Abstract
Corticosteroid treatment is an established therapy for preterm infants, and germline inactivation of the glucocorticoid receptor (GR) gene in the mouse leads to respiratory failure and postnatal lethality. Although glucocorticoids have been thought to critically act in epithelial cells inducing the functional maturation of the lung, inactivation of the GR gene exclusively in the epithelium of the developing murine lung did not impair survival. In contrast, mice lacking GR specifically in mesenchyme-derived cells displayed a phenotype strongly reminiscent of GR knockout animals and died immediately after birth. Detailed analysis of gene expression allows the conclusion that GR acts in cells of the fibroblast lineage controlling their proliferation rate and the composition of the extracellular matrix.
Successful transition to air breathing at birth strictly depends on the adequate functional maturation of the respiratory system in utero. Accordingly, fetal lung development is a complex and highly regulated process orchestrating branching morphogenesis and growth but also differentiation and maturation to ultimately provide the extensive gas exchange area critical for postnatal survival (1). A vast body of evidence from studies in humans and animals illustrates the pivotal role of glucocorticoid signaling during pre- and postnatal lung maturation (2). Clinically, prenatal exposure to therapeutic doses of corticosteroid is employed to reduce incidence and severity of respiratory distress syndrome and bronchopulmonary dysplasia resulting in increased perinatal survival (3). However, although this therapy is now accepted as standard of care, pharmacological doses of glucocorticoids are known to be associated with considerable side effects. Experiments in rodents, primates, and humans have revealed that glucocorticoid treatment may lead to growth retardation, premature differentiation of several organs, and an increased risk for cardiovascular disease as well as mental disorders such as unipolar depression (4, 5). Thus, further insights into the mechanisms of glucocorticoid action in the fetal lung are desirable and might even provide cell-specific alternative strategies to treat preterm infants.
Glucocorticoid effects are mediated by the glucocorticoid receptor (GR), which acts as a ligand-dependent transcription factor and controls target gene expression by DNA-binding-dependent as well as -independent mechanisms (6). In line with its beneficial effects upon pharmacological stimulation, disruption of glucocorticoid signaling by germline inactivation of the GR gene in the mouse leads to respiratory failure and postnatal lethality (7). Intriguingly, mice carrying a point mutation that selectively impairs homodimeric binding of GR to its cognate response elements survive, indicating that the essential functions of GR during murine lung development are mediated via protein-protein interactions rather than DNA binding (8).
In the developing lung, morphogenesis and maturation depend on a delicate signaling network involving various cell types of epithelial and mesenchymal origin (9). To identify the cell type in which GR mediates its critical effects, the Cre/loxP recombination system was used to achieve conditional gene inactivation. A series of mutant mice was generated, lacking GR in the mesenchyme, endothelial cells, or the lung epithelium, respectively, thus allowing the assessment of the relative contribution of these compartments to the phenotype of the germline mutation. We demonstrate that, in contrast to common thinking, GR activity in lung epithelial cells is contributing to murine lung development but not quintessential whereas GR expressed in mesenchymal cells is essential to promote terminal maturation of the respiratory system.
Results
Beneficial effects of corticosteroids for lung development have commonly been attributed to their ability to induce the functional maturation of lung epithelial cells (2). To further investigate their importance, we used the Cre/loxP recombination system to generate mice lacking the GR specifically in epithelial cells. (For a detailed description of the Cre/loxP recombination system and its application see Ref. 10.) To this end, Spc-Cre mice expressing the Cre recombinase under control of the human Sftpc (surfactant-associated protein; c = Spc) promoter were intercrossed with GRflox animals (11, 12). Surprisingly, the resulting GRfl/fl;Spc-Cre mice, abbreviated as GRSpc-Cre mice, were born with Mendelian frequency and survived to adulthood (for detailed breeding record, see Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org).
The efficacy of the Spc-Cre line has been characterized before (11), and the successful ablation of GR protein specifically in epithelial cells of the developing lung was confirmed (Fig. 1, A and B). Despite survival of mutant offspring and their macroscopically unaltered lung development, further histological analysis revealed a delayed progression through lung development in GRSpc-Cre embryos. At embryonic d 18.5 (E18.5), mutant animals displayed less dilated alveolar saccules and correspondingly a higher frequency of both, unexpanded rosettes as well as regions with mesenchymal accumulation (Fig. 1, E and F). Morphometric measurements on semithin sections revealed a significant increase of the mesenchymal compartment at the expense of the alveolar area (Fig. 1I). Furthermore, electron microscopy showed that the thinning of the alveolar septae is incomplete in GRSpc-Cre animals at E18.5, resulting in an increased thickness of the alveolar walls (Fig. 1, J and K). However, Figs. 1, J and K, show as well that despite thickened septae, a rather close apposition of the epithelial and endothelial layers was achieved in GRSpc-Cre lungs generating a diffusion distance sufficient for gas exchange. Expression analysis of epithelial differentiation markers such as proSpc, T1α, or glycogen (the latter only associated with undifferentiated epithelia) revealed no significant differences in mutant compared with control animals (exemplary analysis of proSpc shown in Supplemental Fig. 1; data not shown for T1α and glycogen). In conclusion, these findings demonstrate that in mice, lung epithelial GR is contributing to the functional maturation of the alveolar gas exchange unit but in a modulating and not seminal manner.
Fig. 1.
