Abstract
Aldosterone is a major mineralocorticoid hormone that plays a key role in the regulation of electrolyte balance and blood pressure. Excess aldosterone levels can arise from dysregulation of the renin-angiotensin-aldosterone system and are implicated in the pathogenesis of hypertension and heart failure. Aldosterone synthase (cytochrome P450 11B2, CYP11B2) is the sole enzyme responsible for the production of aldosterone in humans. Blocking of aldosterone synthesis by mediating aldosterone synthase activity is thus a recently emerging pharmacological therapy for hypertension, yet a lack of structural information has limited this approach. Here, we present the crystal structures of human aldosterone synthase in complex with a substrate deoxycorticosterone and an inhibitor fadrozole. The structures reveal a hydrophobic cavity with specific features associated with corticosteroid recognition. The substrate binding mode, along with biochemical data, explains the high 11β-hydroxylase activity of aldosterone synthase toward both gluco- and mineralocorticoid formation. The low processivity of aldosterone synthase with a high extent of intermediates release might be one of the mechanisms of controlled aldosterone production from deoxycorticosterone. Although the active site pocket is lined by identical residues between CYP11B isoforms, most of the divergent residues that confer additional 18-oxidase activity of aldosterone synthase are located in the I-helix (vicinity of the O2 activation path) and loops around the H-helix (affecting an egress channel closure required for retaining intermediates in the active site). This intrinsic flexibility is also reflected in isoform-selective inhibitor binding. Fadrozole binds to aldosterone synthase in the R-configuration, using part of the active site cavity pointing toward the egress channel. The structural organization of aldosterone synthase provides critical insights into the molecular mechanism of catalysis and enables rational design of more specific antihypertensive agents.
Hypertension affects about 25% of adults around the world and is estimated to lead to over 7 million deaths each year (1). It is possible to prevent the development of hypertension by lifestyle changes, and there are numerous effective antihypertensive drugs and their combinations available for patients. The current generally accepted therapy includes drugs targeting proteins of the renin-angiotensin-aldosterone system (2, 3). Nonetheless, hypertension remains inadequately controlled in many patients due to so-called resistant hypertension, one of the causes of which is primary aldosteronism. The developing approach in cardiovascular pathophysiology induced by aldosterone is to suppress its synthesis (i.e. via inhibition of CYP11B2) (4–7). This strategy prevents the nongenomic actions of aldosterone that are not antagonized by mineralocorticoid receptor blockade. However, the development of selective and potent CYP11B2 inhibitors is challenging due to high homology to the CYP11B1 isoform and other steroidogenic enzymes, especially in the absence of structural information.
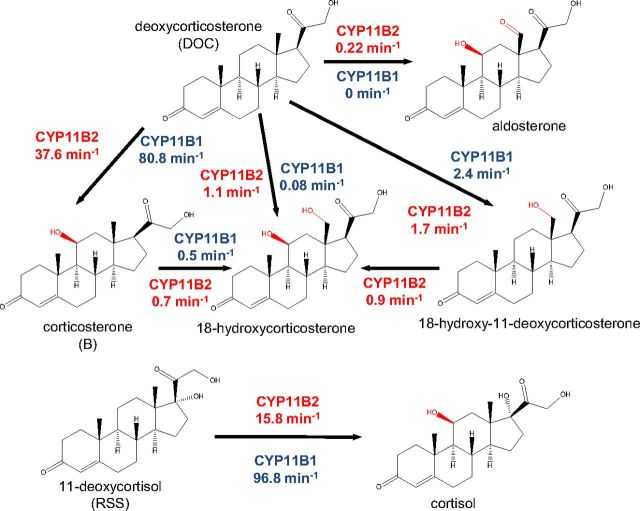
The CYP11B2 enzyme is expressed in the zona glomerulosa of the adrenal cortex, where it catalyzes the 11β-hydroxylation of deoxycorticosterone (DOC) to corticosterone (B), followed by 18-hydroxylation to produce 18-hydroxycorticosterone (18OH-B) with further 18-oxidation of the latter to aldosterone. Impaired corticosterone methyloxidase (CMO or CYP11B2) activity in the conversion of B (type 1) or 18OH-B (type 2) causes respective aldosterone deficiencies (8–12). Aldosterone synthesis is regulated by angiotensin II and potassium levels, as well as ACTH, in an acute or chronic manner (13). CYP11B2 and CYP11B1 genes are tandemly arranged on chromosome 8 (14) with very little sequence divergence (93% amino acid sequence identity) presumably owing to a gene duplication event during evolution (15). Despite high identity, the two proteins differ in terms of their expression patterns within the adrenal cortex, and their regulation and substrate specificities, which accounts for the zone-specific synthesis of gluco- and mineralocorticoids by CYP11B1 and CYP11B2, respectively (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org) (16). This functional diversification and specialization is not well understood at the molecular level. In this study, we compared functional properties of purified human CYP11B enzymes and determined structures of CYP11B2 in complex with DOC and fadrozole to delineate a molecular basis for the isoform specificity and selective inhibition.
Materials and Methods
Protein purification and crystallization
Human CYP11B1 and CYP11B2 cDNAs were purchased from OriGene (accession nos. NM_000497 and NM_000498, respectively; OriGene, Rockville, MD) and cloned into a pCW-LIC vector. The mature forms of CYP11B proteins (without the mitochondrial targeting peptide and with N-terminal tag MAKKTSS added to the 30th residue of CYP11B2 and 26th residue of CYP11B1 and both with C-terminal His6-tag) were coexpressed with GroEL/ES in Escherichia coli JM109 (17, 18). Expression was induced by the addition of 0.5 mm isopropyl-1-thio-D-galactopyranoside, 4 mg/ml arabinose, and 0.5 mm δ-aminolevulinic acid, and the culture was incubated another 48 h at 26 C. Harvested cells were resuspended in 50 mm potassium phosphate buffer (pH 7.4), containing 500 mm NaCl and 20% glycerol. The cells were lysed by passing through an EmulsiFlex-C5 homogenizer (Avestin, Ottawa, Ontario, Canada) and solubilized with 1.5% sodium cholate. The supernatant after centrifugation was loaded onto a 5-ml NiHiTrap chelating column (GE Healthcare, Princeton, NJ). The column was washed with 10 column volumes of 50 mm potassium phosphate buffer (pH 7.4) containing 500 mm NaCl, 0.5% sodium cholate, 20% glycerol, and 25 mm imidazole, and the protein was eluted with the same buffer containing 250 mm imidazole. The protein was further purified to homogeneity by cation-exchange chromatography on a 5ml SourceS column (GE Healthcare) and eluted with 50 mm potassium phosphate buffer (pH 7.4) containing 500 mm NaCl, 0.5% CHAPS (3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate), 20% glycerol. For crystallography, 50 μm DOC or 50 μm fadrozole was added to all buffers. Human CYP17A1, CYP19A1, CYP21A2, adrenodoxin (Adx), Adx reductase, and rat NADPH-cytochrome P450 reductase were purified as described (19, 20). Purified CYP11B2 protein (14 mg/ml) was crystallized using the hanging drop vapor diffusion method. The reservoir solution contained 10% PEG 4000, 0.2 m lithium sulfate, and 0.1 m Tris (pH 8.5). Crystals were soaked in the corresponding mother liquor supplemented with 20% glycerol as cryoprotectant before freezing in liquid nitrogen.
