Follicle Stimulating Hormone and cyclic adenosine 5′-monophosphate enhance the transcription of anti-Müllerian hormone both in human granulosa-luteal cells and in the KK1 granulosa cell line.
Abstract
Anti-Müllerian hormone (AMH), also called Müllerian-inhibiting substance, a member of the TGF-ß family, is responsible for the regression of Müllerian ducts in the male fetus. In females, AMH is synthesized by granulosa cells of preantral and small antral follicles, and production wanes at later stages of follicle maturation. Using RT-PCR in luteal granulosa cells in primary culture and reporter gene techniques in the KK1 granulosa cell line, we show that FSH and cAMP enhance AMH transcription, and LH has an additive effect. Gonadotropins and cAMP act through protein kinase A and p38 MAPK signaling pathways and involve the GATA binding factor-4 and steroidogenic factor-1 transcription factors, among others. The expression profile of AMH and the dynamics of serum AMH after gonadotropin stimulation have been interpreted as a down-regulating effect of FSH upon AMH production by granulosa cells. The specific effect of gonadotropins upon granulosa cells may be obscured in vivo by the effect of FSH upon follicular maturation and by the presence of other hormones and growth factors, acting individually or in concert.
Anti-Müllerian hormone (AMH), also called Müllerian-inhibiting substance, is a member of the TGF-ß family expressed almost exclusively by gonadal somatic cells. Known essentially for its capacity to inhibit the development of Müllerian ducts in male fetuses when secreted by fetal Sertoli cells (reviewed in Ref. 1), AMH has also been detected in granulosa cells, albeit at lower levels (2). AMH expression is initiated in primary follicles, is strongest in preantral and small antral follicles, and wanes subsequently up to ovulation (3–6), becoming nearly undetectable in human follicles larger than 8 mm and disappearing completely in luteal bodies and atretic follicles (7). In large antral follicles, granulosa cells in the cumulus and those close to the antral cavity still express a small variable amount of AMH protein (3, 8–12) and mRNA (5, 6).
Many studies suggest a correlation between the size of the primordial follicle pool and the number of small growing follicles (13), the most active producers of AMH. Indeed, the level of serum AMH is predictive of the age of menopause (14) and of the yield of oocytes after ovarian stimulation (15) and is now considered a reliable marker of ovarian reserve. However, measurement of serum AMH is not an appropriate tool for the study of regulation of AMH production by granulosa cells because its level is modulated not only by the degree of secretion by individual cells but also by the ratio of large vs. small follicles, which varies in response to hormonal stimulation. To achieve our purpose, we chose to study isolated human granulosa-luteal cells obtained from women undergoing in vitro fertilization treatment. However, the low yield does not allow testing of more than one or two treatments per patient. Furthermore, because these cells resist transfection, sensitive reporter gene techniques cannot be used. Therefore, most of our work was carried out using the KK1 mouse luteinized granulosa cell line (16), which has been previously used successfully to study AMH regulation by growth factors (17). A similar reporter gene approach has been instrumental in unraveling the mechanisms of FSH action upon Sertoli cells (18).
Results
AMH expression is up-regulated by cAMP and FSH in human granulosa-luteal cells and in the mouse KK1 granulosa cell line
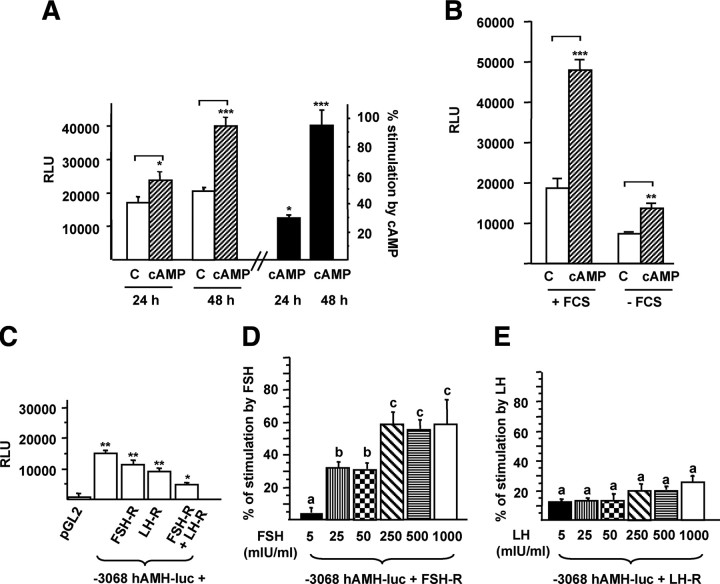
We first investigated by real-time RT-PCR the expression of AMH by human granulosa-luteal cells obtained after ovarian puncture of women undergoing in vitro fertilization treatment. Cells from individual women were studied separately to take into account a variable response to hormonal treatment. Between 6 and 18 × 105 cells were obtained per patient. Because AMH expression by primary culture of Sertoli cells is known to decrease with time (19), we first studied the kinetics of AMH expression. As shown in Fig. 1A, AMH expression fell dramatically after 24 or 72 h of culture in control medium. Addition of FSH or its second messenger cAMP at 24 h increased AMH mRNA by 71 and 94%, respectively, at 72 h (Table 1 and Fig. 1B). LH had no effect (Table 1 and Fig. 1B). Similar results were obtained for cAMP in the granulosa cell line KK1 obtained by targeted oncogenesis (16) (Table 1 and Fig. 1C).
Fig. 1.
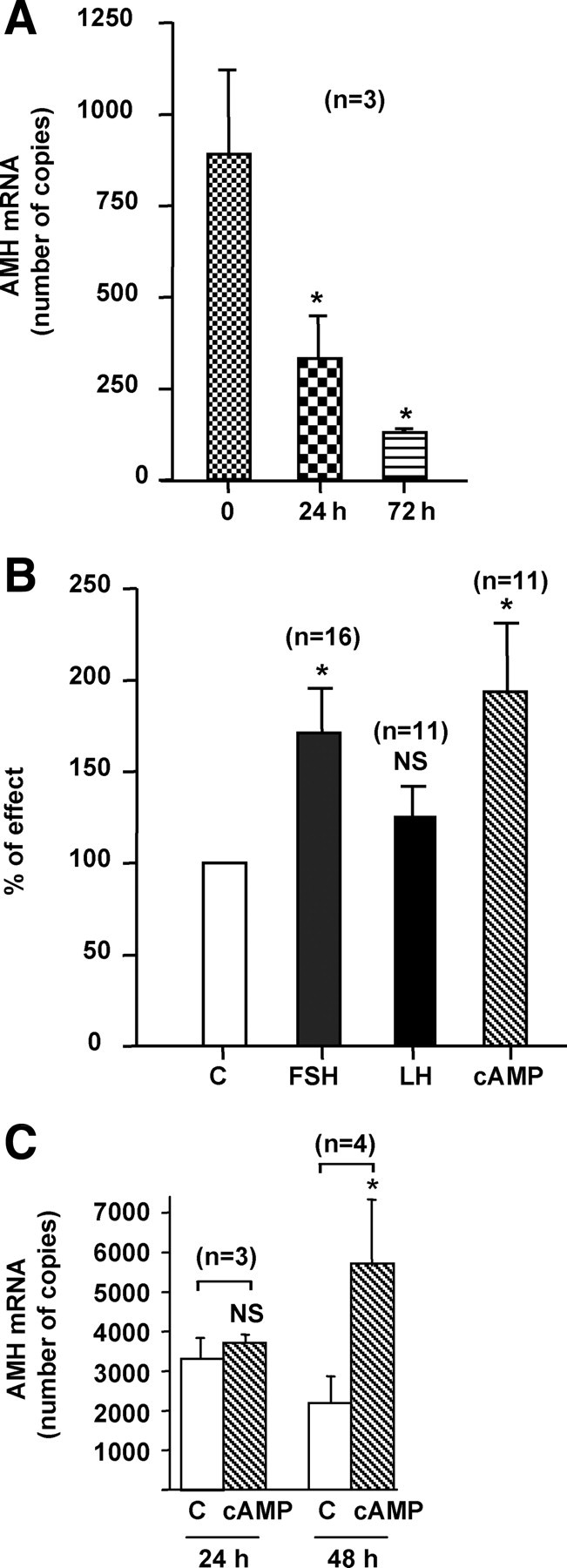
Expression and regulation of AMH mRNAs by gonadotropins in human granulosa-luteal cells and KK1 cells. Panel A, Kinetics of AMH expression in granulosa-luteal cells. Cells were cultured in control medium for 24 or 72 h after seeding. RNAs were extracted at these different time points and AMH expression was analyzed by Real Time RT-PCR. Results are expressed in copy numbers normalized by two reference genes, RPL13a and SDHA and analyzed by the Dunnett test; n represents the number of patients. Panel B, Regulation of AMH expression by gonadotropins in human granulosa-luteal cells. Twenty-four hours after seeding, cells were cultured for 48 h in the presence of FSH (1 IU/ml), LH (1 IU/ml), or cAMP (10 μm) before RNA extraction and analysis of AMH expression by real-time RT-PCR. Results are expressed as a percentage of effect obtained in cells cultured in control medium (C). The effect of each treatment was compared with cells from the same woman cultured in control medium, using the Wilcoxon paired test; n represents the number of patients. Panel C, Regulation of AMH expression by cAMP in KK1 cells. Twenty-four hours after seeding, cells were cultured for 24 and 48 h in control medium (C) or in the presence of cAMP (1 mm) before RNA extraction and analysis of AMH expression by real-time RT-PCR. Results are expressed in copy numbers normalized by HPRT gene expression and analyzed using the Wilcoxon paired test; n represents the number of experiments. Data shown correspond to the mean ± SEM. NS, Not significant; *, P < 0.05.