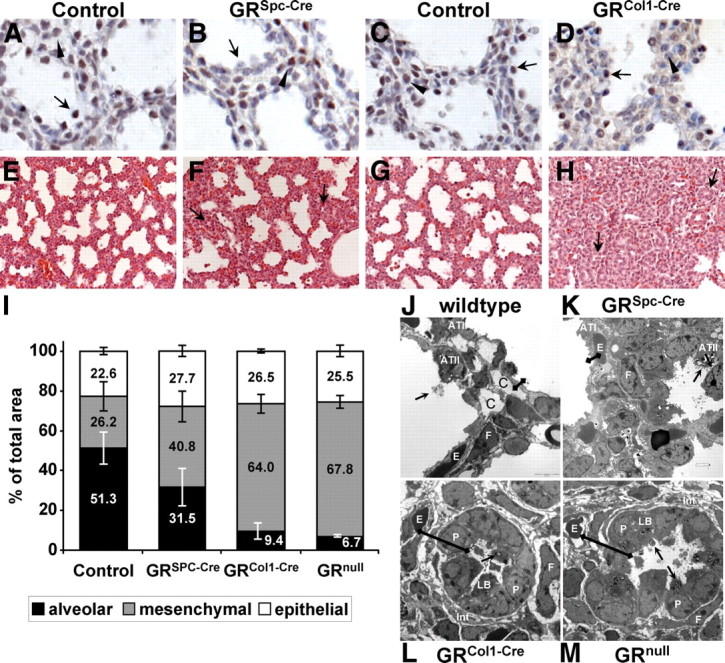
Morphogenetic phenotypes of mice lacking GR in the epithelium and the mesenchyme, respectively. A–D, Immunohistochemistry for GR in epithelium-specific GRSpc-Cre (B) and mesenchyme-specific GRCol1-Cre mutants (D) as well as their littermates (A and C) demonstrates specific inactivation of the GR gene. E17.5 lungs of controls and GRSpc-Cre animals were compared with P0 GRCol1-Cre mutants due to their morphological similarity. Exemplary epithelial cells are indicated by arrows and mesenchymal cells are indicated by arrowheads. E–H, Hematoxylin-eosin staining of paraffin sections from E18.5 GRSpc-Cre (F) and GRCol1-Cre (H) mutants and control littermates (E and G). Unexpanded rosettes are indicated by arrows. I, Morphometric measurements on semithin sections describing the relative contributions of the epithelial, mesenchymal, and alveolar compartments to the total area. Epithelial compartments did not reveal significant differences between any of the genotypes. For the mesenchymal as well as the alveolar compartment, all mutants displayed a significant difference compared with their littermate controls. Moreover, the mesenchyme-specific GRCol1-Cre and the GRnull mutants were significantly different from the epithelium-specific GRSpc-Cre mutants. For each genotype, three to five embryos were analyzed using at least three sections per specimen. J–M, Transmission electron microscopy of lungs from wild-type, conditional GR mutants, and GR knockout animals: P, epithelial precursor cells; F, fibroblasts; E, erythocytes; C, capillaries; LB, lamellar bodies; Int, interstitium with ECM deposition and loose cell contacts; arrows indicate lamellar bodies containing surfactant being secreted into the alveolar lumen; the distance between the alveolar lumen and alveolar capillaries is illustrated by black bars. The entire ring of epithelial progenitor cells in panels L or M reflects the notion of unexpanded rosettes as they have been marked by arrows in panel H.
Consequently, we hypothesized that GR serves critical functions in the lung mesenchyme. Mice lacking GR specifically in mesenchymal cells were generated using a mouse line that expresses the Cre from a BAC containing the locus of the collagen type (I)-α-2 gene (Col1-Cre) (13). Intriguingly, no mutant GRfl/fl;Col1-Cre mice, abbreviated as GRCol1-Cre, mice were obtained at weaning (see Supplemental Table 1 for detailed breeding record), and observation of delivering mothers revealed that mutant neonates died within the first hour after birth displaying respiratory distress and cyanosis reminiscent of the GR-knockout phenotype (7). Importantly, immunohistochemistry demonstrated that GR protein expression in the epithelium of P0 lungs from GRCol1-Cre animals was preserved (Fig. 1, C and D; note that control E17.5 embryos are compared with mutant P0 embryos due to morphological similarity).
Histological examination revealed that lungs of GRCol1-Cre mice failed to progress through the canalicular and saccular phase and still displayed the primitive morphology of the pseudoglandular phase at E18.5 (Fig. 1, G and H). At this stage, alveolar space was virtually absent in GRCol1-Cre lungs and the mostly unexpanded alveolar tubes were surrounded by thick mesenchyme. Morphometry revealed that, in control sections, about 50% of the total area was alveolar space, about 25% was mesenchyme and 25% was epithelium, respectively. In GRCol1-Cre lungs, the alveolar compartment was decreased to less than 10% of the total area whereas the mesenchyme expanded to more than 60% (Fig. 1I) Similar changes were present in GRnull embryos (Fig. 1I), revealing a close qualitative and quantitative similarity of the phenotypes observed in the mesenchyme-specific and the global GR-knockout animals.
Histological analysis by periodic acid Schiff staining and immunostaining for epithelial markers such as T1α demonstrated that the proximo-distal patterning of the respiratory tube was not affected in GRCol1-Cre animals (Supplemental Fig. 2). Similarly, initial quantification of numbers and relative proportions of bronchi and alveolar tubes until E16.5 did not reveal obvious differences, indicating that branching morphogenesis was normal up to this point. However, distal epithelial differentiation was significantly delayed as electron microscopy revealed that alveolar type I epithelial cells (ATI) were completely missing and alveolar type II epithelial cells (ATII) displayed the typical cuboidal morphology of epithelial precursor cells (Fig. 1L and Supplemental Fig. 3 for higher magnifications). In comparison with wild-type or GRSpc-Cre mice, this led to a strongly increased distance between the alveolar space and the pulmonary capillaries (black bars in Fig. 1, J–M) but the ATII cells still possessed the ability to synthesize and secrete surfactant-containing lamellar bodies. Between the unexpanded rosettes of the epithelium, the mesenchymal cells appeared disorganized, formed only loose contacts, and were surrounded by extensive deposition of interstitial extracellular matrix (ECM). Virtually indistinguishable observations were made in lungs of GRnull embryos (Fig. 1M) supporting that GRCol1-Cre animals recapitulate the lung phenotype observed in the germline mutation. It should be noted that no obvious alterations were noted in other organs than the lung upon routine histological examination of GRCol1-Cre mutants.
Having identified the mesenchyme as the critical site for glucocorticoid activity we aimed to further specify the responsible cell type in a next step, considering as most likely candidates either the capillary system, smooth muscle cells, alveolar myofibroblast, or lipofibroblasts.