Structure determination and refinement
Datasets were processed using the program HKL2000 (21); statistics are shown in Table 2. The structure was solved by the molecular replacement method using Molrep in the CCP4 program suit (22) using human CYP11A1 (PDB, 3N9Y) as a search model. Model building was performed using O (23), simulated annealing using XPLOR (24), and restrained refinement using Refmac5 in the CCP4 suite. CYP11B2 crystals contain 12 protein molecules in the asymmetric unit.
Table 2.
Data collection and refinement statistics
| PDB: 4DVQ (DOC) | PDB: 4FDH (fadrozole) | |
|---|---|---|
| Data collection | ||
| Resolution, Å (last shell) | 48.55–2.49 (2.62–2.49) | 49.19–2.71 (2.86–2.71) |
| Space group | P1211 | P1211 |
| Cell dimensions, Å | ||
| a, b, c | 130.4, 199.6, 150.2 | 129.8, 199.1, 150.0 |
| β | 112.2 | 112.08 |
| Molecules in an asymmetric unit | 12 | 12 |
| Rsym, %a | 0.083 (0.940) | 0.090 (1.122) |
| I/σ | 12.7 (1.5) | 10.5 (1.1) |
| Measured reflections | 1,041,277 (137,486) | 678,165 (85,684) |
| Unique reflections | 245,749 (34,366) | 188,505 (26,406) |
| Redundancy | 4.2 (4.0) | 3.6 (3.2) |
| Completeness, % | 99.2 (95.1) | 99.0 (95.2) |
| Refinement | ||
| Rworkb | 0.213 | 0.222 |
| Rfreec | 0.280 | 0.293 |
| No. of residues/atoms | 5551/46,194 | 5568/46,075 |
| No. of waters | 265 | 127 |
| B values (Wilson/refined), Å2 | 61.4/53.6 | 78.7/67.0 |
| Ramachandran plotd | ||
| Most favored, % (residues) | 91.6 (4482) | 89.5 (4395) |
| Additional allowed, % (residues) | 8 (393) | 9.8 (481) |
| Generously allowed, % (residues) | 0.1 (5) | 0.4 (21) |
| Disallowed, % (residues)e | 0.2 (12) | 0.2 (12) |
| RMS deviations | ||
| Bond length, Å | 0.011 | 0.011 |
| Bond angle, degrees | 1.3 | 1.4 |
Rsym =Σhkl [Σi|Ihkl,i − <Ihkl>|]/Σhkl,i <Ihkl>, where Ihkl,i is the intensity of an individual measurement of the reflection with Miller indices h, k, and l, and <Ihkl> is the mean intensity of that reflection.
Rwork = Σ | |Fobs| − |Fcalc| | /Σ |Fobs|, where |Fobs| and |Fcalc| are observed and calculated structure factor amplitudes, respectively.
Rfree is equivalent to Rwork except that 5% of the total reflections were set aside for an unbiased test of the progress of the refinement.
Program PROCHECK (57) was used.
Residues in disallowed region are in the active site. The disallowed conformation of these residues permits proper ligand binding.
Sterol binding spectral determinations
Ligand-induced spectral changes were monitored as a shift of the heme Soret peak: type I (blue shift from 418 nm, this ligand binding induces displacement of water as a sixth ligand) or type II (shift to 425 nm, ligand binding through direct coordination of the heme iron by a nitrogen atom). The apparent dissociation constant of the enzyme-sterol or inhibitor complex (Kd) was determined by differential spectral titration as described (25). The binding of sterols or inhibitor to human CYP11B1 or CYP11B2 was performed in 20 mm HEPES buffer (pH 7.2), 0.1 mm EDTA, and 50 mm NaCl at room temperature, with a final P450 concentration 0.2 μm, the path length was 5.0 cm, the tight binding equation was used for calculations.
Enzyme activity and Ki(app) assays
Activity was measured in a reconstituted system: buffer [25 mm HEPES (pH 7.2), 0.1 mm dithiothreitol, 0.1 mm EDTA, and 4 mm MgCl2], electron transfer chain, either CYP11B1 or CYP11B2 (0.25 μm), human Adx (2 μm), human Adx reductase (0.5 μm), and NADPH regenerating system (glucose-6-phosphate, glucose-6-phosphate dehydrogenase) at 37 C. Activity of aromatase was measured with androstenedione as substrate in the reconstituted system containing human CYP19A1 (0.5 μm) and rat NADPH-cytochrome P450 reductase (1 μm). Steroids were added from stock solutions in ethanol to a final concentration of 100 μm. For Ki(app) determinations, inhibitor concentration was 0–1.5 μm, cytochrome P450 concentration was 20 nm. Steroids were extracted with methylene chloride and analyzed by reverse-phase HPLC with methanol/water as a mobile phase.
LC-MS analysis
Steroids were further analyzed by LC-MS (LCQ Fleet Ion Trap Mass Spectrometer coupled to an Accela HPLC system; Thermo Scientific, Rockford, IL). Chromatography was performed by gradient elution on a Cosmosil 5C18-MS-II column (4.6 × 150 mm; Nacalai Tesque, Inc., San Diego, CA). The mobile phase consisted of water (solvent A) and methanol (solvent B). Mass spectrometry experiments were performed with atmospheric pressure chemical ionization (APCI) at positive ion mode.
