Abstract
Several case reports of autochthonous leishmaniasis in Thailand have been published since 1996. Most of the previous cases presented with visceral leishmaniasis (VL) and were mostly reported in southern part of Thailand. Recently, it has been evident that Leishmania martiniquensis is the main cause of Leishmania infection in Thailand. However, Leishmania siamensis (PCM2 Trang isolate) was found to be of a separate lineage with restricted distribution in southern Thailand and also a cause of disseminated dermal and visceral leishmaniasis in one published case. Here we report the first patient from central Thailand with human immunodeficiency virus infection presenting with disseminated dermal leishmaniasis. Polymerase chain reaction and DNA sequencing analysis (large subunit of RNA polymerase II and 18S ribosomal RNA internal transcribed spacer 1) from the tissue biopsy sample revealed the pathogen sequences to be highly homologous to PCM2 Trang strain previously reported from southern Thailand.
Introduction
Leishmaniasis is a rare tropical disease found in tropical and subtropical countries. Thailand was once considered to be a leishmaniasis-free country. However, during 1960–1986, a few cases of leishmaniasis were described among Thai people who had been to the endemic areas.1 Since 1996, sporadic indigenous cases of leishmaniasis were reported in 1996, 2005, and 2007.2–4 In 2008, a new causative species of autochthonous visceral leishmaniasis Leishmania siamensis was described for the first time by Sukmee and others.5 From then on, more than 10 cases of autochthonous leishmaniasis caused by L. siamensis have been occasionally reported. Until recently, it has been evident that most of the so-called L. siamensis are Leishmania martiniquensis.6
To date, there has been only one published case of PCM2 in southern Thailand.7 Here we report, for the first time, L. siamensis (PCM2 Trang isolate) from a patient living in central Thailand. Furthermore, by large subunit of RNA polymerase II gene and ribosomal RNA internal transcribed spacer 1 (ITS-1) sequencing, we demonstrate that this Leishmania appears to be identical or very similar to an organism previously reported from Trang Province of southern Thailand, namely PCM2 isolate.7
Case Report
In July 2015, a 42-year-old human immunodeficiency virus (HIV)–infected Thai woman with a CD4 count of 89/cm2 presented with 2 weeks of low grade fever and multiple painless erythematous to hyperpigmented plaques and nodules on the skin of her face and lower extremities. These lesions gradually increased in size and number during observation over 3 months.