The capillary system surrounding the alveoli arises through vasculogenesis from the primitive lung mesenchyme and is one of its major derivatives (14). GRTie2-Cre mice lacking GR specifically in the endothelium were therefore generated but showed neither respiratory distress nor other obvious dysfunctions after birth (15). Mutant E18.5 embryos were analyzed for pulmonary maturation in comparison with wild-type embryos, but no significant differences were noted upon histological examination (insets in Fig. 2, A and B). These results exclude that GR serves an essential function for fetal lung maturation through actions in endothelial cells.
Fig. 2.
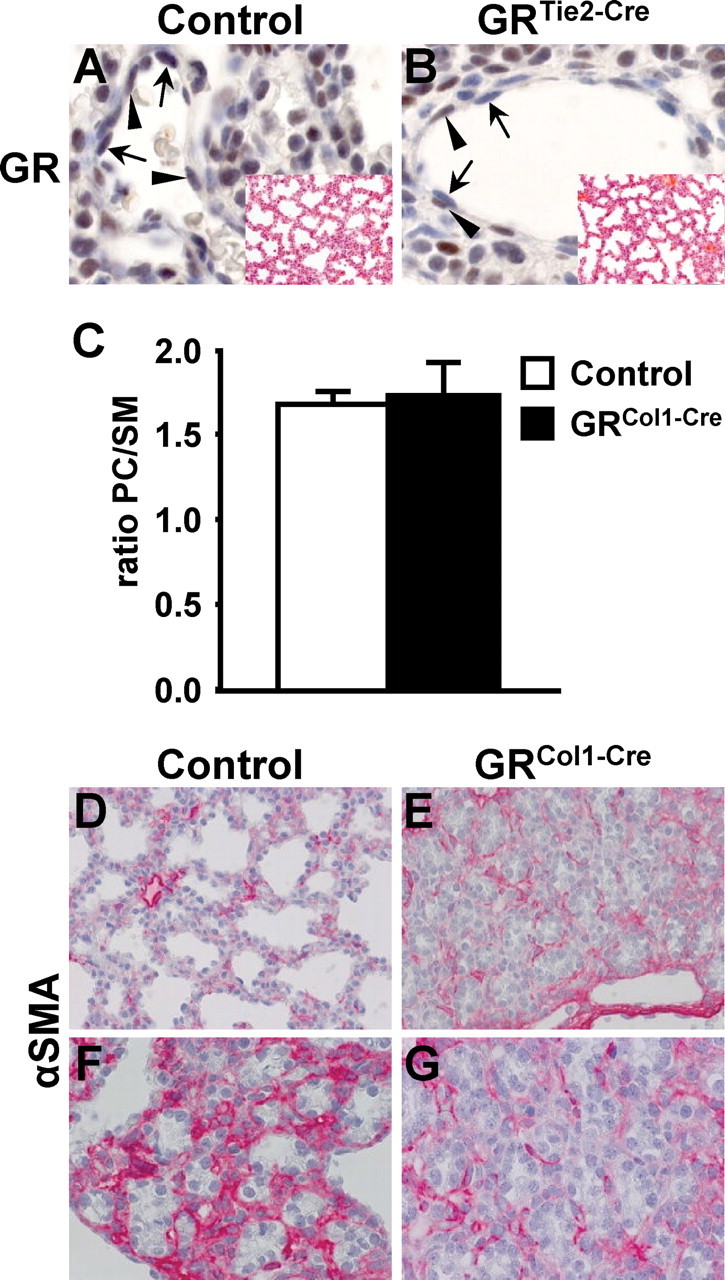
Specification of the cell type providing critical GR activities within the mesenchyme indicates an essential role for alveolar myofibroblast but not endothelial cells or lipofibroblasts. A and B, Immunohistochemistry on sections from E18.5 GRTie2-Cre embryonic lungs demonstrates conditional inactivation of the GR gene in endothelial cells (arrows) whereas GR expression (brown staining) is preserved in pericytes (arrowheads) and other cell types. Insets show hematoxylin-eosin stainings of the same animals, demonstrating normal lung development in GRTie2-Cre embryos in the absence of endothelial GR. C, Phospholipid content of amniotic fluid from E18.5 GRCol1-Cre and control embryos was determined. The graph shows that the ratio of PC to SM is comparable in mutant and control animals. D–G, Immunohistochemistry for α-smooth muscle actin (α-SMA) was applied to label myofibroblast in E18.5 embryos. Overviews (D and E) show continuous weak staining in GRCol1-Cre mutant as well as control animals. Higher magnification images illustrate the lower staining intensity in mutants (G) compared with the distal-most regions in control lungs (F), which resemble the general appearance of mutant lungs in terms of morphology.
Because branching morphogenesis is not altered in GRCol1-Cre animals and the mutation affects primarily the differentiation and dilation of the most distal alveolar saccules, smooth muscle cells are unlikely to participate significantly in the glucocorticoid-mediated maturation of the fetal lung (16).
The exclusion of the above cell types implies that GR exerts its essential function during lung development in alveolar interstitial cells. These cells are still incompletely understood today, but two major cells, alveolar myofibroblasts and lipofibroblasts, are importaht in this context. They have been proposed to be predominantly characterized by their ability to provide contractile forces and lipid supply to the alveolar unit, respectively (17). Although the direct involvement of lipofibroblasts in epithelial surfactant synthesis has not formally been proven, we assumed that a critical role of GR in pulmonary lipid supply should be reflected in an altered lipid composition of the surfactant in mesenchyme-specific GR mutants. In particular, phospholipids form an important constituent of surfactant and the ratio of phosphatidylcholine (PC) to a sphingomyeline (SM) baseline is clinically used to predict the risk of pulmonary complications in preterm infants. During normal pulmonary maturation, the synthesis of PC is induced toward term; however, analysis of the phospholipid content of the amniotic fluid of E18.5 GRCol1-Cre embryos revealed no significant alterations in mutant animals compared with controls (Fig. 2C). This finding indicates that supply and synthesis of surfactant lipids is not dependent on mesenchymal GR; instead, it points toward the alveolar myofibroblast as the predominant site of essential glucocorticoid actions.