Results
Evaluation of functional properties of CYP11B enzymes at protein level
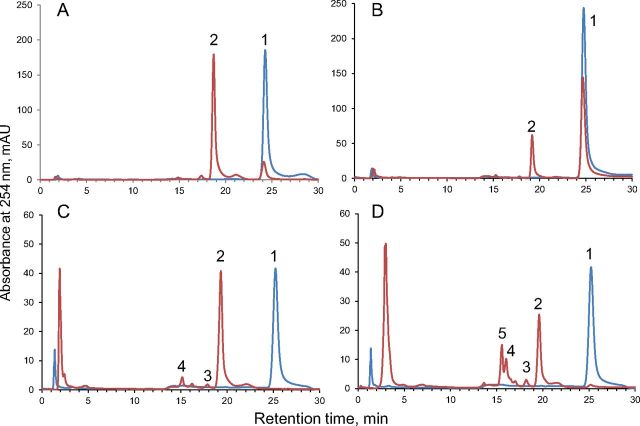
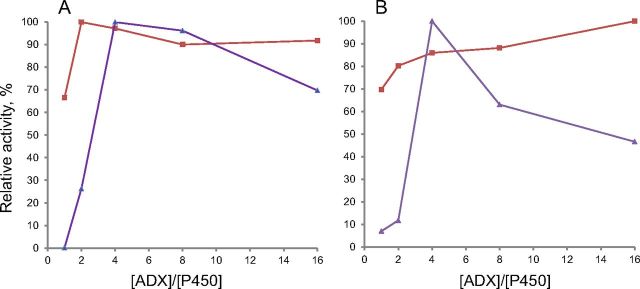
It is well known that human CYP11B1 is an 11β-hydroxylase with minimal 18-hydroxylase activity, whereas CYP11B2 performs both 11- and 18-hydroxylase and also 18-oxidase activities and therefore assigned as aldosterone synthase (26–28). However, little is known about the processivity of the CYP11B2 (i.e. the extent of release of intermediate products) and whether the intermediates can be converted to the final product. On the other hand, information about the ability of CYP11B1 to acquire 18-oxidase activity in vitro without modification at the protein level is currently unavailable. Because human CYP11B1 and CYP11B2 mature proteins differ in only 29 of 479 residues, we reasoned that we might be able to understand their different catalytic properties by measuring their activities side by side in reconstituted systems with substrate/intermediate steroids that both isoforms could use in vivo. The results are summarized in Table 1. Both enzymes worked as efficient 11β-hydroxylases with DOC and 11-deoxycortisol, but CYP11B2 activity was comparably lower (37.6 and 15.8 vs. 80.8 and 96.8 min−1 for CYP11B1, respectively) (Fig. 1). Moreover, CYP11B2 showed low processivity in aldosterone formation because B and 18OH-B intermediates were detected. Fueling the system with electrons by increasing Adx concentration does not change the product CYP11B2 profile (Fig. 2). In the case of CYP11B1, additional products were formed when low substrate concentration and an excess of Adx were used (Fig. 2), although a significant decrease of 11β-hydroxylase activity was observed (Fig. 3). Regardless of the conditions used for CYP11B1, aldosterone was not detected (Fig. 2), which confirms the inability of this enzyme to perform 18-oxidation.
Table 1.
Steroid hydroxylase activity of CYP11B1 and CYP11B2 (min−1)
| Substrates | CYP11B1 | CYP11B2 |
|---|---|---|
| DOC (21-hydroxyprogesterone) | 80.8 ± 7.3 | 37.6 ± 2.4 |
| B | 0.48 ± 0.014 | 0.66 ± 0.073 |
| 11-Deoxycortisol (RSS) | 96.8 ± 9.3 | 15.8 ± 1.82 |
| Cortisol | 0.48 ± 0.053 | 0.23 ± 0,038 |
| 18-Hydroxy-11-DOC (18OH-DOC) | 0 | 0.89 ± 0.014 |
| 11-Dehydrocorticosterone | 0 | 0 |
| Progesterone | 12.3 ± 1.26 | 1.34 ± 0.08 |
| 11β-Hydroxyprogesterone | NM | 0 |
| 21-Hydroxypregnenolone | 0 | 0.032 ± 0.004 |
| Testosterone | 3.62 ± 0.28 | 2.43 ± 0.34 |
| Androstendione | 8.71 ± 1.16 | 1.36 ± 0.23 |
NM, Not measured.
Fig. 1.
Enzymatic activity of two human CYP11B isoforms.
Fig. 2.
HPLC profiles of reactions catalyzed by CYP11B1 (A and C) and CYP11B2 (B and D) using DOC as substrate. Ratio cytochrome P450:substrate, 0.25:100 [the reaction time used: 0 min (blue) and 5 min (red)] (A and B). Ratio cytochrome P450:substrate, 1:20 [the reaction time used: 0 min (blue) and 30 min (red)] (C and D). 1, DOC; 2, B; 3, 18-hydroxy-DOC; 4, 18OH-B; 5, aldosterone.
Fig. 3.
Effect of Adx on steroid 11β-hydroxylase activity with DOC (A) and RSS (B). CYP11B1 marked as triangles and CYP11B2 as squares.
To separate 11β-hydroxylase and 18-hydroxylase activities, C11-hydroxylated (B and cortisol) and C18-hydroxylated (18OH-DOC) steroids were used as substrates for both isoforms (Table 1). As expected, CYP11B1 is a weak 18-hydroxylase and cannot metabolize 18OH-DOC (Table 1 and Fig. 1). Notably, 18-hydroxylase/oxidase activity of CYP11B2 was also weak when one hydroxylation (either at C11 or C18) was preperformed, indicating subtle differences between CYP11B isoforms as 18-hydroxylases. Because CYP11B1 is unable to perform 18-oxidation and no conversion of 18OH-B to aldosterone was very recently shown for CYP11B2 (29), this finding suggests that aldosterone is formed from DOC without dissociation of intermediates from the CYP11B2 active site.
To further explore substrate specificity of CYP11B isoforms, alternative steroids were tested as substrates (Table 1). Both isoforms are specific to the A-ring configuration of steroids, i.e. they cannot metabolize Δ5-steroids with a OH-group at the C3 position, thus preventing direct metabolism of the CYP11A1 product in mitochondria. The ability of both isoforms to metabolize androgens indicates that their specificities are not absolutely strict and that the produced intermediates may have physiological and pathological implications (30, 31).
