Abstract
Recently, reports of delayed hemolytic anemia after treatment with artemisinin and its derivatives have emerged. Here we report two cases of delayed hemolytic anemia in a patient with severe falciparum malaria after treatment with oral artemether–lumefantrine (AL). The first patient, a 20-year-old Japanese male student, was diagnosed with falciparum malaria and was administered AL. As having a high parasitemia rate (20.6%) was the only severe malaria criterion met in this case and his general condition was stable, we continued with AL treatment. Despite disappearance of malarial parasites after 4 days of AL administration, a persistent fever remained. On days 13 and 16, a diagnosis of hemolytic anemia was made (lactate dehydrogenase [LDH]: 1,466 U/L, hemoglobin [Hb]: 7.2 g/dL). A blood smear at that time revealed no parasites. He recovered naturally from delayed hemolysis. The second patient, a 27-year-old Japanese female student, was diagnosed with falciparum malaria (parasitemia: 4.5%) and treated initially with oral quinine hydrochloride and doxycycline. The following day, parasitemia increased to 7.9% and oral AL was initiated. She was discharged on day 4 after achieving parasite clearance and afebrility. However, on day 5, fever (body temperature > 38°C) recurred, and on day 11, a diagnosis of hemolytic anemia was made (LDH: 712 U/L, Hb: 8.8 g/dL). A follow-up confirmed that her condition improved gradually. AL treatment of severe malaria can cause delayed hemolytic anemia. Patients should be followed up for up to 4 weeks to detect signs of hemolysis and provide appropriate symptomatic treatment.
Introduction
The World Health Organization (WHO) has recommended artemisinin-based combination therapies (ACTs) and intravenous artesunate as first-line treatments for uncomplicated Plasmodium falciparum malaria and severe malaria, respectively.1 Accordingly, artemisinin and its derivatives are widely used in both endemic countries and non-endemic developed countries. Numerous studies continue to demonstrate excellent efficacy and safety of artesunate and ACTs, such as artemether–lumefantrine, artesunate–amodiaquine, and dihydroartemisinin–piperaquine in Africa.2 However during 2010 and 2012, a total of 19 cases of post-artesunate delayed hemolytic anemia (PADH) have been reported, mainly in European countries.3 Subsequently, an additional 20 cases of PADH have been reported from malaria non-endemic regions, such as Europe and the United States, as well as endemic countries, such as Gabon, Ghana, and the Democratic Republic of the Congo.4,5 Although most cases of PADH were associated with parenteral artesunate, some case reports have suggested the involvement of intramuscular artemether and oral artemether–lumefantrine.6–8
More recently, 50–80 imported malaria cases have been reported annually in Japan. Among these, P. falciparum malaria accounts for approximately half, followed by Plasmodium vivax malaria. However, only quinine hydrochloride, mefloquine, and atovaquone–proguanil are licensed in Japan for the treatment of malaria; as a result, cases of severe malaria are difficult to treat, and a complete cure cannot be achieved for cases of P. vivax/Plasmodium ovale malaria. In response, the Japanese Research Group on Chemotherapy of Tropical Diseases has begun to import and reserve four antimalarial drugs (quinine gluconate injection, chloroquine phosphate, primaquine phosphate, and artemether–lumefantrine) for emergency therapy. Our hospitals are registered research group facilities, and thus, we are able to use these drugs at our own facilities after obtaining the patient's informed consent, which clearly states that these drugs are not licensed in Japan. Here, we report two cases of delayed hemolytic anemia after treatment with oral artemether–lumefantrine in patients with severe P. falciparum malaria.
Case Presentation
Case 1.
A 20-year-old Japanese male student traveled to Cameroon from February 17 to April 10, 2012 to assist local university students in instructing schoolchildren to use computers. He stayed in a student residence in Douala for the first and last weeks, in Bamenda for 3 weeks, and in Buea for 2 weeks. Before traveling, he received hepatitis A, tetanus, and yellow fever vaccines but not a malaria prophylaxis. From February 26 to 28, 2012, he was admitted to a local hospital because of fever and watery diarrhea. Following a clinical diagnosis of malaria and typhoid fever, he was treated with injected quinine and ciprofloxacin. Laboratory data findings at that time revealed white blood cell count of 6,800/mm3, hemoglobin (Hb) of 14.6 g/dL, and platelet count of 182,000/mm3. A blood smear and blood culture were not performed.
