A pituitary-specific enhancer identified through genomic analyses preferentially directs expression in anterior pituitary corticotropes and contains a highly-conserved target for Tpit homodimers.
Abstract
Cell-specific expression of the pituitary proopiomelanocortin (POMC) gene depends on the combination of tissue- and cell-restricted transcription factors such as Pitx1 and Tpit. These factors act on the proximal POMC promoter together with transcription factors that integrate inputs from signaling pathways. We now report the identification of an upstream enhancer in the POMC locus that is targeted by the same subset of transcription factors, except Pitx1. This enhancer located at −7 kb in the mouse POMC gene is highly dependent on Tpit for activity. Whereas Tpit requires Pitx1 for action on the promoter, it acts on the −7-kb enhancer as homodimers binding to a palindromic Tpit response element (TpitRE). Both half-sites of the TpitRE palindrome and Tpit homodimerization are required for activity. In vivo, the enhancer exhibits preferential activity in corticotrope cells of the anterior lobe whereas the promoter exhibits preference for intermediate lobe melanotropes. The enhancer is conserved among different species with the TpitRE palindrome localized at the center of conserved sequences. However, the mouse and human −7-kb enhancers do not exhibit conservation of hormone responsiveness and may differ in their relative importance for POMC expression. In summary, pituitary expression of the POMC gene relies on an upstream enhancer that complements the activity of the proximal promoter with Tpit as the major regulator of both regulatory regions.
Regulatory mechanisms for gene transcription are very flexible, and many different scenarios can achieve similar outputs of transcription. In particular, recent data have beautifully illustrated the very pliable nature of regulatory sequences such as enhancers and their rapid evolution when comparing related species (1). Whereas the relative contribution of individual regulatory elements vary when comparing homologous genes between species, the nature of their building blocks (i.e., the binding sites for transcription factors) is more conserved. Indeed, it appears that the combination of transcription factor binding sites, and hence their cognate factors, is a more specific signature for individual genes and their regulatory landscape.
Consistent with this emerging picture, the identification of pituitary-restricted transcription factors has been the most instructive on mechanisms for pituitary-specific gene expression, cell differentiation, and organogenesis. While most currently known transcription factors with restricted pituitary expression were initially characterized for their action on cognate binding sites within the promoters of hormone coding genes, the same transcription factors are also acting at multiple distant regulatory elements and enhancers that contribute to pituitary-specific and hormone-regulated transcription. The expression of some of these factors, such as Pitx1, Pitx2, Lhx3, and Lhx4, precede pituitary organogenesis; these factors are important from early phases of pituitary development till adulthood where they contribute to combinatorial programs for transcription of pituitary-specific genes (2). Thus, the Pitx factors were found to act as pan-pituitary transcription factors with DNA binding sites identified in regulatory sequences of all pituitary hormone coding genes (3).
Other factors exhibit expression in a subset of pituitary lineages and accordingly were found to be required for differentiation of these lineages and for sustained expression of the corresponding hormone-coding genes. Indeed, the Prop1 and Pit1 transcription factors are critical regulators of the genetic programs for somatotrope cells expressing GH, lactotropes expressing PRL, and thyrotropes expressing thyrotropin (4–7). Similarly, the highly lineage-restricted transcription factors Tpit and SF1 are required for terminal differentiation of the corticotrope/melanotrope and gonadotrope lineages and for cell-specific transcription of the proopiomelanocortin (POMC) and gonadotropin genes, respectively (8, 9). Combinations of binding sites for these cell-restricted factors, together with targets of ubiquitous transcription factors such as those mediating the action of signaling pathways, are present within regulatory sequences, promoters, and enhancers that direct pituitary-specific gene expression.
The pituitary POMC gene has the unique feature of marking two different pituitary lineages, the corticotropes and melanotropes. However, transcriptional regulation and processing of the polypeptide precursor POMC is unique to each lineage (10). Pituitary transcription of POMC was shown to depend on a −480-bp proximal promoter that targets in vivo expression in both corticotropes and melanotropes (11–15). Extensive characterization of this promoter led to the discovery of the Pitx homeobox transcription factors as well as that of Tpit. The search for other regulatory sequences of the POMC gene has led to the identification of distal upstream enhancers located at −12 kb and −10 kb, which are involved in hypothalamic expression of POMC (16, 17). Although it reproduces the qualitative specificity of pituitary POMC expression in transgenic mice, the proximal promoter does not quantitatively reproduce expression of pituitary POMC, in particular when comparing corticotrope with melanotrope expression (12).
While using genome-wide approaches to investigate regulatory mechanisms active in pituitary corticotrope cells, we identified in vivo binding sites by chromatin immunoprecipitation (ChIP) for various transcription factors in an upstream region of the POMC gene that had not previously been characterized. The present report demonstrates that a region located at about −7 kb upstream of transcription initiation has enhancer properties with preferential activity in pituitary corticotropes compared with melanotropes. The activity of this pituitary-specific enhancer is highly dependent on a palindromic binding site for homodimers of the POMC lineage-restricted factor Tpit. Interestingly, comparison of −7-kb POMC enhancer and promoter sequences in different species revealed a high degree of conservation for the enhancer Tpit palindrome that is not as great as the Tpit binding site of the promoter. Notwithstanding this variability of sequence conservation, both human promoter and enhancer exhibit conserved dependence on Tpit for activity, highlighting the critical importance of cell-specific transcription factors for expression. The variability of regulatory sequences is further illustrated by species differences in hormone responsiveness of the −7-kb enhancer. The present report identifies a novel enhancer for pituitary-specific expression of the POMC gene and identifies species variability in the relative importance of promoters and enhancers.
Results
A candidate regulatory element in the POMC locus
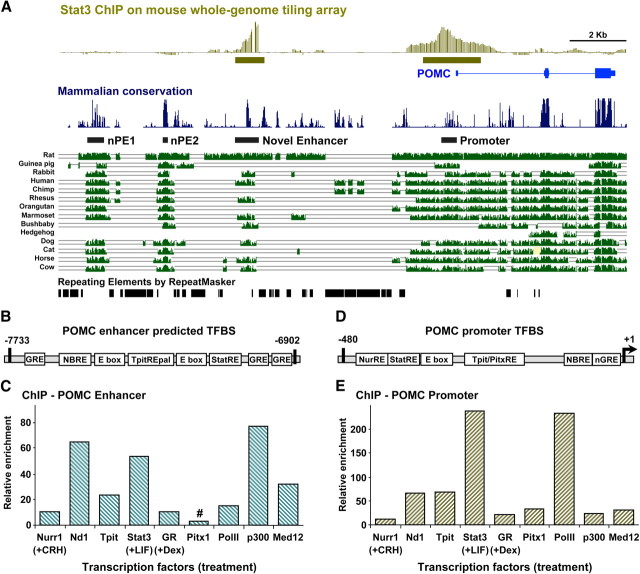
Whole-genome ChIP-chip (chromatin immunoprecipitation analyzed on DNA microarrays) in AtT-20 cells that have corticotrope properties and express POMC revealed two regions of Stat3 recruitment at the POMC locus (18). The most proximal peak corresponds to known Stat3 binding in the promoter region (19, 20). The distal peak is located 7 kb upstream of the transcription start site and coincides with a region that is well conserved in mammals (Fig. 1A). In silico analyses of this region for putative transcription factor binding sites revealed the presence of a binding motif for Stat3, but also predicted glucocorticoid response element sites for the glucocorticoid receptor (GR), NBRE for nuclear receptors of the Nur subfamily, E boxes that are binding sites for transcription factors of the bHLH family, and finally, a palindromic element composed of two binding sites for transcription factors of the T or brachyury family [Tpit response element (TpitRE) pal; Fig. 1B]. We then used ChIP to assess the presence of cognate transcription factors at the predicted sites. We confirmed the occupancy of Nurr1, GR, and Stat3 after treatment with the appropriate stimuli [corticotropin-releasing hormone (CRH), dexamethasone (Dex), and LIF, respectively]. NeuroD1 and Tpit recruitment was also observed (Fig. 1C). It is noteworthy that these same transcription factors are present and known to be important for activity of the POMC promoter (Fig. 1, D and E). A major difference is however the presence of Pitx1 at the promoter and its absolute requirement for action of Tpit (8). In contrast, there are no predicted Pitx binding sites in the −7-kb region, nor is there recruitment of the protein in this region as assessed by ChIP (Fig. 1, B and C). Thus, the −7-kb region of the POMC locus recruits most of the transcription factors previously documented to be important for regulated expression of this gene.
