Abstract
The actions of glucocorticoids at the feto-maternal interface are not well understood. Here, we show that decidualization of human endometrial stromal cells (HESCs) in response to progesterone and cAMP signaling is associated with a strong induction of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) expression and enzyme activity. Decidualization also triggered a gradual decrease in glucocorticoid receptor (GR) expression and reciprocal increase in mineralocorticoid receptor (MR) levels. Gene expression profiling of differentiating HESCs after small interfering RNA (siRNA)-mediated knockdown of either GR or MR identified 239 and 167 significantly regulated genes, respectively. Interestingly, GR-repressed genes were enriched for Krüppel-associated box domain containing zinc-finger proteins, transcriptional repressors involved in heterochromatin formation. In agreement, GR knockdown was sufficient to enhance trimethylated H3K9 levels in decidualizing cells. Conversely, we identified several MR-dependent genes implicated in lipid droplet biogenesis and retinoid metabolism. For example, the induction in differentiating HESCs of DHRS3, encoding a highly conserved enzyme that catalyzes the oxidation/reduction of retinoids and steroids, was enhanced by aldosterone, attenuated in response to MR knockdown, and abolished upon treatment with the MR antagonist RU26752. Furthermore, we demonstrate that decidualization is associated with dynamic changes in the abundance and distribution of cytoplasmic lipid droplets, the formation of which was blocked by RU26752. In summary, progesterone drives local cortisol biosynthesis by decidual cells through induction of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1), leading to transcriptional regulation of distinct GR and MR gene networks involved in epigenetic programming and lipid and retinoid metabolism, respectively.
Glucocorticoids have been implicated in many processes that underpin successful embryo implantation, placentation, and the growth and development of the fetus (1). Consequently, modulation of glucocorticoid action represents a potential strategy for the treatment or prevention of a variety of pregnancy-related disorders. For example, glucocorticoid treatment has been advocated for the prevention of early pregnancy loss based on its ability to reduce the abundance of uterine natural killer cells during the periimplantation window (2), to stimulate human chorionic gonadotropin secretion by cultured human cytotrophoblasts (3), and to accelerate trophoblast growth and invasion (4). However, implantation is an inflammatory process that depends on local release of proinflammatory cytokines and prostaglandins (5–8). By disabling the cytokine-prostaglandin signaling cascade, glucocorticoids potentially could impact adversely on early pregnancy events. Furthermore, compelling evidence suggests that prolonged exposure to high levels of glucocorticoids in pregnancy is detrimental for both placental and fetal development (9–11).
Tissue levels of active endogenous glucocorticoids depend on the expression of 11β-hydroxysteroid dehydrogenase (11βHSD) enzymes. Although the 11βHSD1 isoform is a bidirectional enzyme, it predominantly catalyzes the conversion of inert cortisone (E) to active cortisol (F), thus increasing tissue levels of active glucocorticoids (12). The reduced nicotinamide adenine dinucleotide [NAD(H)]-dependent type 2 isoform (11βHSD2) acts as a dehydrogenase, converting F to E (13). The main cellular targets for F are glucocorticoid receptor (GR) and mineralocorticoid receptor (MR). However, GR normally colocalizes with 11βHSD1 in vivo, whereas 11βHSD2 is commonly found in MR-expressing tissues. Nevertheless, hypercortisolism can result in apparent mineralocorticoid excess as in Cushing syndrome (14). Further, some cell types like adipocytes do not significantly express 11βHSD2, thus enabling F to act through MR (15, 16).
Both 11βHSD isoforms as well as GR and MR are expressed in human endometrium (17). Interestingly, GR expression is confined to stromal cells, whereas MR is reportedly present in both stromal and glandular compartments. Further, 11βHSD1, but not 11βHSD2, is highly up-regulated upon differentiation of primary human endometrial stromal cells (HESCs) into specialist decidual cells in vitro (18). This progesterone (P4)-driven differentiation process bestows unique functions on the endometrium that are essential for pregnancy, including the ability to regulate trophoblast invasion, to modulate local angiogenesis, to recruit specialized uterine natural killer cells and macrophages, and to resist environmental and oxidative stress (19, 20). Based on these observations, we speculated that induction of 11βHSD1 would lead to increased F bioavailability, which in turn regulates the expression of distinct GR- and MR-dependent gene networks in decidualizing HESCs.
Materials and Methods
Patient selection
This study was approved by the Hammersmith and Queen Charlotte's and Chelsea Research Ethics Committee (1997/5065). Written informed consent was obtained from all participating subjects before tissue collection. Endometrial biopsies were timed between 7 and 11 d after the preovulatory LH surge.
Primary cell culture
Endometrial samples were obtained from premenopausal women without uterine pathology, and HESCs were isolated, cultured, and maintained as described previously (16). Samples were collected in Earle's buffered saline containing 100 U/ml penicillin and 100 μg/ml streptomycin. The tissues were washed twice in a 1:1 mixture of DMEM and Ham's F12 (DMEM/F12) (Sigma, Poole, UK), finely minced, and enzymatically digested with collagenase (134 U/ml) and deoxyribonuclease type I (156 U/ml) (Sigma) for 1 h at 37 C. After centrifugation at 400 × g for 4 min, the pellet was resuspended in maintenance medium of DMEM/F12 containing 10% dextran-coated charcoal-treated fetal bovine serum (DCC-FBS), 1% l-glutamine, and 1% antibiotic-antimycotic solution. Proliferating HESCs were cultured in maintenance medium until confluence. In decidualization experiments, confluent monolayers were maintained in DMEM/F12 now containing 2% DCC-FBS and treated with 0.5 mm 8-bromoadenosine cAMP (8-bromo-cAMP) (Sigma, St. Louis, MO) alone or in combination with 1 μm medroxyprogesterone acetate (MPA) (Sigma), 1 μm P4 (Sigma), 0.1 μm dexamethasone (DEX) (Sigma), 0.1 μm E (Sigma), 1 μm aldosterone (Sigma), 10 μm RU26752 (Sigma), 0.1 μm carbenoxolone disodium salt (CBX) (Sigma), or 0.1 μm PF 915275 (PF) (Tocris Bioscience, Abingdon, UK).
Transient transfection
Primary HESCs, cultured in 12-well plates until confluency, were transfected using the ProFection mammalian transfection kit (Promega, Madison, WI), with 100 nm per well of the following small interfering RNA (siRNA) reagents (Dharmacon, Lafayette, CO): siCONTROL nontargeting (NT) siRNA pool, GR siGENOME SMARTpool siRNA, or MR siGENOME SMARTpool siRNA.
Western blot analysis
Whole-cell protein extracts were obtained by direct lysis in Laemmli buffer heated to 100 C. Proteins resolved by SDS-PAGE were transferred to a polyvinyl difluoride membrane (GE Healthcare, Uppsala, Sweden) and probed with antibodies raised against GR, 1:1000 (E-20: SC-1003; Santa Cruz Biotechnology, Inc., Santa Cruz, CA); MR, 1:1000 (H-300: SC-11412; Santa Cruz Biotechnology, Inc.); 11βHSD1, 1:1000 (ab83522; Abcam, Cambridge, UK); 11βHSD2, 1:2000 (ab80317; Abcam); P4 receptor (PR), 1:1000 (NCL-L-PGR-312; Leica Biosystems, Newcastle, UK); histone 3 lysine 9 (H3K9) trimethylation (H3K9me3), 1:1000 (49-1008; Invitrogen Ltd., Paisley, UK); dehydrogenase/reductase superfamily (DHRS)3, 1:1000 (15393-1-AP; Proteintech Group, Manchester, UK); and β-actin, 1:100,000 (A1978; Sigma). After incubation with horseradish peroxidase-conjugated secondary antibodies, 1:2500, (Roche Diagnostics, Mannheim, Germany), chemiluminescence was visualized using the ECL+ chemiluminescence detection kit (Amersham, Little Chalfont, UK).
