Abstract
Primaquine is the only licensed drug available for the elimination of Plasmodium vivax hypnozoites. Methemoglobinemia is currently reported in the course of treatment. There is evidence that metabolites of primaquine formed by the cytochrome pathway are responsible for methemoglobin formation; a genetic polymorphism of cytochrome isoforms; and a potential influence of gender in the activities of these enzymes requiring the establishment of dose × response curves profiles in different population groups. Concentrations of primaquine in plasma and methemoglobin levels were investigated in 54 patients with malaria due to P. vivax during the course of the standard regimen of chloroquine with primaquine (0.25 mg/kg/day for 14 days). All study subjects lived in an endemic area of the Brazilian Amazon Basin. The blood samples were collected before initiation of treatment and 3 hours (range 2–4 hours) after the administration of antimalarial drugs on days 2, 7, and 14. Plasma primaquine concentrations were similar in both genders (males: range = 164–191 ng/mL, females: range = 193–212 ng/mL). Methemoglobin levels ranged from 3.3% to 5.9% in males and from 3.1% to 6.5% in females. There were no significant correlations between the plasma primaquine concentrations or total dose and methemoglobin levels, suggesting that unidentified metabolites rather than parent drug were likely responsible for changes in methemoglobin levels. There was no significant influence of gender on primaquine concentrations in plasma or methemoglobin levels.
Introduction
Malaria due to Plasmodium vivax is a major public health issue worldwide.1 Chloroquine with primaquine are the drugs of choice for treatment of the disease, which aim at radical cure, that is, eliminate all forms of Plasmodium of the blood and of the tissues. The primaquine is the only licensed drug available for the elimination of hypnozoites responsible for relapses, weeks to months after the initial attack. The recommended conventional hypnozoiticidal doses are 0.25–0.5 mg/kg/day for 7 or 14 days.2–4
A major issue in the course of treatment with primaquine is the high incidence of adverse reactions, which are generally dose dependent and include gastrointestinal and hematological disturbances such as nausea, abdominal pain, diarrhea and vomiting, methemoglobinemia, and severe hemolysis in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.3–6
The methemoglobin (MetHB) is an oxidative product of hemoglobin formed when the ferrous iron in the heme moiety of hemoglobin is converted to ferric iron.7 High levels of MetHB are associated with exposure to oxidant agents, with the deficit of enzymes of the redox cycle in red blood cells, with infections, and with the conformational changes in hemoglobin molecule.7,8 The increase in the MetHB levels due to the therapeutic or prophylactic use of primaquine is well documented. Overall, it is autolimited, does not require interruption of the therapy, and returns to normal range within 28 days after commencement of the treatment.9–11
There are considerable evidences that the hydroxylated metabolites formed by the cytochrome P450 (CYP)–dependent pathway, such as 5-hydroxyprimaquine, rather than the parent drug, are responsible for MetHB formation and other adverse reactions, as well as, the hypnozoiticidal action.12–14 The significant interethnic differences in CYP2D6 allele found in studies across many countries may influence the metabolism of the parent drug and, consequently, the MetHB formation in the course of treatment with primaquine, once the genetic polymorphism of CYP2D6 was associated with both the efficacy and the toxicity of primaquine.15–18 Another potential source that influences the MetHB levels is the sex-related difference in the pharmacokinetics of some drugs, which is considered an important determinant for the higher incidence of adverse events in females compared with males.19,20
In this context, the measure of MetHB in the course of the treatment with primaquine becomes relevant in population groups with high degree of miscegenation, such as the riverside population of the Amazon River, where each year about 180,000 cases of P. vivax are reported.21 Despite the extensive use of primaquine in this endemic area, there is a paucity of data on MetHB levels and on the concentrations of the drug in blood components. Therefore, the aim of the present study was to investigate the levels of MetHB and the concentrations of primaquine in the plasma of people living along the Amazon River, with slide-confirmed P. vivax infection and treated with the standard regimen of chloroquine (3 days) with primaquine (14 days).2 MetHB levels were compared between genders and correlated to both plasma concentrations of drug and the total dose administered.
Materials and Methods
Ethical statement.