Table 1.
Sequence of the primers and probes used for real-time RT-PCR experiments
| Genes | Nucleotide sequences (5′–3′) |
Universal Probe Library probes | |
|---|---|---|---|
| Sense | Antisense | ||
| Human AMH | CGCCTGGTGGTCCTACAC | GAACCTCAGCGAGGGTGTT | 69 |
| Human RPL13a | CTGGACCGTCTCAAGGTGTT | GCCCCAGATAGGCAAACTT | 74 |
| Human SDHA | GGACCTGGTTGTCTTTGGTC | CCAGCGTTTGGTTTAATTGG | 80 |
| Mouse AMH | GGCTAGGGGAGACTGGAGAA | AGGTGGAGGCTCTTGGAACT | 41 |
| Mouse SOX9 | CAGCAAGACTCTGGGCAAG | TCCACGAAGGGTCTCTTCTC | 66 |
| Mouse SOX8 | GACCCTAGGCAAGCTGTGG | CTGCACACGGAGCCTCTC | 25 |
| Mouse SF-1 | AGAATTCTCCTTCCGTTCAGC | TCACCACACACTGGACACG | 85 |
| Mouse GATA-4 | GGAAGACACCCCAATCTCG | CATGGCCCCACAATTGAC | 13 |
| Mouse HPRT | TCAACGGGGGACATAAAAGT | CCAGTGTCAATTATATCTTCAACAATC | 22 |
AMH transcription is activated by cAMP, FSH, and LH
We then tested the responsiveness to cAMP of a human AMH promoter-luciferase construct (−3068hAMH-luc) composed of 3068 bp of the human AMH gene 5′-flanking region upstream of the luciferase gene (18) transfected into KK1 cells. As shown Fig. 2A, cAMP stimulated the transcriptional activity of −3068hAMH-luc, this effect growing more significant after 48 h of treatment. Therefore, in additional experiments, luciferase activity of AMH constructs was measured after 48 h of treatment. Similar effects were observed when KK1 cells were cultured without serum, ruling out a possible effect of AMH present in fetal calf serum (Fig. 2B). To compare the effects of cAMP, FSH, and LH, we cotransfected KK1 cells with −3068hAMH-luc and either human FSH-R or LH-R cDNAs. In control medium, luciferase activity was higher in cells cotransfected with the FSH-R or LH-R cDNAs alone or in combination than in cells transfected with the backbone vector (pGL2) (Fig. 2C). Then we tested the activation of the AMH promoter with concentrations of FSH or LH ranging from 5–1000 mIU/ml. FSH did not have any effect at 5 mIU/ml; it enhanced the AMH reporter by approximately 31% at 25 and 50 mIU/ml and by 60% at 250-1000 mIU/ml (Fig. 2D). The stimulation was statistically different between the two ranges of FSH concentration, showing a dose-dependent effect of FSH. LH activated the AMH reporter by 15–25%, without statistical differences between the concentrations (Fig. 2E).
Fig. 2.
Stimulation of the AMH promoter by cAMP, FSH, and LH in KK1 cells. Panel A, Stimulation of the −3068hAMH-luc construct by cAMP. Cells were transfected with 1 μg −3068hAMH-luc, and luciferase activity was assessed 24 and 48 h after treatment with cAMP (1 mm) and compared with cells cultured in control medium (C) using a Student's t test. Results are expressed in RLU or in percentage of stimulation [(RLU of treated cells − RLU of control cells)/RLU of treated cells) × 100]. Panel B, Effect of fetal calf serum (FCS) on the stimulation of −3068hAMH-luc by cAMP. Cells were transfected with 1 μg −3068hAMH-luc, and luciferase activity was assessed 48 h after treatment with cAMP (1 mm) in presence or absence of FCS and compared with cells cultured in control medium (C) using a Student's t test. Results are expressed in RLU. Panel C, Cis-activating capacity of −3068hAMH-luc in the presence of FSH-R and LH-R cDNAs. KK1 cells were cotransfected with 1 μg of the −3068hAMH-luc construct and 1μg of FSH-R and/or LH-R cDNAs, and luciferase activity was assessed after 48 h of culture in control medium. Results are expressed in RLU. Comparisons of means between different experimental conditions were made by repeated-measures ANOVA, followed by Dunnett post hoc test to compare all vs. pGL2. Panels D and E, Stimulation of the −3068hAMH-luc construct by FSH or LH. KK1 cells were cotransfected with 1 μg of the −3068hAMH-luc construct and FSH or LH receptor cDNA (1 μg). Luciferase activity was assessed after 48 h of treatment with FSH (panel D) or LH (panel E) (5–1000 mIU/ml). Results are expressed as percentage of stimulation. Responses to the different FSH and LH doses were compared by repeated-measures ANOVA and subsequently by Student-Newman-Keuls tests. Different superscripts indicate significant differences between the responses. Data shown correspond to the mean ± sem of at least three experiments, each done in triplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Additive effect of FSH and LH on the AMH promoter
Because stimulation of the AMH promoter by either FSH or LH (Fig. 2, D and E) was lower than that obtained with cAMP (Fig. 2A), we cotransfected both receptors (Fig. 2C) together with the −3068hAMH-luc construct, and we treated the cells with a combination of recombinant FSH and LH (1 IU/ml of each) or with human menopausal gonadotropin (HMG) (1 IU/ml), a commercial combination of native FSH and LH. Figure 3A shows that in these conditions, the stimulation of luciferase activity was similar to that obtained with cAMP, indicating an additive effect of FSH and LH combination. Next we tested gonadotropin combinations, with different LH to FSH concentration ratios (0.5, 0.75, 1, 1.5, and 2) and at concentrations similar to those observed in serum at midfollicular phase (10–20 mIU/ml) and at ovulation (50–100 mIU/ml). An additive effect on AMH transcription (42–58% of stimulation) was still found with physiological doses of FSH and LH (compared with Fig. 2, D and E), but it was not different between the two ranges of gonadotropin concentrations (Wilcoxon test, P = 0.07). Interestingly, activation of the AMH reporter was significantly higher for LH to FSH ratios above 1, corresponding to an abnormal clinical situation often encountered in women with polycystic ovary syndrome (PCOS) (Fig. 3B).
Fig. 3.
