Abstract
The GRIN2B (glutamate receptor, ionotropic, N-methyl-d-aspartate 2B) gene, located in the short arm of chromosome 12, encoding the NR2B subunit of the N-methyl-D-aspartate receptor, has recently been recognized to play an important role in corticogenesis and brain plasticity. Deletions in the short arm of chromosome 12 are rare. Hemizygous loss of function of the GRIN2B gene results in developmental delay, whereas gain of function leads to epilepsy, and infantile spasms in particular. In addition, GRIN2B variants have been associated with autism spectrum disorder and schizophrenia. Here the authors report a child with global developmental delay, autistic behavioural features, central hypotonia, dysmorphic features and isolated congenital anomalies of the fingers and toes, and a de novo heterozygous deletion in chromosome locus 12p13.2-p13.1, involving loss of several genes, including GRIN2B. This report and our review of the literature help clarify the distinct phenotypes associated with loss or gain of GRIN2B function.
Keywords: chromosome12p deletion, CDKN1B, GRIN2B, ETV6, neurodevelopment, autism spectrum disorder
While disorders associated with deletions in the short arm of chromosome 12 (12p deletion syndrome) were described prior to the advent of array-based comparative genomic hybridization,1-4 with its emergence, it became possible to correlate clinical phenotypes with particular genes. The 12p13.1 GRIN2B (glutamate receptor, ionotropic, N-methyl-d-aspartate 2B) gene plays a critical role in mammalian brain development, and different mutations are associated with entirely distinct neurodevelopmental, epileptic, and psychiatric disorders.5-12 Here, the authors present a detailed description of a 3-year-old boy with heterozygous de novo interstitial microdeletion at 12p13.2-p13.1 encompassing GRIN2B and review the literature. Our case and review help to clarify and contrast the loss-of-function neurodevelopmental and gain-of-function epileptic GRIN2B phenotypes.
Case Study
Our patient was born at term via spontaneous vaginal delivery to nonconsanguineous parents. Routine antenatal ultrasound, during the 33rd week of gestation, revealed a large head circumference and improper closure of the cerebral ventricles. This was not seen on the postnatal head ultrasound. At delivery, there was light meconium present, however, no resuscitation was required. He developed transient respiratory distress. His birth weight was 8 lbs and 7 oz (90th percentile). Echocardiography at the time showed a patent foramen ovale.
Family history was significant for developmental delay and primary gastrointestinal malignancies in some family members. The patient’s eldest brother, now 6 years old, was diagnosed with autism spectrum disorder and attention-deficit hyperactivity disorder. He had moderate to severe language delay, fine motor delay, motor planning difficulties, and academic challenges. His younger brother, now 5 years old, had isolated speech delay. Our patient’s paternal grandfather had a history of speech and language delay. Paternal grandmother had a benign colon tumor removed at age 21 years, and paternal nephew was found to have an appendiceal tumor at age of 16 years. A paternal uncle was diagnosed with gastrointestinal cancer in his mid-50s.
Our index case was noted to have central hypotonia since the first week of life and global developmental delay since early infancy, predominantly affecting motor and language domains. He never exhibited any regression. He developed adequate head control around 6 months, was able to sit with support by 9 months, and unsupported by 12 months. He could transition from supine to sitting at 18 months and started to pull-to-stand after 24 months. He started ambulating independently at 3 years but still had frequent falls due to poor balance. He required foot orthotics due to abnormalities in foot-ankle alignment during independent ambulation. He developed bilateral pincer grasp at around 15 months and was able to spoon-feed himself by 24 months. At 3 years, he was scribbling with a pen but could not draw discreet lines or shapes. Language development was significantly delayed. At 2 years, his vocabulary consisted of approximately 5 words, which expanded to 10 to 20 single spontaneous words by age 3. His main method of communication was nonverbal with gestures. Receptive language was comparatively better but nonetheless delayed. Cognitively, he could identify his own body parts, was able to follow simple 1-step commands, and knew primary colors and few numbers. He developed a social smile early in infancy and began to laugh around 11 months. He had social interest in peers and was able to initiate and interact in reciprocal play. He engaged in some simple pretend play by 24 months. He also demonstrated some mild repetitive behaviors, such as turning on/off switches and closing doors repeatedly by age 2 along with restricted play themes. Also notable were some sensory aversions, including aversion to certain tactile and auditory stimuli. In terms of his adaptive skills, at age 3, he was not showing any interest in toilet training and was unable to dress himself. He had normal ophthalmological and hearing assessments and had no clinical seizures.
