Dysregulated redox homeostasis driven by elevated NOX4-derived ROS signaling underlies fibroblast-to-myofibroblast differentiation, a hallmark of benign prostatic hyperplasia and prostate cancer.
Abstract
Stromal remodeling, in particular fibroblast-to-myofibroblast differentiation, is a hallmark of benign prostatic hyperplasia (BPH) and solid tumors, including prostate cancer (PCa). Increased local production of TGFβ1 is considered the inducing stimulus. Given that stromal remodeling actively promotes BPH/PCa development, there is considerable interest in developing stromal-targeted therapies. Microarray and quantitative PCR analysis of primary human prostatic stromal cells induced to undergo fibroblast-to-myofibroblast differentiation with TGFβ1 revealed up-regulation of the reactive oxygen species (ROS) producer reduced nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4) and down-regulation of the selenium-containing ROS-scavenging enzymes glutathione peroxidase 3, thioredoxin reductase 1 (TXNRD1), and the selenium transporter selenoprotein P plasma 1. Consistently, NOX4 expression correlated specifically with the myofibroblast phenotype in vivo, and loss of selenoprotein P plasma 1 was observed in tumor-associated stroma of human PCa biopsies. Using lentiviral NOX4 short hairpin RNA-mediated knockdown, pharmacological inhibitors, antioxidants, and selenium, we demonstrate that TGFβ1 induction of NOX4-derived ROS is required for TGFβ1-mediated phosphorylation of c-jun N-terminal kinase, which in turn is essential for subsequent downstream cytoskeletal remodeling. Significantly, selenium supplementation inhibited differentiation by increasing ROS-scavenging selenoenzyme biosynthesis because glutathione peroxidase 3 and TXNRD1 expression and TXNRD1 enzyme activity were restored. Consistently, selenium depleted ROS levels downstream of NOX4 induction. Collectively, this work demonstrates that dysregulated redox homeostasis driven by elevated NOX4-derived ROS signaling underlies fibroblast-to-myofibroblast differentiation in the diseased prostatic stroma. Further, these data indicate the potential clinical value of selenium and/or NOX4 inhibitors in preventing the functional pathogenic changes of stromal cells in BPH and PCa.
Benign prostatic hyperplasia (BPH) and prostate cancer (PCa) are two of the most common diseases affecting aging males (1–3). Although distinct pathologies, BPH and PCa are both associated with changes in the stromal microenvironment that actively promote disease development (4, 5). In particular, the BPH and PCa-adjacent stroma (the latter also termed “reactive stroma”) are characterized by increased extracellular matrix (ECM) deposition, capillary density, and differentiation of fibroblast into myofibroblasts, the mitogenic secretome of which promotes proliferation, angiogenesis, and tumorigenesis (6–9). Initial treatments for BPH and local-confined PCa target androgen signaling/metabolism, which result in apoptosis of androgen-dependent cells and reduced prostate volume (10, 11). However, neither approach specifically addresses the stromal component of disease. Understanding the mechanisms underlying stromal remodeling in particular fibroblast-to-myofibroblast differentiation may facilitate the development of preventive or more effective treatment strategies.
Elevated production of the cytokine TGFβ1 is observed in BPH and pretumorigenic prostatic lesions with tissue and circulating levels positively correlating with disease risk and more rapid PCa progression (12, 13). We and others demonstrated that TGFβ1 induces fibroblast-to-myofibroblast differentiation and stromal remodeling both in vitro and in vivo (14–16). TGFβ1 is thus considered a key inducer of pathogenic stromal reorganization; however, its downstream molecular effectors and hence potential therapeutic targets remain unknown.
Excessive levels of reactive oxygen species (ROS) are associated with the pathology of many human diseases. By contrast, various cellular stimuli (e.g. growth factors, cytokines, and hormones) induce the regulated production of low levels of ROS. In such cellular contexts, ROS act as signaling messengers regulating diverse physiological processes via reversible oxidative modification of lipids, DNA, and specific cysteine residues of susceptible proteins (e.g. transcription factors, protein tyrosine kinases, and protein tyrosine phosphatases) resulting in altered activity and function (17).
The reduced nicotinamide adenine dinucleotide phosphate oxidase (NOX) family is a major source of intracellular ROS (18). NOX enzymes catalyze the reduction of oxygen using cytosolic reduced nicotinamide adenine dinucleotide phosphate as an electron donor generating superoxide, which may undergo subsequent dismutation to hydrogen peroxide. Of the seven NOX enzymes in humans, NOX1 and NOX2 play a role in host defense whereas ROS produced by other NOX enzymes act primarily as signaling molecules (19). Dysregulated NOX4 expression is implicated in differentiation associated with cardiac fibrosis and idiopathic lung pulmonary fibrosis (20, 21). However, the molecular mechanism by which NOX4-derived ROS directed differentiation was not identified.
The potentially damaging effects of ROS are limited by antioxidant systems, such as glutathione peroxidases (GPXs) and thioredoxin reductases (TXNRDs). An integral component of GPX and TXNRD enzymes is the essential trace element selenium (Se), which is incorporated as selenocysteine at their active site (22). The expression and biosynthesis of such selenoproteins are determined by Se status in a strict hierarchical manner (23, 24). Due to its high levels in plasma and an unusually high selenocysteine content, selenoprotein P plasma 1 (SEPP1) is primarily thought to function as a Se transporter (25).
We demonstrate that TGFβ1-mediated fibroblast-to-myofibroblast differentiation of primary human prostatic stromal cells (PrSCs) is driven via induction of NOX4/ROS signaling. NOX4/ROS induce the phosphorylation of c-jun N-terminal kinase (JNK), which subsequently activates the downstream transcriptional program of differentiation. Elevated ROS signaling is supported by the concomitant down-regulation of selenium-containing ROS-scavenging enzymes and the selenium transporter SEPP1. Selenium supplementation restored expression of selenium-containing ROS scavengers, increased thioredoxin reductase 1 (TXNRD1) activity, depleted NOX4-derived ROS levels, and attenuated differentiation. The potential clinical value of selenium and/or NOX4 inhibitors in preventing the transformation of stromal cells in BPH and PCa is indicated.
Results
Dysregulation of redox regulators during prostatic fibroblast differentiation
To investigate the molecular changes during BPH/PCa-associated fibroblast-to-myofibroblast differentiation, the expression profiles of TGFβ1-induced differentiated and nondifferentiated PrSCs were analyzed by Affymetrix microarray; 1611 genes were identified with at least 2.5-fold change in their expression levels. Consistent with previous reports, a significant proportion of regulated genes encoded ECM components or enzymes involved in ECM remodeling (9, 15) (Supplemental Table 1 published on the Endocrine Society's Journals online web site at http://mend.endojournals.org). One of the most strongly induced genes was NOX4 (436.6 ± 20.8-fold). Of the other known NOX and associated genes, the regulatory phox subunit p67phox (NCF2) was also significantly up-regulated. In addition, several genes encoding proteins with ROS-scavenging function were significantly down-regulated, including selenoprotein P plasma 1 (SEPP1), glutathione peroxidase 3 (GPX3), thioredoxin (TXN), and thioredoxin reductase 1 (TXNRD1) (Supplemental Table 1). These data were verified by quantitative PCR (qPCR; Fig. 1). The superior sensitivity of qPCR over microarray for the detection of low-abundance transcripts revealed that despite their very low basal expression (ct value <35) NOX1 and NOX5 were marginally but significantly down-regulated during TGFβ1-induced differentiation (−2.8 ± 0.4 and −2.9 ± 0.4 fold, respectively; P-value = 0.0005). NOX2 or NOX3 was not detectably expressed in PrSCs (data not shown). These data suggest that TGFβ1-induced differentiation of PrSCs is associated with a NOX4-driven pro-oxidant shift in redox homeostasis.
Fig. 1.