The study was submitted and approved by the Ethical Committee of the Instituto Evandro Chagas (protocol 036/10 CEP-IEC). All patients recruited were informed about the goals, as well as the risks and benefits, of the study. The written informed consent was obtained before inclusion in the study in a formulary elaborated according to the National Committee of Ethical and Research.
Study site and patients.
This prospective study was carried out from January 2010 to July 2012 at the Basic Unit Health of Brazil Novo, an area of low transmission along the Amazon River in the state of Amapa, Brazil. The criteria of inclusion were adults with slide-confirmed P. vivax infection, with G6PD activity and body mass index in the normal ranges. The criteria of exclusion included patients with mixed malaria or with signs and symptoms of severe malaria (jaundice, renal or pulmonary impairment, severe anemia, and altered level of consciousness), parasitemia above 5%, hypersensitivity to the drugs, and use of antimalarial drugs 4 weeks before inclusion in the study.
Drug administration and follow-up.
Each patient received multiple oral doses of 600 mg base chloroquine on day 0, and 450 mg base chloroquine on days 2 and 3 (chloroquine phosphate in tablets; 150 mg base/tablet; Farmanguinhos Brazilian Health Office) concurrent with 15 mg base primaquine for 14 days (primaquine phosphate in a tablet with 13.2 mg salt/tablet; Farmanguinhos Brazilian Health Office).2 The drugs were administered within 30 minutes after breakfast under the supervision of clinical staff throughout the study. No additional food or drink was given until 2 hours after the administration of the drugs. The occurrence of vomiting within 30 minutes after the ingestion of the drugs was monitored. After the treatment, the patients were requested to return on days 21, 28, 35, and 42, or at any time whenever signs and symptoms suggestive of malaria occur. A parasite count was performed at each visit.2
Blood sampling.
Venous blood samples (4 mL) were collected from each patient in lithium heparin tubes before commencement of treatment (D0) and after 3 hours (range = 2–4 hours) of administration of the antimalarial drugs on days 2, 7, and 14. One fraction of the blood (1 mL) was used for the determination of MetHB. The remainder (3 mL) was centrifuged at 1,400 × g for 15 minutes for separation of the plasma that was stored at −80°C until the analysis of the drug.
Laboratory analysis.
A reversed-phase high-performance liquid chromatography system with ultraviolet detection (Pro Star; Varian, Walnut, CA) was used for measuring the concentrations of primaquine in plasma. The drug was extracted of the plasma using methyl-tert-butyl-ether at pH 8.0 on a reversed-phase column (ODS C18 4.6 × 250 mm i.d. 5 μm; Supelco Inc., Bellefonte, PA). The mobile phase consisted of acetonitrile/phosphate buffer pH 3.0 (30:70). The flow rate was 1 mL/minute. The wavelength of detection was 250 nm. Quinidine (2.5 μg/mL) was used as internal standard.22 The method was linear in the range of 10–900 ng/mL. The coefficients of variation, within-day and day-to-day, were 8.3% and 12.1%, respectively. The limit of detection was 5 ng/mL and the limit of quantification was 10 ng/mL. The mean recovery of primaquine of plasma was 95.3%. The stability of the plasma sample spiked with the drug was estimated at 120 days.
The quantification of MetHB in whole blood sample was performed according to Hegesh and others.23 Briefly, a total of 1,000 μL of 0.5 M phosphate buffer (pH 6.5) was added to 1,000 μL of cell lysate, and the mixture was centrifuged at 1,600 × g for 2 minutes to sediment debris. The supernatant was used to measure the absorbance at 630 nm (the absorbance maximum for MetHB). Thereafter, a total of 100 μL of KCN 10% was added, and after 5 minutes at room temperature (24°C), a second reading was recorded. KCN converts MetHB to cyanomethemoglobin, which does not absorb at 630 nm. The difference between absorbance readings represents the absorbance due to MetHB. To measure total hemoglobin levels, all of the hemoglobin was converted to MetHB by K3Fe(CN)6 and the absorbance of the sample was recorded at 630 nm. KCN 10% was subsequently added, and a second reading (T2) was recorded. The percentage of MetHB in relation to hemoglobin in the sample was calculated.
Data analysis.