To specifically label myofibroblasts, the expression of α-smooth muscle actin was analyzed by immunohistochemistry (Fig. 2, D–G). At E18.5, virtually all mesenchymal cells showed continuous but weak immunoreactivity with a few cells displaying more prominent signals in both control as well as mutant lungs (Fig. 2, D and E). However, the most distal regions in control lungs were characterized by a strong activation of the mesenchymal fibroblasts (Fig. 2, F and G). This allows the hypothesis that regulation of fibroblast activity is a prerequisite for the subsequent alveolar remodeling and dilation observed in controls and that the failure to influence fibroblast activity in the absence of functional GR contributed to the morphogenetic defects of the GRCol1-Cre mutant lungs.
Because ablation of GR specifically in alveolar myofibroblast was precluded by the lack of Cre-transgenic mouse lines with the desired specificity, further analysis, in particular gene expression profiling, was done on the mesenchyme-specific GRCol1-Cre mutants. Two time points were chosen for expression profile analysis, E16.5 and E18.5, which allowed monitoring the transcriptional changes associated with the progression through the canalicular and saccular phase of murine lung development in control embryos, as well as an assessment to which extent these processes are affected by the mesenchyme-specific loss of GR in the mutants (Fig. 3A). For each of the four conditions, five GeneChips were used, each in turn representing one animal. To identify GR-regulated genes associated with the observed phenotypical changes in GRCol1-Cre mice, probe sets were selected that were differentially regulated in controls from E16.5–E18.5, did not respond with a significant change in mutant lungs during the same period, and furthermore showed a significant difference between the two genotypes with respect to the expression changes between the two time points. (Significance: more than 2-fold change and P value <0.001). Subsequent gene ontology analysis revealed that the majority of these differentially expressed genes could be classified in two major topics, proliferation and ECM and therefore, these aspects were analyzed in more detail.
Fig. 3.
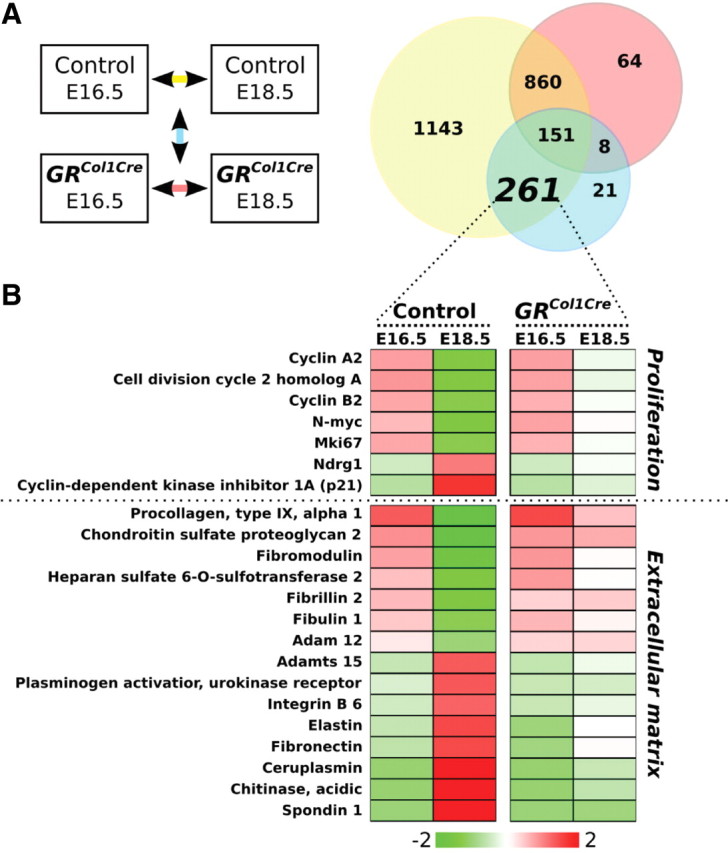
Gene expression profiling in mesenchyme-specific GRCol1-Cre and control lungs. A, Observed expression changes are illustrated in a Venn diagram. The numbers within the circles represent those genes showing a significant change in expression from E16.5–E18.5 in lungs of controls (yellow) and/or in GRCol1-Cre mice (red). In addition, it is taken into consideration whether there is a significant quantitative difference between the changes observed in the two genotypes, and the number of those genes is found in the blue circle. Significance is defined as change more than 2-fold combined with a P value <0.001 (t test) and for each of the four conditions, five GeneChips were used, each representing one embryo. B, Probe sets of the indicated area were subjected to Gene Ontology analysis. The vast majority of gene ontologies could be classified in two topics, proliferation and ECM, and representative examples are given in a heat map.
From E16.5–E18.5, GRCol1-Cre animals failed to down-regulate genes important for the progression through the cell cycle such as cyclins A2, B2. or Cdc2A (cell division cycle 2 homolog A) and Cdc20, whereas the induction of well-described differentiation markers was absent. In particular, the expression of the cyclin-dependent kinase inhibitor p21cip was strongly increased in control animals from E16.5–E18.5 and remained virtually unchanged in mutants. p21cip is regarded as an important regulator promoting terminal differentiation and inhibiting proliferation in many systems (18) and was shown to be regulated by glucocorticoids in lung fibroblasts and other cell types (19).
These findings of cell cycle dysregulation were corroborated by in vivo bromodeoxyuridine (BrdU) labeling of proliferating cells and consecutive histological analysis. The proliferation index was found to be significantly increased in lungs of GRCol1-Cre mice compared with control lungs (12.8% vs. 5.7%, respectively) whereas GRSpc-Cre mice displayed a proliferation rate comparable to their control littermates (5.6% vs. 4.6%, respectively) (Fig. 4 A). Subsequent calculation of separate values for the epithelium and the mesenchyme, respectively, revealed that the proliferation index increased for the GRCol1-Cre mutants in the two compartments to a similar extent (2.3-fold in the epithelium; 2.0-fold in the mesenchyme). This indicates that mesenchymal loss of GR does not exclusively regulate proliferation in the mesenchyme but leads to an increase in the general proliferation rate of the developing lung including the epithelium.
Fig. 4.
