Abstract
TSH is the primary physiological regulator of thyroid gland function. The effects of TSH on thyroid cells are mediated via activation of its membrane receptor [TSH receptor (TSHR)]. In this study, we examined functional thyroid differentiation in zebrafish and characterized the role of TSHR signaling during thyroid organogenesis. Cloning of a cDNA encoding zebrafish Tshr showed conservation of primary structure and functional properties between zebrafish and mammalian TSHR. In situ hybridization confirmed that the thyroid is the major site of tshr expression during zebrafish development. In addition, we identified tpo, iyd, duox, and duoxa as novel thyroid differentiation markers in zebrafish. Temporal analyses of differentiation marker expression demonstrated the induction of an early thyroid differentiation program along with thyroid budding, followed by a delayed onset of duox and duoxa expression coincident with thyroid hormone synthesis. Furthermore, comparative analyses in mouse and zebrafish revealed for the first time a thyroid-enriched expression of cell death regulators of the B-cell lymphoma 2 family during early thyroid morphogenesis. Knockdown of tshr function by morpholino microinjection into embryos did not affect early thyroid morphogenesis but caused defects in later functional differentiation. The thyroid phenotype observed in tshr morphants at later stages comprised a reduction in number and size of functional follicles, down-regulation of differentiation markers, as well as reduced thyroid transcription factor expression. A comparison of our results with phenotypes observed in mouse models of defective TSHR and cAMP signaling highlights the value of zebrafish as a model to enhance the understanding of functional differentiation in the vertebrate thyroid.
In vertebrates, de novo synthesis of the thyroid hormones T4 and T3 is restricted to thyroid follicles, which constitute the functional subunits of thyroid tissue. Thyroid hormones play a critical role for development, growth, and metabolism (1). Biosynthesis of thyroid hormones is a complex multistep process that requires a follicular organization of polarized thyroid cells (2). Iodide uptake from the blood stream is an active process mediated by the sodium-iodide symporter (NIS) (encoded by the Slc5a5 gene), which is localized on the basolateral membrane of thyroid follicular cells (3). At the apical pole of thyroid follicular cells, thyroid peroxidase (TPO) catalyzes the coupling of iodide to tyrosyl residues of thyroglobulin (TG). TG functions as a scaffold for thyroid hormone synthesis and as a storage for thyroid hormones. The H2O2 required for TPO-catalyzed iodide organification is generated by dual oxidase (DUOX), an nicotinamide adenine dinucleotide phosphate dependent oxidase that is colocalized with TPO at the apical membrane (4). Iodinated TG is stored in the lumen of thyroid follicles. Upon demand, iodinated TG is reabsorbed into thyroid follicular cells, and thyroid hormones are liberated by proteolytic cleavage of TG. Central to the process of thyroid hormone synthesis is an efficient intrathyroidal recycling of the trace element iodine (2). One important enzyme in this regard is iodotyrosine dehalogenase (encoded by the Iyd gene), which salvages iodide for intrathyroidal reuse via deiodination of iodotyrosines (5).
TSH is the primary physiological regulator of thyroid gland function, stimulating thyroid hormone synthesis/release, and exerting trophic effects on thyroid tissue (6, 7). A hallmark of TSH action on thyroid follicular cells is that it regulates many of the genes involved in the biosynthetic machinery, intrathyroidal iodide metabolism, and secretory pathways (1, 6, 8). The effects of TSH are mediated through TSH binding and activation of the TSH receptor (TSHR) (6). This receptor is a member of the family of G protein-coupled receptors. Together with the FSH receptor and the LH receptor, the TSHR constitute the subfamily of glycoprotein hormone receptors (GpHR). TSHR signaling in thyroid follicular cells is mediated via several second messenger pathways, the best characterized of which is the activation of adenylate cyclase-cAMP pathway (9).
Congenital hypothyrodism (CH) is the most frequent endocrine disorder in human newborns affecting approximately one of 2000–4000 life births (10). CH is characterized by reduced serum thyroid hormone levels at birth. Approximately 15% of CH cases are due to dyshormonogenesis, including defects in one of the aforementioned steps of thyroid hormone synthesis (11). However, it is of particular interest that the majority of CH cases, approximately 85%, is due to thyroid dysgenesis (TD). The term TD considers a spectrum of developmental abnormalities, including athyreosis (absence of thyroid tissue), thyroid ectopy (most frequently at a sublingual location), hypoplasia (orthotopic thyroid tissue of reduced size), and hemiagenesis (unilateral thyroid tissue). All these phenotypes are considered to be caused by defects during embryonic development. However, the molecular mechanisms underlying TD in human newborns remain still poorly understood (12–14).
The process of thyroid organogenesis has been well investigated in the mouse model (14–16). In the mouse embryo, thyroid development starts between embryonic day (E)8 and E8.5 with the specification of the thyroid anlage, a thickening of the midline endodermal floor of the primitive foregut. At this stage, thyroid precursor cells are characterized by expression of a unique combination of transcription factors comprising NKX2.1, PAX8, HHEX, and FOXE1. Expression of all four genes is maintained throughout morphogenesis and persists in terminally differentiated thyroid cells. The median anlage develops into a diverticulum that descends caudally and loses contact with the pharynx by E11.5. After detachment, the thyroid primordium expands bilaterally and migrates to its final position in front of the trachea until E13.5. Terminal functional differentiation of the thyroid is initiated around E14.5 and involves expression of the functional differentiation markers Tg, Tshr, and Tpo (8, 17). Expression of NIS becomes detectable at E15.5, and functional maturation is evident from thyroid hormone synthesis around E16.5. Using knockout mouse models of Nkx2.1, Pax8, Hhex, and Foxe1, several of the thyroid phenotypes observed in human patients could be reproduced, including athyreosis and thyroid ectopy (14). However, although mouse studies demonstrated the importance of these transcription factors for normal progression of thyroid morphogenesis, mutations in the corresponding human genes were found to explain only a very small percentage of TD cases (12).
Innovative experimental approaches using novel model systems might help to further improve our understanding of morphogenetic processes and gene networks involved in thyroid organogenesis. One promising model is early development in zebrafish. In the recent past, zebrafish has gained much attention as a genetically tractable vertebrate model to study organogenesis (18). Optical clarity of embryos allows direct visualization of developmental processes and its pathological deviations. Small size, high fecundity, external fertilization, rapid development, and a short generation time are important attributes underscoring the utility of zebrafish for studies of early developmental processes. The value of zebrafish for studies on thyroid organogenesis is well supported by the fact that several aspects of thyroid morphogenesis are conserved between zebrafish and mammals (19–23). Early morphogenetic events, such as thyroid specification within the ventral foregut, budding of the primordium, and relocalization into the subpharyngeal mesenchyme, show many similarities in fish and mouse (reviewed in Refs. 16, 24). Moreover, expression of a similar but not identical transcription factor network has been demonstrated in the developing thyroid of zebrafish and mice (19–23). However, differences exist in the timing of specific morphogenetic events and in the anatomy of mature thyroid tissue. Although thyroid follicles are encapsulated in a compact organ in mouse, thyroid follicles are loosely scattered along the pharyngeal midline in zebrafish.
In zebrafish experiments, it is common practice to inhibit pigmentation by addition of 0.003% phenylthiourea (PTU) to the embryo medium (25). However, the use of PTU has the potential to confound studies on thyroid morphogenesis due to TPO inhibition by PTU (26). In this study, we characterize the effects of PTU on thyroid gene expression and examine the utility of the pigmentless casper mutant line (27) for studies on thyroid morphogenesis. Two intriguing aspects of thyroid development in zebrafish are the early onset of tg and slc5a5 expression and the apparent lack of any thyroid phenotype in zebrafish mutants with defective TSH signaling (20). These observations suggested a major difference in the process of functional differentiation between the mouse and zebrafish model. In this study, we aimed at a better characterization of functional thyroid differentiation in zebrafish. We cloned a zebrafish Tshr and characterized its expression profile along with other novel differentiation markers of thyroid development in zebrafish. In addition, we demonstrate a specific thyroid phenotype in a zebrafish Tshr loss-of-function model and propose a model for the role of Tshr signaling during thyroid morphogenesis in zebrafish.
Results
Thyroid morphogenesis proceeds normally in casper mutants
Depigmentation of zebrafish embryos before gene expression analysis by whole-mount in situ hybridization (WISH) is commonly achieved by either bleaching of fixed embryos with hydrogen peroxide solution or by raising embryos in a solution containing 0.003% PTU (25). Bleaching of embryos has several drawbacks, such as reduced detectability of weakly expressed transcripts and impaired integrity of embryos. The addition of PTU to embryo medium, on the other hand, is problematic, because the PTU concentration required for depigmentation is sufficient to block thyroid hormone synthesis due to inhibition of TPO activity by PTU (26). Zebrafish mutant strains that lack pigmentation might therefore offer promising tools for use in WISH experiments, particularly in studies concerning morphogenesis and function of the thyroid gland. In this study, we examined the utility of one such pigment-less mutant line, called casper (27), for studies on thyroid morphogenesis.
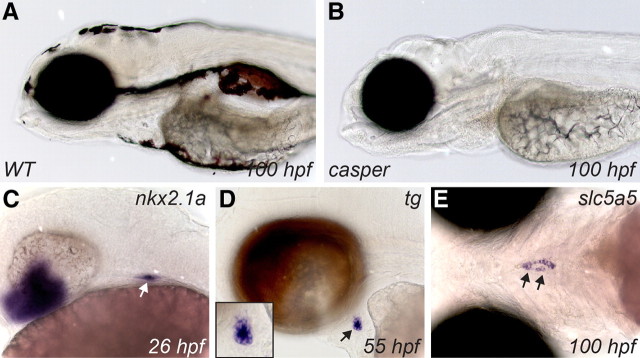
As shown in Fig. 1, A and B, compared with wild-type (WT) larvae, casper larvae are characterized by a complete lack of all melanocytes and iridophores. This line was generated by crossing of two zebrafish lines, nacre (mutation of mitfa) and roy orbison (mutated allele unknown). Casper fish are doubly mutant for nacre and roy (27). Early development of casper embryos is indistinguishable from WT embryos. However, given the unknown identity of the gene mutated in the roy orbison line, we first verified in casper embryos whether thyroid morphogenesis proceeds in a WT manner. Expression patterns for established markers of zebrafish thyroid development were examined in casper embryos between 24 and 100 hours postfertilization (hpf). WISH analyses of spatiotemporal expression profiles for nkx2.1a, tg, and slc5a5 clearly showed that the main morphogenetic processes occur normally in casper embryos. For example, timely specification of the thyroid anlage was detected using the nkx2.1a riboprobe between 24 and 26 hpf (Fig. 1C). Normal thyroid budding, migration, and formation of a first follicle were evident from orthotopic expression of tg (Fig. 1D). Further, the dispersed thyroid tissue forming along the pharyngeal midline during the late phase of thyroid organogenesis was strongly stained by a slc5a5 probe (Fig. 1E). Results from the aforementioned and several additional experiments lead us to conclude that casper mutants display an apparently normal process of thyroid morphogenesis.
Fig. 1.
In contrast to pigmented WT larvae (A), casper larvae are highly transparent due to a lack of melanocytes and iridophores (B). Thyroid marker expression in casper embryos indicate normal progression of thyroid morphogenesis (C–E). WISH of casper embryos with riboprobes for nkx2.1a (C), tg (D), and slc5a5 (E) label the thyroid primordium at 26 hpf (C), demonstrate formation of a first follicle at 55 hpf (D) and stain the dispersed thyroid tissue along the pharyngeal midline at 100 hpf (E), respectively. Inset in D shows a magnified view of the thyroid region. Arrows point to the thyroid. Lateral (A–D) and ventral views (E) are shown with all embryos being oriented with anterior to the left.