While returning to Japan, on April 9, 2012, he visited the airport medical center in Hong Kong with a fever (39.6°C) and was prescribed cephalexin (500 mg thrice daily [tid]), azithromycin (500 mg), metronidazole (500 mg tid), an antipyretic analgesic, and omeprazole for a suspected acute upper respiratory tract infection. Despite receiving these drugs, he had a febrile episode. He then visited our hospital with concerns about imported infectious diseases such as malaria. The patient was alert, and a physical examination found a temperature of 37.8°C, blood pressure of 102/50 mmHg, regular pulse rate of 88/minute, and numerous insect bite scars on his legs. His peripheral capillary oxygen saturation (SpO2) was 97% (room air). A mild wheeze was detected during chest auscultation, but cardiovascular, abdominal, and neurological examinations found no abnormalities. A laboratory examination at admission (day 0) revealed thrombocytopenia, hyperbilirubinemia, and elevated hepatic enzyme levels but no anemia, renal impairment, or hypoglycemia (Table 1). In the absence of a blood smear, a malaria rapid diagnosis test (Malaria Ag Pf/Pan; Standard Diagnostics Inc., Gyeonggi-Do, Korea) detected positivity for both the P. falciparum histidine-rich protein-II antigen and the pLDH antigen from all malaria species. Two sets of blood cultures were negative. We considered uncomplicated falciparum malaria and immediately administered oral artemether–lumefantrine 20/120 mg twice daily.
Table 1.
Laboratory data of case 1 over time
| Day 0 | Day 1 | Day 2 | Day 8 | Day 13 | Day 16 | Day 20 | Day 29 | |
|---|---|---|---|---|---|---|---|---|
| WBC (/mm3) | 3,700 | 4,000 | 7,600 | 7,300 | 5,900 | 4,800 | 4,400 | 36 |
| RBC (× 104/mm3) | 420 | 437 | 384 | 375 | 275 | 213 | 223 | 284 |
| Hb (g/dL) | 14.0 | 14.3 | 12.4 | 11.9 | 9.0 | 7.2 | 7.9 | 10.1 |
| Plt (× 104/mm3) | 2.3 | 1.6 | 2.5 | 18.9 | 22.7 | 25.6 | 28.8 | 22.5 |
| T-bil (mg/dL) | 2.7 | 4.9 | 10.0 | 2.0 | 2.8 | 2.0 | 1.4 | 1.0 |
| I-bil (mg/dL) | 1.8 | 1.6 | 3.3 | 1.3 | 2.4 | 1.7 | 1.2 | 0.9 |
| AST (U/L) | 71 | 87 | 191 | 51 | 67 | 43 | 31 | 25 |
| ALT (U/L) | 62 | 61 | 63 | 57 | 26 | 18 | 13 | 14 |
| LDH (U/L) | 421 | 645 | 1,855 | 511 | 1,466 | 1,238 | 901 | 407 |
| BUN (mg/dL) | 13 | 14 | 15 | 10 | 17 | 14 | 7 | |
| Cre (mg/dL) | 0.79 | 0.82 | 1.21 | 1.04 | 0.98 | 0.97 | 0.83 | |
| Glu (mg/dL) | 138 | |||||||
| Parasitemia (%) | 20.6 | 10.0 | 0 | 0 |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BUN = blood urea nitrogen; Cre = creatinine; Glu = glucose; Hb = hemoglobin; I-bil = indirect bilirubin; LDH = lactate dehydrogenase; Plt = platelets; RBC = red blood cells; T-bil = total bilirubin; WBC = white blood cells.