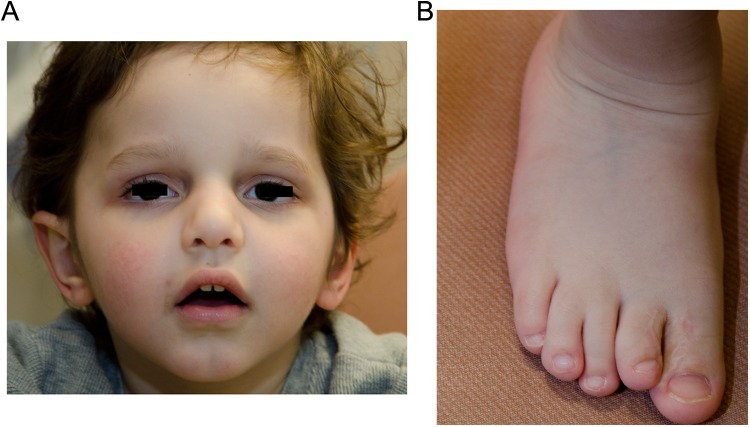
At 36 months, his weight and height were near the 50th percentile, and head circumference was just above the 97th percentile. He had facial dysmorphisms and minor congenital abnormalities of his digits and toes as described in Figure 1. He also had a high anterior hairline with a single posterior hair whorl, along with clinodactyly of his fifth digits bilaterally. On neurological examination, he was interactive with interest in his external environment. He was able to spontaneously produce few meaningful words. His cranial nerves, motor, and sensory examination were unremarkable except for mild truncal hypotonia and poor balance while walking. Other systemic examinations were normal.
Figure 1.
Dysmorphic features. A, Facial features: broad forehead, mild frontal bossing, scaphocephaly, hypoplastic supraorbital ridge, prominent nasal bridge and a bulbous nose, mild micrognathia, slightly wide mouth with downturned corners, deep-set eyes, and posteriorly rotated prominent right ear compared to the left. B, Third and fourth right toe incomplete syndactyly, shorter second toe than first and third.
His complete blood count, liver, renal and thyroid function tests, serum lactate, ammonia, creatine kinase, carnitine, quantitative acylcarnitine profile, amino acid, and urine organic acid were unremarkable. Magnetic resonance imaging of the brain was deferred due to parental concern about risks of anesthesia. Electroencephalography showed spike and wave discharges over the right posterior parietal head region. Array-based comparative genomic hybridization revealed a 3.11 Mb hemizygous loss at chromosomal locus 12p13.2-p13.1 (chr12: 11 147 715 to 14 258 330 bp, hg19). Fluorescence in situ hybridization analysis confirmed the deletion observed by microarray analysis. Fluorescence in situ hybridization analyses of parental samples indicated that the deletion was de novo. Microarray analysis of his eldest brother was normal.
Discussion
The authors describe a child with global developmental delay, central hypotonia, autistic behavioral features, facial dysmorphisms, incomplete syndactyly, thumb duplication, and clinodactyly with focal electroencephalography abnormalities and a de novo deletion at 12p13.2-p13.1. This deletion results in loss of 1 copy of 36 RefSeq genes including 4 OMIM (Online Mendelian Inheritance in Man) morbid genes that include GRIN2B, CDKN1B (cyclin-dependent kinase inhibitor 1B), ETV6 (ETS variant 6), and LRP6 (low density lipoprotein receptor-related protein 6). GRIN2B encodes the NR2 subunit of the N-methyl-D-aspartate receptor and is expressed within striatal and cortical structures. While ours would be the second report of confirmed hemizygous deletion of GRIN2B,7 there are already sufficient descriptions of patients with hemizygous loss of function indicating that most if not all of the neurodevelopmental phenotype of our patient is due to loss of one of the two alleles of this gene. Table 1 summarizes the salient neurodevelopmental features of all the cases to date, including ours, and allows crystallization of the core neurodevelopmental syndrome of hemizygous loss of GRIN2B, which encompasses: delay in motor and speech development, in particular expressive language, with various degree of intellectual disability and behavioral difficulties such as hyperactivity and oppositional behavior, and focal interictal electroencephalography abnormalities predominantly unaccompanied by clinical seizures.