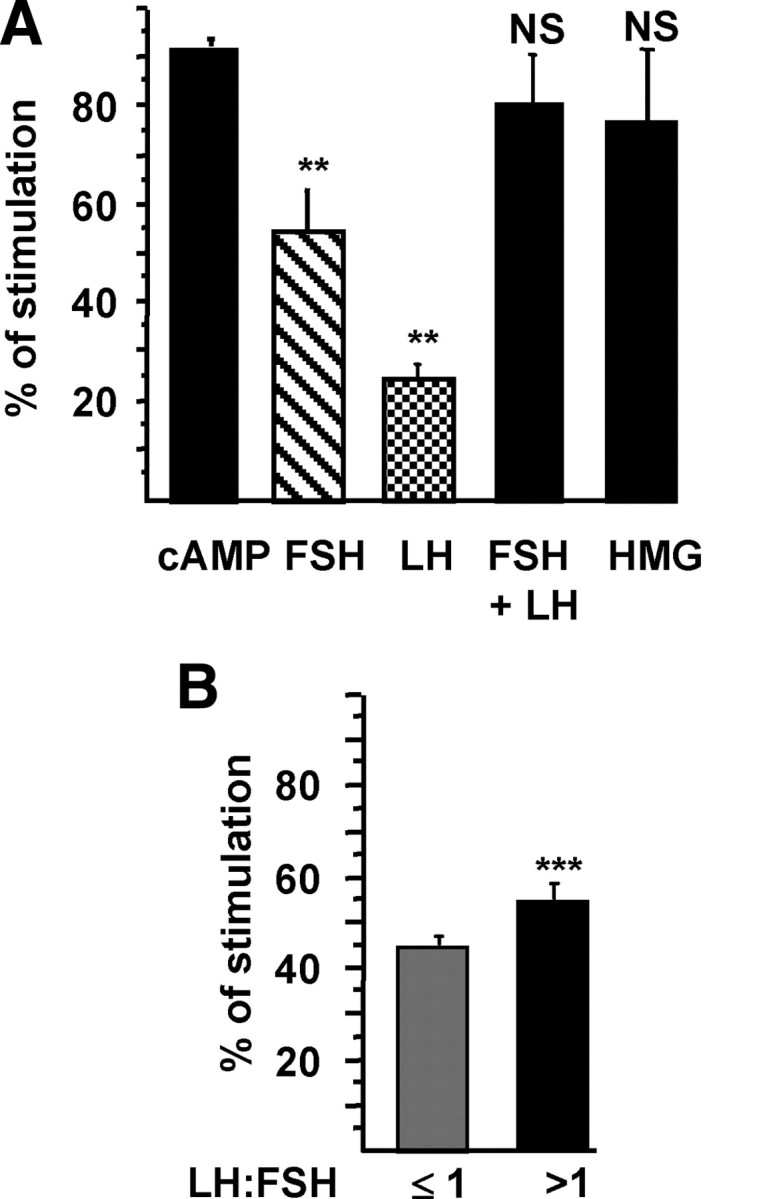
Additive effect of FSH and LH. KK1 cells were cotransfected with 1 μg of the −3068hAMH-luc construct and cDNAs (1 μg) of the FSH and LH receptors. Luciferase activity was assessed 48 h after stimulation by cAMP (1 mm), FSH (1 IU/ml), LH (1 IU/ml), FSH and LH (1 IU/ml of each), or HMG (1 IU/ml) (A) and combinations of FSH and LH at different ratios (0.5, 0.75, 1. 1.5, 2) in two ranges of concentrations (10–20 and 50–100 mIU/ml) (B). Results are expressed as percentage of stimulation. Comparisons of means between different treatments (A) were made by repeated-measures ANOVA, followed by Dunnett post hoc test to compare all vs. cAMP. Comparison of means between LH to FSH ratios (B) were made using the nonparametric Mann and Whitney U test. Data shown correspond to the mean ± sem of at least three experiments, each done in triplicate. NS, Not significant; **, P < 0.01; ***, P < 0.001.
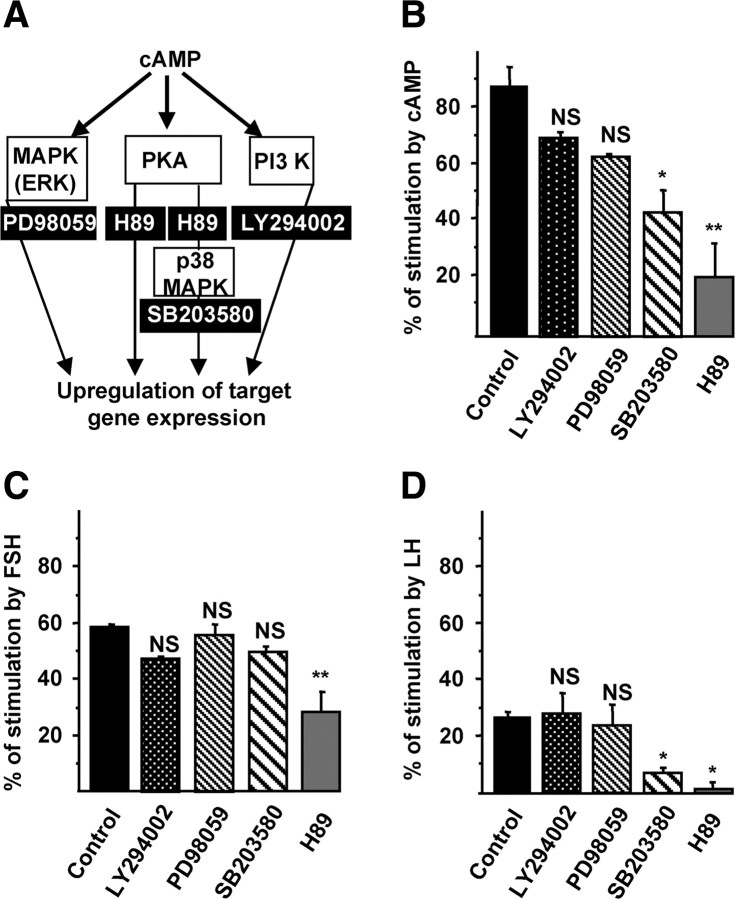
cAMP, FSH, and LH activate the AMH promoter through protein kinase A (PKA)/p38-dependent signaling pathways
Intracellular targets of cAMP include PKA, MAPK p38, ERK, and phosphatidylinositol 3-kinase (PI3K) cascades (20) (Fig. 4A). Using inhibitors of each cascade, we investigated the molecular mechanisms involved in the activation of the AMH promoter by cAMP, FSH, and LH. KK1 cells were transfected with the −3068hAMH-luc construct alone or with the FSH-R or LH-R cDNAs and stimulated for 48 h with cAMP, FSH, or LH in the presence or absence of inhibitors of PI3K (LY29402), MAPK (PD98059), p38 MAPK (SB203580), or PKA (H89). H89 reduced the effects of cAMP, FSH, and LH on AMH promoter (Fig. 4, B–D). SB203580 also decreased cAMP and LH effects but to a lower extent than H89 (Fig. 4, B and D).
Fig. 4.
Signaling pathways used by gonadotropins to activate the AMH promoter. A, Diagram of the signaling pathways activated by cAMP. B–D, Signaling pathways used by cAMP, FSH, and LH to stimulate the AMH promoter. KK1 cells were transfected with 1 μg of −3068hAMH-luc construct alone (B) or with the FSH receptor (C) or LH receptor (D) and stimulated during 48 h, with cAMP (1 mm) (B), FSH (1 IU/ml) (C), or LH (1 IU/ml) (D) in the absence or presence of inhibitors of PI3K (LY29402; 25 μm), MAPK (PD98059; 20 μm), p38 MAPK (SB203580; 20 μm), and PKA (H89; 10 μm). Results are expressed as percentage of stimulation and compared with the effect obtained in the same conditions without inhibitor (control). Data shown correspond to the mean ± sem of at least three experiments, each done in triplicate. Comparisons of means between different experimental conditions were made by repeated-measures ANOVA, followed by Dunnett post hoc test to compare all vs. control. NS, Not significant; *, P < 0.05; **, P < 0.01.
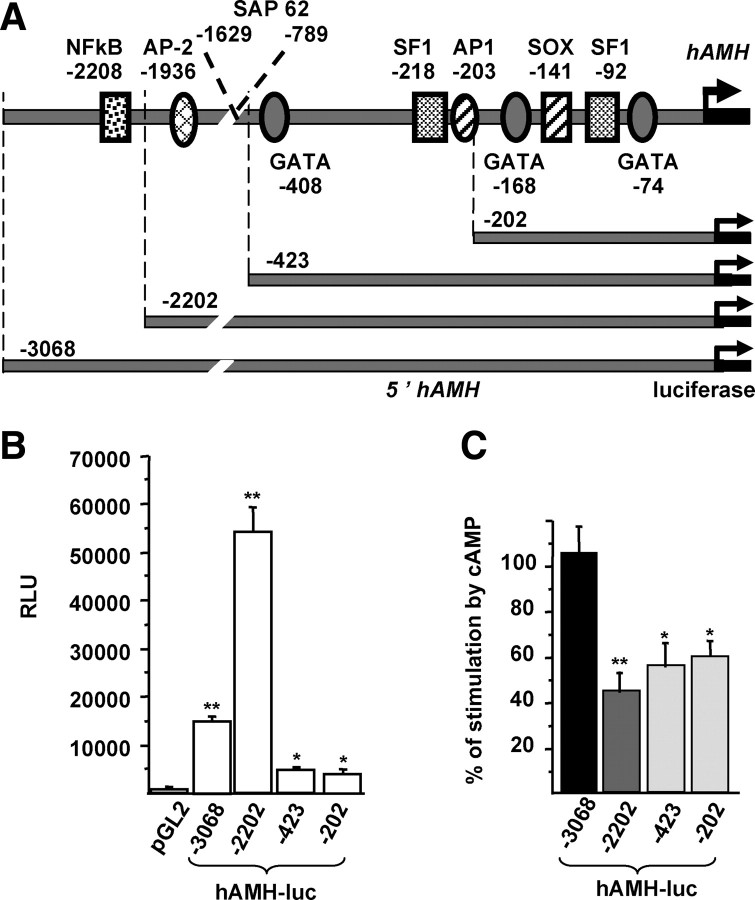
Sequences lying less than 200 bp and more than 2.2 kbp upstream of the AMH transcription start site are involved in the response to cAMP
To identify the transcription factors recruited by gonadotropins to stimulate AMH transcription, we studied the responsiveness to cAMP of constructs containing various lengths of the AMH promoter: 3068, 2202, 423, or 202 bp (Fig. 5A). In control medium, the highest cis-activating capacity was obtained with the 2202-bp promoter (Fig. 5B); the 3068-bp construct showed lower activity, suggesting the presence of a repressor element between 2202 and 3068 bp. The cis-activating capacity of the 423- and 202-bp constructs was low but at least 10-fold higher than that of the backbone vector. The highest cAMP-dependent stimulation of luciferase activity was obtained with the 3068-bp promoter; the 202-bp and 423-bp promoters showed 50–60% of the 3068-bp promoter response, whereas the 2202-bp had a 40–50% response (Fig. 5C). Taken together, these results suggest that more than half of cAMP effect on the AMH promoter involves sequences lying less than 202 bp of the transcription start site but that full activation requires sequences lying more than 2202 bp upstream.