Real-time quantitative (RTQ)-PCR
Total RNA was extracted from primary HESC cultures. After treatment with amplification grade deoxyribonuclease I (Invitrogen Ltd.), cDNA was generated using the SuperScript First-Strand Synthesis System for RT-PCR kit (Invitrogen Ltd.). Template quantification was performed with an ABI Step One System (Applied Biosystems, Foster City, CA) using Power SYBR Green PCR Master Mix (Applied Biosystems) as dye layer and the relative standard curve calculation method. RNA input variances were normalized against the levels of the L19 housekeeping gene, which encodes a ribosomal protein. All measurements were performed in duplicate. Specific primer pairs were designed using Primer3 software (http://frodo.wi.mit.edu): L19 sense, 5′-GCG GAA GGG TAC AGC CAA T-3′ and L19-R antisense, 5′-GCA GCC GGC GCA AA-3′; 11βHSD1 sense, 5′-AGC AAG TTT GCT TTG GAT GG-3′ and 11βHSD1 antisense, 5′-AGA GCT CCC CCT TTG ATG AT-3′; GR sense, 5′-CCC TAC CCT GGT GTC ACT GT-3′ and GR antisense, 5′-GGT CAT TTG GTC ATC CAG GT-3′; MR sense, 5′-GGC ACT CGC TGG CCT GGA TG-3′ and MR antisense, 5′-GTC TCC ATC GCT GCC TCG GC-3′; decidual prolactin (PRL) sense, 5′-AAG CTG TAG AGA TTG AGG AGC AAA C-3′ and decidual PRL antisense, 5′-TCA GGA TGA ACC TGG CTG ACT A-3′; IGF-binding protein 1 (IGFBP1) sense, 5′-CGA AGG CTC TCC ATG TCA CCA-3′ and IGFBP1 antisense, 5′-TGT CTC CTG TGC CTT GGC TAA AC-3′; wingless-type MMTV integration site family, member 4 (WNT4) sense, 5′-TCA GCC CAC AGG GCT TCC AGT-3′ and WNT4 antisense, 5′-CGG CTC GCC AGC ACG TCT TT-3′; F-box only protein 32 (FBXO32) sense, 5′-GCG GCA GTT TCG TGA GCG AC-3′ and FBXO32 antisense, 5′-GGG TGC AAT ATC CAT GGC GCT CTT-3′; glutamate receptor, ionotropic, 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid (GRIA1) sense, 5′-AAG GGG TCT GCC CTG AGA AAT CCA-3′ and GRIA1 antisense, 5′-ACG CCT GCC ACA TTG CTG AGG-3′; zinc-finger (ZNF)486 sense, 5′-CTG GAG GAG TGG CAT TGC CTG G-3′ and ZNF486 antisense, 5′-ACA CAC AAC TGG GGG TTT GGC AAT-3′; and DHRS3 sense, 5′-AGC GCG GCG CCA GAA AGA TT-3′ and DHRS3 antisense, 5′-TCA CCC ACC TTC TCC CGG ACG-3′.
Radio-thin layer chromatography
The activity of 11βHSD1 in HESCs was analyzed essentially as described in literature (21, 22). Briefly, the microsome-containing fraction of undifferentiated and decidualized HESCs was incubated with reduced nicotinamide adenine dinucleotide phosphate (NADPH) (1 mm) and [3H] E. [14C] F (3 × 103 dpm) was added to monitor procedural losses together with 50 μg of unlabeled F. The assay was performed under initial rate conditions, and less than 12% of the substrate was converted to product. Precursor and product steroids were separated by thin layer chromatography using chloroform: methanol (9:1, vol/vol). Retention factor values for F and E were 0.5 and 0.8, respectively. The amount of radioactivity was measured by scintillation spectrometry. Enzyme activity was determined as the amount of product formed per milligram of protein per hour after correction for procedural losses.
Microarray analysis
Genome-wide microarray analysis was performed on primary cultures established from four different patients. Each culture was first transfected either with NT-siRNA or siRNA oligos against MR or GR. The cells were then decidualized for 4 d with 8-bromo-cAMP, P4, and E. Total RNA was extracted using STAT-60 reagent (AMS Biotechnology, Abingdon, UK). RTQ-PCR was performed after first-strand cDNA synthesis to determine the level of GR and MR knockdown. RNA quality was analyzed on an Agilent 2100 bioanalyzer (Agilent Technologies, Waldbronn, Germany). Microarray analysis on total RNA samples was performed by UCL Genomics using Bioconductor version 2.0 and R version 2.9.0. The robust multiarray analysis algorithm was used to obtain normalized data and gene signals. This method performs within-chip and between-chip normalizations in a single step. Gene summaries are generated using the Affymetrix Expression Console software. The criteria used to generate lists of differentially expressed genes are based on standard filtering by fold change (±50% change) and include false discovery rate filter (P ≤ 0.05). Gene ontology annotation was performed using the Database for Annotation, Visualization, and Integrated Discovery Bioinformatics Resources 6.7 (SAIC-Frederick, Inc., Frederick, MD). Microarray data have been deposited in GEO, accession number GSE42538.
Confocal microscopy and lipid droplets staining
Primary HESCs cultured on glass slides were fixed with 4% paraformaldehyde (Sigma) and permeabilized by 0.1% Triton X-100 (BDH Chemicals, London, UK). BODIPY 493/503 (Invitrogen Ltd.) was applied at 1 μg/ml in PBS. BODIPY-stained samples had to be washed with PBS before imaging. 4,6-Diamidino-2-phenylindole was used to identify nuclei. We examined samples under epifluorescent optics, and digital images were obtained with a Zeiss 510 confocal laser scanning microscope (Zeiss, Oberkochen, Germany).
Statistical analysis
Data presented in this study are representative of four or more biological replicates (i.e. primary cultures established from different biopsies). Statistical analysis was performed using a Student's t test after normalization of the data. The level of significance was defined as P < 0.05.
Results
Expression of 11βHSDs, GR, and MR in undifferentiated and decidualized HESCs
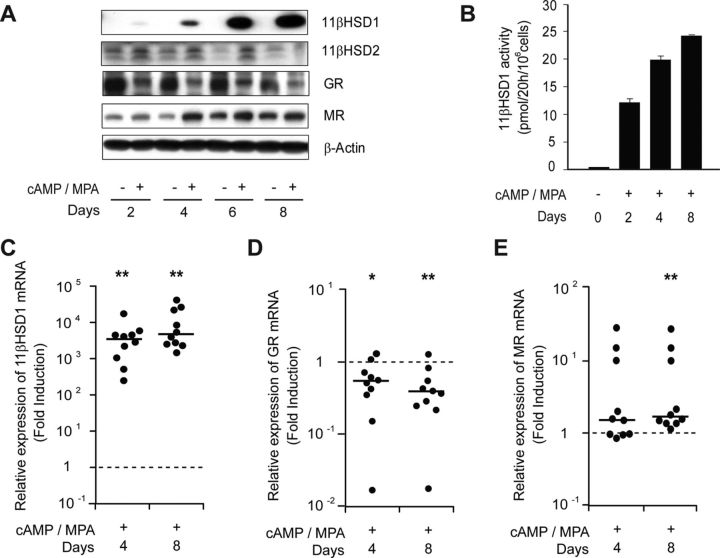
To examine whether corticosteroid signaling plays a role in decidualization, we first examined the expression levels of 11βHSD1, 11βHSD2, GR, and MR in undifferentiated HESCs and cells differentiated with 8-bromo-cAMP and MPA for 2, 4, 6, or 8 d. As shown in Fig. 1A, decidualization of HESCs was associated with a remarkable induction of 11βHSD1 in a time-dependent manner. This was paralleled by a strong increase in 11βHSD1 enzyme activity in decidualizing cells (Fig. 1B). In contrast, 11βHSD2 expression was low in HESCs, and in the absence of treatment with 8-bromo-cAMP and MPA, expression further declined in response to low-serum (2% DCC-FBS) culture conditions. Undifferentiated HESCs also abundantly express GR. However, treatment with 8-bromo-cAMP and MPA down-regulated this nuclear receptor (Fig. 1A). MR followed a reversed pattern with expression gradually rising in differentiating HESCs. Notably, culturing HESCs in low-serum conditions for several days was sufficient to alter MR and GR expression in opposite directions, although the effect was much more pronounced upon 8-bromo-cAMP and MPA treatment (Fig. 1A).