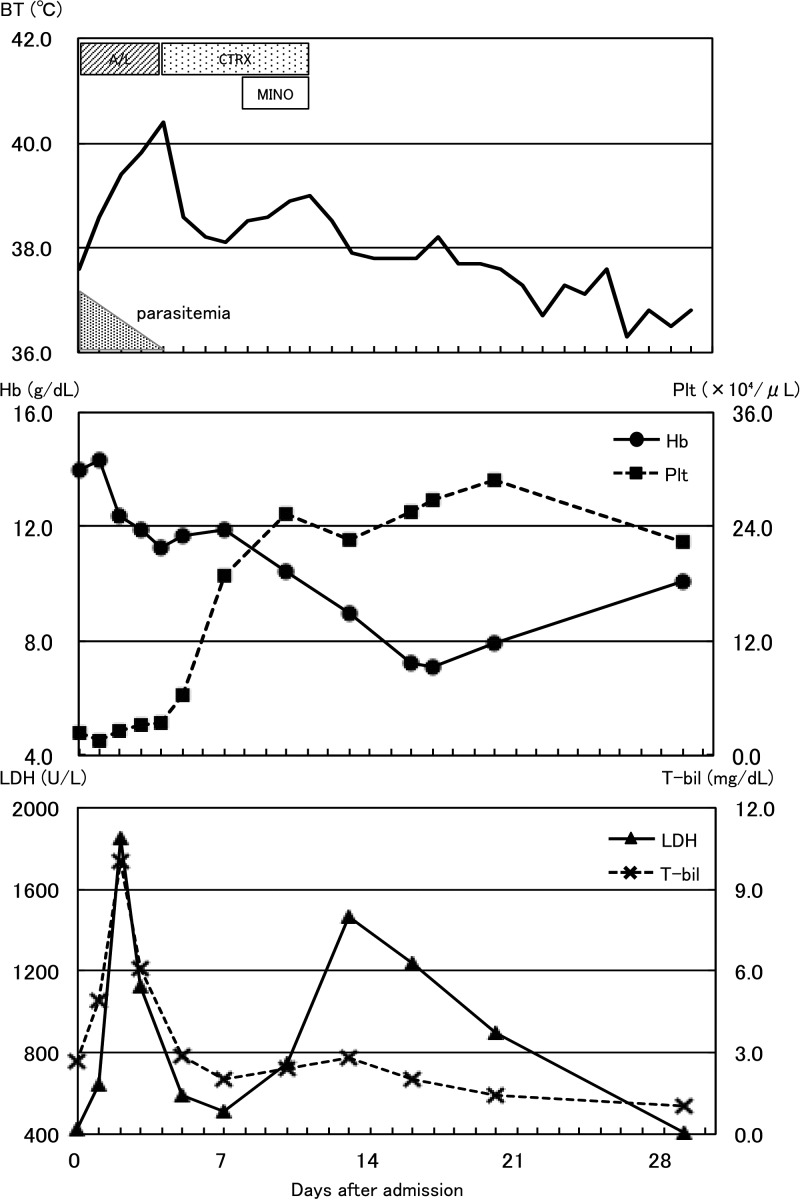
The following day, a blood smear revealed P. falciparum trophozoites, with 20.6% parasitemia. Because his parasitemia satisfied WHO 2010 criteria for severe malaria, optimal management would be to switch treatment from oral to parenteral antimalarials. However, in Japan, intravenous artesunate is not available, and the only available parenteral antimalarial drug is quinine gluconate. We discussed risks and benefits associated with using quinine gluconate injection and decided to continue to administer oral artemether–lumefantrine for 3 days in total. As shown in Figure 1 , although his parasitemia declined gradually and was absent on day 4, he remained febrile (> 38°C). A repeated blood smear revealed no malaria parasites. We suspected partially treated typhoid fever and rickettsiosis. Despite administering ceftriaxone and minocycline, his body temperature increased again. On days 13 and 16, he exhibited laboratory signs of hemolytic anemia (Table 1), with the lowest Hb level, 7.2 g/dL, observed on day 16. No parasites were found in a blood smear, and direct and indirect Coombs test results were negative. His glucose-6-phosphate dehydrogenase activity was normal (G6PD Assay Kit-WST, Dojindo Laboratories, Kumamoto, Japan). His fever gradually decreased in subsequent days. He refused blood transfusions and was discharged on day 29 after his Hb levels increased and lactate dehydrogenase (LDH) decreased.
Figure 1.
Clinical course of case 1. AL = artemether–lumefantrine; BT = body temperature; CTRX = ceftriaxone; MINO = minocycline.
Case 2.