Fig. 1.
Identification of a novel POMC regulatory region. A, Whole-genome Stat3 ChIP-chip analyses in AtT-20 (18) cells reveal recruitment of Stat3 at the POMC promoter and in a region located about 7 kb upstream (green boxes, top diagram). Blue boxes and lines represent the POMC gene exons and introns, respectively. The “mammalian conservation” diagram represents sequence conservation around the POMC locus derived from 19 available mammalian species using mm9 assembly on the UCSC genome browser (32, 33). The position of previously identified hypothalamic regulatory elements nPE1 and nPE2 (16) as well as that of the promoter and −7-kb enhancer are indicated by black boxes. B, Schematic representation of the −7-kb enhancer indicating putative regulatory elements and transcription factor binding sites as identified by in silico analyses with MatInspector software (Genomatix) C, ChIP analyses of the −7-kb POMC enhancer for recruitment of the indicated transcription factors. ChIP experiments performed in AtT-20 cells used hormone-stimulated cells for Nurr1 (+CRH), Stat3 (+LIF), and GR (+Dex). ChIP enrichments are normalized relative to a control IgG ChIP and an unbound control region of the MyoD gene. Data are representative of at least three independent experiments. #Pitx1 ChIP is the only one to exhibit no significant recruitment. D, Schematic representation of the POMC promoter indicating the position of known regulatory elements. E, ChIP analyses of transcription factor recruitment to the POMC promoter in the same experiment as presented for the enhancer in C. All recruitments are significant compared with IgG control.
The −7-kb region has typical enhancer properties
To evaluate the properties of the −7-kb putative regulatory sequences, we inserted an 832-bp fragment encompassing this conserved region into a variety of luciferase reporter plasmids. Previous work had highlighted the importance of the −480-bp proximal promoter and not identified further active sequences up to −4.5-kb (21, 22). We thus compared the activity of a 6.9-kb promoter fragment with a longer one extending to −7.7-kb and encompassing the putative 832-bp enhancer domain: the addition of the −7.7-kb domain increased activity by about 30-fold (Fig. 2A). To determine whether the 862-bp fragment has classical enhancer activity, we assessed its activity on its own and at various positions relative to the proximal promoter. Thus, the −7-kb domain exhibited transcriptional activity when inserted upstream of a minimal promoter or of the proximal promoter, irrespective of its orientation; it was also active when inserted downstream of the reporter (Fig. 2B). The enhancer thus has classical enhancer properties and to assess its cell specificity, we assayed the activity of reporters in various cell lines. In cells of pituitary origin, the αT3 gonadotrope cells, the enhancer had weak activity and it was almost devoid of transcriptional activity in heterologous cells (293T and Cos1) (Supplemental Fig. 1, A–D, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org/). We also tested whether the enhancer sequence may be hormone responsive in addition to exhibiting cell specificity: these experiments did not reveal any significant response of the enhancer to CRH, to the glucocorticoid Dex, to the cytokine LIF, or to combinations of these agents (Fig. 2C). While the cell specificity of the enhancer is consistent with the presence of Tpit at the −7-kb enhancer in AtT-20 cells (Fig. 1C), its inability to respond to CRH, LIF, and Dex is puzzling because the enhancer was also shown by ChIP to recruit Nurr1, Stat3, and GR. The enhancer's unresponsiveness to hormone stimulation was further supported by analysis of the reporter containing the entire −7.7-kb region: this reporter responded to hormone stimulations in much the same way as the proximal −480-bp promoter, and no more (Fig. 2C).
Fig. 2.
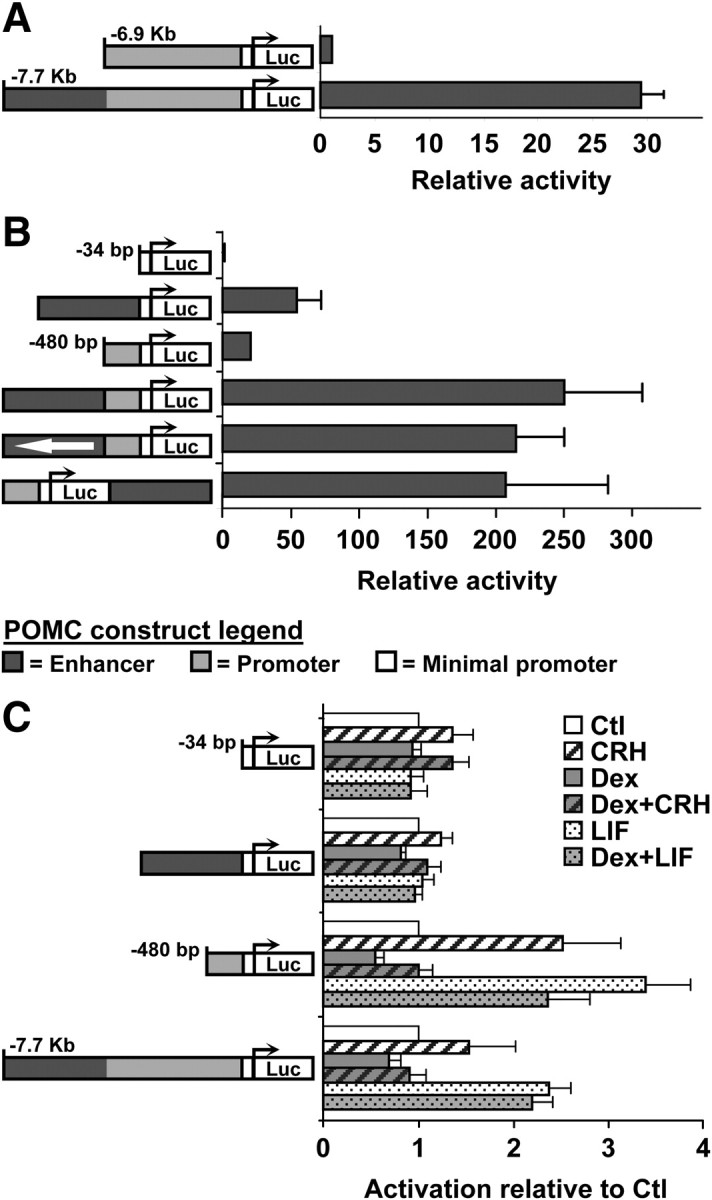
The −7-kb POMC conserved region has enhancer properties. A, Activities of luciferase reporter constructs containing either 6.9- or 7.7-kb upstream 5′ flanking sequences upon transfection in AtT-20 cells. B, Activity (relative to minimal promoter construct) of various luciferase reporters upon transfection of AtT-20 cells. The light gray box represents the −480/−34-bp proximal promoter while the darker gray box represents the enhancer located at −7 kb in the POMC locus. C, Hormone response of POMC luciferase reporters containing either minimal (−34/+63-bp) promoter, −7-kb enhancer, −480-bp proximal promoter or −7.7-kb 5′ flanking sequences. Activation is relative to nontreated cells (Ctl). All data represent the means (± sem) of independent experiments [n = 3 (A), 7 (B), and 4 (C)] each performed in duplicates.