Fig. 1.
Expression of 11βHSD enzymes, GR, and MR in decidualizing HESCs. A, Primary HESCs were treated with 8-bromo-cAMP and MPA in time-course experiments lasting 8 d. Total protein lysates were harvested at the indicated time points and subjected to Western blot analysis for 11βHSD1, 11βHSD2, GR, and MR. β-Actin served as a loading control. B, 11βHSD1 activity was measured using radio-thin layer chromatography in HESCs treated with 8-bromo-cAMP and MPA for the indicated time points. C, Induction of 11βHSD1 mRNA in 10 independent primary cultures treated with 8-bromo-cAMP and MPA for either 4 or 8 d. The data show fold change in expression relative to levels in undifferentiated HESCs (dotted line). Note the logarithmic y-axis. The horizontal bar indicates median. *, P < 0.05; **, P < 0.001. D and E, The same sample set was analyzed for GR and MR, respectively.
We next examined the regulation of 11βHSD1 mRNA in response to differentiation signals in 10 independent primary cultures. As shown in Fig. 1C, 11βHSD1 transcript levels invariably increased by several orders of magnitude in response to 8-bromo-cAMP and MPA treatment. Furthermore, the down-regulation of GR and reciprocal induction of MR at protein level in decidualizing cells were reflected at transcript level (Fig. 1, D and E).
P4 drives the expression of 11βHSD1 in decidualizing HESCs
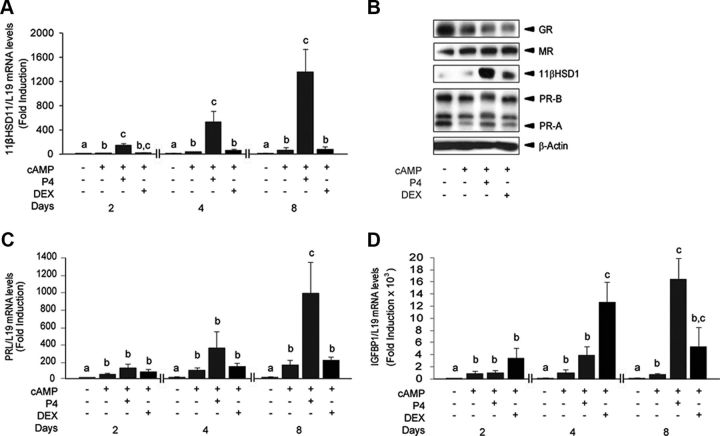
MPA is a progestin with significant glucocorticoid actions. We therefore used P4 and DEX to examine whether either PR or GR drives the induction of 11βHSD1 in differentiating HESCs. Time-course analysis demonstrated that, in contrast to P4, DEX had little or no effect on the induction of 11βHSD1 transcripts in 8-bromo-cAMP-treated cells (Fig. 2A). Compared with treatment with 8-bromo-cAMP alone, addition of DEX did up-regulate 11βHSD1 protein levels, although this response was much more pronounced with 8-bromo-cAMP and P4 (Fig. 2B). Western blot analysis also revealed that cAMP signaling drives the down-regulation of GR and reciprocal induction of MR in decidualizing cells (Fig. 2B). As previously reported (23), 8-bromo-cAMP reduces cellular levels of PR isoforms (PR-A and PR-B). Although cotreatment with P4 induces a mobility shift in PR migration on SDS-PAGE, reflecting ligand-dependent receptor phosphorylation, DEX had no effect (Fig. 2B). We also compared the induction of two decidual markers, PRL and IGFBP1, in primary cultures with 8-bromo-cAMP and either P4 or DEX. Unlike P4, DEX had no effect on cAMP-dependent induction of PRL transcripts in HESCs (Fig. 2C). However, DEX was more potent than P4 in enhancing IGFBP1 expression in cultures stimulated with 8-bromo-cAMP, especially during the early stages of the decidual process (Fig. 2D).
Fig. 2.
Glucocorticoids selectively modulate the expression of decidual marker genes. A, RTQ-PCR analysis of 11βHSD1 transcript levels in HESCs treated with 8-bromo-cAMP with or without P4 or DEX for 2, 4, and 8 d. The results show the fold change (mean ± sem) in 11βHSD1 transcript levels relative to vehicle control of four independent primary cultures. B, Western blot analysis of GR, MR, 11βHSD1, and PR proteins in whole-cell lysates obtained from primary HESCs treated with a combination of 8-bromo-cAMP with or without P4 or DEX for 4 d. β-Actin served as a loading control. C and D, Fold change (mean ± sem) in PRL and IGFBP1 mRNA levels, respectively, relative to vehicle control of four independent primary cultures. Different letters above the error bars indicate that those groups are significantly different from each other at P < 0.05.
To test whether 11βHSD1-dependent F biosynthesis modulates the expression of decidual marker genes, we decidualized primary HESCs with 8-bromo-cAMP and P4 in the presence or absence of E. Addition of E has no effect on the induction of PRL. However, it strongly enhanced the expression of IGFBP1 and, albeit less pronounced, 11βHSD1 transcripts (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Conversely, treatment of primary cultures with an 11βHSD1inhibitor (CBX or PF) attenuated the induction of IGFBP1 and HSD11B1 but not PRL (Supplemental Fig. 2). These observations underscore that 11βHSD1-dependent F biosynthesis impacts selectively on the expression of decidual marker genes.
Identification of GR- and MR-dependent genes in decidualizing HESCs
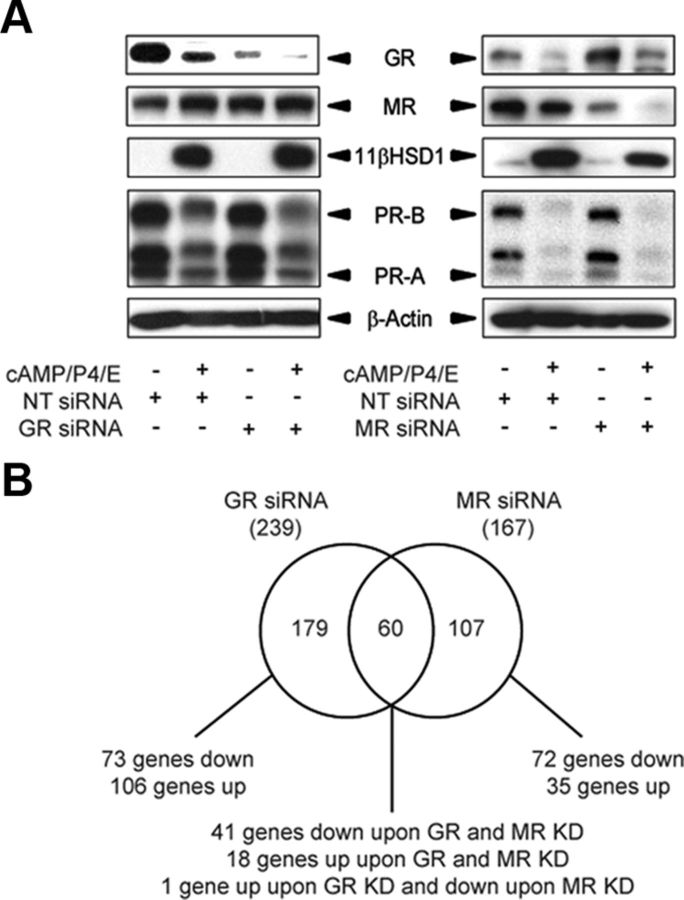
Next, we set out to identify GR- and MR-dependent genes responsive to endogenous F biosynthesis in differentiating HESCs. To do this, four individual primary cultures were first transfected with NT-, GR-, or MR-siRNA and then treated with 8-bromo-cAMP, P4, and E for 4 d. Total mRNA and protein lysates were extracted from parallel cultures. Western blot analysis was used to confirm GR and MR knockdown (Fig. 3A). GR knockdown had no impact on MR, 11βHSD1, or PR levels. In contrast, MR silencing seemed to hinder the induction of 11βHSD1 and modestly up-regulated GR levels.