Structural aspects of substrate binding
To gain insight into the structural basis of substrate recognition, we determined the structure of CYP11B2 in complex with DOC (Table 2). Aldosterone synthase has a typical cytochrome P450 fold around the heme prosthetic group with common positioning of A′ and G′ helices (part of the access channel) for membrane interaction and specific for mitochondrial P450 orientation of the B′ helix (active site) (Fig. 4A) (19, 32). The structure shows that the active site cavity is lined by nonpolar and aromatic residues favorable for binding steroid substrate. The C21-hydroxyl group of DOC makes a hydrogen bond with the main-chain carbonyl of F381. On the other end of DOC, the 3-keto group projects along the I-helix axis but is not involved in direct contact with protein; the closest group for potential hydrogen bond formation is guanidine of R120, which reflects in strict substrate specificity of CYP11B2 to 3-keto-Δ4 steroids (Table 1). A conserved structural link between the I-helix and B-C loop is realized through hydrogen bonding between E310 and the F130 amide nitrogen and borders the active site cavity from this site. The side chains of W116, F130, and F487 ensure the steroid binding geometry from the α-face (Fig. 4B). DOC is bound at an angle of approximately 60° with respect to the porphyrin plane (Fig. 4C), with C18 and C19 angular methyl groups pointing toward the heme. Notably, the distance from the iron is closest for C19 (4Å) (Fig. 4D). In fact, the distance is the same as in aromatase (CYP19A1) in complex with androstenedione (33). This is not very surprising, because these two proteins catalyze sequential hydroxylations of the steroid angular methyl group, CYP19A1 at C19 and CYP11B2 at C18. However, in the case of CYP11B2, an initial 11β-hydroxylation must be performed, as confirmed by the product profile (Fig. 2). When superimposed, the DOC position in the CYP11B2 structure, compared with AD in CYP19A1, is shifted toward the I-helix with the steroid A/B ring junction slightly kinked to avoid steric clash with the I-helix. As a result, the C19-methyl group is placed close to the G314 main chain carbonyl and is not in a favorable geometry for hydroxylation by the iron-bound ferryl species, consistent with recent data showing only trace amounts of C19-hydroxylated product (29). Thus, the enzyme hydroxylates DOC at the kinetically favored C11 position first, as the secondary carbon radical is more stable than a primary radical at C19, assuming that the reaction proceeds via carbon radical intermediates and an “oxygen rebound” mechanism. Consistent with the product profile, 18OH-DOC is also formed because of C18-methyl group proximity, as the distance from the heme iron to C18 and C11 is 4.9 and 4.2 Å, respectively.
Fig. 4.
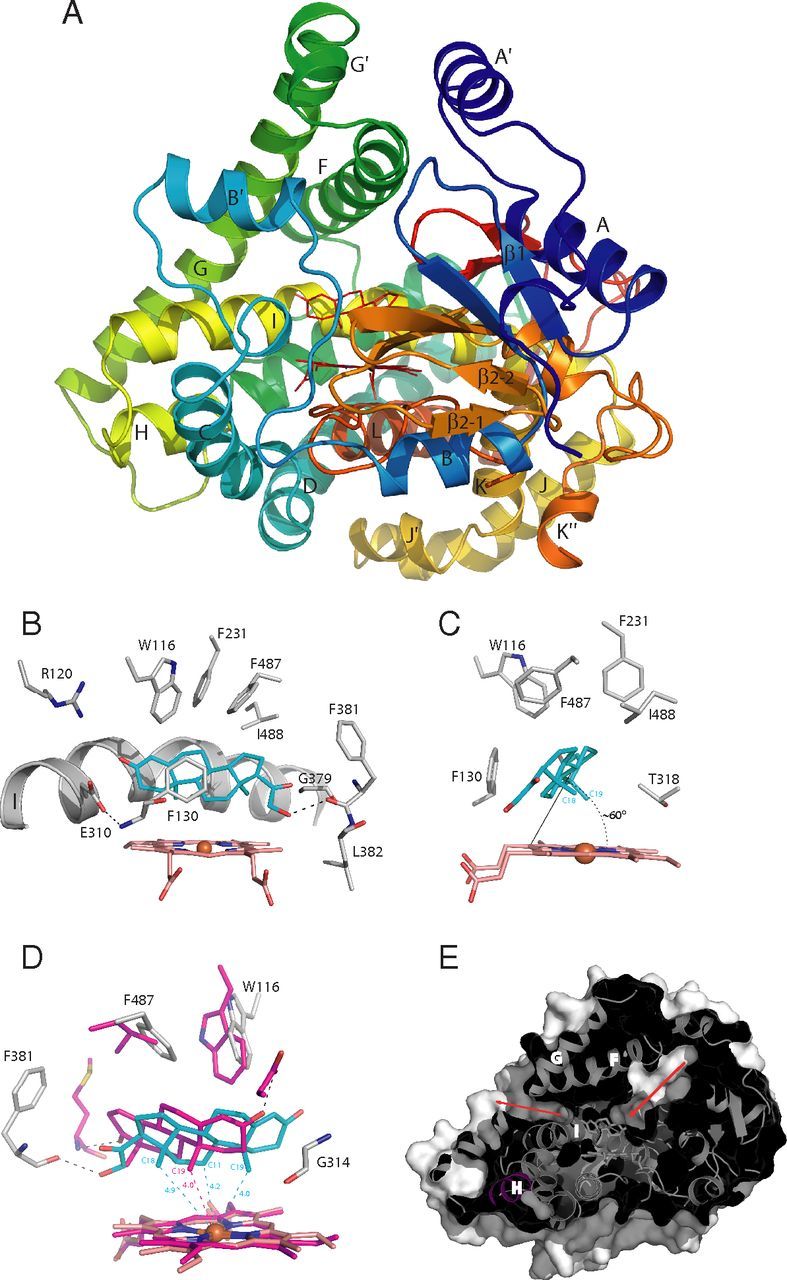
The structure of aldosterone synthase and views of steroid at the active site. A, Overall structure in a ribbon representation rainbow colored from the N terminus (blue) to the C terminus (red). B, Steroid-protein interactions. C, DOC binding angle. D, Superposition of steroids in the active sites of aldosterone synthase (cyan) and aromatase (magenta) (PDB: 3EQM) with the distances from the heme iron to hydroxylation position. E, A slice through the macromolecular surface showing channels to the active site with access and putative egress routes marked by arrows.