Fig. 5.
Determination of cAMP-responsive domains within the AMH promoter. A, Schematic representation of the human AMH gene-promoter-luciferase constructs. The longest construct contained 3068 bp. Localization and nucleotide positions of binding sites for NFκB, AP2, SF-1, AP1, SOX, and GATA-4 in the human promoter are according to Ref. 18 B, Cis-activating capacity of the constructs. KK1 cells were transfected with 1 μg of the previously mentioned constructs, and luciferase activity was analyzed after 48 h culture in control medium. Results are expressed in RLU. Means were compared by repeated-measures ANOVA, followed by Dunnett post hoc test to compare all vs. pGL2. C, Regulation of the constructs by cAMP. KK1 cells were transfected with 1 μg of each construct, and luciferase activity was assessed 48 h after treatment with cAMP (1 mm). Results are expressed as percentage of stimulation by cAMP compared with untreated cells. Means were compared by repeated-measures ANOVA and subsequently by Dunnett post hoc test to compare all vs. −3068hAMH-luc. Data shown correspond to the mean ± sem of at least three experiments, each done in triplicate. *, P < 0.05; **, P < 0.01.
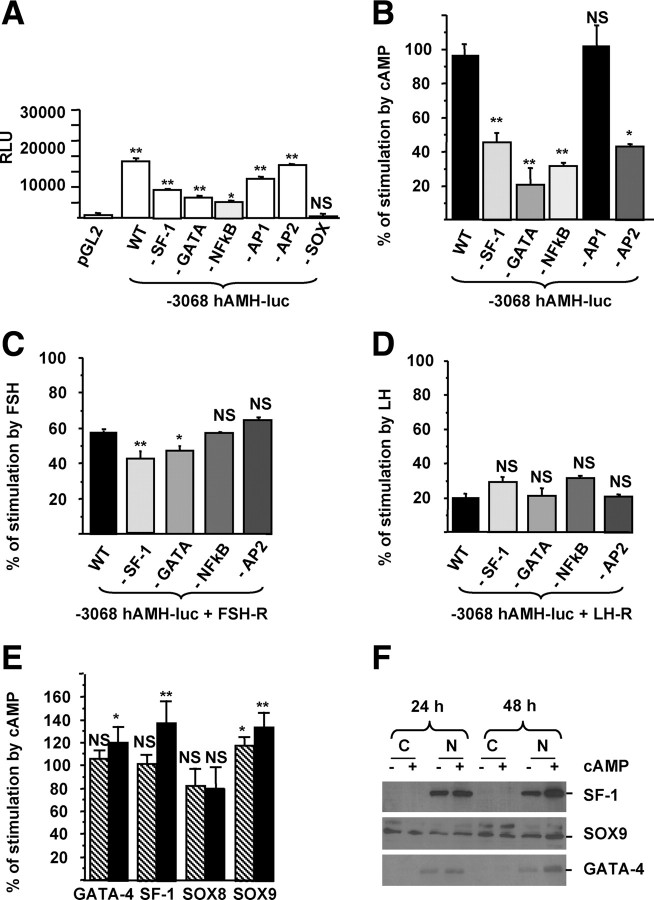
Nuclear factor-κB (NFκB), activation protein-2 (AP2), GATA-4, and SF-1 are involved in gonadotropin activation of the AMH promoter in KK1 cells
To determine which transcription factors are implicated in gonadotropin activation of the AMH promoter, we separately disrupted all the binding sites identified within the 3068 bp of the human AMH gene 5′-flanking region (Fig. 5A). Using targeted mutagenesis, −3068hAMH-luc constructs with mutations in binding sites for NFκB (18), AP2 (18), GATA-4, SF-1, SOX9/8, and AP1 were generated (Table 2). In control medium, only the mutation of the Sex Determining Region Y gene-related high mobility group box protein 9 or 8 (SOX9/8)-binding site resulted in a dramatic decrease of the cis-activating capacity of the construct (Fig. 6A), precluding a study of its regulation. Disruption of all other binding sites, except AP1, namely NFκB, AP2, GATA-4, and SF-1 resulted in a significant decrease of cAMP stimulation of the −3068hAMH-luc reporter gene (Fig. 6B). As shown in Fig. 6C, only the mutation of GATA-4 or SF-1 sites induced a modest but significant decrease of FSH response. Response to LH was not affected (Fig. 6D), probably because the LH effect on the −3068hAMH-luc reporter gene was too low. Because the importance of upstream binding sites for NFκB and AP2 in the activation of the AMH promoter by cAMP had already been shown for Sertoli cells (18), we then focused on the role of transcription factors with binding sites within the first 700 bp of the AMH promoter. All these transcription factors are expressed by KK1 cells. After quantification, only GATA-4, SF-1, and SOX9 but not SOX8 mRNAs were slightly but significantly increased after 48 h of treatment with cAMP (Table 1 and Fig. 6E). We also showed that the level of GATA-4 and SF-1 proteins in the nuclear fraction was increased after 48 h of treatment with cAMP (Fig. 6F). Taken together, these results suggest that NFκB, AP2, GATA-4, and SF-1 participate in the stimulation of the AMH promoter by gonadotropins in KK1 cells.
Table 2.
Sequence of the primers and kits used for targeted mutagenesis
| Mutations | Sequences | Targeted mutagenesis kits |
|---|---|---|
| GATA −408 | TCC TTG GGG TCT CCG GTA CCC CCA CCA GGG GTG G | A |
| SF-1 −218 | GGG GAT GGC CCT GGT ACC CGG CAT GTT GAC ACA TC | B |
| GAT GTG TCA ACA TGC CGG GTA CCA GGG CCA TCC CC | ||
| AP1 −203 | CAA GGA CAG CAT GTG CGC GCA CAG GCC CAG CTC T | A |
| GATA −168 | GCT CTA TCA CTG GGG AGG GGG TAC CGC TGC CAG GGA CAG | B |
| CTG TCC CTG GCA GCG GTA CCC CCT CCC CAG TGA TAG AGC | ||
| SOX9/8 −141 | ACA GAA AGG GCT GGT ACC GAA GGC CAC TCT G | B |
| CAG AGT GGC CTT CGG TAC CAG CCC TTT CTG T | ||
| SF-1 −92 | CGG GCA CTG TCC CGC GCG CAC GCG GCA GAG GAG | B |
| CTC CTC TGC CGC GTG CGC GCG GGA CAG TGC CCG | ||
| GATA −74 | AGG TCG CGG CAG AGG GTA CCG GGG TCT GTC CTG C | B |
A, Altered Sites II in vitro mutagenesis systems kit; B, QuikChange II XL site-directed mutagenesis kit.
Fig. 6.