She was a housewife who lived in the Kanchanaburi Province of central Thailand and had never traveled outside Thailand and other parts of Thailand apart from central area. Ten months ago, she was diagnosed with HIV infection presenting with a CD4 count of 9/cm2 and has had opportunistic infections of Pneumocystis jirovecii pneumonia and pulmonary tuberculosis. Her medications include stavudine, lamivudine, ritonavir-boosted lopinavir, efavirenz, cotrimoxazole, and fluconazole.
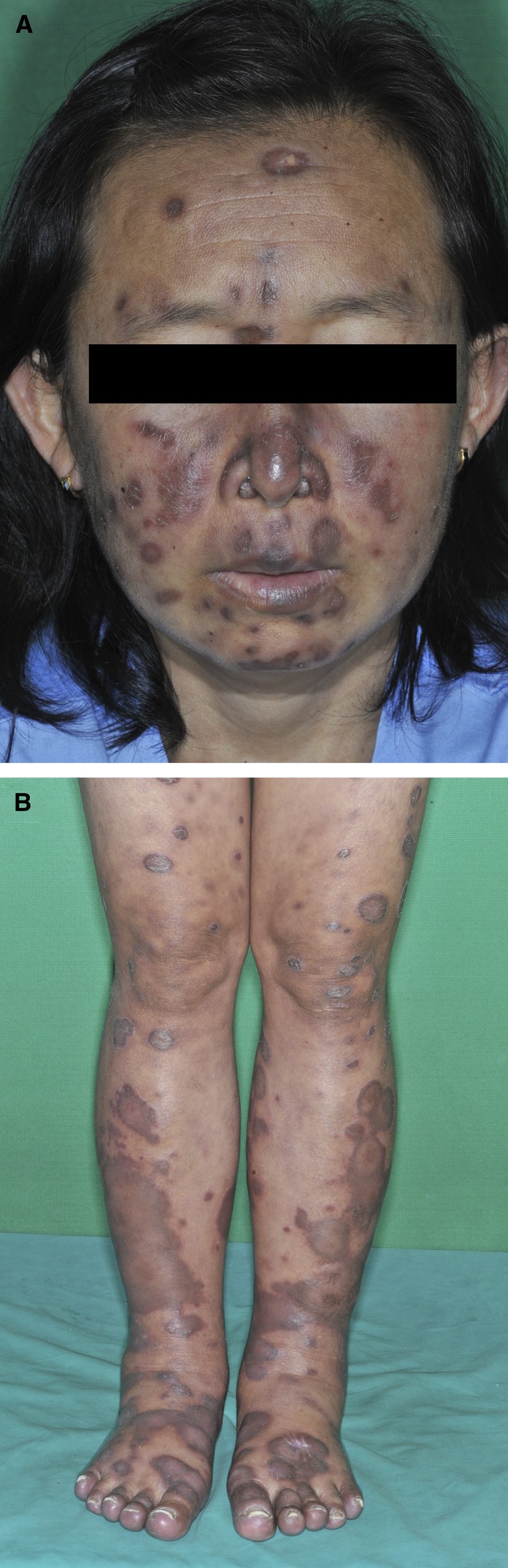
Physical examination showed sign of wasting and body temperature of 37.6°C. The patient had moderate pallor with no jaundice. The examination of abdomen showed mild splenomegaly. There was neither hepatomegaly nor lymphadenopathy. Cutaneous findings showed multiple, well-defined, non-ulcerated, erythematous, indurated hyperpigmented plaques and nodules varying in size on the face and both legs (Figure 1A and B ). Some atrophic hyperpigmented plaques were also noted on the face and few scattered erythematous macules and patches were observed on the torso. There was neither oral thrush nor oral hairy leukoplakia.
Figure 1.
Clinical features of disseminated cutaneous leishmaniasis. (A) Diffuse hyperpigmented plaques and nodules varying in size on the face. (B) Diffuse brownish-to-hyperpigmented plaques at lower extremities.
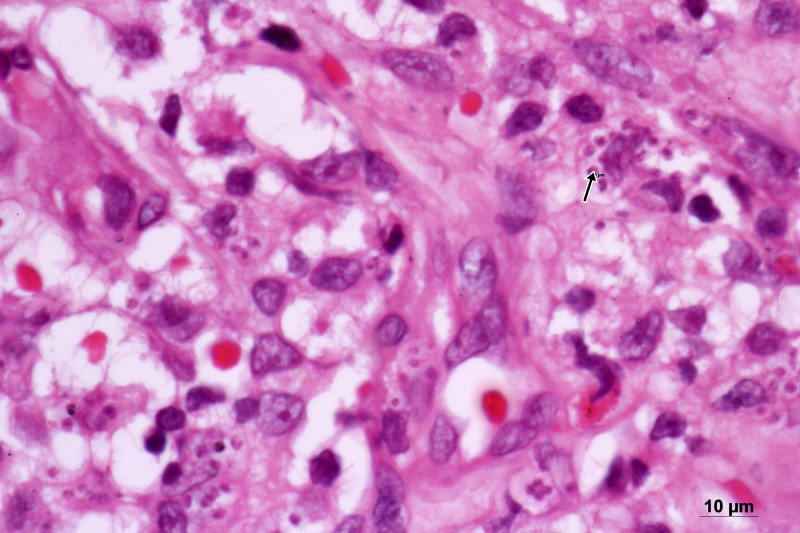
Laboratory investigations showed a hemoglobin level of 7.9 g/dL, a white blood cell count of 5,400 cells/mm3 (neutrophils 71%, lymphocytes 16%, eosinophils 5%, basophils 0%, and monocytes 8%), and a platelet count of 422,000 cells/mm3. Liver function tests gave the following results: an aspartate aminotransferase level of 72 U/L (7–55 U/L), an alanine aminotransferase level of 26 U/L (8–48 U/L), and an alkaline phosphatase level of 314 U/L (45–115 U/L). Her CD4 T-cell count was 89 cells/mm3. Hypoalbuminemia (2.4 g/dL) and hypergammaglobulinemia (6.6 g/dL) were also noted. Other laboratory results were within normal limits. A cutaneous biopsy was performed and histopathology demonstrated normal epidermis. Many nonencapsulated intracytoplasmic yeast-like organisms were noted in the dermis with multiple patchy infiltration of mixed inflammatory cells, particularly, lymphocytes, histiocytes, and neutrophils. Binary fission was not seen (Figure 2 ). All the special stains such as periodic acid–Schiff stain, Giemsa stain, and Gomori methenamine silver stain showed a positive result. Ultrasonography of abdomen revealed mild splenomegaly. Bone marrow study was normal.