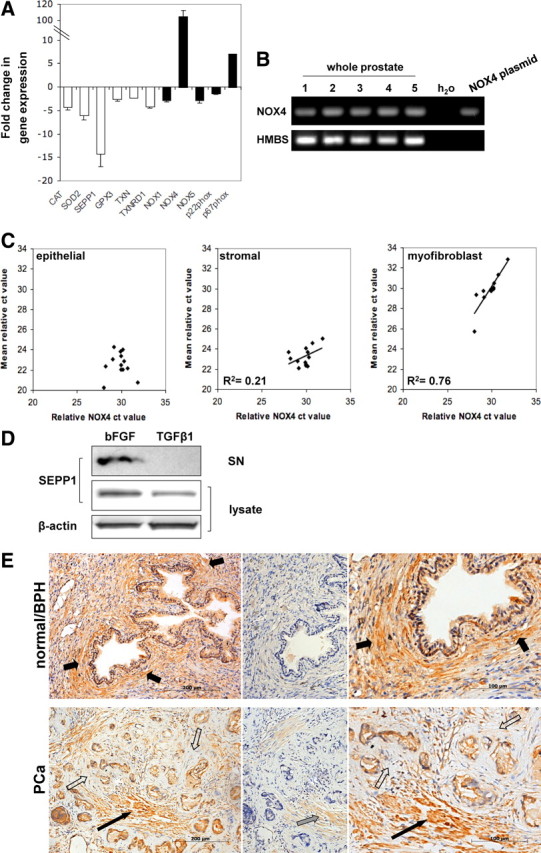
NOX4 and SEPP1 are associated with stromal remodeling in vivo. A, qPCR of ROS-scavenging (white bars) and ROS-producing (black bars) enzymes in PrSCs differentiated with 1 ng/ml TGFβ1 (48 h) relative to control cells incubated with 1 ng/ml bFGF (48 h) to maintain the fibroblast phenotype. Values represent mean fold change (±sem) of four independent experiments using different donors. B and C, NOX4 expression was evaluated in non-tumor-containing human prostate samples. B, RT-PCR of NOX4 (negative control using water as substrate; positive control using plasmid DNA containing full-length NOX4 cDNA). RT-PCR of HMBS is shown as loading control. C, qPCR of NOX4 in prostate specimens (n = 13) relative to the expression of epithelial, stroma, or myofibroblast markers as described in Materials and Methods. D, Western blotting of SEPP1 in lysates and supernatants (SN) of PrSCs treated with 1 ng/ml bFGF or TGFβ1 for 48 h. β-Actin is shown as loading control. A representative blot of three independent experiments is shown. E, SEPP1 immunohistochemistry (left) in normal/BPH and PCa biopsies (Gleason 7), enlarged images are shown (right), preincubation of anti-SEPP1 antibody with blocking peptide (center). Periglandular stromal cells (short black arrows), periglandular tumor stroma (open arrows), SMC bundles (long black arrow), weak immunostaining of SMCs due to incomplete blocking (gray arrow). Sections were counterstained with Mayer's hematoxylin. Tissue specimens were processed in parallel. Images are representative of four independent experiments with specimens from at least eight different donors.
NOX4 expression correlates with the myofibroblast phenotype in vivo
NOX4 expression was verified by qPCR in non-tumor-containing small prostate samples derived from radical prostatectomies (n = 13, Fig. 1B) and compared with the expression of a panel of epithelial-, stromal-, and myofibroblast-specific markers (Fig. 1C). NOX4 exhibited no correlation with eight epithelial markers but weakly correlated with six stromal markers (R2 = 0.21) and more strongly with five different myofibroblast markers (R2 = 0.76). Thus, consistent with our observation from in vitro induced fibroblast-to-myofibroblast differentiation of PrSCs, NOX4 mRNA levels specifically correlate with the myofibroblast phenotype in vivo.
Specific loss of SEPP1 in tumor-associated stroma of human prostate biopsies
Down-regulation of the Se transporter SEPP1 during differentiation (−14.2 ± 2.8-fold by qPCR; Fig. 1A) was confirmed at the protein level in cell lysates by Western blotting (−2.4 ± 0.2 fold; Fig. 1D). Moreover, secreted SEPP1 could be detected in the culture media from prostatic fibroblasts but not in the supernatants from TGFβ1-induced differentiated PrSCs (Fig. 1D).
To determine whether loss of SEPP1 is associated with pathogenic stromal remodeling in vivo, prostate biopsies from normal/BPH and PCa patients were stained for SEPP1 by immunohistochemistry (Fig. 1E). Specificity of the SEPP1 signal was verified by preblocking with a peptide corresponding to residues 244–258 of human SEPP1 against which the antibody was raised (Fig. 1E) (26). In normal prostate (n = 12), strong SEPP1 cytoplasmic staining was observed in basal and luminal epithelial cells and smooth muscle cells (SMCs). Periglandular stromal cells (fibroblasts, perivascular, and endothelial cells) were moderately stained (Fig. 1E). However, in biopsies of PCa patients (Gleason 7, n = 8) SEPP1 immunoreactivity was specifically lost in the periglandular tumor-associated (reactive) stroma whereas adjacent bundles of smooth muscle and tumor cells stained positive (Fig. 1E). Thus, consistent with the reduction of SEPP1 in differentiated PrSCs, the remodeled prostatic stroma in PCa exhibits specific loss of stromal SEPP1.
Elevated ROS production precedes fibroblast differentiation
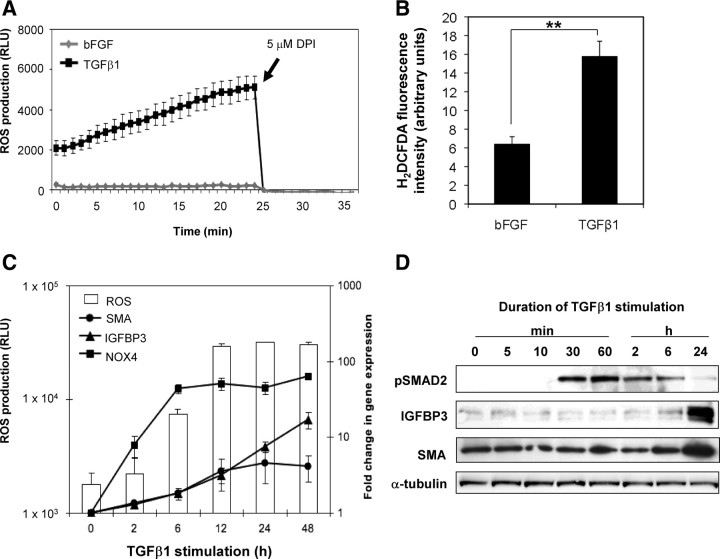
To determine the functional significance of TGFβ1-induced NOX4 expression and suppression of ROS scavengers, ROS production was measured in PrSCs via luminol-based chemiluminescence and using the intracellular probes dihydroethidium (DHE) and 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) (Fig. 2, A and B, and data not shown). In comparison with basic fibroblast growth factor (bFGF)-treated control cells, TGFβ1-differentiated PrSCs produced significantly elevated ROS levels (2.6-fold ± 0.1 by H2DCFDA and 10.2-fold ± 1.7 by luminol), which could be rapidly ablated with the NOX inhibitor diphenylene iodonium (Fig. 2A). No significant change in ROS levels was observed upon PrSC stimulation with phorbol 12-myristate 13-acetate or ionomycin, which induce NOX1 and NOX5 activity, respectively (data not shown). This is consistent with their low expression in PrSCs (as before) and indicates that NOX1 and NOX5 do not significantly contribute to the elevated ROS detected during differentiation.
Fig. 2.
Sustained ROS production precedes fibroblast-to-myofibroblast differentiation. A, ROS production was measured real-time in PrSCs 24 h after stimulation with TGFβ1 or bFGF as control via luminol-based chemiluminescence. Values represent mean of triplicate wells (±sem). A representative example of at least three experiments using independent donors is shown. B, ROS production was measured in PrSCs 48 h after stimulation with TGFβ1or bFGF via H2DCFDA staining and analyzed by fluorescence-activated cell sorting. Values represent mean fluorescence of triplicate samples using three different donors in independent experiments. Significance is indicated (**, P < 0.01). C, Time course assay of ROS production (left y-axis) and qPCR (right y-axis) of PrSCs stimulated for the indicated duration with TGFβ1. Mean values obtained from at least three experiments using independent donors are shown (±sem). D, Western blotting of lysates from PrSCs stimulated with TGFβ1 for the indicated duration with the antibody shown. Blots are representative of three independent experiments using different donors. RLU, Relative light units.
In agreement with tetracycline-inducible NOX4 systems (27), elevated ROS production began 2–6 h after addition of TGFβ1. Peak levels were reached at 12 h and remained steady thereafter (Fig. 2C). Cycloheximide completely abolished TGFβ1-mediated induction of ROS production indicating that de novo protein synthesis is required (data not shown). Elevated ROS production closely correlated with temporal induction of NOX4 expression, whereas up-regulation of differentiation markers smooth muscle cell actin (SMA, ACTG2) and IGF-binding protein 3 (IGFBP3) occurred later (12–24 h; Fig. 2C), a finding confirmed at the protein level (Fig. 2D). Thus, TGFβ1-dependent NOX4 induction and elevated intracellular ROS production precede PrSC differentiation.
