Abstract
Recent studies have emphasized the association between tubulin gene mutations and developmental abnormalities of the cortex. In this study, the authors identified a mutation in the tubulin-encoding class III β-tubulin (TUBB3) gene in a 4-year-old boy presenting with brain abnormalities and unilateral hypohidrosis. The patient showed a left internal strabismus, moderate developmental delay, and congenital hypohidrosis of the right side of the body. Magnetic resonance imaging disclosed gyral disorganization mainly in the left perisylvian region, dysmorphic and hypertrophic basal ganglia with fusion between the putamen and caudate nucleus without affecting the anterior limb of the internal capsule, and moderate hypoplasia of the right brain stem and cerebellum. Diffusion tensor imaging studies revealed disorganization of the pyramidal fibers. The amplitude of the sympathetic skin response was low in the right arm, which led to a diagnosis of focal autonomic neuropathy. Sequencing the TUBB3 gene revealed a de novo missense mutation, c.862G>A (p.E288K).
Keywords: TUBB3, brain abnormality, unilateral hypohidrosis
Microtubules are essential for neuronal migration and axon guidance and are primarily composed of tubulin. Mutations in tubulin genes commonly cause cortical malformations known as tubulinopathies.1 The class III β-tubulin (TUBB3) gene encodes the class III β-tubulin protein, which is specifically expressed in neural tissues. More than 20 heterozygous missense TUBB3 mutations have been associated with 2 distinct clinical tubulinopathies in the past2–6; these are congenital fibrosis of the extraocular muscles type 33,7 and cortical dysplasia, complex, with other brain malformations type 12,4,5 and both.6,8 In this study, the authors identified a de novo heterozygous missense mutation in the TUBB3 gene, which was associated with cortical dysplasia, complex, with other brain malformations type 1 and probably unilateral hypohidrosis.
Case Report
The patient was a 4-year-old boy and the only child of nonconsanguineous parents, with no family history of inherited or neurological diseases. He exhibited dilated lateral ventricles at 30 weeks of gestation. He was born spontaneously at full term. At birth, he weighed 3085 g (50th percentile), was 48 cm long (25th percentile), and occipitofrontal circumference was 32.5 cm (25th percentile). The perinatal history was uneventful. The patient had a left esotropia and moderate developmental delay and was brought into the clinic at age 4 years. His spontaneous movement, sensation, and the deep tendon reflex of the limbs were normal and symmetric. He had no dysmorphic facial features including ptosis and pupil abnormalities. However, reduced sweating was observed on the whole right side of his body, including the face. His parents were aware of this symptom from infancy. This symptom had not deteriorated and caused the patient no disability. Blood and urine screening produced normal results.
Magnetic resonance imaging of the brain disclosed cerebellar vermian dysplasia, dysmorphic and hypertrophic basal ganglia with fusion between the putamen and caudate nucleus, not affecting the anterior limb of the internal capsule, moderate hypoplasia of the right brain stem, and ventriculomegaly (Figure 1A-C). Diffusion tensor imaging revealed disorganization of the pyramidal fibers (Figure 1D). An electroencephalogram showed a few epileptic discharges in the right occipital regions, but no clinical seizures had been reported.
Figure 1.