A 27-year-old Japanese female student traveled to Kenya and Uganda from August 9 to September 14, 2014, to conduct cultural anthropology research. She stayed at a hotel in Nairobi for the first and last week and in Kampala and Gulu for 4 weeks. She received rabies, tetanus, and yellow fever vaccines before traveling but did not receive a malaria prophylaxis. On September 5, she visited a local clinic because of fever and was prescribed analgesics. She became afebrile within a few days but again developed a fever on September 10. She returned to Japan on September 15 and visited the hospital because of continued fever and chills/shaking. She was alert and oriented. Her body temperature was 40.4°C, blood pressure was 79/53 mmHg, pulse rate was a regular 120/minute, respiration rate was 18/minute, and SpO2 was 96% (room air). A physical examination revealed hepatosplenomegaly and many insect bite scars on her legs but otherwise normal findings. A laboratory examination at admission (day 0) revealed elevated liver function levels but no anemia, thrombocytopenia, kidney dysfunction, or hypoglycemia (Table 2). A malaria rapid diagnosis test (BinaxNOW Malaria; Binax, Inc./Alere, Waltham, MA) demonstrated positivity for both the P. falciparum HRP-II antigen and a pan-malarial antigen common to all four malaria species capable of infecting humans; a blood smear examination revealed P. falciparum trophozoites with 4.5% parasitemia. Two sets of blood cultures were negative. The patient was diagnosed with falciparum malaria with hyperparasitemia, and oral quinine hydrochloride and doxycycline were initiated because of lack of immediate availability of parenteral antimalarials. On day 2, a blood smear revealed an increase in parasitemia to 7.9%. We planned a change in therapy to quinine gluconate injection, but the patient complained of severe tinnitus, likely due to quinine, so the treatment was switched to oral artemether–lumefantrine 20/120 mg twice daily for 3 days. As shown in Figure 2 , the parasitemia declined gradually and was absent on day 3; the patient also became afebrile on day 3. She was discharged on day 4. However, on day 5, she developed a fever > 38°C, and returned to the hospital on day 8. Her blood smear examination revealed no malaria parasites, and a blood culture was negative. On day 11, she exhibited laboratory signs of hemolytic anemia (Table 2). No parasites were found on a blood smear, and the direct Coombs test was negative. A telephone follow-up later confirmed that her condition improved gradually.
Table 2.
Laboratory data of case 2 over time
| Day 0 | Day 1 | Day 2 | Day 3 | Day 8 | Day 11 | |
|---|---|---|---|---|---|---|
| WBC (/mm3) | 7,000 | 10,900 | 8,500 | 7,600 | 7,600 | 7,300 |
| RBC (× 104/mm3) | 496 | 420 | 414 | 403 | 375 | 305 |
| Hb (g/dL) | 14.1 | 12.0 | 12.0 | 11.6 | 10.8 | 8.8 |
| Plt (× 104/mm3) | 13.7 | 8.3 | 5.8 | 5.9 | 23.4 | 26.6 |
| T-bil (mg/dL) | 0.8 | 1.1 | 2.1 | 0.9 | 1.7 | 2.2 |
| I-bil (mg/dL) | 0.7 | 1.5 | 2.0 | |||
| AST (U/L) | 97 | 84 | 65 | 44 | 30 | 31 |
| ALT (U/L) | 83 | 63 | 54 | 42 | 18 | 16 |
| LDH (U/L) | 374 | 427 | 451 | 535 | 498 | 712 |
| BUN (mg/dL) | 19.3 | 26.5 | 12.5 | 8.9 | 7.8 | 11 |
| Cre (mg/dL) | 0.92 | 0.78 | 0.71 | 0.71 | 0.63 | 0.56 |
| Glu (mg/dL) | 128 | 101 | 132 | 96 | 104 | 103 |
| Parasitemia (%) | 4.5 | 7.9 | 0.07 | 0 | 0 | 0 |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BUN = blood urea nitrogen; Cre = creatinine; Glu = glucose; Hb = hemoglobin; I-bil = indirect bilirubin; LDH = lactate dehydrogenase; Plt = platelets; RBC = red blood cells; T-bil = total bilirubin; WBC = white blood cells.
Figure 2.