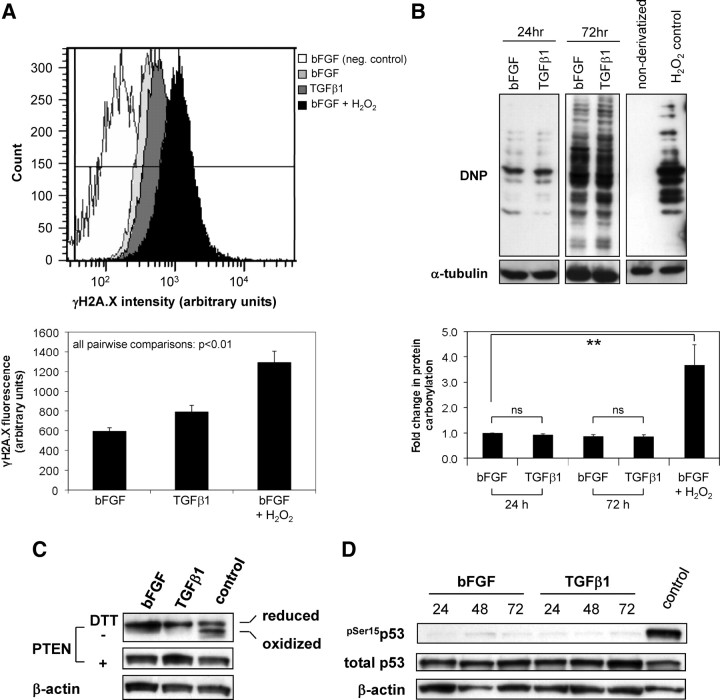
Elevated ROS during differentiation do not impose major global DNA damage or protein oxidation
When cellular ROS-scavenging activity is deficient, high ROS levels may induce nonspecific damage to DNA, proteins, and lipids via irreversible oxidation, termed “oxidative stress” (28). We therefore analyzed the impact of TGFβ1-induced NOX4 activity on γH2A.X levels and the degree of protein carbonylation as markers of genome-wide DNA damage and oxidation in the cellular proteome, respectively (29, 30). Although there was a marginal increase in γH2A.X levels (1.3-fold) during TGFβ1-mediated differentiation, the degree of DNA damage was significantly lower (P = 0.0002) than in hydrogen peroxide control-treated cells (2.2-fold, P = 0.0006) (Fig. 3A). Moreover, no significant change in protein carbonylation was detected in TGFβ1-treated cells relative to bFGF control (Fig. 3B). More specifically, only the reduced (active) form of the readily oxidized protein tyrosine phosphatase family member phosphatase and tensin analog (PTEN), which migrates slower under nonreducing SDS-PAGE relative to the oxidized (inactive) phosphatase (31), was present in lysates of PrSCs stimulated for 24 h with bFGF or TGFβ1 (Fig. 3C). Furthermore, in PrSCs incubated for 24–72 h with TGFβ1, there was no significant increase in phosphorylation of p53 at Ser15, which serves as an early indicator of oxidative stress-induced DNA damage (32) (Fig. 3D). Thus, despite sustained elevated ROS levels and reduced expression of ROS-scavenging enzymes, ROS produced in response to TGFβ1 do not impose major global DNA damage or protein oxidation.
Fig. 3.
Elevated ROS production during differentiation do not induce major global DNA damage or protein oxidation. A, H2A.X phosphorylated at Ser139 was quantified via flow cytometry in PrSCs stimulated for 48 h with either bFGF or TGFβ1. Top panel, Histograms from a single experiment of γH2A.X staining intensity in PrSCs treated as indicated (negative control, omission of primary antibody in bFGF-treated samples). Note the increased (rightward) shift in staining intensity in H2O2 relative to bFGF and TGFβ1-treated samples. Lower panel, Mean values (±sem) of triplicate samples using different donors in three independent experiments. B (top panel), PrSCs were treated for the indicated duration with either bFGF or TGFβ1 before detection of total protein carbonyl levels via immunoblotting for anti-2,4-Dinitrophenol (DNP) immunoreactive proteins in cell extracts derivatized with 2,4-dinitrophenylhydrazine (DNPH). Negative control, nonderivatized cell lysate from H2O2 treated PrSCs. B (lower panel), Densitometric quantification of total protein carbonyl levels in PrSCs treated as before. Mean values (±sem) of three independent experiments using different donors are shown. Significance is indicated (**, P < 0.01; ns, not significant). C and D, Western blotting of lysates from PrSCs stimulated with bFGF or TGFβ1 for the indicated duration (panel C, 24 h; panel D, hours) with the antibody shown. Blots are representative of three independent experiments using different donors. A–D, As positive control, PrSCs were incubated with bFGF for 24 (panels B–D) or 48 h (panel A) before subsequent treatment with 250 μm H2O2 for 60 min. DTT, Dithiothreitol.
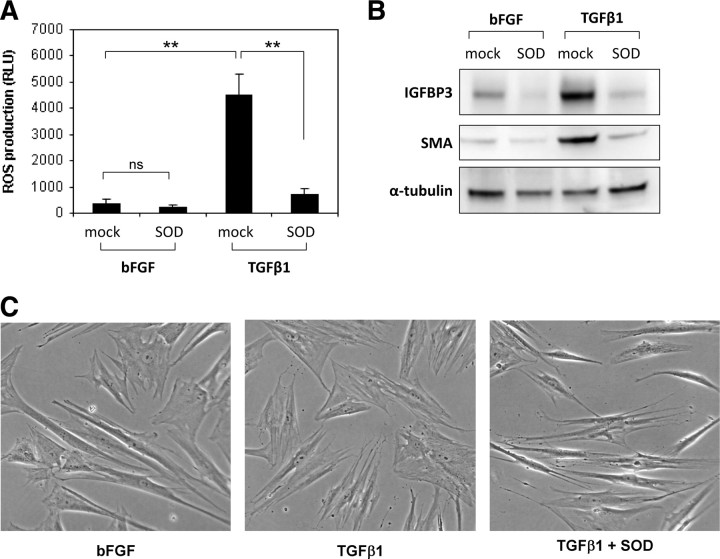
Elevated ROS are essential for fibroblast-to-myofibroblast differentiation
ROS produced in response to growth factors and cytokines are emerging as important second signaling messengers. We therefore investigated whether the elevated ROS produced in response to TGFβ1 are required for PrSC differentiation. To this end, the antioxidant enzyme superoxide dismutase (SOD) conjugated to polyethylene glycol (PEG) to enhance cell permeation was employed. SOD, which catalyzes the dismutation of superoxide into H2O2 and O2, significantly reduced TGFβ1-induced ROS levels as determined by luminol-based chemiluminescence (Fig. 4A). Moreover, SOD inhibited induction of the differentiation markers IGFBP3 and SMA and phenotypic switching (Fig. 4, B and C). These data provide key evidence that ROS, most likely superoxide, are essential for TGFβ1-induced differentiation in PrSCs.
Fig. 4.
ROS are essential for fibroblast-to-myofibroblast differentiation. PrSCs were incubated with PEG-conjugated SOD (PEG-SOD, 60 U/ml) and bFGF or TGFβ1 as indicated for 24 h before (panel A) luminol-based chemiluminescent detection of ROS production (panel A), Western blotting using the indicated antibodies (panel B) or phase contrast microscopy (panel C) (magnification ×40). A, Values represent the mean (±sem) of triplicate wells in three independent experiments using different donors. Significance is indicated (**, P < 0.01; ns, not significant). C, Note the thin, elongated, and light-refractive phenotype of bFGF-treated PrSCs (fibroblasts) in comparison with the flattened and less light-refractive morphology of TGFβ1-differentiated PrSCs (myofibroblasts). B and C, Images are representative of at least four independent experiments using different donors. RLU, Relative light units.