Fadrozole as a probe to identify protein-inhibitor interactions
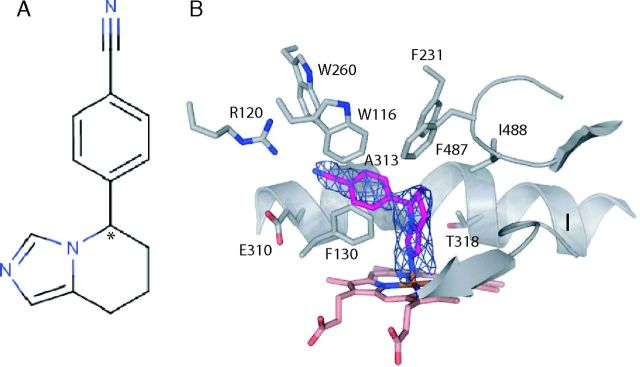
As the sole producer of aldosterone, CYP11B2 is a novel therapeutic target for antihypertensive agents. Thus, to explore the structural requirements for aldosterone suppression via CYP11B2 inhibition, we used nonsteroid molecule and solved the structure of CYP11B2 in complex with fadrozole (Fig. 5A and Table 2). Fadrozole was initially developed as an aromatase (CYP19A1) inhibitor and used as a drug to treat breast cancer (34). The R-enantiomer of fadrozole (FAD286) was later discovered to be a potent aldosterone synthase inhibitor (35) but was also found to inhibit CYP11B1 (36, 37). The addition of fadrozole to CYP11B2 induced characteristic type II spectral changes in the Soret region, which indicates the displacement of a water ligand and the direct coordination of heme iron by the nitrogen atom of fadrozole (Fig. 5B). According to the spectral data, fadrozole binds with high affinity to CYP11B2 (Kd 370 nm) but with even higher affinity to CYP11B1 (Kd 9 nm) and to CYP19A1 (Kd 16 nm) (Table 3). Notably, fadrozole also bound tightly to CYP17A1 (Kd 162 nm), but no binding was observed for CYP21A2. These data are consistent with the steroid regiospecificity of these P450s. The apparent inhibition constant Ki(app) values for fadrozole are 14.8 and 354 nm for CYP11B1 and CYP11B2, respectively, and 45 nm for CYP19A1 (Table 3). In the CYP11B2 active site, fadrozole binds using most of the residues for DOC binding (Fig. 5B). The imidazopyridine moiety of fadrozole makes van der Waals contacts with F130, F487, I488, and the methyl group of T318. The benzonitrile ring of fadrozole is enclosed by W116, F231, W260, A313, and E310, whereas the nitrile group is involved in π-π interactions with W260 but not close enough to make a hydrogen bond with R120. The bound fadrozole configuration corresponds to the R-enantiomer, which fits perfectly into the density. Its binding mode toward the egress channel, where isoform-specific differences appear to provide flexibility, is in good agreement with the binding characteristics for CYP11B2.
Fig. 5.
Inhibitor binding to CYP11B2. A, Fadrozole structure with the chiral center marked by an asterisk. B, Active site residues engaged in interaction with inhibitor (Fo-Fc density at 2.5σ, blue mesh).
Table 3.
Inhibition of CYP11B enzymes by fadrozole in comparison with other steroidogenic P450s
| Cytochrome P450 | Kd, nm | Ki(app), nm |
|---|---|---|
| CYP11B1 (steroid 11β-hydroxylase) | 9 ± 1 | 14.8 ± 2 |
| CYP11B2 (aldosterone synthase) | 370 ± 53 | 354 ± 39 |
| CYP19A1 (aromatase) | 16 ± 2 | 45 ± 7 |
| CYP17A1 (steroid 17α-hydroxylase/17,20-lyase) | 162 ± 29 | NM |
| CYP21A2 (steroid 21-hydroxylase) | ND | NM |
The apparent dissociation constant Kd was determined by differential spectral titration using low-spin P450s. NM, Not measured; ND, not detected.
Discussion
Our data indicate that both human CYP11B isoforms can be defined as 11β- and 18-hydroxylases, which is consistent with their high sequence similarity and previously published data. The differences between two isoforms are in several amino acid substitutions, which define the ability of only one isoform, CYP11B2, to further catalyze C18-oxidation reaction, thus enabling the conversion of DOC in three successive reactions to aldosterone, although with low yield. It is well known that CYP11B genes are regulated differently (16); however, it was not previously confirmed that the amount of aldosterone produced is low due to low processivity of the CYP11B2 enzyme. The catalytic properties of purified CYP11B1 and CYP11B2 (high 11β- and low 18-hydroxylase activities for both enzymes and low 18-oxidase activity specific to CYP11B2) could explain the many-fold higher physiological concentration of cortisol and corticosterone than aldosterone in serum and tissues (16, 38). The catalytic efficiency of CYP11B2 does not exclude the existence of physiological effectors of 18-hydroxylase and/or 18-oxidase activity as suggested earlier (39–42) but as yet unidentified. It might be similar as observed for CYP17A1, where its lyase activity is regulated by cytochrome b5 (43, 44). Notably, there is no current model explaining the requirement of one or more CYP11B isoforms in different species, which would obviously also require a systematic analysis of the enzymes' expression patterns and final products of steroidogenic pathways in different taxonomic groups. In the case of the human enzymes, our results suggest that both CYP11B isoforms could also be involved in the androgen metabolic pathway (30, 31), and this aspect of their function needs further examination.
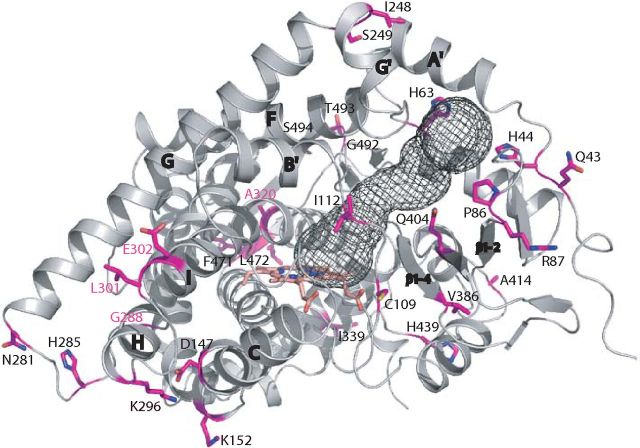
Mapping amino acid differences between CYP11B2 and CYP11B1 to the structure shows that nonidentical residues are located outside of the active site, and neither residue is interacting with substrate and inhibitor (Fig. 6). Studies of the chimeric enzymes, encoded by genes with CYP11B1 sequence at 5′ and CYP11B2 sequence at 3′ end and found in patients with glucocorticoid-remediable aldosteronism (GRA) (45, 46), have led to the conclusion that residues encoded by exon 5 and downstream exons of CYP11B2 are critical for aldosterone production. Follow-up studies have narrowed down to 10 amino acids located between positions 248 and 339 and proposed the requirement of G288, L301, E302, and A320 for 18-hydroxylation/oxidation (47–49). These isoform-specific residues are clustered around the H-helix (G/S288) and in the I-helix (L/P301, E/D302, and A/V320) in CYP11B2 structure (Fig. 6). Our structures provide insights into how additional 18-oxygenation can be achieved. The different hydroxylation status of CYP11B products suggests different retention times of the substrate/intermediates in the active site, perhaps due to relatively increased intrinsic flexibility of one of the isoforms. Two channels connect the CYP11B2 active site to the surface (Figs. 4E and 6). One channel is well defined between strand β1-4 and helices A′, G′, and B′ and likely serves as a substrate access channel, similarly to CYP11A1 and CYP21A2 (19, 50). A second channel is found between helices G, I, and B-C loop, although too narrow for steroid product egress without forced opening. This channel extends toward the N terminus of I-helix. CYP11B2-specific residues may provide conformational flexibility, which affects the dissociation rates of hydroxylated intermediates. The opening of the egress channel may be critical in the functioning of CYP11B enzymes: it allows for product exit after the first hydroxylation step in CYP11B1 (i.e. CYP11B1-like constitutive channel opening) but remains closed to ensure efficient 18-oxidation due to enhanced protein dynamics in CYP11B2 (i.e. CYP11B2-like inducible channel opening). This idea is consistent with the role of the H-helix region as a hinge that participates in opening and closing the F-G helix region over the active site in other cytochrome P450s (51, 52).