Data are reported as mean ± standard deviation. The plasma primaquine concentrations and the MetHB levels were compared among days of blood sampling by one-way analysis of variance and between genders using Student's t test. The Pearson's coefficient of correlation was used to determine the correlations between the plasma primaquine concentrations or the total dose and the MetHB levels on each day of blood sampling. Statistical analyses were performed with STATISTICA software package (version 6; StatSoft, Tulsa, OK). Changes were accepted as significant at P = 0.05 or below.
Results
A total of 54 patients met the criteria to inclusion in the study. All of the patients completed the 42-day follow-up period. The parasites were cleared from peripheral blood within the first 2 days of study, and there was no reappearance of parasites during the follow-up period. There were no reports of serious adverse reactions in the course of the study. The baseline characteristics of patients are provided in Table 1.
Table 1.
Baseline characteristics of subjects with Plasmodium vivax malaria
| Characteristic | Male (N = 27) | Female (N = 27) |
|---|---|---|
| Ages, years | 39 (15–49) | 29 (20–42) |
| Parasite count, mm3 | 3,190 (500–4,300) | 4,120 (1,500–9,300) |
| Parasite clearance, hour | 50 (12–60) | 52 (14–62) |
| Previous episodes of infection, % | 100 | 100 |
| Hemoglobin, g/dL | 12.1 (9.3–15.6) | 11.9 (11–15) |
| Hematocrit, % | 39.2 (27–46) | 38.7 (34–42) |
| Erythrocytes count, 106 | 4.32 (3.17–5.6) | 4.21 (3.47–5.4) |
| White blood cells, mm3 | 4,900 (4,200–7,200) | 4,200 (2,800–7,700) |
Results are expressed as mean and range.
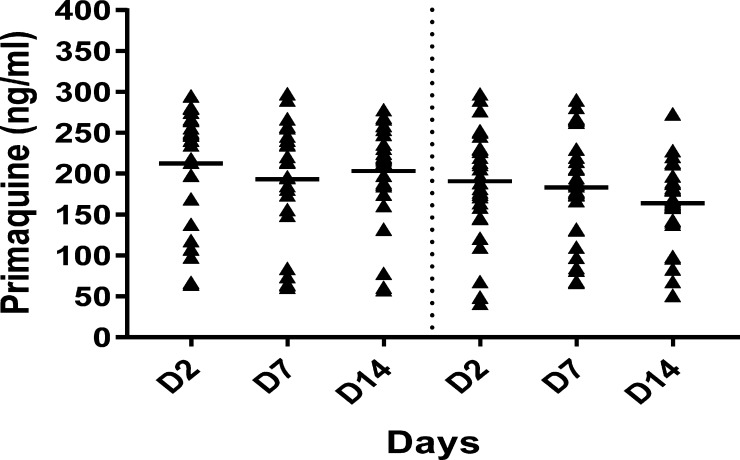
There were no measurable concentrations of primaquine in the baseline samples of all patients. The means of the plasma primaquine concentrations on days 2, 7, and 14 were 212 (68) ng/mL, 193 (70) ng/mL, and 203 (60) ng/mL in female patients and 191 (66) ng/mL, 183 (64) ng/mL, and 164 (48) ng/mL in male patients, respectively (Figure 1 ). There were no significant differences in the concentrations of primaquine in the plasma among days of blood sampling in both male (F = 1.421; P = 0.253) and female (F = 0.286; P = 0.759) patients. The concentrations of primaquine also were similar in both genders on days 3 (t = 0.499; P = 0.480), 7 (t = 0.437; P = 0.333), and 14 (t =0.896; P = 0.191).
Figure 1.
Plasma primaquine concentrations of female (left side) and male (right side) patients with Plasmodium vivax. Horizontal lines indicate mean values.