Fig. 3.
Identification of GR- and MR-regulated genes in decidualizing HESCs. A, Western blot analysis of GR, MR, 11βHSD1, and PR expression in protein lysates extracted from primary cultures first transfected with NT-, GR-, or MR-siRNA and then treated with 8-bromo-cAMP, P4, and E for 4 d. β-Actin served as a loading control. C, Venn diagram showing the number of differentially expressed genes in decidualizing cells treated with 8-bromo-cAMP, P4, and E for 4 d in response to GR or MR knockdown.
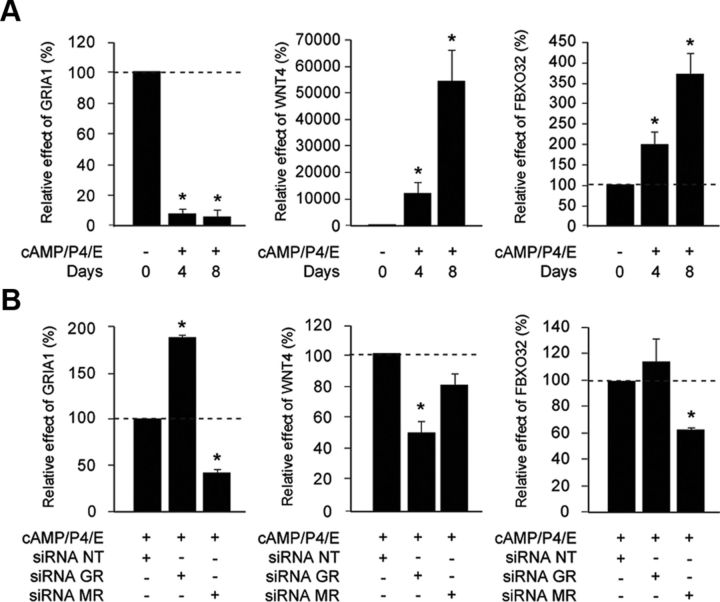
Total RNA extracted from decidualizing cultures transfected with NT-, GR-, or MR-siRNA was processed for whole genome microarray analysis. Using a cut-off of more than or equal to 1.5-fold change, 179 and 107 GR- and MR-selective genes, respectively, were identified (P < 0.05) (Fig. 3B). Interestingly, GR knockdown resulted in significantly more up- than down-regulated genes (106 vs. 73, respectively; χ2 test, P < 0.001), whereas MR knockdown had the opposite effect (up- and down-regulated genes: 35 vs. 72, respectively; χ2 test, P < 0.001). Supplemental Tables 1–4 list the genes induced or repressed by GR and MR. We also identified 60 genes under control of both nuclear receptors in decidualizing HESCs with only a single gene, GRIA1, regulated in an opposing manner (Supplemental Table 5). We chose GRIA1 as well as WNT4 and FBXO32 (putative GR- and MR-dependent genes, respectively) for initial validation of the array findings. Using independent cultures, we first monitored the expression of these genes in response to treatment with 8-bromo-cAMP, P4, and E for 4 or 8 d and then examined the impact of either GR or MR knockdown. GRIA1, which encodes for one of the four ionotropic 2-amino-3-(3-hydroxyl-5-methyl-isoxazol-4-yl)propanoicacid receptor subunits (24), is profoundly repressed in differentiating cells with transcript levels declining more than 90% after 4 d of treatment (Fig. 4A). This repression was partially relieved upon GR knockdown but enhanced in response to MR silencing (Fig. 4B). WNT4 and FBXO32 are both induced upon decidualization predominantly in a GR- and MR-dependent manner, respectively (Fig. 4). Interestingly, FBXO32, which encodes the E3 ubiquitin ligases Atrogin-1, was recently shown to be an 11βHSD1-dependent gene in skeletal muscle cells (25). In agreement, treatment of primary cultures with either CBX or PF inhibited FBXO32 and GRIA1 expression in decidualizing cells PRL (Supplemental Fig. 2). In case of GRIA1, this suggests that MR-dependent induction may be dominant over GR-dependent inhibition. However, 11βHSD1 inhibition had no significant effect on the induction of WNT4 in decidualizing cells. Thus, like other steroid hormone receptors, GR and MR modify decidual gene expression in a ligand-dependent and ligand-independent manner.
Fig. 4.
Expression and validation of putative GR- and MR-dependent genes in decidualizing HESCs. A, Expression of the putative GR- and MR-dependent genes in primary cultures treated 8-bromo-cAMP, P4, and E for the indicated time points. The results show mean fold change (±sem) relative to levels in undifferentiated cells (dotted lines). B, Independent primary cultures (n = 3) not used in the array analysis were first transfected with NT-, GR-, or MR-siRNA and subsequently treated with 8-bromo-cAMP, P4, and E for 4 d. Transcript levels were measured by RTQ-PCR and expressed as fold-induction (±sem) relative to expression levels in transfected HESCs with NT-siRNA before differentiation (dotted lines). *, P < 0.05.
GR limits the expression Krüppel-associated box domain containing ZNF (KRAB-ZNF) transcriptional repressors
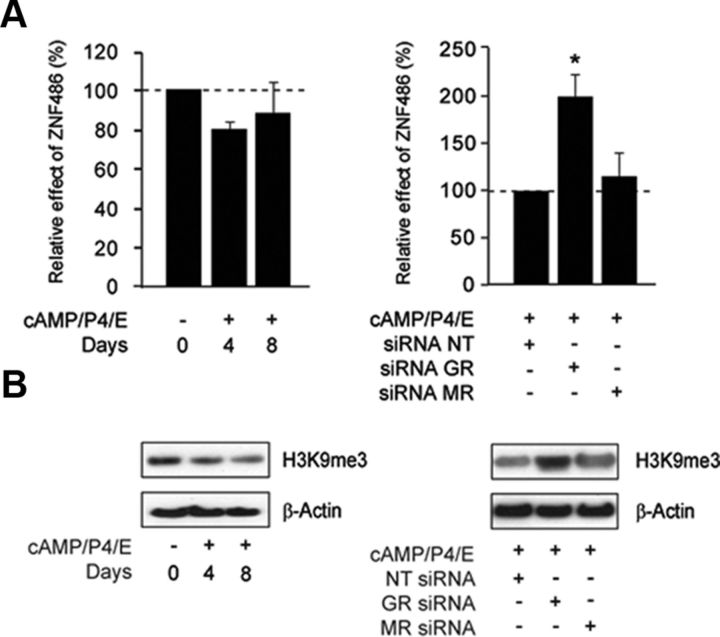
After correction for multiple testing (Benjamini and Hochberg false discovery rate), gene ontology annotation revealed that the genes repressed by GR were strongly enriched for KRAB-ZNF proteins (P < 0.0001). In fact, 18 members of this family of transcriptional repressors were up-regulated upon GR knockdown: ZNF91, ZNF92, ZNF100, ZNF253, ZNF254, ZNF311, ZNF420, ZNF484, ZNF486, ZNF585A, ZNF586, ZNF613, ZNF624, ZNF625, ZNF626, ZNF669, ZNF738, and ZNF791. Notably, the expression of five (ZNF181, ZNF223, ZNF254, ZNF625, and ZNF669) also increased upon MR knockdown (Supplemental Tables 4 and 5). Validation analyses demonstrated that the expression of ZNF486 transcripts declines modestly upon treatment of HESCs with 8-bromo-cAMP, P4, and E (Fig. 5A, left panel). In agreement with the array findings, GR knockdown selective up-regulated ZNF486 mRNA levels in differentiating HESCs by approximately 2-fold (Fig. 5A, right panel).
Fig. 5.