PTU treatment of embryos alters thyroid gene expression
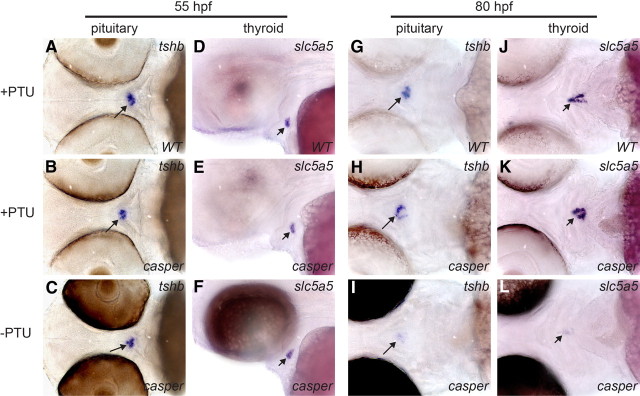
During in situ hybridizations with the slc5a5 riboprobe, we noticed that casper larvae at late stages (≥80 hpf) required prolonged incubation in staining solution (∼12 h) to achieve a similar signal intensity relative to what we routinely observed in experiments with PTU-treated WT larvae (∼5–6 h). These observations indicate a higher level of slc5a5 expression in thyroids of PTU-treated WT larvae compared with casper larvae. Previous studies concluded that PTU treatment does not affect thyroid morphogenesis in zebrafish (26), but to the best of our knowledge, possible effects of PTU treatment on differentiation marker expression have not yet been addressed. To examine the effects of PTU treatment on thyroid marker expression in more detail, we performed comparative WISH analyses of PTU-treated WT embryos, PTU-treated casper, and untreated casper embryos. Although PTU treatment leads to a similar loss of eye pigmentation in WT and casper embryos, PTU-treated WT and casper embryos can still be unequivocally identified after WISH experiments due to very faint dorsal skin pigmentation of WT embryos. These differences allowed us to process age-matched embryos from all three treatment groups in the same test tube during WISH. At 55 hpf, we found no differences between the three groups of embryos regarding tshb expression in pituitary thyrotrophs (n = 17–19) (Fig. 2, A–C) or thyroidal expression of slc5a5 (n = 22–24) (Fig. 2, D–F). Similarly, no differences were observed for nkx2.1a and tg expression in the thyroid of 55-hpf embryos (data not shown). By 80 hpf, however, pituitary tshb expression was increased in all WT (n = 18) and casper (n = 21) larvae treated with PTU (Fig. 2, G and H) relative to untreated casper larvae (n = 25) (Fig. 2I). Interestingly, concurrent with the up-regulation of pituitary tshb expression, we observed a strongly increased expression of slc5a5 in all WT (n = 22) and casper (n = 28) larvae treated with PTU (Fig. 2, J and K) relative to untreated casper larvae (n = 26) (Fig. 2L). Thyroidal expression of nkx2.1a and tg mRNA was also enhanced by PTU treatment but less strongly compared with slc5a5 (data not shown). Similar PTU effects on tshb and slc5a5 expression were observed in additional independent experiments examining WT larvae raised in the presence or absence of PTU (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Numerous in vitro and in vivo studies have shown that Slc5a5 mRNA and NIS expression in thyrocytes is tightly controlled by TSH (3) and that goitrogens up-regulate thyroidal Slc5a5 mRNA and NIS expression in vivo secondary to increased pituitary TSH synthesis/release (28–30). To our knowledge, this is the first demonstration of altered thyroid gene expression in response to PTU treatment during early zebrafish development. Thus, the expression pattern observed in the thyroid of PTU-treated zebrafish larvae at 80 hpf corresponds well with the classical goitrogenic response reported in adult mammalian thyroid tissue. What is surprising is the early onset of a putative pituitary-thyroid feedback signaling in zebrafish, because PTU- induced alterations of tshb and slc5a5 expression were apparent at developmental stages, when immunoreactivity for T4 just starts to become detectable in zebrafish thyroid tissue (22).
Fig. 2.
Embryo treatment with 0.003% PTU affects pituitary and thyroid gene expression as demonstrated by WISH. At 55 hpf, pituitary expression of tshb (see ventral views in A–C) and thyroidal expression of slc5a5 (see lateral views in D–F) does not differ between PTU-treated WT, PTU-treated casper, and nontreated casper embryos. However, by 80 hpf, expression of tshb (see ventral views in G–I) and slc5a5 (see ventral views in J–L) is markedly increased in PTU-treated larvae relative to nontreated larvae. All embryos are oriented with anterior to the left. Long arrows point to pituitary, and short arrows point to thyroid.
Cloning and functional characterization of zebrafish Tshr
Using a combination of bioinformatics and RT-PCR, we cloned a cDNA encoding for zebrafish Tshr. Sequencing predicts an open reading frame of 2271 bp encoding for a 757-amino acid (aa) polypeptide. From the cDNA-deduced primary structure, we identified the typical traits characteristic for members of the GpHR subfamily, including a large amino-terminal extracellular domain of 420 aa (including the signal peptide), a 260-aa heptahelical transmembrane domain, and a C-terminal 77-aa cytoplasmic domain (Supplemental Fig. 2). Nine leucine-rich repeats important for TSH binding of the human TSHR were found to be conserved in the extracellular domain of zebrafish Tshr. A phylogenetic tree, constructed using the neighbor joining algorithm based on full polypeptide sequences, showed a tight clustering of zebrafish Tshr with TSHR from other teleosts and tetrapods in a clade, which is well separated from a clade containing FSH receptor and LH receptor sequences (Supplemental Fig. 3). Including the zebrafish Tshr described in this study, TSHR are now described in seven teleosts (31–36). Collectively, these studies demonstrate that predicted structural characteristics are strongly conserved between teleost and mammalian TSHR.
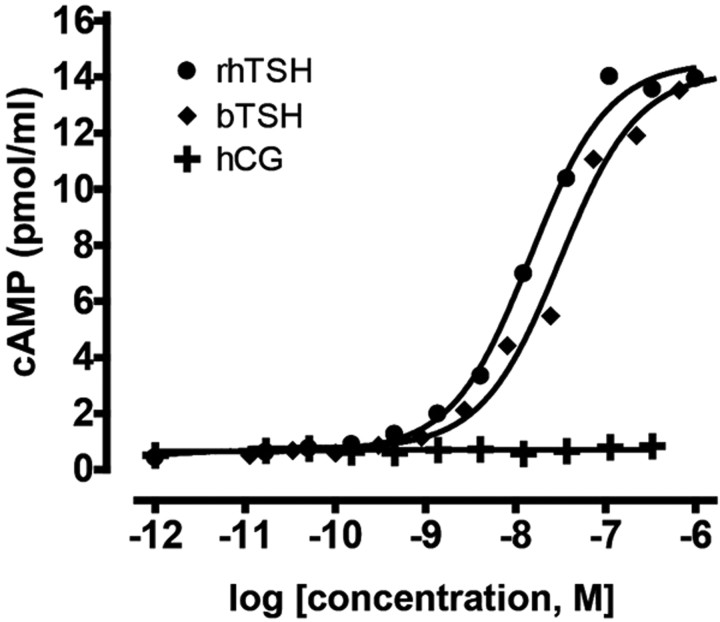
Functional characterization of the zebrafish Tshr was carried out in HEK293T cells transiently transfected with tshr/pcDNA3.1 expression vector. cAMP accumulation was measured after stimulation of transfected cells with several glycoprotein hormones. Although purified TSH from zebrafish was not available for these experiments, we found that bovine and human recombinant TSH but not human chorionic gonadotropin (hCG) stimulated cAMP production in a concentration-dependent manner in HEK293T cells transfected with zebrafish Tshr (Fig. 3). No effects of these hormones were observed in mock-transfected cells (data not shown). Thus, zebrafish Tshr responds functionally to mammalian TSH preparations.
Fig. 3.
Functional characterization of zebrafish TSHR. Intracellular cAMP production was assessed in HEK293T cells transiently transfected with zebrafish tshr/pcDNA3.1 expression vector after stimulation with bovine TSH (bTSH), recombinant human TSH (rhTSH), and hCG. cAMP generation was measured by homogeneous time-resolved fluorescence. Data are expressed as means of triplicate measurements.
Thyroid differentiation marker expression in zebrafish embryos
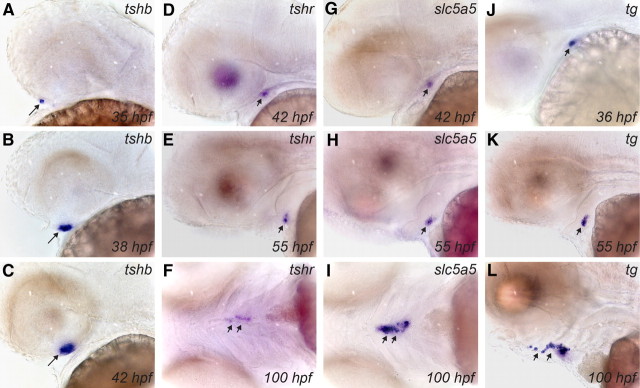
The spatiotemporal expression pattern of tshr mRNA was examined in whole-mount preparations of WT and casper embryos between 24 and 100 hpf and was compared with the expression profiles of the previously described thyroid markers tg and slc5a5 (20), as well as with the early expression pattern of zebrafish tshb (37). Results obtained for all four genes were highly consistent when comparing WT and casper embryos. In agreement with previous reports (37), we found that expression of tshb mRNA is confined to the developing pituitary (Fig. 4, A–C, and data not shown). Our WISH analyses of tshb expression revealed first specific staining of the pituitary anlage already between 34 and 36 hpf (Fig. 4A), implicating an earlier onset of tshb expression than the previously reported onset between 40 and 42 hpf (37). Strong tshb expression was observed from 38 hpf onwards (Fig. 4, B and C). Given the reported onset of cga expression (encoding for the α-subunit of TSH) around 32 hpf (38), our expression data implicate concurrent expression of both TSH subunits by 36 hpf in the zebrafish pituitary.
Fig. 4.
Developmental expression of tshb and thyroid differentiation markers in zebrafish embryos as demonstrated by WISH. Lateral views of the head region show the early expression pattern of tshb in pituitary (A–C) and of tshr (D and E), slc5a5 (G and H), and tg (J and K) in thyroid primordium. Note the earlier onset of tg expression relative to tshr and slc5a5 and the strong staining of the thyroid at the one-follicle stage (55 hpf) by all three thyroid markers. At 100 hpf, dispersed thyroid tissue along the pharyngeal midline is strongly labeled by riboprobes for tshr (ventral view in F), slc5a5 (ventral view in I), and tg (lateral view in L). Long arrows point to pituitary, and short arrows point to thyroid. All embryos are oriented with anterior to the left.
Compared with tshb, the onset of tshr expression was slightly delayed, because tshr mRNA was not detected before 40 hpf by WISH. Between 40 and 42 hpf, tshr mRNA expression became detectable as a faint staining of the thyroid primordium (Fig. 4D). Thyroidal levels of tshr expression increased subsequently, and from 46 hpf onwards, tshr mRNA was readily detectable in developing thyroid tissue at all stages examined (Fig. 4, E and F). Weak extrathyroidal expression of tshr mRNA was noted in lens and brain. Similar to previous studies (20), slc5a5 expression in the thyroid primordium became detectable between 40 and 42 hpf (Fig. 4G), and strong thyroidal slc5a5 expression was observed at all subsequent stages (Fig. 4, H and I). In contrast to tshr and slc5a5, expression of tg was detectable in the thyroid primordium as early as 34 hpf (Fig. 4J). From 42 hpf onwards, tg expression was very high in thyroid tissue (Fig. 4, K and L). Thus, our data demonstrate that tshr mRNA is already expressed during the process of thyroid budding.
Apart from tg and slc5a5, no information was available so far concerning the expression of still other markers of functional thyroid differentiation. Our analyses of the zebrafish genome database identified zebrafish orthologs of mammalian TPO, IYD, DUOX, and DUOXA. In situ hybridization experiments demonstrate expression of tpo, iyd, duox, and duoxa in zebrafish thyroid tissue but also reveal differences in temporal expression of these four genes. Weak expression of tpo mRNA was first detected between 40 and 42 hpf in the thyroid primordium (Fig. 5A). tpo expression levels increased at subsequent stages, and strong staining of thyroid tissue was observed from 46 to 100 hpf (Fig. 5, B–D). Between 40 and 100 hpf, tpo expression was confined to the developing thyroid (Fig. 5D). In the developing thyroid, iyd was weakly detectable as early as 38 hpf (Fig. 5E). Expression of iyd mRNA increased at subsequent stages, and strong staining of thyroid tissue was observed from 42 to 100 hpf (Fig. 5, F and G). In addition to the thyroid, iyd expression was also detected in liver and gut (Fig. 5H).
Fig. 5.