The means of the MetHB levels on days 0, 2, 7, and 14 were 1.1 (0.5) %, 4.7 (1.6) %, 6.5 (2) %, and 3.1 (0.8) % in female patients and 1.0 (0.3) %, 4.2 (1.6) %, 5.9 (2.5) %, and 3.2 (1.1) % in male patients, respectively (Figure 2 ). There was an increase in MetHB levels after commencement of treatment on days 2 and 7, and then a decrease on day 14 in male (F = 14.813; P < 0.001) and in female (F = 5.567; P = 0.013) patients. The MetHB levels were similar in both genders in predose samples (t = 0.724; P = 0.182) and on days 3 (t = 0.749; P = 0.231), 7 (t = 0.826; P = 0.209), and 14 (t = 0.239; P = 0.406). There were no significant correlations between the plasma primaquine concentrations and MetHB levels in male patients on days 2 (r = −0.391; P = 0.136), 7 (r = −0.097; P = 0.738), and 14 (r = −0.163; P = 0.614). A similar result was found in female patients on days 2 (r = −0.702; P = 0.441), 7 (r = −0.728; P = 0.481), and 14 (r = −0.785; P = 0.425). There were no significant correlations between the total dose of primaquine administered and MetHB levels in male (r = 0,412; P = 0.587) and in female (r = 0.240; P = 0.753) patients (Figure 3 ).
Figure 2.
Methemoglobin levels (%) in female (left side) and male (right side) in the course of treatment with primaquine 15 mg/kg/day. Horizontal lines indicate mean values.
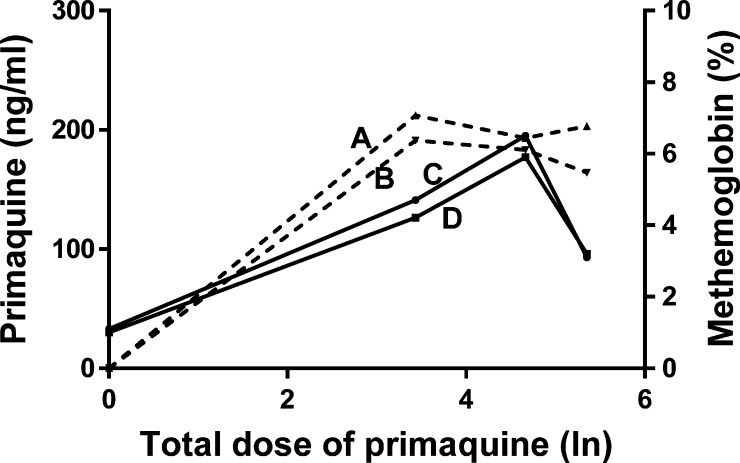
Figure 3.
Total dose of primaquine administrated (mg/kg), plasma primaquine concentrations (ng/mL) in female (line A) and male (line B) patients and methemoglobin levels (%) in female (line C) and male (line D) patients.
Discussion
In this study, the levels of MetHB and the plasma primaquine concentrations were examined in uncomplicated cases of P. vivax from the Brazilian Amazon Basin. Moreover, the MetHB levels were correlated with the total dose and with the concentrations of primaquine in plasma, in both genders, to confirm that the parent drug is not responsible by MetHB formation. Compared with other studies, the plasma primaquine concentrations found in this study were higher than those reported in healthy Thai volunteers, Thai male patients, and Indian patients in the course of the same standard regimen with primaquine,24–26 but they were lower than those in Korean patients with P. vivax.27 These findings corroborate a probable interracial difference of the pharmacokinetics parameters of primaquine. Furthermore, there was a slight increase in the concentrations of primaquine on day 2 in both genders that can be justified by the concurrent administration of chloroquine or by the influence of the Plasmodium on the pharmacokinetics disposition of the drug.26–28
The plasma primaquine concentrations were slightly higher in female patients in all days of blood sampling with the highest difference on day 14. The sex-related difference in the pharmacokinetics of the primaquine was associated with the duration of the exposure. For instance, there was lack of effect of sex in the pharmacokinetics parameters of the drug after short exposure time in healthy volunteers from different ethnicities,29,30 but the chronic exposure to the drug reduced the volume of distribution and the oral clearance in female volunteers.31,32
As expected, there was a significant increase in the MetHB levels after commencement of treatment in both genders, but the values were not clinically relevant to the study, because the threshold of occurrence of clinical signs and symptoms of methemoglobinemia is generally above 20%.7,8 Moreover, the levels of MetHB found in the study are in line to reports from other endemic areas, which suggests the probable absence of interracial difference in MetHB formation in the course of treatment with primaquine.9–11