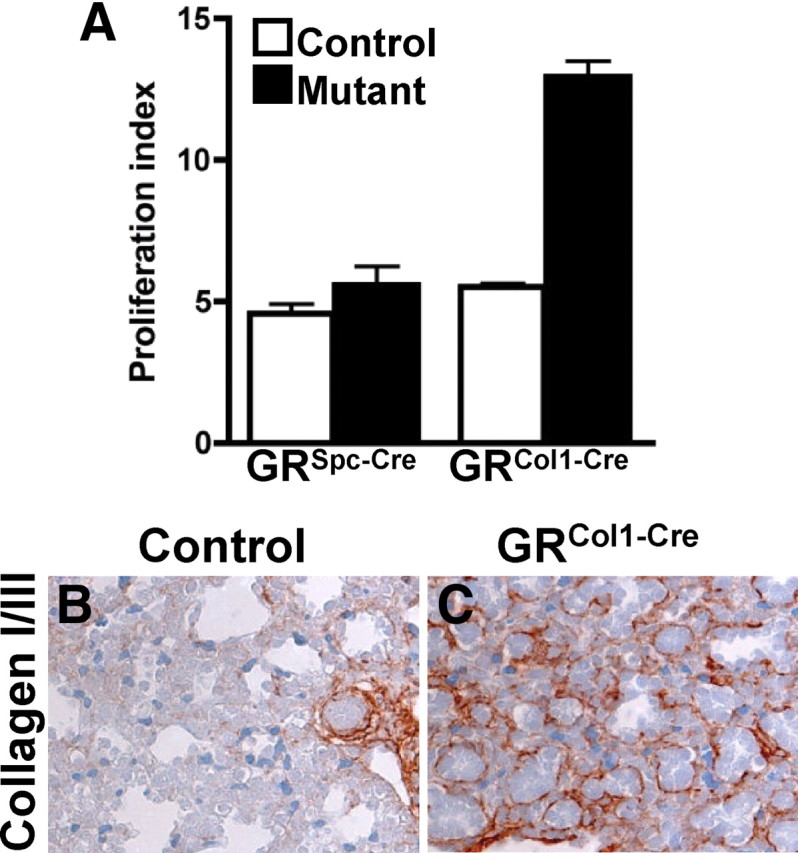
Lungs of GRCol1-Cre animals show an increase in the proliferation rate and changes in the composition of the ECM. A, Mothers were injected with BrdU 2 h before embryo collection, and proliferating cells were identified by immunohistochemistry. The proliferation index is defined as the percentage of BrdU-positive cells of the total cell number. Each bar represents three animals. B and C, Immunohistochemistry shows continuous expression of collagens I and III until E18.5 in GRCol1-Cre mutant lungs. In contrast, maturation coincides with loss of detectable collagen fibers in lungs of control littermates.
Gene expression profiling also revealed a massive dysregulation of ECM components as well as ECM-modifying enzymes such as chitinase 3 or lysyloxidase 1 (Fig. 3B). The observed changes affected all major constituents of the lung ECM, i.e. collagens (e.g. collagen type VI), elastic fibers (elastin, fibrillin 2, and lysyl oxidase 1), and proteoglycans (chondroitin sulfate proteoglycan 2), in addition numerous cell surface molecules that interact with the ECM, for instance integrins, were significantly increased. Immunohistochemically, these changes in the overall composition of the ECM were exemplified using an antibody against collagens I/III, demonstrating persistent and strong staining of collagen fibers in GRCol1-Cre animals (Fig. 4C). In control animals instead, pulmonary maturation coincided with reduced immunoreactivity for these collagens, and prominent staining was only observed around blood vessels and airways (Fig. 4B).
For many of the ECM genes identified by the expression profiling, important functions during lung development have been identified by in vivo models. For example, the induced expression of elastin as well as its major cross-linking enzyme lysyloxidase 1 was significantly attenuated in GRCol1-Cre animals, and knockout studies have demonstrated an essential role during alveolar maturation for both genes (20, 21). In line with this, Goldner Trichrome-Elastica staining revealed that the formation of elastic fibers could not be detected in the distal part of the lungs of GRCol1-Cre animals whereas elastogenesis around larger bronchi and vessels was unaffected (Supplemental Fig. 4).
Finally, it deserves mentioning that the gene expression profiling was in line with our above findings that surfactant synthesis was not significantly affected by mesenchymal GR loss. Although some of the proteins involved in or regulating phospholipid synthesis such as fatty acid synthase or phosphocholine cytidyltransferase displayed a mildly attenuated expression, neither of them showed a significant change. Similarly, none of the surfactant-associated proteins showed a statistically significant differential expression in GRCol1-Cre animals compared with their wild-type littermates (data not shown).
Discussion
Progression through the later phases of murine lung development requires a developmental switch from the pseudoglandular phase characterized by proliferation and growth, toward growth arrest and differentiation, which are necessary for terminal maturation during the canalicular and saccular stage (22). This process coincides with an increase of pulmonary GR expression as well as a surge in fetal corticosteroid levels observed from E16.5 until birth. Gene targeting experiments in the present study confirm that GR exerts its key function during lung morphogenesis by initiating the transition to the maturational phase of terminal differentiation.
In most studies, GR has been considered to exert its critical functions during the maturation of the lung in epithelial cells (2). Glucocorticoids have been shown to induce the synthesis and secretion of pulmonary surfactant in epithelial ATII cells, reducing the surface tension within the alveolus and thus preventing alveolar collapse (23). Moreover, corticosteroid receptors are known to induce the transepithelial sodium and water transport via regulation of the epithelial sodium channel (24, 25), which is essential for the rapid clearance of lung liquid during the perinatal transition from a fluid-filled lung to air breathing (26). However, the present work demonstrated that epithelial GR only has a positive modulating role for murine lung development, demonstrated by a delayed progression through lung development observed in mice lacking lung epithelial GR.