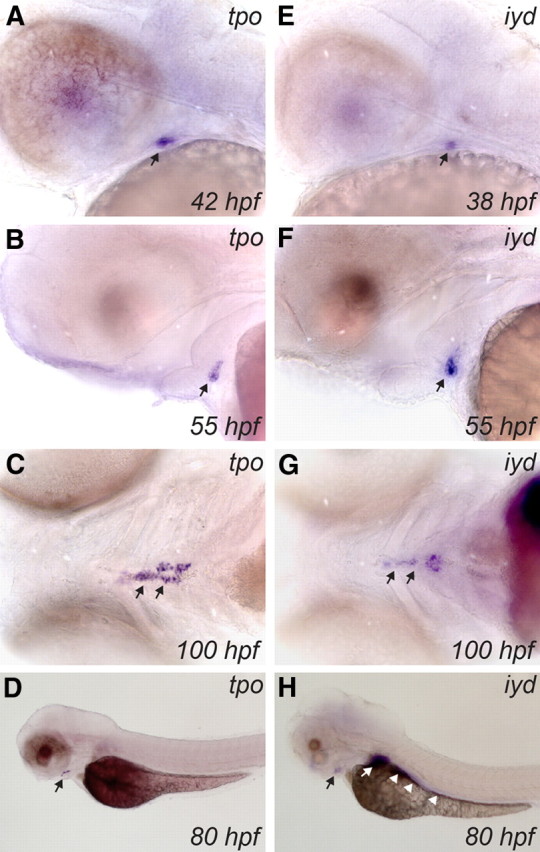
Developmental expression of tpo and iyd in zebrafish embryos as demonstrated by WISH. Lateral views of the head region show the early expression pattern of tpo (A and B) and iyd (E and F) in thyroid primordium. Note the earlier onset of iyd thyroid expression (∼38 hpf) relative to tpo (40–42 hpf) and the strong expression of both genes in the thyroid at the one-follicle stage (55 hpf). Ventral views show that tpo (C) and iyd (G) are strongly expressed in differentiated thyroid tissue at 100 hpf. Black arrows point to thyroid. Although the tpo riboprobe (lateral view in D) specifically stains the thyroid, iyd (lateral view in H) is also strongly expressed in liver (white arrow) and gut (white arrowheads). All embryos are oriented with anterior to the left.
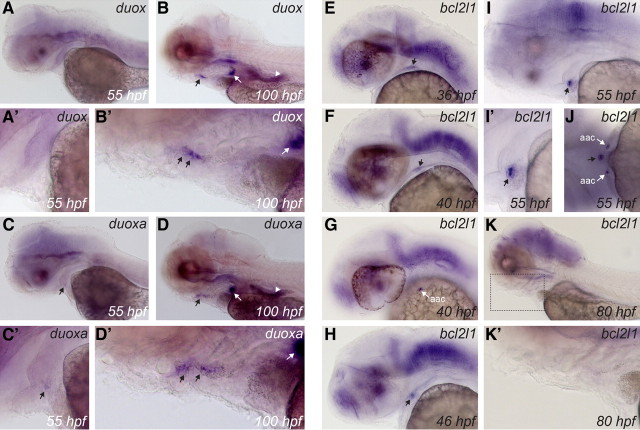
Although two Duox (Duox1 and Duox2) and two Duoxa genes (Duoxa1 and Duoxa2) have been identified in mammals, the genome of zebrafish and other teleosts contains only a single duox gene (39) and a single duoxa gene (40). Consistent with recently published data (41), our WISH experiments demonstrate duox mRNA expression in various tissues, including epidermis, brain, intestine, and ultimobranchial bodies (Fig. 6, A and B). From 80 hpf, weak duox expression was also observed in thyroid tissue (Fig. 6, B and B'), an expression domain not recognized in previous studies. No duox expression was found in the thyroid of embryos younger than 60 hpf. Expression of duox in the zebrafish thyroid tissue appears to be very low. A moderate staining was only obtained after prolonged incubation in staining solution (usually 72 h). Furthermore, so far, we could only demonstrate a specific staining in casper and WT larvae treated with PTU. No specific staining was obtained in untreated casper embryos due to increasing background staining upon further incubation in staining solution. Expression of duoxa was found in most tissues expressing duox, including thyroid, brain, intestine, and ultimobranchial bodies (Fig. 6, C and D). However, duoxa mRNA was not detected in the epidermis, which was strongly labeled by the duox probe at early stages (data not shown). Furthermore, although not analyzed in detail, the expression pattern of duoxa in brain was distinct from that of duox (data not shown). In the developing thyroid, duoxa expression was detectable at earlier stages than duox. Weak expression was detected around 55 hpf (Fig. 6, C and C'). Concurrent expression of duoxa and duox in thyroid tissue was demonstrated at least from 80 hpf onwards (compare B' and D' in Fig. 6).
Fig. 6.
Developmental expression of duox, duoxa, and bcl2l1 in zebrafish embryos as demonstrated by WISH. All panels show lateral views of embryos and larvae oriented with anterior to the left. A, Absence of detectable duox expression in thyroid primordium at 55 hpf (see also magnified view of thyroid region in A'). Moderate duox expression was detected in differentiated thyroid tissue at later stages as shown in B (see magnified view of subpharyngeal region in B') but only after prolonged stain development. Black arrows point to thyroid. Using the duoxa riboprobe, a weak staining of thyroid was observed at 55 hpf (C and magnified view of the thyroid region in C'). As shown in D, stronger duoxa expression was detected in differentiated thyroid tissue at later stages (see also magnified view of the subpharyngeal region in D'). Extrathyroidal tissues expressing duox and duoxa include brain, ultimobranchial bodies (white arrows in B, B', D, and D'), and intestine (white arrowhead). E, F, and H, Expression of bcl2l1 during early stages of thyroid morphogenesis. bcl2l1 is strongly expressed in the thyroid at the one-follicle stage (I and magnified view of thyroid region in I'). In addition to the brain, the bcl2l1 riboprobe also strongly labels the arch-associated cells (aac), which are located bilaterally to the thyroid primordium as shown in G and J. Thyroidal expression of bcl2l1 ceases after 60 hpf and is undetectable by 80 hpf (K). K', Magnified view of the boxed region in K highlighting the absence of thyroid-specific staining even after prolonged stain development. Black arrows point to developing thyroid tissue.
Taken together, these data demonstrate an early onset of mRNA expression for important functional differentiation markers such as tg, slc5a5, tshr, tpo, and iyd around the time of thyroid budding, whereas induction of duox was delayed. In larvae more than or equal to 80 hpf, staining of the thyroid using probes for slc5a5, tshr, tpo, and iyd occurred much faster in larvae treated with PTU, suggesting enhanced expression of these markers. Notably, PTU treatment did not obviously alter expression levels of iyd mRNA in liver and gut.
Identification of a novel gene transiently expressed in zebrafish thyroid
In addition to identifying zebrafish orthologs of known mammalian thyroid markers, we recently initiated a screen for novel genes expressed in the thyroid of zebrafish embryos. Candidate genes for this screen are identified based on results from ongoing transcriptome analyses of mouse embryonic thyroid tissue. One such gene with thyroid-enriched expression in mouse embryos is Bcl2 (Supplemental Fig. 4). WISH analyses of the corresponding zebrafish ortholog (bcl2, NM_001030253) did not reveal elevated expression in zebrafish thyroid (data not shown). However, for a related zebrafish gene, bcl2-like 1 (bcl2l1, NM_131807), we detected strong expression in zebrafish thyroid tissue. When using a bcl2l1-specific riboprobe, staining of the thyroid primordium was first detected between 34 and 36 hpf (Fig. 6E). Thyroidal bcl2l1 expression became stronger by 40 hpf and was maintained until 60 hpf, when staining of the thyroid started to fade (Fig. 6, F, H, and I). As shown in Fig. 6K, bcl2l1 expression was no longer detectable in thyroid tissue by 80 hpf. Thus, bcl2l1 is only transiently expressed at high levels in the thyroid primordium. Extrathyroidal expression domains of bcl2l1 include the central nervous system and the arch-associated cells (42), a small group of cells located bilaterally to the thyroid between 36 and 60 hpf (see Fig. 6, G and J).
tshr knockdown reduces the number of functional follicles
Mouse models of defective TSHR signaling display hypothyroidism and develop thyroid gland hypoplasia postnatally (17, 43). Similar loss-of-function phenotypes have been reported in human patients harboring homozygous inactivating TSHR mutations (44, 45). To assess the role of Tshr during zebrafish thyroid development in more detail, we used the morpholino antisense oligonucleotide (MO) technology to knock down Tshr protein expression in zebrafish embryos.
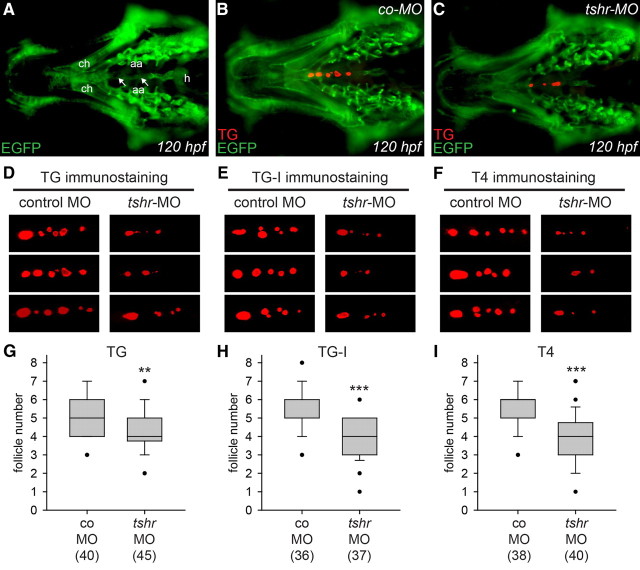
For a first set of microinjection experiments, we used Tg(fli1a:EGFP) transgenic embryos expressing enhanced green fluorescent protein (EGFP) in endothelial cells and neural crest-derived mesenchyme (46). As shown in Fig. 7A, whole-mount immunofluorescence staining of EGFP expression highlights the anatomical position of various craniofacial structures, including major blood vessels, such as the ventral aorta and aortic arch arteries, and cartilage elements, such as the ceratohyal. Exploiting these anatomical landmarks, we first analyzed the localization of thyroid follicles in tshr morphants and control larvae. Double immunostaining of EGFP and TG at 120 hpf clearly demonstrated localization of thyroid follicles along the ventral aorta in control larvae (Fig. 7B) and tshr morphants (Fig. 7C). Follicles immunoreactive for TG were located in the region between the heart outflow tract and the anterior end of the ceratohyal cartilage. Similar staining patterns of thyroid tissue were observed when antibodies for iodide-rich TG (TG-I) and T4 were used in whole-mount immunofluorescent experiments (Fig. 7, D–F). The staining patterns seen for all three thyroid markers are consistent with the previously reported strong immunostaining of follicular lumina by TG and T4 antibodies (20, 26). In control larvae, TG, TG-I, and T4 immunostaining each labeled four to seven follicles along the pharyngeal midline. In tshr morphants, however, we observed a statistically significant reduction in the number of follicles immunoreactive for TG, TG-I, and T4 (Fig. 7, G–I). Moreover, many follicles detected in tshr morphants had a greatly reduced size, irrespective of whether the immunostaining was performed for TG, TG-I, or T4 (Fig. 7, D–F). The markedly reduced immunostaining for TG-I and T4 implicates impaired iodide organification and thyroid hormone synthesis in tshr morphants. Taken together, these data demonstrate that knockdown of tshr function does not prevent follicle formation in zebrafish but causes a significant reduction in the number and size of functional follicles.
Fig. 7.
Effects of tshr knockdown on whole-mount immunofluorescence staining for TG, TG-I, and T4 in Tg(fli1a:EGFP) transgenic larvae. A–C, Ventral views of the head region of 120 hpf larvae oriented with anterior to the left. A, The anatomical positions of the ceratohyal (ch), ventral aorta (white arrows), aortic arch arteries (aa), and heart (h) are highlighted by EGFP immunofluorescence. TG immunofluorescence shows the presence of immunoreactive thyroid follicles in embryos injected with control morpholino (co-MO) (B) and tshr morpholino (tshr-MO) (C). D–F, Representative selection of immunostainings (magnified ventral views of thyroid region, anterior to the left) obtained at 120 hpf for TG, TG-I, and T4 in embryos injected with co-MO and tshr-MO. Thyroid tissue of tshr morphants was characterized by a reduced number of functional follicles, many of which displaying a strongly reduced size. Boxplots in G–I show results from quantification of the number of follicles immunoreactive for TG, TG-I, and T4, respectively. Black dots mark outliers and the number of larvae analyzed is indicated in parentheses. Asterisks denote statistically significant differences (Mann-Whitney-test: **, P < 0.01; ***, P < 0.001).