Clinical course of case 2. AL = artemether–lumefantrine; BT = body temperature; DOXY = doxycycline.
Discussion
The following definition of PADH has been proposed: a > 10% decrease in Hb levels associated with a haptoglobin level < 0.1 g/L and an increase in LDH to > 390 U/L, or an increase of > 10% at 7 days after the initiation of parenteral artemisinin treatment.9,10 A common feature of PADH cases was a high initial parasitemia level.11 In case 1, the initial parasitemia was 20.6%, the LDH level increased from 511 U/L on day 8 to 1,466 U/L on day 13, and the Hb level decreased from 11.9 g/dL on day 8 to 7.2 g/dL on day 16. In case 2, the initial parasitemia was 4.5%, the LDH level increased from 498 U/L on day 8 to 712 U/L on day 11, and the Hb level decreased from 10.8 g/dL on day 8 to 8.8 g/dL on day 11. Accordingly, both cases fulfilled the proposed definition of PADH.
Whether artemisinin derivatives other than artesunate are related to delayed-onset hemolytic anemia remains controversial. Among the 39 previously reported PADH cases, 14 had switched to oral artemether–lumefantrine following initial treatments such as parenteral artesunate or parenteral artesunate plus tetracyclines.3,12–14 Other combinations of ACTs included artesunate–amodiaquine following artesunate injection,5 dihydroartemisinin–piperaquine following intramuscular artemether,6 and artemether–lumefantrine following quinine injection plus doxycycline.8 Only one previous case of PADH was associated with artemether–lumefantrine as the sole regimen.7 Therefore, we believed that all artemisinin derivatives and all routes of administration could potentially induce delayed hemolytic anemia when used to treat severe malaria11 and that our present cases were likely attributable to oral artemether–lumefantrine.
We could not rule out other causes of hemolysis. In case 1, we administered ceftriaxone for 7 days (days 4–10) and minocycline for 3 days (days 8–10) for persistent fever after parasite clearance under the respective suspicions of typhoid fever and rickettsiosis. Ceftriaxone is known to cause drug-induced immune hemolytic anemia (DIIHA).15 However, DIIHA is rare, with an estimated incidence of approximately one per million individuals.16 Furthermore, cases of ceftriaxone-induced immune hemolytic anemia occur mostly in children and rarely in adults.15,17 To date, there has been only a single case report of minocycline-induced hemolytic anemia in Japanese children.18 From these observations, it is unlikely that ceftriaxone or minocycline caused the delayed hemolytic anemia observed in the patient from case 1, although additional investigations of DIIHA were not conducted. In case 2, the patient was treated with quinine hydrochloride and doxycycline for the first 12 hours, followed by oral artemether–lumefantrine for 3 days. Massive hemolysis accompanied by renal impairment, commonly known as blackwater fever (BWF), is a known complication of malaria, often associated with quinine therapy. However, the onset of BWF after quinine use is typically much more rapid than that observed in our case. In a Vietnamese study, the median time between quinine use and BWF onset was 24 hours.19 Moreover, BWF commonly occurs in malaria patients with very low parasitemia or an absence of blood-stage parasites.20
According to a French prospective study, intravenous artesunate-related delayed hemolytic anemia occurred in 22% (13 of 60) of returning travelers with severe malaria.9 In another prospective study of Ghanaian and Gabonese children aged 6 months to 10 years, five of 72 (7%) patients exhibited delayed hemolysis at approximately day 14 after treatment with artesunate and artemether–lumefantrine.9 However, little is known about the occurrence of artemether-lumefantrine-related delayed hemolysis. As previously described, neither parenteral artesunate nor artemether–lumefantrine is licensed in Japan; the latter is used only at registered facilities after obtaining informed consent from the patient. After treatment, attending physicians fill in and return the patient records formulated by our research group. From April 2003 to March 2014, 66 patients were treated with artemether–lumefantrine. Among them, a total of four patients (6%), including the two patients described herein, developed delayed hemolytic anemia (manuscript in preparation). As in our case, Japanese physicians tend to choose artemether–lumefantrine to treat malaria, even with hyperparasitemia, because intravenous artesunate is not available in Japan, we expect rapid parasite clearance after treatment with artemisinin derivatives, and we are concerned about side effects of quinine gluconate injection. However, we should reaffirm the principle that the standard of care is to treat all cases of severe malaria with a parenteral regimen.