Identification of the transcription factors involved in cAMP and gonadotropin stimulation of the AMH gene. A, Cis-activating capacity of the −3068hAMH-luc constructs with mutations in binding sites for NFκB, AP2, GATA-4, SF-1, AP1, and SOX. KK1 cells were transfected with 1 μg of the constructs, and luciferase activity was analyzed after 48 h of culture in control medium. Results are expressed in RLU. B–D, Regulation of the mutant constructs by cAMP (B), FSH (C), and LH (D). KK1 cells were transfected with 1 μg of mutated constructs and 1 μg of FSH-R (C) or LH-R (D), and luciferase activity was assessed 48 h after treatment with cAMP (1 mm) (B), FSH (1 IU/ml) (C), or LH (1 IU/ml) (D). Results are expressed as percentage of stimulation. Data shown correspond to the mean ± sem of three experiments, each done in triplicate. Comparisons of means between different experimental conditions were made by repeated-measures ANOVA, followed by Dunnett post hoc test to compare all vs. control (pGL2 for A and −3068hAMH-luc (WT) for B– D). E, GATA-4, SF-1, SOX9, and SOX8 mRNAs were analyzed by RT-PCR, and their regulation by cAMP in KK1 cells was quantified by real-time RT-PCR after 24 (hatched bars) and 48 (black bars) hours of treatment by cAMP (1 mm). Results are expressed as percentage of response compared with untreated cells using the Student's t test. Data shown correspond to the mean ± sem of three experiments, each done in triplicate. F, Nuclear translocation of SF-1, SOX9, and GATA-4 in the presence of cAMP. SF-1, SOX9, and GATA-4 proteins in cytosol and nuclear fractions (50 μg of each) were analyzed by Western blotting after 24 and 48 h of treatment with cAMP. NS, Not significant; *, P < 0.05; **, P < 0.01.
Discussion
Despite increasing recognition of the clinical importance of AMH in female reproductive endocrinology, little information is available on its regulation. AMH transcription in granulosa cells is up-regulated by bone morphogenetic proteins (21, 22) and by coculture with oocytes of preantral or preovulatory follicles (23) and down-regulated by testosterone (24). For estradiol, opposite effects have been reported (see below).
Studies of the relationship of FSH with AMH have also yielded conflicting results. Clinical data could be interpreted as supporting the hypothesis that FSH inhibits AMH expression, but their value is open to discussion. Obviously, the inverse correlation between low serum AMH and high FSH in infertile women is meaningless, because both are independent markers of decreased ovarian reserve (25, 26). The fall of serum AMH levels during controlled ovarian hyperstimulation with FSH can be at least partly attributed to the acceleration of follicle maturation, leading to the loss of small antral follicles, the main producers of AMH (27, 28). Patients harboring granulosa cell tumors secrete high amounts of AMH that are related to the volume of the tumor, (29–31) whereas endogenous production of FSH is suppressed; however, cancer cells cannot be taken as models, due to disruption of regulatory mechanisms (32).
Granulosa cells express FSH receptors already by the secondary stage of follicle maturation (33) at the time of rising AMH production. The decreased expression of AMH during late follicle maturation has been interpreted as supporting a negative effect of FSH upon AMH expression (5). At that stage, local changes in the environment surrounding the follicle may modulate or mask the effect of gonadotropins (34–36). For example, estrogens, whose production is stimulated by FSH, may inhibit AMH production (5, 37), but this is controversial (38). Many other hormones and growth factors emanating from the follicle, oocyte, or theca could be involved too.
The same caveats apply to the outcome of various treatment protocols and may explain some of the conflicting results. On one hand, Baarends et al. observed a decrease of AMH expression in some, but not all, preantral and small antral follicles in prepubertal rats treated with GnRH antagonist and FSH (5). On the other hand, several reports are consistent with a positive effect of FSH and/or LH upon AMH expression. Injection of a GnRH antagonist decreases AMH expression in the marmoset (10). Suppression of gonadotropin secretion results in a drop of AMH serum level in women (39). Xu et al. (40) have detected an increased production of AMH by isolated human secondary follicles, cultured in the presence of FSH.
Culture of granulosa cells appears the method of choice for specifically investigating the regulation of ovarian AMH by FSH; however, even with this method, agreement has not been reached. Pellatt and co-workers (9) failed to detect an effect of FSH upon AMH concentration of the culture medium of human granulosa-luteal cells, but the medium also contained testosterone, a potent repressor of AMH production in Sertoli cells (41). Rico et al. (22) observed an inhibitory effect of FSH upon the expression of AMH by bovine granulosa cells. Voutilainen and Miller (42) detected no effect of FSH but observed a stimulatory effect of cAMP upon AMH mRNA of pooled human granulosa cells by Northern blotting. Technical issues and species differences may explain some of the variability. In the present work, we find that FSH and its second messenger cAMP up-regulate AMH expression.
The AMH production of the granulosa-luteal cells obtained after in vitro fertilization procedures is extremely low. We therefore switched to a reporter gene approach to highlight modest effects, not detectable by conventional expression studies, and also to investigate the mechanism of action of gonadotropins on the AMH promoter. We employed KK1 cells, which have been successfully used to study GATA-4 regulation of AMH (17), in which we transfected the human FSH-R or/and LH-R cDNAs, and a reporter gene constituted by 3068 bp of the human AMH gene 5′-flanking region driving the luciferase gene. We found that both FSH and cAMP stimulate AMH trans-activation, the effect of FSH being dose dependent (Fig. 2). A similar reporter gene has been used to demonstrate the up-regulation of AMH by FSH in SMAT-1 Sertoli cells (18) because in vivo, the strong inhibitory action of testosterone masks the stimulatory effect of FSH upon Sertoli cell secretion of AMH (43, 44).
We also demonstrate, using the reporter gene approach, a significant (25%, P < 0.01) enhancement of AMH transcription by LH in KK1 cells (Fig. 2). The increase of AMH mRNA seen in human granulosa-luteal cells was not significant (25.8%, P < 0.07), in keeping with the results of Pellatt et al. (9) (28% stimulation, P = 0.06). In contrast, Voutilainen and Miller (42) showed an increase of AMH mRNAs by human chorionic gonadotropin (hCG) in granulosa-luteal cells. This effect is lower than that obtained with cAMP. In line with these results, we observed a stimulation of the AMH reporter gene comparable to that induced by cAMP only when KK1 cells were incubated with both FSH and LH (Fig. 3).
Normally, in large follicles, the LH receptor is highly expressed by granulosa cells close to the basement membrane but not by the ones in the cumulus (45), which express AMH; thus, the modest effects of LH reported are not physiological in normal women. However, they could apply to PCOS, the most common cause of infertility, characterized by an overexpression of AMH by granulosa cells (9, 46). Granulosa cells from PCOS follicles are at a prematurely advanced stage of development (47, 48). Furthermore, most PCOS patients present with increased GnRH/LH pulsatility (49, 50) and a LH to FSH ratio above 1. Interestingly, when we used a similar combination, gonadotropin stimulation of AMH transcription was more effective (Fig. 3). LH stimulation might thus contribute to overexpression of AMH by PCOS granulosa cells. Because AMH can activate the expression of FSH and LH ß-subunits (51), it may amplify the syndrome.
Gonadotropins induce cAMP-mediated events predominantly through the PKA-signaling pathway (20). In keeping with this notion, H89, an inhibitor of PKA, prevents FSH, LH, and cAMP stimulation of AMH transcription in KK1 granulosa cells (Fig. 4). LH and cAMP effects are also reduced in the presence of SB303580, an inhibitor of p38 MAPK (Fig. 4). However, the negative effect of SB303580 is less potent than that of H89, indicating that PKA is the major pathway involved in AMH up-regulation in response to LH. Relatively few gonadotropin/cAMP-responsive genes are regulated through the p38 MAPK pathway in granulosa cells. FSH-stimulated activation of p38 MAPK leads to the phosphorylation of the actin-capping protein heat shock protein-27 (52), triggers Crtl1 expression (53), and regulates estradiol production (54). LH uses the p38 MAPK signaling pathway to activate epoxide hydroxylase 2 (55) and the transcription factor Runx1 (56).
The best known transcription factor regulated by PKA is the cAMP-response element-binding protein, but its involvement in FSH and LH effects on AMH transcription seems unlikely because no cAMP-response element has been identified in the AMH promoter. Based upon reporter gene and expression studies, we demonstrate that AMH up-regulation by FSH in KK1 granulosa cells mobilizes GATA-4 and SF-1 transcription factors; NFκB, AP2 are involved in the cAMP response. The involvement of NFκB and AP2 on the stimulation of AMH transcription by cAMP has also been reported in SMAT-1 Sertoli cells (18). Several in vitro and in vivo studies have demonstrated the importance of GATA-4, SF-1, and SOX9 transcription factors for AMH expression in Sertoli cells and in heterologous systems, SOX9 being absolutely required to trigger AMH transcription (57). All directly bind to the AMH promoter but also interact with each other and with several cofactors (58, 59). In granulosa cells, Anttonen et al. (17) have shown that GATA-4 trans-activates the AMH promoter and that its cofactor Friend of GATA-2 inhibits this effect. GATA-4 is frequently used by FSH and hCG to regulate their target genes (60), in keeping with our results (Fig. 5 and 6).