The incidence of partial thyroid ectopy is not altered by tshr knockdown
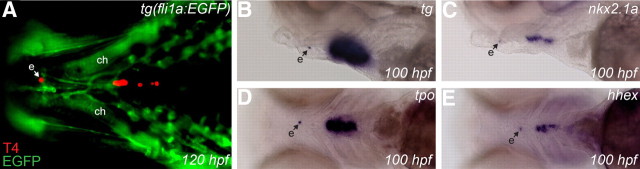
Interestingly, in addition to the orthotopic follicular domain, we also noted immunoreactivity for TG, TG-I, and T4 at a site located anterior to the ceratohyal cartilage in approximately 5% of all immunostained larvae (Fig. 8A). Subsequent in situ hybridizations (using a protocol with long incubation in staining solution) revealed the presence of a small cluster of cells at this site expressing the thyroid markers tg, tpo, nkx2.1a, and hhex (Fig. 8, B–E). The ectopic expression domain was located in the midline and ventral to the floor of the pharynx. In zebrafish, the thyroid primordium is initially located in the ventral pharynx at a level between the first and second pharyngeal arch. Interestingly, the ectopic domain was detected in later stage larvae approximately halfway between Meckel's cartilage and ceratohyal cartilage, which are derived from first and second pharyngeal arch mesenchyme, respectively. Thus, the ectopic site is positioned in close vicinity to the pharyngeal region that was initially populated by thyroid precursor cells. Collectively, these observations indicate that the most anterior domain of immunostaining very likely corresponds to ectopic yet functional thyroid tissue remnants that likely failed to detach from the pharyngeal floor earlier during zebrafish embryogenesis. Notably, the incidence of ectopic thyroid tissue at this site did not differ between control larvae and tshr morphants. Ectopic thyroid tissue was detected by immunostaining in 5.8% of control larvae (6/104) and 4.3% of tshr morphants (5/117) and by in situ hybridization using the tg riboprobe in 21.8% of control larvae (21/110) and 19.4% of tshr morphants (20/98).
Fig. 8.
Identification of ectopic thyroid tissue by whole-mount immunofluorescence staining for T4 and WISH of thyroid marker genes. A, Ventral view of the head region of 120 hpf Tg(fli1a:EGFP) transgenic larvae oriented with anterior to the left. T4 immunofluorescence revealed the presence of an ectopic immunoreactive domain (e) anterior to the ceratohyal cartilage (ch). In situ hybridization of 100-hpf larvae with various thyroid markers, including tg, nkx2.1a, tpo, and hhex, also labels a small cluster of cells in the same region (B–E). Lateral views of the pharyngeal region show that this anterior expression domain is located close to the base of the pharyngeal floor (B and C). Ventral views demonstrate the pharyngeal midline position of this ectopic domain (D and E). All larvae are oriented with anterior to the left.
tshr knockdown alters thyroid marker gene expression
We next performed WISH analyses using an expanded panel of thyroid markers to characterize in more detail the defects occurring during thyroid morphogenesis in tshr morphants. Given the aforementioned potential of PTU treatment to enhance TSH stimulation of thyroid tissue, all analyses were done in parallel for casper embryos raised in the presence or absence of PTU. As judged from expression analyses of nkx2.1a, hhex, and pax2a at 26 hpf, both timing of specification and morphology of the early thyroid anlage appeared unaffected in embryos injected with tshr-MO (Supplemental Fig. 5).
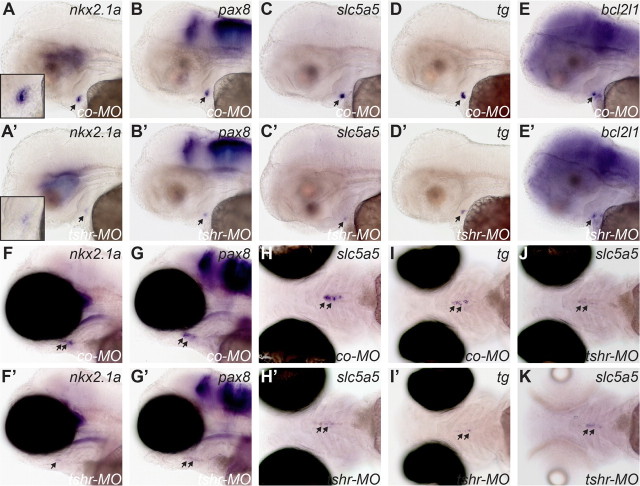
A thyroid phenotype in tshr morphants was first detectable at the one-follicle stage (55 hpf), when thyroidal expression of several transcription factors was reduced in 28–42% of tshr morphants relative to age-matched control embryos (Table 1). Representative specimen with reduced nkx2.1a and pax8 expression are shown in Fig. 9, A' and B'. Results obtained for pax2a, hhex, and foxe1 are shown in Supplemental Fig. 6. The reduction in transcription factor expression was thyroid-specific, because no such effects were observed for the various extrathyroidal expression domains of nkx2.1a (ventral forebrain), pax2a (hindbrain, midbrain-hindbrain boundary, and otic vesicle), pax8 (hindbrain and midbrain-hindbrain boundary), and foxe1 (pharyngeal epithelium). In affected tshr morphants, formation of a first follicle was barely detectable (see Fig. 9, A and A', insets). Although the thyroid primordium appeared smaller in tshr morphants, this might be due to the lack of organization of cells into a follicular structure.
Table 1.
Quantitative analysis of the number of embryos and larvae showing reduced gene expression resulting from microinjection of tshr-MO
| Molecular marker | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stagea | PTUb | nkx2.1a | hhex | pax2a | pax8 | foxe1 | tg | tpo | slc5a5 | iyd | bcl2l1 |
| 55 hpf | None | 37%c | 41% | 28% | 42% | 39% | 51% | 44% | 59% | 49% | 40% |
| 25/68d | 24/58 | 21/74 | 31/73 | 21/54 | 31/61 | 33/75 | 41/69 | 37/75 | 23/57 | ||
| 0.003% | 42% | 39% | 31% | 40% | 40% | 48% | 55% | 71% | 58% | 36% | |
| 33/77 | 24/61 | 20/64 | 22/55 | 24/60 | 30/62 | 34/62 | 52/73 | 38/65 | 24/67 | ||
| 100 hpf | None | 100% | 46% | n.q.e | 78% | n.d.f | 31% | 100% | 100% | 100% | n.d. |
| 78/78 | 29/63 | 51/65 | 20/64 | 84/84 | 81/81 | 74/74 | |||||
| 0.003% | 99% | 81% | n.q. | 97% | n.d. | 61% | 100% | 100% | 100% | n.d. | |
| 70/71 | 48/59 | 59/61 | 46/76 | 78/78 | 76/76 | 72/72 | |||||
Staining characteristics of thyroid tissue were compared between embryos injected with 2.5 ng tshr-MO or equimolar amounts of standard control MO. Sample size in the control group was n = 57–81 embryos per gene and time point. All analyses were performed with casper embryos. n.q., Not quantified; n.d., not detectable.
Developmental stage in hpf at which embryos were sampled and fixed for analyses.
Analyses were performed for embryos raised in the absence or presence of 0.003% PTU in embryo medium.
Percentage of tshr morphants displaying reduced expression of a given thyroid marker.
Number of tshr morphants displaying reduced expression/total number of tshr morphants analyzed.
Effects not quantified (n.q.) due to very low or absent staining in control larvae at 100 hpf.
Expression not detectable (n.d.) in thyroid of tshr morphants and control larvae at 100 hpf.
Fig. 9.
tshr morphants display reduced thyroid marker expression as demonstrated by WISH. Lateral views of control embryos (A–E) and tshr morphants (A'–E') analyzed for nkx2.1a, pax8, slc5a5, tg, and bcl2l1 expression at 55 hpf show reduced thyroid expression in tshr morphants. From the staining patterns, timely formation of a first follicle is barely detectable in tshr morphants (see insets in A and A'). Qualitatively similar effects were observed in embryos raised in the presence or absence of 0.003% PTU. For a better visualization of staining in the thyroid region, photomicrographs in A–E and A'–E' show casper larvae raised in PTU. At 100 hpf, analyses of nkx2.1a, pax8, slc5a5, and tg expression in control larvae (F–I) and tshr morphants (F'–I') showed strongly reduced expression of all genes in tshr morphants. Lateral (F, F', G, and G') and ventral views (H, H', I, and I') are shown. Thyroid staining was barely detectable in many tshr morphants. On the other hand, tg staining indicates that the anterior-posterior extension of the pharyngeal midline region populated by thyroid cells is similar between control larvae and tshr morphants (compare I and I'). All photomicrographs in F–I and F'–I' show casper larvae raised in the absence of PTU. Qualitatively similar effects were observed in PTU-treated larvae, but due to enhanced expression of thyroid markers in PTU-treated larvae, the differences in staining intensities were more pronounced. Ventral views in J and K show slc5a5 expression in tshr morphants raised in the absence (J) or presence (K) of PTU. Note that slc5a5 expression was not increased in PTU-treated tshr morphants relative to untreated tshr morphants. All larvae are oriented with anterior to the left. Arrows point to thyroid. co-MO, Embryos injected with standard control morpholino; tshr-MO, embryos injected with tshr morpholino.
Reduced expression of the functional differentiation markers tg, tpo, slc5a5, and iyd was observed in a somewhat higher percentage of tshr morphants, ranging from 44 to 71% (Table 1). Representative specimens with reduced slc5a5 and tg expression are shown in Fig. 9, C' and D'. Results obtained for iyd and tpo are shown in Supplemental Fig. 6. Expression of bcl2l1 was reduced in up to 40% of tshr morphants (Table 1 and Fig. 9E'). For most of the markers analyzed, a comparison of embryos raised in the presence or absence of PTU revealed no difference in the effects pattern caused by tshr knockdown (see Table 1). Notable exceptions were tpo, slc5a5, and iyd, for which we observed a modest increase in the percentage of tshr morphants displaying reduced marker expression. Among all genes analyzed in tshr morphants at 55 hpf, the expression of slc5a5 was most strongly affected.
By 100 hpf, the effects of tshr knockdown became more severe for most of the genes analyzed (Table 1). At this time point, almost 100% of tshr morphants displayed strongly reduced expression of nkx2.1a (Fig. 9, F and F'), slc5a5 (Fig. 9, H and H'), tpo (Supplemental Fig. 7), and iyd (Supplemental Fig. 7). The expression of tg (Fig. 9, I and I'), hhex (Supplemental Fig. 7), and pax8 mRNA (Fig. 9, G and G') was reduced in only 31, 46, and 78% of tshr morphants raised in the absence of PTU. Although effects of tshr knockdown on expression of these three genes were stronger in PTU-treated larvae (see Table 1), it is worth noting that still 39% of tshr morphants displayed no difference in tg expression relative to embryos injected with control MO. In addition, we performed WISH experiments for pax2a, foxe1, and bcl2l1, the expression of which is either very weak (pax2a) or undetectable (foxe1 and bcl2l1) in the thyroid of control larvae at 100 hpf. Injection of tshr MO did not alter the expression of these genes in 100 hpf larvae (data not shown).
A final experiment aimed at a more direct comparison of thyroidal expression levels in tshr morphants raised in the presence or absence of PTU until 100 hpf. slc5a5 was selected as a thyroid marker for this experiment because its marked up-regulation in PTU-treated control larvae. Because thyroid staining by the slc5a5 probe was very weak or absent in tshr morphants examined in the previous experiments, the staining step was extended to 72 h for the tshr morphants analyzed in this WISH experiment. No differences in slc5a5 expression could be identified between tshr morphants raised in the presence or absence of PTU (Fig. 9, J and K). Thus, a stimulating effect of PTU treatment on slc5a5 expression was absent in tshr morphants.
Overexpression of tshr mRNA partially rescues the thyroid phenotype of tshr morphants
A conventional attempt to rescue the thyroid defects in tshr morphants, that is by injection of tshr mRNA at the one-cell stage, proved to be not possible due to an early onset of gross developmental abnormalities. As an alternative approach of phenotypic rescue, we coinjected tshr-MO with transposase mRNA and a Transposable élément of medake fish Oryzias latipes, N° 2 (Tol2) construct, pTol2hsp70:tshr, in which a heat-shock promoter drives expression of tshr. To circumvent the gross developmental effects resulting from early tshr mRNA misexpression, embryos were heat shocked starting from 2 d postfertilization. More than 80% of pTol2hsp70:tshr-injected embryos showed normal overall development (Supplemental Fig. 8, A–C), with tshr misexpression being dependent upon heat-shock treatment (Supplemental Fig. 8, E–H). However, we observed overt developmental effects in 5–20% of pTol2hsp70:tshr-injected embryos in six independent injection experiments. WISH analyses revealed that such grossly affected embryos misexpressed high levels of tshr mRNA even in the absence of heat shock (Supplemental Fig. 8D). Only embryos displaying overall normal development were considered for the following analyses of phenotypic rescue.
WISH analyses of thyroid marker expression in heat-shocked embryos at 100 hpf revealed a partial rescue of slc5a5 (Fig. 10, A–D) and nkx2.1a (Fig. 10, I–L) expression in pTol2hsp70:tshr-injected tshr morphants relative to noninjected tshr morphants. Although noninjected tshr morphants consistently displayed strongly reduced thyroidal staining of slc5a5 and nkx2.1a mRNA, approximately 30–50% of pTol2hsp70:tshr-injected tshr morphants displayed, if any, only a moderately reduced staining for slc5a5 and nkx2.1a mRNA relative to control embryos. Expression of tg mRNA was moderately reduced in one third of noninjected tshr morphants but was indistinguishable from controls in tshr morphants injected with pTol2hsp70:tshr (Fig. 10, E–H). Consistent with these gene expression data, the number of TG-immunoreactive thyroid follicles in rescued tshr morphants was not significantly different from control embryos (Fig. 10M). On the other hand, only a partial rescue was observed for the number of T4-immunoreactive thyroid follicles (Fig. 10N). Although pTol2hsp70:tshr-injected tshr morphants had significantly more T4-immunoreactive follicles than noninjected tshr morphants, the number of T4-immunoreactive follicles in pTol2hsp70:tshr-injected tshr morphants was still significantly reduced compared with control embryos.