Enhancer activity is highly dependent on a palindromic Tpit binding site
We focused first on the molecular basis for cell specificity of the −7-kb enhancer. The in silico binding site predictions identified a putative palindromic Tbox response element (Fig. 3A). The putative TpitREpal is completely conserved in mice and humans and highly homologous to a consensus TbxRE. We mutated each half site individually and in combination to assess their importance for enhancer activity (Fig. 3A). When assessed together with the minimal POMC promoter, enhancer activity was severely reduced by mutagenesis of either half site and completely abrogated by their combined mutation (Fig. 3B). Similarly, the loss of enhancer activity was severe when the TpitREpal mutations were assessed in a reporter construct that contained the proximal promoter (Fig. 3C); in this context, the single and double mutants were as effective as each other and abolished enhancer activity. We have previously shown that Tpit requires Pitx1 to form heterodimers that bind and act on the Tpit/PitxRE of the proximal POMC promoter (8). We thus tested the effect of mutations introduced over either the Tpit or Pitx binding sites of the proximal promoter and their combination on the activity of the reporter that contains both promoter and enhancer (Fig. 3C). All three mutants had similar effects and decreased reporter activity by 2- to 3-fold. Combined mutations of the TpitREpal of the enhancer and of the Tpit/PitxRE of the promoter completely abolished activity of enhancer and promoter (Fig. 3C). Finally, we compared the activity of oligonucleotide reporters containing the TpitREpal from the POMC enhancer with similar oligonucleotides containing the consensus TbxRE and found both to be similarly active (Fig. 3D). In this isolated context, mutagenesis of each half site again had a dramatic effect on TpitREpal activity (Fig. 3D).
Fig. 3.
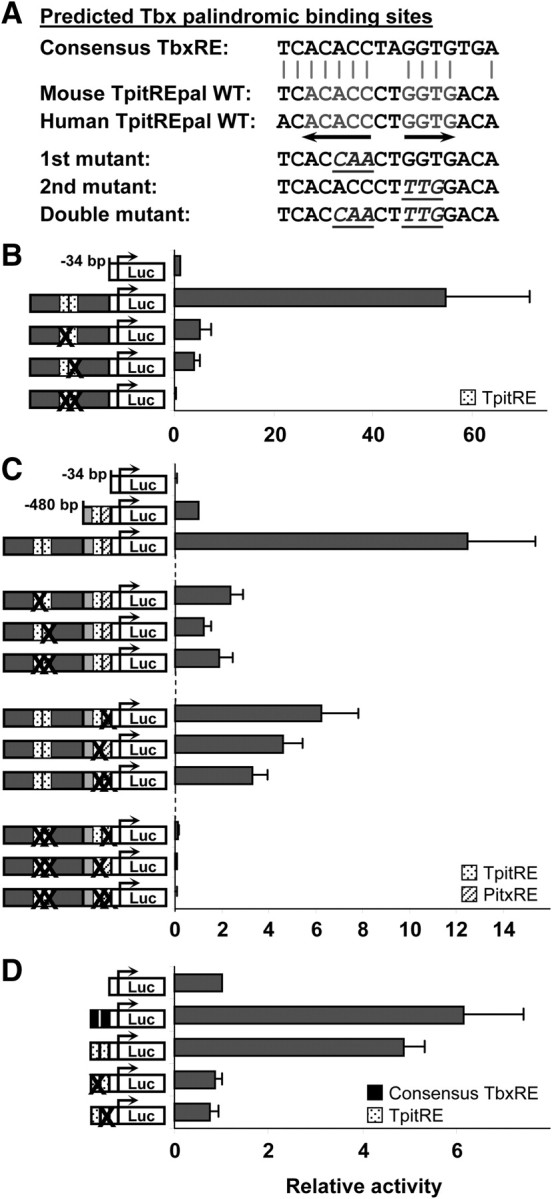
Enhancer activity is dependent on palindromic Tpit binding site (TpitREpal). A, DNA sequence of the mouse and human TpitREpal elements of the −7-kb enhancer compared with a consensus TbxRE. The nucleotide changes used to create mutations in each or both half sites of the TpitREpal are indicated. B, Activity of enhancer reporters upon transfection into AtT-20 cells. The X over the TpitRE boxes indicates mutagenesis of either or both half sites. C, Activity of various reporters upon transfection in AtT-20 cells. The X in the reporter diagram indicates the element(s) that have been mutated in either enhancer and/or promoter. D, Activity of oligonucleotide luciferase reporters upon transfection into AtT-20 cells. The reporters contain either consensus TbxRE or TpitREpal oligonucleotides or their mutants as indicated. All data represent the means (± sem) of independent experiments [n = 5 (B), 3 (C), and 3 (D)] each performed in duplicates.
We then assessed Tpit's ability to activate enhancer reporters because the high degree of conservation between TbxRE and TpitREpal might be taken to suggest that any Tbox factor may account for the activity of the TpitREpal in AtT-20 cells. Cotransfection experiments were thus conducted in αT3 cells, a model of pituitary gonadotrope cells that do not express Tpit. In this heterologous system, increasing doses of Tpit activate the proximal POMC promoter up to about 3-fold whereas the enhancer construct was stimulated up to 6-fold by Tpit expression. Together, promoter and enhancer resulted in a synergistic activation of about 15-fold. Mutations of the TpitREpal half sites completely abrogated the ability to respond to Tpit (Fig. 4A). In further support of the importance of Tpit dimers for TpitRE activation, we used a natural TPIT mutant identified in isolated ACTH deficiency patients, Tpit M86R (23). Tpit M86R was previously shown to bind a TbxRE as monomer but it is not able to dimerize and form homodimers on this sequence or to dimerize with Pitx1 for binding to the Tpit/PitxRE of the promoter. The ability of Tpit M86R to activate either enhancer, promoter or enhancer+promoter constructs is severely curtailed (Fig. 4, B and C) in agreement with a requirement on Tpit dimers for activation of the TpitRE. When we compared the ability of Tpit or Tpit M86R to activate the consensus TbxRE or TpitREpal reporters, it appeared that the TpitREpal of the POMC enhancer is more sensitive to the dimerization defect of Tpit M86R than the consensus TbxRE (Fig. 4D).
Fig. 4.
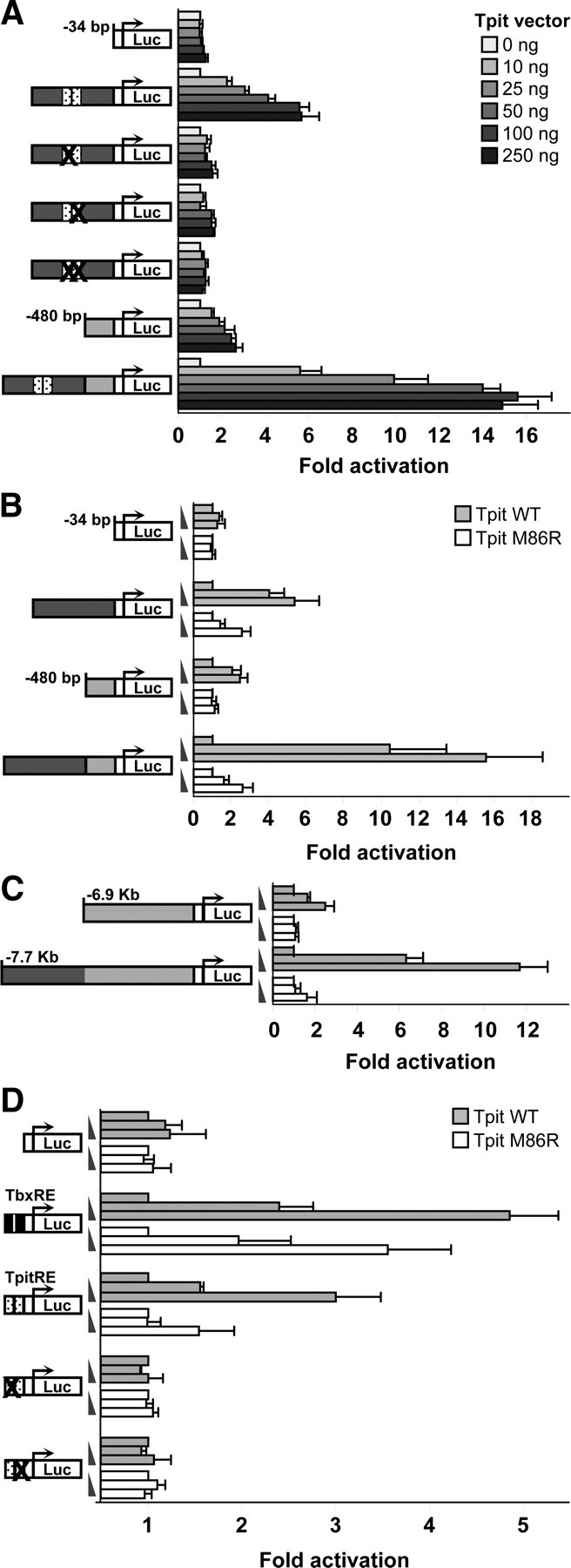
Activation of luciferase reporter constructs by Tpit in Tpit-deficient mouse αT3 cells. A, Tpit dose-response curves for activation of POMC luciferase reporters represented schematically on left. B, Effect of wild-type or dimerization-deficient M86R Tpit (50 or 250 ng of expression vectors) on activity of indicated reporters. C, Dose-responses to Tpit and Tpit M86R of −7.7-kb at −6.9-kb POMC promoter reporters. D, Responsiveness of oligonucleotide reporters to either Tpit or Tpit M86R. All data are presented as means (± sem) of at least three independent experiments, each performed in duplicates.