Fig. 6.
Mapping residues different between CYP11B1 and CYP11B2 on the structure. Access channel was calculated using CAVER and shown as a mesh. Residues affecting activity at C18 are labeled in magenta.
Alternatively, A320 (V320 in CYP11B1), which is located near the proton transfer path required for O2 activation, could be crucial for the methyloxidase reaction (53) and as well for 11β-hydroxylation efficiency. In CYP11A1 and in CYP19A1 structures (PDB: 3N9Y and 3EQM, respectively), a serine residue occupies the respective position, where it provides a hydrogen bond to the residue on an adjacent I-helical turn. The network around conserved acidic-alcohol pair mediating proton delivery to heme-bound dioxygen might be essential in P450s performing multistep catalysis. Notably, other isoform-specific residues are more similar between CYP11A1 and CYP11B2 than within the CYP11B subfamily (Supplemental Fig. 2). More likely, the aforementioned factors collectively contribute to 18-oxidase activity of CYP11B2. The possibility of induced conformational changes in CYP11B2 (by mitochondrial component/s), which may stimulate aldosterone formation, remains to be demonstrated but is plausible.
The obtained structural information allows rationalizing the effect of mutations in CYP11B2 leading to aldosterone deficiencies. The majority of these mutations are distant from the active site but important for structural integrity, substrate binding, or interaction with the redox-partner (Supplemental Table 1). The molecular basis for the enzyme's specificity can be used for isoform-specific type I inhibitor development in hypertension treatment. Efficient inhibitors of this class were synthesized previously (54), and this direction can further be explored based on the CYP11B2 substrate-bound structure. On the other hand, the fadrozole-bound structure shows that small inhibitor binding does not induce conformational changes, and the unoccupied space within a portion of the access channel can be exploited to achieve greater isoform specificity of type II inhibitors (Figs. 5B and 6). New structures also provide a basis for rational design of dual selective inhibitors of CYP19A1 and CYP11B2 (55) or CYP17A1 and CYP11B1 (56) targeting side effects of breast or prostate cancer treatment, respectively.
The atomic coordinates and structure factors have been deposited in the Protein Data Bank under the accession codes 4DVQ(CYP11B2 in complex with DOC) and 4FDH (CYP11B2 in complex with fadrozole).
Acknowledgments
We thank W. Tempel, T. S. Cherkesova, I. P. Grabovec, T. A. Sushko, A. V. Yantsevich, and D. V. Mukha for technical assistance.
The Structural Genomics Consortium is a registered charity (no. 1097737) that receives funds from the Canadian Institutes for Health Research, the Canadian Foundation for Innovation, Genome Canada through the Ontario Genomics Institute, GlaxoSmithKline, Karolinska Institutet, the Knut and Alice Wallenberg Foundation, the Ontario Innovation Trust, the Ontario Ministry for Research and Innovation, Merck & Co., the Novartis Research Foundation, the Swedish Agency for Innovation Systems, the Swedish Foundation for Strategic Research, and the Wellcome Trust.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Adx
- Adrenodoxin
- B
- corticosterone
- DOC
- deoxycorticosterone
- 18OH-B
- 18-hydroxycorticosterone.
References
- 1. Fagard RH. 2012. Resistant hypertension. Heart 98:254–261 [DOI] [PubMed] [Google Scholar]
- 2. Brown NJ. 2005. Aldosterone and end-organ damage. Curr Opin Nephrol Hypertens 14:235–241 [DOI] [PubMed] [Google Scholar]
- 3. Tomaschitz A , Pilz S , Ritz E , Obermayer-Pietsch B , Pieber TR. 2010. Aldosterone and arterial hypertension. Nat Rev Endocrinol 6:83–93 [DOI] [PubMed] [Google Scholar]
- 4. Hartmann RW , Müller U , Ehmer PB. 2003. Discovery of selective CYP11B2 (aldosterone synthase) inhibitors for the therapy of congestive heart failure and myocardial fibrosis. Eur J Med Chem 38:363–366 [DOI] [PubMed] [Google Scholar]
- 5. Jansen PM , van den Meiracker AH , Jan Danser AH. 2009. Aldosterone synthase inhibitors: pharmacological and clinical aspects. Curr Opin Investig Drugs 10:319–326 [PubMed] [Google Scholar]
- 6. Paulis L , Unger T. 2010. Novel therapeutic targets for hypertension. Nat Rev Cardiol 7:431–441 [DOI] [PubMed] [Google Scholar]
- 7. Laurent S , Schlaich M , Esler M. 2012. New drugs, procedures, and devices for hypertension. Lancet 380:591–600 [DOI] [PubMed] [Google Scholar]
- 8. Nomoto S , Massa G , Mitani F , Ishimura Y , Miyahara K , Toda K , Nagano I , Yamashiro T , Ogoshi S , Fukata J , Onishi S , Hashimoto K , Doi Y , Imura H , Shizuta Y. 1997. CMO I deficiency caused by a point mutation in exon 8 of the human CYP11B2 gene encoding steroid 18-hydroxylase (P450C18). Biochem Biophys Res Commun 234:382–385 [DOI] [PubMed] [Google Scholar]
- 9. Pascoe L , Curnow KM , Slutsker L , Rösler A , White PC. 1992. Mutations in the human CYP11B2 (aldosterone synthase) gene causing corticosterone methyloxidase II deficiency. Proc Natl Acad Sci USA 89:4996–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mitsuuchi Y , Kawamoto T , Miyahara K , Ulick S , Morton DH , Naiki Y , Kuribayashi I , Toda K , Hara T , Orii T , et al. 1993. Congenitally defective aldosterone biosynthesis in humans: inactivation of the P-450C18 gene (CYP11B2) due to nucleotide deletion in CMO I deficient patients. Biochem Biophys Res Commun 190:864–869 [DOI] [PubMed] [Google Scholar]