Fig. 10.
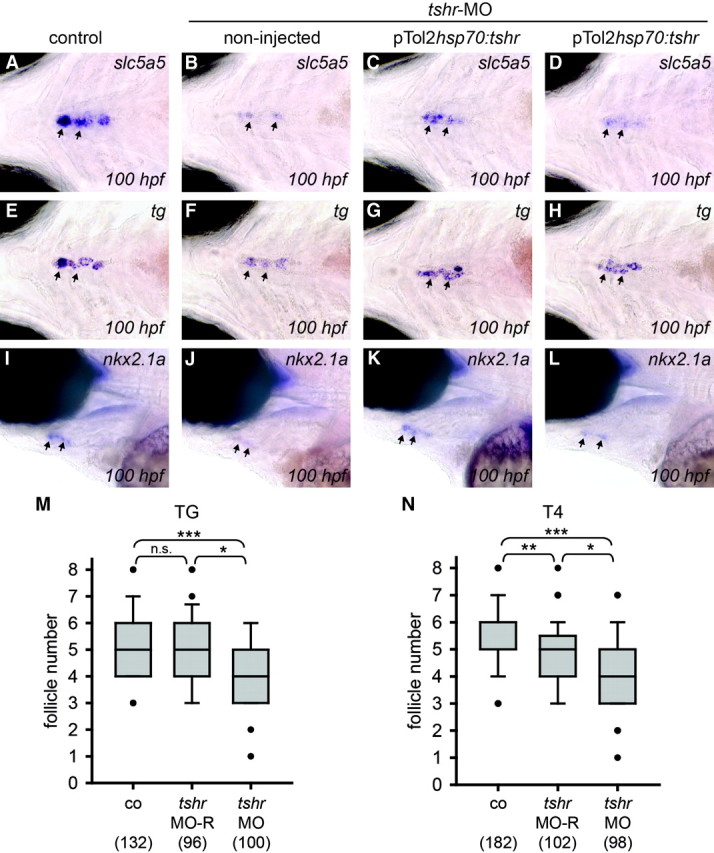
The thyroid phenotype of tshr morphants is partially rescued by tshr mRNA overexpression. For phenotypic rescue, tshr morphants were coinjected with pTol2hsp70:tshr plasmid and transposase mRNA and heat shocked from 2 d postfertilization onwards. A–L, Results from in situ hybridization analyses of thyroid marker expression in heat-shocked embryos at 100 hpf. Ventral (A–H) and lateral views (I–L) of the thyroid region are shown with all embryos being oriented with anterior to the left. Arrows point to thyroid tissue. Compared with noninjected tshr morphants, pTol2hsp70:tshr-injected tshr morphants showed a partial rescue of slc5a5 (A–D) and nkx2.1a (I–L) mRNA expression. Expression of tg mRNA in pTol2hsp70:tshr-injected morphants after heat shock was indistinguishable from control embryos (E–H). Boxplots in M and N summarize results from quantification of the number of follicles immunoreactive for TG and T4 in 120 hpf Tg(fli1a:EGFP) embryos, respectively. Immunostaining was evaluated in control embryos (co), tshr morphants (tshr-MO), and tshr morphants rescued by coinjection of pTol2hsp70:tshr (MO-R). Black dots mark outliers, and the number of larvae analyzed is indicated in parentheses. Asterisks denote statistically significant differences (Dunn's test: *, P < 0.05; **, P < 0.01; ***, P < 0.001). n.s., Not significant.
Discussion
Recently, zebrafish embryonic development emerged as a promising new model to delineate morphogenetic processes and molecular mechanisms of thyroid organogenesis (19–22, 47). However, findings in previous zebrafish studies also raised some questions, particularly with respect to the process of functional differentiation of the zebrafish thyroid. Although TSH is regarded the primary physiological regulator of thyroid gland function (7), conclusive information was not available concerning the onset of TSH-TSHR signaling in zebrafish and its role during thyroid morphogenesis. During the initial studies on the validation of casper mutant embryos, we readily observed PTU-induced changes along the pituitary-thyroid axis from 80 hpf onwards. The effects pattern observed is compatible with the classical model of increased TSH synthesis and elevated TSH stimulation of thyroid tissue in response to thyroid hormone synthesis inhibitors (28, 29, 48). For slc5a5, the paradigm of a TSH-dependent thyroid gene in mammals (3), we observed a marked up-regulation in the thyroid of PTU-treated larvae, supporting the view that thyroid gene expression is TSH-dependent at least during late thyroid development in zebrafish.
To gain greater insights into the developmental role of TSH-TSHR signaling, we cloned the zebrafish Tshr and showed a high conservation in its primary structure when compared with mammalian TSHR. Our results are in line with previous studies on teleost TSHR (31–36). Recent reports have shown a preferential retention of duplicate genes in some G protein-coupled receptor subfamilies after the ray-finned fish lineage-specific whole-genome duplication event (49). However, it is worth noting that only a single tshr gene has been described in all but one teleost species investigated to date. Thus, retention of duplicate tshr genes is not commonly observed in teleost species. The two tshr genes in amago salmon likely represent paralogs arising from a recent genome duplication in the salmonid lineage (33). Functional properties have been previously assessed in four other teleosts, and combined with our zebrafish data, studies on teleost TSHR consistently show that TSH preparations are most potent in stimulating cAMP accumulation, whereas gonadotropins and hCG show, if any, rather weak activity at high concentrations (31–33, 36).
Expression profiles for teleost TSHR have been limited in most studies to RT-PCR analyses of adult tissues, whereas data on spatiotemporal expression profiles during embryonic development were completely lacking. In previous studies, tshr expression has been detected at high levels in grossly dissected tissue fragments comprising the thyroid region, in brain and at very high levels in gonadal tissues (31–33, 35, 36). Specific expression in thyroid follicles has only been demonstrated by in situ hybridization in amago salmon smolts and parrs (33).
The present study is the first to address tshr expression during early development in a teleost. We show that the developing thyroid is indeed the major site of expression in zebrafish embryos and that tshr mRNA is already expressed during the process of thyroid budding. Thus, although still limited to the mRNA level, coincident expression of tshr in thyroid and cga and tshb in pituitary can be detected as early as 40–42 hpf in zebrafish embryos. In addition to tshr, we identified tpo, iyd, duox, and duoxa as novel thyroid differentiation marker in zebrafish. In situ hybridization revealed thyroid-specific expression for tpo, whereas iyd was expressed in thyroid, liver, and gut. To the best of our knowledge, zebrafish is the first vertebrate where thyroidal iyd expression has been demonstrated during embryonic development. Interestingly, similar to tg, slc5a5, and tshr, expression of iyd and tpo mRNA was observed during early stages of thyroid morphogenesis in zebrafish. Moreover, the finding of enhanced expression of tshr, tpo, and iyd after embryo treatment with PTU implicates regulation of these genes by TSH in zebrafish, similar to what has been observed in mammals (28, 29) and amphibians (48).
These new data allowed us to propose a refined model of functional thyroid differentiation during zebrafish development. Collectively, the expression profiles of tg, tshr, slc5a5, iyd, and tpo demonstrate an early onset of a molecular differentiation program along with the process of thyroid budding. A similar induction of functional differentiation markers occurs much later during mouse thyroid morphogenesis. For example, thyroid budding in mice occurs between E9.5 and E10.5 (15), whereas differentiation marker expression becomes detectable between E14 and E16 (8, 17). However, in both zebrafish and mice, functional differentiation markers are induced shortly before functional differentiation becomes manifest at the morphological level by formation of thyroid follicles. In zebrafish, expression of molecular differentiation markers (between 32 and 42 hpf) precedes formation of a first follicle by 55 hpf, whereas in mice, the onset of Tg, Tshr, Tpo, and NIS expression between E14.5 and E16 precedes formation of thyroid follicles around E16.5 (8, 17).
What differs between zebrafish and mouse is that in mice, a series of morphogenetic events (migration, bilobulation, and growth) separates the process of thyroid budding and the onset of follicle formation by several days (14, 15). In the case of the zebrafish thyroid, the time elapsed between completion of thyroid budding and formation of a first follicle is very short (∼10 h). Bilobulation does not occur in zebrafish, and in contrast to mice, a distinct growth phase of the thyroid primordium before initiation of functional differentiation is lacking in zebrafish. A massive growth of thyroid tissue occurs only after a first follicle has formed, suggesting a model in which functional differentiation is initially favored over thyroid growth in zebrafish. Thus, although similar morphogenetic processes (e.g. thyroid budding, migration, growth, and functional differentiation) are involved in thyroid organogenesis in zebrafish and mouse, the sequence of these events is asynchronous in the two models. Compared with mouse, distinct morphogenetic processes occur rather concurrently in zebrafish. The coincidence of thyroid budding and induction of tshr and other functional differentiation markers represents one example corroborating this contention. The distinct schedule of morphogenetic events in zebrafish might be necessary to allow for the rapidity of thyroid organogenesis, where a few follicles are formed within 48 h after specification of the thyroid anlage.
With respect to the apparent lack of thyroid growth before 55 hpf, one novel finding of our study points to a so far unrecognized role of apoptosis regulators during early thyroid morphogenesis. By searching for novel genes expressed in the thyroid primordium of zebrafish embryos, we observed thyroid-enriched expression of bcl2l1, a gene which encodes for a member of the BCL2 family of cell death regulators. Similar to mammalian BCL2L1 proteins, zebrafish Bcl2l1 has been shown to exert prosurvival activity (50). At present, the function of bcl2l1 during thyroid development in zebrafish remains obscure. From the developmental expression profile, one might anticipate a role during early morphogenesis as bcl2l1 is strongly expressed in the thyroid primordium from 36 hpf to 60 hpf but is subsequently down-regulated. What is particularly interesting is that high bcl2l1 expression coincides with a phase of limited thyroid growth. It is therefore tempting to suggest that high expression of a potent prosurvival factor may support the maintenance of a small nonproliferating organ primordium during early development. The conservation of thyroid-enriched expression of antiapoptotic proteins during zebrafish and mouse development warrants further studies on the role of apoptosis during thyroid morphogenesis in these models.
The onset of thyroid hormone synthesis is the hallmark of functional maturation of thyroid tissue, and H2O2 is essential for TPO-catalyzed iodination of TG (51). Recently, DUOX2 has been identified as a core component of the thyroidal H2O2 generator (52, 53). Functional maturation and membrane targeting of DUOX2 is dependent on the DUOX maturation factor DUOXA2 (40). The identification of biallelic mutations of DUOX2 and DUOXA2 in CH patients support the essential role of DUOX activity for thyroid hormone synthesis (54). In this study, we show for the first time the expression of duox and duoxa in zebrafish thyroid. In zebrafish, duox and duoxa are less strongly expressed in thyroid compared with tg, tshr, slc5a5, iyd, and tpo, at least during the developmental period examined. The low level expression might be one reason why thyroidal expression of duox has not been recognized in previous studies (41). Interestingly, coincident expression of duox and duoxa in thyroid was not detectable at the stage of first follicle formation. By 55 hpf, the colloid of this follicle shows immunoreactivity for TG (20) but not yet for T4 (21). T4 immunoreactivity of thyroid follicles becomes detectable between 72 and 80 hpf (21), coincident with weak yet detectable duox and duoxa expression in the zebrafish thyroid. This temporal correlation raises the possibility that the onset of duox expression might represent a developmentally regulated factor for the initiation of de novo synthesis of thyroid hormones. The finding that zebrafish expresses only a single duox gene could facilitate future studies aiming at a knockdown of all DUOX activity to elucidate its role for thyroid hormone synthesis in zebrafish.