In conclusion, using complementary approaches, we show that FSH and cAMP stimulate AMH transcription by granulosa cells. FSH and LH have an additive effect, which may be important in PCOS. Gonadotropins and cAMP act through the PKA and p38 MAPK signaling pathways and the GATA-4 and SF-1 transcription factors among others. These data are in keeping with those already demonstrated for Sertoli cells (reviewed in Ref. 61) and unify the concept of regulatory mechanisms in male and female gonads, despite the sharp differences in the timing of their AMH expression.
Materials and Methods
Reagents
Recombinant human FSH (Gonal-F) and LH (Luveris) were obtained from Serono Pharmaceuticals (Boulogne, France) and HMG from Ferring Pharmaceuticals (Gentilly, France). Rabbit polyclonal antibodies against SOX9 (ref AB5535) and SF-1 were purchased, respectively, from Millipore (Temecula, CA) and Upstate Biotechnology (Waltham, MA), goat polyclonal anti-GATA-4 was from Santa Cruz Biotechnology (Santa Cruz, CA); and peroxidase-labeled antigoat and antirabbit antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). KK1 cells and the LH-R cDNA construct were a generous gift of Dr. Ilpo Huthaniemi.
Targeted mutagenesis
Most AMH reporter constructs were obtained as previously described (18). Plasmids with mutations in binding sites for GATA-4 (at positions −408, −168, and −74), SF-1 (at position −218 and −92), and SOX9/8 (at position −141) of the human AMH promoter were generated using the QuikChange II XL site-directed mutagenesis (Stratagene, La Jolla, CA) or Altered Sites II in vitro mutagenesis systems (Promega, Charbonnières, France) kits according to the manufacturer's instructions, and mutagenic oligonucleotide primers (Table 2) were synthesized by Eurogentec (Liège, Belgium).
Subjects
Thirty-two infertile in vitro fertilization-embryo transfer patients, 20–40 yr of age, were studied prospectively. All of them met the following inclusion criteria: 1) both ovaries present, with no morphological abnormalities, adequately visualized in transvaginal ultrasound scans; 2) menstrual cycle length range between 25 and 35 d; 3) no current or past diseases affecting ovaries or gonadotropin or sex steroid secretion, clearance, or excretion; 4) no clinical signs of hyperandrogenism; and 5) body mass indexes ranging between 18 and 25 kg/m2. Infertility was due to either tubal or sperm abnormalities. In agreement with the inclusion criteria, no patient suffering from PCOS has been enrolled. An informed consent was obtained from all women, and this investigation received the approval of our internal institutional review board.
Ovarian stimulation
All women received a time-release GnRH agonist (Decapeptyl 3 mg; Organon Pharmaceuticals, Saint-Denis, France) from cycle d 2 onward. About 3 wk after gonadotropin suppression confirmed by the detection of low serum levels of estradiol and gonadotropins, recombinant FSH therapy (Gonal-F; Serono Pharmaceuticals) was initiated at a dosage of 150–225 IU/d. Daily FSH doses and timing of hCG administration were adjusted according to the usual criteria of follicular maturation. Administration of hCG (Gonadotrophine Chorionique ‘Endo,’ 10,000 IU im; Organon Pharmaceuticals) was performed when at least four follicles were larger than 16 mm in diameter and estradiol levels per mature follicle (>16 mm in diameter) were higher than 200 pg/ml. Oocytes were retrieved 36 h after hCG administration by transvaginal ultrasound-guided aspiration.
Collection of human granulosa-luteal cells
After oocyte isolation, follicular fluids from each patient were centrifuged separately through a one-step density Percoll gradient (vol/vol, PBS/Percoll) at 350 × g for 15 min to remove red blood cells. Granulosa-luteal cells were collected at the interface, washed with PBS, and seeded at 3 × 105 cells per well in six-well plates in DMEM/F-12 (Invitrogen, Carlsbad, CA) containing 10% fetal calf serum (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen).
Cell culture and transfection assays
KK1 cell line was cultured at 37 C under 5% CO2 in DMEM/F-12 (Invitrogen) containing 10% fetal calf serum (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Twenty-four hours before transfection, KK1 cells were seeded in six-well plates at 3.5 × 105 cells per well in the same medium except that fetal calf serum was charcoal stripped (Invitrogen). Transient transfection was performed with the Lipofectamine Plus kit (Invitrogen).
Western blotting
After 24 or 48 h of treatment in presence of cAMP, KK1 cells were washed and lysed to prepare nuclear and cytosol extracts according to the ProteoExtract subcellular proteome extraction kit (Calbiochem, La Jolla, CA). Protein concentration was determined using the bicinchoninic acid assay (Pierce, Rockford, IL). Proteins were subjected to SDS-PAGE (Bio-Rad Laboratories, Hercules, CA) and transferred onto a Protran BA85 nitrocellulose membrane (Whatman, Dassel, Germany). Membranes were first incubated with antibodies against SOX9, SF-1, or GATA-4 as described (62). Then blots were probed with a peroxidase-labeled antirabbit or antigoat antibody at 1:5000 (Jackson ImmunoResearch). Bands were visualized with the enhanced chemiluminescence detection reagent (GE Healthcare, Buckinghamshire, UK).
RNA extraction and reverse transcription
Twenty hours after seeding, human granulosa-luteal cells were serum starved for 1 h and cultured for 48 h without serum in the presence of FSH (1 IU/ml), LH (1 IU/ml), or N,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (cAMP) (10 μm), and KKI cells were cultured for either 48 or 72 h in the presence of cAMP (1 mm). Total RNAs were extracted with the RNA Plus extraction kit (QIAGEN, Valencia, CA).
Reverse transcription was performed in a total of 20 μl with the Omniscript reverse transcription kit for RT-PCR (QIAGEN) using 1 μg RNA, Omniscript reverse transcriptase, and oligo-deoxythymidine primers and random hexamers as recommended by the manufacturer.
Quantitative real-time PCR
Quantification of the content of AMH, RPL13a (ribosomal protein L13a), SDHA (succinate dehydrogenase complex subunit A), SF-1, GATA-4, SOX8, SOX9, and hprt (hypoxanthine guanine phosphoribosyl transferase) mRNA was performed by real-time PCR using the TaqMan PCR method. The primers and the Universal Probe Library probes (Roche Diagnostics, Indianapolis, IN) used to amplify these genes are indicated in Table 1. Real-time PCR was performed with 1/5 dilution of the cDNAs using the Lightcycler 480 Probes Master kit (Roche Diagnostics, Mannheim, Germany). The PCR protocol used an initial denaturing step at 95 C for 10 min followed by 45 cycles of 95 C for 10 sec, 60 C for 30 sec, and 72 C for 1 sec. To generate external standard curves, different concentrations of the purified and quantified PCR products were amplified. Copy numbers were normalized by two reference genes, RPL13a and SDHA for human AMH, and one, HPRT, for mouse AMH.
Reporter assays
KK1 cells were transfected with 1 μg of the different AMH reporter genes with or without 1 μg of the human FSH-R or/and LH-R cDNAs. At the end of the transfection, cells were stimulated with cAMP (1 mm) or human recombinant FSH or LH at different concentrations. Forty-eight hours later, cells were washed twice with PBS and lysed for 20 min under rocking in 500 μl passive lysis buffer (Luciferase Assay System; Promega). Twenty microliters were analyzed for firefly luciferase activity according to the manufacturer's instructions using a Lumat LB95507 luminometer (EG&G Berthold, Thoiry, France). Results are expressed in relative light units (RLU) or in percentage of stimulation [(RLU of treated cells − RLU of control cells)/RLU of treated cells) × 100]. Data shown correspond to the mean ± SEM of at least three experiments each done in triplicate.
Statistics
Results were analyzed by the Wilcoxon test for paired comparisons between control and treated cells, Student's t test or by nonparametric Mann and Whitney U test to compare two means, and repeated-measures ANOVA followed by Dunnett post hoc tests to compare all vs. control or by Student-Newman-Keuls tests to compare the different conditions to each other.
Acknowledgments
We thank Arnaud Leclerc and the in vitro fertilization laboratory of Antoine Béclère Hospital for technical assistance. We thank Richard Cate for critical reading of the manuscript.