During preparation of this manuscript, Manwani et al. (27) reported that lung epithelium-specific loss of GR in mouse embryos leads to reduced viability at birth. The authors used a doxycycline-inducible system and found an increase in neonatal lethality from apparently 15% in doxycycline-treated littermate controls to about 55% in epithelium-specific GR mutants. Given the similarity of the observed morphological phenotypes to results in our study, the differences in susceptibility may be explained by strain effects (C57B/6 in our case; information not available for Manwani et al.) or a combined effect of doxycycline and gene ablation. Morimoto and Kopan (28) have shown specifically for the lung epithelium that expression of the reverse tetracycline transactivator and doxycycline treatment during pregnancy have adverse effects on embryonic lung development including impaired alveologenesis, loss/reduction in expression of surfactant-associated proteins, and death. Although Manwani et al. used a doxycycline regimen considered to be safe by Whitsett and Perl (29) the adverse effects of the doxycycline-inducible system or other unfavorable conditions together with the delayed progression through lung development caused by lung epithelium-specific GR ablation may have led to the observed lethality.
In contrast, the present study uses conditional gene inactivation in the mesenchyme to recapitulate the striking morphological phenotype observed in GRnull animals, establishing that GR serves its essential role in the mesenchyme and more precisely in cells of the fibroblast lineage. Interestingly, historic experiments by Smith (30) showed that maximal induction of surfactant synthesis by glucocorticoids in lung epithelial cell lines was dependant on the coculture of lung fibroblasts, leading to the suggestion of a so-called fibroblast pneumocyte factor. Although the latter has never been identified, a number of signaling molecules with appropriate properties have been described. In particular, fibroblast growth factor 7 is expressed in mesenchymal cells and has been shown to be induced by glucocorticoids in cultured pulmonary fibroblasts. Conditioned medium of these cells, in turn, exerted a stimulating effect on the surfactant synthesis in cultured lung epithelial cells (31). Fibroblast growth factor 7 was found to be differentially regulated in GRCol1-Cre lungs compared with control littermates; however, these changes did not reach significance. In a comparable way, other signaling pathways important for lung development and known to interact with glucocorticoid signaling such as TGFβ and Shh signaling showed considerable tendencies and alterations but were not significantly changed as a whole. Hence, our results did not allow identification of a single pathway responsible for the observed changes but rather point toward GR exerting its critical role via numerous interactions within the intensive signaling network governing proliferation and differentiation in the developing lung.
We propose an additional, potentially critical mechanism by which glucocorticoid signaling might influence lung development. The present study revealed that glucocorticoid signaling in lung mesenchyme exerted a major impact on the composition of the ECM and the activation of alveolar myofibroblasts. Because this affected all major constituents of the lung ECM as well as numerous cell surface molecules that interact with the ECM, the morphogenetic development of the lungs might have been influenced in various ways: The ECM provides structural support, which is particularly critical for a largely hollow organ as the lung, and becomes additionally important during processes such as cell adhesion and migration (32–34). Moreover, lung maturation involves aspects of remodeling such as the attenuation of the epithelium or the thinning of the alveolar septae, including the close apposition of basement membranes of capillaries and alveoli. These processes can be expected to depend on the capacity of the interstitial matrix to change with regard to elasticity and contraction. In addition, ECM functions have more recently been extended to the modulation of growth factor signaling by multiple modes such as enzymatic activation or inactivation of signaling molecules but also their sequestration or release. Thereby, the composition of the ECM influences the availability and activity of these molecules and hence determines their range of action (35).
In summary, glucocorticoid signaling may interact with a number of signaling pathways critically modulating epithelial-mesenchymal interactions in the developing lung. These results provide important new aspects of glucocorticoid action during lung development and may warrant careful analysis of the mesenchymal components during corticosteroid therapy in preterm infants.
Materials and Methods
Mouse work
Mice were housed according to international standard conditions, and all experiments conformed to local and international guidelines for the use of experimental animals. All employed mouse lines, GRflox, GRnull, Spc-Cre, Col1-Cre, and Tie2-Cre, have been described previously and were maintained on a C57Bl6 background (7, 11–13, 15). Heterozygous GRnull/+ animals or GRflox/+/Cre and GRflox/flox animals were bred to generate mutants (GRnull/null, denotated GRnull, or GRflox/flox/Cre, denotated GRCre, respectively) and littermate controls (GR+/+ and GRflox/flox, respectively). Staged matings were set up to harvest embryonic organs at desired stages of fetal development. In the case of a vaginal plug in the morning, noon of the same day was designated E0.5. At the embryonic day of interest, mothers were killed by CO2 and the pups were collected by Caesarean section. Complete uteri were removed and transferred to ice-cold PBS, and embryonic lungs were dissected out.
Histology and immunohistochemistry
Specimens were fixed in 4% paraformaldehyde overnight, dehydrated, and embedded in paraffin for sectioning. Hematoxylin-eosin, periodic-acid-Schiff, and Goldner Trichrome-Elastica stainings were performed according to standard protocols. For immunohistochemistry, the following primary antibodies were used: polyclonal rabbit anti-GR (1:200) (M-20, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), monoclonal hamster anti-T1α (1:500) (clone 8.1.1, Developmental Studies Hybridoma Bank), polyclonal rabbit anti-Spc (1:500) (Upstate Biotechnology, Inc., Lake Placid, NY), polyclonal rabbit anticollagen I/III (1:20) (no. 2150–2555; BIOTREND Chemicals, LLC, Destin, FL); mouse anti-α-smooth muscle actin (mouse ascites fluid, clone 1A4, no.2547; Sigma, St. Louis, MO). Sections were further processed using appropriate secondary antibodies and the VECTASTAIN ABC system (Vector Laboratories) followed by staining with diaminobenzidine (Sigma).
Morphometric measurements
Specimen were fixed in Karnovsky's solution for 24 h at room temperature, dehydrated in an increasing ethanol gradient and embedded in Araldit. Semithin sections were prepared on a Leica Ultracut UCT with a thickness of 500 nm and stained with toluidine blue. Per genotype, three to five embryos were analyzed and for each, at least three pictures of different localizations within the lung were acquired and evaluated using the processing and analysis software imageJ (available at http://rsb.info.nih.gov/ij/index.html). Note that the alveolar area comprises only airspace and excludes the epithelial layer.