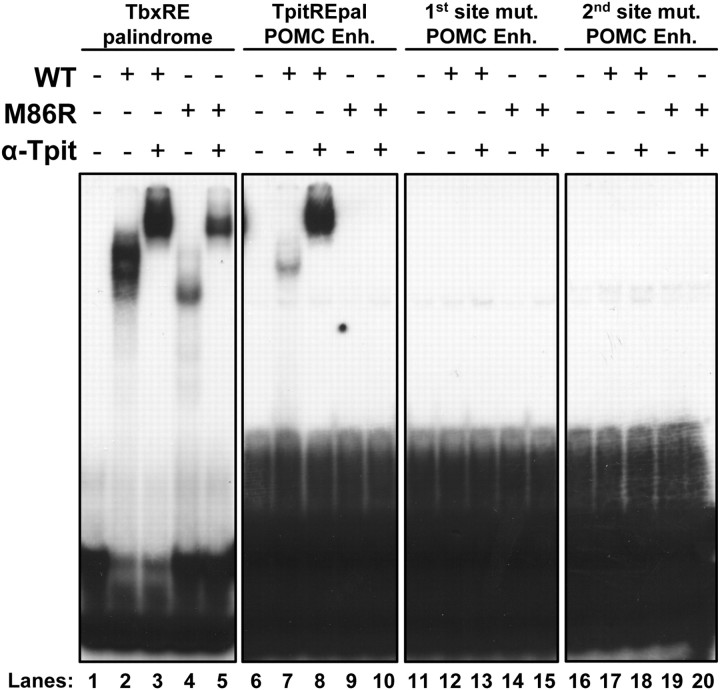
We next assessed the in vitro binding properties of wild-type and M86R Tpit on the TbxRE probe and compared it to the TpitREpal of the POMC enhancer. We had previously shown that Tpit binds the TbxRE as monomer and homodimer (9) (Fig. 5, lanes 2 and 3). Similarly, we had shown that Tpit M86R only binds this probe as monomer (23) (Fig. 5, lanes 4 and 5). Interestingly, the TpitREpal probe only revealed a single band with wild-type Tpit that comigrates with the dimers observed with the TbxRE probe (Fig. 5, lane 7). This band is supershifted by the Tpit antibody (lane 8). And significantly, the TpitREpal probe is not bound in similar in vitro conditions by Tpit M86R (Fig. 5, lanes 9 and 10), in agreement with the greater sensitivity of this target sequence to the dimerization mutation in transfection assays (Fig. 4D). Finally, mutagenesis of either half site of the TpitREpal completely prevented in vitro binding of Tpit or Tpit M86R (Fig. 5, lanes 12–15 and 17–20). In summary, the TpitREpal is highly dependent on Tpit dimers for binding and either mutagenesis of one half site or the Tpit M86R dimerization-deficient mutant completely abolished its in vitro binding.
Fig. 5.
Tpit dimers bind the TpitREpal in vitro. A, Gel retardation was used to assess binding of either wild-type (WT) Tpit or mutant M86R to the consensus TbxRE palindrome and to TpitREpal probes, or to probes mutated for each half-site of the TpitREpal, as indicated. Equivalent amounts of 32P-labeled probes were used. The first panel is an overnight film exposure, whereas the last three panels are 3 d exposures of the same gel. Tpit binding to the consensus TbxRE probe is stronger than the TpitREpal (compare lanes 2 and 7), but Tpit:DNA complexes are supershifted by Tpit antibody in both cases (lanes 3 and 8). The M86R Tpit dimerization-mutant exhibit monomeric binding on the consensus TbxRE, contrasting with an absence of TpitREpal probe retardation (lanes 4 and 9). Mutant probes for either half-site of the TpitREpal do not exhibit any binding (lanes 11–20).
The −7-kb POMC enhancer exhibits preference for anterior pituitary corticotropes
The in vivo properties of the −7-kb enhancer were assessed by transient transgenesis in mice. For this purpose, we used LacZ reporter transgenes and assessed their activity at the cellular level in e17.5 founder mouse embryos. The −480-bp POMC promoter was previously shown to direct expression only in the pituitary and in both corticotropes and melanotropes (11–15). In agreement with previous reports, we found significant penetrance of the promoter transgene in pituitary corticotropes and melanotropes (Fig. 6A) as assessed by colabeling for Tpit and the β-galactosidase (βGal) marker from the transgene. A reporter containing the −7-kb enhancer upstream of the minimal promoter was also expressed in pituitary and in no other tissue (Fig. 6A and data not shown), and within the pituitary expression of this transgene was limited to Tpit-positive cells. Similarly, a transgene containing both −7-kb enhancer and proximal promoter exhibited corticotrope/melanotrope specificity (Fig. 6A). We and others have previously noticed that the −480-bp POMC promoter has a slight preference for expression in intermediate lobe melanotropes compared with anterior lobe. To assess this preference and compare it to enhancer activity, we quantified the ratio of βGal/Tpit-double positive cells over the total number of Tpit-positive cells in pituitaries of mice harboring the three transgenes. This analysis (Fig. 6B) confirmed a preference of the −480-bp promoter for IL melanotropes whereas the enhancer construct exhibited the reverse preference with a 2-fold more frequent coexpression with Tpit in the anterior relative to intermediate lobe. The transgene containing both had a somewhat intermediate preference for anterior corticotropes. These data indicated that the POMC enhancer and promoter have opposite preferences when assessed in vivo and together they balance each other such that the resulting combined transgene shows little lineage preference. These data clearly support a role for both promoter and enhancer for in vivo expression of the POMC gene. Further support for the in vivo activity of the −7-kb enhancer came from ChIP analyses of the Mediator complex protein Med12 at the POMC promoter and enhancer (Fig. 1, C and E). Indeed, a recent study has identified the Mediator complex and proteins of the cohesin family as critical components for bridging active enhancers with their corresponding promoter (24). The presence of Med12 at the −7-kb enhancer is thus in support of the activity of this enhancer in AtT-20 cells. The activity of the −7-kb enhancer is also supported by ChIP detection of p300 (25) at the enhancer (Fig. 1C).
Fig. 6.