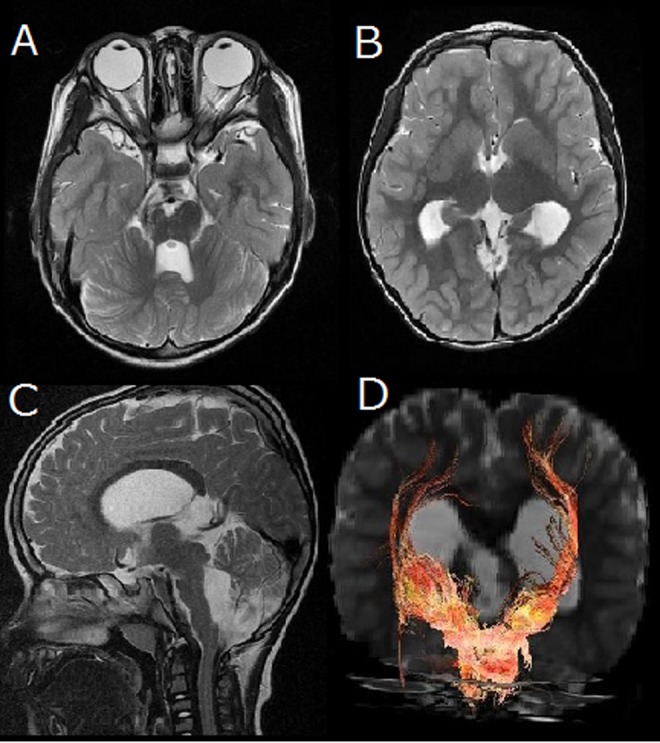
Brain magnetic resonance imaging (MRI) disclosed moderate hypoplasia of the right brain stem, cerebellar vermian dysplasia (A), dysgyria, enlarged lateral ventricle, hypertrophic basal ganglia with fusion between the putamen and caudate nucleus without affecting the anterior limb of the internal capsule (B). Midline sagittal MRI shows corpus callosum dysgenesis, mild dilatation of the third and fourth ventricles, and hypoplasia of the pons (C). Frontal view of corticospinal tracts in the patient shows that the right corticospinal tracts appear to be thinner and less widely distributed in the cerebral hemispheres (D).
These clinical features are similar to those that have commonly been described in patients with TUBB3 mutations, particularly patients with cortical dysplasia, complex, with other brain malformations type 1. Therefore, the authors performed a mutation screening of TUBB3 after approval from the institutional review board of Yamagata University, Faculty of Medicine, and obtaining written informed consent from the parents. Sequencing was done by polymerase chain reaction–direct sequencing according to the standard methods using originally designed primers. The results showed a missense mutation, c.862G>A (p.E288K), which was not detected in both parents, indicating a de novo mutation.
Since he showed unilateral hypohidrosis, the authors surveyed his autonomic nerve function. Orthostatic hypotension was not detected in the patient; the heart rate was normal and increased on standing. Thermoregulatory sweat testing was performed by applying a starch and iodine paste to the thigh, which turns purple in the presence of sweat. This test revealed a decrease in sweating on the right thigh. The amplitude of the sympathetic skin response was low on the right thigh, which led to a diagnosis of right autonomic dysfunction associated with reduced sweating (Figure 2A). Tests of heart rate and blood pressure showed normal cardiovascular autonomic functioning (Figure 2B). For cardiac autonomic tests, myocardial metaiodobenzylguanidine uptake was measured and found to be normal.
Figure 2.
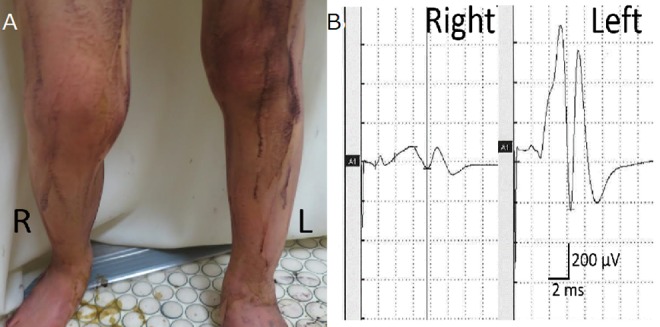
Thermoregulatory sweat testing was performed by applying a starch and iodine paste to the thigh, which turns purple in the presence of sweat. This test revealed hypohidrosis on the right thigh (A). Sympathetic skin response test demonstrated reduced amplitude in the right side, which demonstrated right autonomic dysfunction (B).
Discussion
p.E288K mutation has already been described by Oegema et al.4 The patient with the mutation demonstrated the same clinical and imaging features as this patient: strabismus, asymmetric pons, basal ganglia dysgenesis, and dysgyria.