We are grateful to the Association pour la Recherche sur le Cancer for its grant (no. 4253) and to l'Assistance-Publique Hôpitaux de Paris for the Contrat d'Interface to Nathalie di Clemente.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMH
- Anti-Müllerian hormone
- AP2
- activation protein-2
- hCG
- human chorionic gonadotropin
- HMG
- human menopausal gonadotropin
- NFκB
- nuclear factor-κB
- PCOS
- polycystic ovary syndrome
- PI3K
- phosphatidylinositol 3-kinase
- PKA
- protein kinase
- RLU
- relative light units
- SOX8/9
- Sex Determining Region Y gene-related high mobility group box protein 9 or 8.
References
- 1. Josso N , Picard JY , Rey R , di Clemente N. 2006. Testicular anti-Müllerian hormone: history, genetics, regulation and clinical applications. Pediatr Endocrinol Rev 3:347–358 [PubMed] [Google Scholar]
- 2. Vigier B , Picard JY , Tran D , Legeai L , Josso N. 1984. Production of anti-Müllerian hormone: another homology between Sertoli and granulosa cells. Endocrinology 114:1315–1320 [DOI] [PubMed] [Google Scholar]
- 3. Bézard J , Vigier B , Tran D , Mauléon P , Josso N. 1987. Immunocytochemical study of anti-Müllerian hormone in sheep ovarian follicles during fetal and postnatal development. J Reprod Fertil 80:509–516 [DOI] [PubMed] [Google Scholar]
- 4. Hirobe S , He WW , Gustafson ML , MacLaughlin DT , Donahoe PK. 1994. Mullerian inhibiting substance gene expression in the cycling rat ovary correlates with recruited or graafian follicle selection. Biol Reprod 50:1238–1243 [DOI] [PubMed] [Google Scholar]
- 5. Baarends WM , Uilenbroek JT , Kramer P , Hoogerbrugge JW , van Leeuwen EC , Themmen AP , Grootegoed JA. 1995. Anti-Müllerian hormone and anti-Müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 136:4951–4962 [DOI] [PubMed] [Google Scholar]
- 6. Modi D , Bhartiya D , Puri C. 2006. Developmental expression and cellular distribution of Mullerian inhibiting substance in the primate ovary. Reproduction 132:443–453 [DOI] [PubMed] [Google Scholar]
- 7. Weenen C , Laven JS , Von Bergh AR , Cranfield M , Groome NP , Visser JA , Kramer P , Fauser BC , Themmen AP. 2004. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 10:77–83 [DOI] [PubMed] [Google Scholar]
- 8. Rajpert-De Meyts E , Jørgensen N , Graem N , Müller J , Cate RL , Skakkebaek NE. 1999. Expression of anti-Müllerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab 84:3836–3844 [DOI] [PubMed] [Google Scholar]
- 9. Pellatt L , Hanna L , Brincat M , Galea R , Brain H , Whitehead S , Mason H. 2007. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab 92:240–245 [DOI] [PubMed] [Google Scholar]
- 10. Thomas FH , Telfer EE , Fraser HM. 2007. Expression of anti-Mullerian hormone protein during early follicular development in the primate ovary in vivo is influenced by suppression of gonadotropin secretion and inhibition of vascular endothelial growth factor. Endocrinology 148:2273–2281 [DOI] [PubMed] [Google Scholar]
- 11. Takahashi M , Hayashi M , Manganaro TF , Donahoe PK. 1986. The ontogeny of Müllerian inhibiting substance in granulosa cells of the bovine ovarian follicle. Biol Reprod 35:447–453 [DOI] [PubMed] [Google Scholar]
- 12. Ueno S , Kuroda T , Maclaughlin DT , Ragin RC , Manganaro TF , Donahoe PK. 1989. Müllerian inhibiting substance in the adult rat ovary during various stages of the estrous cycle. Endocrinology 125:1060–1066 [DOI] [PubMed] [Google Scholar]
- 13. Scheffer GJ , Broekmans FJ , Looman CW , Blankenstein M , Fauser BC , teJong FH , teVelde ER. 2003. The number of antral follicles in normal women with proven fertility is the best reflection of reproductive age. Hum Reprod 18:700–706 [DOI] [PubMed] [Google Scholar]
- 14. de Vet A , Laven JS , de Jong FH , Themmen AP , Fauser BC. 2002. Anti-Müllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril 77:357–362 [DOI] [PubMed] [Google Scholar]
- 15. van Rooij IA , Broekmans FJ , te Velde ER , Fauser BC , Bancsi LF , de Jong FH , Themmen AP. 2002. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod 17:3065–3071 [DOI] [PubMed] [Google Scholar]
- 16. Kananen K , Markkula M , Rainio E , Su JG , Hsueh AJ , Huhtaniemi IT. 1995. Gonadal tumorigenesis in transgenic mice bearing the mouse inhibin α-subunit promoter/Simian virus T-antigen fusion gene: characterization of ovarian tumors and establishment of gonadotropin-responsive granulosa cell lines. Mol Endocrinol 9:616–627 [DOI] [PubMed] [Google Scholar]
- 17. Anttonen M , Ketola I , Parviainen H , Pusa AK , Heikinheimo M. 2003. FOG-2 and GATA-4 are coexpressed in the mouse ovary and can modulate Müllerian-inhibiting substance expression. Biol Reprod 68:1333–1340 [DOI] [PubMed] [Google Scholar]
- 18. Lukas-Croisier C , Lasala C , Nicaud J , Bedecarrás P , Kumar TR , Dutertre M , Matzuk MM , Picard JY , Josso N , Rey R. 2003. Follicle stimulating hormone increases testicular anti-Müllerian hormone (AMH) production through Sertoli cell proliferation and a non-classical AMP-mediated activation of the AMH gene. Mol Endocrinol 17:550–561 [DOI] [PubMed] [Google Scholar]
- 19. Vigier B , Picard JY , Campargue J , Forest MG , Heyman Y , Josso N. 1985. Secretion of anti-Müllerian hormone by immature bovine Sertoli cells in primary culture, studied by a competition type radio-immunoassay: lack of modulation by either FSH or testosterone. Mol Cell Endocrinol 43:141–150 [DOI] [PubMed] [Google Scholar]
- 20. Sands WA , Palmer TM. 2008. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal 20:460–466 [DOI] [PubMed] [Google Scholar]
- 21. Shi J , Yoshino O , Osuga Y , Koga K , Hirota Y , Hirata T , Yano T , Nishii O , Taketani Y. 2009. Bone morphogenetic protein-6 stimulates gene expression of follicle-stimulating hormone receptor, inhibin/activin β-subunits, and anti-Mullerian hormone in human granulosa cells. Fertil Steril 92:1794–1798 [DOI] [PubMed] [Google Scholar]
- 22. Rico C , Medigue C , Fabre S , Jarrier P , Bontoux M , Clement F , Monniaux D. 10 November 2010. Regulation of anti-Mullerian hormone production in the cow: a multi-scale study at endocrine, ovarian, follicular, and granulosa cell levels. Biol Reprod 10.1095/biolreprod.110.088187 [DOI] [PubMed] [Google Scholar]
- 23. Salmon NA , Handyside AH , Joyce IM. 2004. Oocyte regulation of anti-Mullerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol 266:201–208 [DOI] [PubMed] [Google Scholar]
- 24. Crisosto N , Sir-Petermann T , Greiner M , Maliqueo M , Moreno M , Aedo P , Lara HE. 2009. Testosterone-induced downregulation of anti-Mullerian hormone expression in granulosa cells from small bovine follicles. Endocrine 36:339–345 [DOI] [PubMed] [Google Scholar]
- 25. Singer T , Barad DH , Weghofer A , Gleicher N. 2009. Correlation of anti-Müllerian hormone and baseline follicle-stimulating hormone levels. Fertil Steril 91:2616–2619 [DOI] [PubMed] [Google Scholar]
- 26. Seifer DB , MacLaughlin DT , Christian BP , Feng B , Shelden RM. 2002. Early follicular serum Müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril 77:468–471 [DOI] [PubMed] [Google Scholar]
- 27. Fanchin R , Schonäuer LM , Righini C , Frydman N , Frydman R , Taieb J. 2003. Serum anti-Müllerian hormone dynamics during controlled ovarian hyperstimulation. Hum Reprod 18:328–332 [DOI] [PubMed] [Google Scholar]
- 28. La Marca A , Malmusi S , Giulini S , Tamaro LF , Orvieto R , Levratti P , Volpe A. 2004. Anti-Mullerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum Reprod 19:2738–2741 [DOI] [PubMed] [Google Scholar]
- 29. Rey RA , Lhommé C , Marcillac I , Lahlou N , Duvillard P , Josso N , Bidart JM. 1996. Anti-Müllerian hormone as a serum marker of granulosa-cell tumors of the ovary: comparative study with serum α-inhibin and estradiol. Am J Obstet Gynecol 174:958–965 [DOI] [PubMed] [Google Scholar]