GR signaling plays a role in chromatin remodeling in decidualizing HESCs. A, ZNF486 transcript levels were measured in four primary cultures treated 8-bromo-cAMP, P4, and E for the indicated time points (left panel) as well as in cultures decidualized for 4 d after GR or MR knockdown (right panel). The results show mean fold change (±sem) relative to levels in undifferentiated cells (dotted lines). *, P < 0.05. B, Western blot analysis of H3K9me3 expression in total protein lysates from primary cultures treated 8-bromo-cAMP, P4, and E for the indicated time points (left panel) as well as from cultures decidualized for 4 d upon GR or MR knockdown (right panel). β-Actin served as a loading control.
KRAB-ZNF proteins block transcriptional initiation by recruiting a variety of chromatin modifiers to promoters of target genes, resulting in an increase in H3K9me3 (26, 27). We therefore decided to monitor this repressive chromatin mark to determine whether GR plays a role in chromatin remodeling that underpins the decidual phenotype (28). Western blot analysis revealed a gradual decline in global H3K9me3 levels in response to differentiation cues (Fig. 5B, left panel). Knockdown of GR, and perhaps to a lesser extent of MR, was sufficient to reverse this response (Fig. 5B, right panel).
MR is essential for retinoid metabolism and lipid droplet biogenesis
In contrast to GR, MR acts primarily as a transcriptional activator in decidualizing HESCs (Supplemental Tables 1 and 5). Perhaps the most striking observation is that several MR-induced genes encode for key enzymes involved in retinoid metabolism and cholesterol homeostasis, including retinol saturase, members of the short-chain DHRS (DHRS3, DHRS4, and DHRS4L2), the dehydrocholesterol reductase DHCR7, and the steroidogenic acute regulatory protein-related lipid transfer protein domain containing protein STARD5. To validate the array findings, we focused on DHRS3 (also known as retinal short-chain dehydrogenase/reductase 1). This enzyme was highly induced at both mRNA and protein level in a time-dependent manner upon treatment of primary HESCs with 8-bromo-cAMP, P4, and E (Fig. 6A). MR knockdown attenuated the induction of DHRS3 transcripts in decidualizing cells (Fig. 6B, right panel). To further explore MR dependency of DHRS3 expression in decidualizing cells, primary cultures were treated with 8-bromo-cAMP, P4, and either aldosterone or RU26752, a MR antagonist (29, 30). Although addition of aldosterone potentiated the induction DHRS3 in cells differentiated with 8-bromo-cAMP and P4, RU26752 completely abolished this response (Fig. 6B, right panel).
Fig. 6.
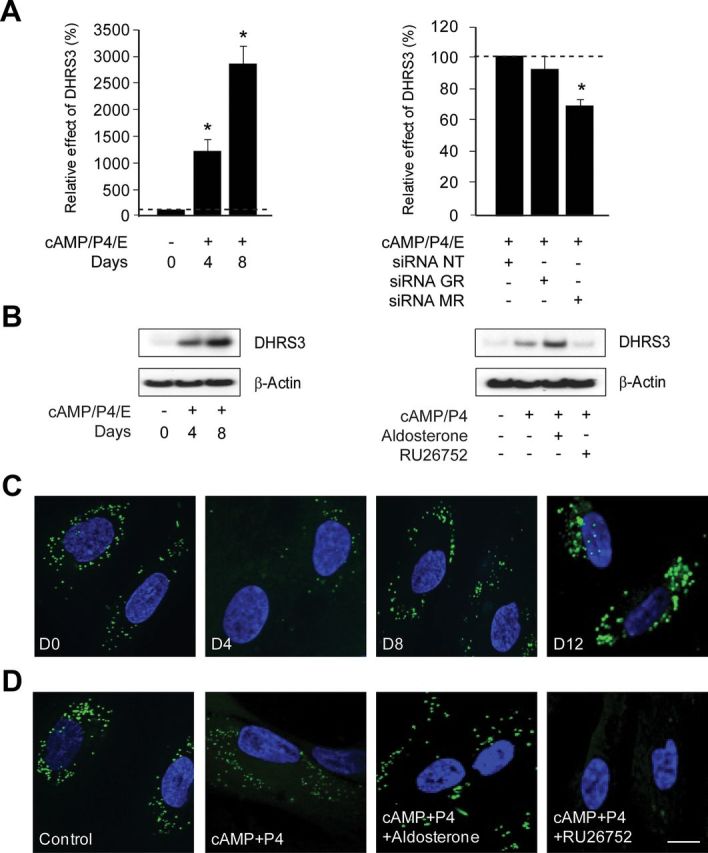
MR-dependent induction of DHRS3 in decidualizing HESCs is associated with dynamic changes in cytoplasmic lipid droplets. DHRS3 expression was examined in parallel primary cultures treated with 8-bromo-cAMP, P4, and E for the indicated time points as well as after GR or MR siRNA knockdown by RTQ-PCR (A) or Western blot analysis (B). DHRS3 expression was analyzed in four independent primary cultures. The left panel shows percentage change (±sem) in mRNA expression relative to levels in undifferentiated cells (dotted lines). C, Confocal micrographs showing lipid droplets stained with BODIPY (green) and nuclei stained with 4,6-diamidino-2-phenylindole (DAPI) (blue) in HESCs treated with 8-bromo-cAMP, P4, and E for the indicated time points. D, Representative confocal images of HESCs decidualized with 8-bromo-cAMP and P4 in the presence or absence of aldosterone or RU26752 for 8 d. Scale bar, 10 μm.
DHRS3 has recently been implicated in endoplasmic reticulum (ER)-derived lipid droplet formation (31). Loading of primary HESCs with a cell-permeable lipophilic fluorescence dye, BODIPY 493/503, followed by confocal microscopy demonstrated that decidualization is associated with highly dynamic changes in the appearance of these cytoplasmic lipid droplets. Undifferentiated HESCs contain numerous small droplets. Unexpectedly, these lipid droplets were consistently less abundant after 4 d of differentiation (Fig. 6C). By d 8, the droplets had reaccumulated especially near the periphery of the cells. Four days later, fewer but larger and more centrally localized lipid droplets were present in decidualizing HESCs (Fig. 6C). Next, we treated primary cultures with 8-bromo-cAMP, P4, and either aldosterone or RU26752 for 8 d. Interestingly, although addition of aldosterone favored the formation of larger droplets, RU26752 virtually abolished their presence all together (Fig. 6D).
Discussion
Elevated circulating P4 levels maintain the maternal decidual response in pregnancy and are therefore indispensable for survival of the fetus. However, circulating levels of androgens, aldosterone, and F also increase in early pregnancy (10, 32–34). Furthermore, the endometrium expresses the full complement of cognate receptors as well as a host of steroidogenic enzymes that will determine the local bioavailability of different ligands. For example, increased expression accounts for the higher conversion of testosterone to dihydrotestosterone in secretory compared with proliferative endometrium (35, 36). We now show that P4 drives by 11βHSD1 expression, which is further reinforced by F production and signaling. This positive feedback mechanism renders HSD11B1 one of the most highly induced genes upon HESC differentiation (18). We further show that decidualization is also associated with a rebalancing of corticosteroid receptors in favor of MR.
MR and GR show significant amino acid homology in their ligand- and DNA-binding domains with PR and androgen receptor (AR) (37). Together, these four receptors constitute the 3-ketosteroid receptor subfamily of nuclear receptors. The structural homology between these receptors implies that a degree of promiscuity may exist in the binding of various natural or synthetic ligands. This is indeed the case. For example, P4 binds MR, although the physiological consequences of this interaction, if any, are still unknown (38). Similarly, MPA, which unlike P4 does not have the propensity to partition onto glass and plastic in culture, is widely used to study PR responses in vitro, yet it is also a potent activator of GR and AR (35, 39). Structural homology also raises the possibility of functional redundancy between these receptors. In a previous study, we showed that this is not the case for PR and AR (35). By combining siRNA-mediated knockdown with genome-wide expression profiling, we showed that AR signaling is essential for cytoskeletal organization and cell cycle regulation in decidualizing cells. PR knockdown deregulated approximately nine times more genes than AR silencing (92 vs. 860 genes, respectively). Interestingly, a significant number of PR-regulated genes encode for membrane-bound receptors and intermediates in various signal transduction pathways, suggesting that P4 dependency of the decidual phenotype is, at least in part, accounted for by PR-dependent reprogramming of pathways activated by growth factors and cytokines (35).