Electron microscopy
Specimen were fixed in Karnovsky's solution for 24 h at room temperature, dehydrated in an increasing ethanol gradient, and embedded in Araldit. Sections were prepared on a Leica Ultracut UCT with a thickness of 70 nm and contrasted with uranyl acetate and lead citrate before inspection with the Zeiss EM900 (Carl Zeiss, Thornwood, NY).
Lipid analysis
Amniotic fluid was collected from E18.5 embryos, and lipid extractions were performed in the presence of internal PC and SM standards as described elsewhere (36). After solvent evaporation, samples were resuspended in 10 mm ammonium acetate in methanol. Nano-ESI-tandem mass spectrometry analysis was performed on a Micromass QII triple-stage quadrupol tandem mass spectrometer, equipped with a nano-ESI source (Z spray) from Micromass/Waters (Manchester, UK). Argon was used as collision gas at a nominal pressure of 2.5 × 10−3 millibar. The cone voltage was set to 30 V. Resolution of Q1 and Q3 was set to achieve isotope resolution. Detection of PC and SM was performed by precursor ion scanning for fragment ion m/z 184 (positive ion mode) at collision energy 32 eV.
Determination of the proliferation index
Pregnant mothers were injected ip with 300 mg BrdU/kg body weight 2 h before the embryo collection by Caesarean section. Specimens were processed for immunohistochemistry as described above, and proliferating cells were detected with a monoclonal mouse anti-BrdU antibody (Bu20a; DAKO, Carpinteria, CA). The proliferation index is defined as the percentage of BrdU-positive cells per total cell number. For both mutations, three embryos were analyzed for mutants and controls, respectively, and per specimen, in total nine photographs from three different sections were used for counting.
RNA analyses: gene expression profiling
Profiling of gene expression was performed on lungs from GRCol1-Cre animals and controls that were collected as described in Mouse work. Left lung lobes were immersed in RNALater solution (Sigma-Aldrich Chemie GmbH, Munich, Germany), snap frozen in liquid nitrogen, and stored at −80 C. Total RNA was prepared with the RNeasy Mini Kit (QIAGEN, Hilden, Germany) and its quality was assessed on RNA LabChips (Agilent Technologies, Santa Clara, CA). Microarray experiments were carried out using GeneChip Mouse Genome 430A 2.0 arrays (Affymetrix, Santa Clara, CA). Labeling of the target RNA (1–2 μg total RNA), hybridization, and scanning of the microarrays were performed according to manufacturer's instruction.
Two time points, E16.5 and E18.5, were analyzed for mutants and controls, respectively. For each of the four conditions, five GeneChips were used, each representing one animal. Analysis of array data was performed using the R/Bioconductor and MeV 4.0. Data were normalized, and expression values were computed using the GeneChip robust multi-array average method. To identify GR-regulated genes associated with the observed phenotypical changes in GRCol1-Cre mice, probe sets were selected that were differentially regulated in controls from E16.5–E18.5, did not respond with a significant change in mutant lungs during the same period, and furthermore showed a significant difference between the two genotypes with respect to the expression changes between the two time points. Significance was defined as a change greater than 2-fold combined with a P value <0.001 (t test). This gene set was subsequently subjected to gene ontology (GO) analysis using GOstat (http://gostat.wehi.edu.au/).
Acknowledgments
This work was supported by the “Deutsche Forschungsgemeinschaft” through Collaborative Research Centres SFB 488 and SFB 636, the European Union through grant LSHM-CT-2005-018652 (CRESCENDO), the Bundesministerium für Bildung und Forschung (BMBF) through NGFNplus grants FZK 01GS08153 and 01GS08142 and project 0313074C (HepatoSys), the Helmholtz Gemeinschaft Deutscher Forschungszentren through Initiative CoReNe (Network III, E2) and Alliance HelMA (HA-215), and the Deutsche Krebshilfe through project 108567.
Disclosure Summary: The authors declare no conflict of interest.
Footnotes
- ATI
- Alveolar type I epithelial cells
- BrdU
- bromodeoxyuridine
- ECM
- extracellular matrix
- GR
- glucocorticoid receptor
- PC
- phosphatidylcholine
- SM
- sphingomyeline.
References
- 1. Cardoso WV , Lü J. 2006. Regulation of early lung morphogenesis: questions, facts and controversies. Development 133:1611–1624 [DOI] [PubMed] [Google Scholar]
- 2. Whitsett JA , Matsuzaki Y. 2006. Transcriptional regulation of perinatal lung maturation. Pediatr Clin North Am 53:873–887, viii [DOI] [PubMed] [Google Scholar]
- 3. Gilstrap LC , Christensen R , Clewell WH , Dalton ME , Davidson EC , Escobedo MB , Gjerdingen DK , Goddardfinegold J , Goldenberg RK , Grimes DA , Hansen TN , Kauffman RE , Keeler EB , Oh W , Susman EJ , Vogel MG , Avery ME , Ballard PL , Ballard RA , Crowley P , Garite T , Goldenberg RL , Hankins GDV , Jobe AH , Koppe JG , et al. 1995. Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 273:413–4187823388 [Google Scholar]
- 4. Seckl JR. 2004. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol 151(Suppl 3):U49–U62 [DOI] [PubMed] [Google Scholar]
- 5. Reinisch JM , Simon NG , Karow WG , Gandelman R. 1978. Prenatal exposure to prednisone in humans and animals retards intrauterine growth. Science 202:436–438 [DOI] [PubMed] [Google Scholar]
- 6. Beato M , Herrlich P , Schütz G. 1995. Steroid hormone receptors: many actors in search of a plot. Cell 83:851–857 [DOI] [PubMed] [Google Scholar]
- 7. Cole TJ , Blendy JA , Monaghan AP , Krieglstein K , Schmid W , Aguzzi A , Fantuzzi G , Hummler E , Unsicker K , Schütz G. 1995. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 9:1608–1621 [DOI] [PubMed] [Google Scholar]