Table 1.
Phenotypic Spectrum of Mutations in the GRIN2B Gene Causing Loss or Gain of Function.
| Reference | Mutation | Origin | Functional Effect | Age | Sex | Phenotype | Congenital Anomalies | Electroencephalography | Seizures | Intellectual Disability | Magnetic Resonance Imaging |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Our patient | Interstitial deletion including entire GRIN2B gene 12p13.2-p13.1 (chr12:11 147 715-14 258 330) × 1 | De novo | Loss of function | 3 | M | Global developmental delay (motor and speech expressive > receptive), central hypotonia, facial dysmorphism | Sacro-coccygeal dimple, incomplete syndactyly, clinodactyly of fifth digits | Spike and wave discharges over the right posterior parietal head region | No | Moderate | Not performed |
| Dimassi et al7 | Interstitial deletion including entire GRIN2B gene 12p13.1-p12.3 (chr12:13 116 327-17 251 654) | De novo | Loss of function | 12 | F | Developmental delay (motor and speech), intellectual disability, mild dysmorphic features | Sacro-coccygeal dimple | Not available | Single complex partial | Moderate | Normal |
| Dimassi et al7 | Interstitial deletion including promoter region and first exon of GRIN2B 12p13.1-p12.3 (chr12:14 132 839-16 785 705) | De novo | Loss of function | 24 | M | Developmental delay (motor and speech), mental retardation, visual-constructive difficulties, dysmorphic features | Small toenails | Not available | No | Moderate | Not available |
| Dimassi et al7 | Interstitial deletion including promoter region and first exon of GRIN2B 12p13.1-p12.3 (chr12:14 063 896-14 651 132) | De novo | Loss of function | 3 | M | Developmental delay (motor and speech), behavioral problems, axial hypotonia | None | Not available | No | Moderate | Normal |
| Endele et al5 and Freunscht et al6 | Breakpoint in 12p13.1 disrupts the GRIN2B gene in exon 4 | De novo | Loss of function | 6 | M | Developmental delay (motor and speech expressive>receptive), mild intellectual disability and behavioral problems | None | Left-sided sharp wave complexes | No | Mild | Normal |
| Endele et al5 and Freunscht et al6 | GRIN2B is disrupted within exon 2 in 12p13.1 | De novo | Loss of function | 14 | M | Severe cognitive impairment, no expressive speech sensory stimulation and self-injurious behaviors | Cryptorchidis, choanal atresia, inguinal hernia, bilateral optic atrophy | Slow dysrhythmia, occipital abortive sharp waves | No | Severe | Hydrocep- alusexternus |
| Endele et al5 and Freunscht et al6 | Missense mutation in the GRIN2B gene (c.2044C > T; p.R682C). | De novo | Loss of function | 14 | M | Global developmental delay (motor and speech expressive > receptive), fine motor coordination impairment and behavioral problems | None | Normal | No | Mild | Normal |
| Endele et al5 and Freunscht et al6 | Heterozygous deletion of a dinucleotide in exon 3 c.803_804delCA, p.T268SfsX15). | De novo | Loss of function | 14 | F | Global developmental delay (motor and speech expressive > receptive), behavioral problems, sleep problems | Mild limited extension at the elbows | Normal | No | Moderate | Not performed |
| Freunscht et al6 | Missense mutation within the GRIN2B gene (c.1906G > C; p.A636P). | De novo | Loss of function | 3 | F | Mild intellectual disability, behavioral problems | None | Sharp wave complexes temporoparietal | No | Mild | Normal |
| Lemke et al8 | c.1853T>G, p.Val618Gly | De novo | Gain of function | 2 | M | West syndrome | Not available | Multifocal bursts of irregular spike waves as well as rhythmic bilateral generalized spike waves with a frequency of 4 to 5 per second reminiscent of modified hypsarrhythmia | Infantile spasms | Severe | Not available |
| Lemke et al8 | c.1844A>T, p.Asn615Ile | De novo | Gain of function | 5 | F | West syndrome | Not available | Hypsarrhythmia | Infantile spasms, generalized tonic-clonic seizure | Severe | Normal |