Figure 2.
Histopathological examination of the skin biopsy, hematoxylin–eosin staining reveals many intracellular yeast-like organisms sized 2 μm (× 1,000).
After culture on Sabouraud dextrose agar for 2 weeks showed no fungal growth, molecular characterization was performed from tissue biopsy using polymerase chain reaction (PCR) amplification and sequencing and comparing the database from GenBank. Pan-fungal PCR for ITS-1 and ITS-2 appeared negative but positive for D1/D2.8 Subsequently, DNA sequencing was done for D1/D2. The sequences revealed 99% homology to Leishmania amazonensis (accession no. JX030045). Therefore, another PCR and DNA sequencing were performed using primer specific to Leishmania for its identification.6,9 Sequences from two independent loci were examined: the ITS-1 region of the 18S ribosomal RNA gene and the large subunit of RNAPolII. The large subunit of RNAPolII sequences of our parasite appeared to be 1,181/1,184 base pairs (99%) identical to L. siamensis previously reported (GenBank accession no. KM820664) while ITS-1 sequence showed 282/295 base pairs (96%) similarity (JX195640).6 GenBank accession nos. for the new sequences are KX347438 for ITS-1 and KX347439 for RNAPolII.
The sequences obtained from our case and PCM2 from Trang (JX195640) were the most similar while lower identity (95%) of ITS-1 sequence was observed between a pair of our Leishmania and Ghanaian Leishmania (EF524071). In addition, the lower identity (98%) of RNAPolII sequence between our strain and Leishmania enriettii (AF151727) was observed. Therefore, we concluded that patient was infected with L. siamensis (PCM2 Trang). Intravenous amphotericin B deoxycholate 0.6 mg/kg/day was administered daily for 4 weeks, and then she was referred back to Samutsakorn general hospital for further treatment with intravenous amphotericin (total dose 1,260 mg) followed by itraconazole 400 mg/day indefinitely due to the patient's immunocompromised status. Her general condition was improved, and her skin lesions decreased in size at the time of referral.
Discussion
To the best of our knowledge, this is the second report of PCM2 isolate. However, the name “Leishmania siamensis” remains confusing due to mistaking L. martiniquensis for L. siamensis in the past. In 2013, Leelayoova and others discovered that the so-called L. siamensis has indeed two separate lineages: the TR lineage and PG lineage.9 Further works by Pothirat and others clarified using sequence analysis that a novel isolate from northern Thailand LSCM1 (accession no. JX898938) and all other strains except PCM2 Trang were L. martiniquensis (PG lineage), as previously stated from the Martinique island.6 This reflected that L. martiniquensis is the most common cause of autochthonous leishmaniasis in Thailand. Therefore, we can draw conclusion that autochthonous leishmaniasis in Thailand caused by two species of Leishmania: L. martiniquensis and L. siamensis. Although L. martiniquensis has worldwide distribution, our current strain appeared more restricted to Thailand.6 However, due to small number of PCM2 cases, further reports need to be collected for further clarification of various strains of Leishmania.
As Thailand is an endemic area for penicilliosis and other fungal HIV infections, the culture for leishmaniasis is not routinely performed in such cases. In terms of molecular diagnosis, we used primers designed from loci previously used for Leishmania identification.10–13 ITS-1 region is the most variable marker that can be used to analyze the genetic difference among Leishmania species.9
The clinical manifestations of novel leishmaniasis in Thailand can be categorized into three clinical forms: visceral, diffused cutaneous, and diffused cutaneous combined with visceral.14 The infection has been mostly reported in immunocompromised patients, particularly those with HIV infection. Other than L. martiniquensis and L. siamensis, Leishmania infantum was also reported to be the cause of autochthonous leishmaniasis in Thailand.3 Our patient had low-grade, subacute fever and disseminated cutaneous lesions without other visceral involvement.
