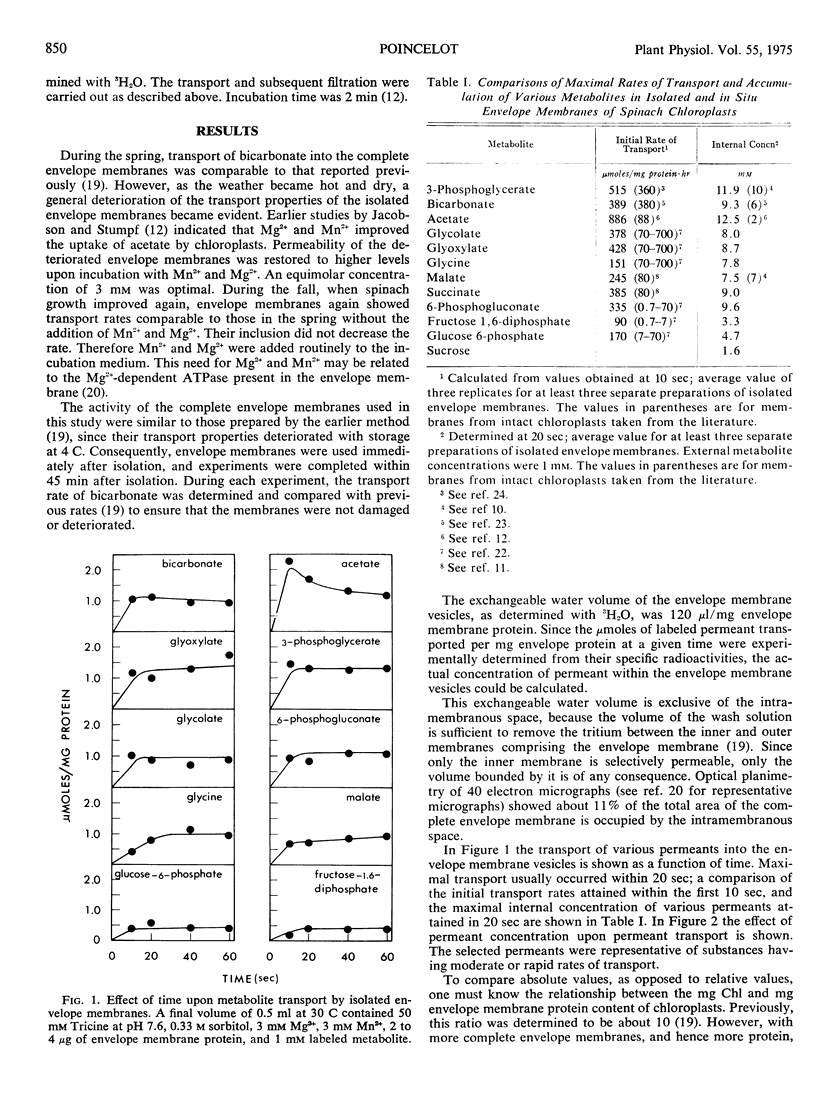
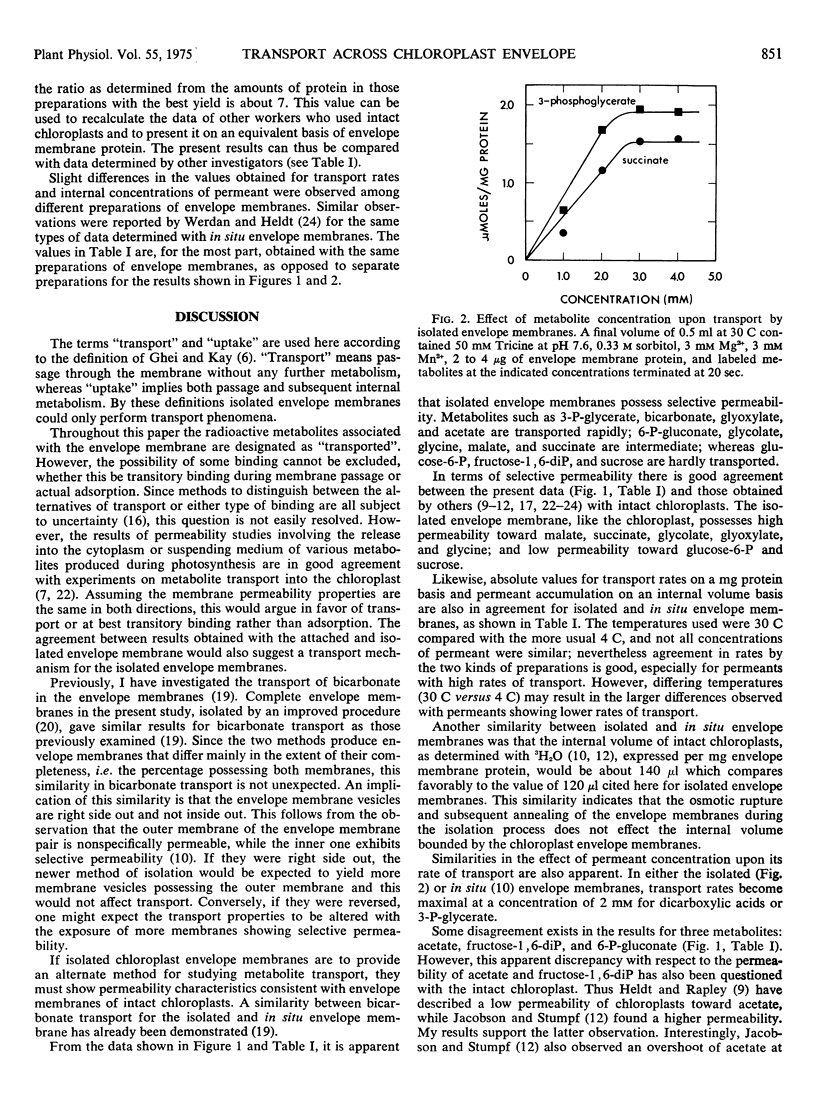

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldry C. W., Walker D. A., Bucke C. Calvin-cycle intermediates in relation to induction phenomena in photosynthetic carbon dioxide fixation by isolated chloroplasts. Biochem J. 1966 Dec;101(3):642–646. doi: 10.1042/bj1010642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham J. A., Kirk M., Jensen R. G. Photosynthesis by isolated chloroplasts. I. Diffusion of labeled photosynthetic intermediates between isolated chloroplasts and suspending medium. Biochim Biophys Acta. 1968 Jan 15;153(1):211–218. doi: 10.1016/0005-2728(68)90162-x. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P. Regulation of ribulose 1,5-diphosphate carboxylase in the photosynthetic assimilation of carbon dioxide. J Biol Chem. 1973 Jul 25;248(14):4956–4964. [PubMed] [Google Scholar]
- Chu D. K., Bassham J. A. Inhibition of ribulose 1,5-diphosphate carboxylase by 6-phosphogluconate. Plant Physiol. 1972 Aug;50(2):224–227. doi: 10.1104/pp.50.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Holtz R. B., Benson A. A. Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem. 1973 Oct 25;248(20):7215–7222. [PubMed] [Google Scholar]
- Ghei O. K., Kay W. W. Properties of an inducible C 4 -dicarboxylic acid transport system in Bacillus subtilis. J Bacteriol. 1973 Apr;114(1):65–79. doi: 10.1128/jb.114.1.65-79.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Jacobson B. S., Stumpf P. K. Fat metabolism in higher plants. LV. Acetate uptake and accumulation by class I and II chloroplasts from Spinacia oleracea. Arch Biochem Biophys. 