Abstract
From September 2013 to July 2014, several gold miners working in the tropical forest consulted the Maripasoula Health Center in French Guiana for edema and findings consistent with right-sided cardiac failure. Of the 42 cases of beriberi that were diagnosed, one patient died. The laboratory and clinical investigation demonstrated vitamin B1 deficiency in most of the patients tested. Furthermore, 30 of 42 patients responded favorably to 500 mg of intravenous or intramuscular thiamine supplementation. In addition, dietary investigation showed insufficient thiamine intake in these patients. We concluded that patients had acquired beriberi because of diet restrictions, hard labor, and infectious diseases, notably malaria. In 2016, cases were still being reported. We recommend screening for compatible symptoms in gold miners, thiamine supplementation, and nutritional intervention.
Introduction
In French Guiana, a French overseas territory in South America, several thousands of illegal gold miners, mostly from Brazil, are mining in the rain forest.1 Gold camps are isolated and represent a risk for the health and safety of not only the local populations, who depend on the forest for their livelihoods, but also for gold miners who themselves are the first victims of the precarious conditions they live in.2–5
Beriberi is a clinical syndrome caused by vitamin B1 (thiamine) deficiency. The principal manifestations are cardiovascular (cardiac failure, lactic acidosis, and death due to cardiac and vasodilatory shock) and neurological (peripheral neuropathy or Wernicke–Korsakoff syndrome). Beriberi has been associated with a thiamine-deficient diet, mostly in vulnerable populations such as refugees, prisoners, and populations dependent on polished rice.6–8 Beriberi has also been described among Amerindian populations living in the Amazon region in Brazil.9,10 Interestingly, the last case of beriberi was in French Guiana in the mid-19th century when the penal colony was still operational.11,12
In September 2013, several gold miners with lower extremity edema and signs and symptoms compatible with right heart failure were identified at the health center of Maripasoula. One death preceded by similar manifestations was also reported in Cayenne hospital. This outbreak was investigated between September 2013 and the end of July 2014, to establish the diagnosis and identify the risk factors, and to implement control and prevention interventions.
Description of the Investigation
Epidemiological investigation.
In this study, a suspected case of beriberi was defined as any patient who was living in a gold mine who presented an acute onset of any or all of the following signs within the past 6 months: lower limb edema, cardiac failure, peripheral neuropathy, and/or Wernicke–Korsakoff syndrome. A confirmed case was defined as a suspected case with laboratory confirmation of thiamine deficiency. Patients with a medical history of diabetes, hypertension, cardiomyopathy, neuropathy, and peripheral vascular disease were excluded.
All cases were interviewed with a standard questionnaire for symptoms, risk factors, place of residence and work, consumption of drugs, and use of toxic chemicals. The type and quantity of food consumed were also collected to calculate the calorie intake.
Clinical and laboratory investigations.
We reviewed medical charts for all patients using a standardized form. Cases of beriberi were classified according to World Health Organization definitions.7 Dry beriberi was defined by the presence of polyneuropathy of the extremities, reduced tendon reflexes and progressive weakness, and wasting of muscles without cardiac signs. Wet beriberi was characterized by edema, pulmonary congestion with pleural effusion, and signs of high cardiac output. Mixed beriberi was defined by the presence of both wet and dry signs and symptoms. Shoshin beriberi cases were classified as a fulminant form with lactic acidosis, hypotension, tachycardia, and pulmonary edema. Recovery was defined by a complete resolution of signs and symptoms at the medical examination during the 2-month follow-up after the end of the treatment. Relapse was defined as a complete resolution of signs and symptoms after the treatment followed by a relapse of signs and symptoms during the 2-month follow-up after the end of the treatment.
Electrocardiograms were performed to look for conduction, rhythm, or repolarization abnormalities.