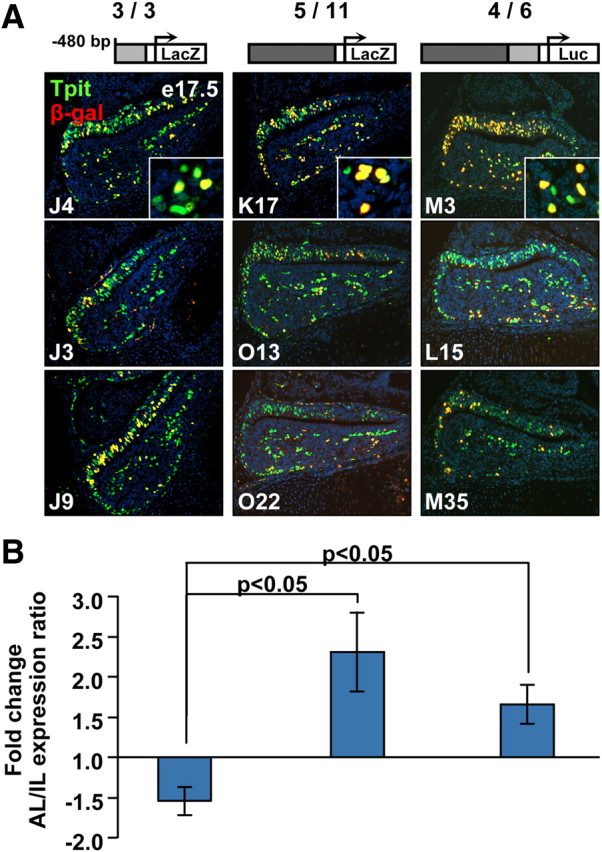
Preferential activity of −7-kb enhancer transgene in corticotropes compared with promoter bias toward melanotropes. A, The different LacZ transgene reporters used to assess activities of POMC promoter, enhancer, or both together, are represented schematically at the top together with the number of transgenic e17.5 pups that express the transgene over the total number of transgenic pups analyzed. Coimmunofluorescence for Tpit (green) and βGal transgene (red) is shown for three different pituitaries harboring each of the three transgenes analyzed. Insets show magnifications of colocalization in anterior lobe. B, Quantitation of βGal transgene reporter expression in AL corticotropes relative to IL melanotropes. The expression of βGal transgenes was scored over the total number of Tpit-positive cells in AL and IL separately, and their ratio is presented for each transgene. For each expressing transgenic embryo, at least four pituitary sections were scored. Pairwise P values were calculated using t test for the enhancer constructs relative to the −480-bp promoter construct. Whereas the −480-bp promoter exhibits a preference for IL melanotrope expression, both reporters containing the enhancer exhibit preference for AL corticotropes.
Species differences in POMC regulatory sequences
The high degree of conservation of the TpitREpal in human and mouse POMC enhancers contrasts with the weaker conservation of the Tpit binding motif in the human Tpit/PitxRE (Fig. 7A and Supplemental Fig. 2). In fact, the putative human Tpit binding site of the promoter Tpit/PitxRE is so poorly conserved that is not recognized by any in silico algorithm, and one may be justified to challenge the importance of Tpit for activity of the human promoter. To assess the relative importance of the human promoter and enhancer compared with rodents, we made similar luciferase reporters containing the human promoter and/or enhancer. The activity of these reporters was assessed by transfection in AtT-20 cells and compared with the rodent sequences that were previously characterized. This analysis revealed that both human enhancer and promoter are active and exhibit synergism (much like the rodent regulatory sequences) but they are less active when assessed in the mouse AtT-20 cells (Fig. 7B). The species differences between the cognate transcription factors may account for the lesser activity of human sequences. Complementation of Tpit activity in αT3 cells stimulated both human promoter and enhancer (Fig. 7C). The data thus support action of Tpit on the human Tpit/PitxRE despite its poor conservation. A direct assessment of the Tpit/PitxRE potency was carried out using reporters containing three copies of Tpit/PitxRE from rat, mouse, and human. This analysis (Fig. 7D) showed that all three are responsive to Tpit but that their relative activities correlate well with sequence conservation of the Tpit binding site by comparison to a canonical Tbox factor binding site.
Fig. 7.
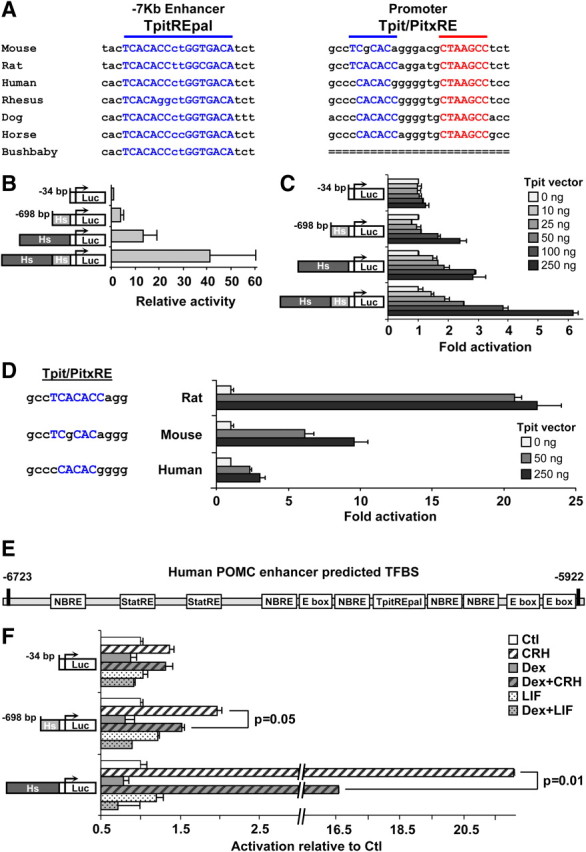
Properties of human −7-kb POMC enhancer. A, Sequence comparison of conserved Tpit regulatory elements of the promoter and −7-kb enhancer. The TpitREpal of the −7-kb enhancer is more conserved across species than the Tpit/PitxRE of the promoter. TpitRE sequences are shown in blue and PitxRE in red. B, Activity of luciferase reporters containing either human (Hs) POMC promoter, −7-kb enhancer or both upon lipofection into AtT-20 cells. Results are the means ± sem of four independent experiments each performed in duplicate. C, Activation of indicated luciferase reporters by increasing amounts of Tpit expression vector upon lipofection into αT3 cells. A representative of two independent experiments is shown. D, Activation of rat, mouse, and human Tpit/PitxRE reporters by indicated amounts of Tpit expression plasmid after lipofection in αT3 cells. Conserved nucleotides within the Tpit binding site are indicated by capital letters. E, Schematic representation of the human −7-kb enhancer indicating the position of predicted regulatory elements/transcription factor binding sites as identified by in silico analyses with MatInspector software (Genomatix). F, Hormone response of the indicated luciferase reporters upon lipofection into AtT-20 cells. Representative data of four independent experiments, each performed in duplicate (± sem).
The in silico analyses of the human orthologous enhancer region revealed predicted transcription factor binding sites (Fig. 7E) that are similar to those that were predicted and found to recruit cognate factors in the mouse (Fig. 1B). Surprisingly and in contrast to the mouse enhancer (Fig. 2C), the human enhancer reporter was found to be responsive to CRH but not to LIF (Fig. 7F). Whereas the enhancer is not responsive to Dex alone, Dex significantly repressed CRH-stimulated activity. It is striking that the human enhancer is predicted to have very good Stat3 binding sites as well as multiple NBRE sequences, but no glucocorticoid response element (Fig. 7E). As for the mouse enhancer, it thus appears that the presence of either in silico predicted site or occupancy as revealed by ChIP are not good predictors of the activity of the enhancer regulatory sequences because the mouse enhancer was found to be unresponsive to hormones despite the presence of appropriate binding sites and the human enhancer responded to CRH and Dex but not to LIF despite the presence of bona fide Stat3 sites. Collectively, these data suggest that protein:protein interactions and protein context at regulatory sequences are critical to determine the potency of specific transcription factors to modulate transcription. These data suggest that POMC promoter and enhancer may play different roles depending on the species.