| Lemke et al8 | c.1619G>A, p.Arg540HIs | De novo | Gain of function | 10 | F | Focal epilepsy and intellectual disability | Not available | Slowing over the left frontoparietal region | Focal dyscognitive with postictal paresis of the right arm, generalized tonic-clonic seizure, status epileptics | Mild | Postictal diffusion restriction - resolved |
| Lemke et al8 | c.2011-5_2011-4delTC | Paternal | Potential splice defect | 4 | M | West syndrome | Not available | Hypsarrhythmia | Infantile spasms, tonic, focal motor | Severe | Normal |
A gain of function in the GRIN2B gene has also recently been implicated to cause a severe form of epileptic encephalopathy manifesting as West syndrome.8 Although our patient has focal electroencephalography abnormalities he has not had any clinical seizures at the time of the submission of this manuscript. This may be due to a loss of function, as opposed to a gain of function in the GRIN2B gene manifesting as two separate phenotypes, the latter consisting mainly of neurodevelopmental disorder with global developmental delay and intellectual disability and the former causing an epileptic encephalopathy. Other types of mutations in GRIN2B gene—nonsense, splice-site, and frameshift—have also been identified in patients with cognitive and intellectual impairments.5 Various neurobehavioral disorders statistically associated with GRIN2B gene variants including autistic spectrum disorder,9,10 obsessive-compulsive disorder,11 schizophrenia,10,12 and bipolar disorder12 have been described.
N-Methyl-D-aspartate receptors are tetrameric ligand-gated ion channels composed of 2 glycine-binding NR1 subunits and 2 glutamate-binding NR2 subunits (NR2A, NR2B, NR2C, and NR2D).13 GRIN2B represents a rate-limiting genetic factor in gating the N-methyl-D-aspartate receptor’s function in the developing and mature mammalian brain.5 The subunit composition of N-methyl-D-aspartate receptors is spatially and temporally regulated. It undergoes a maturational change from heterotetramers containing predominantly NR2B early in life to those containing NR2B, NR2A, or both subunits in the adult brain.13 GRIN2B has also been shown to be involved in long-term potentiation of synaptic transmission—the mechanism purported in memory and learning processes.14 Mutations in the GRIN2B gene leads to abnormal subunit functioning affecting neuronal ion flux resulting in developmental abnormalities and epilepsy.5
Deletions in the short arm of chromosome 12 are also associated with a wide variety of hematopoietic malignancies due to mutation of the 2 known tumor-suppressor genes: ETV6 and CDKN1B, both of which are also part of our patient’s deletion. Germline heterozygous mutations in CDKN1B have been described in patients with multiple endocrine neoplasia.15 Hemizygous deletions encompassing CDKN1B have also been reported with small intestine neuroendocrine tumors.16 Similarly, several somatic heterozygous mutations of ETV6 gene involving translocation and fusion with various other genes have been reported with acute and chronic leukemia.17 Germline variants of both CDKN1B and ETV6 are associated with adult predisposition to prostate cancer.18,19 As such, our patient and children with similar chromosomal 12 deletion GRIN2B developmental delay should be monitored for neoplastic risk.
In conclusion, hemizygous loss of function of GRIN2B leads to intellectual disability, developmental delays, particularly in language, hyperactivity and oppositional behavior, and abnormal electroencephalography, but rare or no seizures. In contrast, gain of function in this gene is one cause of West syndrome. Likely due to its central neurological role as part of the N-methyl-D-aspartate receptor, variants likely affecting its expression are associated with common neurobehavioral disorders including schizophrenia and autism.
Acknowledgments
The authors thank the patient, his parents, and the members of the Cytogenetics and Microarray Laboratory at The Hospital For Sick Children.