There was no significant correlation between the plasma primaquine concentrations and the MetHB levels, suggesting that other compounds were responsible for the increase in the MetHB levels. This finding is corroborated by the nonsignificant correlation between the total dose of primaquine and the MetHB levels, suggesting that the compounds responsible for the increase in MetHB levels have a short half-life and do not show significant accumulation in tissue, which is in accordance with the reports on the profiles of the hydroxylated metabolites of the primaquine.12–15,33
The profiles curves of total dose × MetHB and plasma primaquine × MetHB in both genders have two main characteristics: 1) A prominent lag phase accompanied by the high concentration of primaquine in plasma. This finding can be justified either by a probable reduction of hydroxylated metabolites formation via CYP-2D6, resulting in a pharmacokinetics interaction between primaquine and chloroquine, or by the physiopathology of the disease.14,15,26,28 2) A decline of MetHB levels after half of the treatment following the clearance of parasites and the cessation of signals and symptoms of the disease. This finding is justified by the partial restoration of the redox balance inside red blood cells and consequent reduction of the MetHB levels. In fact, besides hydroxylated metabolites of the primaquine, there are other sources that promote a pro-oxidant environment within the erythrocytes that increase the MetHB levels, for instance, the host's immune response to Plasmodium evolves phagocytosis, the production of oxygen radicals and nitric oxide, as well as the degradation of hemoglobin in the acidic food vacuole of parasites, which provides nutrients and maintains the osmotic stability within the host cell producing the redox active by-products, free heme, and hydrogen peroxide.3–5,11,13,33,34
The levels of MetHB were similar in both genders, but were slightly higher in females on days 2 and 7 after commencement of treatment. This finding may be due to sex-related differences in the formation, or in susceptibility to adverse reaction, of these hydroxylated metabolites. Further studies should elucidate if the gender has a significant impact on the pharmacokinetics or pharmacodynamics of these metabolites.
The main limitation of the study was the collection of blood samples in predetermined days, instead of the serial blood sampling. However, both sampling methods were valuable to estimate the exposure to the antimalarial drugs.35 Another two potential limitations of the study are as follows: 1) The concentrations of carboxyprimaquine were not measured in the study, because there were no evidences to support pharmacological or toxicological activities of the metabolite.3–6 2) The blood was sampled between 2 and 3 hours after the administration of the drugs, which is relatively in accordance with the reported Tmax of 2.0 hours with the range of 0.5–4 hours for chloroquine–primaquine combination therapy.26
According to our results, the MetHB levels and the plasma primaquine concentrations of people living in the Brazilian Amazon Basin were similar in both genders, but both were slightly higher in female patients. The curves for time and total dose versus MetHB levels show a lag phase followed by decline midway through the treatment period. No significant correlations were found between plasma primaquine concentrations or total dose and MetHB levels, suggesting that primaquine metabolites are involved in the formation of MetHB.
ACKNOWLEDGMENTS
We would like to acknowledge World Wide Antimalarial Resistance Network (WWARN) for providing standard for mefloquine analysis.
Footnotes
Authors' addresses: José Luiz Vieira, Michelli E. S. Ferreira, and Michelle V. D. Ferreira, Health Science Institute, Para Federal University, Belem, Brazil, E-mails: jvieira@ufpa.br and latoxufpa@gmail.com. Margarete M. Gomes, Central Laboratory of Public Health, Amapa, Brazil.
Reprint requests: José Luiz Vieira, Laboratório de Toxicologia, Faculdade de Farmácia, Universidade Federal do Pará, Campus do Guamá, Av. Augusto Corrêa 01, Belém 66075-110, Brazil, E-mail: jvieira@ufpa.br.