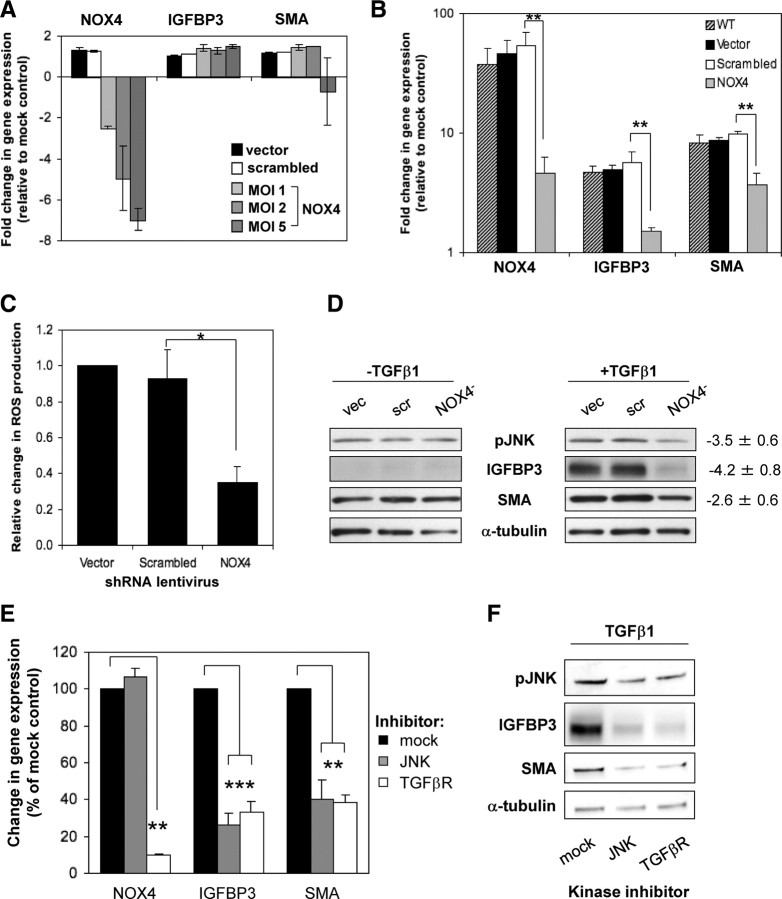
NOX4 is essential for fibroblast-to-myofibroblast differentiation
To confirm that NOX4 is the ROS-producing source in response to TGFβ1, NOX4-specific lentiviral-delivered shRNA was employed (Fig. 5A). NOX4 short hairpin RNA (shRNA) reduced basal NOX4 expression in a dose-dependent manner and significantly attenuated TGFβ1-induced NOX4 expression (45.9 ±4.7-fold in vector and scrambled control cells) to just 8.3 ± 2.8-fold [multiplicity of infection (MOI) 2; Fig. 5B]. Expression of the weakly detectable NOX1 and NOX5 was not significantly altered (data not shown). Due to the limited availability of NOX4-specific immunological agents (33), it was not possible to verify NOX4 knockdown at the protein level.
Fig. 5.
NOX4-derived ROS mediate differentiation via increased JNK phosphorylation. A, qPCR of PrSCs infected with the indicated shRNA-expressing lentivirus at MOI 2 [vector (vec) and scrambled (scr)] or the indicated MOI (NOX4) for 96 h. B, qPCR of PrSCs infected as above (MOI 2) and subsequently stimulated for 24 h with TGFβ1. A and B, Mean values (±sem) of at least three experiments using independent donors are shown relative to nontransduced mock-treated PrSCs. C, Luminol-based chemiluminescent detection of ROS production by PrSCs treated as in panel B. Values represent mean fold change in ROS production (±sem) from triplicate wells in at least three experiments using independent donors relative to vector control cells. D, Western blotting of total cell lysates from PrSCs treated as in panel B in the presence or absence of TGFβ1 for 24 h. A representative example of four independent experiments using different donors is shown. Values denote densitometric quantification of bands from NOX4 shRNA-treated lysates relative to combined scores from vector and scrambled shRNA-treated lysates (mean ± sem). E and F, PrSCs were treated with TGFβ1 and the indicated inhibitor (JNK, 1 μm SP600125; ALK5/TGFβR1, 1 μm SB431542) for 24 h before qPCR of the indicated genes (panel E) or Western blotting of total cell lysates using the antibodies indicated (panel B). E, Mean values from at least three independent experiments using different donors are shown expressed as percentage (±sem) relative to mock control treated with TGFβ1 and dimethylsulfoxide (DMSO) equivalent. F, A representative example of three independent experiments using different donors is shown. Significance is indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
We next investigated whether NOX4 silencing reduced TGFβ1-induced ROS production. Indeed, NOX4 knockdown reduced TGFβ1-induced ROS levels by 64.9% ± 9.1 (Fig. 5C). Residual ROS levels were most likely due to incomplete silencing of NOX4 because higher levels of NOX4 lentivirus (MOI 5) further reduced TGFβ1-induced ROS production (data not shown). However, cell viability was impaired at MOI > 6, which is consistent with a threshold basal level of NOX4-derived ROS being essential for cell survival (34, 35). Subsequent experiments thus employed lentivirus at MOI 2. Under these conditions, NOX4 knockdown significantly attenuated TGFβ1 induction of differentiation markers IGFBP3 and SMA at the mRNA (−3.7 ± 0.2- and −2.5 ± 0.4-fold, respectively; Fig. 5B) and protein level (Fig. 5D) compared with vector and scrambled control cells. Basal IGFBP3 and SMA mRNA and protein levels were not affected by NOX4 knockdown (Fig. 5, A and D, respectively). The morphological changes of PrSC fibroblast-to-myofibroblast differentiation (15) were also inhibited upon NOX4 silencing (data not shown). Collectively, these data establish NOX4 as the predominant ROS-producing source induced by TGFβ1 in PrSCs and an essential mediator of fibroblast-to-myofibroblast differentiation.
NOX4 induces JNK phosphorylation to mediate differentiation
The intracellular response to cytokines including TGFβ1 is transduced by the concerted action of numerous kinases and phosphatases, the activity of which is frequently redox sensitive (17). We therefore examined the effect of NOX4 silencing on the phosphorylation status of different kinases during differentiation. TGFβ1-induced phosphorylation of PKC and PKB/AKT was not perturbed by NOX4 knockdown, and p38 MAPK was not detectably phosphorylated in PrSCs before or after differentiation (data not shown). However, NOX4 silencing reduced TGFβ1-stimulated but not basal phosphorylation of JNK (Fig. 5D).
Using a JNK-specific inhibitor (SP600125), we examined the requirement of JNK during differentiation. Although there was no significant change in TGFβ1 induction of NOX4 mRNA (Fig. 5E), TGFβ1-induction of IGFBP3 and SMA and morphological differentiation were inhibited by SP600125 (Fig. 5F and data not shown). Collectively, these data indicate that NOX4 is required for JNK phosphorylation, which in turn coordinates the downstream differentiation response to TGFβ.
Selenium attenuates differentiation by restoring ROS-scavenging selenoenzyme activity
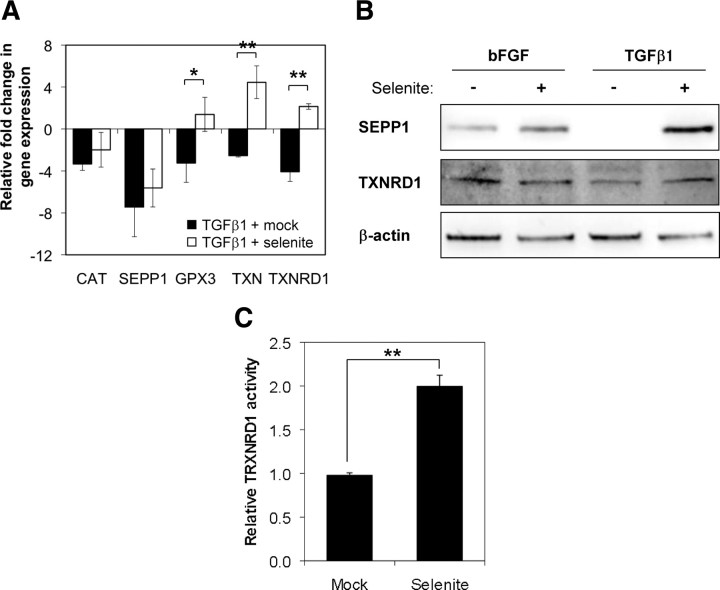
The above data suggest that abrogating NOX4-derived ROS signaling may represent a therapeutic strategy to inhibit fibroblast-to-myofibroblast differentiation in BPH and PCa; however, there are currently no NOX4-specific inhibitors. We therefore examined whether exogenous Se was sufficient to restore expression/activity of selenium-containing ROS-scavenging enzymes and thereby abrogate NOX4-derived ROS signaling to inhibit differentiation. Subcytotoxic concentrations (5 nm) of selenium as inorganic sodium selenite significantly increased basal expression of TXN and the selenoenzymes GPX3 and TXNRD1 but not that of non-selenium-containing CAT (data not shown). Moreover, differentiation-associated down-regulation of GPX3, TXN, and TXNRD1 was completely inhibited, whereas CAT expression remained comparable to cells treated with TGFβ1 alone (Fig. 6A). Despite SEPP1 mRNA levels being unchanged by selenite treatment (Fig. 6A), SEPP1 protein levels increased upon addition of selenite (Fig. 6B). In addition, TXNRD1 mRNA and protein levels and enzyme activity were significantly increased upon selenite treatment (TXNRD1 activity 2.0 ± 0.1; P = 0.004) (Fig. 6, B and C).