Author Contributions: NM was involved in patient’s management, literature review, initial drafting, and revision of the manuscript and interpretation of data. EK performed literature review, drafting the manuscript, and interpretation of data. AO was involved in patient’s management, revising the manuscript, and interpretation of data. BM supervised the literature search and was involved in patient’s management, revising the manuscript, and interpretation of data.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Written consent was obtained from the patient’s parents.
References
- 1. Kivlin JD, Fineman RM, Williams MS. Phenotypic variation in the del(12p) syndrome. Am J Med Genet. 1985;22(4):769–779. [DOI] [PubMed] [Google Scholar]
- 2. Romain DR, Goldsmith J, Columbano-Green LM, Chapman CJ, Smythe RH, Parfitt RG. Partial monosomy 12p13.1-13.3. J Med Genet. 1987;24(7):434–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fryns JP, Kleczkowska A, Van den Berghe H. Interstitial deletion of the short arm of chromosome 12. Report of a new patient and review of the literature. Ann Genet. 1990;33(1):43–45. [PubMed] [Google Scholar]
- 4. Magnelli N, Therman E. Partial 12p deletion: a cause for a mental retardation, multiple congenital abnormality syndrome. J Med Genet. 1975;12(1):105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Endele S, Rosenberger G, Geider K, et al. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat Genet. 2010;42(11):1021–1026. [DOI] [PubMed] [Google Scholar]
- 6. Freunscht I, Popp B, Blank R, Endele S, et al. Behavioral phenotype in five individuals with de novo mutations within the GRIN2B gene. Behav Brain Funct. 2013;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dimassi S, Andrieux J, Labalme A, et al. Interstitial 12p13.1 deletion involving GRIN2B in three patients with intellectual disability. Am J Med Genet A. 2013;161A(10):2564–2569. [DOI] [PubMed] [Google Scholar]
- 8. Lemke JR, Hendrickx R, Geider K, et al. GRIN2B mutations in west syndrome and intellectual disability with focal epilepsy. Ann Neurol. 2014;75(1):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Roak BJ, Deriziotis P, Lee C, et al. Exome sequencing in sporadic autism spectrum disorder identifies de novo mutation. Nat Genet. 2011;43(6):585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tarabeux J, Kebir O, Gauthier J, et al. Rare mutations in N-methyl-D-aspartate glutamate receptors in autism spectrum disorders and schizophrenia. Transl Psychiatry. 2011;1:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnold PD, Rosenberg DR, Mundo E, Tharmalingam S, Kennedy JL, Richter MA. Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: a preliminary study. Psychopharmacology (Berl). 2004;174(4):530–538. [DOI] [PubMed] [Google Scholar]
- 12. Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32(9):1888–1902. [DOI] [PubMed] [Google Scholar]
- 13. Matta JA, Ashby MC, Sanz-Clemente A, Roche KW, Isaac JT. mGluR5 and NMDA receptors drive the experience and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron. 2011;70(2):339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. deQuervain DJ, Papassotiropoulos A. Identification of a genetic cluster influencing memory performance and hippocampal activity in humans. Proc Natl AcadSci U S A. 2006;103(11):4270–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pellegata NS, Quintanilla-Martinez L, Siggelkow H, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl AcadSci U S A. 2006;103(42):15558–15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Francis JM, Kiezun A, Ramos AH, et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat Genet. 2013;45(12):1483–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wall M, Rayeroux KC, MacKinnon RN, Zordan A, Campbell LJ. ETV6 deletion is a common additional abnormality in patients with myelodysplastic syndromes or acute myeloid leukemia and monosomy 7. Haematologica. 2012;97(12):1933–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang BL, Zheng SL, Isaacs SD, et al. A polymorphism in the CDKN1B gene is associated with increased risk of hereditary prostate cancer. Cancer Res. 2004;64(6):1997–1999. [DOI] [PubMed] [Google Scholar]
- 19. Kibel AS, Faith DA, Bova GS, Isaacs WB. Mutational Analysis of ETV6 in Prostate Carcinoma. Prostate. 2002;52(4):305–310. [DOI] [PubMed] [Google Scholar]