References
- 1.World Health Organization . World Malaria Report. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 2.World Health Organization . Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization; 2010. 2010. [Google Scholar]
- 3.Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg. 2006;65:402–415. [PubMed] [Google Scholar]
- 4.Vale N, Moreira R, Gomes P. Primaquine revised six decades after its discovery. Eur J Med Chem. 2009;44:937–953. doi: 10.1016/j.ejmech.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 6.Robert W, Taylor J, White NJ. Antimalarials drug toxicity: a review. Drug Saf. 2004;27:25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hsiech HS, Jaffe ER. The metabolism of methemoglobin in human erythrocytes. In: Surgenor DMN, editor. The Red Blood Cell. New York, NY: Academic Press II; 1975. pp. 799–824. [Google Scholar]
- 8.Kiese M. Methemoglobinemia: A Comprehensive Treatise. Cleveland, OH: CRC Press; 1974. [Google Scholar]
- 9.Carmona-Fonseca J, Álvarez G, Maestre A. Methemoglobinemia and adverse events in Plasmodium vivax malaria patients associated with high doses of primaquine treatment. Am J Trop Med Hyg. 2009;80:188–193. [PubMed] [Google Scholar]
- 10.Greaves J, Evans DAP, Fletcher KA. Urinary primaquine excretion and red cell methaemoglobin levels in man following a primaquine: chloroquine regimen. Br J Clin Pharmacol. 1980;10:293–295. doi: 10.1111/j.1365-2125.1980.tb01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen RJ, Sachs JR, Wicker DJ, Conrad ME. Methemoglobinemia provoked by malarial chemoprophylaxis in Vietnam. N Engl J Med. 1968;279:1127–1131. doi: 10.1056/NEJM196811212792102. [DOI] [PubMed] [Google Scholar]
- 12.Mihaly GW, Ward SA, Edwards G, Orme ML, Breckenridge AM. Pharmacokinetics of primaquine in man: identification of the carboxylic acid derivative as a major plasma metabolite. Br J Clin Pharmacol. 1984;17:441–446. doi: 10.1111/j.1365-2125.1984.tb02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganesan S, Tekwani BL, Sahu R, Tripathi LM, Walker LA. Cytochrome P450-dependent toxic effects of primaquine on human erythrocytes. Toxicol Appl Pharmacol. 2009;241:14–22. doi: 10.1016/j.taap.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, Deye G, Melendez V, Ockenhouse CF. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med. 2013;369:1381–1382. doi: 10.1056/NEJMc1301936. [DOI] [PubMed] [Google Scholar]
- 15.Potter BM, Xie LH, Vuong C, Zhang J, Zhang P, Duan D, Luong TL, Bandara Herath HM, Dhammika Nanayakkara NP, Tekwani BL, Walker LA, Nolan CK, Sciotti RJ, Zottig VE, Smith PL, Paris RM, Read LT, Li Q, Pybus BS, Sousa JC, Reichard GA, Marcsisin SR. Differential CYP 2D6 metabolism alters primaquine pharmacokinetics. Antimicrob Agents Chemother. 2015;59:2380–2387. doi: 10.1128/AAC.00015-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pybus BS, Marcsisin SR, Jin X, Deye G, Sousa JC, Li Q, Caridha D, Zeng Q, Reichard GA, Ockenhouse C, Bennett J, Walker LA, Ohrt C, Melendez V. The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar J. 2013;12:212–221. doi: 10.1186/1475-2875-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma MK, Woo MH, McLeod HL. Genetic basis of drug metabolism. Am J Health Syst Pharm. 2002;59:2061–2069. doi: 10.1093/ajhp/59.21.2061. [DOI] [PubMed] [Google Scholar]
- 18.Bertilsson L. Geographical/interracial differences in polymorphic drug oxidation. Current state of knowledge of cytochromes P450 (CYP) 2D6 and 2C19. Clin Pharmacokinet. 1995;29:192–209. doi: 10.2165/00003088-199529030-00005. [DOI] [PubMed] [Google Scholar]