To evaluate variation in these sequence motifs in evolution, we examined UCSC multi-species comparisons of POMC locus (Supplemental Fig. 3A). Interestingly this comparison reveals conservation of the POMC enhancer (Supplemental Fig. 3B) across mammals, with some exceptions like guinea pig, chimp, orangutan, and cat. This uneven conservation contrasts with the more global conservation of the hypothalamic enhancers nPE1 and nPE2 and the promoter (Supplemental Fig. 3, A and C).
Discussion
The quest to identify relevant regulatory sequences has been ongoing since the advent of gene regulation studies, and it is still a challenge. Very recent genomic studies have identified markers of chromatin structure that appear to present the most hopeful and generally applicable approach to this quest, but it is still a relatively challenging trial-and-error endeavor. It is now clear that most mammalian genes have multiple regulatory enhancers that may be very distant from protein-coding sequences. Further, these regulatory modules/enhancers are used in different combinations to target cell-specific expression of genes that are expressed in multiple cells or tissues. In the present work, we have queried the relevance of sequences located about 7 kb upstream of the POMC gene transcription start site because of the identification through ChIP of different transcription factors in this region, initially Stat3 followed by many other factors known to be critical for the corticotrope-specific program of gene expression. The −7-kb enhancer is highly conserved among mammalian species but that in itself is not always a sufficient criterion to discriminate relevant regulatory sequences. The presence of general coactivators such as p300 at most active enhancers is a good predictor of regulatory sequences (25, 26). Further, the recently observed link provided between active tissue-specific enhancers and promoters by the Mediator complex and cohesins (24) provides the most powerful tool to identify enhancers that are active in a cell-specific context. Hence, demonstration of strong recruitment of p300 and of the Mediator protein Med12 at the POMC −7-kb enhancer is a strong argument to suggest that this enhancer is active in AtT-20 cells. In this context, it is noteworthy that Stat3, or any of the other proteins that we have assessed, is not present at two other upstream enhancers of the POMC locus, namely the nPE1 and nPE2 elements that have been otherwise associated with neuronal expression of POMC (16). The chromatin marks studied in the present work are therefore consistent with activity of the −7-kb enhancer in AtT-20 cells (and in pituitary) whereas the upstream nPE1 and nPE2 appear irrelevant for pituitary expression, in contrast to their importance for driving POMC expression in the hypothalamus and arcuate nucleus.
The activity of the −7-kb enhancer is highly dependent on the palindromic TpitREpal (Figs. 3 and 4). The TpitREpal is the first reported example of a natural palindromic response element for transcription factors of the Tbox family. Indeed, the consensus TbxRE palindromic element was defined by in vitro analyses (27, 28), but no sequences of this type were yet reported in natural regulatory sequences. It is noteworthy that the central peak of conservation (Fig. 1A and Supplemental Fig. 3B) observed within the −7-kb enhancer is centered around the TpitREpal. The TpitREpal is thus at the core of the conserved sequences and interestingly, it is activated by dimers of Tpit, a most highly cell-restricted transcription factor because it is only expressed in the two pituitary POMC lineages (8). The importance of the TpitREpal for POMC expression is consistent with the identification of numerous Tpit mutations in children with congenital early onset isolated ACTH deficiency. We have previously shown that many of these Tpit mutant proteins are deficient in DNA binding, and most interestingly we identified one mutation that blocks dimerization of Tpit, the M86R mutation (23). In agreement with the ACTH deficiency caused by this mutation, the Tpit M86R mutant protein fails to homodimerize and to efficiently activate reporters containing the TpitREpal. By comparison to the Tpit/PitxRE of the promoter, the TpitREpal of the −7-kb enhancer may thus be regarded as the most specific target of the highly cell-restricted transcription factor Tpit, particularly in the human gene where the promoter Tpit/PitxRE is less conserved (Fig. 7, A and D). The cell-specific activity of the enhancer in vivo is thus not surprising and consistent with a unique role of the −7-kb in ensuring pituitary cell-specific expression of POMC.
The −7-kb regulatory sequences exhibit all the hallmarks of enhancers, namely their activity at a distance from the promoter, including downstream of the reporter gene and in either orientation (Fig. 2). The mouse −7-kb enhancer does not appear to contribute to hormone responsiveness, despite the identification by ChIP of CRH-activated Nurr1, LIF-activated Stat3, and Dex-dependent GR (Fig. 1C). Thus, a contribution of the enhancer for hormonal regulation of POMC transcription may not be essential because the −480-bp promoter recapitulates responsiveness to CRH, LIF, and Gc by comparison to regulation of the endogenous POMC gene. This issue is further confounded by the fact that the human −7-kb enhancer does exhibit responsiveness to CRH and Dex but not to LIF (Fig. 7E). Because both −7-kb enhancer and promoter are conserved in many species, it is possible that many regulatory motifs are conserved within these regulatory modules across species but they are not used in all species.
It is noteworthy that the −7-kb enhancer harbors putative binding sites for all but one critical transcription factors of the POMC promoter and that in all cases, the putative activity of these binding sites is supported by ChIP data (Fig. 1). The only exception is Pitx1 that is critical for promoter activity and in particular, activity of the Tpit/PitxRE that represents the corner stone of cell specificity of the promoter (8, 29). Interestingly, the poor conservation of the Tpit binding site in the human and mouse Tpit/PitxRE compared with rat is correlated with their intrinsic activity (Fig. 7D) but not so directly with promoter activity (Figs. 2B and 7B) or with promoter/enhancer occupancy by Tpit (Fig. 1, C and E). Because enhancer activity is so critically dependent on the highly cell-restricted Tpit (Fig. 3), it is possible that it serves a lineage-restricted amplification role whereas the POMC promoter that also depends on Pitx1 may have a more important role in initial activation of the locus because Pitx1 is expressed before pituitary organogenesis. Be that as it may, both promoter and enhancer exhibit highly pituitary-specific activity in transgenic mouse assays without detectable expression in any other tissue including the hypothalamus where expression appears to be driven by the nPE1 and nPE2 enhancers that lie further upstream. At this point, it is not clear what may be the molecular basis for the preferential activity of the −7-kb enhancer in AL corticotropes compared with the promoter preference for IL melanotropes. In all likelihood, both promoter and −7-kb enhancer participate in activity of the POMC gene in both cell types.
The elegant studies of David Stern in drosophila (1) have recently exemplified the frequent redundancy of regulatory modules for construction of various tissue-specific gene expression patterns. We may speculate that the relative importance of enhancer relative to promoter may differ between species, such as in men and mice, but such conclusion would require analyses in homologous systems.
Materials and Methods
Cell culture and transfection
AtT-20, αT3, 293T, and Cos1 cells were maintained in DMEM supplemented with 10% fetal bovine serum and antibiotics. The cells were plated in 12-well plates and transfected with 500 ng of luciferase reporter construct using Lipofectamine reagent (Invitrogen, Carlsbad, CA). The second day after transfection, cells were stimulated for 4 h with either PBS as vehicle, CRH 10−7 m, dexamethasone (Dex) 10−7 m (Sigma-Aldrich, St-Louis, MO), and/or LIF 10 ng/ml (Chemicon, Billerica, MA), as indicated. To assess Tpit-responsiveness, we cotransfected 500 ng of luciferase reporters with increasing concentration (0–250 ng) of WT and M86R mutant Tpit effector plasmid (23) and empty pSP64 plasmid to make up the total amount.