Previous studies of mouse lines with defective TSH and TSHR signaling have demonstrated that early steps of thyroid morphogenesis are independent of TSHR function (17, 43). Snell dwarf (dw/dw) mutant mice do not express TSH, whereas hyt/hyt mutant mice harbor a TSHR mutation that prevents TSH binding (8). In both mutant lines, embryos have a thyroid of normal size at E17.5, but thyroid hypoplasia will develop postnatally (17). A similar phenotype was observed in genetically engineered TSHR knockout mice (17, 43). A tshr mutant line has not yet been described in zebrafish, and the limited information on the possible role of TSHR signaling during thyroid organogenesis is derived from observations in lia mutants. The zebrafish lia mutant has a mutation in the fgf3 gene that causes defective adenohypophysis development and loss of thyrotrophs (55). Therefore, lia mutants have been considered as a model for defective TSH signaling. Intriguingly, Alt et al. (20) reported that formation of thyroid follicles is not defective in lia mutant larvae. The thyroid follicles displayed nearly normal size and shape and showed immunoreactivity to T4. These data suggested that TSH is not required for formation, growth, and function of thyroid follicles in zebrafish (20). However, it was unclear whether basal activity of a zebrafish Tshr might be sufficient to ensure normal follicle development.
To study the role of TSHR signaling more directly, we used microinjection of embryos with a tshr-specific MO to generate a loss-of-function model for TSHR signaling. In contrast to the aforementioned studies with the lia mutant, our analyses of tshr morphants revealed a clear thyroid phenotype compared with controls. In fact, our data demonstrate that loss of TSHR signaling impairs functional differentiation. First, tshr morphants have a significantly reduced number of functional follicles, many of which displaying a reduced size. Second, the observation of reduced immunoreactivity for TG-I and T4 implicates compromised iodide organification and thyroid hormone synthesis in tshr morphants. Third, expression of tpo, slc5a5, and iyd is strongly reduced in tshr morphants, whereas effects on tg expression are less marked. Given that the proteins encoded by these genes are all integral components of the biosynthetic machinery required for thyroid hormone production (2), their down-regulation most likely accounts for the impaired functional capacity of thyroid follicles. Furthermore, a moderate thyroid phenotype characterized by delayed formation of a first follicle was detectable already by 55 hpf in tshr morphants.
The question whether tshr knockdown also affected thyroid growth remains less clear. Our whole-mount immunofluorescence analyses are limited to the detection of follicular colloid and are not expected to detect thyroid follicular cells. Thus, these analyses do not provide an estimate of the total amount of thyroid cells present in tshr morphants. Among the in situ hybridizations performed at 100 hpf, those conducted with the tg probe were most instructive concerning possible growth effects. Here, the thyroid staining of up to 69% of tshr morphants was indistinguishable from control larvae. Although the three-dimensional dispersal of stained cells makes quantitative estimations very difficult, a marked reduction in the size of the stained domain was not observed. Thus, we conclude cautiously that a growth defect is not readily detectable in tshr morphants.
By using a transient transgenic system of heat-shock-inducible tshr overexpression, the thyroid phenotype resulting from knockdown of endogenous tshr was partially rescued, corroborating the specificity of the morpholino loss-of-function approach. Because we used a transient transgenesis approach, mosaicism of transgene expression due to delayed transgene integration most likely accounted for the incomplete rescue. However, our data show that Tol2-based transient transgenesis provides a viable approach for use in phenotypic rescue experiments during later stages of zebrafish development.
Collectively, the thyroid phenotype observed in tshr morphants resembles in many aspects the phenotype described for the hyt/hyt mutant mice. hyt/hyt mutant mice present with a relatively normal thyroid size at birth, but display hypothyroidism, reduced intralumenal colloid, reduced but detectable iodinated TG, reduced intrathyroidal T4, moderately reduced TG expression, and greatly reduced NIS and Tpo expression (17, 56, 57). Similar to hyt/hyt mutant mice, partial functionality of the thyroid was detected in tshr morphants. In the case of the zebrafish model, the residual activity might be due to a partial independence of thyroid function from TSHR signaling or from incomplete knockdown of TSHR function by the tshr-MO used in this study. At present, we cannot exclude either of these two possibilities. In general, most morpholino-mediated knockdowns are incomplete, and ongoing dilution due to cell division will decrease intracellular concentrations during the course of development (58). On the other hand, the less severe effects of tshr knockdown on differentiation marker expression at 55 hpf compared with 100 hpf might imply differences in the dependence from TSHR signaling between early and late stages of thyroid development.
Our analyses of thyroid gene expression in tshr morphants also revealed an important difference compared with mouse models of defective TSHR signaling. Impaired TSHR signaling caused only in the zebrafish model a marked down-regulation of the transcription factors nkx2.1a and pax8. Although in vitro models suggested that Pax8 expression is dependent on TSH stimulation (59), neither NKX2.1 nor PAX8 protein expression was affected in mouse models with defective TSHR signaling (17).
TSHR signaling in thyroid cells is largely mediated via activation of the adenylate cyclase-cAMP pathway. Given this, we also compared the thyroid phenotype of tshr morphants with that observed in transgenic mice expressing a dominant negative Creb (cAMP response element-binding protein) isoform specifically in the thyroid (60). Interestingly, the thyroid phenotype of the latter mouse model resembles in some aspects the one in the zebrafish model. Of particular interest is the coincidence of a strong reduction in Nkx2.1 and Pax8 expression along with the down-regulation of functional differentiation factors in mice with defective cAMP signaling and in tshr morphants. The results obtained in transgenic mouse with defective cAMP signaling support the view that the down-regulation of functional differentiation factors might be mediated in part by reduced expression of thyroid transcription factors. Preliminary observations in zebrafish embryos injected with nkx2.1a-MO support this view by showing reduced expression of tshr and slc5a5 at low submaximal nkx2.1a-MO concentrations (data not shown). Collectively, these data raise the possibility of a positive feedback signaling between TSHR signaling and thyroid transcription factor expression in the zebrafish thyroid. The details of this interesting regulatory circuit are currently under investigation. From the aforementioned mouse-zebrafish comparisons, we can conclude that the thyroid phenotype in tshr morphants is unique in that it combines several aspects of thyroid phenotypes observed in different mouse models with defective TSHR and cAMP signaling. This finding corroborates the value of the zebrafish model to provide unique information complementing results obtained with different mammalian models.
Thyroid ectopy represents the most common cause of CH (13). Ectopic thyroid tissue has been reported anywhere along the route of thyroid descent from the foramen cecum to its normal position in the neck. However, in approximately 90% of cases, the thyroid tissue is present at a sublingual position, that is at the site of thyroid origin. The literature on thyroid gland anatomy in teleosts is replete with observations of ectopic thyroid follicles located in tissues as diverse as ocular choroid, kidney, spleen, intestine, liver, or heart (61). The embryological origin of these ectopic thyroid tissues has not yet been clarified. In zebrafish larvae, however, we observed ectopic thyroid tissue exclusively at a midline position in close vicinity to the site of thyroid origin within the ventral pharynx. Due to the differential relative movements during development of the pharynx and the subpharyngeal thyroid tissue, the part of the pharynx from which the thyroid originates becomes located more anteriorly relative to the orthotopic thyroid domain. The finding of ectopic thyroid tissue in zebrafish at a site that can be considered anatomically equivalent to the sublingual thyroid in human suggests a so far unrecognized potential of zebrafish for studies addressing the mechanisms of developmental defects resulting in thyroid ectopy. Although we observed so far only cases of partial ectopy in zebrafish, one possible application might be the use of zebrafish embryos as a tool to screen orthotopic and ectopic thyroid tissue for differential expression of the candidate genes recently identified in human ectopic thyroids (62). Notably, our analyses of tshr morphants did not detect a difference in the prevalence of ectopic thyroid tissue compared with controls.
In conclusion, we have provided novel expression data on functional differentiation markers during thyroid morphogenesis in zebrafish. These data indicate the induction of an early molecular differentiation program during thyroid budding preceding the formation of a first follicle shortly thereafter. A delayed induction of duox expression occurs coincidently with the onset of thyroid hormone synthesis raising the possibility that duox expression represents a developmentally regulated factor of functional maturation. Finally, we demonstrate that loss of Tshr function causes a specific thyroid phenotype that is characterized by a reduction in the number and size of functional follicles, strong down-regulation of differentiation markers, and reduced expression of thyroid transcription factors. From an evolutionary perspective, our phenotype analyses revealed aspects of TSHR signaling that are conserved between mouse and zebrafish, such as the down-regulation of functional differentiation markers, but also revealed some differences, particularly with regard to the regulation of thyroid transcription factors. Collectively, our data demonstrate that TSHR signaling is required for functional differentiation of the zebrafish thyroid.
Materials and Methods
Zebrafish husbandry and embryo culture
Zebrafish (Danio rerio) embryos were obtained from natural spawning of adult fish. Three zebrafish lines were used in this study, including WT, casper mutant (obtained from Leonard Zon, Howard Hughes Medical Institute, Boston, MA), and Tg(fli1a:EGFP) transgenic fish lines (obtained from Brant M. Weinstein, National Institutes of Health, Bethesda, MD). Zebrafish embryos were raised at 28.5 C according to Westerfield (25) and staged in hpf as described by Kimmel et al. (63). Embryos and larvae were fixed in 4% phosphate-buffered paraformaldehyde (PFA) (Sigma, St. Louis, MO) overnight at 4 C, washed in PBS containing 0.1% Tween 20 (PBST), gradually transferred to 100% methanol, and stored at −20 C until use for in situ hybridization or immunofluorescence analyses. If indicated, pigmentation of embryos was inhibited by addition of 0.003% PTU (Sigma) to the embryo medium at 24 hpf.
Identification of thyroid differentiation markers
BLAST searches of the zebrafish genome database (www.ensembl.org/Danio_rerio) and the GenBank database (www.ncbi.nlm.nih.gov/genbank) were performed to identify zebrafish orthologs of mammalian Tshr, Tpo, Iyd, Duox, and Duoxa genes. For each gene, our searches retrieved only a single zebrafish ortholog. No duplicates were found. Zebrafish orthologs of Tshr, Tpo, Iyd, Duox, and Duoxa were found on chromosome 14 (tshr; NM_001145763), chromosome 17 (tpo; XM_692744), chromosome 23 (iyd; XM_691419), chromosome 25 (duox; XM_001919359), and chromosome 25 (duoxa; XM_002666907), respectively. Results from reciprocal best blast hit analyses supported the identity of the zebrafish orthologs.
RNA probe synthesis
Synthesis of dioxygenin-labeled RNA probes for in situ hybridization was performed using the PCR-based approach as described by David and Wedlich (64) with the following modifications. DNA templates for riboprobes were generated by PCR using gene-specific primers containing the T3 promoter sequence and were purified with MinElute Reaction Cleanup kit (QIAGEN, Valencia, CA) followed by phenol-chloroform extraction. PCR primer sequences are listed in Supplemental Table 1. DNA was reconstituted in ribonuclease-free water, template purity was verified by gel electrophoresis, and DNA concentrations were measured using a NanoDrop ND-1000 UV/VIS spectrophotometer (Thermo Scientific, Rockford, IL). Dioxygenin-labeled riboprobes were synthetized from 500 ng of DNA by in vitro transcription using DIG RNA labeling kit (Roche, Indianapolis, IN) and T3 RNA-Polymerase (Promega, Madison, WI) according to the manufacturers recommendation. After deoxyribonuclease I treatment, riboprobes were purified using High Pure PCR Product Purification kit (Roche), integrity was checked by gel electrophoresis, and probes were stored at −80 C until use.
Whole-mount in situ hybridization
WISH with zebrafish embryos and larvae was performed as described in Thisse and Thisse (65) with the following modifications. In the standard WISH protocol, riboprobe hybridization was performed at 65 C overnight, and probes were detected using antidioxygenin antibody conjugated to alkaline phosphatase (1:6000; Roche). For detection of duox and duoxa mRNA, the standard protocol was changed in that 5% dextran sulfate was added to the hybridization mix, and hybridization was performed at 63 C. Staining reactions were performed with the alkaline phosphatase substrates BM Purple (Roche) and NBT/BCIP (Roche). Stained embryos were washed in PBST, postfixed in 4% PFA, and embedded in 90% glycerol for light microscopy. Images of stained specimens were captured using an Axiocam digital camera mounted on an Axioplan 2 microscope (Zeiss, Oberkochen, Germany). Whenever appropriate, photomicrographs of stained embryos show results obtained in PTU-treated casper embryos as visualization of staining patterns in the pharyngeal region is facilitated due to the lack of eye pigmentation.