- 30. Long WQ , Ranchin V , Pautier P , Belville C , Denizot P , Cailla H , Lhommé C , Picard JY , Bidart JM , Rey R. 2000. Detection of minimal levels of serum anti-Müllerian hormone during follow-up of patients with ovarian granulosa cell tumor by means of a highly sensitive ELISA. J Clin Endocrinol Metab 85:540–544 [DOI] [PubMed] [Google Scholar]
- 31. Gustafson ML , Lee MM , Scully RE , Moncure AC , Hirakawa T , Goodman A , Muntz HG , Donahoe PK , MacLaughlin DT , Fuller AF. 1992. Müllerian inhibiting substance as a marker for ovarian sex-cord tumor. New Engl J Med 326:466–471 [DOI] [PubMed] [Google Scholar]
- 32. Fuller PJ , Chu S , Fikret S , Burger HG. 2002. Molecular pathogenesis of granulosa cell tumours. Mol Cell Endocrinol 191:89–96 [DOI] [PubMed] [Google Scholar]
- 33. McGee EA , Hsueh AJW. 2000. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 21:200–214 [DOI] [PubMed] [Google Scholar]
- 34. Glister C , Tannetta DS , Groome NP , Knight PG. 2001. Interactions between follicle-stimulating hormone and growth factors in modulating secretion of steroids and inhibin-related peptides by nonluteinized bovine granulosa cells. Biol Reprod 65:1020–1028 [DOI] [PubMed] [Google Scholar]
- 35. Knight PG , Glister C. 2006. TGF-β superfamily members and ovarian follicle development. Reproduction 132:191–206 [DOI] [PubMed] [Google Scholar]
- 36. Hsueh AJ , McGee EA , Hayashi M , Hsu SY. 2000. Hormonal regulation of early follicle development in the rat ovary. Mol Cell Endocrinol 163:95–100 [DOI] [PubMed] [Google Scholar]
- 37. Andersen CY , Byskov AG. 2006. Estradiol and regulation of anti-Mullerian hormone, inhibin-A, and inhibin-B secretion: analysis of small antral and preovulatory human follicles' fluid. J Clin Endocrinol Metab 91:4064–4069 [DOI] [PubMed] [Google Scholar]
- 38. Ikeda Y , Nagai A , Ikeda MA , Hayashi S. 2002. Increased expression of Müllerian-inhibiting substance correlates with inhibition of follicular growth in the developing ovary of rats treated with E2 benzoate. Endocrinology 143:304–312 [DOI] [PubMed] [Google Scholar]
- 39. Arbo E , Vetori DV , Jimenez MF , Freitas FM , Lemos N , Cunha-Filho JS. 2007. Serum anti-Müllerian hormone levels and follicular cohort characteristics after pituitary suppression in the late luteal phase with oral contraceptive pills. Hum Reprod 22:3192–3196 [DOI] [PubMed] [Google Scholar]
- 40. Xu M , Barrett SL , West-Farrell E , Kondapalli LA , Kiesewetter SE , Shea LD , Woodruff TK. 2009. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod 24:2531–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rey R , Lukas-Croisier C , Lasala C , Bedecarrás P. 2003. AMH/MIS: what we know already about the gene, the protein and its regulation. Mol Cell Endocrinol 211:21–31 [DOI] [PubMed] [Google Scholar]
- 42. Voutilainen R , Miller WL. 1987. Human Müllerian inhibitory factor messenger ribonucleic acid is hormonally regulated in the fetal testis and in adult granulosa cells. Mol Endocrinol 1:604–608 [DOI] [PubMed] [Google Scholar]
- 43. Rey R , Mebarki F , Forest MG , Mowszowicz I , Cate RL , Morel Y , Chaussain JL , Josso N. 1994. Anti-Müllerian hormone in children with androgen insensitivity. J Clin Endocrinol Metab 79:960–964 [DOI] [PubMed] [Google Scholar]
- 44. Al-Attar L , Noël K , Dutertre M , Belville C , Forest MG , Burgoyne PS , Josso N , Rey R. 1997. Hormonal and cellular regulation of Sertoli cell anti-Müllerian hormone production in the postnatal mouse. J Clin Invest 100:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peng XR , Hsueh AJ , LaPolt PS , Bjersing L , Ny T. 1991. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology 129:3200–3207 [DOI] [PubMed] [Google Scholar]
- 46. Catteau-Jonard S , Jamin SP , Leclerc A , Gonzalès J , Dewailly D , di Clemente N. 2008. Anti-Müllerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles from women with polycystic ovary syndrome. J Clin Endocrinol Metab 93:4456–4461 [DOI] [PubMed] [Google Scholar]
- 47. Jonard S , Dewailly D. 2004. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update 10:107–117 [DOI] [PubMed] [Google Scholar]
- 48. Willis DS , Watson H , Mason HD , Galea R , Brincat M , Franks S. 1998. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab 83:3984–3991 [DOI] [PubMed] [Google Scholar]
- 49. Taylor AE , McCourt B , Martin KA , Anderson EJ , Adams JM , Schoenfeld D , Hall JE. 1997. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab 82:2248–2256 [DOI] [PubMed] [Google Scholar]
- 50. Arroyo A , Laughlin GA , Morales AJ , Yen SS. 1997. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab 82:3728–3733 [DOI] [PubMed] [Google Scholar]
- 51. Bédécarrats GY , O'Neill FH , Norwitz ER , Kaiser UB , Teixeira J. 2003. Regulation of gonadotropin gene expression by Mullerian inhibiting substance. Proc Natl Acad Sci USA 100:9348–9353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maizels ET , Cottom J , Jones JC , Hunzicker-Dunn M. 1998. Follicle stimulating hormone (FSH) activates the p38 mitogen-activated protein kinase pathway, inducing small heat shock protein phosphorylation and cell rounding in immature rat ovarian granulosa cells. Endocrinology 139:3353–3356 [DOI] [PubMed] [Google Scholar]
- 53. Sun GW , Kobayashi H , Suzuki M , Kanayama N , Terao T. 2003. Follicle-stimulating hormone and insulin-like growth factor I synergistically induce up-regulation of cartilage link protein (Crtl1) via activation of phosphatidylinositol-dependent kinase/Akt in rat granulosa cells. Endocrinology 144:793–801 [DOI] [PubMed] [Google Scholar]
- 54. Inagaki K , Otsuka F , Miyoshi T , Yamashita M , Takahashi M , Goto J , Suzuki J , Makino H. 2009. p38-Mitogen-activated protein kinase stimulated steroidogenesis in granulosa cell-oocyte cocultures: role of bone morphogenetic proteins 2 and 4. Endocrinology 150:1921–1930 [DOI] [PubMed] [Google Scholar]
- 55. Shkolnik K , Ben-Dor S , Galiani D , Hourvitz A , Dekel N. 2007. A novel ovary-specific and ovulation-associated variant of epoxide hydrolase 2. FEBS Lett 581:4891–4898 [DOI] [PubMed] [Google Scholar]
- 56. Jo M , Curry TE. 2006. Luteinizing hormone-induced RUNX1 regulates the expression of genes in granulosa cells of rat periovulatory follicles. Mol Endocrinol 20:2156–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arango NA , Lovell-Badge R , Behringer RR. 1999. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell 99:409–419 [DOI] [PubMed] [Google Scholar]
- 58. Watanabe K , Clarke TR , Lane AH , Wang X , Donahoe PK. 2000. Endogenous expression of Müllerian inhibiting substance in early postnatal rat Sertoli cells requires multiple steroidogenic factor-1 and GATA-4-binding sites. Proc Natl Acad Sci USA 97:1624–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tremblay JJ , Viger RS. 2001. Nuclear receptor Dax-1 represses the transcriptional cooperation between GATA-4 and SF-1 in Sertoli cells. Biol Reprod 64:1191–1199 [DOI] [PubMed] [Google Scholar]
- 60. Viger RS , Guittot SM , Anttonen M , Wilson DB , Heikinheimo M. 2008. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol 22:781–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grinspon RP , Rey RA. 2010. Anti-Müllerian hormone and Sertoli cell function in paediatric male hypogonadism. Horm Res Paediatr 73:81–92 [DOI] [PubMed] [Google Scholar]
- 62. Gouédard L , Chen YG , Thevenet L , Racine C , Borie S , Lamarre I , Josso N , Massague J , di Clemente N. 2000. Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Müllerian hormone and its type II receptor. J Biol Chem 275:27973–27978 [DOI] [PubMed] [Google Scholar]