- 19.Meibohm B, Beierle I, Derendorf H. How important are gender differences in pharmacokinetics? Clin Pharmacokinet. 2002;41:329–342. doi: 10.2165/00003088-200241050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira-Ferreira J, Lacerda MV, Brasil P, Ladislau JL, Tauil PL, Daniel Ribeiro CT. Malaria in Brazil: an overview. Malar J. 2010;9:115–132. doi: 10.1186/1475-2875-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dua VK, Kar EK, Sarina R, Sharma VP. High-performance liquid chromatographic determination of primaquine and carboxyprimaquine concentrations in plasma and blood cells in Plasmodium vivax malaria cases following chronic dosage with primaquine. J Chromatogr B Biomed Sci Appl. 1996;657:93–98. doi: 10.1016/0378-4347(95)00357-6. [DOI] [PubMed] [Google Scholar]
- 23.Hegesh E, Grener N, Cohen OS, Bochkousky R, Shuval HI. A sensitive micromethod for the determination of methemoglobin in blood. Clin Chim Acta. 1972;30:679–683. doi: 10.1016/0009-8981(70)90260-3. [DOI] [PubMed] [Google Scholar]
- 24.Ward SA, Mihaly GW, Edwards G, Looareesuwan S, Phillips RE, Chanthavanich P, Warrell DA, Orme ML, Breckenridge AM. Pharmacokinetics of primaquine in man. II. Comparison of acute vs chronic dosage in Thai subjects. Br J Clin Pharmacol. 1985;19:751–755. doi: 10.1111/j.1365-2125.1985.tb02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bangchang KN, Songsaeng W, Thanavibul A, Chroenlarp P, Karbwang J. Pharmacokinetics of primaquine in G6PD deficient and G6PD normal patients with vivax malaria. Trans R Soc Trop Med Hyg. 1994;88:220–222. doi: 10.1016/0035-9203(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 26.Pukrittayakamee S, Tarning J, Jittamala P, Charunwatthana P, Lawpoolsri S, Lee SJ, Hanpithakpong W, Hanboonkunupakarn B, Day NP, Ashley EA, White NJ. Pharmacokinetic interactions between primaquine and chloroquine. Antimicrob Agents Chemother. 2014;58:3354–3359. doi: 10.1128/AAC.02794-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YR, Kuh HJ, Kim MY, Kim YS, Ghung W-C, Kim S, II, Kang MW. Pharmacokinetics of primaquine and carboxyprimaquine in Korean patients with vivax malaria. Arch Pharm Res. 2004;27:576–580. doi: 10.1007/BF02980134. [DOI] [PubMed] [Google Scholar]
- 28.Bhatia SC, Saraph YS, Revankar SN, Doshi KJ, Bharuca ED, Desai ED, Vaidya AB, Subrahmanyam D, Gupta KC, Satoskar RS. Pharmacokinetics of primaquine in patients with P. vivax malaria. Eur J Clin Pharmacol. 1986;31:205–210. doi: 10.1007/BF00606660. [DOI] [PubMed] [Google Scholar]
- 29.Elmes NJ, Bennett SM, Abdalla H, Carthew TL, Edstein MD. Lack of sex effect on the pharmacokinetics of primaquine. Am J Trop Med Hyg. 2006;74:951–952. [PubMed] [Google Scholar]
- 30.Cuong BT, Binh VQ, Dai B, Duy DN, Lovell CM, Rieckmann KH, Edstein MD. Does gender, food or grapefruit juice alter the pharmacokinetics of primaquine in healthy subjects? Br J Clin Pharmacol. 2006;61:682–689. doi: 10.1111/j.1365-2125.2006.02601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singhasivanon V, Sabcharoen A, Attanath P, Chongsuphajaisiddhi T, Diquet B, Turk P. Pharmacokinetics of primaquine in healthy volunteers. Southeast Asian J Trop Med Public Health. 1991;22:527–533. [PubMed] [Google Scholar]
- 32.Binh VC, Chinh NT, Thanh NX, Cuong BT, Quang NN, Dai B, Travers T, Edstein MD. Sex affects the steady-state pharmacokinetics of primaquine but not doxycycline in healthy subjects. Am J Trop Med Hyg. 2009;81:747–753. doi: 10.4269/ajtmh.2009.09-0214. [DOI] [PubMed] [Google Scholar]
- 33.Bolchoz LJC, Budinsky RA, McMillan DC, Jollow DJ. Primaquine-induced hemolytic anemia: formation and hemotoxicity of the arylhydroxylamine metabolite 6-methoxy-8-hydroxylaminoquinoline. J Pharmacol Exp Ther. 2001;297:509–515. [PubMed] [Google Scholar]
- 34.Percario S, Moreira D, Gomes BAQ, Ferreira MED, Gonçalves ACM, Laurindo PSOC, Vilhena TC, Dolabela MF, Green MD. Oxidative stress in malaria. Int J Mol Sci. 2012;13:16346–16372. doi: 10.3390/ijms131216346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization . Methods and Techniques for Assessing Exposure to Antimalarials Drugs in Clinical Field Studies. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]