- 8. Reichardt HM , Kaestner KH , Tuckermann J , Kretz O , Wessely O , Bock R , Gass P , Schmid W , Herrlich P , Angel P , Schütz G. 1998. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 93:531–541 [DOI] [PubMed] [Google Scholar]
- 9. Shannon JM , Hyatt BA. 2004. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol 66:625–645 [DOI] [PubMed] [Google Scholar]
- 10. Tronche F , Kellendonk C , Reichardt HM , Schütz G. 1998. Genetic dissection of glucocorticoid receptor function in mice. Curr Opin Genet Dev 8:532–538 [DOI] [PubMed] [Google Scholar]
- 11. Okubo T , Hogan BL. 2004. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tronche F , Kellendonk C , Kretz O , Gass P , Anlag K , Orban PC , Bock R , Klein R , Schütz G. 1999. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23:99–103 [DOI] [PubMed] [Google Scholar]
- 13. Florin L , Alter H , Gröne HJ , Szabowski A , Schütz G , Angel P. 2004. Cre recombinase-mediated gene targeting of mesenchymal cells. Genesis 38:139–144 [DOI] [PubMed] [Google Scholar]
- 14. Stenmark KR , Gebb SA. 2003. Lung vascular development: breathing new life into an old problem. Am J Respir Cell Mol Biol 28:133–137 [DOI] [PubMed] [Google Scholar]
- 15. Constien R , Forde A , Liliensiek B , Gröne HJ , Nawroth P , Hämmerling G , Arnold B. 2001. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis 30:36–44 [DOI] [PubMed] [Google Scholar]
- 16. Kim N , Vu TH. 2006. Parabronchial smooth muscle cells and alveolar myofibroblasts in lung development. Birth Defects Res C Embryo Today 78:80–89 [DOI] [PubMed] [Google Scholar]
- 17. McGowan SE , Torday JS. 1997. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol 59:43–62 [DOI] [PubMed] [Google Scholar]
- 18. Besson A , Dowdy SF , Roberts JM. 2008. CDK Inhibitors: cell cycle regulators and beyond. Dev Cell 14:159–169 [DOI] [PubMed] [Google Scholar]
- 19. Yang JQ , Rudiger JJ , Hughes JM , Goulet S , Gencay-Cornelson MM , Borger P , Tamm M , Roth M. 2008. Cell density and serum exposure modify the function of the glucocorticoid receptor C/EBP complex. Am J Respir Cell Mol Biol 38:414–422 [DOI] [PubMed] [Google Scholar]
- 20. Mäki JM , Sormunen R , Lippo S , Kaarteenaho-Wiik R , Soininen R , Myllyharju J. 2005. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol 167:927–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wendel DP , Taylor DG , Albertine KH , Keating MT , Li DY. 2000. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol 23:320–326 [DOI] [PubMed] [Google Scholar]
- 22. Warburton D , Bellusci S , De Langhe S , Del Moral PM , Fleury V , Mailleux A , Tefft D , Unbekandt M , Wang K , Shi W. 2005. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr Res 57:26R–37R [DOI] [PubMed] [Google Scholar]
- 23. Mendelson CR. 2000. Role of transcription factors in fetal lung development and surfactant protein gene expression. Annu Rev Physiol 62:875–915 [DOI] [PubMed] [Google Scholar]
- 24. Itani OA , Auerbach SD , Husted RF , Volk KA , Ageloff S , Knepper MA , Stokes JB , Thomas CP. 2002. Glucocorticoid-stimulated lung epithelial Na(+) transport is associated with regulated ENaC and sgk1 expression. Am J Physiol Lung Cell Mol Physiol 282:L631–L641 [DOI] [PubMed] [Google Scholar]
- 25. Nakamura K , Stokes JB , McCray PB. 2002. Endogenous and exogenous glucocorticoid regulation of ENaC mRNA expression in developing kidney and lung. Am J Physiol Cell Physiol 283:C762–C772 [DOI] [PubMed] [Google Scholar]
- 26. Hummler E , Barker P , Gatzy J , Beermann F , Verdumo C , Schmidt A , Boucher R , Rossier BC. 1996. Early death due to defective neonatal lung liquid clearance in α-ENaC-deficient mice. Nat Genet 12:325–328 [DOI] [PubMed] [Google Scholar]
- 27. Manwani N , Gagnon S , Post M , Joza S , Muglia L , Cornejo S , Kaplan F , Sweezey NB. 2010. Reduced viability of mice with lung epithelial-specific knockout of glucocorticoid receptor. Am J Respir Cell Mol Biol 43:599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morimoto M , Kopan R. 2009. rtTA toxicity limits the usefulness of the SP-C-rtTA transgenic mouse. Dev Biol 325:171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whitsett JA , Perl AK. 2006. Conditional control of gene expression in the respiratory epithelium: a cautionary note. Am J Respir Cell Mol Biol 34:519–520 [DOI] [PubMed] [Google Scholar]
- 30. Smith BT. 1979. Lung maturation in the fetal rat: acceleration by injection of fibroblast-pneumonocyte factor. Science 204:1094–1095 [DOI] [PubMed] [Google Scholar]
- 31. Chelly N , Henrion A , Pinteur C , Chailley-Heu B , Bourbon JR. 2001. Role of keratinocyte growth factor in the control of surfactant synthesis by fetal lung mesenchyme. Endocrinology 142:1814–1819 [DOI] [PubMed] [Google Scholar]
- 32. Francis SE , Goh KL , Hodivala-Dilke K , Bader BL , Stark M , Davidson D , Hynes RO. 2002. Central roles of α5β1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol 22:927–933 [DOI] [PubMed] [Google Scholar]
- 33. Nguyen NM , Senior RM. 2006. Laminin isoforms and lung development: all isoforms are not equal. Dev Biol 294:271–279 [DOI] [PubMed] [Google Scholar]
- 34. Sakai T , Larsen M , Yamada KM. 2003. Fibronectin requirement in branching morphogenesis. Nature 423:876–881 [DOI] [PubMed] [Google Scholar]
- 35. ten Dijke P , Arthur HM. 2007. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol 8:857–869 [DOI] [PubMed] [Google Scholar]
- 36. Brügger B , Sandhoff R , Wegehingel S , Gorgas K , Malsam J , Helms JB , Lehmann WD , Nickel W , Wieland FT. 2000. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J Cell Biol 151:507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]