The variety of clinical presentations of cutaneous leishmaniasis does not depend only on the causative strains, but also on the nature of the host immune status, exclusively immune suppression caused by HIV infection.15 It is possible that PCM2 isolate is uniquely able to cause these cutaneous manifestations in patients with acquired immune deficiency syndrome. In immunocompetent hosts, a T-helper cell 1 (Th1) response provides protective effect against Leishmania, whereas Th2 cytokines causes susceptibility to infection and disease progression. HIV infection facilitates a Th2 immune response that promotes the progression of leishmaniasis.16
Recently, cavernicolous species of phlebotomine sand flies from Kanchanaburi Province has been studied providing the diversity of sand flies potential to be leishmaniasis vector.17 However, limitation of this study was identification of sand flies vector. Epidemiological studies are necessary to determine life cycle of novel Leishmania with potential vectors and reservoir hosts for developing further disease control and prevention in Thailand.
In summary, this is the report of disseminated autochthonous cutaneous leishmaniasis in central Thailand, and the causative organism is identified as L. siamensis (PCM2 Trang). It is evident that autochthonous leishmaniasis in Thailand is caused by two species of Leishmania: L. martiniquensis and L. siamensis. Our report supports the existence of L. siamensis (PCM2 Trang). Although, Thailand is not regarded as endemic area for leishmaniasis, diffuse cutaneous infiltration with yeast-like organism in histopathology especially in immunocompromised patient should raise the suspicion of cutaneous leishmaniasis. Moreover, molecular diagnosis has been proved to be fast and effective method for diagnosis of leishmaniasis.
Supplementary Material
Footnotes
Authors' addresses: Chavalit Supsrisunjai, Tanawatt Kootiratrakarn, Thareena Bunnag, Prapaipit Chaowalit, and Vesarat Wessagowit, Molecular Genetics Laboratory, Institute of Dermatology, Bangkok, Thailand, E-mails: chervilius@hotmail.com, tanawatt_k@hotmail.com, thareena@hotmail.com, prapaipit_cw@hotmail.com, and vesarat@hotmail.com. Pailin Puangpet, Department of Occupational and Contact Dermatitis, Institute of Dermatology, Bangkok, Thailand, E-mail: pailin.samutrapong@gmail.com.
References
- 1.Suttinont P, Thammanichanont C, Chantarakul N. Visceral leishmaniasis: a case report. Southeast Asian J Trop Med Public Health. 1987;18:103–106. [PubMed] [Google Scholar]
- 2.Kongkaew W, Siriarayaporn P, Leelayoova S, Supparatpinyo K, Areechokchai D, Duang-ngern P, Chanachai K, Sukmee T, Samung Y, Sridurongkathum P. autochthonous visceral leishmaniasis: a report of a second case in Thailand. Southeast Asian J Trop Med Public Health. 2007;38:8–12. [PubMed] [Google Scholar]
- 3.Maharom P, Siripattanapipong S, Mungthin M, Naaglo T, Sukkawee R, Pudkorn R, Wattana W, Wanachiwanawin D, Areechokchai D, Leelayoova S. Visceral leishmaniasis caused by Leishmania infantum in Thailand. Southeast Asian J Trop Med Public Health. 2008;39:988–990. [PubMed] [Google Scholar]
- 4.Thisyakorn U, Jongwutiwes S, Vanichsetakul P, Lertsapcharoen P. Visceral leishmaniasis: the first indigenous case report in Thailand. Trans R Soc Trop Med Hyg. 1999;93:23–24. doi: 10.1016/s0035-9203(99)90166-9. [DOI] [PubMed] [Google Scholar]