- 11. Peter M , Fawaz L , Drop SL , Visser HK , Sippell WG. 1997. Hereditary defect in biosynthesis of aldosterone: aldosterone synthase deficiency 1964–1997. J Clin Endocrinol Metab 82:3525–3528 [DOI] [PubMed] [Google Scholar]
- 12. Lee PD , Patterson BD , Hintz RL , Rosenfeld RG. 1986. Biochemical diagnosis and management of corticosterone methyl oxidase type II deficiency. J Clin Endocrinol Metab 62:225–229 [DOI] [PubMed] [Google Scholar]
- 13. Hattangady NG , Olala LO , Bollag WB , Rainey WE. 2012. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol 350:151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mornet E , Dupont J , Vitek A , White PC. 1989. Characterization of two genes encoding human steroid 11β-hydroxylase (P-450(11)β). J Biol Chem 264:20961–20967 [PubMed] [Google Scholar]
- 15. Colombo L , Dalla Valle L , Fiore C , Armanini D , Belvedere P. 2006. Aldosterone and the conquest of land. J Endocrinol Invest 29:373–379 [DOI] [PubMed] [Google Scholar]
- 16. Rainey WE. 1999. Adrenal zonation: clues from 11β-hydroxylase and aldosterone synthase. Mol Cell Endocrinol 151:151–160 [DOI] [PubMed] [Google Scholar]
- 17. Williams PA , Cosme J , Sridhar V , Johnson EF , McRee DE. 2000. Mammalian microsomal cytochrome P450 monooxygenase: structural adaptations for membrane binding and functional diversity. Mol Cell 5:121–131 [DOI] [PubMed] [Google Scholar]
- 18. Wester MR , Stout CD , Johnson EF. 2002. Purification and crystallization of N-terminally truncated forms of microsomal cytochrome P450 2C5. Methods Enzymol 357:73–79 [DOI] [PubMed] [Google Scholar]
- 19. Strushkevich N , MacKenzie F , Cherkesova T , Grabovec I , Usanov S , Park HW. 2011. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc Natl Acad Sci USA 108:10139–10143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pechurskaya TA , Harnastai IN , Grabovec IP , Gilep AA , Usanov SA. 2007. Adrenodoxin supports reactions catalyzed by microsomal steroidogenic cytochrome P450s. Biochem Biophys Res Commun 353:598–604 [DOI] [PubMed] [Google Scholar]
- 21. Otwinowski Z , Minor W. 1997. Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol 276:307–326 [DOI] [PubMed] [Google Scholar]
- 22. Collaborative Computational Project, Number 4 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50:760–763 [DOI] [PubMed] [Google Scholar]
- 23. Jones TA , Zou JY , Cowan SW , Kjeldgaard M. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A 47:110–119 [DOI] [PubMed] [Google Scholar]
- 24. Brünger AT , Adams PD , Clore GM , DeLano WL , Gros P , Grosse-Kunstleve RW , Jiang JS , Kuszewski J , Nilges M , Pannu NS , Read RJ , Rice LM , Simonson T , Warren GL. 1998. Crystallography, NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54:905–921 [DOI] [PubMed] [Google Scholar]
- 25. Strushkevich N , Usanov SA , Park HW. 2010. Structural basis of human CYP51 inhibition by antifungal azoles. J Mol Biol 397:1067–1078 [DOI] [PubMed] [Google Scholar]
- 26. Kawainoto T , Mitsuuchi Y , Ohnishi T , Ichikawa Y , Yokoyama Y , Sumimoto H , Toda K , Miyahara K , Kuribayashi I , Nakao K , et al. 1990. Cloning and expression of a cDNA for human cytochrome P-450aldo as related to primary aldosteronism. Biochem Biophys Res Commun 173:309–316 [DOI] [PubMed] [Google Scholar]
- 27. Curnow KM , Tusie-Luna MT , Pascoe L , Natarajan R , Gu JL , Nadler JL , White PC. 1991. The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol Endocrinol 5:1513–1522 [DOI] [PubMed] [Google Scholar]
- 28. Kawamoto T , Mitsuuchi Y , Toda K , Yokoyama Y , Miyahara K , Miura S , Ohnishi T , Ichikawa Y , Nakao K , Imura H , et al. 1992. Role of steroid 11β-hydroxylase and steroid 18-hydroxylase in the biosynthesis of glucocorticoids and mineralocorticoids in humans. Proc Natl Acad Sci USA 89:1458–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hobler A , Kagawa N , Hutter MC , Hartmann MF , Wudy SA , Hannemann F , Bernhardt R. 2012. Human aldosterone synthase: recombinant expression in E. coli and purification enables a detailed biochemical analysis of the protein on the molecular level. J Steroid Biochem Mol Biol 132:57–65 [DOI] [PubMed] [Google Scholar]
- 30. Parr MK , Zöllner A , Fusshöller G , Opfermann G , Schlörer N , Zorio M , Bureik M , Schänzer W. 2012. Unexpected contribution of cytochrome P450 enzymes CYP11B2 and CYP21, as well as CYP3A4 in xenobiotic androgen elimination. Insights from metandienone metabolism. Toxicol Lett 213:381–391 [DOI] [PubMed] [Google Scholar]
- 31. Yazawa T , Uesaka M , Inaoka Y , Mizutani T , Sekiguchi T , Kajitani T , Kitano T , Umezawa A , Miyamoto K. 2008. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: conservation and evolution of the androgen metabolic pathway. Endocrinology 149:1786–1792 [DOI] [PubMed] [Google Scholar]
- 32. Annalora AJ , Goodin DB , Hong WX , Zhang Q , Johnson EF , Stout CD. 2010. Crystal structure of CYP24A1, a mitochondrial cytochrome P450 involved in vitamin D metabolism. J Mol Biol 396:441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghosh D , Griswold J , Erman M , Pangborn W. 2009. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature 457:219–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thürlimann B , Beretta K , Bacchi M , Castiglione-Gertsch M , Goldhirsch A , Jungi WF , Cavalli F , Senn HJ , Fey M , Löhnert T. 1996. First-line fadrozole HCI (CGS 16949A) versus tamoxifen in postmenopausal women with advanced breast cancer. Prospective randomised trial of the Swiss Group for Clinical Cancer Research SAKK 20/88. Ann Oncol 7:471–479 [DOI] [PubMed] [Google Scholar]