We adopted a similar approach to identify MR- and GR-dependent genes in differentiating HESCs. However, we decided against using MPA, because it introduces a bias in favor of GR responses. Instead, we combined 8-bromo-cAMP with P4 and added E as the substrate for 11βHSD1 conversion. After 4 d of treatment, we identified 179 and 107 deregulated genes upon GR or MR knockdown, respectively. Like PR (35), GR represses significantly more genes than it induces in decidualizing cells. The number of up- and down-regulated genes upon AR knockdown is comparable, whereas MR functions primarily to promote the expression of certain decidual genes. Cross-referencing of the array data identified only 12 genes that are regulated by PR or AR as well as GR or MR (Supplemental Table 6).
Mining of the GR-dependent genes yielded some unexpected results. For example, GR represses the expression of SPP1 (osteopontin), a major component of the embryo-endometrial interactome (40), suggesting that glucocorticoid exposure during the window of implantation may interfere with embryo implantation. GR stimulated the induction of WNT4, a key component of P4 responses in both the uterus and breast (41, 42). Further, GR as well as MR signaling may be important for sustained cAMP activity in decidualizing cells by up-regulating the α-catalytic subunit of protein kinase A (PRKACA) (Supplemental Table 5).
However, the most striking observation was that GR activity attenuates the induction of 18 KRAB-ZNF proteins. With 675 encoding genes, the C2H2 zinc-finger proteins comprise the largest family of regulatory proteins in mammals, and 36% contain a KRAB domain (27, 43, 44). Over 50% of all human KRAB-ZFP genes are located in clusters on chromosome 19, including 15 of the 18 genes found to be regulated by GR in this study. The functions of these GR-repressed KRAB-ZFP genes are unknown with the exception of ZNF420, which encodes ATM and p53-associated krüppel type zinc finger protein, a negative regulator of p53-mediated apoptosis (45). However, it is well established that the KRAB domain confers a potent transcriptional repressor function by mediating specific interactions with a corepressor protein, krüppel-associated protein 1 (encoded by TRIM28), which in turn serves to recruit chromatin deacetylation machinery (46–48), as well as methyltransferase complexes (27). Because KRAB-ZNF transcription factors have been implicated in trimethylating H3K9 (27), we decided to monitor the cellular levels of this histone mark upon differentiation of HESCs. Perhaps somewhat fortuitously, this line of inquiry showed that decidualization is associated with a decline in global cellular H3K9me3 levels, which is disrupted upon GR knockdown.
Analysis of genes deregulated upon MR knockdown highlighted the dynamic changes in lipid droplet formation and retinoid metabolism that occur upon decidual transformation of HESCs. Although the MR dependency of these metabolic functions in decidualizing HESCs was unanticipated, it is in keeping with the observation that silencing of this nuclear receptor in murine adipocytes completely prevents lipid accumulation (49). In contrast, GR knockout only mildly impairs adipogenesis. Aldosterone has been shown to promote adipose conversion of 3T3-L1 and 3T3-F442A cells, whereas DEX inhibits terminal adipocyte maturation (50). Further, two MR-dependent genes in decidualizing HESCs, RETSAT and DHRS3, are strongly implicated in both intracellular lipid accumulation and retinoid metabolism (31, 51).
Retinoic acid (RA), the biologically active metabolite of vitamin A (retinol), is essential for embryogenesis and maintenance of pregnancy. Both RA deficiency and excess cause severe fetal malformation, suggesting that retinoid metabolism must be tightly controlled at the feto-maternal interface (52–54). RA is derived from oxidation of all-trans-retinaldehyde (retinal), an unstable intermediate that fluxes between retinol and RA. Because DHRS3 is a retinaldehyde reductase that promotes storage of retinol in lipid droplets, its level of expression may be an important mechanism to modulate local RA availability (31, 55). We now show that DHRS3 levels are low in undifferentiated HESCs, but expression increases markedly upon decidualization, in parallel with the induction of 11βHSD1. In fact, both enzymes are structurally related members of the SDR superfamily that localize to the ER (56). The terminal enzymes involved in the synthesis of lipid droplets also localize to the ER and often to droplets themselves, as is the case for DHRS3 (31). Thus, the dynamic changes in the appearance and abundance of lipid droplets in decidualizing HESCs may at least partly reflect the changing nature of ER-resident enzymes. Lipid droplets are often viewed as mere energy storage facilities, containing predominantly neutral lipids and various proteins (57). Based on our observations, it is tempting to speculate that the constituents and functions of these lipid droplets also change upon decidualization of HESCs, perhaps becoming more akin to the retinyl ester storage particles (retinosomes) present in the eye (58).
In summary, our observations suggest that decidualization of the endometrium promotes the formation of a corticosteroid gradient at the feto-maternal interface. Although F is virtually absent in the placenta due to the abundant expression of 11βHSD2 (59, 60), its production on the maternal side may exert important autocrine as well as paracrine functions. For example, local F biosynthesis could constitute a major mechanism that protects the fetal allograft against a potential maternal immune response. Importantly, our data also suggest that MR is a central regulator of the metabolic functions of the maternal decidua. This may be of particular importance to human pregnancy, because perfusion of the placenta is not established before 10–12 wk of gestation (61). Consequently, throughout the process of organogenesis (3–8 wk of pregnancy), fetal nutrition depends entirely on secretions produced by endometrial glands and decidualizing stroma (62, 63). The role of the endometrial 11βHSD1/GR/MR pathway in reproductive failure clearly warrants further investigation.
Acknowledgments
We thank all participating women. We also thank Mr. Kunal Shah for his valuable advice, Dr. Atul Purohit for his assistance with the radio-thin layer chromatography, and University College London Genomics for carrying out the microarray analysis.
This work was supported by the Biomedical Research Unit in Reproductive Health, a joint initiative between the University Hospitals Coventry and Warwickshire National Health Service Trust and Warwick Medical School. K.K. was supported by a Uehara Memorial Foundation Research fellowship and a Naito Foundation subsidy for interinstitutional researchers.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- 8-bromo-cAMP
- 8-bromoadenosine cAMP
- CBX
- carbenoxolone disodium salt
- DCC-FBS
- dextran-coated charcoal-treated fetal bovine serum
- DEX
- dexamethasone
- DHRS
- dehydrogenase/reductase superfamily
- E
- cortisone
- ER
- endoplasmic reticulum
- F
- cortisol
- FBXO32
- F-box only protein 32
- GR
- glucocorticoid receptor
- GRIA1
- glutamate receptor, ionotropic, 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid
- HESC
- human endometrial stromal cell
- H3K9
- histone 3 lysine 9
- H3K9me3
- H3K9 trimethylation
- 11βHSD
- 11β-hydroxysteroid dehydrogenase
- IGFBP1
- IGF-binding protein 1
- KRAB-ZNF
- Krüppel-associated box domain containing ZNF
- MPA
- medroxyprogesterone acetate
- MR
- mineralocorticoid receptor
- NT
- nontargeting
- P4
- progesterone
- PF
- PF 915275
- PR
- P4 receptor
- PRL
- prolactin
- RA
- retinoic acid
- RTQ
- real-time quantitative
- siRNA
- small interfering RNA
- WINT4
- wingless-type MMTV integration site family, member 4
- ZNF
- zinc-finger.