Blood specimens were collected according to the standard procedures of the laboratory and were tested for vitamin B1 levels, which was possible by measuring the erythrocyte transketolase activity coefficient. Blood tubes were drawn and kept refrigerated at 4°C at the health center. For transketolase activity, the standardized protocol required the use of heparin tubes that were centrifuged to remove the plasma, keeping the tube in darkness, and then freezing it. The samples were then sent daily to the main laboratory in Cayenne, from where they were dispatched to specialized laboratories. The analysis was performed at the CERBA Laboratory in Paris. Thiamine deficiency was defined by vitamin B1 concentration < 66.5 nmol/L (CERBA Laboratory, Paris) or erythrocyte transketolase activity below 124 U/L with a thiamin diphosphate stimulation effect greater than 20% in the analysis performed on erythrocytes by Beaujon Hospital, Paris.
Malaria was diagnosed using thin and thick blood smears and rapid diagnostic tests SD Bioline Malaria Ag P.f/Pan (Gyeonggi-do, Republic of Korea). For parasitological examination of stools, a single sample was taken. Fecal examination was performed combining direct examination of fresh feces with the quantitative techniques of Kato-Katz method, Baermann, and Merthiolate iodine formalin staining.
Environmental investigation.
The French army conducted toxicological analyses in sand and dirt samples sampled from “Eaux Claires,” the main gold mine, in June 2014.
Rice and bean samples from gold miners were cultured to detect citreoviridin-producing Penicillium citreonigrum suspected in a previous outbreak in neighboring Brazil.13 Citreoviridin is a mycotoxin produced by molds common in rice, which causes a syndrome similar to wet beriberi. However, its role has not been proven in humans yet.
Ethical and regulatory approval.
The study was a retrospective analysis of anonymized monocentric data, which is permitted by French regulations. Commission Nationale de l'Informatique et des Libertés registration number was N°1939018.
Results
Outbreak description.
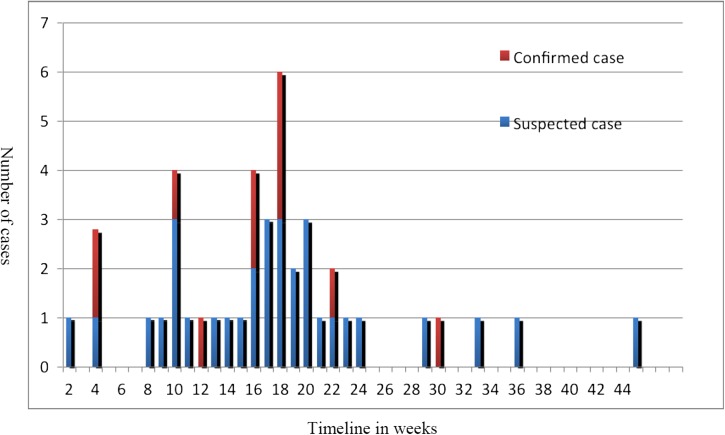
From September 2013 to the end of July 2014, a total of 10 confirmed and 32 suspected cases were reported. All cases, except one, resided in illegal mining camps located in the rainforest in the interior of French Guiana. The median age was 36 years (range: 22–65 years). There were 37 men and five women. The outbreak started in September, with a sharp increase in cases in October, peaking in December and January 2014 (Figure 1 ). Of the 42 patients, 16 were referred to the capital city, Cayenne, by helicopter, for further treatment in Cayenne General Hospital, the main referral hospital located on the coastal part of French Guiana, where one patient died in September. In October, after concluding that the patients' clinical signs and symptoms were compatible with beriberi, physicians refrained from using dextrose solutions, and gave thiamine supplementation administered at 500 mg/day intravenously or, when impossible, intramuscularly. This was followed by oral supplementation (500 mg/week) for gold miners. Supplementation was followed by the rapid regression of symptoms.
Figure 1.
Epidemic curve for the outbreak of thiamine deficiency in Maripasoula.