Reporter constructs
The previously described (29) minimal (−34/+63bp) and proximal (−480/+63bp) POMC promoter luciferase reporter constructs were used as starting point for enhancer constructs. The enhancer region (832-bp PCR amplified fragment) was inserted upstream the minimal promoter and in different location and orientation in the proximal promoter reporter. The 6.9-kb and 7.7-kb POMC locus constructs were created by insertion of two Pfu Turbo amplified fragments into the previous reporter plasmid after complete removal of the proximal POMC promoter. The TpitREpal mutants were generated according to QuickChange site-directed mutagenesis protocol (Stratagene, La Jolla, CA), whereas the Tpit/PitxRE mutant were previously described (8). Consensus TbxRE and TpitREpal oligonucleotides were inserted upstream the minimal POMC promoter to assess the importance of Tpit homodimer binding. Oligonucleotides used for cloning are: TbxRE GATCCAATTTCACACCTAGGTGTGAAATTG), TpitREpal (GATCCGTACTCACACCCTGGTGACATCTCG/GATCCGAGATGTCACCAGGGTGTGAGTACG), TpitREpal first mutant and second mutant used the same oligos as TpitREpal with the appropriate modifications. The rat, mouse, and human Tpit/PitxRE constructs were created using similar oligonucleotides that encompass their respective Tpit/Pitx binding site. Cohesive double-stranded oligonucleotides were inserted in the same orientation as trimers upstream of the minimal POMC promoter to evaluate the effect of sequence divergence at the Tpit binding site on activity. We used the following oligonucleotides to create the constructs: rat (GATCCTGCCTCACACCAGGATGCTAAGCCTCTGTCCAGTA/GATCTACTGGACAGAGGCTTAGCATCCTGGTGTGAGGCAG), mouse (GATCCTGCCTCGCACAGGGACGCTAAGCCTCTGTCCAGTA/GATCTACTGGACAGAGGCTTAGCGTCCCTGTGCGAGGCAG) and human (GATCCTGCCCCACACGGGGGTGCTAAGCCTCCCGCCCGTA/GATCTACGGGCGGGAGGCTTAGCACCCCCGTGTGGGGCAG). Human promoter and enhancer reporter constructs were designed with sequences corresponding to the mouse reporters. All reporter constructs were sequenced to ensure accuracy of DNA sequences.
ChIP and QPCR
AtT-20 cells were grown to 60–70% confluence and stimulated with 10 ng/ml LIF, CRH 10−7 m, or Dex 10−7 m for 20 min when required. ChIP were performed as described previously (30), with little modifications. Briefly, chromatin was crosslinked with 1% formaldehyde added directly to the culture medium (5 min at room temperature). Crosslinking was stopped with glycine 125 mm in PBS for 5 min. Nuclei were prepared by sequential incubation and purification by centrifugation on ice for 5 min in buffer A [10 mm Tris-HCl (pH 8), 10 mm EDTA, 0.25% Triton X-100], and for 30 min in buffer B [10 mm Tris-HCl (pH 8), 1 mm EDTA, 200 mm NaCl] (all buffers include protease inhibitors). Nuclei were resuspended in sonication buffer [10 mm Tris-HCl (pH 8), 1 mm EDTA, 0.5% SDS, 0.5% Triton X-100, 0.05% NaDOC, 140 mm NaCl] and sonicated to obtain chromatin fragments of an average length of 500 bp. Sonicated chromatin was immunoprecipitated with either rabbit IgG (G2018, Sigma-Aldrich), GR (sc-1004), a combination of phospho-Stat3 (sc-7993) and Stat3 (sc-482x) antibodies, RNA Pol II (sc-899), Nurr1 (sc-991x), p300 (sc-585x) from Santa Cruz Biotechnology (Santa Cruz, CA), Med12 (A300–774A, Bethyl, Montgomery, TX) or homemade Pitx1 (31), NeuroD1 (12) and Tpit (8) antibodies. The immunoprecipitate was collected using protein-A/G beads (Santa Cruz Biotechnology). After washes and decrosslinking, DNA was purified using QIAquick columns following manufacturer's directives (Qiagen, Mississauga, On). Enrichment was assed by QPCR with Perfecta SYBR green PCR kit (Quanta Biosciences, Gaithersburg, MD). The oligonucleotides used for QPCR are: POMC promoter (TGGTTTCACAAGATATCACACTTTCCC/TCGGAGTGGAATTACCTATGTGCG) POMC enhancer (TTCCCATGCAGGTCACAAGACTCA/AAGGCAGAGGGTGGAAGGAAGAAA) and MyoD as control (TGCTCCTTTGAGACAGCAGA/TTTCAGGAGGGCTCCCATGT).
Binding motif analyses
The mouse and human POMC enhancer sequences were challenged against all known transcription factor binding motifs using the MatInspector software (Genomatix).
Electromobility shift assay
The gel retardation assay was performed as previously described (23). The retardation assay with the consensus TbxRE palindrome (GATCCAATTTCACACCTAGGTGTGAAATT), TpitREpal (GATCCGTACTCACACCCTGGTGACATCTC; GATCCGAGATGTCACCAGGGTGTGAGTAC), TpitREpal first mutant (GATCCGTACTCACCAACTGGTGACATCTC; GATCCGAGATGTCACCAGTTGGTGAGTAC) and TpitREpal second mutant (GATCCGTACTCACACCCTTTGGACATCTC; GATCCGAGATGTCCAAAGGGTGTGAGTAC) was performed using 5 μl of in vitro transcribed mouse WT and M86R Tpit proteins produced with the rabbit reticulocyte lysate system following the manufacturer's protocol (Promega, Madison, WI). The proteins were incubated for 1 h on ice with 50,000 CPM of 32P-dATP labeled probes of similar specific activity in presence of 1 μg of salmon sperm DNA. Homemade rabbit anti-Tpit antibody was added 20 min before loading for the supershifted experiments.
Transgenic mice
We inserted the POMC promoter and −7-kb enhancer region into pWHERE plasmid (InvivoGen, San Diego, Ca). This reporter was modified to excise the injected fragments with NotI restriction enzyme. The transgenes containing a LacZ reporter gene with a nuclear localization signal were injected into B6C3 fertilized eggs. Mouse embryos (e17.5) were genotyped by PCR with the following oligonucleotides: AATGCTGTTCCTGCAGACCTCTCT/GCGCTGGTGGTTAGGAAGAACTTA for the promoter construct, and TTCCCATGCAGGTCACAAGACTCA/CAGTCTCTCCTTTGCAGCACAACA for the −7kb enhancer constructs.
Immunofluorescence and cell counts
Paraffin-embedded formalin-fixed transgenic embryonic tissue sections were analyzed by coimmunofluorescence with rabbit anti-Tpit and goat βGal (56028, MP Biomedicals, Solon, OH); Hoechst was used for nuclear staining. Complete embryo sections were analyzed for each transgenics, and Tpit and/or βGal expression was found to be restricted to the pituitary POMC-expressing cells. The number of βGal expressing Tpit-positive cells as well as the total number of Tpit cells was determined for anterior (AL) and intermediate (IL) pituitary lobe separately. To evaluate the relative tissue penetrance of the different transgenes, the AL/IL ratio was then calculated on the proportion Tpit cells expressing βGal reporter gene.
Acknowledgments
We are grateful to Lise Laroche for her expert secretarial assistance.
Address all correspondence and requests for reprints to: Jacques Drouin, Laboratoire de génétique moléculaire, Institut de recherches cliniques de Montréal (IRCM), 110, avenue des Pins Ouest, Montréal QC H2W 1R7 Canada. E-mail: jacques.drouin@ircm.qc.ca.
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and National Cancer Institute of Canada (NCIC). G.S.-D. was supported by a COPSE scholarship. D.L. was supported by a fellowship from Université de Montréal.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- βGal
- β-Galactosidase
- ChIP
- chromatin immunoprecipitation
- CRH
- corticotropin-releasing hormone
- Dex
- dexamethasone
- GR
- glucocorticoid receptor
- POMC
- proopiomelanocortin
- TpitRE
- Tpit response element.