The mechanism by which artemisinin and its derivatives induce delayed hemolytic anemia in patients with severe malaria is not fully understood. Initially, toxicity related to the use of non-good manufacturing practice artesunate or higher total doses of artesunate was thought to induce delayed hemolytic anemia, although this hypothesis has been recently discredited.21–23 A recently published report suggested that delayed hemolytic anemia is caused by the delayed clearance of previously infected erythrocytes that continue to circulate despite the antiparasitic effects of artemisinin.9 Delayed hemolysis might also be attributable to an immune mechanism because several cases have included positive direct Coombs tests or direct antiglobin tests, although our patients had negative direct Coombs test results.23 Furthermore, artesunate-dependent antibodies were recently reported in a French patient.13
Similar to our patients, most patients with delayed hemolysis described to date have recovered naturally. Several patients with severe anemia, however, required blood transfusions to support their recovery. Other reports have described the administration of corticosteroids to an artesunate-induced antibody-positive patient13 and some patients with positive Coombs tests24,25; however, it is unclear whether this treatment influenced the course of delayed hemolytic anemia, and therefore, corticosteroids cannot be recommended for the treatment of delayed hemolysis.23 Accordingly, the main focus of clinical delayed hemolysis management is an intensified follow-up. Rolling and others recommend that attending physicians monitor patients with severe malaria weekly for up to 4 weeks after artemisinin treatment23 for symptoms such as prolonged fever, observed in our case 1, and relapsing fever.
Lastly, we would like to emphasize the importance of chemoprophylaxis. The two cases we reported could have been prevented by a simple intervention, malaria chemoprophylaxis. Health-care providers should give appropriate advice on prevention to people who travel to endemic countries.
In conclusion, delayed hemolytic anemia is known to occur in a certain percentage of severe malaria patients after treatment with artemisinin and its derivatives. Notably, even oral artemether–lumefantrine can cause these hemolytic events. An unusual clinical course in successfully treated malaria, such as persistent or recurrent fever, as seen in several case reports of PADH,6,7,26 might offer a clue suggesting the occurrence of delayed hemolysis. We should therefore closely monitor artemisinin-treated patients for up to 4 weeks to monitor the symptoms and signs of hemolysis and provide appropriate supportive treatment.
Footnotes
Consent for publication: Written informed consent was obtained from the patients for the publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor of this journal.
Financial support: This research is supported by grants from Ministry of Health, Labour and Welfare (H25-Iryogijutsu-Shitei-012), and the Emerging/Re-emerging Infectious Diseases Project of Japan (15fk0108046h0003) from Japan Agency for Medical Research and development (AMED).
Authors' addresses: Yasuhiro Tsuchido, Kentaro Tochitani, Koh Shinohara, and Tsunehiro Shimizu, Department of Infectious Diseases, Kyoto City Hospital, Kyoto, Japan, E-mails: tutti.zero@gmail.com, tochiken@me.com, shinoharakoh@gmail.com, and tsunetak915@yahoo.co.jp. Fukumi Nakamura-Uchiyama, Yukiteru Ouji, and Masahide Yoshikawa, Department of Pathogen, Infection and Immunity, Nara Medical University, Nara, Japan, E-mails: fukumi_nakamura@tokyo-hmt.jp, oujix@naramed-u.ac.jp, and myoshika@naramed-u.ac.jp. Kasumi Toyoda, Naokuni Hishiya, Taku Ogawa, Kenji Uno, Kei Kasahara, and Keiichi Mikasa, Center for Infectious Diseases, Nara Medical University, Nara, Japan, E-mails: toyodakasumi@hotmail.co.jp, hissy22@hotmail.com, t.ogawammx@gmail.com, hello_kenji2004@yahoo.co.jp, kassan@naramed-u.ac.jp, and mikasak1@naramed-u.ac.jp. Moritoshi Iwagami and Shigeyuki Kano, Department of Tropical Medicine and Malaria, Research Institute, National Center for Global Health and Medicine, Tokyo, Japan, E-mails: miwagami@ri.ncgm.go.jp and kano@ri.ncgm.go.jp. Haruhiko Maruyama, Department of Infectious Diseases, Division of Parasitology, Faculty of Medicine, University of Miyazaki, Miyazaki, Japan, E-mail: hikomaru@med.miyazaki-u.ac.jp.