Fig. 6.
Selenite restores expression and activity of ROS-scavenging selenoenzymes. A, qPCR of the indicated genes in PrSCs pretreated for 12 h with 5 nm sodium selenite or mock control before stimulation with TGFβ1 for a further 24 h. Values represent mean fold change in gene expression (±sem) relative to bFGF control (without selenite). B, Western blotting of total cell lysates from cells preincubated with selenite as in panel A and subsequently stimulated either with bFGF or TGFβ1 as indicated in the presence or absence of selenite for a further 24 h. Blots are representative of three independent experiments using different donors. C, Mean fold change in TXNRD1 enzyme activity (±sem) in cell extracts from PrSCs treated with 5 nm selenite relative to mock-treated controls. A–C, Data are derived from at least three independent experiments using different donors. Significance is indicated (**, P < 0.01; *, P < 0.05).
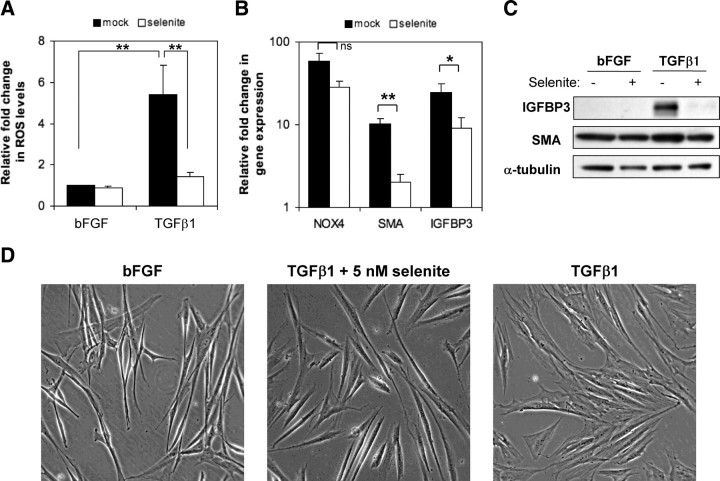
Consistent with increased selenoenzyme ROS-scavenging activity, selenite strongly reduced TGFβ1-induced ROS levels (9.0 ± 3.8-fold; P = 0.01) without significantly attenuating TGFβ1 induction of NOX4 mRNA (−2.1 ± 0.3 fold, p-value = 0.07) (Fig. 7, A and B). Basal levels of the differentiation markers IGFBP3 and SMA were unaffected by selenite treatment (Fig. 7C and data not shown). However, the attenuation of ROS induction by selenite was sufficient to inhibit TGFβ1-mediated induction of IGFBP3 and SMA at the mRNA and protein level (Fig. 7, B and C). In addition, selenite reduced pJNK levels as observed upon NOX4 knockdown. Moreover, selenite inhibited phenotypic switching associated with TGFβ1-induced differentiation (Fig. 7D). Collectively, these data indicate that selenite abrogates the initiated TGFβ1-induced differentiation cascade by restoring the biosynthesis and activity of ROS-scavenging selenoenzymes, thereby depleting NOX4-derived ROS and attenuating ROS signaling.
Fig. 7.
Selenite inhibits TGFβ1-mediated fibroblast-to-myofibroblast differentiation. PrSCs were pretreated for 12 h with 5 nm sodium selenite or mock control before stimulation with 1 ng/ml bFGF or TGFβ1 in the presence or absence of selenite for a further 24 h. Cells were subsequently processed for (A) ROS determination via luminol-based chemilumiscence (panel A), qPCR of the indicated genes (panel B), Western blotting of total cell lysates using the antibodies indicated (panel C) or phase contrast microscopy (panel D) (magnification ×40). Note the thin, elongated, and light-refractive phenotype of bFGF-treated PrSCs (fibroblasts) in comparison with the flattened and less light-refractive morphology of TGFβ1-differentiated PrSCs (myofibroblasts). C and D, Images are representative of at least four independent experiments using different donors. A and B, Values represent mean fold change (±sem) relative to bFGF control (without selenite) from four independent experiments using different donors. Significance is indicated (**, P < 0.01; *, P < 0.05; ns not significant).
Discussion
Stromal remodeling via fibroblast-to-myofibroblast differentiation promotes the development of BPH and PCa. Elevated production of TGFβ1, a potent inducer of fibroblast differentiation in vitro and in vivo, is considered the inducing stimulus (15, 16, 36, 37). We demonstrate that ROS signaling by NOX4 induces fibroblast-to-myofibroblast differentiation in PrSCs by increasing phosphorylation of JNK, which coordinates downstream cytoskeletal remodeling and phenotypic differentiation. NOX4 specifically correlated in vivo with the myofibroblast phenotype, the predominant stromal cell type in BPH and PCa. Moreover, loss of the Se transporter SEPP1 was observed in the tumor-associated stroma of PCa biopsies. To our knowledge this is the first report demonstrating dysregulation of redox homeostasis in stromal remodeling in BPH and PCa.
NOX4 is the major source of elevated ROS during TGFβ1-mediated PrSC differentiation as demonstrated by isoform-specific knockdown. The abrogation of differentiation upon depletion of superoxide by SOD demonstrated the critical role of NOX4-derived ROS as mediators of differentiation and, moreover, suggested that superoxide is the primary ROS signaling mediator rather than its dismutation product H2O2.
In contrast to many peptide growth factors that induce transient ROS production, PrSCs undergoing differentiation produce sustained elevated levels of intracellular ROS as demonstrated using the intracellular redox-sensitive probes DHE and H2DCFDA. Nonetheless, TGFβ1-differentiated PrSCs do not exhibit major global DNA damage or protein oxidation, indicating that ROS produced in response to TGFβ1 in PrSCs act primarily as intracellular signaling molecules to coordinate differentiation. In addition to the prostate, TGFβ1, as well as other peptide growth factors, induces NOX4 expression and ROS production in cells from diverse tissues, including liver, lung, heart, and kidney (20, 21, 38). This suggests that NOX4-derived ROS are a common mediator of TGFβ/peptide growth factor signal transduction.
The signaling functions of ROS are primarily mediated by oxidative modification of redox-sensitive proteins, including transcription factors (e.g. nuclear factor-κB, activator protein 1, hypoxia inducible factor 1, p53), protein tyrosine phosphatases, and protein tyrosine kinases (17). Typically, ROS inactivate protein tyrosine phosphatases but activate protein tyrosine kinases and thereby promote kinase cascades. Consistently, PrSC differentiation was associated with NOX4/ROS-dependent phosphorylation of JNK, which was confirmed using pharmacological inhibition to be essential for transducing the TGFβ1 differentiation signal downstream of NOX4. The precise NOX4/ROS target(s) that is responsible for elevated JNK phosphorylation remain to be identified; however, during differentiation we observed NOX4/ROS-dependent down-regulation of DUSP10, which encodes a dual-specificity phosphatase that selectively dephosphorylates JNK and p38 (39) (data not shown). These data would be consistent with the sustained NOX4/ROS-dependent phosphorylation of JNK during differentiation and suggest that NOX4 modulates pJNK levels, at least in part, by targeting transcription factor(s) that regulate the expression of DUSP phosphatase(s).
Although targeting NOX4-derived ROS signaling directly for therapeutic intervention of PCa/BPH remains a possibility, there are currently no specific NOX4 inhibitors. We therefore explored the alternative strategy of increasing ROS-scavenging activity. The primary function of SEPP1 is considered the transport of Se to peripheral tissues, which is required for the expression and biosynthesis of selenoproteins (23, 24, 40–42). Thus, down-regulation of SEPP1 during differentiation, a direct transcriptionally suppressed target of TGFβ1/SMAD (43), may result in cellular Se deficiency and decreased selenoenzyme ROS- scavenging activity and thereby potentiate NOX4-derived ROS signaling. Indeed, selenite-mediated inhibition of differentiation was associated with 1) reduced TGFβ-induced ROS without a reduction in NOX4 mRNA levels; 2) elevated mRNA levels of the selenoenzymes GPX3 and TXNRD1 as reported previously (41, 42, 44); 3) induced TXNRD1 protein levels; and 4) increased TXNRD1 enzyme activity. Selenite had no effect on SEPP1 mRNA levels, most likely due to upstream inhibition by TGFβ1/SMAD (43); however, SEPP1 protein levels were increased presumably via posttranslational mechanisms (45). Collectively, these data suggest that selenite attenuates fibroblast-to-myofibroblast differentiation via enhanced biosynthesis of ROS-scavenging selenoenzymes, which depletes TGFβ1-induced NOX4-derived ROS, thereby preventing dysregulated NOX4/ROS signaling.