The epidemiological investigation demonstrated that most patients worked in the illegal gold mines surrounding Maripasoula (Table 1). The women did not work in the mines; most were cooks and some declared sex work. The majority of men worked in the mines or in water with water pumps. Only one quarter of patients reported handling and using mercury (N = 3/12). A large proportion (24/29) reported a history of malaria, with 11 cases reporting antimalarial treatment in the past 3 months. The reported food intake was insufficient, that is, 1,075 Kcal/day and 0.44 mg of thiamine/day, whereas the recommendations for physically active populations are 3,500 Kcal and 0.4 mg of thiamine/1,000 Kcal.7 The main reported foods were beans and chicken. Washing of rice grains before cooking was frequently reported. The main antithiamine factors were sugar and coffee consumption. Only two people declared alcohol consumption and none declared using drugs.
Table 1.
Characteristics of beriberi outbreak cases (N = 42), September 2013–July 2014, French Guiana
| N | Percent | |
|---|---|---|
| Demographics | ||
| Median age (range) | 36 (22–65) | |
| Sex (male) | 37 | 88 |
| Country of birth | ||
| Brazil | 42 | 100 |
| State of Maranhao | 38 | 90 |
| State of Piaui | 2 | 4.7 |
| State of Grande Norte | 2 | 4.7 |
| Duration of stay in French Guiana Amazonian forest before admission | ||
| Median in months (range) | 9 (2–360) | |
| Duration of stay in the last gold mine camp | ||
| Median in months (range) | 6 (2–60) | |
| Name of gold mine camp | ||
| Eaux Claires | 29 | 69 |
| Marodeio | 3 | 7.1 |
| Pied de Limon | 3 | 7.1 |
| La Gresia | 1 | 2.3 |
| Benzdorp | 2 | 4.6 |
| Bafadim | 1 | 2.3 |
| Antonio do Brinco | 1 | 2.3 |
| Saint Elie | 1 | 2.3 |
| Missing data | 2 | 4.6 |
| Occupational exposures | ||
| Uses mercury in mining | 3 (N = 13) | 23 |
| Working hours > 12 hours per day | 8 (N = 12) | 66.6 |
| Working hours < 12 hours per day | 4 (N = 12) | 33 |
| Classification/type of beriberi* | ||
| Wet beriberi | 28 | 66.6 |
| Dry beriberi | 0 | 0 |
| Mixed beriberi | 13 | 31 |
| Shoshin beriberi | 1 | 2.3 |
| Outcome† | ||
| Recovery after thiamine supplementation | 30 | 71.4 |
| Relapse after thiamine supplementation | 2 | 4.7 |
| Death before thiamine supplementation | 1 | 2.3 |
| Missing follow-up data | 9 | 21.4 |
Dry beriberi was defined by the presence of polyneuropathy of the extremities, reduced tendon reflexes and progressive weakness, and wasting of muscles without cardiac signs. Wet beriberi was characterized by edema, pulmonary congestion with pleural effusions, and signs of high cardiac output. Mixed beriberi was defined by the presence of both wet and dry symptoms. Shoshin beriberi cases were classified as a fulminant form with lactic acidosis, hypotension, tachycardia, and pulmonary edema.
Recovery was defined by a complete resolution of signs and symptoms at the medical examination during the 2-month follow-up after the end of the treatment.
Clinical and laboratory findings.
The most frequent syndromes were edema (N = 40/42), paresthesia (N = 21/42), and dyspnea (N = 10/42).
Thiamine levels were measured in 14 patients, and were found to be lower than normal values in 10 individuals; the normal laboratory range is 35.8–90.5 nmol/L.
Other nutritional deficiencies were observed. The majority of cases presented folic acid deficiency (14/21). Hypoalbuminemia (7/13), vitamin B12 deficiency (2/20), and selenium deficiency (2/6) were also found in some patients. Seven of 13 tested patients had hypoalbuminemia, two of the 20 tested had vitamin B12 deficiency, and two of the six tested had selenium deficiency. NT-proBNP levels were above normal in 24 of the 37 tested cases and had significantly decreased (P = 0.043) after thiamine supplementation.
Twelve of 33 patients were diagnosed with malaria (Plasmodium falciparum [7/33] and Plasmodium vivax [5/33]) infections. Seven patients had hookworm eggs in their stool (7/10). No other intestinal helminths were found. Parasitological examination of stools did not find Giardia. Entamoeba coli was found in 2/10 patients.