- 35. Ménard J , Pascoe L. 2006. Can the dextroenantiomer of the aromatase inhibitor fadrozole be useful for clinical investigation of aldosterone-synthase inhibition? J Hypertens 24:993–997 [DOI] [PubMed] [Google Scholar]
- 36. LaSala D , Shibanaka Y , Jeng AY. 2009. Coexpression of CYP11B2 or CYP11B1 with adrenodoxin and adrenodoxin reductase for assessing the potency and selectivity of aldosterone synthase inhibitors. Anal Biochem 394:56–61 [DOI] [PubMed] [Google Scholar]
- 37. Roumen L , Peeters JW , Emmen JM , Beugels IP , Custers EM , de Gooyer M , Plate R , Pieterse K , Hilbers PA , Smits JF , Vekemans JA , Leysen D , Ottenheijm HC , Janssen HM , Hermans JJ. 2010. Synthesis, biological evaluation, and molecular modeling of 1-benzyl-1H-imidazoles as selective inhibitors of aldosterone synthase (CYP11B2). J Med Chem 53:1712–1725 [DOI] [PubMed] [Google Scholar]
- 38. Williams GH , Cain JP , Dluhy RG , Underwood RH. 1972. Studies of the control of plasma aldosterone concentration in normal man. I. Response to posture, acute and chronic volume depletion, and sodium loading. J Clin Invest 51:1731–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brandon DD , Markwick AJ , Chrousos GP , Loriaux DL. 1989. Glucocorticoid resistance in humans and nonhuman primates. Cancer Res 49:2203s–2213s [PubMed] [Google Scholar]
- 40. Ho MM , Vinson GP. 1993. 11β-Hydroxylase gene expression in the rat adrenal cortex. J Endocrinol 139:301–306 [DOI] [PubMed] [Google Scholar]
- 41. Min L , Strushkevich NV , Harnastai IN , Iwamoto H , Gilep AA , Takemori H , Usanov SA , Nonaka Y , Hori H , Vinson GP , Okamoto M. 2005. Molecular identification of adrenal inner zone antigen as a heme-binding protein. FEBS J 272:5832–5843 [DOI] [PubMed] [Google Scholar]
- 42. MacKenzie SM , Connell JM , Davies E. 2012. Non-adrenal synthesis of aldosterone: a reality check. Mol Cell Endocrinol 350:163–167 [DOI] [PubMed] [Google Scholar]
- 43. Gilep AA , Sushko TA , Usanov SA. 2011. At the crossroads of steroid hormone biosynthesis: the role, substrate specificity and evolutionary development of CYP17. Biochim Biophys Acta 1814:200–209 [DOI] [PubMed] [Google Scholar]
- 44. Miller WL , Auchus RJ , Geller DH. 1997. The regulation of 17,20 lyase activity. Steroids 62:133–142 [DOI] [PubMed] [Google Scholar]
- 45. Lifton RP , Dluhy RG , Powers M , Rich GM , Cook S , Ulick S , Lalouel JM. 1992. A chimaeric 11β-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 355:262–265 [DOI] [PubMed] [Google Scholar]
- 46. Pascoe L , Curnow KM , Slutsker L , Connell JM , Speiser PW , New MI , White PC. 1992. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc Natl Acad Sci USA 89:8327–8331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Curnow KM , Mulatero P , Emeric-Blanchouin N , Aupetit-Faisant B , Corvol P , Pascoe L. 1997. The amino acid substitutions Ser288Gly and Val320Ala convert the cortisol producing enzyme, CYP11B1, into an aldosterone producing enzyme. Nat Struct Biol 4:32–35 [DOI] [PubMed] [Google Scholar]
- 48. Mulatero P , Curnow KM , Aupetit-Faisant B , Foekling M , Gomez-Sanchez C , Veglio F , Jeunemaitre X , Corvol P , Pascoe L. 1998. Recombinant CYP11B genes encode enzymes that can catalyze conversion of 11-deoxycortisol to cortisol, 18-hydroxycortisol, and 18-oxocortisol. J Clin Endocrinol Metab 83:3996–4001 [DOI] [PubMed] [Google Scholar]
- 49. Böttner B , Schrauber H , Bernhardt R. 1996. Engineering a mineralocorticoid- to a glucocorticoid-synthesizing cytochrome P450. J Biol Chem 271:8028–8033 [DOI] [PubMed] [Google Scholar]
- 50. Zhao B , Lei L , Kagawa N , Sundaramoorthy M , Banerjee S , Nagy LD , Guengerich FP , Waterman MR. 2012. Three-dimensional structure of steroid 21-hydroxylase (cytochrome P450 21A2) with two substrates reveals locations of disease-associated variants. J Biol Chem 287:10613–10622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Graham SE , Peterson JA. 2002. Sequence alignments, variabilities, and vagaries. Methods Enzymol 357:15–28 [DOI] [PubMed] [Google Scholar]
- 52. Gay SC , Roberts AG , Halpert JR. 2010. Structural features of cytochromes P450 and ligands that affect drug metabolism as revealed by x-ray crystallography and NMR. Future Med Chem 2:1451–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Böttner B , Denner K , Bernhardt R. 1998. Conferring aldosterone synthesis to human CYP11B1 by replacing key amino acid residues with CYP11B2-specific ones. Eur J Biochem 252:458–466 [DOI] [PubMed] [Google Scholar]
- 54. Johnston JO , Wright CL , Holbert GW. 1995. Enzyme-activated inhibitors of steroidal hydroxylases. J Steroid Biochem Mol Biol 52:17–34 [DOI] [PubMed] [Google Scholar]
- 55. Hu Q , Yin L , Hartmann RW. 2012. Selective dual inhibitors of CYP19 and CYP11B2: targeting cardiovascular diseases hiding in the shadow of breast cancer. J Med Chem 55:7080–7089 [DOI] [PubMed] [Google Scholar]
- 56. Hu Q , Jagusch C , Hille UE , Haupenthal J , Hartmann RW. 2010. Replacement of imidazolyl by pyridyl in biphenylmethylenes results in selective CYP17 and dual CYP17/CYP11B1 inhibitors for the treatment of prostate cancer. J Med Chem 53:5749–5758 [DOI] [PubMed] [Google Scholar]
- 57. Laskowski RA , MacArthur MW , Moss DS , Thornton JM. 1993. PROCHECK—a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291 [Google Scholar]