References
- 1. Frankel N , Davis GK , Vargas D , Wang S , Payre F , Stern DL. 2010. Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature 466:490–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drouin J. 2010. Pituitary development. In: , Melmed S (ed) The Pituitary, 3rd Edition. Elsevier-Academic Press, 3–19 [Google Scholar]
- 3. Tremblay JJ , Lanctôt C , Drouin J. 1998. The pan-pituitary activator of transcription, Ptx-1 (pituitary homeobox1), acts in synergy with SF-1 and Pit1 and is an upstream regulator of the Lim-homeodomain gene Lim3/Lhx3. Mol Endocrinol 12:428–441 [DOI] [PubMed] [Google Scholar]
- 4. Sornson MW , Wu W , Dasen JS , Flynn SE , Norman DJ , O'Connell SM , Gukovsky I , Carriere C , Ryan AK , Miller AP , Zuo L , Gleiberman AS , Andersen B , Beamer WG , Rosenfeld MG. 1996. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384:327–333 [DOI] [PubMed] [Google Scholar]
- 5. Camper SA , Saunders TL , Katz RW , Reeves RH. 1990. The Pit-1 transcription factor gene is a candidate for the murine snell dwarf mutation. Genomics 8:586–590 [DOI] [PubMed] [Google Scholar]
- 6. Li S , Crenshaw EBI , Rawson EJ , Simmons DM , Swanson LW , Rosenfeld MG. 1990. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347:528–533 [DOI] [PubMed] [Google Scholar]
- 7. Zhu X , Gleiberman AS , Rosenfeld MG. 2007. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87:933–963 [DOI] [PubMed] [Google Scholar]
- 8. Lamolet B , Pulichino AM , Lamonerie T , Gauthier Y , Brue T , Enjalbert A , Drouin J. 2001. A pituitary cell-restricted T-box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 104:849–859 [DOI] [PubMed] [Google Scholar]
- 9. Pulichino AM , Vallette-Kasic S , Tsai JPY , Couture C , Gauthier Y , Drouin J. 2003. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev 17:738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Proulx-Ferland L , Meunier H , Côté J , Dumont D , Gagné B , Labrie F. 1983. Multiple factors involved in the control of ACTH and a-MSH secretion. J steroid Biochem 19:439–445 [DOI] [PubMed] [Google Scholar]
- 11. Tremblay Y , Tretjakoff I , Peterson A , Antakly T , Zhang CX , Drouin J. 1988. Pituitary-specific expression and glucocorticoid regulation of proopiomelanocortin fusion gene in transgenic mice. Proc Natl Acad Sci USA 85:8890–8894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lavoie PL , Budry L , Balsalobre A , Drouin J. 2008. Developmental dependence on NurRE and EboxNeuro for expression of pituitary POMC. Mol Endocrinol 22:1647–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hammer GD , Fairchild-Huntress V , Low MJ. 1990. Pituitary-specific and hormonally regulated gene expression directed by the rat proopiomelanocortin promoter in transgenic mice. Mol Endocrinol 4:1689–1697 [DOI] [PubMed] [Google Scholar]
- 14. Liu B , Hammer GD , Rubinstein M , Mortrud M , Low MJ. 1992. Identification of DNA elements cooperatively activating proopiomelanocortin gene expression in the pituitary gland of transgenic mice. Mol Cell Biol 12:3978–3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rubinstein M , Mortrud M , Liu B , Low MJ. 1993. Rat and mouse proopiomelanocortin gene sequences target tissue-specific expression to the pituitary gland but not to the hypothalamus of transgenic mice. Neuroendocrinology 58:373–380 [DOI] [PubMed] [Google Scholar]
- 16. de Souza FS , Santangelo AM , Bumaschny V , Avale ME , Smart JL , Low MJ , Rubinstein M. 2005. Identification of neuronal enhancers of the proopiomelanocortin gene by transgenic mouse analysis and phylogenetic footprinting. Mol Cell Biol 25:3076–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young JI , Otero V , Cerdan MG , Falzone TL , Chan EC , Low MJ , Rubinstein M. 1998. Authentic cell-specific and developmentally regulated expression of pro-opiomelanocortin genomic fragments in hypothalamic and hindbrain neurons of transgenic mice. J Neurosci 18:6631–6640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Langlais D , Couture C , Balsalobre A , Drouin J. 2008. Regulatory network analyses reveal genome-wide potentiation of LIF signaling by glucocorticoids and define an innate cell defense response. PLoS Genet e1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bousquet C , Zatelli MC , Melmed S. 2000. Direct regulation of pituitary proopiomelanocortin by STAT3 provides a novel mechanism for immuno-neuroendocrine interfacing. J Clin Invest 106:1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mynard V , Guignat L , Devin-Leclerc J , Bertagna X , Catelli MG. 2002. Different mechanisms for leukemia inhibitory factor-dependent activation of two proopiomelanocortin promoter regions. Endocrinology 143:3916–3924 [DOI] [PubMed] [Google Scholar]
- 21. Jeannotte L , Trifiro MA , Plante RK , Chamberland M , Drouin J. 1987. Tissue-specific activity of the pro-opioomelanocortin gene promoter. Mol Cell Biol 7:4058–4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Therrien M , Drouin J. 1991. Pituitary pro-opiomelanocortin gene expression requires synergistic interactions of several regulatory elements. Mol Cell Biol 11:3492–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vallette-Kasic S , Couture C , Balsalobre A , Gauthier Y , Metherell L , Dattani M , Drouin J. 2007. The TPIT gene mutation M86R associated with isolated adrenocorticotropin deficiency interferes in protein: protein interactions. J Clin Endocrinol Metab 92:3991–3999 [DOI] [PubMed] [Google Scholar]
- 24. Kagey MH , Newman JJ , Bilodeau S , Zhan Y , Orlando DA , van Berkum NL , Ebmeier CC , Goossens J , Rahl PB , Levine SS , Taatjes DJ , Dekker J , Young RA. 2010. Mediator and cohesin connect gene expression and chromatin architecture. Nature 467:430–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heintzman ND , Hon GC , Hawkins RD , Kheradpour P , Stark A , Harp LF , Ye Z , Lee LK , Stuart RK , Ching CW , Ching KA , Antosiewicz-Bourget JE , Liu H , Zhang X , Green RD , Lobanenkov VV , Stewart R , Thomson JA , Crawford GE , Kellis M , Ren B. 2009. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 459:108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heintzman ND , Stuart RK , Hon G , Fu Y , Ching CW , Hawkins RD , Barrera LO , Van Calcar S , Qu C , Ching KA , Wang W , Weng Z , Green RD , Crawford GE , Ren B. 2007. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39:311–318 [DOI] [PubMed] [Google Scholar]
- 27. Kispert A , Hermann BG. 1993. The Brachyury gene encodes a novel DNA binding protein. EMBO J 12:4898–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muller CW , Herrmann BG. 1997. Crystallographic structure of the T domain-DNA complex of the Brachyury transcription factor. Nature 389:884–888 [DOI] [PubMed] [Google Scholar]
- 29. Lamonerie T , Tremblay JJ , Lanctôt C , Therrien M , Gauthier Y , Drouin J. 1996. PTX1, a bicoid-related homeobox transcription factor involved in transcription of pro-opiomelanocortin (POMC) gene. Genes Dev 10:1284–1295 [DOI] [PubMed] [Google Scholar]
- 30. Batsche E , Desroches J , Bilodeau S , Gauthier Y , Drouin J. 2005. Rb enhances p160/SRC coactivator-dependent activity of nuclear receptors and hormone responsiveness. J Biol Chem 280:19746–19756 [DOI] [PubMed] [Google Scholar]
- 31. Lanctôt C , Gauthier Y , Drouin J. 1999. Pituitary homeobox 1 (Ptx1) is differentially expressed during pituitary development. Endocrinology 140:1416–1422 [DOI] [PubMed] [Google Scholar]
- 32. Kent WJ , Sugnet CW , Furey TS , Roskin KM , Pringle TH , Zahler AM , Haussler D. 2002. The human genome browser at UCSC. Genome Res 12:996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blanchette M , Tompa M. 2002. Discovery of regulatory elements by a computational method for phylogenetic footprinting. Genome Res 12:739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]