A broad range of diagnostic explorations was conducted to look for potential infectious causes of myocarditis: Treponema pallidum hemagglutination assay, venereal disease research laboratory test for syphilis and serology using enzyme-linked immunosorbent assay for toxoplasmosis, leptospirosis, trichinellosis, Q fever, Legionella, Bartonella, Rickettsia infection, echinococcosis, arboviruses (dengue and chikungunya), herpes viruses (Epstein–Barr virus, cytomegalovirus, varicella-zoster virus, and herpes simplex virus), trypanosomiasis, hepatitis B and C, and Coxsackie virus were performed and found to be either negative or showing signs of previous immunization. One patient was positive for human immunodeficiency virus and another had biopsy-confirmed leprosy. Penicillium citreonigrum was negative in food samples.13 Toxicological soil analyses were normal.
Patient outcome.
The majority of patients (71.5%, N = 30/42) recovered after injected and oral thiamine supplementation (Table 1). The mean average recovery duration (disappearance of edema, paresthesia, and right-sided cardiac failure signs and symptoms) was 3 days. One patient with an associated selenium deficiency recovered after selenium and thiamine cosupplementation. One patient, with Shoshin beriberi, died before thiamine supplementation could be administered.
Discussion
The imputation of this outbreak to beriberi was based on the evocative clinical presentation and context, the good therapeutic response in the majority of cases, and the low thiamine levels in the majority of tested cases.
Given the clinical chronology and the average durations of stay in gold mines, it seemed very unlikely that cases were imported. The reasons why the first onset occurred in 2013 are still unknown. This outbreak maybe is the result of some changes in the population's thiamine intake or other risk factors, or simply, improved detection. However, our findings suggest that this gold miner population experienced beriberi because of chronic low dietary thiamine intake that was exacerbated by known causes of increased thiamine requirement. The conditions requiring increased thiamine included physical efforts, chronic intestinal parasitic infections, and recurrent fever, either caused by malaria or by other multiple infections inherent to living in conditions conducive to the transmission of water-borne and vector-borne diseases. In favor of this, our study showed that the majority of patients were living in “Eau Claire” camp, where an outbreak of multiple coinfections was reported the year before our investigation.5 Malaria is often associated with beriberi because it increases glucose demand and requirement for lactate elimination, both of which are affected by the lack of thiamine.14
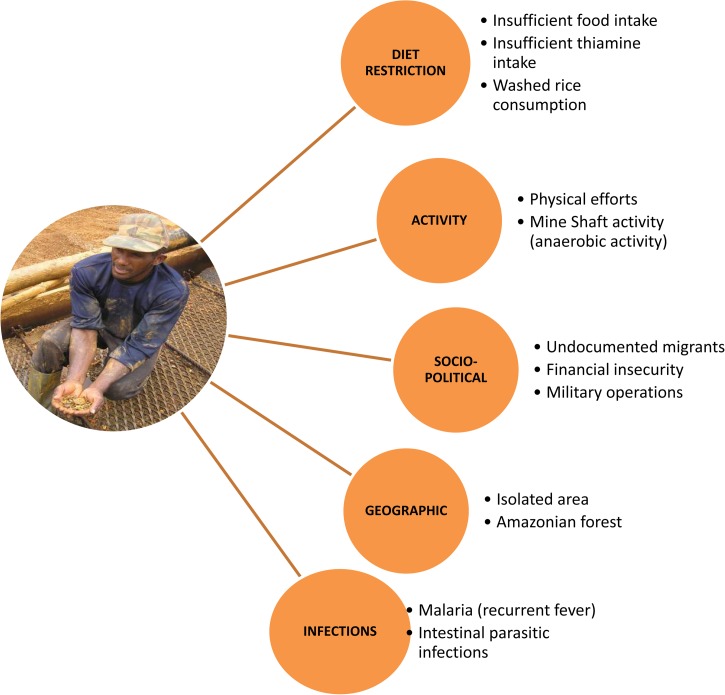
Finally, the consumption of thiamine antagonists, such as sugar and coffee, was also reported, which possibly contributed to the patients' thiamine deficiency. Citreoveridin-producing P. citreonigrum was not found, but its causative role in beriberi has not been proven in humans. The beginning of this epidemic may have been triggered by the increased number of military missions aiming at destroying camps and food reserves, which may have worsened nutritional deficiencies. Another factor which might have caused the outbreak was the recent return of mine shafts with an increase of anaerobic working conditions due to low oxygen concentrations in the pits (Figure 2 ).