Interestingly, this patient presented with hypohidrosis, which has not been reported in patient with the same mutation in TUBB3 or other cases of tubulinopathies described to date.1,4 TUBB3 mutations are associated with peripheral axonal neuropathies via axonal protein trafficking defects,8 but he had no obvious evidence of peripheral neuropathy. This patient had a reduced sympathetic skin response without other autonomic dysfunction, such as pupil impairment and unilateral hemiparesis or sensory deficits. These findings suggest dysfunction of the sympathetic sudomotor neurons alone. Hypohidrosis is caused by disrupted function of the cortex, brain stem, and spine. Because the sympathetic sudomotor neurons descend ipsilaterally from the hypothalamus via the brain stem to the intermediolateral column of the spinal cord, unilateral brain stem dysfunction could cause ipsilateral hypohidrosis.9 Indeed, this patient presented with right brain stem dysplasia. The authors hypothesize that the TUBB3 mutation disturbed the guidance of projection fibers, resulting in right brain dysplasia and dysfunction of right sudomotor neurons. The hypohidrosis on the right side of the body may have been a consequence of this ipsilateral dysfunction.
TUBB3 and TUBB2B mutations are usually associated with asymmetric clinical findings, such as right brain stem hypoplasia.5,10 TUBA1A mutations have also been described in cases with perisylvian asymmetrical polymicrogyria. Therefore, unilateral dysfunction may represent a phenotypic feature of tubulinopathies. Concordantly, this patient also showed asymmetrical phenotypes. The presence of these phenotypes in tubulin-related disorders could be related to a differential expression of tubulin genes in different sides of the brain.10
Footnotes
Author Contributions: SF, KK, and AT worked up the case, under the supervision of HT. MK was responsible for genetic analysis. The first draft of the manuscript was prepared by SF, RT, and HT, which was revised critically and finally approved by all authors.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Informed consent was taken from the parents for publication of the case report.
References
- 1. Bahi-Buisson N, Poirier K, Fourniol F, et al. The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain. 2014;137(pt 6):1676–1700. [DOI] [PubMed] [Google Scholar]
- 2. Poirier K, Saillour Y, Bahi-Buisson N, et al. Mutations in the neuronal beta-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum Mol Genet. 2010;19(22):4462–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Demer JL, Clark RA, Tischfield MA, Engle EC. Evidence of an asymmetrical endophenotype in congenital fibrosis of extraocular muscles type 3 resulting from TUBB3 mutations. Invest Ophthalmol Vis Sci. 2010;51(9):4600–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oegema R, Cushion TD, Phelps IG, et al. Recognizable cerebellar dysplasia associated with mutations in multiple tubulin genes. Hum Mol Genet. 2015;24(18):5313–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimojima K, Okamoto N, Yamamoto T. A novel TUBB3 mutation in a sporadic patient with asymmetric cortical dysplasia. Am J Med Genet A. 2016;170A(4):1076–1079. [DOI] [PubMed] [Google Scholar]
- 6. Whitman MC, Andrews C, Chan WM, et al. Two unique TUBB3 mutations cause both CFEOM3 and malformations of cortical development. Am J Med Genet A. 2016;170A(2):297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chew S, Balasubramanian R, Chan WM, et al. A novel syndrome caused by the E410K amino acid substitution in the neuronal beta-tubulin isotype 3. Brain. 2013;136(pt 2):522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tischfield MA, Baris HN, Wu C, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140(1):74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Korpelainen JT, Sotaniemi KA, Myllyla VV. Ipsilateral hypohidrosis in brain stem infarction. Stroke. 1993;24(1):100–104. [DOI] [PubMed] [Google Scholar]
- 10. Tischfield MA, Cederquist GY, Gupta ML, Jr, Engle EC. Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr Opin Genet Dev. 2011;21(3):286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]