References
- 1. Michael AE , Papageorghiou AT. 2008. Potential significance of physiological and pharmacological glucocorticoids in early pregnancy. Hum Reprod Update 14:497–517 [DOI] [PubMed] [Google Scholar]
- 2. Quenby S , Kalumbi C , Bates M , Farquharson R , Vince G. 2005. Prednisolone reduces preconceptual endometrial natural killer cells in women with recurrent miscarriage. Fertil Steril 84:980–984 [DOI] [PubMed] [Google Scholar]
- 3. Ringler GE , Kallen CB , Strauss JF. 1989. Regulation of human trophoblast function by glucocorticoids: dexamethasone promotes increased secretion of chorionic gonadotropin. Endocrinology 124:1625–1631 [DOI] [PubMed] [Google Scholar]
- 4. Mandl M , Ghaffari-Tabrizi N , Haas J , Nöhammer G , Desoye G. 2006. Differential glucocorticoid effects on proliferation and invasion of human trophoblast cell lines. Reproduction 132:159–167 [DOI] [PubMed] [Google Scholar]
- 5. Bazer FW , Wu G , Spencer TE , Johnson GA , Burghardt RC , Bayless K. 2010. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod 16:135–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chard T. 1995. Cytokines in implantation. Hum Reprod Update 1:385–396 [DOI] [PubMed] [Google Scholar]
- 7. Kelly RW , King AE , Critchley HO. 2001. Cytokine control in human endometrium. Reproduction 121:3–19 [DOI] [PubMed] [Google Scholar]
- 8. Sharkey A. 1998. Cytokines and implantation. Rev Reprod 3:52–61 [DOI] [PubMed] [Google Scholar]
- 9. Aufdenblatten M , Baumann M , Raio L , Dick B , Frey BM , Schneider H , Surbek D , Hocher B , Mohaupt MG. 2009. Prematurity is related to high placental cortisol in preeclampsia. Pediatr Res 65:198–202 [DOI] [PubMed] [Google Scholar]
- 10. Gennari-Moser C , Khankin EV , Schüller S , Escher G , Frey BM , Portmann CB , Baumann MU , Lehmann AD , Surbek D , Karumanchi SA , Frey FJ , Mohaupt MG. 2011. Regulation of placental growth by aldosterone and cortisol. Endocrinology 152:263–271 [DOI] [PubMed] [Google Scholar]
- 11. Seckl JR , Meaney MJ. 2004. Glucocorticoid programming. Ann NY Acad Sci 1032:63–84 [DOI] [PubMed] [Google Scholar]
- 12. Courtney R , Stewart PM , Toh M , Ndongo MN , Calle RA , Hirshberg B. 2008. Modulation of 11β-hydroxysteroid dehydrogenase (11βHSD) activity biomarkers and pharmacokinetics of PF-00915275, a selective 11βHSD1 inhibitor. J Clin Endocrinol Metab 93:550–556 [DOI] [PubMed] [Google Scholar]
- 13. Ferrari P , Smith RE , Funder JW , Krozowski ZS. 1996. Substrate and inhibitor specificity of the cloned human 11β-hydroxysteroid dehydrogenase type 2 isoform. Am J Physiol 270:E900–E904 [DOI] [PubMed] [Google Scholar]
- 14. Arriza JL , Weinberger C , Cerelli G , Glaser TM , Handelin BL , Housman DE , Evans RM. 1987. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237:268–275 [DOI] [PubMed] [Google Scholar]
- 15. Yang K , Khalil MW , Strutt BJ , Killinger DW. 1997. 11β-hydroxysteroid dehydrogenase 1 activity and gene expression in human adipose stromal cells: effect on aromatase activity. J Steroid Biochem Mol Biol 60:247–253 [DOI] [PubMed] [Google Scholar]
- 16. Engeli S , Böhnke J , Feldpausch M , Gorzelniak K , Heintze U , Janke J , Luft FC , Sharma AM. 2004. Regulation of 11β-HSD genes in human adipose tissue: influence of central obesity and weight loss. Obes Res 12:9–17 [DOI] [PubMed] [Google Scholar]
- 17. McDonald SE , Henderson TA , Gomez-Sanchez CE , Critchley HO , Mason JI. 2006. 11β-hydroxysteroid dehydrogenases in human endometrium. Mol Cell Endocrinol 248:72–78 [DOI] [PubMed] [Google Scholar]
- 18. Takano M , Lu Z , Goto T , Fusi L , Higham J , Francis J , Withey A , Hardt J , Cloke B , Stavropoulou AV , Ishihara O , Lam EW , Unterman TG , Brosens JJ , Kim JJ. 2007. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol 21:2334–2349 [DOI] [PubMed] [Google Scholar]
- 19. Gellersen B , Brosens IA , Brosens JJ. 2007. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25:445–453 [DOI] [PubMed] [Google Scholar]
- 20. Leitao B , Jones MC , Fusi L , Higham J , Lee Y , Takano M , Goto T , Christian M , Lam EW , Brosens JJ. 2010. Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB J 24:1541–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Su X , Lawrence H , Ganeshapillai D , Cruttenden A , Purohit A , Reed MJ , Vicker N , Potter BV. 2004. Novel 18β-glycyrrhetinic acid analogues as potent and selective inhibitors of 11β-hydroxysteroid dehydrogenases. Bioorg Med Chem 12:4439–4457 [DOI] [PubMed] [Google Scholar]
- 22. Su X , Vicker N , Lawrence H , Smith A , Purohit A , Reed MJ , Potter BV. 2007. Inhibition of human and rat 11β-hydroxysteroid dehydrogenase type 1 by 18β-glycyrrhetinic acid derivatives. J Steroid Biochem Mol Biol 104:312–320 [DOI] [PubMed] [Google Scholar]
- 23. Brosens JJ , Hayashi N , White JO. 1999. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 140:4809–4820 [DOI] [PubMed] [Google Scholar]
- 24. Sugimoto M , Sasaki S , Watanabe T , Nishimura S , Ideta A , Yamazaki M , Matsuda K , Yuzaki M , Sakimura K , Aoyagi Y , Sugimoto Y. 2010. Ionotropic glutamate receptor AMPA 1 is associated with ovulation rate. PLoS One 5:e13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biedasek K , Andres J , Mai K , Adams S , Spuler S , Fielitz J , Spranger J. 2011. Skeletal muscle 11β-HSD1 controls glucocorticoid-induced proteolysis and expression of E3 ubiquitin ligases atrogin-1 and MuRF-1. PLoS One 6:e16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Groner AC , Meylan S , Ciuffi A , Zangger N , Ambrosini G , Dénervaud N , Bucher P , Trono D. 2010. KRAB-zinc finger proteins and KAP1 can mediate long-range transcriptional repression through heterochromatin spreading. PLoS Genet 6:e1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frietze S , O'Geen H , Blahnik KR , Jin VX , Farnham PJ. 2010. ZNF274 recruits the histone methyltransferase SETDB1 to the 3′ ends of ZNF genes. PLoS One 5:e15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grimaldi G , Christian M , Steel JH , Henriet P , Poutanen M , Brosens JJ. 2011. Down-regulation of the histone methyltransferase EZH2 contributes to the epigenetic programming of decidualizing human endometrial stromal cells. Mol Endocrinol 25:1892–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agarwal MK , Mirshahi M. 1999. General overview of mineralocorticoid hormone action. Pharmacol Ther 84:273–326 [DOI] [PubMed] [Google Scholar]
- 30. Kelly SP , Chasiotis H. 2011. Glucocorticoid and mineralocorticoid receptors regulate paracellular permeability in a primary cultured gill epithelium. J Exp Biol 214:2308–2318 [DOI] [PubMed] [Google Scholar]
- 31. Deisenroth C , Itahana Y , Tollini L , Jin A , Zhang Y. 2011. p53-Inducible DHRS3 is an endoplasmic reticulum protein associated with lipid droplet accumulation. J Biol Chem 286:28343–28356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Castracane VD , Stewart DR , Gimpel T , Overstreet JW , Lasley BL. 1998. Maternal serum androgens in human pregnancy: early increases within the cycle of conception. Hum Reprod 13:460–464 [DOI] [PubMed] [Google Scholar]
- 33. Cloke B , Christian M. 2012. The role of androgens and the androgen receptor in cycling endometrium. Mol Cell Endocrinol 358:166–175 [DOI] [PubMed] [Google Scholar]