These findings are consistent with a large body of data in experimental animals that Se deficiency or supplementation increases or reduces tumor incidence, respectively (46–48). However, several large-scale clinical and epidemiological studies yielded conflicting results relating plasma Se levels to the risk of PCa and the protective effect of Se supplementation on PCa incidence (49–52). Clearly, further well-designed studies are required to encompass a number of factors that may have contributed to these inconsistencies, e.g. the source and dose of the Se supplement employed, baseline Se levels, individual Se requirements, and genetic variations within antioxidant and selenoprotein genes (53, 54). However, together with the data herein the significant reduction in PCa incidence observed in the Nutritional Prevention of Cancer study suggests that Se supplementation may benefit subpopulations in whom activity of disease-relevant selenoenzymes are suboptimal, perhaps due to environmental and/or genetic factors (52, 53).
In summary, NOX4-derived ROS are essential TGFβ1 signaling effectors that induce the phosphorylation of JNK. Thereby, downstream transcriptional cascades are activated leading to prostatic fibroblast-to-myofibroblast differentiation. ROS signaling and differentiation are supported by the concomitant down-regulation of ROS-scavenging selenoenzymes, which can be attenuated by the addition of Se. To our knowledge, these data are the first to demonstrate dysregulation of redox homeostasis in pathogenic activation of stromal fibroblasts in age-related proliferative diseases of the prostate and point to the potential clinical benefit of Se supplementation and/or local NOX4 inhibition in stromal-targeted therapy. Given that TGFβ signaling and myofibroblast activation are associated with numerous fibrotic disorders (e.g. idiopathic lung pulmonary fibrosis, nephrogenic systemic fibrosis, hypertrophic scarring, proliferative vitreoretinopathies, atherosclerotic lesions) and tumorigenesis, it will be interesting to determine whether similar NOX4-dependent processes are at work.
Materials and Methods
Reagents
Reagents were from Sigma Aldrich (St. Louis, MO) unless otherwise specified. Human recombinant TGFβ1 was from R&D Systems (Minneapolis, MN); kinase inhibitors and concentrations employed were as follows: TGFβ type 1 receptor activin receptor-like kinase ALK5 inhibitor SB431542 (1 μm; Tocris Bioscience, Ellisville, MO), JNK inhibitor SP600125 (1 μm; Calbiochem, La Jolla, CA). Antibodies were obtained as follows: p53, phospho-JNK, TXNRD1, and α-tubulin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); IGFBP3 and phospho-SMAD2/3 (R&D Systems); β-actin and α-SMA (Sigma), phospho-p53, -H2A.X, and PTEN (Cell Signaling Technology, Danvers, MA); and horseradish peroxidase-conjugated secondary antibodies (Promega Corp., Madison, WI) SEPP1 was a kind gift from Holger Steinbrenner (Düsseldorf, Germany).
Primary cell culture
Human primary prostatic fibroblasts (PrSCs) were established from prostate organoids as described previously (15). PrSCs were maintained for routine culture in stromal cell growth medium (Lonza, Basel, Switzerland) at 37 C in a humidified atmosphere of 5% CO2. For all experiments, cells of passage 2–4 were used directly from culture (not previously frozen). For differentiation, PrSCs were incubated for 12 h in RPMI 1640 (Lonza) supplemented with 1% charcoal-treated bovine calf serum (ctBCS) (Hyclone Laboratories, Logan, UT) and antibiotics. Cells were subsequently stimulated with either 1 ng/ml bFGF as mock control or 1 ng/ml TGFβ1 for the indicated duration. For kinase/antioxidant inhibition, cells were pretreated for 1 h with the appropriate kinase inhibitor/antioxidant or dimethylsulfoxide/PEG equivalent before stimulation with bFGF or TGFβ1 as indicated. All experiments were performed at least three times with primary cells from different donors.
RNA isolation, cDNA synthesis and qPCR
Prostate samples from the ventral part of the prostate were obtained after radical prostatectomy (n = 13), snap frozen, and stored in liquid nitrogen before homogenization and total RNA isolation using TriZol reagent (Invitrogen, Carlsbad, CA). Total RNA from PrSCs was isolated using TriFast reagent (PeqLab, Erlangen, Germany). cDNA synthesis and qPCR were performed as described elsewhere (15). Primer sequences are given in Supplemental Table 2. For PrSC experiments cDNA concentrations were normalized by the internal standard hydroxymethylbilane synthase (HMBS), a moderate copy number housekeeping gene not regulated under the experimental conditions employed. Relative changes in gene expression were calculated as described elsewhere (55). For prostate samples cDNA concentrations were normalized to HMBS and EEF1A1. NOX4 expression was compared with the geometric mean expression (ct) value of epithelial markers (KLK3, KLK2, DPP4, EHF, CDH1, TMPRSS2, CORO2A, and KRT5), stromal markers (SMA, IGF-I, TGFB1I1, OGN, CNN1, PAGE4), or myofibroblast markers (COMP, PLN, RARRES1, COL4A1, TNC).
Microarrays
PrSCs from three independent donors incubated overnight in 1% charcoal-treated FBS/RPMI were stimulated either with 1 ng/ml bFGF as mock control or with 1 ng/ml TGFβ1 for 48 h. From each donor 2 μg total RNA were pooled and hybridization to Affymetrix Human Genome U133 Plus 2.0 GeneChips was performed at the Microarray Facility (Tübingen, Germany). A technical replicate array was performed. Raw expression data were normalized using the GRCMA algorithm at CARMAweb (56, 57). The complete microarray dataset is available at ArrayExpress (E-MEXP-2167).
Lentiviral-mediated knockdown of NOX4
NOX4, scrambled, and empty vector shRNA lentiviral particles were generated as described elsewhere (58). For viral transduction, PrSCs were seeded in appropriate vessels in stromal cell growth medium. The following day, media were replenished supplemented with 8 μg/ml polybrene and virus-containing supernatant at the MOI indicated. After 96 h, cells were incubated overnight in 1% ctBCS-supplemented RPMI containing antibiotics before stimulation with 1 ng/ml TGFβ1 for the duration indicated. In all experiments, empty pLKO.1 vector and/or scramble shRNA vector (Addgene plasmid 1864) was used as controls.
Determination of ROS production
For luminol-based chemiluminescent ROS detection, 20,000 PrSCs in triplicate in 24-well plates were incubated overnight in 1% ctBCS in RPMI before stimulation as indicated. Cell monolayers were rinsed with prewarmed Hanks' Buffered Salt Solution (HBSS, Lonza) without Ca2+ and Mg2+ and incubated with 4 U/ml horseradish peroxidase and 10 μg/ml luminol in HBSS. Luminescence was measured on a Chameleon luminescence counter (HVD Life Sciences, Vienna, Austria) at 37 C. Values were normalized against cell number using the Cell Titer Glo Luminescence assay reagent (Promega).
ROS production was also measured via CM-H2DCFDA in 2 × 105 PrSCs seeded in triplicate in 6-cm dishes and differentiated as above. Cells were trypsinized and rinsed in prewarmed HBSS before loading with 10 μm CM-H2DCFDA (Invitrogen) in HBSS for 30 min at 37 C. After washing, cells were resuspended in 500 μl HBSS and analyzed by flow cytometry on a FACSCanto II (BD Biosciences, Palo Alto, CA).
Western blotting and immunohistochemistry
Isolation of total cell lysates and Western blotting were performed as described previously (15) and normalized for total protein content via Bradford assay (Bio-Rad Laboratories, Hercules, CA). Detection of protein carbonylation was performed as described elsewhere (59). For analysis of PTEN oxidation, lysates were prepared in the presence of 10 mm N-ethylmaleimide to prevent cysteine oxidation during lysis. Prostate tissue sections from paraffin blocks of formalin-fixed whole-biopsy specimens (obtained from the archives of the Institute of Pathology at the University Hospital Basel, Switzerland) were processed for immunohistochemistry as described elsewhere (60). Where indicated SEPP1 antibody (1:500) was preblocked overnight at 4 C in 1% BSA/PBS containing 50 μg/ml blocking peptide (244–258 amino acids, AltaBioscience, Birmingham, UK).