References
- 1.World Health Organization World Malaria Report. 2009. http://apps.who.int/iris/bitstream/10665/44234/1/9789241563901_eng.pdf Available at. Accessed June 9, 2016.
- 2.Sylla K, Abiola A, Tine RC, Faye B, Sow D, Ndiaye JL, Ndiaye M, Lo AC, Folly K, Ndiaye LA, Gaye O. Monitoring the efficacy and safety of three artemisinin based-combinations therapies in Senegal: results from two years surveillance. BMC Infect Dis. 2013;13:598–607. doi: 10.1186/1471-2334-13-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briggs M, Arguin PM. Published reports of delayed hemolytic anemia after treatment with artesunate for severe malaria–worldwide, 2010–2012. MMWR Morb Mortal Wkly Rep. 2013;62:5–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Paczkowski MM, Landman KL, Arguin PM. Update on cases of delayed hemolysis after parenteral artesunate therapy for malaria, United States, 2008 and 2013. MMWR Morb Mortal Wkly Rep. 2014;63:753–755. [PMC free article] [PubMed] [Google Scholar]
- 5.Burri C, Ferrari G, Ntuku HM, Kitoto AT, Duparc S, Hugo P, Mitembo DK, Lengeler C. Delayed anemia after treatment with injectable artesunate in the Democratic Republic of the Congo: a manageable issue. Am J Trop Med Hyg. 2014;91:821–823. doi: 10.4269/ajtmh.14-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarvis JN, Coltart CE, Pule M, Chiodini PL, Doherty T. Artemisinin therapy and severe delayed haemolysis. Lancet. 2013;382:180. doi: 10.1016/S0140-6736(13)60812-0. [DOI] [PubMed] [Google Scholar]
- 7.De Nardo P, Oliva A, Giancola ML, Ghirga P, Mencarini P, Bibas M, Nicastri E, Antinori A, Corpolongo A. Haemolytic anemia after oral artemether-lumefantrine treatment in a patient affected by severe imported falciparum malaria. Infection. 2013;41:863–865. doi: 10.1007/s15010-013-0451-x. [DOI] [PubMed] [Google Scholar]
- 8.Corpolongo A, De Nardo P, Ghirga P, Gentilotti E, Bellagamba R, Tommasi C, Paglia MG, Nicastri E, Narciso P. Haemolytic anemia in an HIV-infected patient with severe falciparum malaria after treatment with oral artemether-lumefantrine. Malar J. 2012;11:91–94. doi: 10.1186/1475-2875-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jauréguiberry S, Ndour PA, Roussel C, Ader F, Safeukui I, Nguyen M, Biligui S, Ciceron L, Mouri O, Kendjo E, Bricaire F, Vray M, Angoulvant A, Mayaux J, Haldar K, Mazier D, Danis M, Caumes E, Thellier M, Buffet P. French Artesunate Working Group Postartesunate delayed hemolysis is a predictable event related to the lifesaving effect of artemisinins. Blood. 2014;124:167–175. doi: 10.1182/blood-2014-02-555953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arguin PM. Case definition: postartemisinin delayed hemolysis. Blood. 2014;124:157–158. doi: 10.1182/blood-2014-06-578922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rehman K, Lötsch F, Kremsner PG, Ramharter M. Haemolysis associated with the treatment of malaria with artemisinin derivatives: a systematic review of current evidence. Int J Infect Dis. 2014;29:268–273. doi: 10.1016/j.ijid.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Laura R, Phillipe L, Marie-Pierre H. Scientific Study Group for Travel Medicine . A Case of Hemolytic Anemia after Severe Malaria Successfully Treated with Artesunate. 2013. http://www.sbimc.org/posters%202013/Abstract5.pdf Joint Symposium belgische vereniging voor infectiologie en klinische microbiologie. Available at. Accessed June 9, 2016. [Google Scholar]