Figure 2.
Coexisting risk factors of Beriberi outbreak, September 2013–July 2014, French Guiana.
Our findings are comparable to previously reported beriberi outbreaks in working-class men, such as those reported in farmers in Africa and in fishermen in Asia.15,16 Furthermore, an outbreak of beriberi has previously been described in Roraima State in Brazil, near French Guiana, in a different population (Amerindians), which suggested that the cause of the disease was not connected with ethnicity, but probably with the precarious conditions of food intake in this area.9
The limitations of our study include the lack of a miner control group, and missing thiamine values due to the geographical challenges of French Guiana. Two “verbal autopsies” reporting deaths during transport of patients with “swollen bodies,” and other unverifiable reports of recent deaths in gold mines suggest the number of cases and deaths from beriberi was probably underestimated.
In March 2016, the outbreak was still not considered to be controlled. Detailed epidemiological investigations have stopped due to staff shortages. However, physicians in Maripasoula continue thiamine supplementation for miners. Some miners are now familiar with this disease and thus self-medicate with thiamine in the camps. Although beriberi is a preventable illness, this outbreak is another reminder of the challenges of access to prevention and care for this specific vulnerable population.
ACKNOWLEDGMENTS
We acknowledge the physicians and microbiologists of Cayenne hospital and Maripasoula Health Center whose collaboration made this investigation possible.
Footnotes
Authors' addresses: Emilie Mosnier, Unité de Maladie Infectieuses et Tropicales, Centre Hospitalier Andrée Rosemon, Cayenne, French Guiana, France, E-mail: emilie.mosnier@gmail.com. Florence Niemetzky, Juliette Stroot, Paul Brousse, Basma Guarmit, and Muriel Ville, Departement des Centres de Santé, Centre Hospitalier de Cayenne, Cayenne, French Guiana, France, E-mails: florenceniem@gmail.com, juliette1046@hotmail.com, paul.brousse@ch-cayenne.fr,basma.guarmit@ch-cayenne.fr, and muriel.ville@ch-cayenne.fr. Vincent Pommier de Santi, Direction Inter-armées du Service de Santé des Armées (Diass), Cayenne, French Guiana, France, E-mail: vincent.pommierdesanti@gmail.com. Denis Blanchet, Laboratoire Hospitalo Universitaire de Parasitologie et Mycologie, Centre hospitalier de Cayenne, Cayenne, French Guiana, France, E-mail: denis.blanchet@ch-cayenne.fr. Philippe Abboud and Felix Djossou, Department of Infectious and Tropical Diseases, Cayenne General Hospital, Cayenne, French Guiana, France, E-mails: philippe.abboud@ch-cayenne.fr and felix.djossou@ch-cayenne.fr. Mathieu Nacher, Epidémiologie des Parasitoses et Mycoses Tropicales (EPAT), Université des Antilles et de la Guyane, Cayenne, French Guiana, France, Centre d'Investigation Clinique Epidémiologie Clinique Antilles Guyane (CIC-EC INSERM CIE 802), Cayenne General Hospital, Cayenne, French Guiana, France, E-mail: mathieu.nacher@ch-cayenne.fr.