Whole-mount immunofluorescence
After rehydration into PBST, 120-hpf larvae were treated with 10 μg/ml proteinase K (Roche) for 30 min, rinsed shortly in PBST, and postfixed in 4% PFA for 20 min. After further rinsing in PBST, larvae were immersed in blocking buffer (PBST containing 1.0% dimethylsulfoxide, 1% BSA, 5% horse serum, and 0.8% Triton X-100) for 2 h. Larvae were then incubated overnight in blocking buffer containing primary antibodies at 4 C. After several washing steps in PBST containing 1% BSA, larvae were incubated with secondary antibodies overnight at 4 C. The following antibodies were used: rabbit anti-TG polyclonal antibody (1:1000; Dako, Glostrup, Denmark), mouse anti-TG-I monoclonal antibody (1:1000; C. Ris-Stalpers, Amsterdam, The Netherlands), rabbit anti-T4 polyclonal antibody (1:2000; MP Biochemicals, Solon, OH), chicken anti-GFP polyclonal antibody (1:2000; Abcam, Cambridge, MA), cyanine 3-conjugated antirabbit IgG antibody (1:250; Jackson ImmunoResearch, West Grove, PA), cyanine 3-conjugated antimouse IgG antibody (1:250; Jackson ImmunoResearch), and Alexa Fluor 488-conjugated antichicken IgG antibody (1:300; Invitrogen, Carlsbad, CA). Stained larvae were washed in PBST and embedded in 90% glycerol for fluorescence microscopy. Images were acquired by an Axiocam MR3 camera on an Axio Observer Z1 microscope using Axiovision software (Zeiss). All image series for quantification of follicle number were acquired the same day, using identical acquisition settings.
Cloning of zebrafish tshr full coding sequence
For amplification of the complete coding region of zebrafish tshr, primers were designed based on the predicted cDNA sequence, and restriction enzyme sites were added to the forward (BamHI) and reverse (XbaI) primer sequences. Final primer sequences were: tshr- forward 5′-CGCGGATCCGAGCCTACATTTATTCTTTATACCA-3′ and tshr-reverse 5′-TGCTCTAGAGTTGCAGAAGTGTATTCTCTCCGTTA-3′. RT-PCR amplification was performed using total RNA from 72-hpf zebrafish embryos. Amplicons were cloned into pcr4-TOPO (Invitrogen) and sequenced. BLAST analyses of the sequences identified a clone containing the complete coding sequence of 2271 bp and sharing 99% nucleotide homology to the sequence predicted from genome mapping. Full-length GpHR sequences were collected from GenBank (accession nos. are reported in Supplemental Table 2), and multiple alignments were performed using ClustalW. A phylogenetic tree was constructed using the neighbor joining algorithm based on full polypeptide sequences.
Functional characterization
The entire coding region of zebrafish tshr was inserted into pcDNA3.1 giving tshr/pcDNA3.1 expression vector (Invitrogen). Human embryonic kidney 293 cells (HEK393T) were used for transient transfection experiments and were cultured in DMEM supplemented with 10% fetal bovine serum, sodium pyruvate, 100 unit/ml penicillin/streptomycin, and fungizone at 37 C in a humidified atmosphere at 95% air and 5% CO2. Briefly, cells were trypsinized, counted, and transfected with tshr/pcDNA3.1 expression vector in suspension with DNA-polyethylene imine complexes. Then, 20000 cells were seeded in 96-well plates. After 24 h, medium was replaced, and 48 h after transfection, the intracellular accumulation of cAMP was measured by homogeneous time-resolved fluorescence (CisBio, Bedford, MA). The medium was removed and replaced by Krebs-Ringer-HEPES buffer supplemented with 25 μm of the phosphodiesterase inhibitor Rolipram. After 30 min of incubation with various concentrations of bovine TSH (Sigma), recombinant human TSH (Genzyme Corp., Cambridge, MA), or hCG (Sigma) at room temperature, the stimulation was stopped by adding lysis buffer; 10 μl of each well were transferred in a 384-well plate by using the Biomek Laboratory Automation Workstation (Beckman Coulter, Inc., Brea, CA). The workstation was used to transfer in each well 5 μl of diluted cAMP labeled with d2 fluorophore and 5 μl diluted monoclonal antibody against cAMP labeled with Europium cryptate fluorophore. After 1 h of incubation at room temperature, the plate was read using RUBYstar reader (BMG LABTECH GmbH, Ortenberg, Germany). cAMP concentration was extrapolated using a standard curve treated in parallel. cAMP measurements were done in triplicate. Results are expressed as picomoles cAMP per milliliter.
Morpholino microinjection
For knockdown of tshr function, a MO was obtained from Gene Tools (Philomath, OR). The tshr-MO was designed as a translation-blocking MO targeting the 5′-untranslated region immediately upstream of the start codon of the tshr gene. The sequence of the tshr-MO was 5′-ATGTAGGCTATTTTCTTCCACACGA-3′. Database searches did not identify any sequence with significant similarity of known zebrafish genes to the tshr-MO. The control MO, designed by Gene Tools as a standard control MO with no target specificity, had the following sequence: 5′-CCTCTTACCTCAGTTACAATTTATA-3′. Working solutions of morpholinos were prepared in 0.12 m KCl containing phenol red to check injection efficiency, and approximately 1 nl of MO solution was microinjected into the yolk sac of one- to four-cell stage embryos. In initial dose-finding experiments, embryos were injected with tshr-MO at doses ranging from 1.0 to 10 ng/embryo along with injections of equimolar amounts of control MO and saline (mock injection). Although development of embryos injected with control MO and saline did not differ from noninjected siblings, injection of tshr-MO at concentrations more than or equal to 3 ng/embryo caused dose-dependent increases in mortality and severe overall developmental defects (e.g. retarded development and growth, edema formation, craniofacial malformations, and deformed body shape). Based on expression patterns of tshr mRNA, we attributed the developmental phenotypes observed at concentrations more than or equal to 3 ng/embryo to nonspecific and toxic effects of the tshr-MO. The highest tshr-MO concentration not causing gross developmental defects was 2.5 ng/embryo. This concentration was used in all morpholino injection experiments reported in this study.
Rescue experiments
The pTol2hsp70:tshr construct was generated by Gateway cloning using the Tol2kit according to Kwan et al. (66). pME-tshr was constructed by subcloning the tshr coding sequence (lacking the morpholino target sequence) into pME-MCS between BamHI and XbaI restriction sites. The pTol2hsp70:tshr vector was assembled from the entry clones p5E-hsp70, pME-tshr, and the destination vector pTol2DestR4R2pA (67). Capped mRNA encoding for transposase was generated by in vitro transcription using mMessage mMachine SP6 kit (Ambion) and NotI-linearized pCS-zT2TP plasmid (68) as a template.
Each rescue experiment comprised three experimental groups, including embryos injected with 2.5 ng tshr-MO only (tshr morphants), embryos coinjected with 2.5 ng tshr-MO, 25 pg of transposase mRNA and 25 pg of pTol2hsp70:tshr plasmid (pTol2hsp70:tshr-injected tshr morphants), and embryos not injected with tshr-MO (controls). All injections were made at the one-cell stage. Starting from E2, embryos from all three experimental groups were heat shocked twice per day. For heat-shock treatment, embryos were transferred to prewarmed embryo medium (40 C), incubated for 30 min at 40 C, and subsequently transferred back to embryo medium at 28.5 C. A total of six independent rescue experiments were performed, three with Tg(fli1a:EGFP) embryos (used for TG and T4 immunostaining at 120 hpf) and three with casper embryos (used for gene expression analyses at 100 hpf).
Statistics
For the statistical analyses of follicle number measurements, pair-wise comparisons were conducted using the nonparametric Mann-Whitney test (initial morphant characterization) or Dunn's multiple comparison test (rescue experiments). Statistical analyses were performed using the software package GraphPad Prism 4.0 (GraphPad, San Diego, CA). Differences were considered significant at P < 0.05.
Acknowledgments
We thank Leonard Zon (Howard Hughes Medical Institute, Boston, MA) for providing the casper mutant line, Brant M. Weinstein (National Institutes of Health, Bethesda, MD) for providing the Tg(fli1a:EGFP) transgenic line, Koichi Kawakami (National Institute of Genetics, Shizuoka, Japan) for Tol2 reagents, E. Urizar and F. Miot for fruitful discussions and comments on the Manuscript, and V. Janssens, D. Masse, and G. Pottier for technical assistance.
This work was supported by the Belgian Fonds de la Recherche Scientifique Medicale (FRSM[2]3_4_557_08), by Action de Recherche Concertée de la Communauté Française de Belgique (ARC N°AUWB-08/13-ULB10), by Fonds d'Encouragement à la Recherche (Université Libre de Bruxelles), and by grants from the Belgian National Fund for Scientific Research (FNRS). R.O. is FNRS Short Term Foreign Postdoctoral Fellow., E.M. is FNRS Research Fellow., and S.C. is FNRS Senior Research Associate.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aa
- Amino acid
- Bcl
- B-cell lymphoma
- CH
- congenital hypothyrodism
- DUOX
- dual oxidase
- E
- embryonic day
- EGFP
- enhanced green fluorescent protein
- GpHR
- glycoprotein hormone receptor
- hCG
- human chorionic gonadotropin
- hpf
- hours postfertilization
- MO
- morpholino antisense oligonucleotide
- NIS
- sodium-iodide symporter
- PBST
- PBS containing 0.1% Tween 20
- PFA
- paraformaldehyde
- PTU
- phenylthiourea
- TD
- thyroid dysgenesis
- TG
- thyroglobulin
- TG-I
- iodide-rich TG
- Tol2
- Transposable élément of medaka fish Oryzias latipes, N° 2
- TPO
- thyroid peroxidase
- TSHR
- TSH receptor
- WISH
- whole-mount in situ hybridization
- WT
- wild type.