Analysis of oxidative damage to DNA
PrSCs (5 × 105) seeded in triplicate in 10-cm dishes were incubated overnight in 1% ctBCS in RPMI before stimulation with bFGF or TGFβ1 for 48 h. Histone H2A.X phosphorylated at Ser139 (γH2A.X) was detected via flow cytometry on a FACSCanto II (BD Biosciences) after immunostaining according to the manufacturer's instructions (Cell Signaling Technology). PrSCs treated with non-apoptosis-inducing concentrations of H2O2 (250 μm for 60 min) served as positive control.
TXNRD1 enzyme activity
PrSCs (4.5 × 105) seeded in duplicate in 6-cm dishes were differentiated for 48 h. Cell monolayers were rinsed in ice-cold PBS before being resuspended in 150 μl lysis buffer (0.5% Triton X-100; 0.5% deoxycholate; 150 mm NaCl; 10 mm Tris-HCl, pH 7.5; 5 mm EDTA; and protease inhibitors). Samples were incubated on ice for 30 min before centrifugation at 13,000 rpm for 15 min at 4 C. Cleared supernatants were normalized for total protein content via Bradford assay before determination of TRXND1 activity by a TXNRD1 activity assay kit (Abcam, Cambridge, MA) according to the manufacturer's instructions.
Statistical analysis
Numerical data are presented as mean ± sem from at least three independent experiments using independent donors. Statistical evaluation was performed using a Student's t test (not significant (ns) P > 0.05; *, P < 0.05; **, P < 0.01).
Acknowledgments
We gratefully acknowledge Professor Stephan Dirnhofer (University Hospital Basel, Switzerland) for assistance with immunohistochemistry and Dr Barbara Lener for technical assistance with the generation of NOX4 shRNA lentivirus.
This work was supported by the Austrian Science Fund (FWF; NRN S9307-B05 and S9305-B05); N.S. is the recipient of a Lise Meitner Scholarship (FWF; M903-B05).
Disclosure Summary: The authors have no potential conflicts of interest.
Footnotes
- bFGF
- Basic fibroblast growth factor
- BPH
- benign prostatic hyperplasia
- CM-H2DCFDA 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
- acetyl ester
- ctBCS
- charcoal-treated bovine calf serum
- ECM
- extracellular matrix
- GPX3
- glutathione peroxidase 3
- HBSS
- Hanks’ Buffered Salt Solution
- HMBS
- hydroxymethylbilane synthase
- IGFBP3
- IGF binding protein 3
- MOI
- multiplicity of infection
- NOX
- reduced nicotinamide adenine dinucleotide phosphate oxidase
- PCa
- prostate cancer
- PEG
- polyethylene glycol
- PrSC
- primary prostatic stromal cell
- PTEN
- phosphatase and tensin analog
- qPCR
- quantitative PCR
- SEPP1
- selenoprotein P plasma 1
- shRNA
- short hairpin RNA
- SMA
- smooth muscle cell actin
- SMC
- smooth muscle cell
- SOD
- superoxide dismutase
- TXN
- thioredoxin
- TXNRD1
- thioredoxin reductase 1.
References
- 1. Sampson N , Untergasser G , Plas E , Berger P. 2007. The ageing male reproductive tract. J Pathol 211:206–218 [DOI] [PubMed] [Google Scholar]
- 2. Isaacs JT. 1994. Etiology of benign prostatic hyperplasia. Eur Urol 25(Suppl 1):6–9 [DOI] [PubMed] [Google Scholar]
- 3. Jemal A , Siegel R , Ward E , Murray T , Xu J , Smigal C , Thun MJ. 2006. Cancer statistics, 2006. CA Cancer J Clin 56:106–130 [DOI] [PubMed] [Google Scholar]
- 4. Olumi AF , Grossfeld GD , Hayward SW , Carroll PR , Tlsty TD , Cunha GR. 1999. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59:5002–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barclay WW , Woodruff RD , Hall MC , Cramer SD. 2005. A system for studying epithelial-stromal interactions reveals distinct inductive abilities of stromal cells from benign prostatic hyperplasia and prostate cancer. Endocrinology 146:13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ao M , Franco OE , Park D , Raman D , Williams K , Hayward SW. 2007. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res 67:4244–4253 [DOI] [PubMed] [Google Scholar]
- 7. Yang F , Tuxhorn JA , Ressler SJ , McAlhany SJ , Dang TD , Rowley DR. 2005. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res 65:8887–8895 [DOI] [PubMed] [Google Scholar]
- 8. Tuxhorn JA , McAlhany SJ , Yang F , Dang TD , Rowley DR. 2002. Inhibition of transforming growth factor-β activity decreases angiogenesis in a human prostate cancer-reactive stroma xenograft model. Cancer Res 62:6021–6025 [PubMed] [Google Scholar]
- 9. Verona EV , Elkahloun AG , Yang J , Bandyopadhyay A , Yeh IT , Sun LZ. 2007. Transforming growth factor-β signaling in prostate stromal cells supports prostate carcinoma growth by up-regulating stromal genes related to tissue remodeling. Cancer Res 67:5737–5746 [DOI] [PubMed] [Google Scholar]
- 10. Tiwari A. 2007. Advances in the development of hormonal modulators for the treatment of benign prostatic hyperplasia. Expert Opin Invest Drugs 16:1425–1439 [DOI] [PubMed] [Google Scholar]
- 11. Taplin ME. 2008. Androgen receptor: role and novel therapeutic prospects in prostate cancer. Expert Rev Anticancer Ther 8:1495–1508 [DOI] [PubMed] [Google Scholar]
- 12. Li Z , Habuchi T , Tsuchiya N , Mitsumori K , Wang L , Ohyama C , Sato K , Kamoto T , Ogawa O , Kato T. 2004. Increased risk of prostate cancer and benign prostatic hyperplasia associated with transforming growth factor-β 1 gene polymorphism at codon 10. Carcinogenesis 25:237–240 [DOI] [PubMed] [Google Scholar]
- 13. Wikström P , Stattin P , Franck-Lissbrant I , Damber JE , Bergh A. 1998. Transforming growth factor β1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate 37:19–29 [DOI] [PubMed] [Google Scholar]
- 14. Tuxhorn JA , Ayala GE , Smith MJ , Smith VC , Dang TD , Rowley DR. 2002. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 8:2912–2923 [PubMed] [Google Scholar]
- 15. Untergasser G , Gander R , Lilg C , Lepperdinger G , Plas E , Berger P. 2005. Profiling molecular targets of TGF-β1 in prostate fibroblast-to-myofibroblast transdifferentiation. Mech Ageing Dev 126:59–69 [DOI] [PubMed] [Google Scholar]
- 16. Roberts AB , Sporn MB , Assoian RK , Smith JM , Roche NS , Wakefield LM , Heine UI , Liotta LA , Falanga V , Kehrl JH , Fauci AS. 1986. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 83:4167–4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trachootham D , Lu W , Ogasawara MA , Nilsa RD , Huang P. 2008. Redox regulation of cell survival. Antioxid Redox Signal 10:1343–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bedard K , Krause KH. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313 [DOI] [PubMed] [Google Scholar]
- 19. Torres M. 2003. Mitogen-activated protein kinase pathways in redox signaling. Front Biosci 8:d369–d391 [DOI] [PubMed] [Google Scholar]
- 20. Cucoranu I , Clempus R , Dikalova A , Phelan PJ , Ariyan S , Dikalov S , Sorescu D. 2005. NAD(P)H oxidase 4 mediates transforming growth factor-β1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97:900–907 [DOI] [PubMed] [Google Scholar]
- 21. Hecker L , Vittal R , Jones T , Jagirdar R , Luckhardt TR , Horowitz JC , Pennathur S , Martinez FJ , Thannickal VJ. 2009. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15:1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bellinger FP , Raman AV , Reeves MA , Berry MJ. 2009. Regulation and function of selenoproteins in human disease. Biochem J 422:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Low SC , Grundner-Culemann E , Harney JW , Berry MJ. 2000. SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. EMBO J 19:6882–6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crane MS , Howie AF , Arthur JR , Nicol F , Crosley LK , Beckett GJ. 2009. Modulation of thioredoxin reductase-2 expression in EAhy926 cells: implications for endothelial selenoprotein hierarchy. Biochim Biophys Acta 1790:1191–1197 [DOI] [PubMed] [Google Scholar]