References
- 1.Guyane World Wildlife Fund. Estimation de l'emprise de l'activité Aurifère Illégale en Guyane–Année 2008. French Guiana; France: World Wildlife Fund: 2013. [Google Scholar]
- 2.Fréry N, Maury-Brachet R, Maillot E, Deheeger M, de Mérona B, Boudou A. Gold-mining activities and mercury contamination of native Amerindian communities in French Guiana: key role of fish in dietary uptake. Environ Health Perspect. 2001;109:449–456. doi: 10.1289/ehp.109-1240303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nacher M, Guérin PJ, Demar-Pierre M, Djossou F, Nosten F, Carme B. Made in Europe: will artemisinin resistance emerge in French Guiana? Malar J. 2013;12:152. doi: 10.1186/1475-2875-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musset L, Pelleau S, Girod R, Ardillon V, Carvalho L, Dusfour I, Gomes MSM, Djossou F, Legrand E. Malaria on the Guiana Shield: a review of the situation in French Guiana. Mem Inst Oswaldo Cruz. 2014;109:525–533. doi: 10.1590/0074-0276140031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosnier E, Carvalho L, Mahamat A, Chappert J-L, Ledrans M, Ville M. Multiple outbreaks in gold washing camps in the Amazon forest (French Guiana): what are lessons learned to improve access to prevention and care? Bull Epidemiol Hebd (Paris) 2015;11:181–189. [Google Scholar]
- 6.Luxemburger C, White NJ, ter Kuile F, Singh HM, Allier-Frachon I, Ohn M, Chongsuphajaisiddhi T, Nosten F. Beri-beri: the major cause of infant mortality in Karen refugees. Trans R Soc Trop Med Hyg. 2003;97:251–255. doi: 10.1016/s0035-9203(03)90134-9. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization, United Nations High Commissioner for Refugees Thiamine Deficiency and Its Prevention and Control in Major Emergencies. 1999. http://www.who.int/nutrition/publications/emergencies/WHO_NHD_99.13/en/ Report No. WHO/NHD/99.13. Available at. Accessed August 15, 2015.
- 8.Barennes H, Sengkhamyong K, René JP, Phimmasane M. Beriberi (thiamine deficiency) and high infant mortality in northern Laos. PLoS Negl Trop Dis. 2015;9:e0003581. doi: 10.1371/journal.pntd.0003581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerroni MP, Barrado JCS, Nobrega AA, Lins ABM, da Silva IP, Mangueira RR, da Cruz RH, Mendes SM, Sobel J. Outbreak of beriberi in an Indian population of the upper Amazon region, Roraima State, Brazil, 2008. Am J Trop Med Hyg. 2010;83:1093–1097. doi: 10.4269/ajtmh.2010.10-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.San Sebastian M, Jativa R. Beriberi in a well-nourished Amazonian population. Acta Trop. 1998;70:193–196. doi: 10.1016/s0001-706x(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 11.Dufougeré W. Ankylostomiase et Béribéri en Guyane française. Bull Soc Pathol Exot. 1920;13:603–617. [Google Scholar]
- 12.Coudreau H. Journal des Voyages et des Aventures de Terre et de mer. 1895;2:22. [Google Scholar]
- 13.Da Rocha MW, Resck IS, Caldas ED. Purification and full characterisation of citreoviridin produced by Penicillium citreonigrum in yeast extract sucrose (YES) medium. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2015;32:584–595. doi: 10.1080/19440049.2014.961177. [DOI] [PubMed] [Google Scholar]
- 14.Mayxay M, Taylor AM, Khanthavong M, Keola S, Pongvongsa T, Phompida S, Phetsouvanh R, White NJ, Newton PN. Thiamin deficiency and uncomplicated falciparum malaria in Laos. Trop Med Int Health. 2007;12:363–369. doi: 10.1111/j.1365-3156.2006.01804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doung-ngern P, Kesornsukhon S, Kanlayanaphotporn J, Wanadurongwan S, Songchitsomboon S. Beriberi outbreak among commercial fishermen, Thailand 2005. Southeast Asian J Trop Med Public Health. 2007;38:130–135. [PubMed] [Google Scholar]
- 16.Thurnham DI, Cathcart AE, Livingstone MB. A retrospective investigation of thiamin and energy intakes following an outbreak of beriberi in The Gambia. Nutrients. 2011;3:135–151. doi: 10.3390/nu3010135. [DOI] [PMC free article] [PubMed] [Google Scholar]