References
- 1. Yen PM. 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- 2. Dunn JT , Dunn AD. 2001. Update on intrathyroidal iodine metabolism. Thyroid 11:407–414 [DOI] [PubMed] [Google Scholar]
- 3. Dohán O , De la Vieja A , Paroder V , Riedel C , Artani M , Reed M , Ginter CS , Carrasco N. 2003. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev 24:48–77 [DOI] [PubMed] [Google Scholar]
- 4. Song Y , Ruf J , Lothaire P , Dequanter D , Andry G , Willemse E , Dumont JE , Van Sande J , De Deken X. 2010. Association of duoxes with thyroid peroxidase and its regulation in thyrocytes. J Clin Endocrinol Metab 95:375–382 [DOI] [PubMed] [Google Scholar]
- 5. Moreno JC , Visser TJ. 2010. Genetics and phenomics of hypothyroidism and goiter due to iodotyrosine deiodinase (DEHAL1) gene mutations. Mol Cell Endocrinol 322:91–98 [DOI] [PubMed] [Google Scholar]
- 6. Vassart G , Dumont JE. 1992. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 13:596–611 [DOI] [PubMed] [Google Scholar]
- 7. Dumont JE , Lamy F , Roger P , Maenhaut C. 1992. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev 72:667–697 [DOI] [PubMed] [Google Scholar]
- 8. De Felice M , Postiglione MP , Di Lauro R. 2004. Minireview: thyrotropin receptor signaling in development and differentiation of the thyroid gland: insights from mouse models and human diseases. Endocrinology 145:4062–4067 [DOI] [PubMed] [Google Scholar]
- 9. Dremier S , Milenkovic M , Blancquaert S , Dumont JE , Døskeland SO , Maenhaut C , Roger PP. 2007. Cyclic adenosine 3′,5′-monophosphate (cAMP)-dependent protein kinases, but not exchange proteins directly activated by cAMP (Epac), mediate thyrotropin/cAMP-dependent regulation of thyroid cells. Endocrinology 148:4612–4622 [DOI] [PubMed] [Google Scholar]
- 10. Rastogi MV , LaFranchi SH. 2010. Congenital hypothyroidism. Orphanet J Rare Dis 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grasberger H , Refetoff S. 2010. Congenital defects of thyroid hormone synthesis. In: , Weiss RE , Refetoff S eds. Genetic diagnosis of endocrine disorders. London: Elsevier; 87–96 [Google Scholar]
- 12. Pohlenz J , Van Vliet G. 2010. Developmental Abnormalities of the Thyroid. In: , Weiss RE , Refetoff S eds. Genetic diagnosis of endocrine disorders. London: Elsevier; 97–104 [Google Scholar]
- 13. Van Vliet G. 2003. Development of the thyroid gland: lessons from congenitally hypothyroid mice and men. Clin Genet 63:445–455 [DOI] [PubMed] [Google Scholar]
- 14. De Felice M , Di Lauro R. 2004. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev 25:722–746 [DOI] [PubMed] [Google Scholar]
- 15. Fagman H , Andersson L , Nilsson M. 2006. The developing mouse thyroid: embryonic vessel contacts and parenchymal growth pattern during specification, budding, migration, and lobulation. Dev Dyn 235:444–455 [DOI] [PubMed] [Google Scholar]
- 16. Fagman H , Nilsson M. 2010. Morphogenesis of the thyroid gland. Mol Cell Endocrinol 323:35–54 [DOI] [PubMed] [Google Scholar]
- 17. Postiglione MP , Parlato R , Rodriguez-Mallon A , Rosica A , Mithbaokar P , Maresca M , Marians RC , Davies TF , Zannini MS , De Felice M , Di Lauro R. 2002. Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc Natl Acad Sci USA 99:15462–15467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lieschke GJ , Currie PD. 2007. Animal models of human disease: zebrafish swim into view. Nat Rev Genet 8:353–367 [DOI] [PubMed] [Google Scholar]
- 19. Alt B , Elsalini OA , Schrumpf P , Haufs N , Lawson ND , Schwabe GC , Mundlos S , Grüters A , Krude H , Rohr KB. 2006. Arteries define the position of the thyroid gland during its developmental relocalisation. Development 133:3797–3804 [DOI] [PubMed] [Google Scholar]
- 20. Alt B , Reibe S , Feitosa NM , Elsalini OA , Wendl T , Rohr KB. 2006. Analysis of origin and growth of the thyroid gland in zebrafish. Dev Dyn 235:1872–1883 [DOI] [PubMed] [Google Scholar]
- 21. Elsalini OA , von Gartzen J , Cramer M , Rohr KB. 2003. Zebrafish hhex, nk2.1a, and pax2.1 regulate thyroid growth and differentiation downstream of Nodal-dependent transcription factors. Dev Biol 263:67–80 [DOI] [PubMed] [Google Scholar]
- 22. Wendl T , Lun K , Mione M , Favor J , Brand M , Wilson SW , Rohr KB. 2002. Pax2.1 is required for the development of thyroid follicles in zebrafish. Development 129:3751–3760 [DOI] [PubMed] [Google Scholar]
- 23. Rohr KB , Concha ML. 2000. Expression of nk2.1a during early development of the thyroid gland in zebrafish. Mech Dev 95:267–270 [DOI] [PubMed] [Google Scholar]
- 24. Porazzi P , Calebiro D , Benato F , Tiso N , Persani L. 2009. Thyroid gland development and function in the zebrafish model. Mol Cell Endocrinol 312:14–23 [DOI] [PubMed] [Google Scholar]
- 25. Westerfield M. 2000. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). 4th ed. Eugene, OR: University of Oregon Press [Google Scholar]
- 26. Elsalini OA , Rohr KB. 2003. Phenylthiourea disrupts thyroid function in developing zebrafish. Dev Genes Evol 212:593–598 [DOI] [PubMed] [Google Scholar]
- 27. White RM , Sessa A , Burke C , Bowman T , LeBlanc J , Ceol C , Bourque C , Dovey M , Goessling W , Burns CE , Zon LI. 2008. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levy O , Dai G , Riedel C , Ginter CS , Paul EM , Lebowitz AN , Carrasco N. 1997. Characterization of the thyroid Na+/I− symporter with an anti-COOH terminus antibody. Proc Natl Acad Sci USA 94:5568–5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Milenkovic M , De Deken X , Jin L , De Felice M , Di Lauro R , Dumont JE , Corvilain B , Miot F. 2007. Duox expression and related H2O2 measurement in mouse thyroid: onset in embryonic development and regulation by TSH in adult. J Endocrinol 192:615–626 [DOI] [PubMed] [Google Scholar]
- 30. Opitz R , Trubiroha A , Lorenz C , Lutz I , Hartmann S , Blank T , Braunbeck T , Kloas W. 2006. Expression of sodium-iodide symporter mRNA in the thyroid gland of Xenopus laevis tadpoles: developmental expression, effects of antithyroidal compounds, and regulation by TSH. J Endocrinol 190:157–170 [DOI] [PubMed] [Google Scholar]
- 31. Goto-Kazeto R , Kazeto Y , Trant JM. 2009. Molecular cloning, characterization and expression of thyroid-stimulating hormone receptor in channel catfish. Gen Comp Endocrinol 161:313–319 [DOI] [PubMed] [Google Scholar]
- 32. Kumar RS , Ijiri S , Kight K , Swanson P , Dittman A , Alok D , Zohar Y , Trant JM. 2000. Cloning and functional expression of a thyrotropin receptor from the gonads of a vertebrate (bony fish): potential thyroid-independent role for thyrotropin in reproduction. Mol Cell Endocrinol 167:1–9 [DOI] [PubMed] [Google Scholar]
- 33. Oba Y , Hirai T , Yoshiura Y , Kobayashi T , Nagahama Y. 2000. Cloning, functional characterization, and expression of thyrotropin receptors in the thyroid of amago salmon (Oncorhynchus rhodurus). Biochem Biophys Res Commun 276:258–263 [DOI] [PubMed] [Google Scholar]
- 34. Ponce M , Infante C , Manchado M. 2010. Molecular characterization and gene expression of thyrotropin receptor (TSHR) and a truncated TSHR-like in Senegalese sole. Gen Comp Endocrinol 168:431–419 [DOI] [PubMed] [Google Scholar]
- 35. Rocha A , Gómez A , Galay-Burgos M , Zanuy S , Sweeney GE , Carrillo M. 2007. Molecular characterization and seasonal changes in gonadal expression of a thyrotropin receptor in the European sea bass. Gen Comp Endocrinol 152:89–101 [DOI] [PubMed] [Google Scholar]
- 36. Vischer HF , Bogerd J. 2003. Cloning and functional characterization of a testicular TSH receptor cDNA from the African catfish (Clarias gariepinus). J Mol Endocrinol 30:227–238 [DOI] [PubMed] [Google Scholar]
- 37. Herzog W , Zeng X , Lele Z , Sonntag C , Ting JW , Chang CY , Hammerschmidt M. 2003. Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Dev Biol 254:36–49 [DOI] [PubMed] [Google Scholar]
- 38. Nica G , Herzog W , Sonntag C , Hammerschmidt M. 2004. Zebrafish pit1 mutants lack three pituitary cell types and develop severe dwarfism. Mol Endocrinol 18:1196–1209 [DOI] [PubMed] [Google Scholar]
- 39. Kawahara T , Quinn MT , Lambeth JD. 2007. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol 7:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grasberger H , Refetoff S. 2006. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem 281:18269–18272 [DOI] [PubMed] [Google Scholar]
- 41. Flores MV , Crawford KC , Pullin LM , Hall CJ , Crosier KE , Crosier PS. 2010. Dual oxidase in the intestinal epithelium of zebrafish larvae has anti-bacterial properties. Biochem Biophys Res Commun 400:164–168 [DOI] [PubMed] [Google Scholar]
- 42. Guo S , Wilson SW , Cooke S , Chitnis AB , Driever W , Rosenthal A. 1999. Mutations in the zebrafish unmask shared regulatory pathways controlling the development of catecholaminergic neurons. Dev Biol 208:473–487 [DOI] [PubMed] [Google Scholar]
- 43. Marians RC , Ng L , Blair HC , Unger P , Graves PN , Davies TF. 2002. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci USA 99:15776–15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abramowicz MJ , Duprez L , Parma J , Vassart G , Heinrichs C. 1997. Familial congenital hypothyroidism due to inactivating mutation of the thyrotropin receptor causing profound hypoplasia of the thyroid gland. J Clin Invest 99:3018–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tonacchera M , Agretti P , Pinchera A , Rosellini V , Perri A , Collecchi P , Vitti P , Chiovato L. 2000. Congenital hypothyroidism with impaired thyroid response to thyrotropin (TSH) and absent circulating thyroglobulin: evidence for a new inactivating mutation of the TSH receptor gene. J Clin Endocrinol Metab 85:1001–1008 [DOI] [PubMed] [Google Scholar]
- 46. Lawson ND , Weinstein BM. 2002. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 248:307–318 [DOI] [PubMed] [Google Scholar]
- 47. Wendl T , Adzic D , Schoenebeck JJ , Scholpp S , Brand M , Yelon D , Rohr KB. 2007. Early developmental specification of the thyroid gland depends on han-expressing surrounding tissue and on FGF signals. Development 134:2871–2879 [DOI] [PubMed] [Google Scholar]
- 48. Opitz R , Schmidt F , Braunbeck T , Wuertz S , Kloas W. 2009. Perchlorate and ethylenethiourea induce different histological and molecular alterations in a non-mammalian vertebrate model of thyroid goitrogenesis. Mol Cell Endocrinol 298:101–114 [DOI] [PubMed] [Google Scholar]
- 49. Semyonov J , Park JI , Chang CL , Hsu SY. 2008. GPCR genes are preferentially retained after whole genome duplication. PLoS One 3:e1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kratz E , Eimon PM , Mukhyala K , Stern H , Zha J , Strasser A , Hart R , Ashkenazi A. 2006. Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ 13:1631–1640 [DOI] [PubMed] [Google Scholar]
- 51. Song Y , Driessens N , Costa M , De Deken X , Detours V , Corvilain B , Maenhaut C , Miot F , Van Sande J , Many MC , Dumont JE. 2007. Roles of hydrogen peroxide in thyroid physiology and disease. J Clin Endocrinol Metab 92:3764–3773 [DOI] [PubMed] [Google Scholar]
- 52. De Deken X , Wang D , Dumont JE , Miot F. 2002. Characterization of ThOX proteins as components of the thyroid H2O2-generating system. Exp Cell Res 273:187–196 [DOI] [PubMed] [Google Scholar]
- 53. Ameziane-El-Hassani R , Morand S , Boucher JL , Frapart YM , Apostolou D , Agnandji D , Gnidehou S , Ohayon R , Noël-Hudson MS , Francon J , Lalaoui K , Virion A , Dupuy C. 2005. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem 280:30046–30054 [DOI] [PubMed] [Google Scholar]
- 54. Grasberger H. 2010. Defects of thyroidal hydrogen peroxide generation in congenital hypothyroidism. Mol Cell Endocrinol 322:99–106 [DOI] [PubMed] [Google Scholar]
- 55. Herzog W , Sonntag C , Walderich B , Odenthal J , Maischein HM , Hammerschmidt M. 2004. Genetic analysis of adenohypophysis formation in zebrafish. Mol Endocrinol 18:1185–1195 [DOI] [PubMed] [Google Scholar]
- 56. Stein SA , Shanklin DR , Krulich L , Roth MG , Chubb CM , Adams PM. 1989. Evaluation and characterization of the hyt/hyt hypothyroid mouse. II. Abnormalities of TSH and the thyroid gland. Neuroendocrinology 49:509–519 [DOI] [PubMed] [Google Scholar]
- 57. Stein SA , Zakarija M , McKenzie JM , Shanklin DR , Palnitkar MB , Adams PM. 1991. The site of the molecular defect in the thyroid gland of the hyt/hyt mouse: abnormalities in the TSH receptor-G protein complex. Thyroid 1:257–266 [DOI] [PubMed] [Google Scholar]
- 58. Bill BR , Petzold AM , Clark KJ , Schimmenti LA , Ekker SC. 2009. A primer for morpholino use in zebrafish. Zebrafish 6:69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mascia A , Nitsch L , Di Lauro R , Zannini M. 2002. Hormonal control of the transcription factor Pax8 and its role in the regulation of thyroglobulin gene expression in thyroid cells. J Endocrinol 172:163–176 [DOI] [PubMed] [Google Scholar]
- 60. Nguyen LQ , Kopp P , Martinson F , Stanfield K , Roth SI , Jameson JL. 2000. A dominant negative CREB (cAMP response element-binding protein) isoform inhibits thyrocyte growth, thyroid-specific gene expression, differentiation, and function. Mol Endocrinol 14:1448–1461 [DOI] [PubMed] [Google Scholar]
- 61. Fournie JW , Wolfe MJ , Wolf JC , Courtney LA , Johnson RD , Hawkins WE. 2005. Diagnostic criteria for proliferative thyroid lesions in bony fishes. Toxicol Pathol 33:540–551 [DOI] [PubMed] [Google Scholar]
- 62. Abu-Khudir R , Paquette J , Lefort A , Libert F , Chanoine JP , Vassart G , Deladoëy J. 2010. Transcriptome, methylome and genomic variations analysis of ectopic thyroid glands. PLoS One 5:e13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kimmel CB , Ballard WW , Kimmel SR , Ullmann B , Schilling TF. 1995. Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310 [DOI] [PubMed] [Google Scholar]
- 64. David R , Wedlich D. 2001. PCR-based RNA probes: a quick and sensitive method to improve whole mount embryo in situ hybridizations. Biotechniques 30:769–774 [PubMed] [Google Scholar]
- 65. Thisse C , Thisse B. 2008. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3:59–69 [DOI] [PubMed] [Google Scholar]
- 66. Kwan KM , Fujimoto E , Grabher C , Mangum BD , Hardy ME , Campbell DS , Parant JM , Yost HJ , Kanki JP , Chien CB. 2007. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236:3088–3099 [DOI] [PubMed] [Google Scholar]
- 67. Villefranc JA , Amigo J , Lawson ND. 2007. Gateway compatible vectors for analysis of gene function in the zebrafish. Dev Dyn 236:3077–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kawakami K , Takeda H , Kawakami N , Kobayashi M , Matsuda N , Mishina M. 2004. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell 7:133–144 [DOI] [PubMed] [Google Scholar]