- 34. Jensen E , Wood C , Keller-Wood M. 2002. The normal increase in adrenal secretion during pregnancy contributes to maternal volume expansion and fetal homeostasis. J Soc Gynecol Investig 9:362–371 [PubMed] [Google Scholar]
- 35. Cloke B , Huhtinen K , Fusi L , Kajihara T , Yliheikkilä M , Ho KK , Teklenburg G , Lavery S , Jones MC , Trew G , Kim JJ , Lam EW , Cartwright JE , Poutanen M , Brosens JJ. 2008. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology 149:4462–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ito K , Suzuki T , Akahira J , Moriya T , Kaneko C , Utsunomiya H , Yaegashi N , Okamura K , Sasano H. 2002. Expression of androgen receptor and 5α-reductases in the human normal endometrium and its disorders. Int J Cancer 99:652–657 [DOI] [PubMed] [Google Scholar]
- 37. Funder JW. 1997. Glucocorticoid and mineralocorticoid receptors: biology and clinical relevance. Annu Rev Med 48:231–240 [DOI] [PubMed] [Google Scholar]
- 38. Myles K , Funder JW. 1996. Progesterone binding to mineralocorticoid receptors: in vitro and in vivo studies. Am J Physiol 270:E601–E607 [DOI] [PubMed] [Google Scholar]
- 39. Selman PJ , Wolfswinkel J , Mol JA. 1996. Binding specificity of medroxyprogesterone acetate and proligestone for the progesterone and glucocorticoid receptor in the dog. Steroids 61:133–137 [DOI] [PubMed] [Google Scholar]
- 40. Altmäe S , Reimand J , Hovatta O , Zhang P , Kere J , Laisk T , Saare M , Peters M , Vilo J , Stavreus-Evers A , Salumets A. 2012. Research resource: interactome of human embryo implantation: identification of gene expression pathways, regulation, and integrated regulatory networks. Mol Endocrinol 26:203–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brisken C , Heineman A , Chavarria T , Elenbaas B , Tan J , Dey SK , McMahon JA , McMahon AP , Weinberg RA. 2000. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev 14:650–654 [PMC free article] [PubMed] [Google Scholar]
- 42. Franco HL , Dai D , Lee KY , Rubel CA , Roop D , Boerboom D , Jeong JW , Lydon JP , Bagchi IC , Bagchi MK , DeMayo FJ. 2011. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J 25:1176–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rousseau-Merck MF , Koczan D , Legrand I , Möller S , Autran S , Thiesen HJ. 2002. The KOX zinc finger genes: genome wide mapping of 368 ZNF PAC clones with zinc finger gene clusters predominantly in 23 chromosomal loci are confirmed by human sequences annotated in EnsEMBL. Cytogenet Genome Res 98:147–153 [DOI] [PubMed] [Google Scholar]
- 44. Vaquerizas JM , Kummerfeld SK , Teichmann SA , Luscombe NM. 2009. A census of human transcription factors: function, expression and evolution. Nat Rev Genet 10:252–263 [DOI] [PubMed] [Google Scholar]
- 45. Tian C , Xing G , Xie P , Lu K , Nie J , Wang J , Li L , Gao M , Zhang L , He F. 2009. KRAB-type zinc-finger protein Apak specifically regulates p53-dependent apoptosis. Nat Cell Biol 11:580–591 [DOI] [PubMed] [Google Scholar]
- 46. Moosmann P , Georgiev O , Le Douarin B , Bourquin JP , Schaffner W. 1996. Transcriptional repression by RING finger protein TIF1β that interacts with the KRAB repressor domain of KOX1. Nucleic Acids Res 24:4859–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schultz DC , Ayyanathan K , Negorev D , Maul GG , Rauscher FJ. 2002. SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev 16:919–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ayyanathan K , Lechner MS , Bell P , Maul GG , Schultz DC , Yamada Y , Tanaka K , Torigoe K , Rauscher FJ. 2003. Regulated recruitment of HP1 to a euchromatic gene induces mitotically heritable, epigenetic gene silencing: a mammalian cell culture model of gene variegation. Genes Dev 17:1855–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hoppmann J , Perwitz N , Meier B , Fasshauer M , Hadaschik D , Lehnert H , Klein J. 2010. The balance between gluco- and mineralo-corticoid action critically determines inflammatory adipocyte responses. J Endocrinol 204:153–164 [DOI] [PubMed] [Google Scholar]
- 50. Caprio M , Fève B , Claës A , Viengchareun S , Lombès M , Zennaro MC. 2007. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J 21:2185–2194 [DOI] [PubMed] [Google Scholar]
- 51. Schupp M , Lefterova MI , Janke J , Leitner K , Cristancho AG , Mullican SE , Qatanani M , Szwergold N , Steger DJ , Curtin JC , Kim RJ , Suh MJ , Suh M , Albert MR , Engeli S , Gudas LJ , Lazar MA. 2009. Retinol saturase promotes adipogenesis and is downregulated in obesity. Proc Natl Acad Sci USA 106:1105–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Niederreither K , Dollé P. 2008. Retinoic acid in development: towards an integrated view. Nat Rev Genet 9:541–553 [DOI] [PubMed] [Google Scholar]
- 53. Xia HF , Ma JJ , Sun J , Yang Y , Peng JP. 2010. Retinoic acid metabolizing enzyme CYP26A1 is implicated in rat embryo implantation. Hum Reprod 25:2985–2998 [DOI] [PubMed] [Google Scholar]
- 54. Han BC , Xia HF , Sun J , Yang Y , Peng JP. 2010. Retinoic acid-metabolizing enzyme cytochrome P450 26a1 (cyp26a1) is essential for implantation: functional study of its role in early pregnancy. J Cell Physiol 223:471–479 [DOI] [PubMed] [Google Scholar]
- 55. Cerignoli F , Guo X , Cardinali B , Rinaldi C , Casaletto J , Frati L , Screpanti I , Gudas LJ , Gulino A , Thiele CJ , Giannini G. 2002. retSDR1, a short-chain retinol dehydrogenase/reductase, is retinoic acid-inducible and frequently deleted in human neuroblastoma cell lines. Cancer Res 62:1196–1204 [PubMed] [Google Scholar]
- 56. Maser E , Oppermann UC. 1997. Role of type-1 11β-hydroxysteroid dehydrogenase in detoxification processes. Eur J Biochem 249:365–369 [DOI] [PubMed] [Google Scholar]
- 57. Goodman JM. 2008. The gregarious lipid droplet. J Biol Chem 283:28005–28009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Orban T , Palczewska G , Palczewski K. 2011. Retinyl ester storage particles (retinosomes) from the retinal pigmented epithelium resemble lipid droplets in other tissues. J Biol Chem 286:17248–17258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shams M , Kilby MD , Somerset DA , Howie AJ , Gupta A , Wood PJ , Afnan M , Stewart PM. 1998. 11β-hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction. Hum Reprod 13:799–804 [DOI] [PubMed] [Google Scholar]
- 60. McCalla CO , Nacharaju VL , Muneyyirci-Delale O , Glasgow S , Feldman JG. 1998. Placental 11β-hydroxysteroid dehydrogenase activity in normotensive and pre-eclamptic pregnancies. Steroids 63:511–515 [DOI] [PubMed] [Google Scholar]
- 61. Jauniaux E , Watson AL , Hempstock J , Bao YP , Skepper JN , Burton GJ. 2000. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 157:2111–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Burton GJ , Scioscia M , Rademacher TW. 2011. Endometrial secretions: creating a stimulatory microenvironment within the human early placenta and implications for the aetiopathogenesis of preeclampsia. J Reprod Immunol 89:118–125 [DOI] [PubMed] [Google Scholar]
- 63. Spiegler E , Kim YK , Wassef L , Shete V , Quadro L. 2012. Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim Biophys Acta 1821:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]