- 5.Sukmee T, Siripattanapipong S, Mungthin M, Worapong J, Rangsin R, Samung Y, Kongkaew W, Bumrungsana K, Chanachai K, Apiwathanasorn C, Rujirojindakul P, Wattanasri S, Ungchusak K, Leelayoova S. A suspected new species of Leishmania, the causative agent of visceral leishmaniasis in a Thai patient. Int J Parasitol. 2008;38:617–622. doi: 10.1016/j.ijpara.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Pothirat T, Tantiworawit A, Chaiwarith R, Jariyapan N, Wannasan A, Siriyasatien P, Supparatpinyo K, Bates MD, Kwakye-Nuako G, Bates PA. First isolation of Leishmania from northern Thailand: case report, identification as Leishmania martiniquensis and phylogenetic position within the Leishmania enriettii complex. PLoS Negl Trop Dis. 2014;8:e3339. doi: 10.1371/journal.pntd.0003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bualert L, Charungkiattikul W, Thongsuksai P, Mungthin M, Siripattanapipong S, Khositnithikul R, Naaglor T, Ravel C, El Baidouri F, Leelayoova S. Autochthonous disseminated dermal and visceral leishmaniasis in an AIDS patient, southern Thailand, caused by Leishmania siamensis. Am J Trop Med Hyg. 2012;86:821–824. doi: 10.4269/ajtmh.2012.11-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Fu YF, Wang RY, Li L, Cao YH, Chen YQ, Zhao HZ, Zhang QQ, Wu JQ, Weng XH, Cheng XJ, Zhu LP. Identification of clinically relevant fungi and Prototheca species by rRNA gene sequencing and multilocus PCR coupled with electrospray ionization mass spectrometry. PLoS One. 2014;9:e98110. doi: 10.1371/journal.pone.0098110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leelayoova S, Siripattanapipong S, Hitakarun A, Kato H, Tan-ariya P, Siriyasatien P, Osatakul S, Mungthin M. Multilocus characterization and phylogenetic analysis of Leishmania siamensis isolated from autochthonous visceral leishmaniasis cases, southern Thailand. BMC Microbiol. 2013;13:60. doi: 10.1186/1471-2180-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croan DG, Morrison DA, Ellis JT. Evolution of the genus Leishmania revealed by comparison of DNA and RNA polymerase gene sequences. Mol Biochem Parasitol. 1997;89:149–159. doi: 10.1016/s0166-6851(97)00111-4. [DOI] [PubMed] [Google Scholar]
- 11.Dougall AM, Alexander B, Holt DC, Harris T, Sultan AH, Bates PA, Rose K, Walton SF. Evidence incriminating midges (Diptera: Ceratopogonidae) as potential vectors of Leishmania in Australia. Int J Parasitol. 2011;41:571–579. doi: 10.1016/j.ijpara.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Garcia L, Kindt A, Bermudez H, Llanos-Cuentas A, De Doncker S, Arevalo J, Wilber Quispe Tintaya K, Dujardin JC. Culture-independent species typing of neotropical Leishmania for clinical validation of a PCR-based assay targeting heat shock protein 70 genes. J Clin Microbiol. 2004;42:2294–2297. doi: 10.1128/JCM.42.5.2294-2297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spanakos G, Piperaki ET, Menounos PG, Tegos N, Flemetakis A, Vakalis NC. Detection and species identification of Old World Leishmania in clinical samples using a PCR-based method. Trans R Soc Trop Med Hyg. 2008;102:46–53. doi: 10.1016/j.trstmh.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Siriyasatien P, Chusri S, Kraivichian K, Jariyapan N, Hortiwakul T, Silpapojakul K, Pym AM, Phumee A. Early detection of novel Leishmania species DNA in the saliva of two HIV-infected patients. BMC Infect Dis. 2016;16:89. doi: 10.1186/s12879-016-1433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhary RG, Bilimoria FE, Katare SK. Diffuse cutaneous leishmaniasis: co-infection with human immunodeficiency virus (HIV) Indian J Dermatol Venereol Leprol. 2008;74:641–643. doi: 10.4103/0378-6323.45111. [DOI] [PubMed] [Google Scholar]
- 16.Clerici M, Shearer GM. A TH1 → TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 17.Apiwathnasorn C, Samung Y, Prummongkol S, Phayakaphon A, Panasopolkul C. Cavernicolous species of phlebotomine sand flies from Kanchanaburi Province, with an updated species list for Thailand. Southeast Asian J Trop Med Public Health. 2011;42:1405–1409. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.