- 25. Saito Y , Sato N , Hirashima M , Takebe G , Nagasawa S , Takahashi K. 2004. Domain structure of bi-functional selenoprotein P. Biochem J 381:841–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mostert V , Lombeck I , Abel J. 1998. A novel method for the purification of selenoprotein P from human plasma. Arch Biochem Biophys 357:326–330 [DOI] [PubMed] [Google Scholar]
- 27. Serrander L , Cartier L , Bedard K , Banfi B , Lardy B , Plastre O , Sienkiewicz A , Fórró L , Schlegel W , Krause KH. 2007. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Valko M , Leibfritz D , Moncol J , Cronin MT , Mazur M , Telser J. 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84 [DOI] [PubMed] [Google Scholar]
- 29. Rogakou EP , Pilch DR , Orr AH , Ivanova VS , Bonner WM. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273:5858–5868 [DOI] [PubMed] [Google Scholar]
- 30. Amici A , Levine RL , Tsai L , Stadtman ER. 1989. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J Biol Chem 264:3341–3346 [PubMed] [Google Scholar]
- 31. Lee SR , Yang KS , Kwon J , Lee C , Jeong W , Rhee SG. 2002. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem 277:20336–20342 [DOI] [PubMed] [Google Scholar]
- 32. Nair VD , Yuen T , Olanow CW , Sealfon SC. 2004. Early single cell bifurcation of pro- and antiapoptotic states during oxidative stress. J Biol Chem 279:27494–27501 [DOI] [PubMed] [Google Scholar]
- 33. Nauseef WM. 2008. Biological roles for the NOX family NADPH oxidases. J Biol Chem 283:16961–16965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peshavariya H , Dusting GJ , Jiang F , Halmos LR , Sobey CG , Drummond GR , Selemidis S. 2009. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn-Schmiedebergs Arch Pharmacol 380:193–204 [DOI] [PubMed] [Google Scholar]
- 35. Shono T , Yokoyama N , Uesaka T , Kuroda J , Takeya R , Yamasaki T , Amano T , Mizoguchi M , Suzuki SO , Niiro H , Miyamoto K , Akashi K , Iwaki T , Sumimoto H , Sasaki T. 2008. Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int J Cancer 123:787–792 [DOI] [PubMed] [Google Scholar]
- 36. Kyprianou N , Tu H , Jacobs SC. 1996. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol 27:668–675 [DOI] [PubMed] [Google Scholar]
- 37. Cardillo MR , Petrangeli E , Perracchio L , Salvatori L , Ravenna L , Di Silverio F. 2000. Transforming growth factor-beta expression in prostate neoplasia. Anal Quant Cytol Histol 22:1–10 [PubMed] [Google Scholar]
- 38. Sturrock A , Huecksteadt TP , Norman K , Sanders K , Murphy TM , Chitano P , Wilson K , Hoidal JR , Kennedy TP. 2007. Nox4 mediates TGF-β1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292:L1543–L1555 [DOI] [PubMed] [Google Scholar]
- 39. Theodosiou A , Smith A , Gillieron C , Arkinstall S , Ashworth A. 1999. MKP5, a new member of the MAP kinase phosphatase family, which selectively dephosphorylates stress-activated kinases. Oncogene 18:6981–6988 [DOI] [PubMed] [Google Scholar]
- 40. Saito Y , Takahashi K. 2002. Characterization of selenoprotein P as a selenium supply protein. Eur J Biochem 269:5746–5751 [DOI] [PubMed] [Google Scholar]
- 41. Barnes KM , Evenson JK , Raines AM , Sunde RA. 2009. Transcript analysis of the selenoproteome indicates that dietary selenium requirements of rats based on selenium-regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activity. J Nutr 139:199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clarke C , Baghdadi H , Howie AF , Mason JI , Walker SW , Beckett GJ. 2010. Selenium supplementation attenuates procollagen-1 and interleukin-8 production in fat-loaded human C3A hepatoblastoma cells treated with TGFβ1. Biochim Biophys Acta 1800:611–618 [DOI] [PubMed] [Google Scholar]
- 43. Mostert V , Dreher I , Kohrle J , Wolff S , Abel J. 2001. Modulation of selenoprotein P expression by TGF-β(1) is mediated by Smad proteins. BioFactors 14:135–142 [DOI] [PubMed] [Google Scholar]
- 44. Gallegos A , Berggren M , Gasdaska JR , Powis G. 1997. Mechanisms of the regulation of thioredoxin reductase activity in cancer cells by the chemopreventive agent selenium. Cancer Res 57:4965–4970 [PubMed] [Google Scholar]
- 45. Yang JG , Hill KE , Burk RF. 1989. Dietary selenium intake controls rat plasma selenoprotein P concentration. J Nutr 119:1010–1012 [DOI] [PubMed] [Google Scholar]
- 46. Pence BC , Delver E , Dunn DM. 1994. Effects of dietary selenium on UVB-induced skin carcinogenesis and epidermal antioxidant status. J Invest Dermatol 102:759–761 [DOI] [PubMed] [Google Scholar]
- 47. Diwadkar-Navsariwala V , Prins GS , Swanson SM , Birch LA , Ray VH , Hedayat S , Lantvit DL , Diamond AM. 2006. Selenoprotein deficiency accelerates prostate carcinogenesis in a transgenic model. Proc Natl Acad Sci USA 103:8179–8184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Selenius M , Rundlof AK , Olm E , Fernandes AP , Bjornstedt M. 2010. Selenium and the selenoprotein thioredoxin reductase in the prevention, treatment and diagnostics of cancer. Antioxid Redox Signal 12:867–880 [DOI] [PubMed] [Google Scholar]
- 49. Li H , Stampfer MJ , Giovannucci EL , Morris JS , Willett WC , Gaziano JM , Ma J. 2004. A prospective study of plasma selenium levels and prostate cancer risk. J Natl Cancer Inst 96:696–703 [DOI] [PubMed] [Google Scholar]
- 50. Allen NE , Appleby PN , Roddam AW , Tjonneland A , Johnsen NF , Overvad K , Boeing H , Weikert S , Kaaks R , Linseisen J , Trichopoulou A , Misirli G , Trichopoulos D , Sacerdote C , Grioni S , Palli D , Tumino R , Bueno-de-Mesquita HB , Kiemeney LA , Barricarte A , Larrañaga N , Sánchez MJ , Agudo A , Tormo MJ , Rodriguez L , et al. 2008. Plasma selenium concentration and prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Clin Nutr 88:1567–1575 [DOI] [PubMed] [Google Scholar]
- 51. Lippman SM , Klein EA , Goodman PJ , Lucia MS , Thompson IM , Ford LG , Parnes HL , Minasian LM , Gaziano JM , Hartline JA , Parsons JK , Bearden JD , Crawford ED , Goodman GE , Claudio J , Winquist E , Cook ED , Karp DD , Walther P , Lieber MM , Kristal AR , Darke AK , Arnold KB , Ganz PA , Santella RM , et al. 2009. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 301:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Duffield-Lillico AJ , Dalkin BL , Reid ME , Turnbull BW , Slate EH , Jacobs ET , Marshall JR , Clark LC. 2003. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int 91:608–612 [DOI] [PubMed] [Google Scholar]
- 53. Rayman MP. 2009. Selenoproteins and human health: insights from epidemiological data. Biochim Biophys Acta 1790:1533–1540 [DOI] [PubMed] [Google Scholar]
- 54. Zhuo P , Diamond AM. 2009. Molecular mechanisms by which selenoproteins affect cancer risk and progression. Biochim Biophys Acta 1790:1546–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rainer J , Sanchez-Cabo F , Stocker G , Sturn A , Trajanoski Z. 2006. CARMAweb: comprehensive R- and bioconductor-based web service for microarray data analysis. Nucleic Acids Res 34:W498–W503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu Z , Irizarry R , Gentlemen R , Martinez-Murillo F , Spencer F. 2004. A model-based background adjustment for oligonucleotide expression arrays. J Am Stat Assoc 99:909–917 [Google Scholar]
- 58. Lener B , Koziel R , Pircher H , Hutter E , Greussing R , Herndler-Brandstetter D , Hermann M , Unterluggauer H , Jansen-Durr P. 2009. The Nadph Oxidase Nox4 restricts the replicative lifespan of human endothelial cells. Biochem J 423:363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Unterluggauer H , Micutkova L , Lindner H , Sarg B , Hernebring M , Nystrom T , Jansen-Dürr P. 2009. Identification of Hsc70 as target for AGE modification in senescent human fibroblasts. Biogerontology 10:299–309 [DOI] [PubMed] [Google Scholar]
- 60. Zenzmaier C , Untergasser G , Hermann M , Dirnhofer S , Sampson N , Berger P. 2008. Dysregulation of Dkk-3 expression in benign and malignant prostatic tissue. Prostate 68:540–547 [DOI] [PubMed] [Google Scholar]