1972 Dec;153(2):656–663. doi: 10.1016/0003-9861(72)90384-0. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nobel P. S., Wang C. T. Amino acid permeability of pea chloroplasts as measured by osmotically determined reflection coefficients. Biochim Biophys Acta. 1970 Jul 7;211(1):79–87. doi: 10.1016/0005-2736(70)90125-2. [DOI] [PubMed] [Google Scholar]
- Poincelot R. P., Day P. R. An improved method for the isolation of spinach chloroplast envelope membranes. Plant Physiol. 1974 Nov;54(5):780–783. doi: 10.1104/pp.54.5.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincelot R. P. Isolation and lipid composition of spinach chloroplast envelope membranes. Arch Biochem Biophys. 1973 Nov;159(1):134–142. doi: 10.1016/0003-9861(73)90437-2. [DOI] [PubMed] [Google Scholar]
- Poincelot R. P. Uptake of bicarbonate ion in darkness by isolated chloroplast envelope membranes and intact chloroplasts of spinach. Plant Physiol. 1974 Oct;54(4):520–526. doi: 10.1104/pp.54.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenn J. D., Lilley R. M., Walker D. A. Inorganic pyrophospatase and photosynthesis by isolated chloroplasts. I. Characterisation of chloroplast pyrophosphatase and its relation to the response to exogenous pyrophosphate. Biochim Biophys Acta. 1973 Dec 14;325(3):586–595. doi: 10.1016/0005-2728(73)90218-1. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim Biophys Acta. 1972 Dec 14;283(3):430–441. doi: 10.1016/0005-2728(72)90260-5. [DOI] [PubMed] [Google Scholar]
- ZELITCH I. THE RELATION OF GLYCOLIC ACID SYNTHESIS TO THE PRIMARY PHOTOSYNTHETIC CARBOXYLATION REACTION IN LEAVES. J Biol Chem. 1965 May;240:1869–1876. [PubMed] [Google Scholar]