- 13.Raffray L, Receveur MC, Beguet M, Lauroua P, Pistone T, Malvy D. Severe delayed autoimmune haemolytic anaemia following artesunate administration in severe malaria: a case report. Malar J. 2014;13:398–403. doi: 10.1186/1475-2875-13-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolling T, Agbenyega T, Issifou S, Adegnika AA, Sylverken J, Spahlinger D, Ansong D, Löhr SJ, Burchard GD, May J, Mordmüller B, Krishna S, Kremsner PG, Cramer JP. Delayed hemolysis after treatment with parenteral artesunate in African children with severe malaria–a double-center prospective study. J Infect Dis. 2014;209:1921–1928. doi: 10.1093/infdis/jit841. [DOI] [PubMed] [Google Scholar]
- 15.Neuman G, Boodhan S, Wurman I, Koren G, Bitnun A, Kirby-Allen M, Ito S. Ceftriaxone-induced immune hemolytic anemia. Ann Pharmacother. 2014;48:1594–1604. doi: 10.1177/1060028014548310. [DOI] [PubMed] [Google Scholar]
- 16.Garratty G. Immune hemolytic anemia associated with drug therapy. Blood Rev. 2010;24:143–150. doi: 10.1016/j.blre.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Imam SN, Wright K, Bhoopalam N, Choudhury A. Hemolytic anemia from ceftriaxone in an elderly patient: a case report. J Am Med Dir Assoc. 2008;9:610–611. doi: 10.1016/j.jamda.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Kudoh T, Nagata N, Suzuki N, Nakata S, Chiba S, Takahashi T. Minocycline-induced hemolytic anemia. Acta Paediatr Jpn. 1994;36:701–704. doi: 10.1111/j.1442-200x.1994.tb03274.x. [DOI] [PubMed] [Google Scholar]
- 19.Tran TH, Day NP, Ly VC, Nguyen TH, Pham PL, Nguyen HP, Bethell DB, Dihn XS, Tran TH, White NJ. Blackwater fever in southern Vietnam: a prospective descriptive study of 50 cases. Clin Infect Dis. 1996;23:1274–1281. [PubMed] [Google Scholar]
- 20.Van den Ende J, Coppens G, Verstraeten T, Van Haegenborgh T, Depraetere K, Van Gompel A, Van den Enden E, Clerinx J, Colebunders R, Peetermans WE, Schroyens W. Recurrence of blackwater fever: triggering of relapses by different antimalarials. Trop Med Int Health. 1998;3:632–639. doi: 10.1046/j.1365-3156.1998.00287.x. [DOI] [PubMed] [Google Scholar]
- 21.Zoller T, Junghanss T, Kapaun A, Gjorup I, Richter J, Hugo-Persson M, Mørch K, Foroutan B, Suttorp N, Yürek S, Flick H. Intravenous artesunate for severe malaria in travelers, Europe. Emerg Infect Dis. 2011;17:771–777. doi: 10.3201/eid1705.101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yansouni CP, Libman MD. Does post-artesunate delayed haemolysis change practice? Travel Med Infect Dis. 2015;13:122–123. doi: 10.1016/j.tmaid.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Rolling T, Agbenyega T, Krishna S, Kremsner PG, Cramer JP. Delayed haemolysis after artesunate treatment of severe malaria: review of the literature and perspective. Travel Med Infect Dis. 2015;13:143–149. doi: 10.1016/j.tmaid.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Kreeftmeijer-Vegter AR, van Genderen PJ, Visser LG, Bierman WF, Clerinx J, van Veldhuizen CK, de Vries PJ. Treatment outcome of intravenous artesunate in patients with severe malaria in The Netherlands and Belgium. Malar J. 2012;11:102–112. doi: 10.1186/1475-2875-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eder M, Farne H, Cargill T, Abbara A, Davidson RN. Intravenous artesunate versus intravenous quinine in the treatment of severe falciparum malaria: a retrospective evaluation from a UK centre. Pathog Glob Health. 2012;106:181–187. doi: 10.1179/2047773212Y.0000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caramello P, Balbiano R, De Blasi T, Chiriotto M, Deagostini M, Calleri G. Severe malaria, artesunate and haemolysis. J Antimicrob Chemother. 2012;67:2053–2054. doi: 10.1093/jac/dks139. [DOI] [PubMed] [Google Scholar]